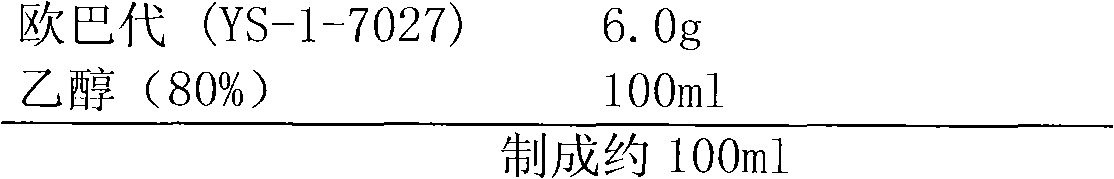Febuxostat tablet and preparation method thereof
A technology of febuxostat and tablets, which is applied in the field of medicine, can solve the problems of febuxostat that the medicinal effect cannot meet the ideal requirements, high medicinal content of febuxostat, and low bioavailability, and achieve improved Effects of bioavailability, quality control, enhanced solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Tablet prescription:
[0032]
[0033] Adhesive
[0034]
[0035] Coating Solution Prescription
[0036]
[0037] Preparation Process:
[0038] 1. Mix the prescribed amount of febuxostat, lactose and sodium lauryl sulfate evenly, and then pulverize it with an ultrafine pulverizer to control the average particle size below 50 μm;
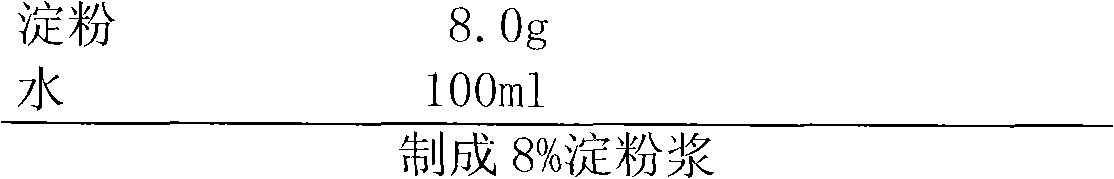
[0039] 2. Mix the pulverized mixture with the prescribed amount of microcrystalline cellulose and 10 g of carboxymethyl starch sodium evenly, then add 8% of the binder to make soft material from starch slurry, and pass through a 18-mesh sieve to make wet granules;
[0040] 3. Put the wet granules in a drying oven, and dry them with blast at 50-60°C;
[0041] 4. Pass the dry granules through a 18-mesh sieve for granulation, add the prescribed amount of lubricant and the remaining sodium starch glycolate, mix well, and press into tablets to obtain tablet cores;
[0042] 5. Coating with Opadry ethanol solution, the weight gain of the c...
example 2
[0047] Tablet core prescription
[0048]
[0049] Adhesive
[0050]
[0051] Coating Solution Prescription:
[0052]
[0053]
[0054] Preparation Process
[0055] 1. Mix the prescribed amount of febuxostat, pre-crossed starch and Tween evenly, and then pulverize it with an ultrafine pulverizer, and control the average particle size below 30 μm;
[0056] 2. After mixing the pulverized mixture with the prescription amount of microcrystalline cellulose and 10g sodium starch glycolate / , add adhesive 8% starch slurry to make a soft material, and pass through a 18-mesh sieve to make wet granules;
[0057] 3. Put the wet granules in a drying oven, and dry them with blast at 50-60°C;
[0058] 4. Pass the dry granules through a 18-mesh sieve for granulation, add the prescribed amount of lubricant and the remaining sodium starch glycolate, mix well, and press into tablets to obtain tablet cores;
[0059] 5. Coating with Opadry ethanol solution, the weight gain of the co...
example 3
[0064] Tablet prescription:
[0065]
[0066] Adhesive
[0067]
[0068] Coating Solution Prescription
[0069]
[0070] craft
[0071] 1. Mix the prescribed amount of febuxostat, lactose and sodium lauryl sulfate evenly, and then pulverize it with an ultrafine pulverizer, and control the average particle size below 30 μm;
[0072] 2. Mix the pulverized mixture with the prescribed amount of microcrystalline cellulose and 10 g of carboxymethyl starch sodium evenly, then add 8% of the binder to make soft material from starch slurry, and pass through a 18-mesh sieve to make wet granules;
[0073] 3. Put the wet granules in a drying oven, and dry them with blast at 50-60°C;
[0074] 4. Pass the dry granules through a 18-mesh sieve for granulation, add the prescribed amount of lubricant and the remaining sodium starch glycolate, mix well, and press into tablets to obtain tablet cores;
[0075] 5. Coating with Opadry ethanol solution, the weight gain of the coating is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com