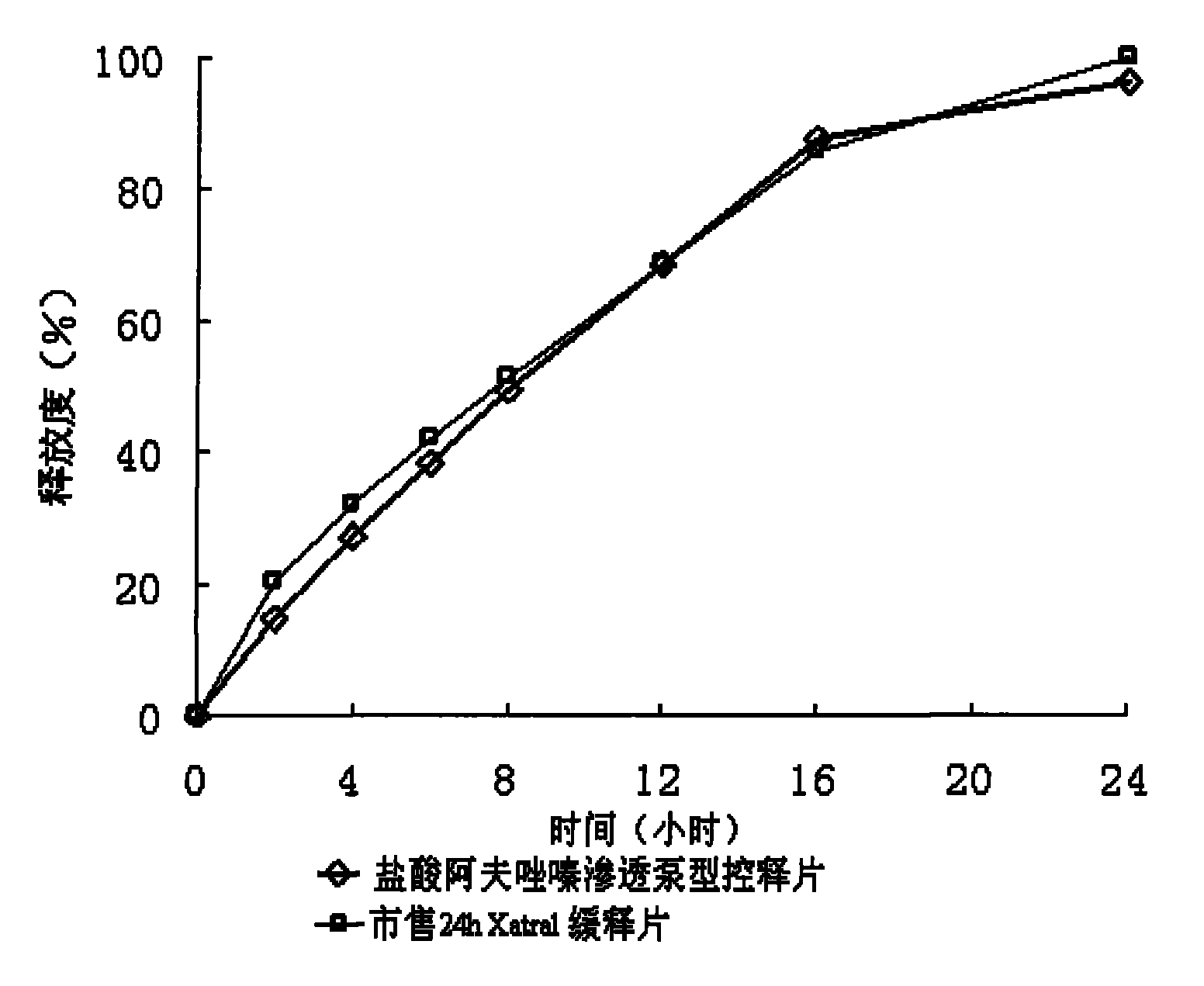
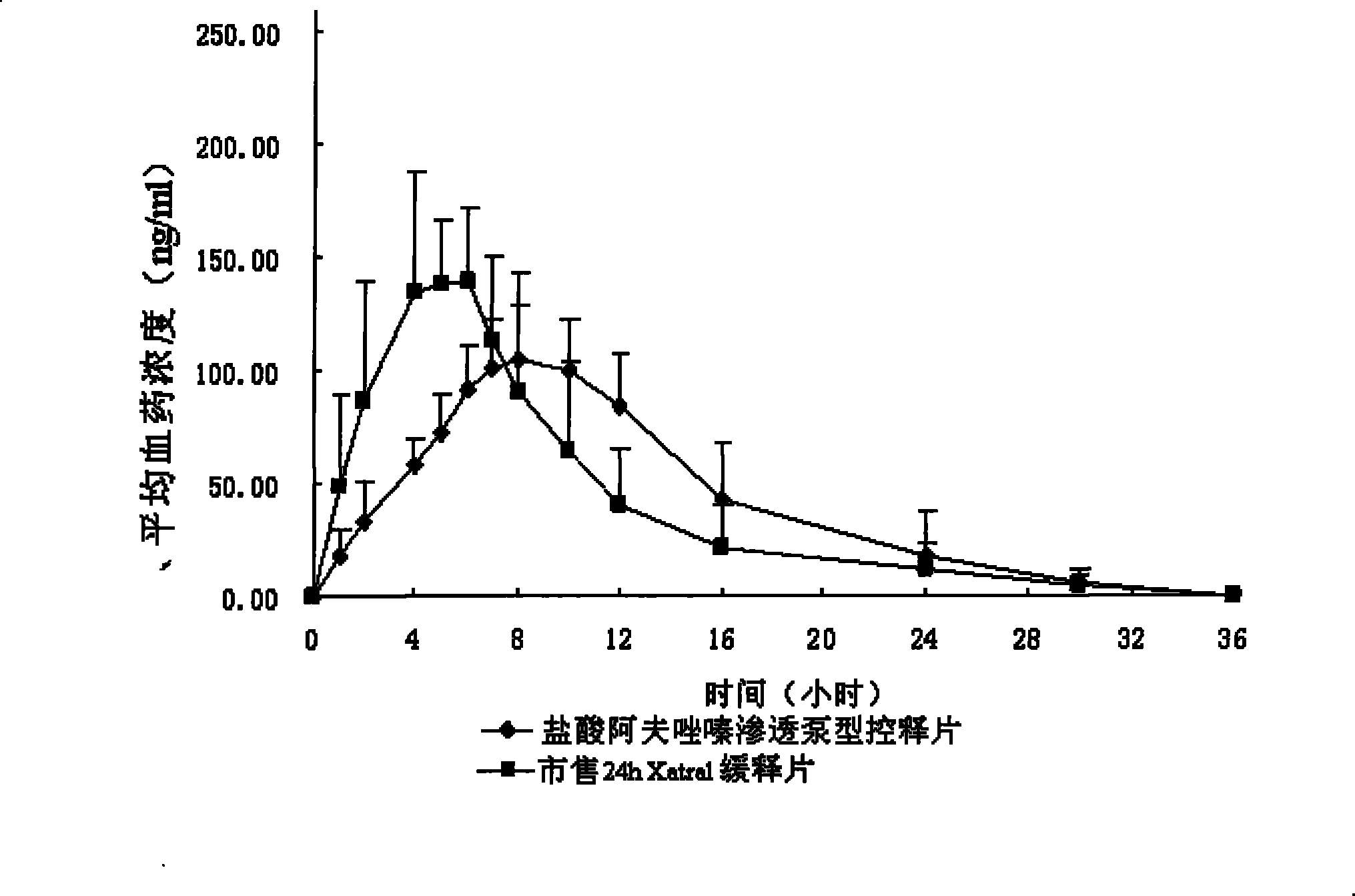
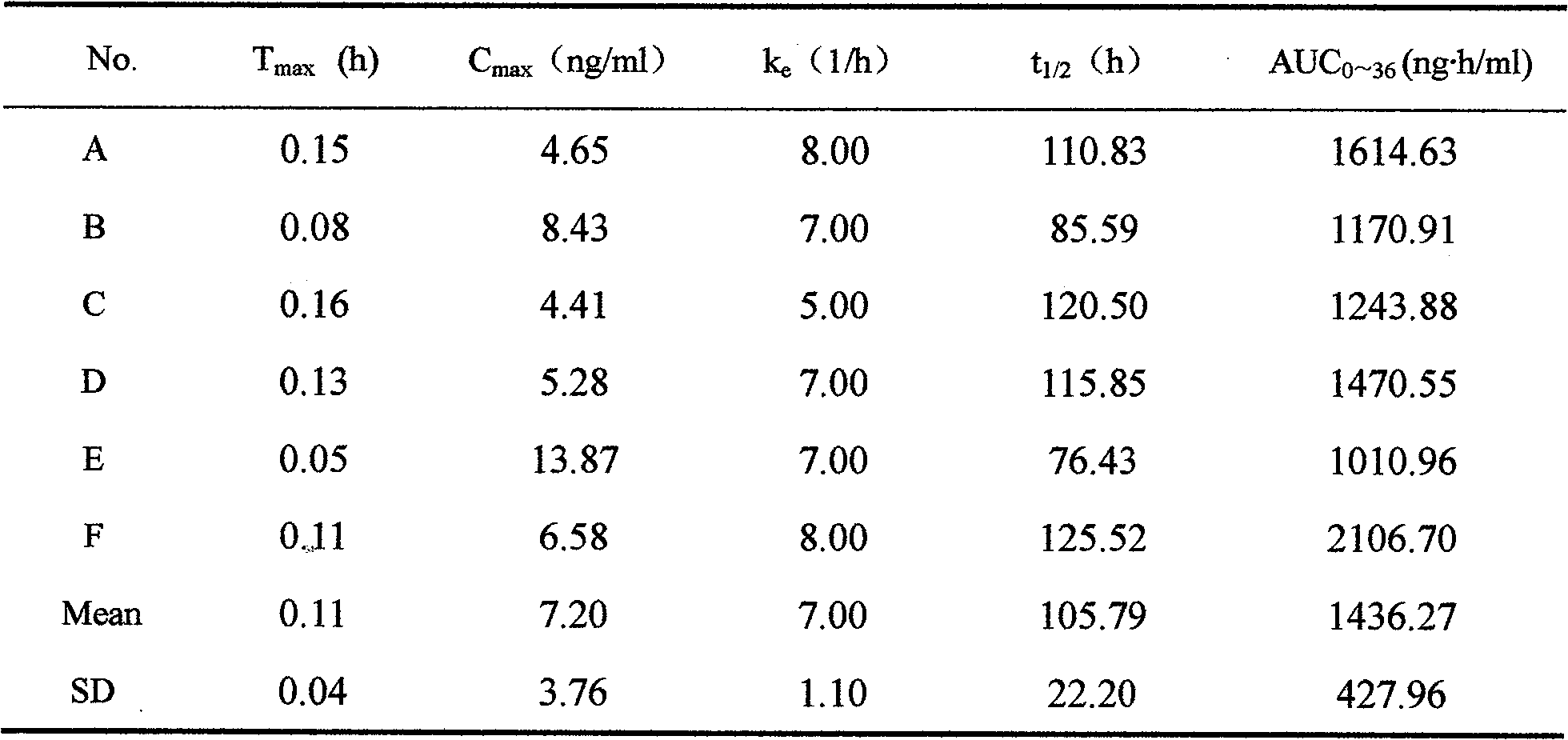
Alfuzosin Hydrochloride permeating pump type controlled-release preparation and method for preparing the same
A controlled-release technology of alfuzosin hydrochloride and osmotic pump, which is applied in the field of medicine, can solve the problems of good water solubility of alfuzosin hydrochloride, difficulty in controlling the drug release rate, and large batch-to-batch differences to achieve drug efficacy Long maintenance time, convenient administration and taking, and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Tablet prescription:
[0029] Alfuzosin Hydrochloride 10.0g
[0030] Lactose 40.0g
[0031] Microcrystalline Cellulose 40.0g
[0032] Magnesium stearate 0.25g
[0033] Tablet core preparation: mix the excipients and main ingredients through a 100-mesh sieve, make a soft material with 95% ethanol, granulate through a 18-mesh sieve, ventilate and dry in an oven at 40-45°C, add magnesium stearate, pass through a 16-mesh sieve, mix and prepare Granules, compressed tablets.
[0034] Coating Solution Prescription:
[0035] Cellulose acetate 100g
[0036] Macrogol 400 4g
[0037]Dibutyl phthalate 4g
[0038] Acetone 5L
[0039] Coating process: Put the tablet core in the coating pan, blow in hot air, and coat the tablet core when the temperature is about 45°C. The flow rate of the coating solution is 5-7mL / min, and the temperature in the coating pan is about 45°C until the thickness of the outer coating film of the tablet core reaches a weight gain of 4.0-4.5%, contin...
Embodiment 2
[0041] Tablet prescription:
[0042] Alfuzosin Hydrochloride 10.0g
[0043] Sucrose 35.0g
[0044] Lactose 45.0g
[0045] Magnesium stearate 0.25g
[0046] Tablet core preparation, with embodiment 1;
[0047] Coating Solution Prescription:
[0048] Cellulose acetate 100g
[0049] Macrogol 1500 5g
[0050] Dibutyl phthalate 5g
[0051] Acetone 5L
[0052] Coating process, with embodiment 1.
Embodiment 3
[0054] Tablet prescription:
[0055] Alfuzosin Hydrochloride 10.0g
[0057] Pregelatinized starch 40.0g
[0058] Magnesium stearate 0.25g
[0059] Tablet core preparation: same as Example 1.
[0060] Coating Solution Prescription:
[0061] Cellulose acetate 100g
[0062] Macrogol 400 4.5g
[0063] Dibutyl phthalate 4g
[0064] Acetone 5L
[0065] Coating process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com