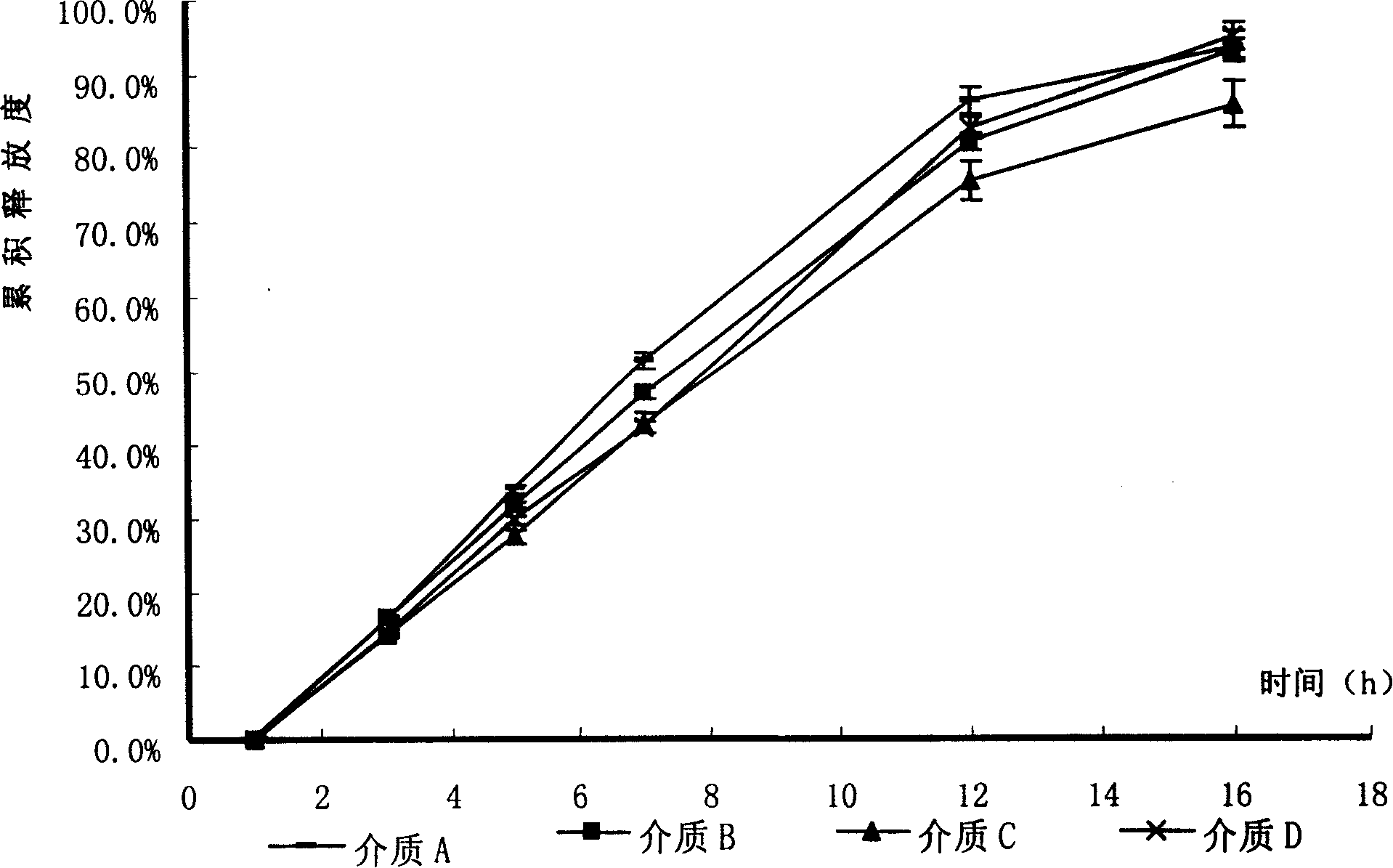
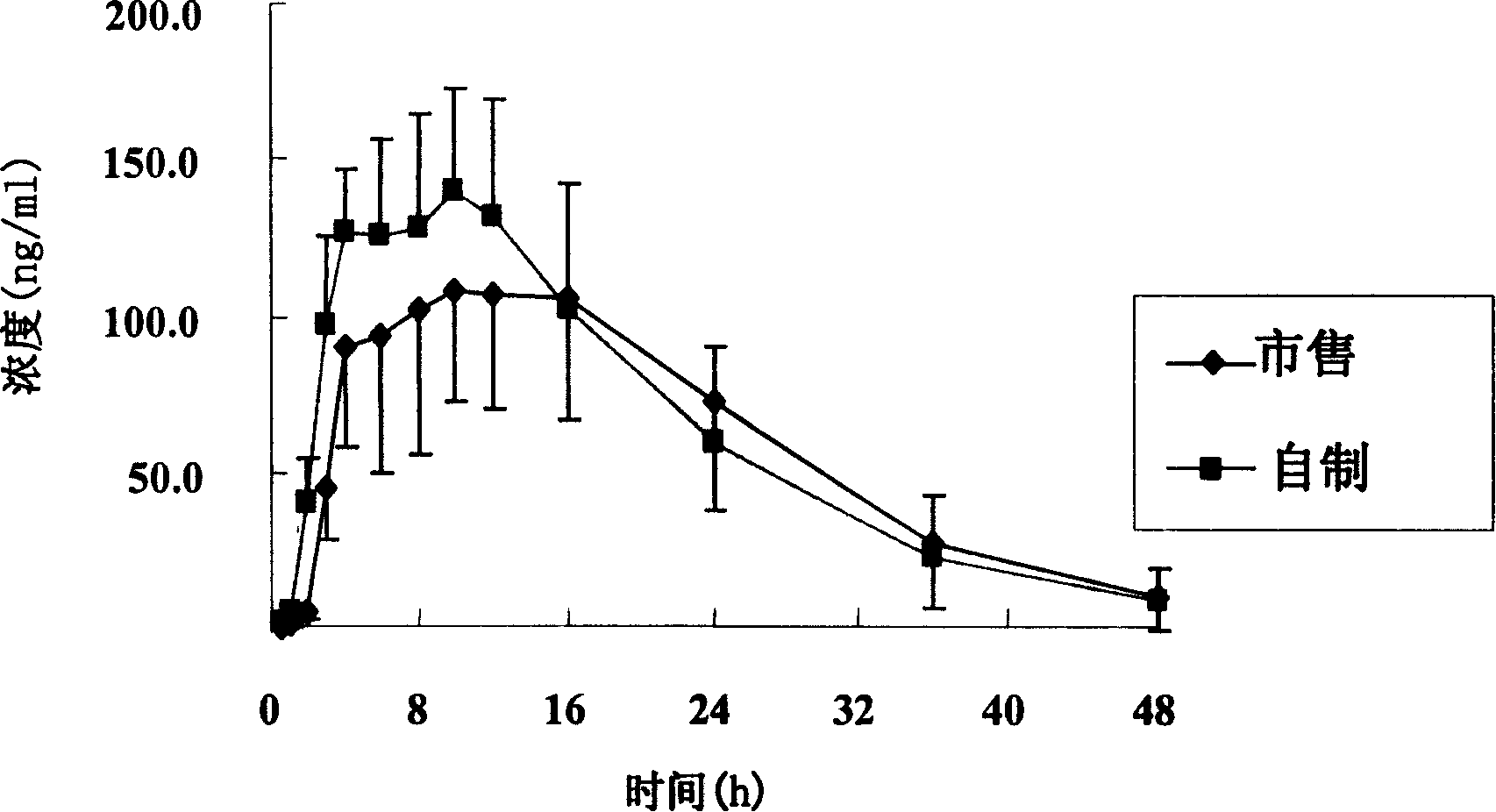
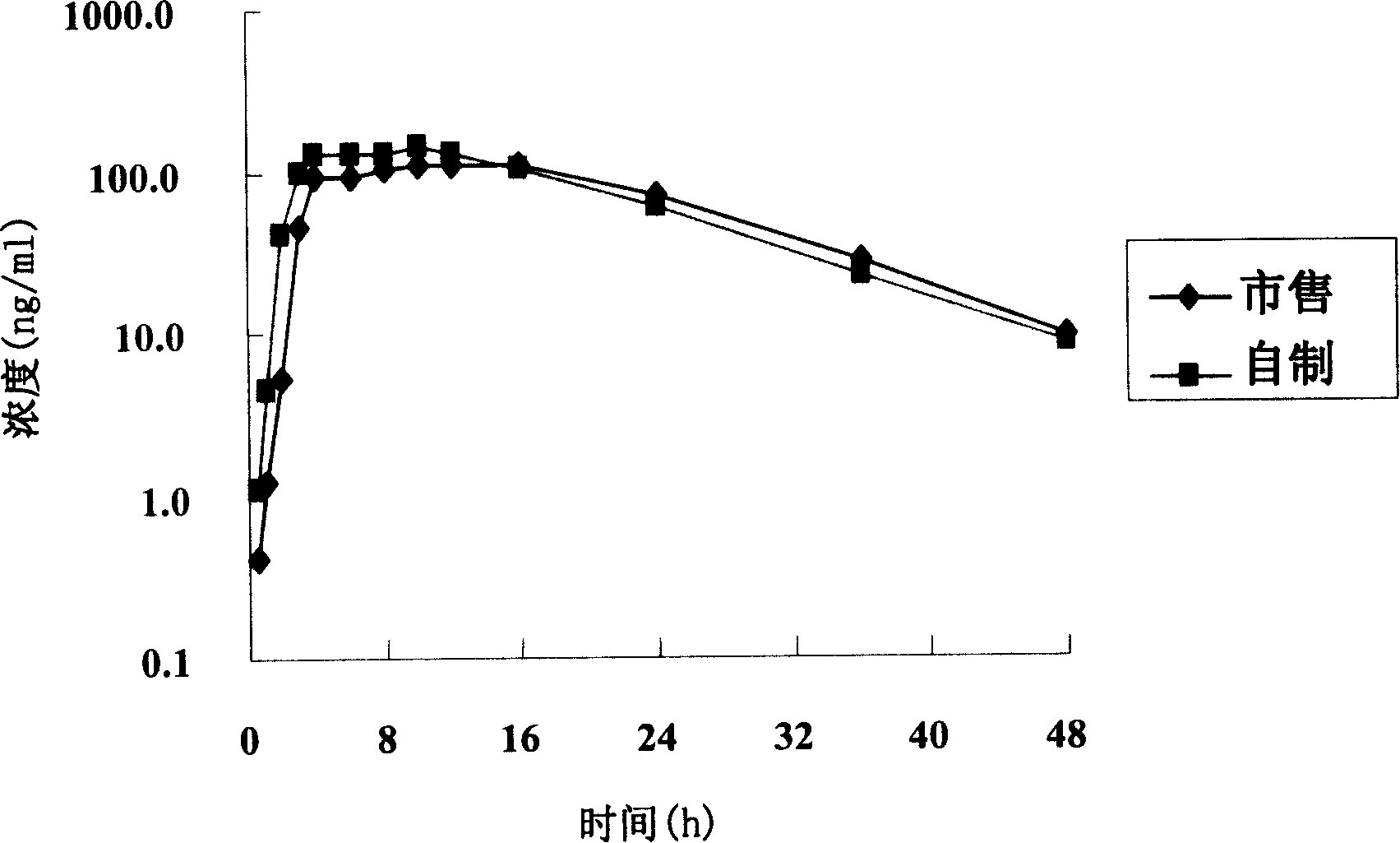
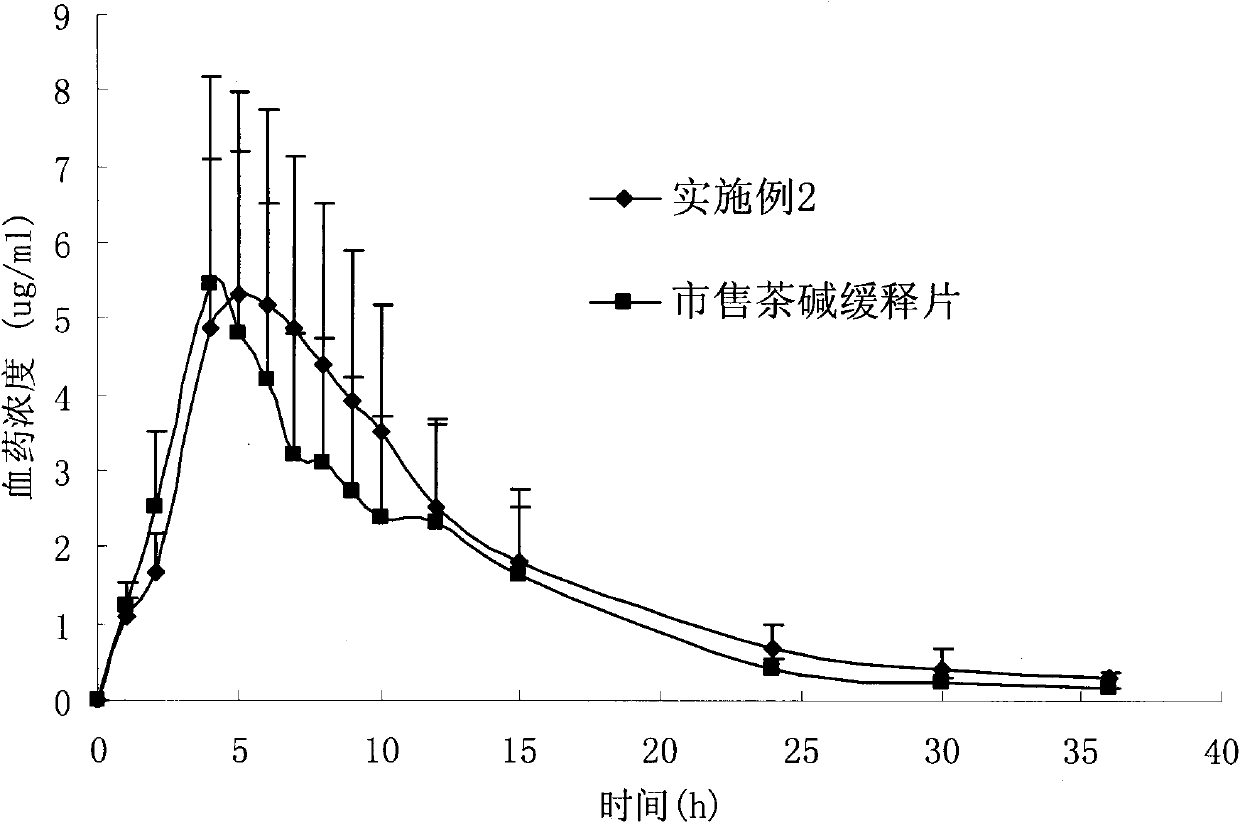
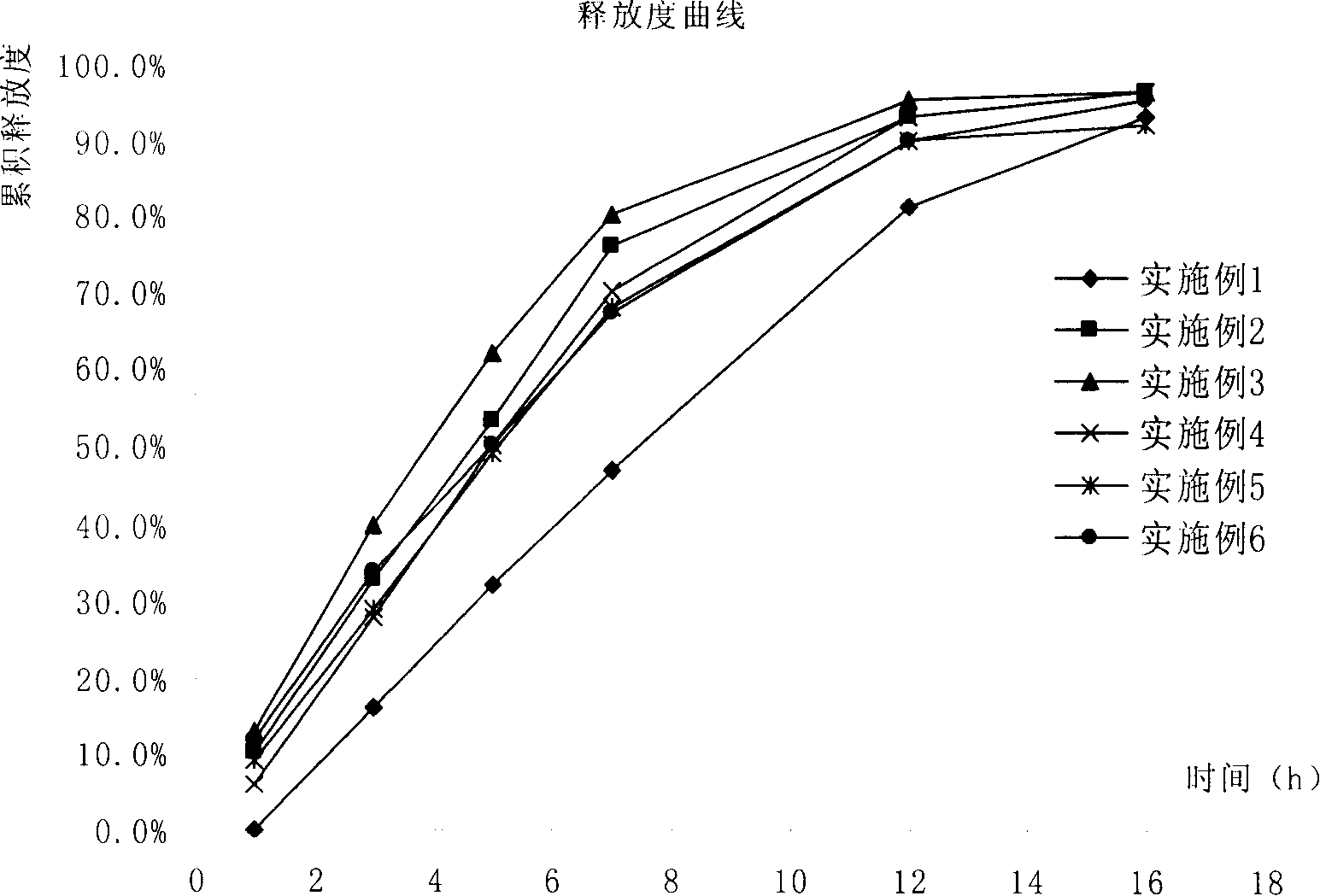
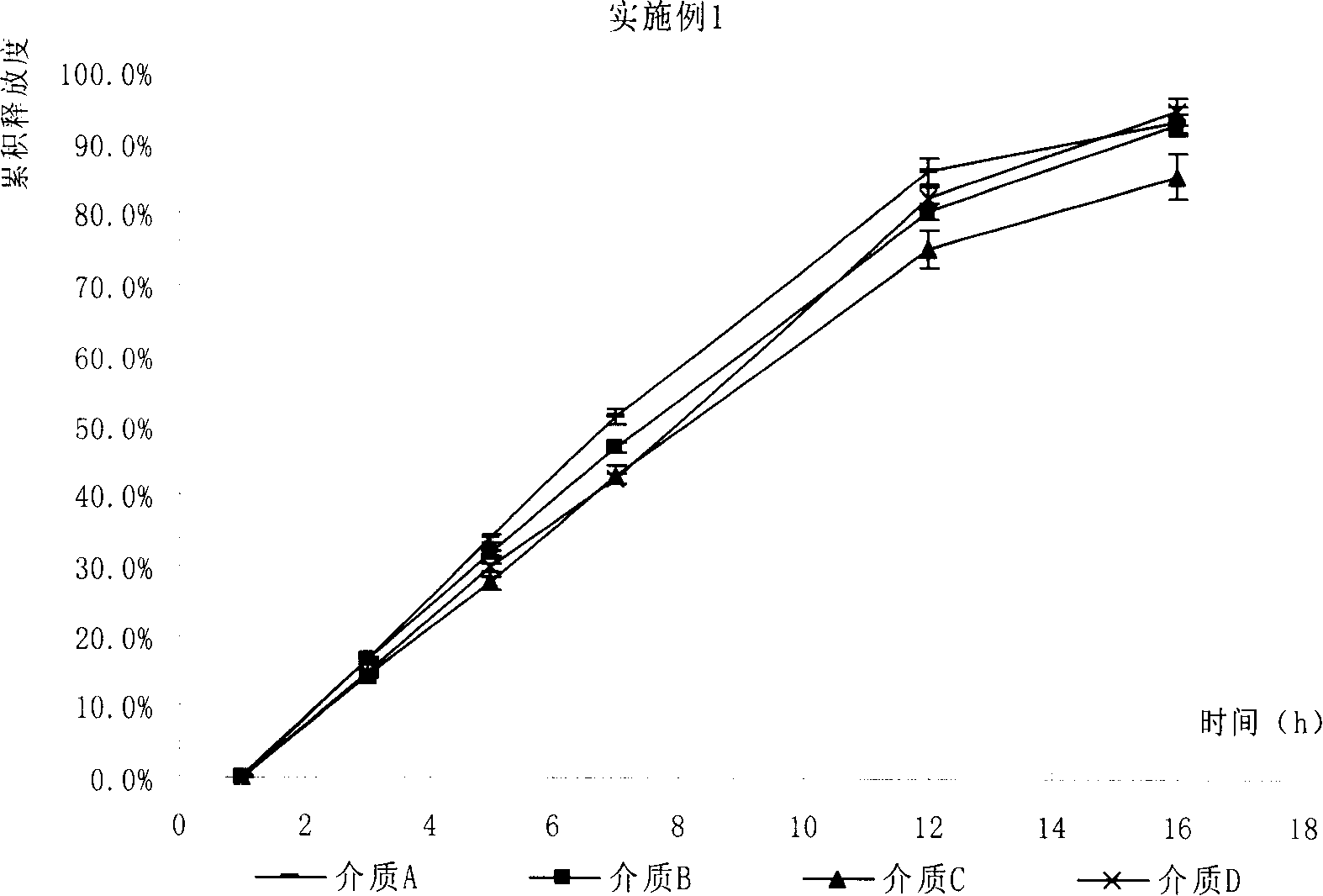
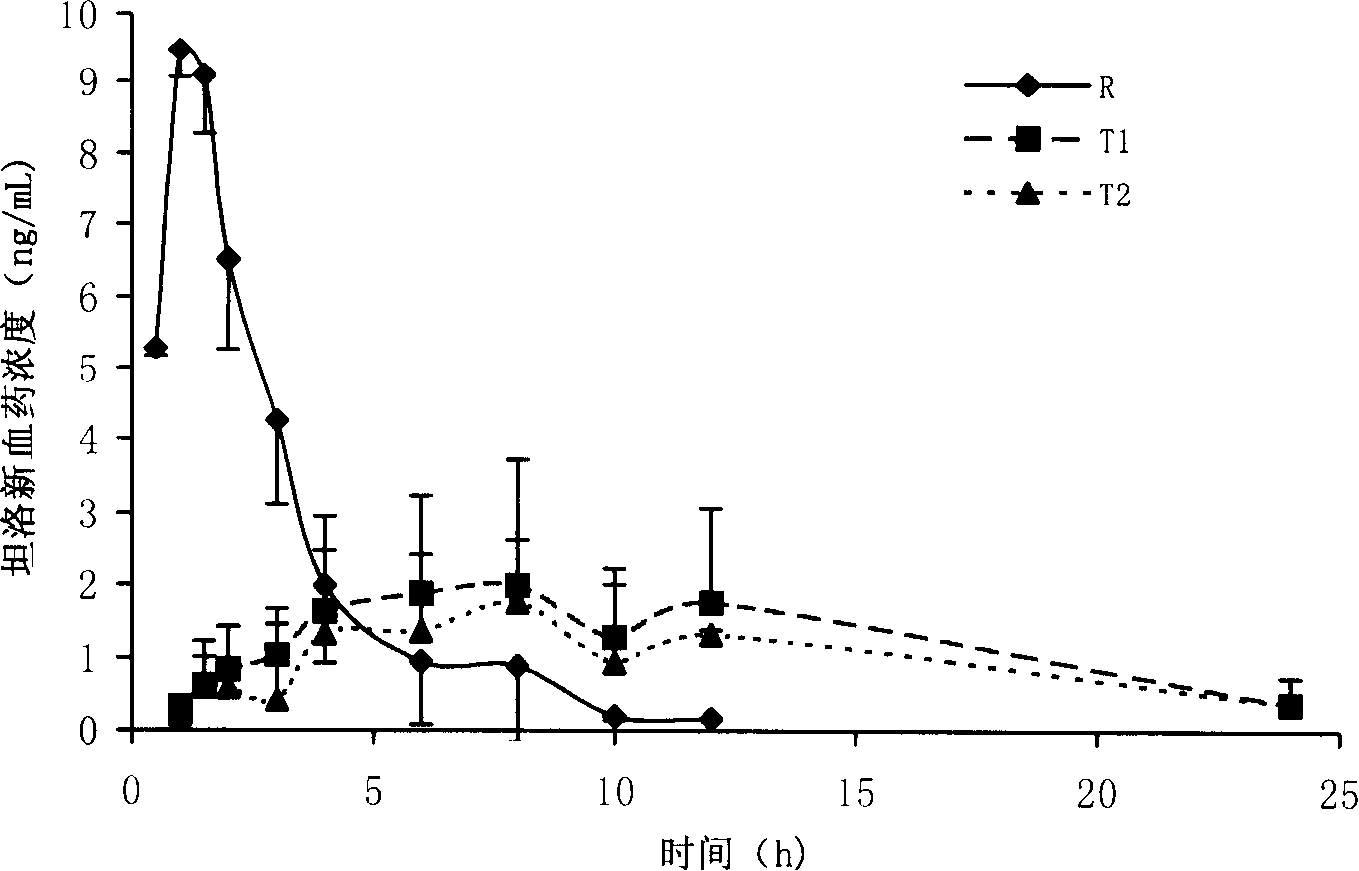
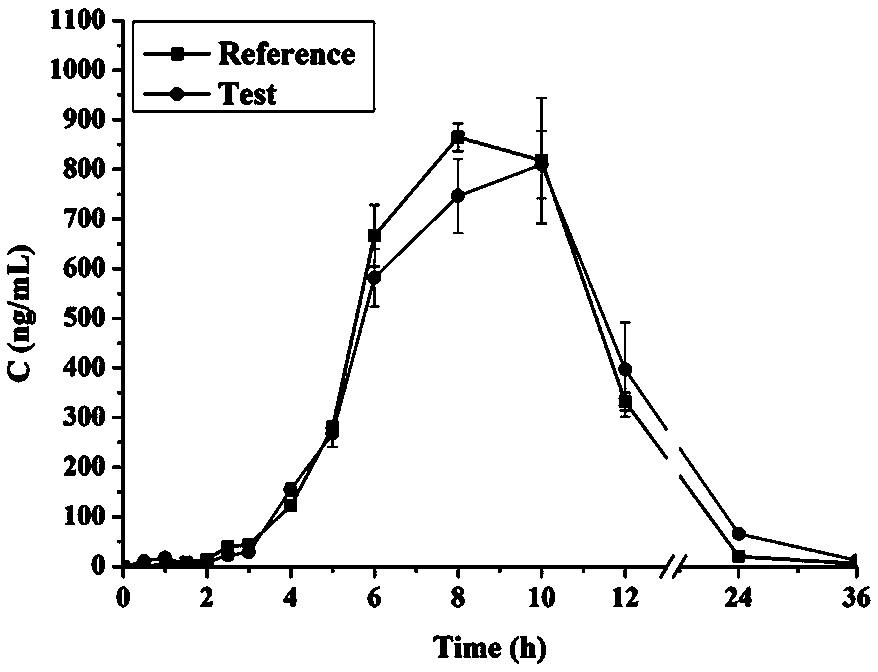
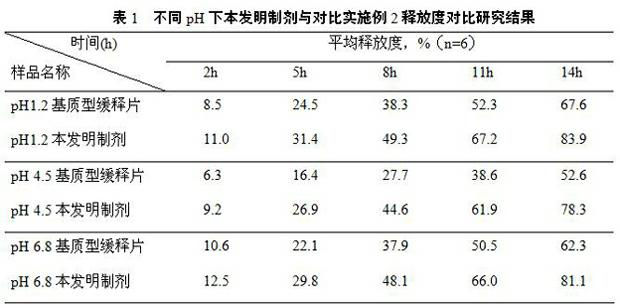
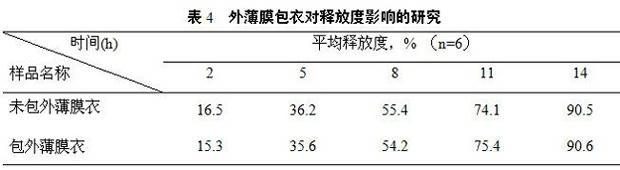
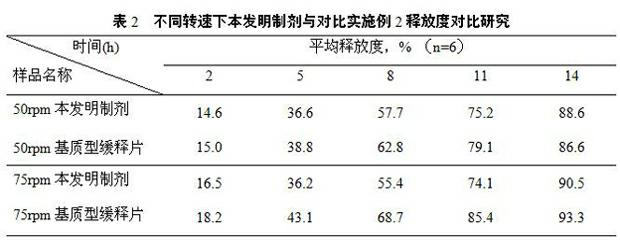
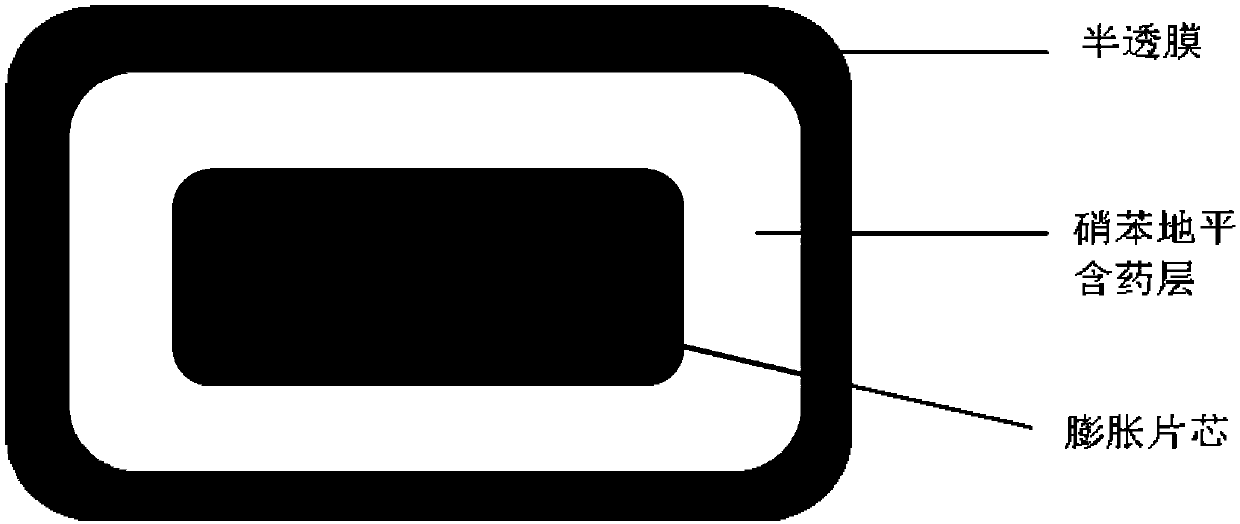
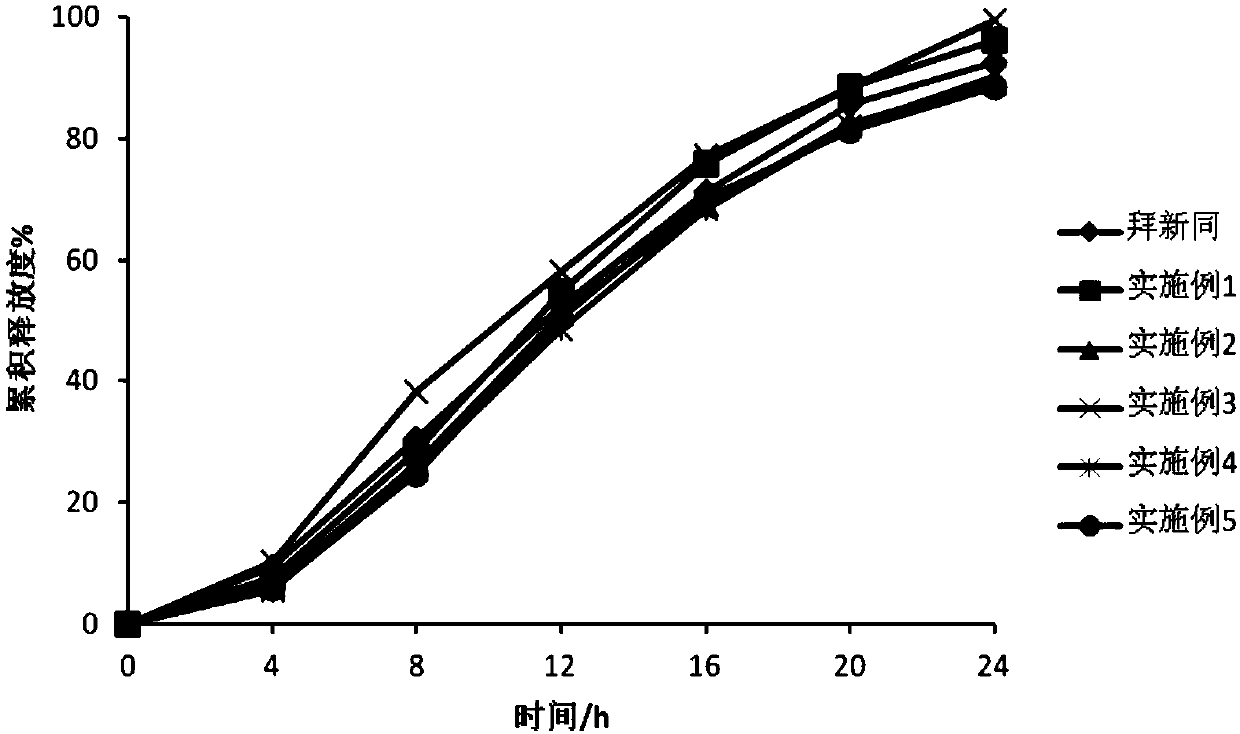
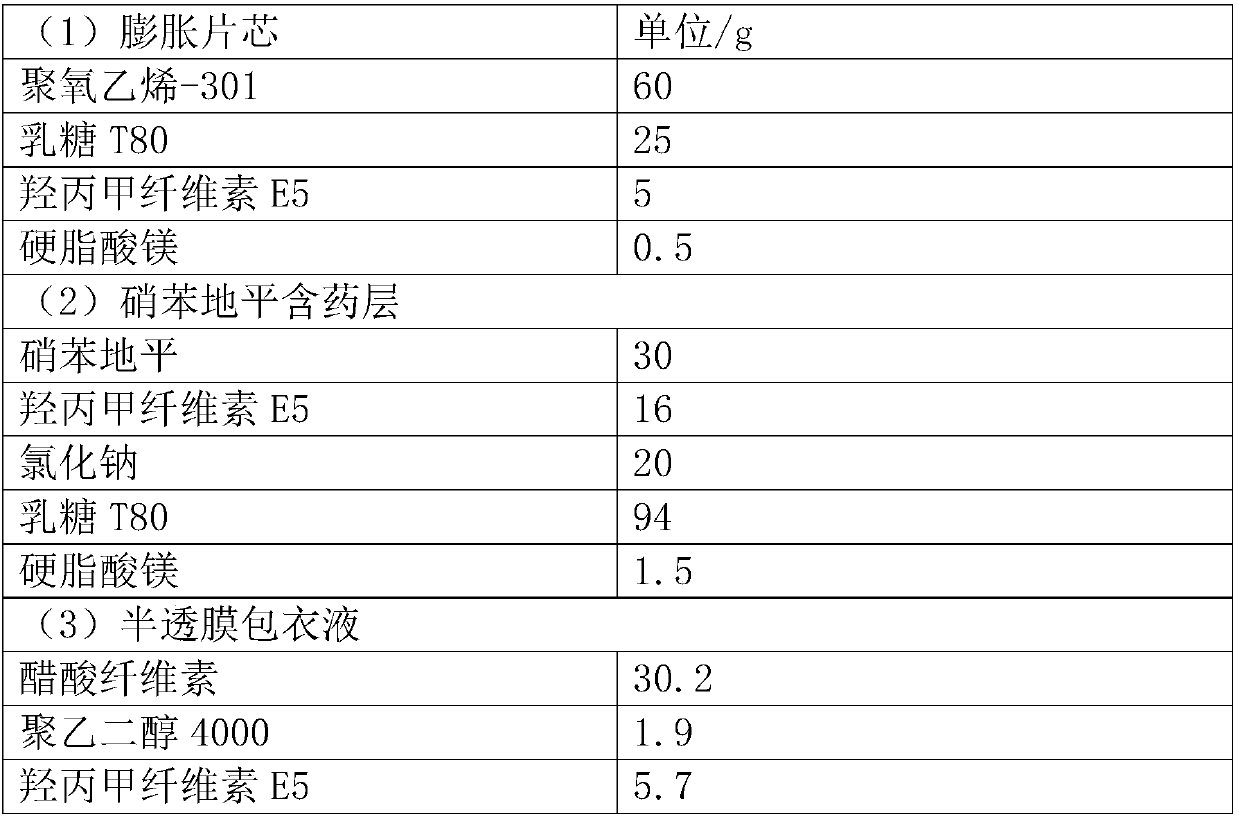
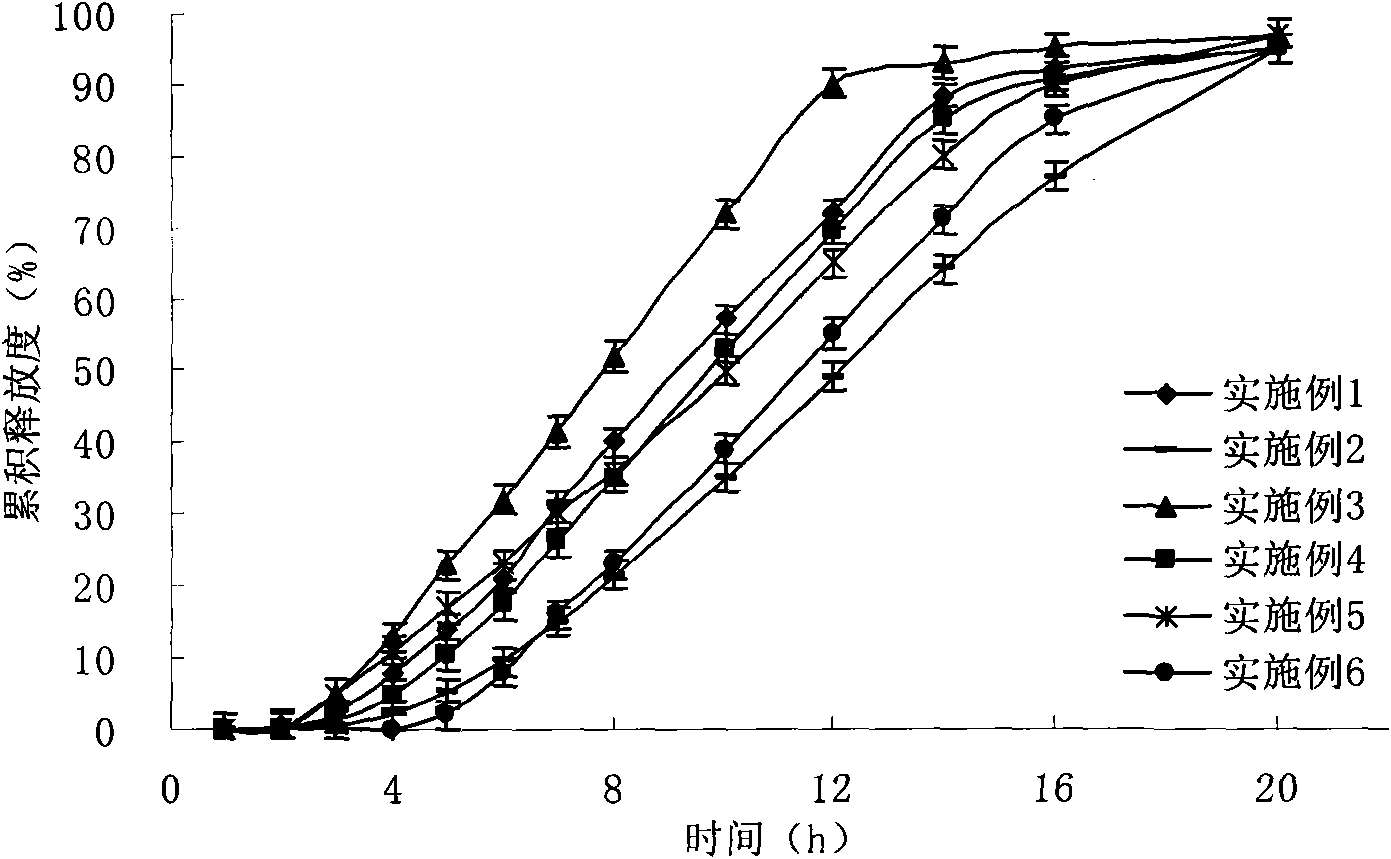
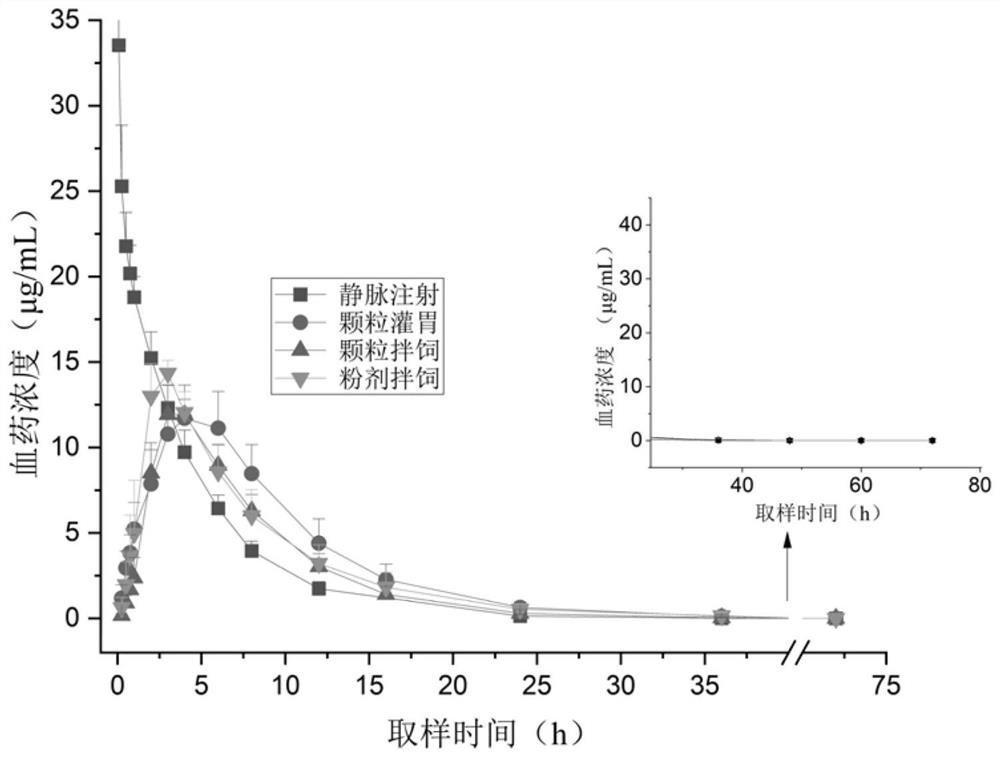
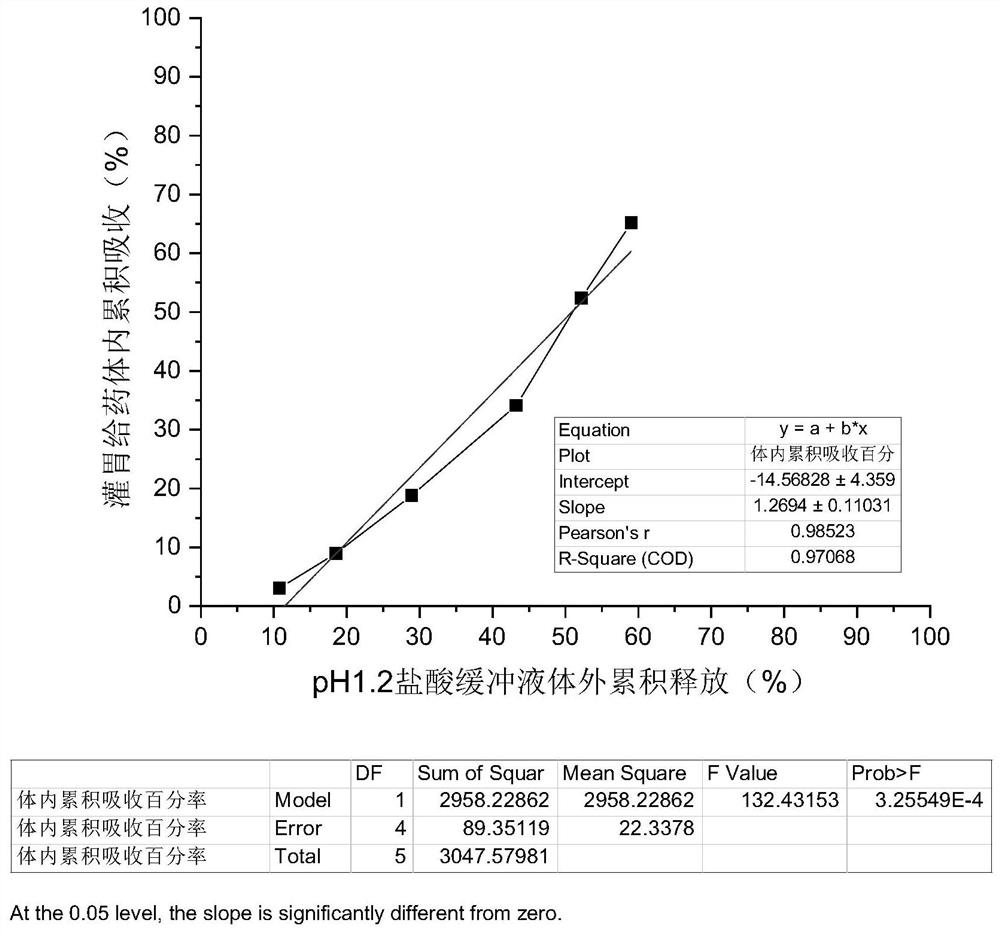
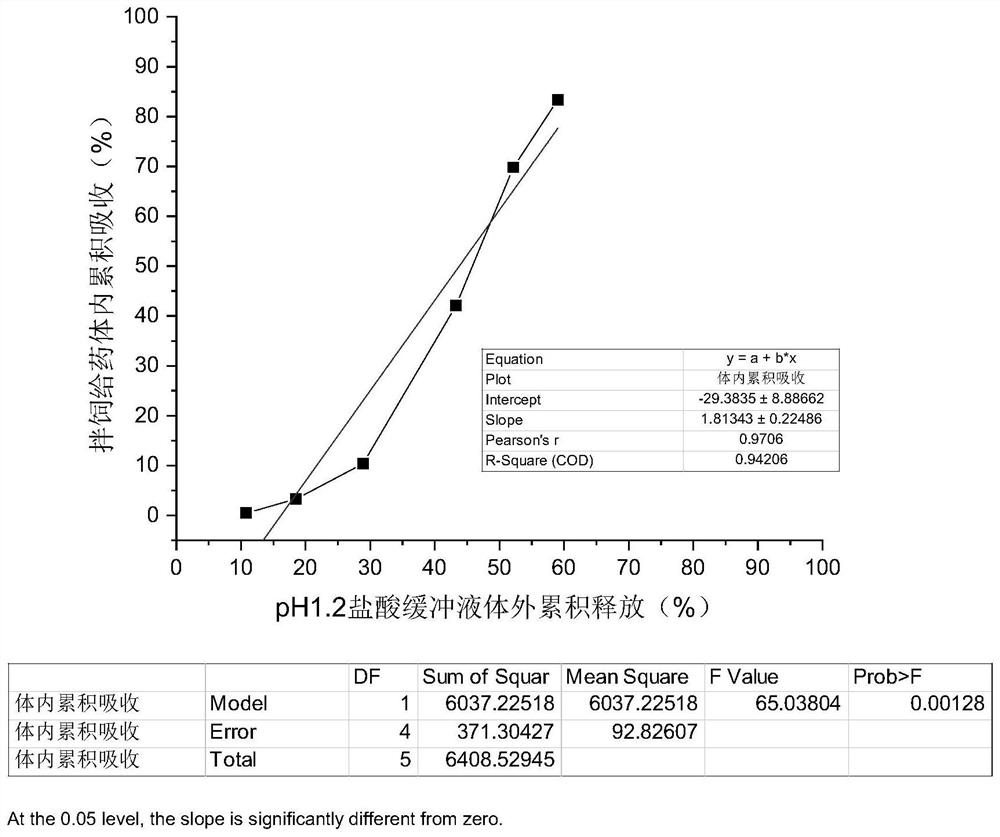
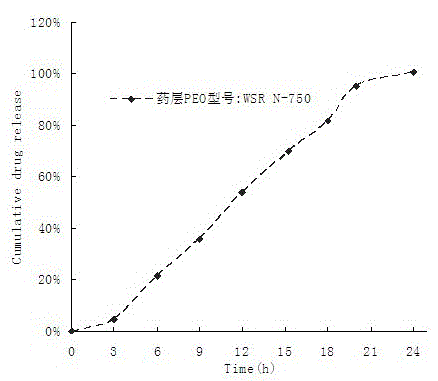
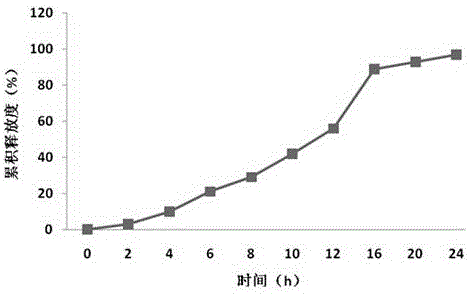
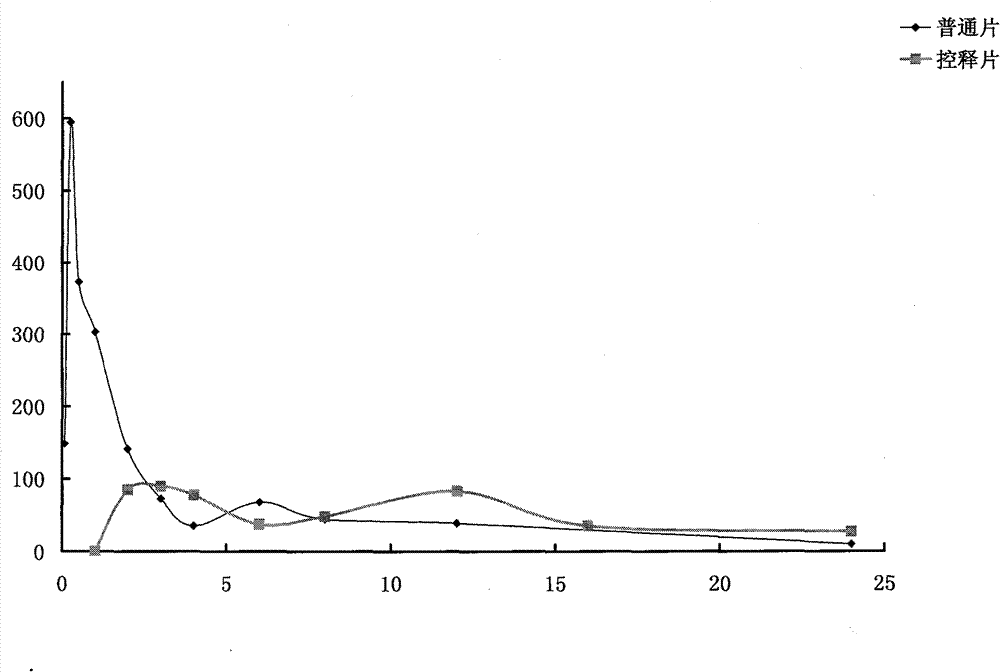
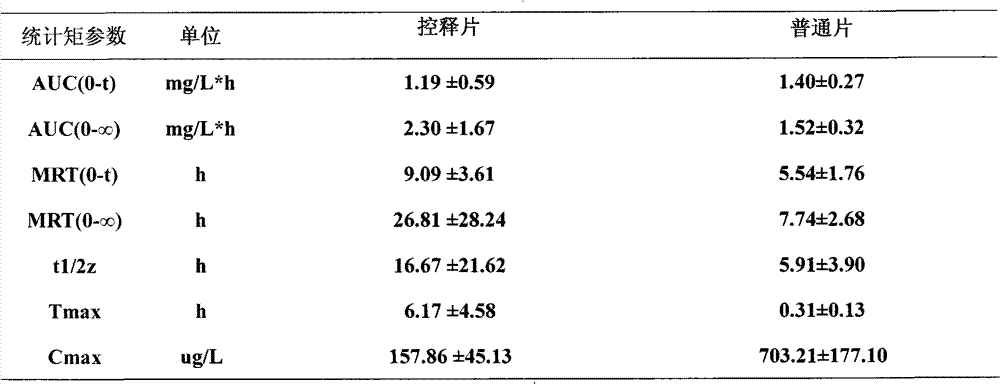
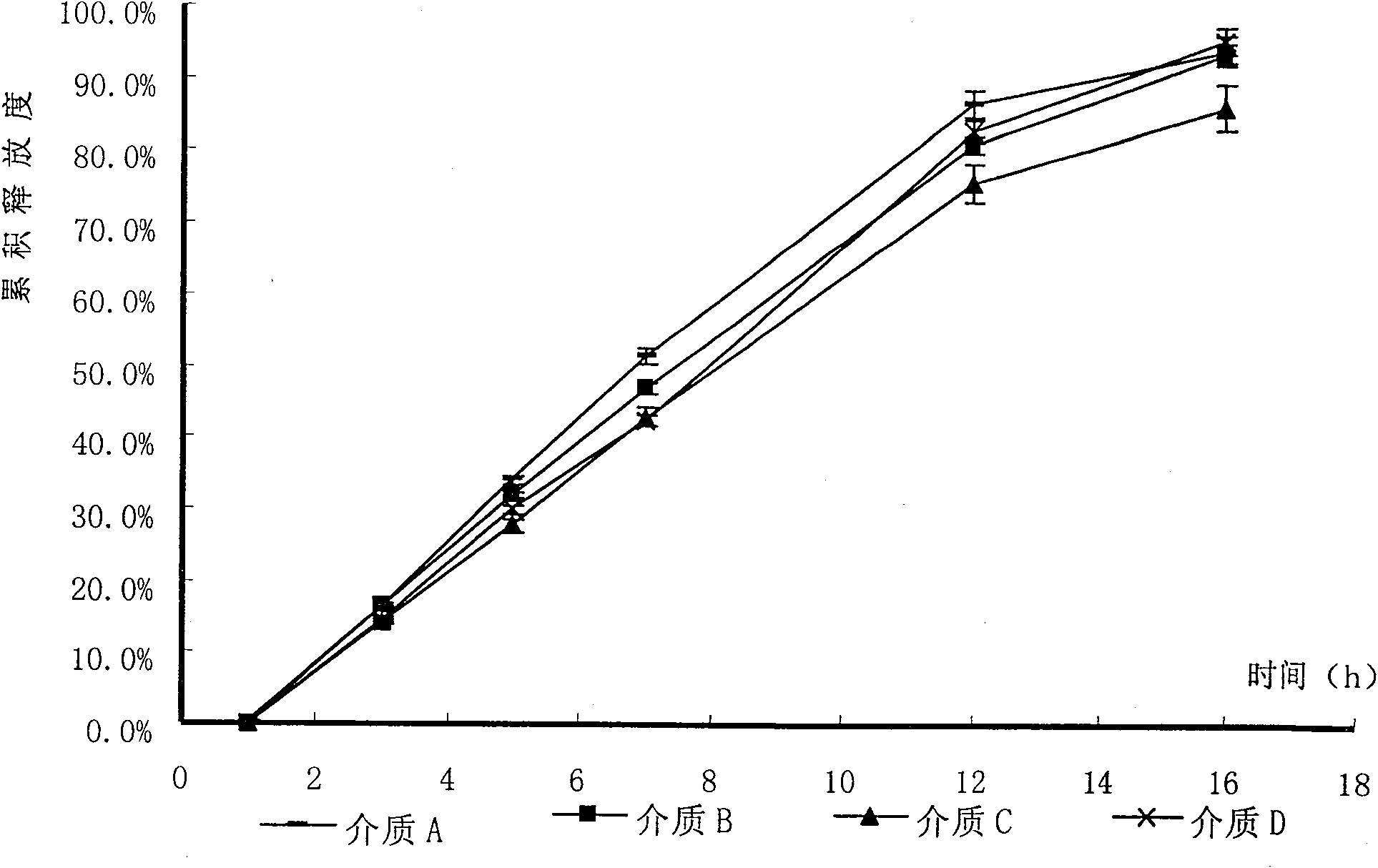
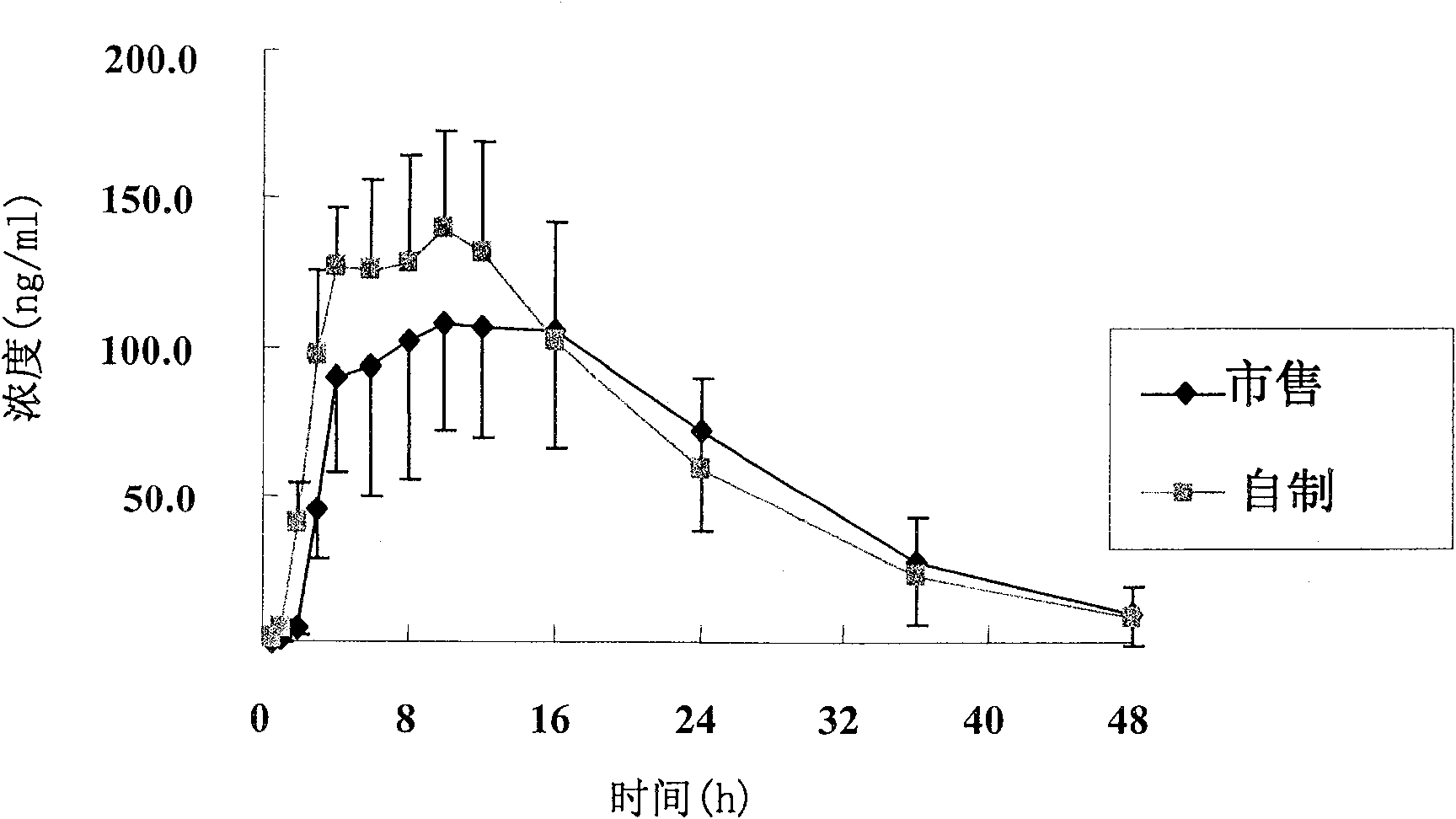
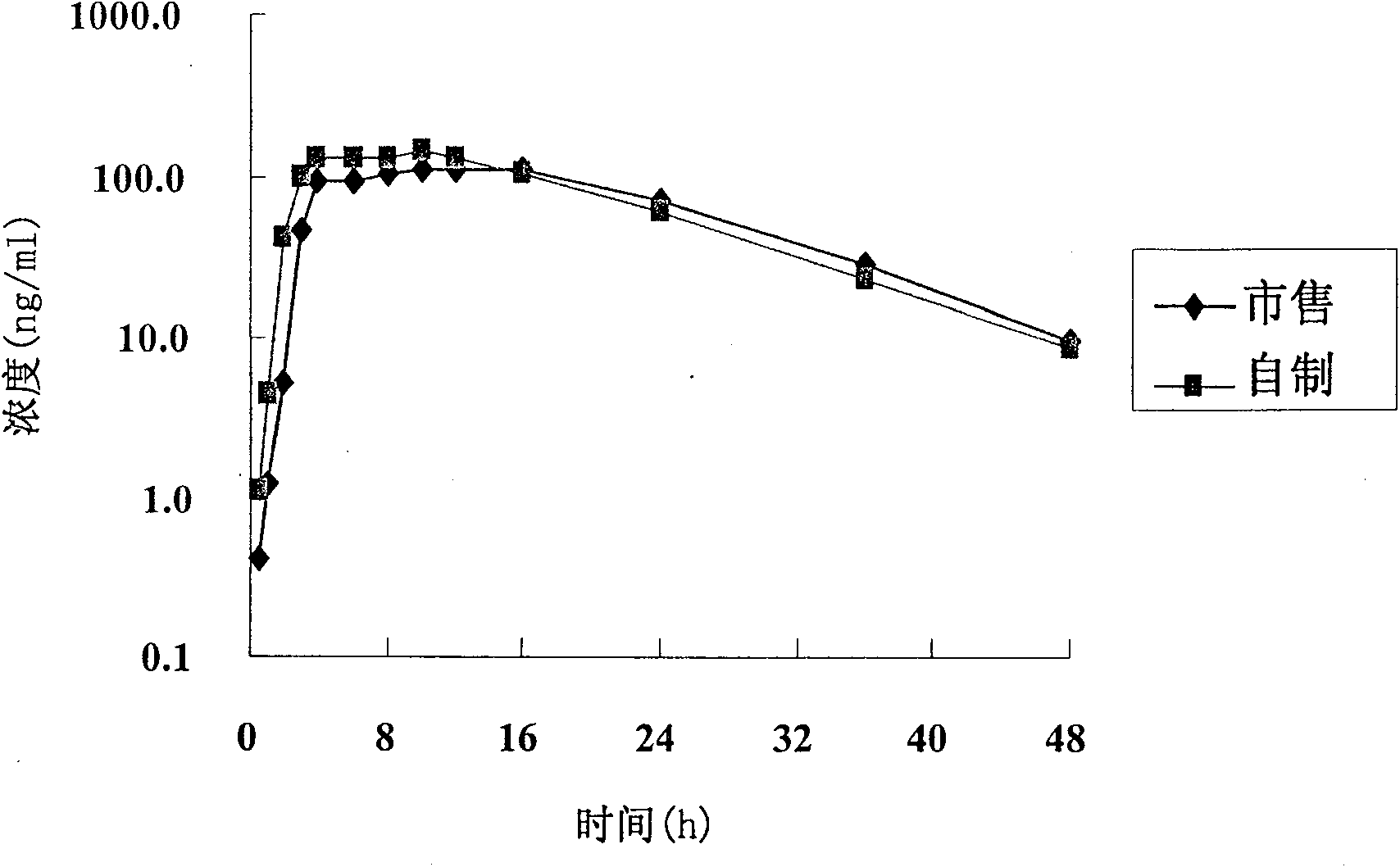
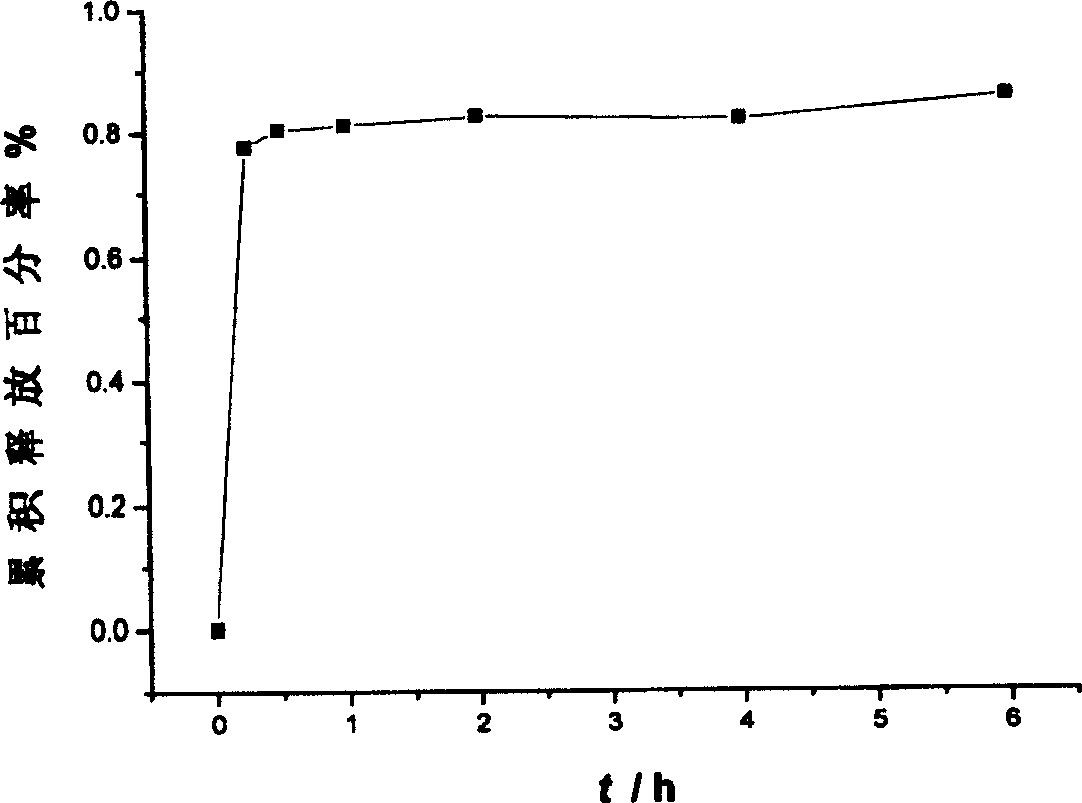
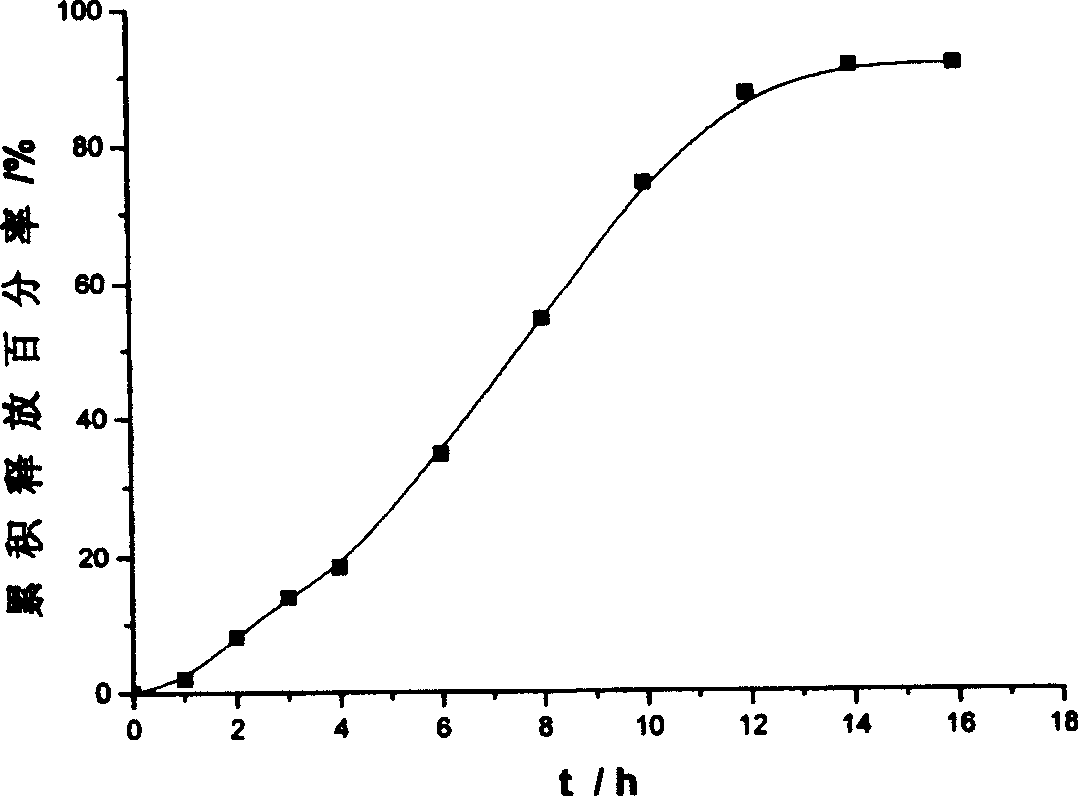
Patents
Literature
36results about How to "Good correlation between in vitro and in vivo" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A explosive core composition of controlled release administer drug and controlled release preparation as well as its preparing method
ActiveCN101161242AImprove securityImprove effectivenessInorganic non-active ingredientsOsmotic deliverySolubilityMedicine
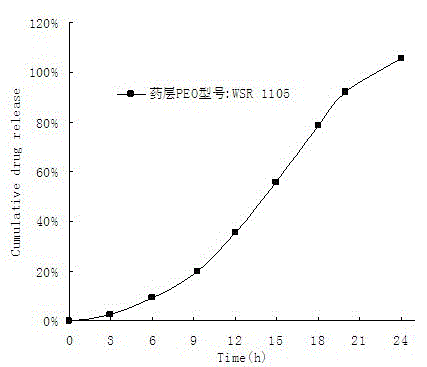
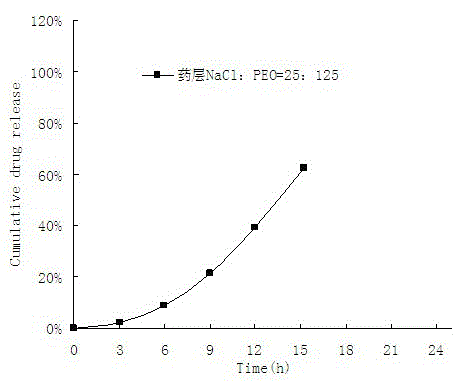
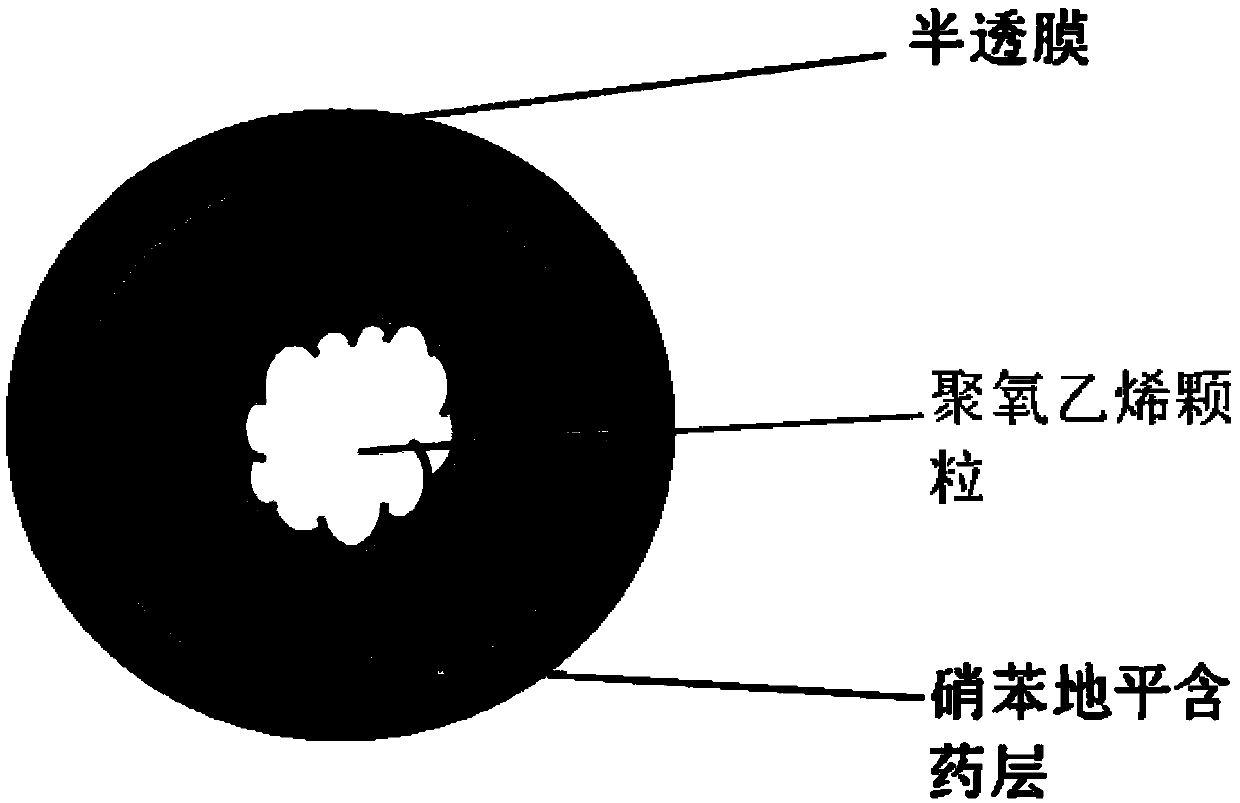
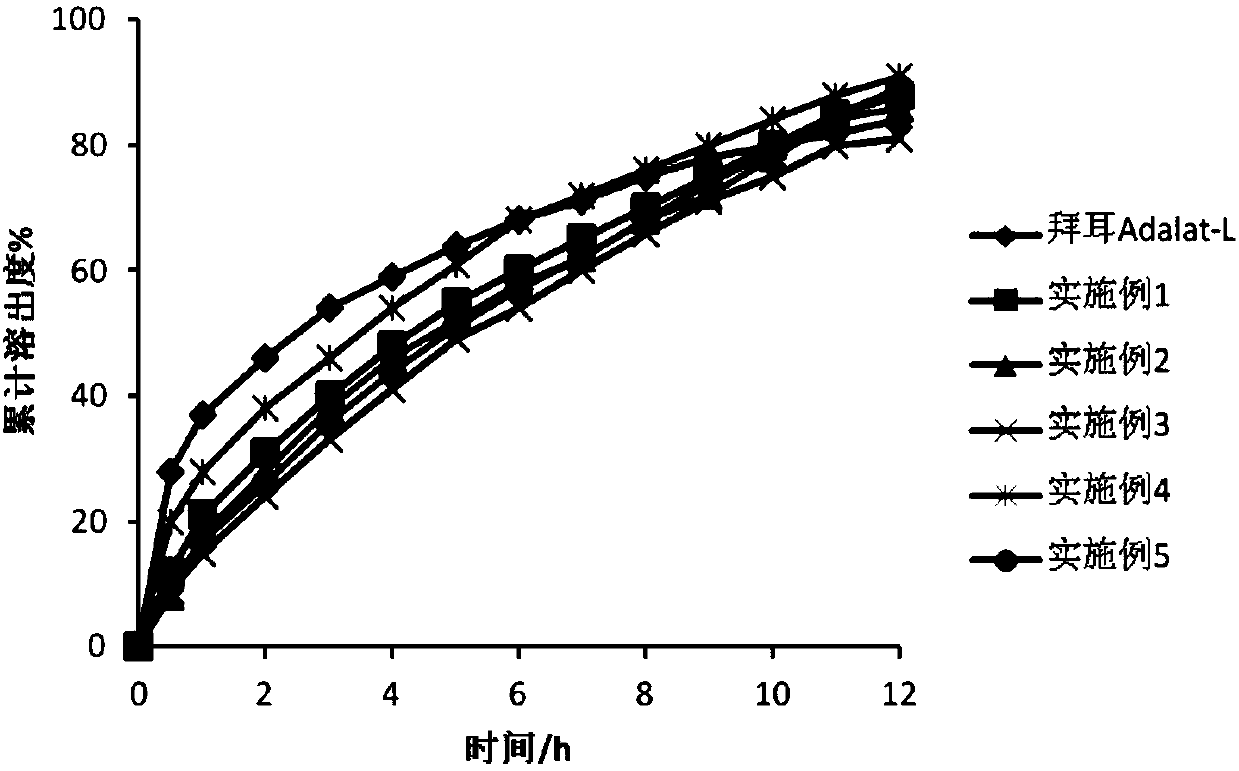
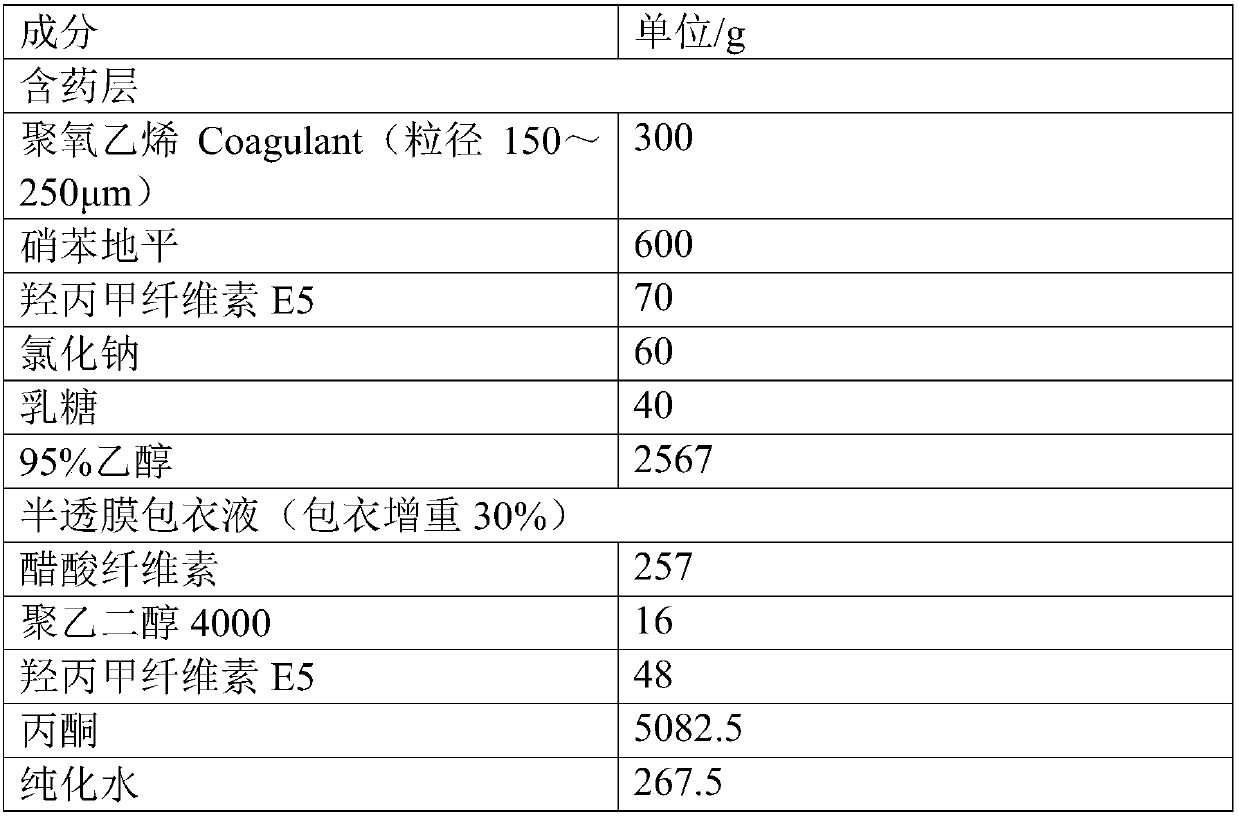
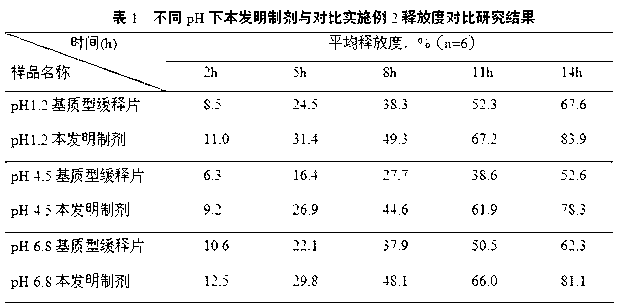
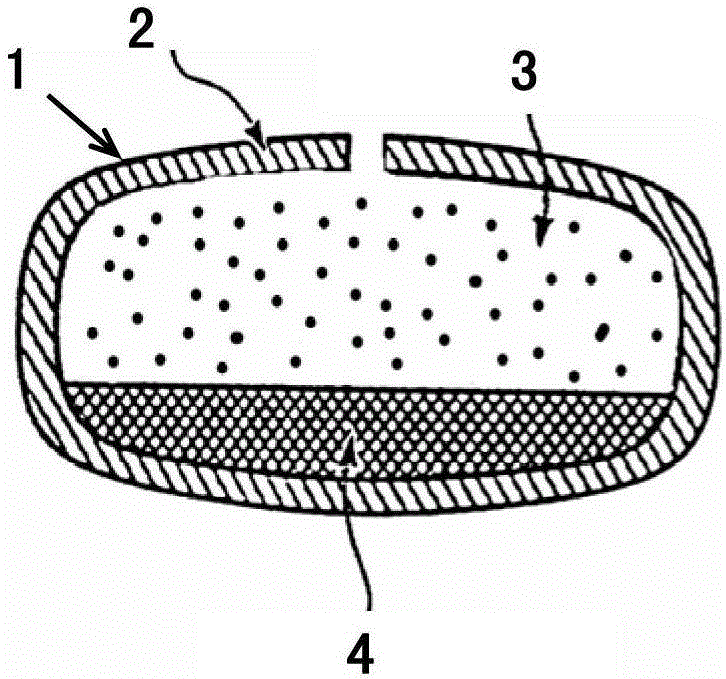
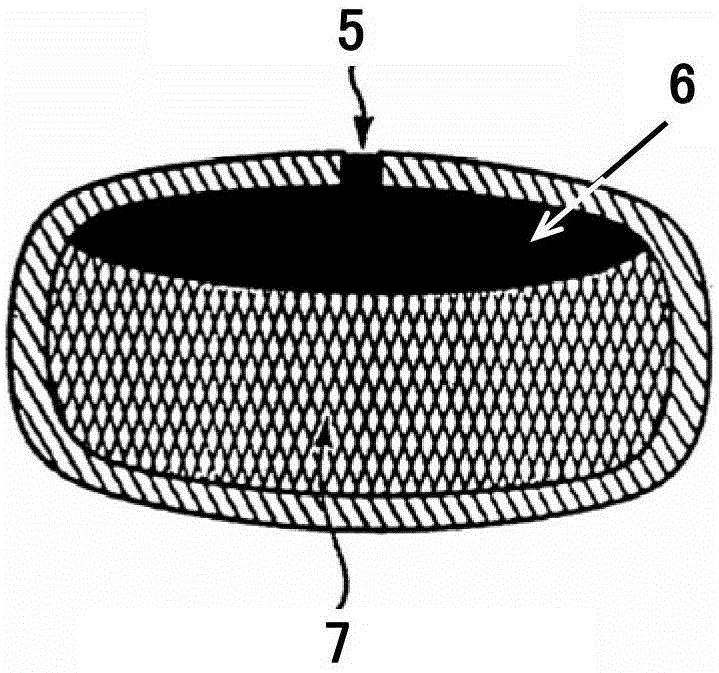
A medicine core composition used for low solubility active medicine controlling release administration is provided in the present invention, wherein, at least comprising medicine-containing layer and boosting layer, the medicine-containing layer comprises low solubility active medicine and hydrophilic polymer carrier, the boosting layer at least comprises penetration enhancing polymer, insoluble polymer and osmotic pressure promoter; a osmotic pump containing the medicine core composition is also provided in the present invention, wherein also comprising semi-transparent material film coating outer of the medicine core; the medicine core composition of the present invention can controlled speed release medicine, leading the preparation containing the medicine core to achieve aim of administration one time every day, that is about in 24 hours to release active medicine.
Owner:OCEAN STAR INT
Intelligent numerical control bionic drug dissolubility tester
InactiveCN102313795AGood correlation between in vitro and in vivoGood reproducibilityPreparing sample for investigationFlexible member pumpsEngineeringHuman gastrointestinal tract
Relating to the technical field of drug examination equipment, the invention especially relates to an intelligent numerical control bionic drug dissolubility tester, which comprises a stomach and intestine bionic device, a loading slot and a controller. The stomach and intestine bionic device includes a bionic stomach, a bionic intestinal tract communicated with the bionic stomach, a peristaltic pump and an extruding gear. The peristaltic pump and the extruding gear are against the external surface of the bionic stomach and the external surface of the bionic intestinal tract. The invention makes bionic design for the stomach and intestine in vitro, and simulates human body gastrointestinal peristalsis and the process of digestion and absorption. With the advantages of high automation degree, good detection result reappearance, and good?in vitro and in vivo correlation, the tester can better predict the drug dissolubility in vivo.
Owner:GUANGDONG MEDICAL UNIV
Self-assembled composite membrane controlled sustained-release preparation and preparation method thereof
InactiveCN101904818AGood correlation between in vitro and in vivoPromote absorptionPharmaceutical non-active ingredientsGranular deliveryCarrageenanPharmaceutical formulation
The invention belongs to the technical field of medicinal preparations, and in particular relates to a self-assembled composite membrane controlled sustained-release preparation and a preparation method thereof. The self-assembled composite membrane controlled sustained-release preparation comprises 2.5 to 67 percent of medicament, 12 to 76 percent of cationic polymer, 12 to 76 percent of anionic polymer and the balance of other auxiliary materials. Through the traditional process, matrix tablets or granules are prepared from polymers such as chitosan, sodium alga acid, carrageenan, sodium carboxymethylcellulose and the like with different charges; and in the releasing process, based on physiology pH change of a human body, two or more polymers with the different charges are reacted with each other to form an insoluble composite membrane on the surface of the tablet, and the matrix system is automatically converted into self-assembled composite membrane controlled sustained-release tablets or granules. The method can prepare medicaments with good controlled sustained-release effect by a simple process.
Owner:SHENYANG PHARMA UNIVERSITY
Tamsulosin hydrochloride double-layer osmotic pump controlled-releasing tablet and preparation method thereof
InactiveCN101167701AStable concentrationRelease constant speed evenlyInorganic non-active ingredientsPharmaceutical delivery mechanismExcipientMoisture
The invention provides double-layer osmotic pump tablets of tamsulosin ehydrochloride and a process for preparation. The medicament contains tamsulosin ehydrochloride and acceptable medical polymeric excipient, and is characterized in that the invention has excellent zero-level controlled releasing, pH level of environment, movements of the stomach and intestine, and food, has little effect on releasing action and food, and has no effect on the internal pharmacokinetics parameter. According to the percentage by weight, the preparation contains tamsulosin ehydrochloride 0-2%, excipient in pastille layer with the function of controlled-releasing 30-70% excipient in boosting layer with the function of controlled releasing 30-70%, and the rest percentage of other excipient. The of process for preparation the double layer permeable pump controlled-release tablets of tamsulosin ehydrochloride comprises (1) the preparation of pastille layer, (2) the preparation of boosting layer, (3) the compressing of the two layers, (4) the coating of the double layer tablets, (5) the perforating of the coated tablets, (6) the packing of moisture proof cost. The invention is clinically used for the treatment of paruria symptom like frequent micturition, diuresis at night, dysuria caused by prostatic hyperplasia.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
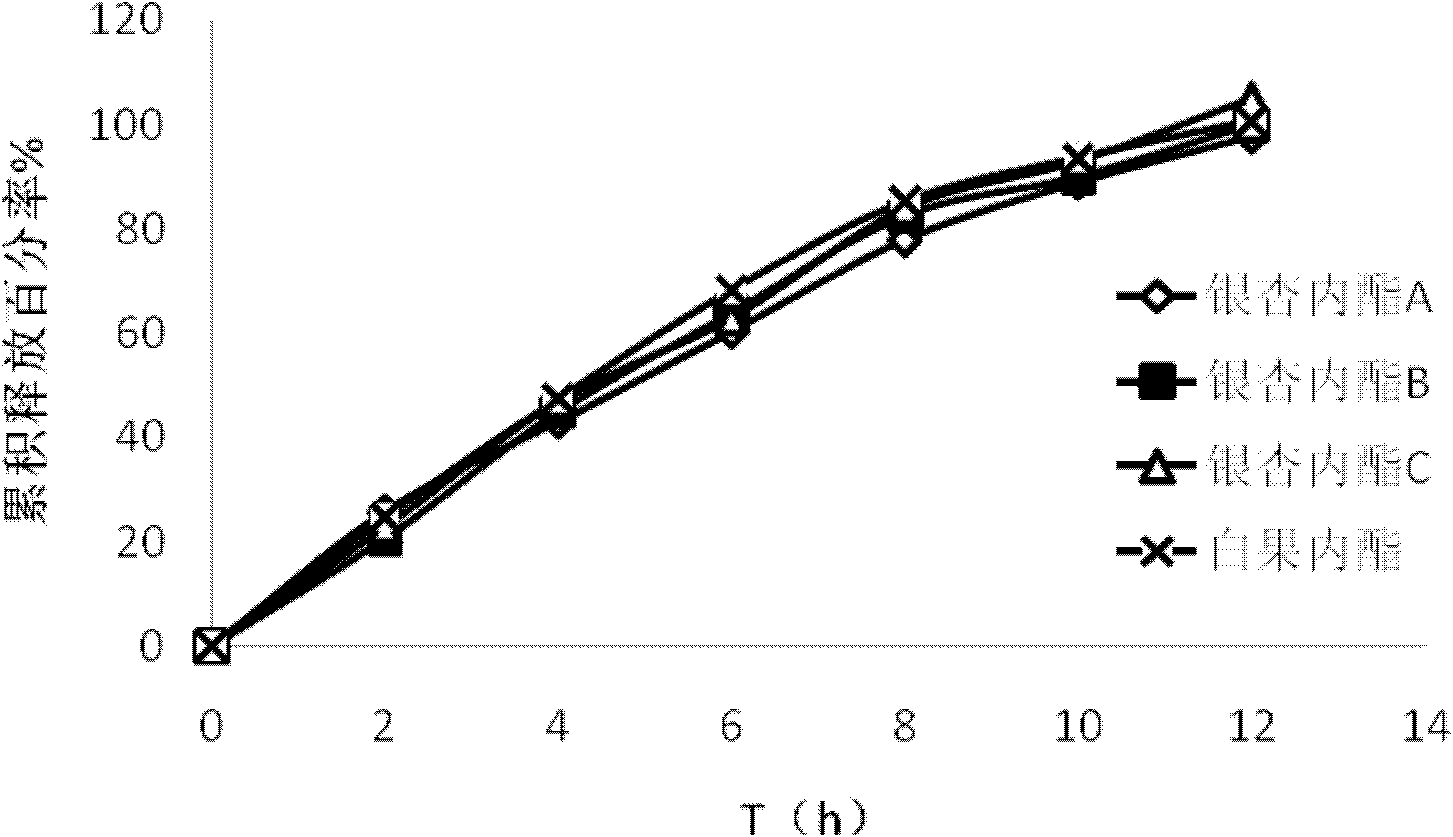
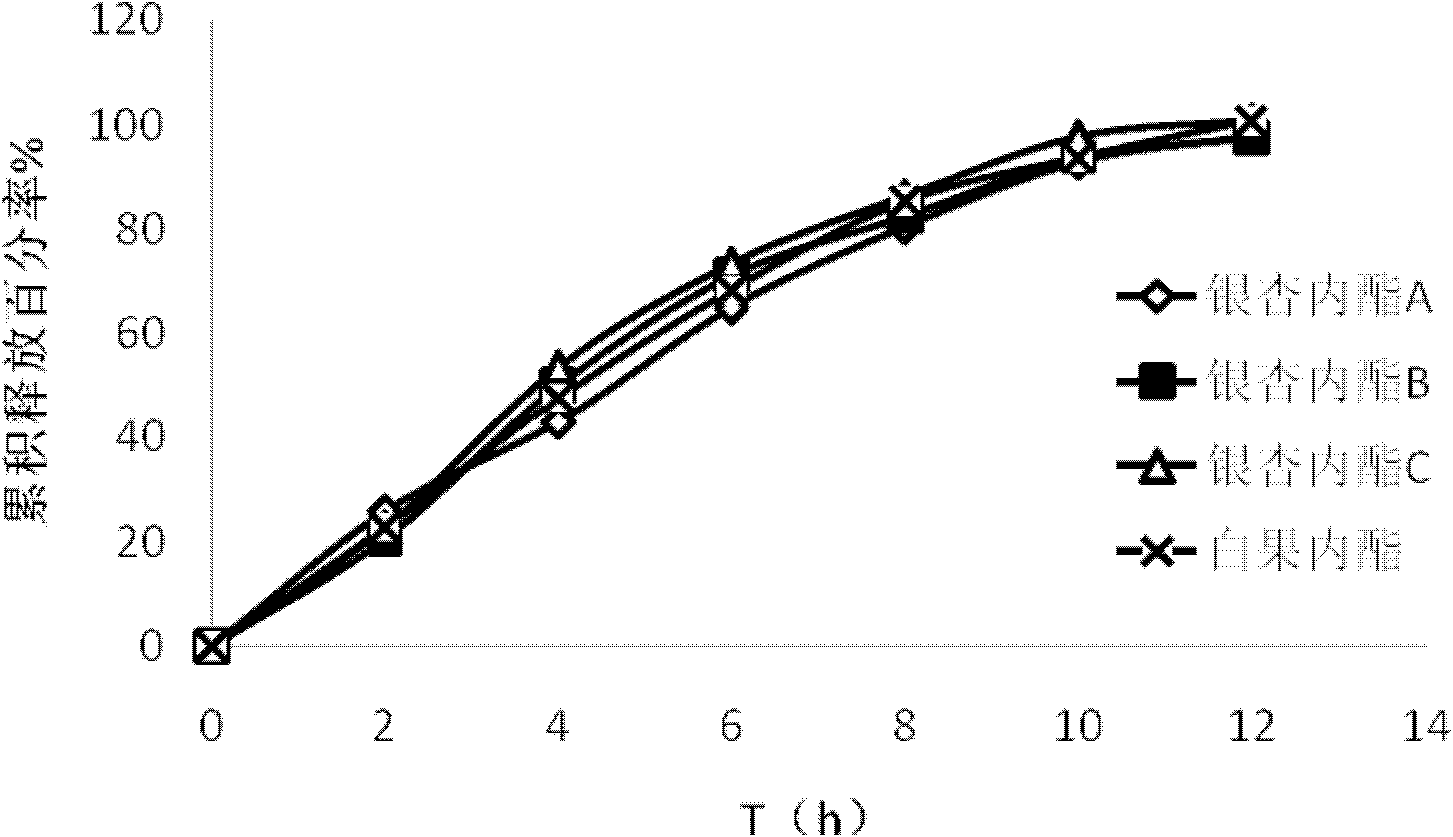
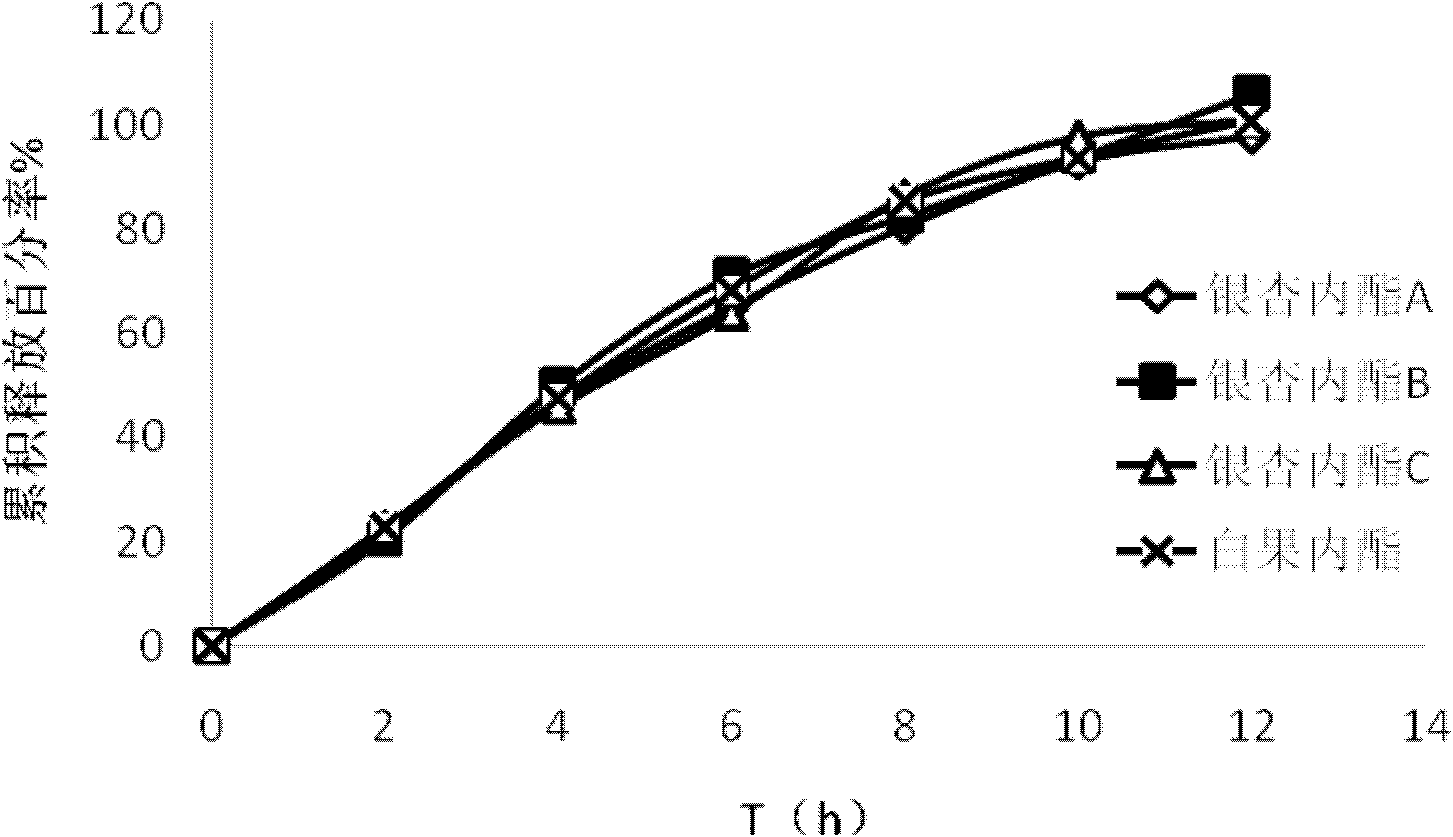
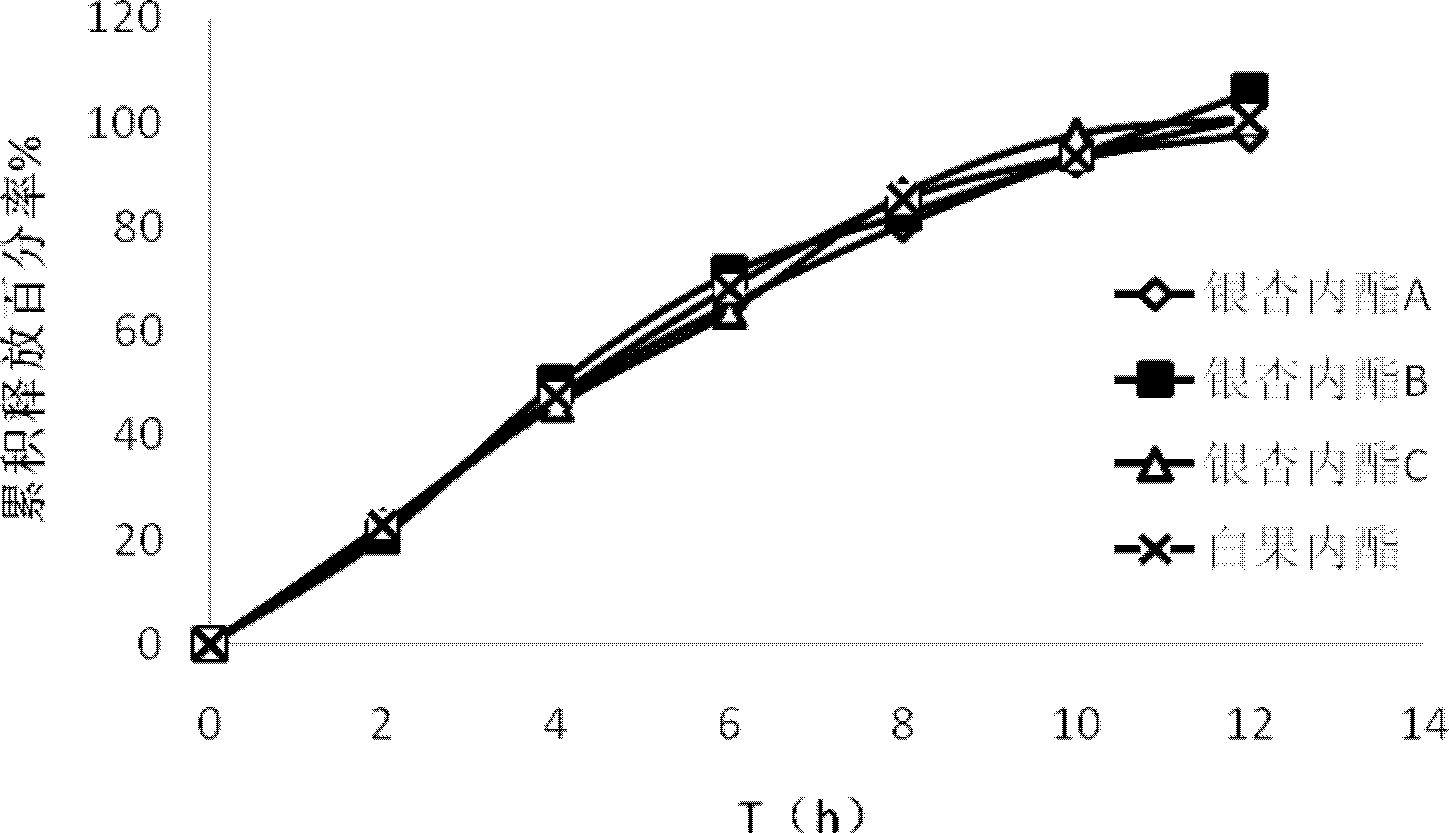
Ginkgolide osmotic pump tablet and preparation method thereof
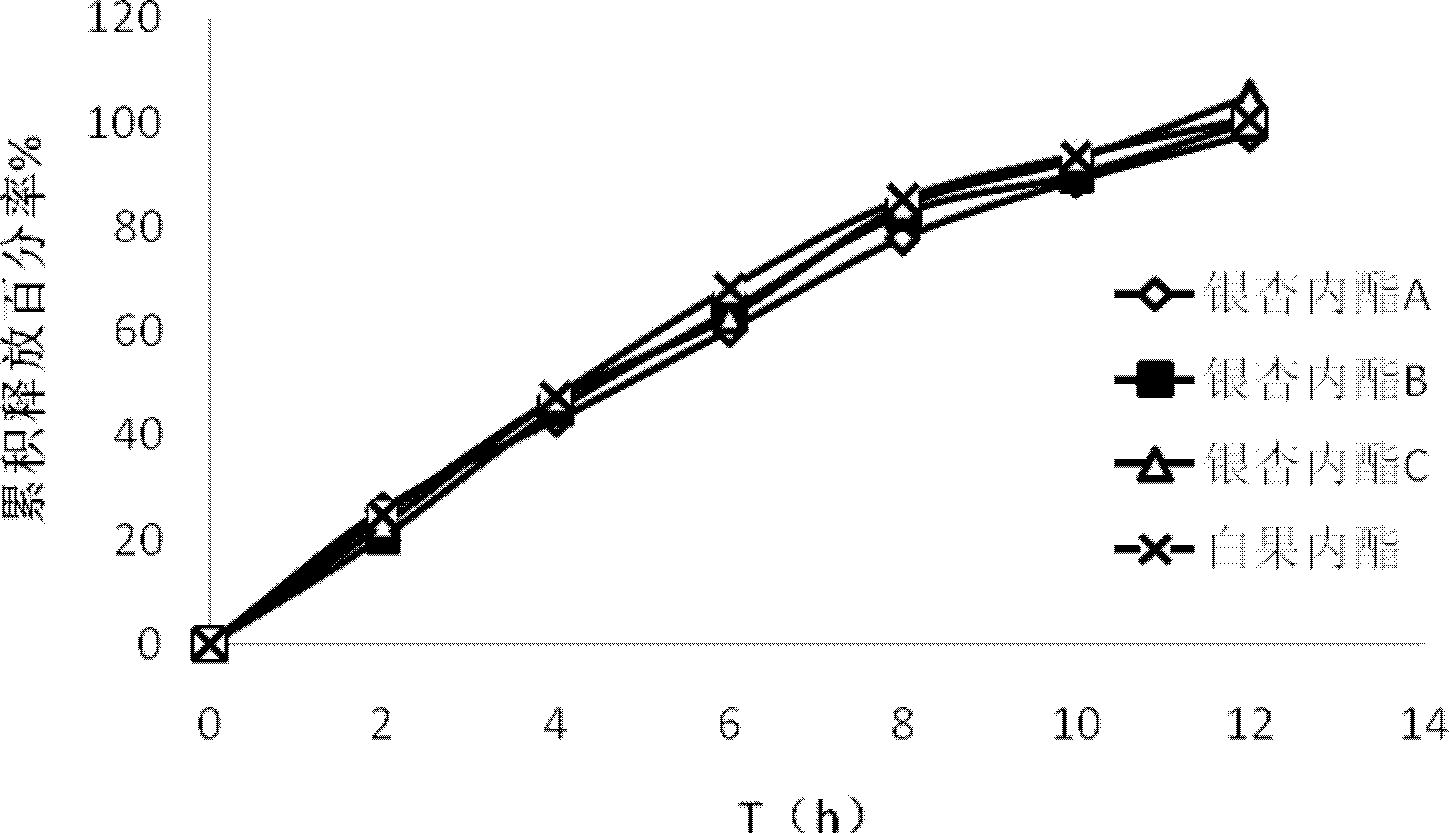
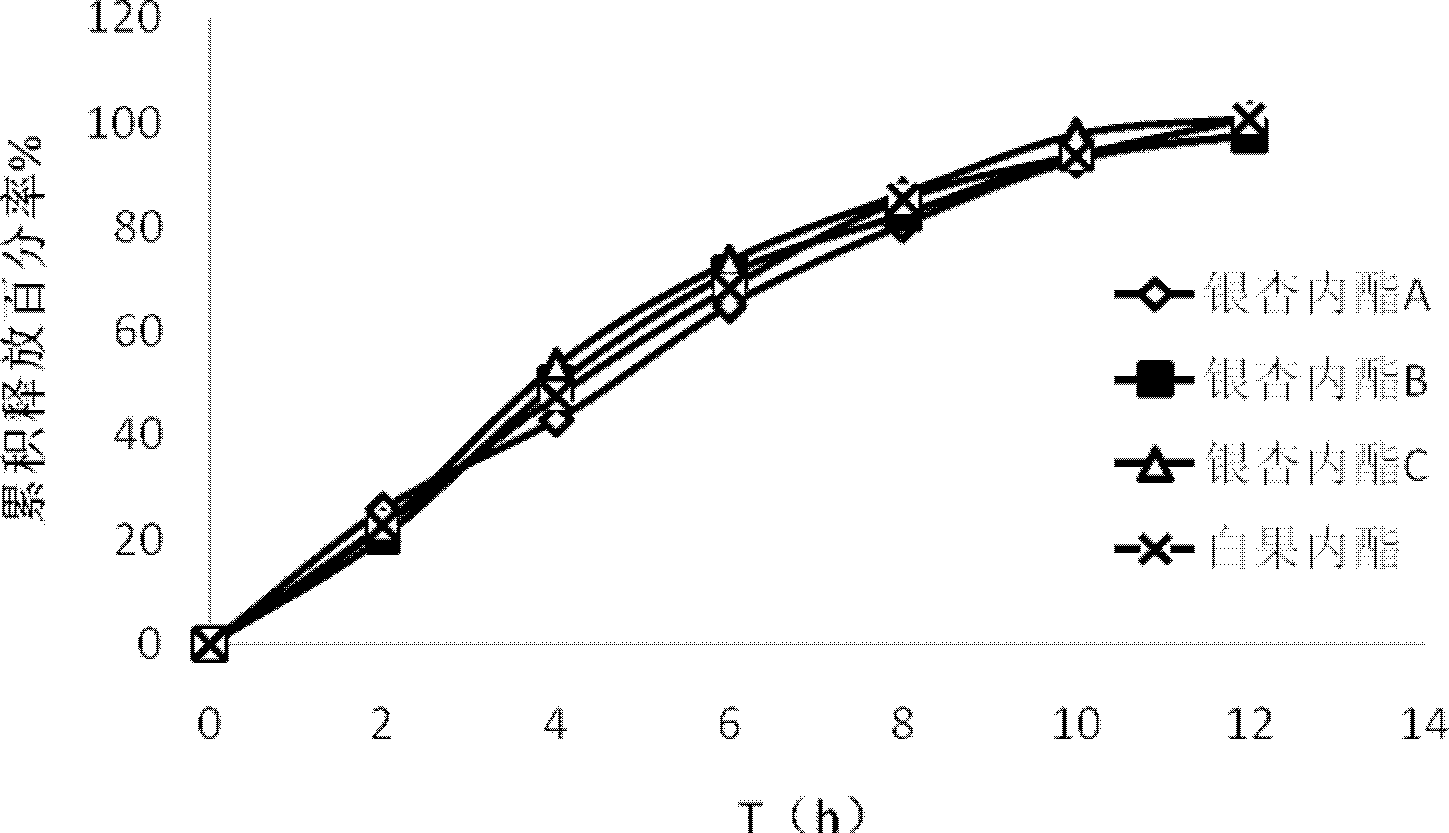
InactiveCN103142532AImplement synchronous releaseImprove solubilityOrganic active ingredientsNervous disorderSolubilityPatient compliance
The invention provides a ginkgolide osmotic pump tablet and a preparation method thereof. The ginkgolide osmotic pump tablet provided by the invention is a double-layer tablet composed of a drug-containing layer and a push layer, and is coated, wherein the drug-containing layer comprises drug release holes. The ginkgolide osmotic pump tablet uses ginkgolide at effective positions of the ginkgo biloba extract as an active component, and a total amount of ginkgolide A, ginkgolide B, ginkgolide Cand bilobalide is not less than 70 %. According to the invention, by combining a solid dispersion solubilising technique and an osmotic pump tablets control-release technology, the ginkgolide is firstly prepared into the solid dispersion, and then further prepared into the ginkgolide osmotic pump tablet on a basis of improving solubility of poorly soluble components, thereby realizing synchronous slow release of complex components in the ginkgolide, reducing drug administration times and improving patient compliance.
Owner:FUDAN UNIV
Novel paliperidone progressively-increased release osmotic pump preparation and preparation method thereof
ActiveCN103271889AAvoid titrationImprove complianceOrganic active ingredientsNervous disorderControlled Release TabletOsmotic pump
The invention belongs to the technical field of pharmaceutical preparations and discloses a paliperidone bi-layer osmotic pump progressively-increased controlled release tablet and a preparation method thereof. The osmotic pump preparation comprises a tablet core and a semipermeable coating film, wherein the tablet core comprises a medicine containing layer and a boosting layer, the semipermeable coating film envelops the tablet core and is provided with medicine release holes, the medicine containing layer contains middle molecular polyoxyethylene (average molecular weight is 900, 000-1, 000,,000) and an osmotic active substance in a certain proportion so that the hydration rate of the medicine containing layer is proper, the release of the active medicine basically presents a progressively-increased release trend and the progressively-increased release can be kept for 14-24 hours by using the bi-layer tablet core and the single-layer coating. By adopting the preparation, the adverse reaction of the medicine can be reduced greatly, the compliance of a patient can be improved, simultaneously, the preparation is simple to prepare, the preparation process of the progressively-increased osmotic pump preparation is simplified greatly, and the preparation is more conductive to industrialized production.
Owner:SHENYANG PHARMA UNIVERSITY
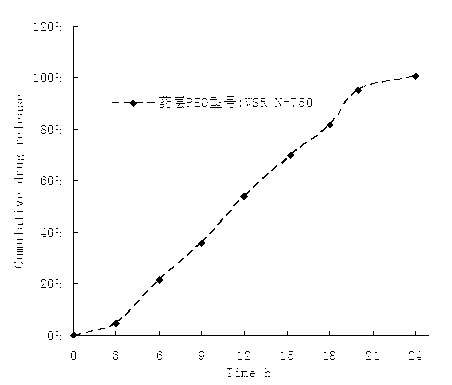
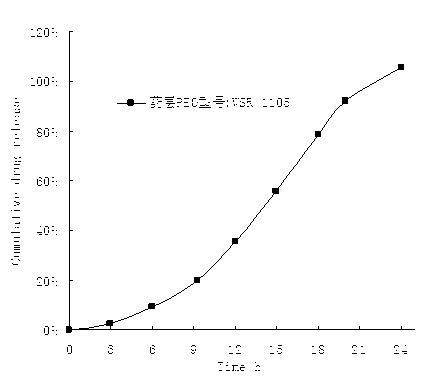
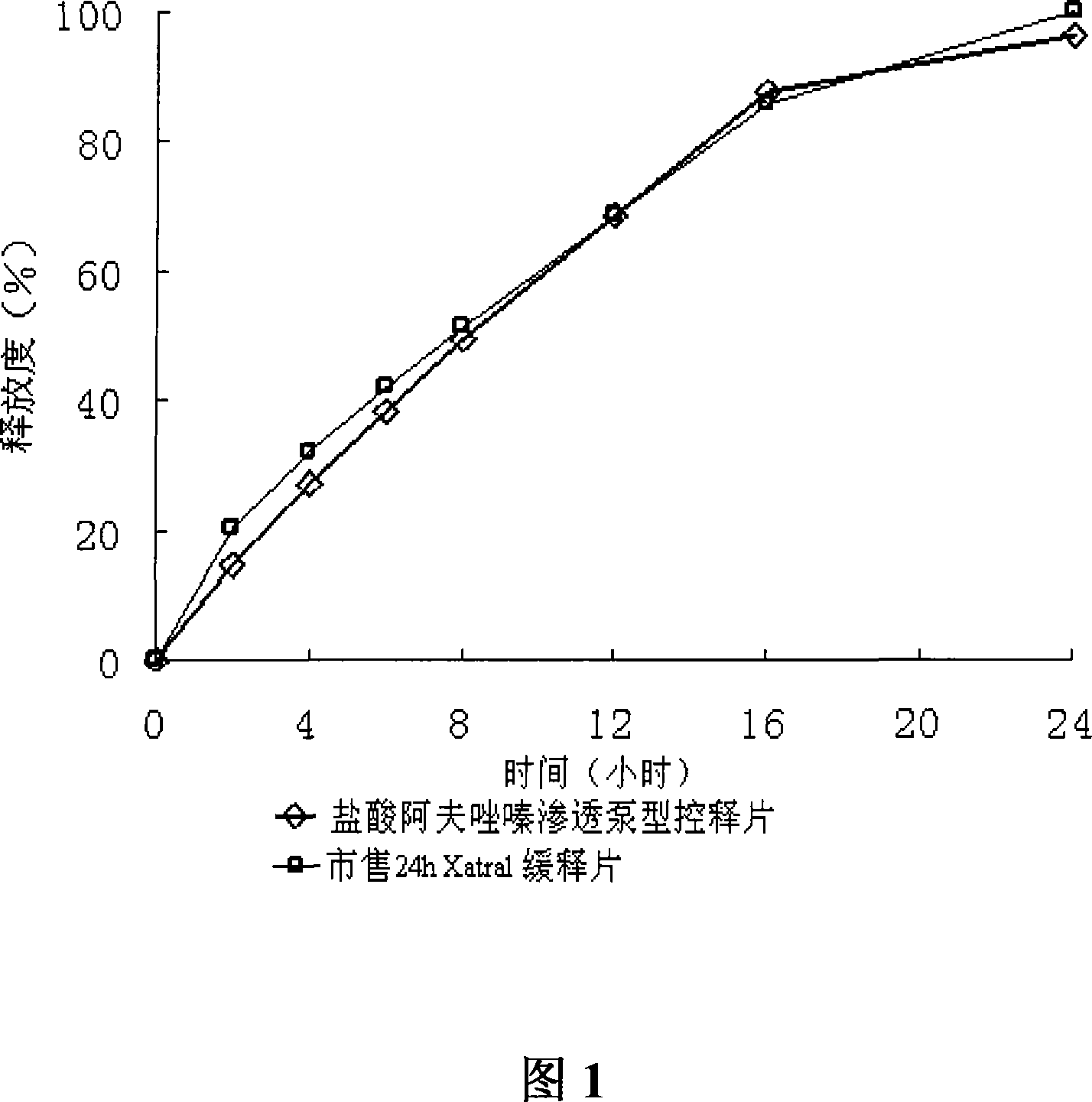
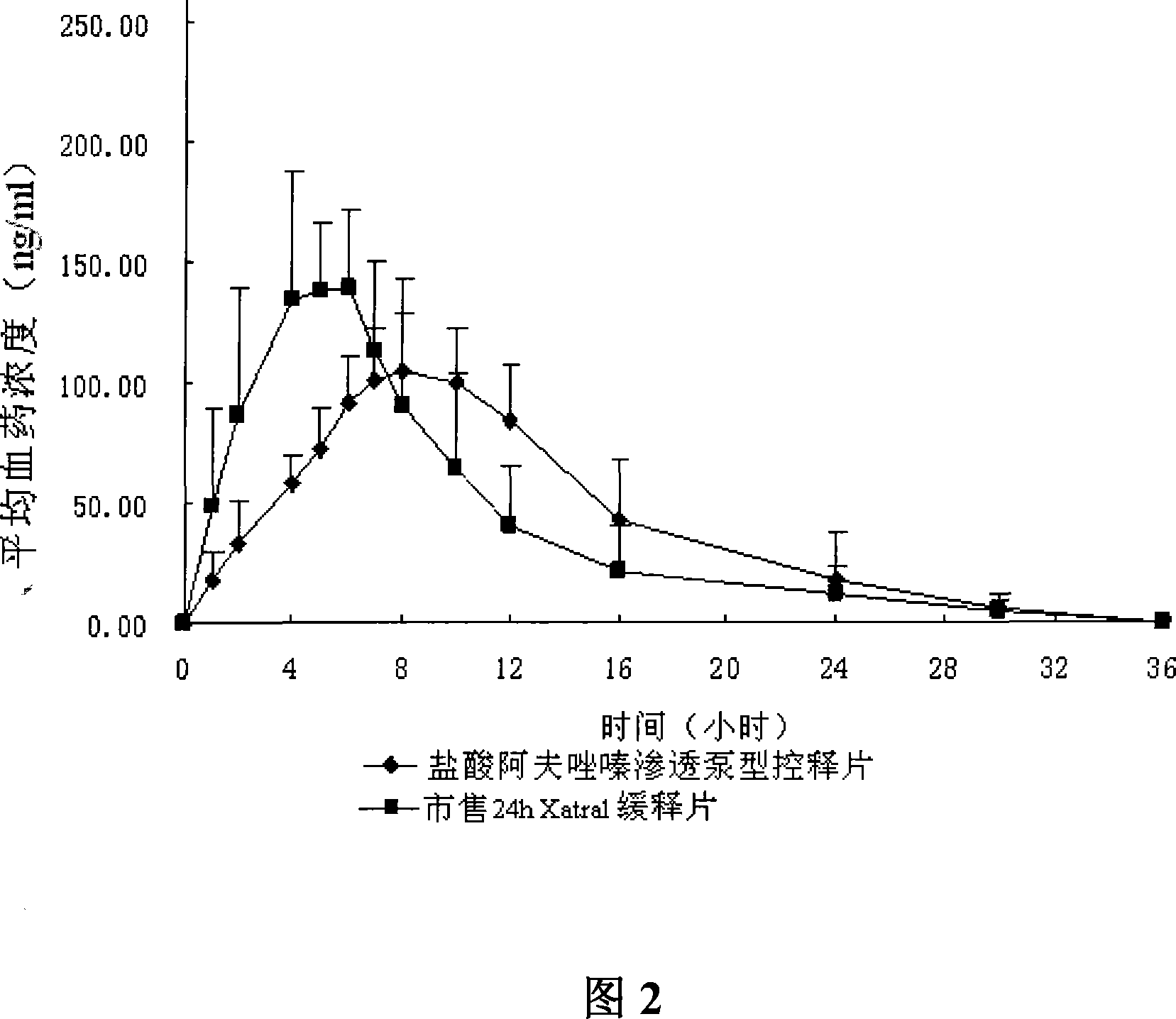
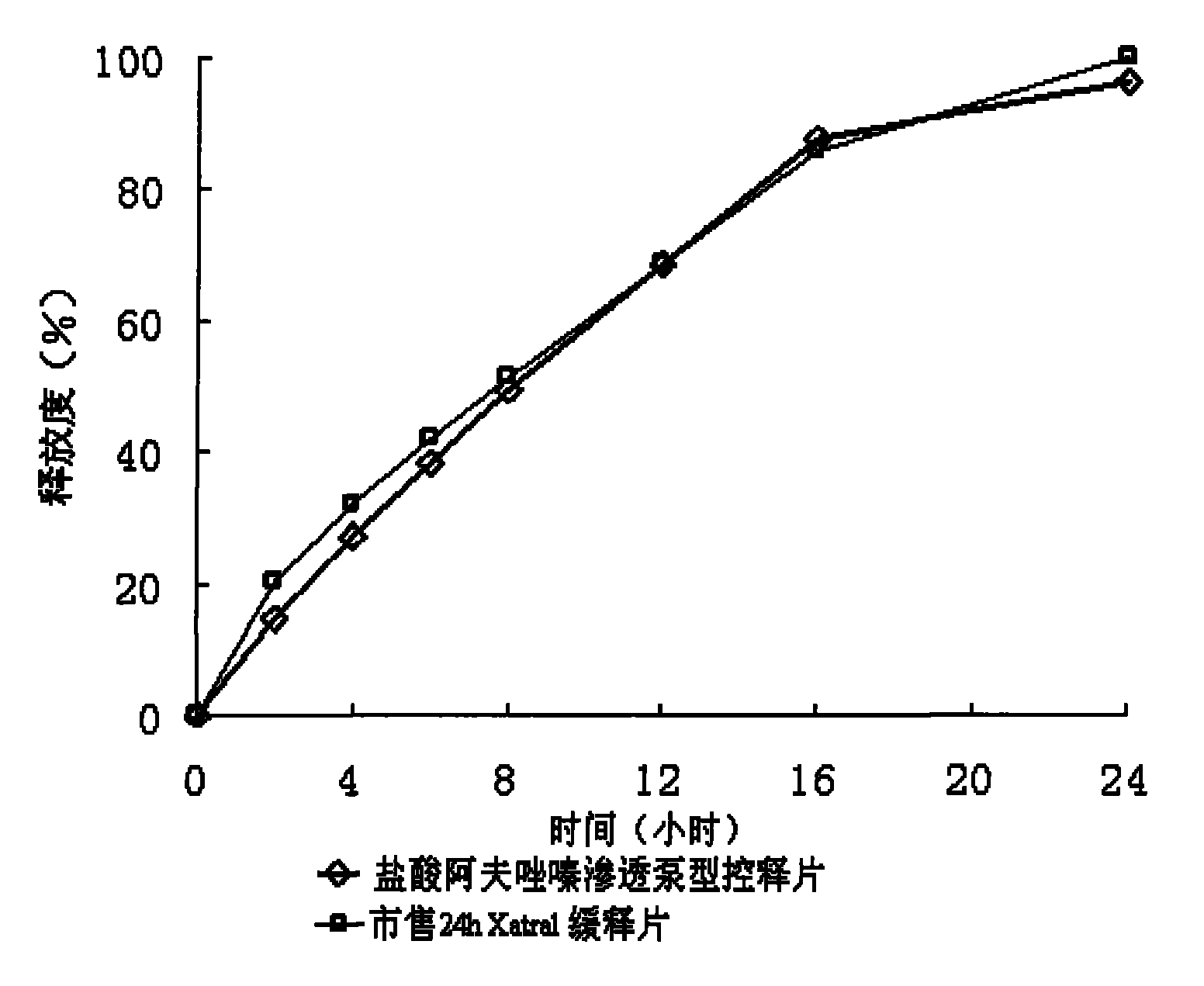
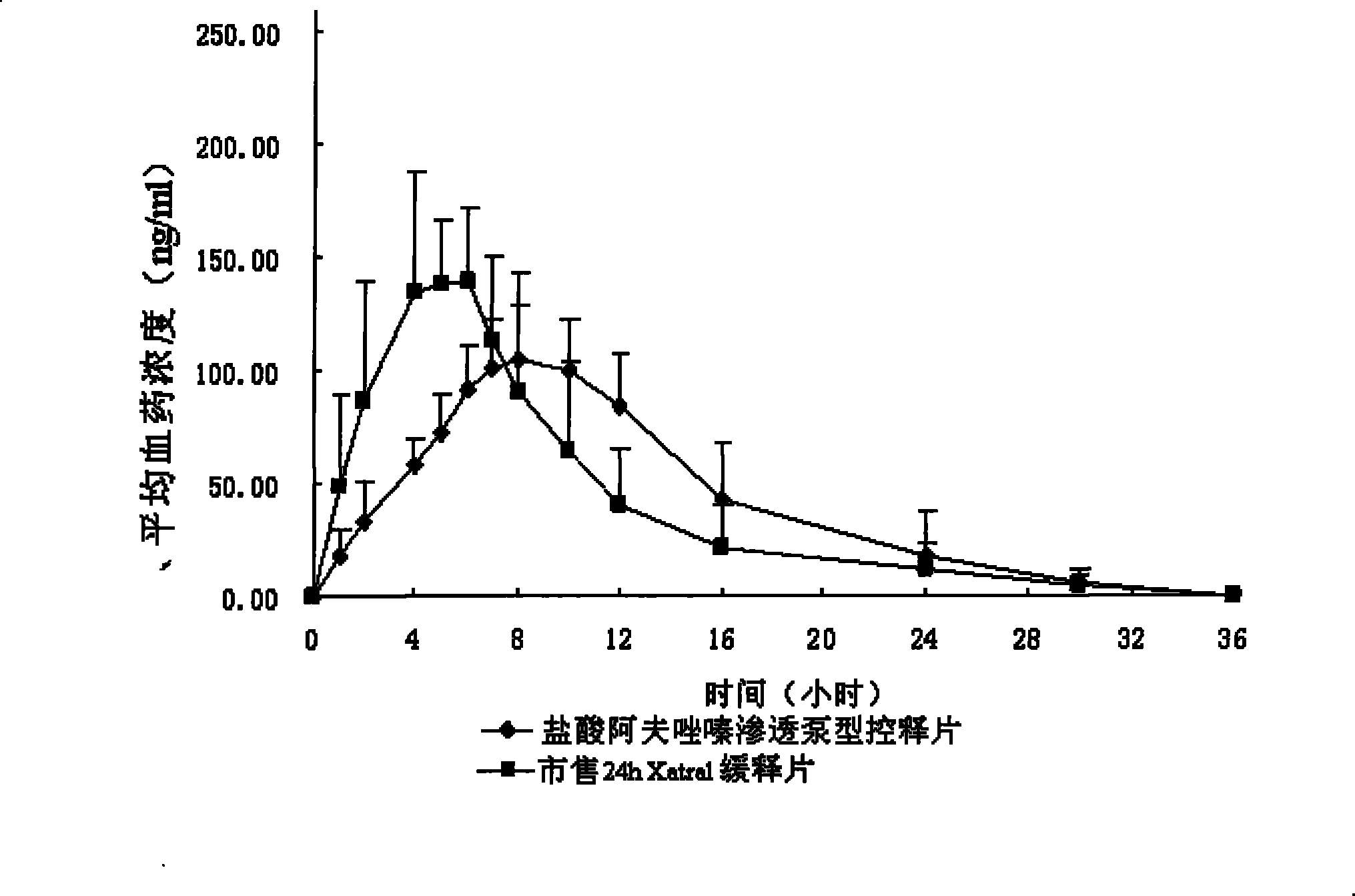
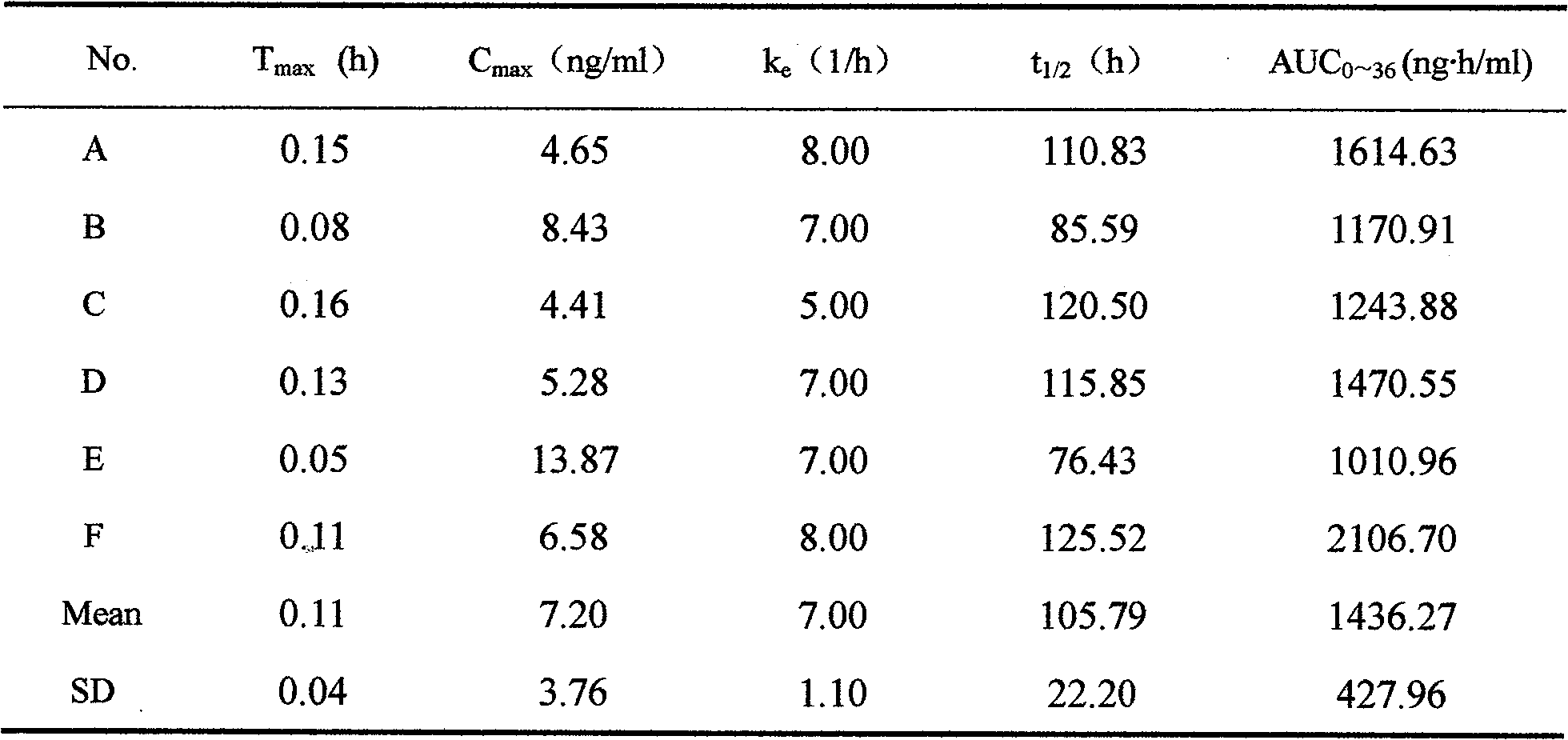
Alfuzosin Hydrochloride permeating pump type controlled-release preparation and method for preparing the same
InactiveCN101095681AStable blood concentrationLong lastingOrganic active ingredientsPharmaceutical delivery mechanismControlled Release TabletOsmotic pump
The invention belongs to medical technique field, and relates to osmotic pump controlled-release tablets of alfuzosin hydrochloride and the preparation method, which comprises tablet core, clothing sheet membrane and controlled-release pore. The solid medicine and osmotic active substance and shaping agent forms the tablet core; the coating membrane is insoluble controlled-release clothing sheet that is water permeable but not medical permeable. Said pore is on the clothing sheeting membrane. The comprised raw material of tablet core and clothing sheet membrane and proportion by weight are as follows: table core: alfuzosin hydrochloride 0.5-50%, packing agent 5-80%, penetration -promoting agent 5-80%, lubricating agent 0.2-3%, adhesive agent 0.01-10%; clothing sheet membrane: semi-permeable membrane clothing sheet high polymer material 70-99%, plasticizing agent 1-30%. The invention is characterized by stable and continuous release, long retention time for effective blood concentration and reduced peak-to-valley of blood concentration phenomena.
Owner:KANGYA OF NINGXIA PHARMA +1
Phencynonate hydrochloride double-layer osmotic pump controlled release tablet and preparation method thereof
ActiveCN103284974AAvoid large fluctuations in blood concentrationImprove effectivenessOrganic active ingredientsSenses disorderMotion sicknessSide effect
The invention provides a phencynonate hydrochloride double-layer osmotic pump controlled release tablet and a preparation method thereof. The tablet comprises, from interior to exterior, a double-layer tablet core prepared through compaction of a drug-containing layer and a boosting layer, a semipermeable coating film with drug releasing holes and a moistureproof coating film, wherein the drug-containing layer contains an effective amount of phencynonate hydrochloride, a suspending aid, an osmotic pressure promoter and other pharmaceutic adjuvants, the boosting layer contains a propellant, an osmotic pressure promoter, a sustained releasing agent and other pharmaceutic adjuvants, and the semipermeable coating film is perforated with more than one drug releasing hole in one side close to the drug-containing layer. According to the invention, the phencynonate hydrochloride double-layer osmotic pump controlled release tablet has a substantial zero order drug release characteristic and enables drugs to enter into a human body at a constant speed; drug release behavior is not affected by gastrointestinal peristalses, pH values, food effects and the like, individual difference of the drug release behavior is small, and blood concentration is steady; the tablet has long-lasting drug effects, is taken once per day, has little toxic and side effects and good compliance in administration and can be used for preventing or treating motion sickness syndrome, Parkinson' disease, Parkinson' disease syndrome, acute attack of vertigo and epilepsy.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Simvastatin osmotic pump preparation and preparation method thereof
ActiveCN102512398AImprove securityImprove effectivenessOrganic active ingredientsMetabolism disorderOsmotic pumpSucrose
The invention discloses simvastatin monolayer osmotic pump sustained release tablets. Each simvastatin monolayer osmotic pump sustained release tablet comprises a tablet core and a controlled-release film coated layer, wherein the tablet core contains the following components in percentage by weight: 1 to 20 percent of simvastatin serving as a main medicine, 1 to 25 percent of filling agent, 50 to 90 percent of penetrating agent, 1 to 15 percent of adhesive, 0.5 to 5 percent of release conditioning agent and 0.5 to 3 percent of lubricating agent; the controlled-release film coated layer comprises a controlled-release film-forming material, a plasticizer and / or a pore-forming agent; the filling agent comprises microcrystalline cellulose and dextrin; and the penetrating agent contains cane sugar and lactose.
Owner:HEFEI LIFEON PHARMA
Nifedipine controlled release capsule and preparation method thereof
InactiveCN111184699AWell mixedRelease constantOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseDrug release rate
The invention provides a nifedipine controlled release capsule and a preparation method thereof. The controlled release capsule is prepared by filling a capsule shell with nifedipine, polyoxyethyleneand organic acid and coating the capsule shell. The mass ratio of nifedipine, polyoxyethylene and organic acid is (10-90):(1-15):(1-30); the coating is prepared from cellulose, a plasticizer and a pore-forming agent by mixing in a weight ratio of (10-50):(0-15):(1-25). Double-layer tabletting, laser or mechanical punching are not required, the preparation process is simple, the cost is low, the drug release rate is stable, zero-order release is basically achieved within 24 hours, and drug release is complete; the capsule is administered once a day, and thus, patient compliance is improved.
Owner:HEBEI UNIVERSITY
Indapamide osmotic pump preparation and preparation method thereof
ActiveCN102429886AImprove securityImprove effectivenessOrganic active ingredientsPharmaceutical delivery mechanismRelease modulatorProlonged-release tablet
The invention discloses an indapamide monolayer osmotic pump sustained-release tablet, which comprises a tablet core and a controlled-release film coating layer, wherein the tablet core contains 0.5-2% of indapamide as a monarch drug, 1-20% of filling agent, 50-90% of penetration enhancer, 1-15% of adhesive, 1-20% of release regulator and 0.5-3% of lubricant; the controlled-release film coating layer comprises a controlled-release film forming material, a plasticizer and / or a pore-forming agent; and the filling agent comprises agar powder.
Owner:HEFEI LIFEON PHARMA
Osmotic pump controlled release tablet of solid dispersion of hydroxy camptothecin and prepn. method
InactiveCN1872031AImprove solubilityImprove absorption rateOrganic active ingredientsPharmaceutical delivery mechanismControlled Release TabletOsmotic pump
A tablet of the dispersed hydroxy camptothecine solid released under the control of osmotic pump is composed of a tablet core containing dispersed hydroxy camptothecine solid, release controlling auxiliaries, and carrier and a perforated coated layer. Its preparing process includes preparing tablet core, coating and perforating.
Owner:TSINGHUA UNIV
Posaconazole double-layer osmotic pump controlled release tablet and preparation method thereof
InactiveCN103948558AReduced responseDelay drug resistanceOrganic active ingredientsAntimycoticsIn vivoPharmaceutical formulation
The invention belongs to the field of medicinal preparation and specifically provides a posaconazole double-layer osmotic pump controlled release tablet and a preparation method thereof. The tablet comprises a tablet core and a semipermeable controlled release film. The tablet core comprises a drug containing layer and a boosting layer. The drug containing layer is located inside the controlled release film which is provided with a drug releasing hole. The boosting layer is located inside the controlled release film at a place far away from the drug releasing hole. One end of the water-permeable controlled release film is provided with one or more drug releasing holes. The release curve of the preparation drug shows a zero-order release pattern, and thus the problem of drug resistance caused by long-term use of posaconazole dosage forms on the market is solved. Besides, the problems that the preparation technology is complex, the preparation cost is high, and the drug is unsuitable for industrialized mass production due to the adoption of a hot-melt extrusion technology are solved. Simultaneously, the in vivo-in vitro correlation of the drug is better ensured and the clinical monitoring of plasma concentration is better facilitated.
Owner:JINAN KANGHE MEDICAL TECH
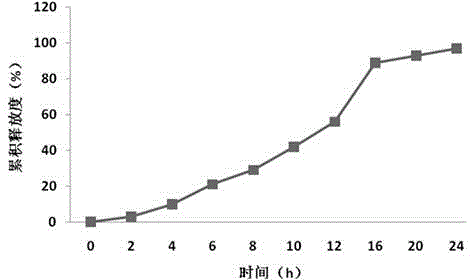
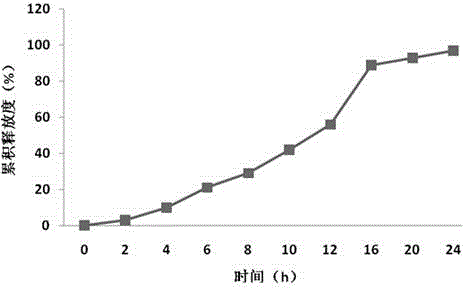
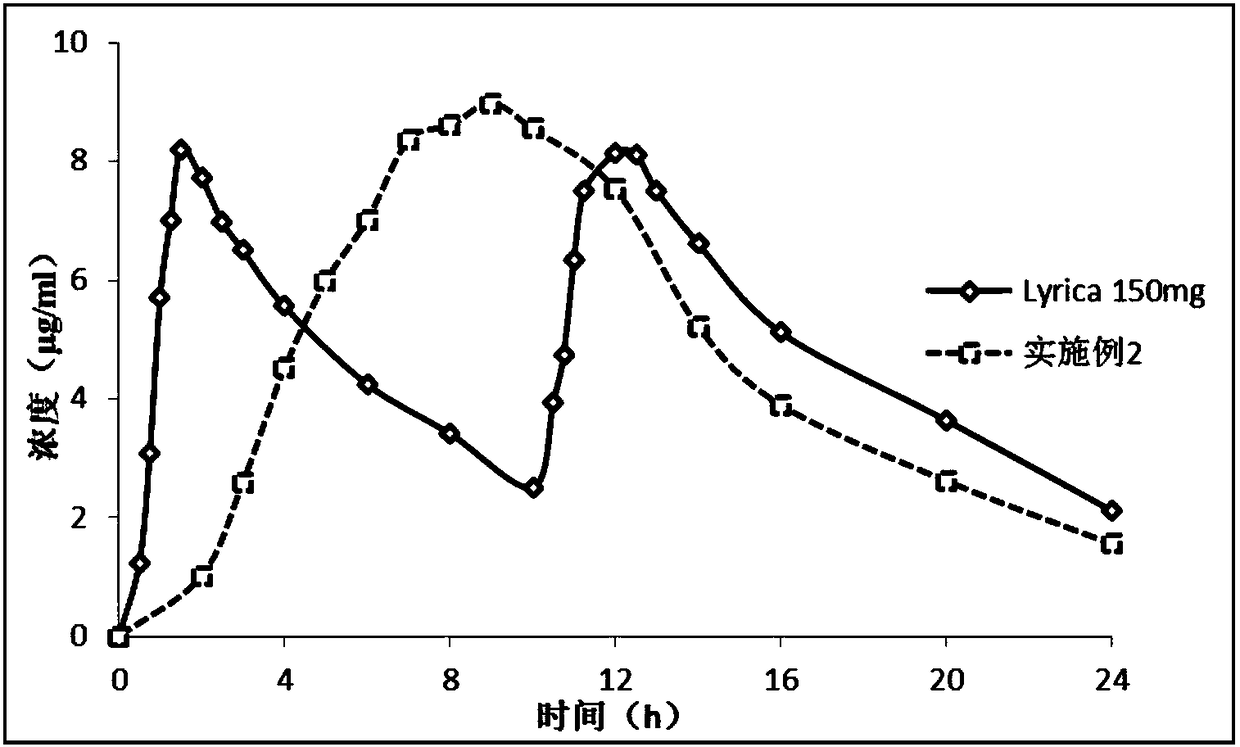
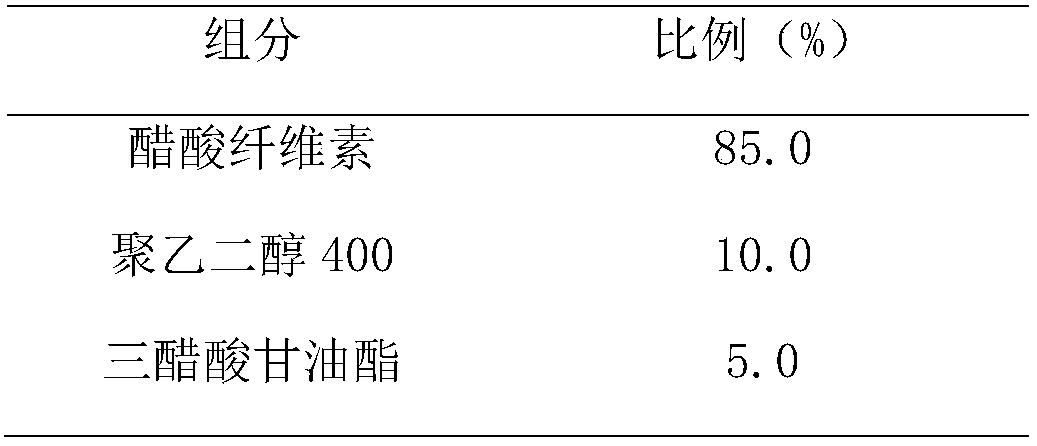
Single-chamber osmotic pump controlled release tablet containing pregabalin
InactiveCN108478537AGood correlation between in vitro and in vivoReduce peak and valley effectOrganic active ingredientsNervous disorderTime rangeWater soluble
The invention discloses a single-chamber osmotic pump controlled release tablet containing pregabalin. The tablet comprises a single-layer piece core, a controlled release film coating layer and at least one laser hole in a controlled release film. The basic composition of the piece core includes pregabalin or salt or hydrate thereof, diluent, adhesive and lubricant, and does not include an expansible polymer. The adopted accessories are simple and economical, the tablet can be prepared through a simple direct tabletting process, and the controlled release film coating layer includes a film forming material, a pore-foaming agent and plasticizer. According to the drug release mechanism, water and a physiological medium dissolve water-soluble active components and the diluent in the piece core through the controlled release film to form trans-film osmotic pressure, the dissolved active components are released out of the piece core through the small holes in the controlled release film, the controlled release tablet can release a therapeutic drug within a certain time range at a constant drug release rate, and the influence of the stomach and intestine difference between different individuals on drug absorption can be minimized.
Owner:南京易亨制药有限公司
Nifedipine microporous osmotic pump core-encapsulating tablet with expanding tablet core and preparation method thereof
InactiveCN107753457ANot easy to produce concentrationNo cloggingOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineSemipermeable membrane
The invention belongs to the technical field of medicines, and particularly relates to a nifedipine microporous osmotic pump core-encapsulating tablet with an expanding tablet core and a preparation method thereof. The expanding tablet core is contained in the microporous osmotic pump core-encapsulating tablet, a nifedipine medicine-containing layer and a semipermeable membrane are sequentially arranged on the surface of the expanding tablet core, and the weight proportion of all the components is: expanding tablet core:nifedipine medicine-containing layer:semipermeable membrane=(5-55):(5-75):(5-30). The nifedipine microporous osmotic pump core-encapsulating tablet containing the expanding tablet core which is prepared by adopting the invention is almost equivalent to a medicine released at a zero-order release rate, moreover, perforation is not required, and the production process is mature, and can be industrially scaled up.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Three-stage pulsed release controlled release tablet and preparation method thereof
ActiveCN101773482AImprove curative effectLittle side effectsOrganic active ingredientsPharmaceutical delivery mechanismControlled Release TabletOsmotic pump
The invention provides a metoprolol pulse osmotic pump controlled release tablet and a preparation method thereof. The controlled release tablet comprises a tablet core, a time lag coating layer and a controlled release coating layer with a release orifice from inside to outside, wherein the tablet core consists of a drug containing layer and a boosting layer, based on the gross weight of the drug containing layer, the drug containing layer comprises 5-65 wt% of metoprolol as active material and 35-95 wt% of pharmaceutically acceptable carrier; based on the gross weight of the boosting layer, the boosting layer comprises 30-85 wt% of swelling agent and 15-70 wt% of osmotic pressure promoting agent; the weight gain of the time lag coating layer is 5-50 wt% of the tablet core; and the weight gain of the controlled release coating layer is 3-30 wt% of the tablet core. The metoprolol pulse osmotic pump controlled release tablet has three-stage release behavior, and is characterized in that the tablet has 2-5 hours time lag at the initial period after being taken orally, increased release is showed at the intermediate period, and release at constant speed is showed at the later period.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Calcium rosuvastatin and fenofibric acid choline salt time-selecting osmotic pump controlled release tablet and preparation method thereof
InactiveCN103356500ASmall toxicityImprove complianceOrganic active ingredientsMetabolism disorderFENOFIBRIC ACIDControlled Release Tablet
The invention provides a compound preparation consisting of calcium rosuvastatin and a fenofibric acid choline salt time-selecting osmotic pump controlled release tablet and a preparation method thereof. The compound preparation structurally comprises the following components from inside to outside in sequence: a tablet chip consisting of a medicine containing layer and a boosting layer, a controlled release coating for a medicine release hole, a calcium rosuvastatin quick release layer and a protective gastric soluble thin film coating. The preparation method comprises the following steps of: (1) preparing the medicine containing layer; (2) preparing the boosting layer; (3) pressing the tablet chip; (5) coating a control release coating; (6) punching the coating tablet; (6) coating the calcium rosuvastatin quick release layer; and (7) coating the protective gastric soluble thin film coating.
Owner:肖广常
Quality evaluation and control method of florfenicol sustained-release particles
ActiveCN113288876AGood correlation between in vitro and in vivoEnsure consistencyAntibacterial agentsOrganic active ingredientsBiochemical engineeringPharmaceutical drug
The invention discloses a quality evaluation and control method of florfenicol sustained-release granules, which comprises the following steps of: obtaining in-vitro disintegration and release processes of florfenicol sustained-release granule medicines through a dissolution test, and establishing correlation with the in-vivo process of the medicines, so that the in-vitro dissolution limit of the medicines is formulated as follows: in simulated gastric juice, the in-vitro disintegration and release processes of the florfenicol sustained-release granules are subjected to the in-vitro disintegration and release processes of the florfenicol sustained-release granules; the cumulative release rate within 30 minutes does not exceed 20%, and the cumulative release rate within 2 hours does not exceed 50%; and in simulated intestinal juice, the cumulative release rate within 30 minutes does not exceed 25%, the cumulative release rate within 4 hours does not exceed 75%, and the cumulative release rate within 8 hours is greater than 85%. By taking the dissolution limit of the florfenicol sustained-release product as a quality standard and evaluating the in-vitro dissolution of the florfenicol sustained-release product, the product quality of preparation production is controlled, the consistency of in-vivo metabolic processes of products of different production batches is ensured, and a basis is provided for product quality control and evaluation.
Owner:SOUTH CHINA AGRI UNIV
Novel incremental release osmotic pump preparation of paliperidone and preparation method thereof
ActiveCN103271889BAvoid titrationImprove complianceOrganic active ingredientsNervous disorderControlled Release TabletOsmotic pump
Owner:SHENYANG PHARMA UNIVERSITY
Ginkgolide osmotic pump tablet and preparation method thereof
InactiveCN103142532BImplement synchronous releaseImprove solubilityPharmaceutical delivery mechanismGinkgophyta medical ingredientsSolubilityPatient compliance
The invention provides a ginkgolide osmotic pump tablet and a preparation method thereof. The ginkgolide osmotic pump tablet provided by the invention is a double-layer tablet composed of a drug-containing layer and a push layer, and is coated, wherein the drug-containing layer comprises drug release holes. The ginkgolide osmotic pump tablet uses ginkgolide at effective positions of the ginkgo biloba extract as an active component, and a total amount of ginkgolide A, ginkgolide B, ginkgolide Cand bilobalide is not less than 70 %. According to the invention, by combining a solid dispersion solubilising technique and an osmotic pump tablets control-release technology, the ginkgolide is firstly prepared into the solid dispersion, and then further prepared into the ginkgolide osmotic pump tablet on a basis of improving solubility of poorly soluble components, thereby realizing synchronous slow release of complex components in the ginkgolide, reducing drug administration times and improving patient compliance.
Owner:FUDAN UNIV
Alfuzosin Hydrochloride permeating pump type controlled-release preparation and method for preparing the same
InactiveCN101095681BReduce toxic and side effectsStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismRetention timeContinuous release
The invention belongs to medical technique field, and relates to osmotic pump controlled-release tablets of alfuzosin hydrochloride and the preparation method, which comprises tablet core, clothing sheet membrane and controlled-release pore. The solid medicine and osmotic active substance and shaping agent forms the tablet core; the coating membrane is insoluble controlled-release clothing sheet that is water permeable but not medical permeable. Said pore is on the clothing sheeting membrane. The comprised raw material of tablet core and clothing sheet membrane and proportion by weight are asfollows: table core: alfuzosin hydrochloride 0.5-50%, packing agent 5-80%, penetration -promoting agent 5-80%, lubricating agent 0.2-3%, adhesive agent 0.01-10%; clothing sheet membrane: semi-permeable membrane clothing sheet high polymer material 70-99%, plasticizing agent 1-30%. The invention is characterized by stable and continuous release, long retention time for effective blood concentration and reduced peak-to-valley of blood concentration phenomena.
Owner:KANGYA OF NINGXIA PHARMA +1
Nifedipine micro-porous osmotic pump particle and preparation method thereof
InactiveCN107773555AImprove securityImprove effectivenessOrganic active ingredientsPill deliveryNifedipineMedicine
The invention belongs to the technical field of medicines and in particular discloses a nifedipine micro-porous osmotic pump particle taking polyoxyethylene-containing particles as an expanded pill core and a preparation method thereof. The micro-porous osmotic pump particle takes the polyoxyethylene particles as the expanded pill core. The nifedipine micro-porous osmotic pump particle prepared bythe invention is capable of releasing drugs at a nearly zero release rate and is simple in production process and capable of easily realizing industrialized enlargement.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Simulation liquid and method for in-vitro detection of zinc-containing medical instrument
PendingCN111381018AQuick collectionGood correlation between in vitro and in vivoBiological testingWhole blood unitsMicrobiology
The invention relates to a simulation liquid for in-vitro detection of a zinc-containing medical instrument. The simulation liquid comprises 1-50g / L of a DMEM culture medium, 0-80% of serum or whole blood or plasma and a pH buffer substance in volume ratio. In vitro, the zinc-containing medical instrument is placed in the simulation liquid, the zinc content in the simulation liquid and the zinc content in the cleaning liquid are tested, the biological risk can be evaluated, the zinc corrosion speed can be obtained, and the result can correspond in vivo and in vitro. The testing method can be used for detecting whether the zinc corrosion speed of a product in production is stable or not, and can also be used for preliminarily judging whether the support design meets the requirements or notin the research and development process.
Owner:BIOTYX MEDICAL (SHENZHEN) CO LTD
Indapamide osmotic pump preparation and preparation method thereof
ActiveCN102429886BImprove securityImprove effectivenessOrganic active ingredientsPharmaceutical delivery mechanismRelease modulatorProlonged-release tablet
The invention discloses an indapamide monolayer osmotic pump sustained-release tablet, which comprises a tablet core and a controlled-release film coating layer, wherein the tablet core contains 0.5-2% of indapamide as a monarch drug, 1-20% of filling agent, 50-90% of penetration enhancer, 1-15% of adhesive, 1-20% of release regulator and 0.5-3% of lubricant; the controlled-release film coating layer comprises a controlled-release film forming material, a plasticizer and / or a pore-forming agent; and the filling agent comprises agar powder.
Owner:HEFEI LIFEON PHARMA
A kind of posaconazole double-layer osmotic pump controlled-release tablet and preparation method thereof
InactiveCN103948558BReduced responseDelay drug resistanceOrganic active ingredientsAntimycoticsIn vivoPharmaceutical formulation
Owner:JINAN KANGHE MEDICAL TECH
Bicyclol double-layer osmotic pump control-released tablet and preparation method thereof
ActiveCN102091052BImprove clinical efficacyReduce peak and trough plasma concentration differencesDigestive systemAntiviralsSide effectBlood concentration
Owner:BEIJING UNION PHARMA FACTORY
A medicine core composition of controlled release drug delivery and controlled release preparation as well as its preparing method
ActiveCN100563637CImprove securityImprove effectivenessInorganic non-active ingredientsOsmotic deliverySolubilityControlled drugs
The present invention provides a drug core composition for controlled-release administration of active drugs with low solubility, which at least includes a drug-containing layer and a booster layer, and the drug-containing layer includes active drugs with low solubility and hydrophilic A polymer carrier, the booster layer at least includes a permeation-promoting polymer, an insoluble polymer and an osmotic pressure enhancer; the present invention also provides an osmotic pump preparation containing the drug core composition, which also includes The thin film of semipermeable material; drug core composition of the present invention can release drug at a controlled rate, so that the formulation containing the drug core composition can release the active drug in about 24 hours once a day. .
Owner:OCEAN STAR INT
An osmotic pump controlled release tablet of solid dispersion of hydroxy camptothecin and a preparation method thereof
InactiveCN100464740CHas zero-order drug release behaviorSmall individual differencesOrganic active ingredientsPharmaceutical delivery mechanismControlled Release TabletOsmotic pump
A tablet of the dispersed hydroxy camptothecine solid released under the control of osmotic pump is composed of a tablet core containing dispersed hydroxy camptothecine solid, release controlling auxiliaries, and carrier and a perforated coated layer. Its preparing process includes preparing tablet core, coating and perforating.
Owner:TSINGHUA UNIV
Pitavastatin calcium double-layer osmotic pump controlled-release tablet and preparation method thereof
InactiveCN101829069BAvoid large fluctuations in blood concentrationImprove securityMetabolism disorderPill deliveryControlled Release TabletDrug release
The invention discloses a pitavastatin calcium double-layer osmotic pump controlled-release tablet and a preparation method thereof. The controlled-release tablet comprises a double-layer slab core consisting of a medicament-containing layer and a driving layer, and a layer of coating film which is wrapped on the outer surface of the double-layer slab core, belongs to semi-transparent films, and is provided with a medicament-releasing pore on the surface of the medicament-containing layer, wherein the medicament-containing layer in the slab core contains pitavastatin calcium which accounts for 0.1 to 1.0 percent of the weight of the double-layer slab core, and also contains a basic auxiliary material, an osmotic active substance, a penetration enhancer, a bonding agent and a lubricating agent; the driving layer contains a penetration enhancer, an osmotic active substance, a bonding agent and a lubricating agent; and the coating film comprises one or more of a film forming material or a pore-forming agent, a plasticizing agent and the like. The pitavastatin calcium double-layer osmotic pump controlled-release tablet has the characteristics of stable medicament releasing rate after being taken and easily controlled accumulative release rates in a specific time, can effectively reduce the medicament taking times of patients so as to improve adaptability of the patients, and has high safety and effectiveness.
Owner:北京华禧联合科技发展有限公司
Phencynonate hydrochloride double-layer osmotic pump controlled-release tablet and preparation method thereof
ActiveCN103284974BAvoid large fluctuations in blood concentrationImprove effectivenessOrganic active ingredientsSenses disorderMotion sicknessHuman body
The invention provides a phencynonate hydrochloride double-layer osmotic pump controlled release tablet and a preparation method thereof. The tablet comprises, from interior to exterior, a double-layer tablet core prepared through compaction of a drug-containing layer and a boosting layer, a semipermeable coating film with drug releasing holes and a moistureproof coating film, wherein the drug-containing layer contains an effective amount of phencynonate hydrochloride, a suspending aid, an osmotic pressure promoter and other pharmaceutic adjuvants, the boosting layer contains a propellant, an osmotic pressure promoter, a sustained releasing agent and other pharmaceutic adjuvants, and the semipermeable coating film is perforated with more than one drug releasing hole in one side close to the drug-containing layer. According to the invention, the phencynonate hydrochloride double-layer osmotic pump controlled release tablet has a substantial zero order drug release characteristic and enables drugs to enter into a human body at a constant speed; drug release behavior is not affected by gastrointestinal peristalses, pH values, food effects and the like, individual difference of the drug release behavior is small, and blood concentration is steady; the tablet has long-lasting drug effects, is taken once per day, has little toxic and side effects and good compliance in administration and can be used for preventing or treating motion sickness syndrome, Parkinson' disease, Parkinson' disease syndrome, acute attack of vertigo and epilepsy.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com