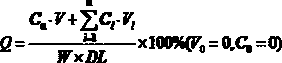Ivermectin controlled-release capsule and preparation method and application thereof
A slow-release technology for ivermectin, which is applied in the field of preparation of animal drugs, can solve the problems of only oral bioavailability, low blood drug concentration, and poor treatment effect on ectoparasites, so as to improve the drug protection rate and enhance Therapeutic, particle uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Follow the steps below to prepare ivermectin sustained-release microcapsules:
[0047] S1. Weigh 0.2% ivermectin, 38.8% hydroxypropyl-β-cyclodextrin, 60% PVAP (polyethylene phthalate) according to the proportion, heat and melt hydroxypropyl-β-cyclodextrin at 90°C , mixed evenly to obtain a mixture;
[0048] S2. The ivermectin raw material is dissolved in ethanol and added to the mixture described in step S1, and stirred evenly to obtain the mixture;
[0049] S3. Add PVAP (polyethylene phthalate) to the mixture described in step S2, and stir evenly at 40°C to obtain a mixture;
[0050] S4. Cool the mixture described in step S3 to 65-80°C, and carry out fluidized bed spray granulation, the temperature of fluidized air is 20°C, and the linear velocity is 55 m / s;
[0051] S5. Cool the powder described in step S4, sieve through a 40-mesh sieve, and collect to obtain 0.2% ivermectin sustained-release microcapsules with a particle size of about 400 μm.
Embodiment 2
[0053] Follow the steps below to prepare ivermectin sustained-release microcapsules:
[0054] S1. According to the proportion, weigh 0.4% ivermectin, 44.6% HPMC (hydroxypropyl methylcellulose), 55% acrylic resin No. II, heat and melt HPMC (hydroxypropylmethylcellulose) at 95°C, and mix evenly to obtain a mixture ;
[0055] S2. The ivermectin raw material is dissolved in ethanol and added to the mixture described in step S1, and stirred evenly to obtain the mixture;
[0056] S3. Add acrylic resin No. II to the mixture described in step S2, and stir evenly at 50° C. to obtain a mixture;
[0057] S4. Cool the mixture described in step S3 to 65-80°C, and carry out fluidized bed spray granulation, the temperature of fluidized air is 18°C, and the linear velocity is 135 m / s;
[0058] S5. Cool the powder described in step S4, sieve through a 40-mesh sieve, and collect to obtain 0.4% ivermectin sustained-release microcapsules with a particle size of about 80 μm.
Embodiment 3
[0060] Follow the steps below to prepare ivermectin sustained-release microcapsules:
[0061] S1. Weigh 0.6% ivermectin, 45.4% bile salt / phospholipid mixed micelles, 54% HPMCAS (hydroxypropyl methylcellulose acetate succinate) according to the proportion, heat and melt the bile salt / phospholipid mixed micelles at 100°C , mixed evenly to obtain a mixture;
[0062] S2. The ivermectin raw material is dissolved in ethanol and added to the mixture described in step S1, and stirred evenly to obtain the mixture;
[0063] S3. Add HPMCAS (hydroxypropylmethylcellulose acetate succinate) to the mixture described in step S2, and stir evenly at 50°C to obtain a mixture;
[0064] S4. Cool the mixture described in step S3 to 65-80°C, and carry out fluidized bed spray granulation, the temperature of fluidized air is 25°C, and the linear velocity is 100 m / s;
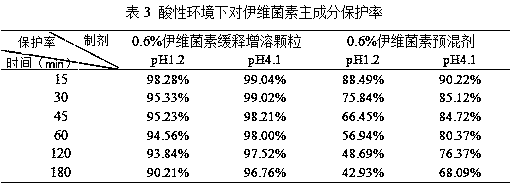
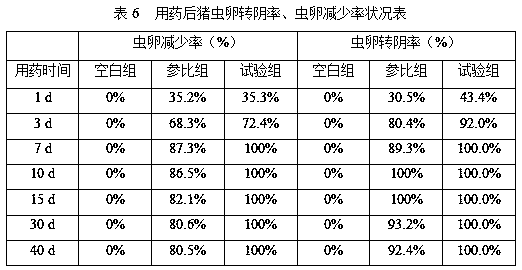
[0065] S5. Cool the powder described in step S4, sieve through a 40-mesh sieve, and collect to obtain 0.6% ivermectin sustained-relea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com