Liquid preparation containing Vonoprazan
A liquid preparation, the technology of vonoprazan fumarate, which is applied in the field of medicine, can solve the problems that the drug effect is not as obvious as the injection, and can not achieve good results, so as to achieve easy access to excipients, improved stability, stable and reliable process control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment one (anti-metal ion complexation-pH investigation)
[0021] prescription
[0022] Name of raw material
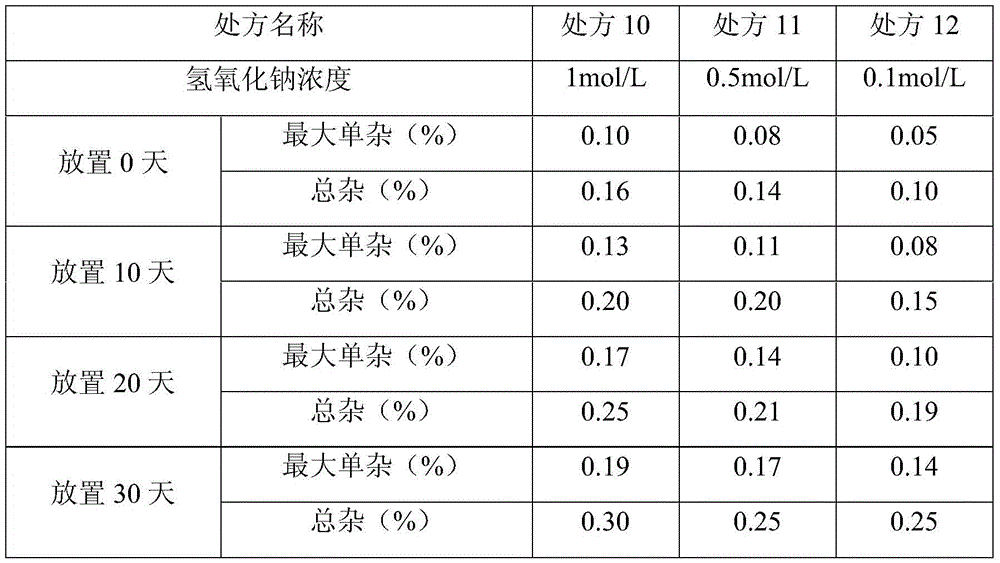
[0023] Prepare injections according to the above prescriptions, and investigate the anti-metal ion activity at different pHs. ), prescription 5 (pH8.0).
[0024] craft
[0025] The processes of prescription 1 to prescription 5 are all the same, only the difference in pH, the specific process is as follows:
[0026] Weigh the vinoprazan fumarate in a glass beaker, add water for injection, and stir the mixture with a stirrer to dissolve them. Add 1 mol / L sodium hydroxide aqueous solution, and adjust the mixture to the corresponding value of the prescription.
[0027] This experiment investigated the content precipitation of vinoprazan fumarate injection after 30 days storage.
[0028] prescription name
[0029] From the above results, it can be seen that the anti-metal ion activity of the preparations at pH 7.0 and pH 8.0 is higher t...
Embodiment 2
[0030] Embodiment two (anti-metal ion complexation-EDTA-Na dosage investigation)
[0031] prescription
[0032] Name of raw material
[0033] The process of prescription 6 to prescription 7 is the same, only the amount of EDTA-Na is different, the specific process is as follows:
[0034] Weigh vinorazan fumarate and EDTA-Na in a glass beaker, add water for injection, and stir the mixture with a stirrer to dissolve them. A 1 mol / L aqueous sodium hydroxide solution was added to adjust the mixture to 7.0.
[0035] This experiment investigated the content precipitation of vinoprazan fumarate injection after 30 days storage.
[0036] prescription name
[0037] As can be seen from the above results, when the pH was 7.0, the EDTA-Na dosage only needed 5 mg (0.1% w / v) to satisfy the anti-metal ion complexation, so the present invention adopts EDTA-Na dosage as 0.1% w / v.
Embodiment 3
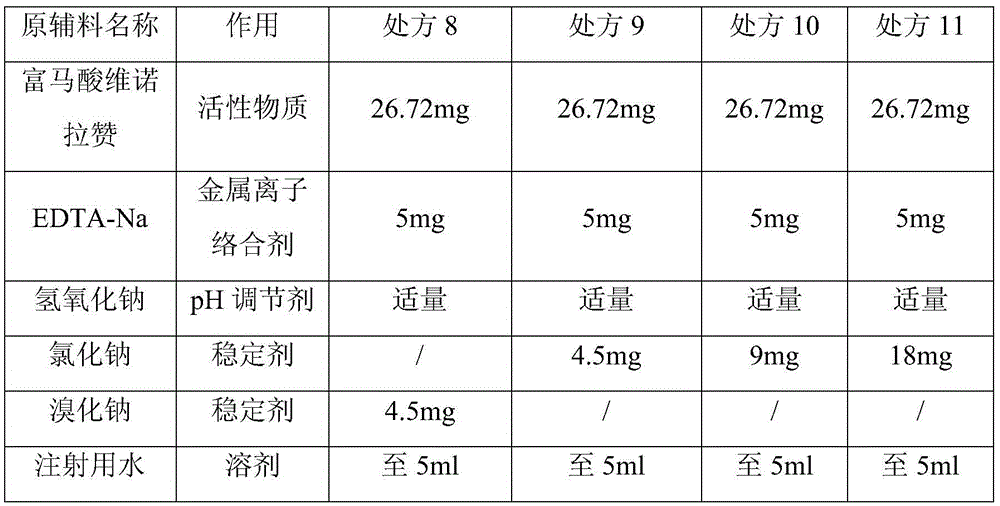
[0038] Embodiment three (stabilizer-inorganic salt dosage investigation)
[0039] prescription
[0040]
[0041] Weigh vinorazan fumarate, EDTA-Na, and halides in a glass beaker, add water for injection, and stir the mixture with a stirrer to dissolve them. A 1 mol / L aqueous sodium hydroxide solution was added to adjust the mixture to 7.0.
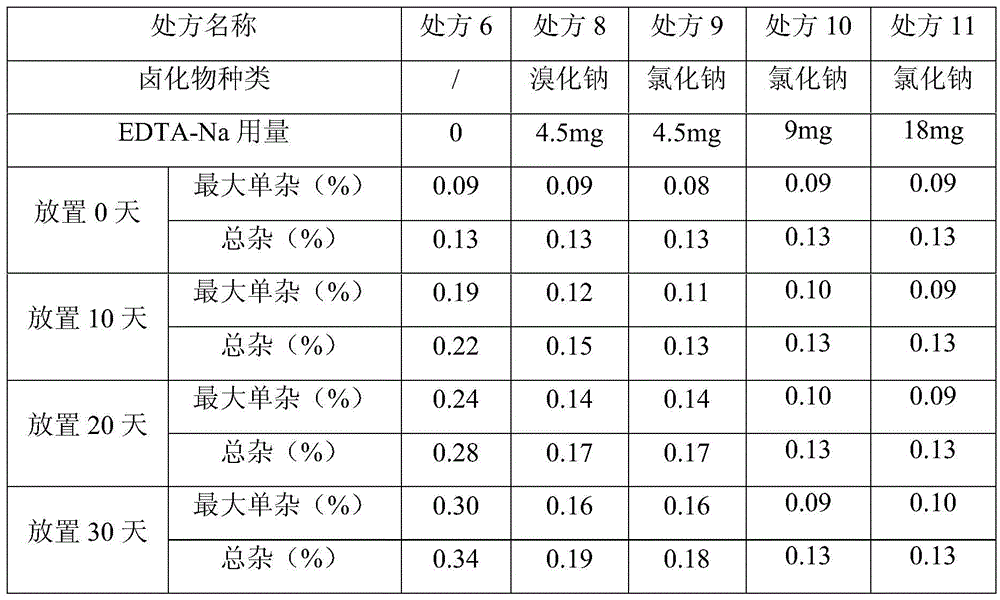
[0042] This experiment investigated the changes of related substances in vinoprazan fumarate injection placed at 60°C for 30 days.
[0043]
[0044] As can be seen from the above results, the use of sodium bromide and sodium chloride has no significant impact on the stability of the preparation, and the amount of sodium chloride has a significant impact on the stability. When the amount of sodium chloride is 9mg (0.18%), good stability can be achieved. Effect, so the present invention adopts sodium chloride consumption to be 0.18%w / v.
[0045]The Wonorazan liquid preparation of the present invention, by investigating the high-temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com