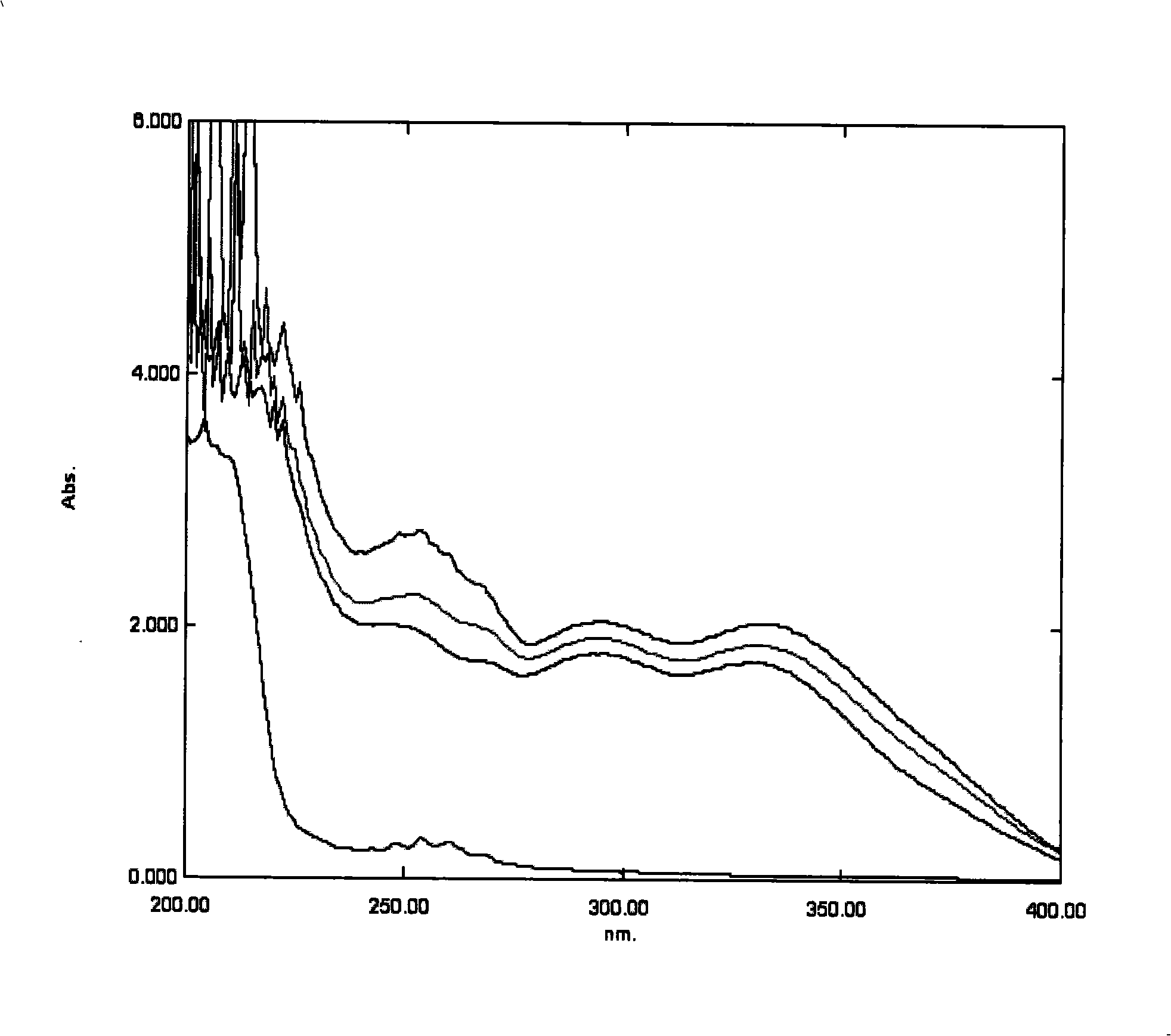
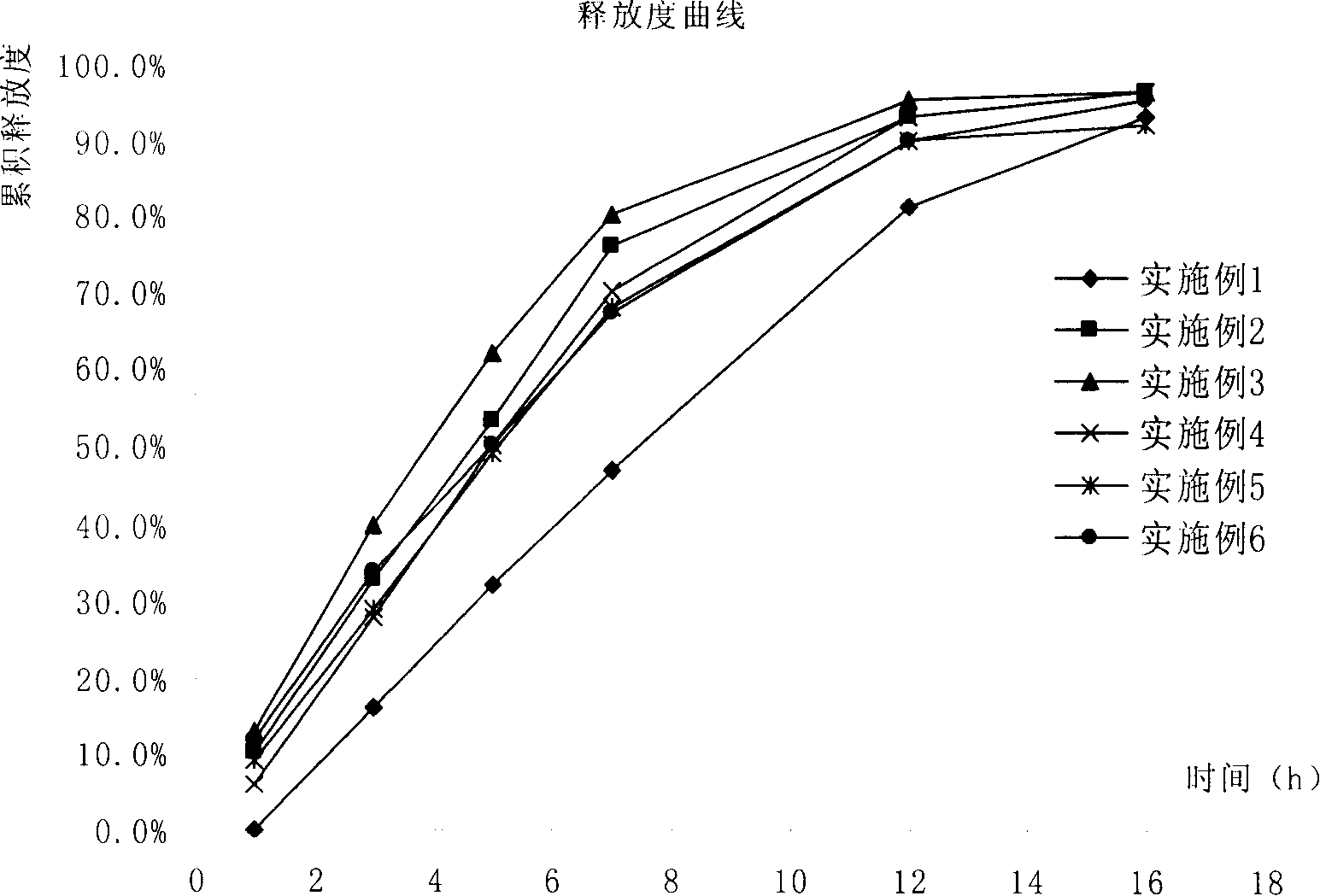
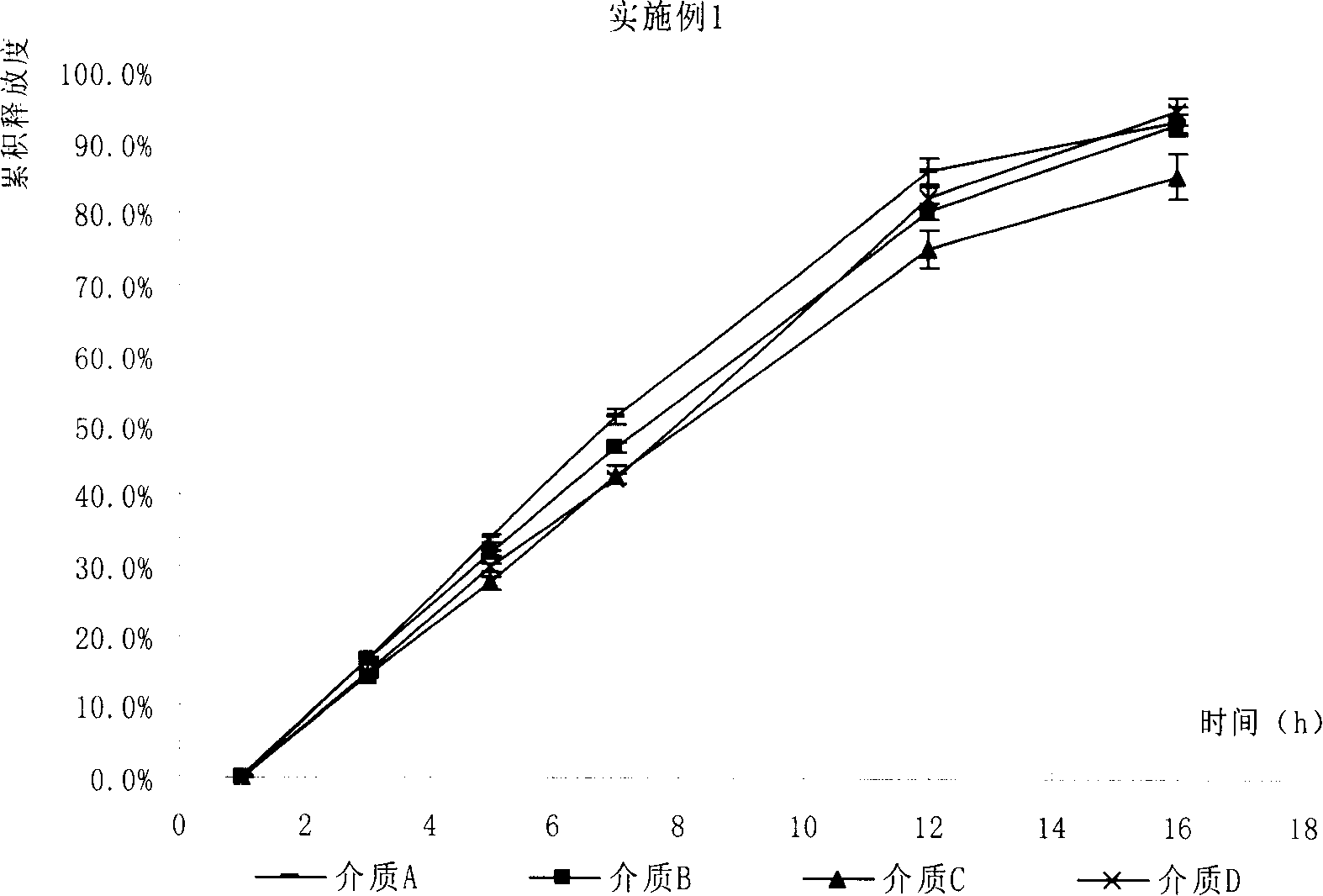
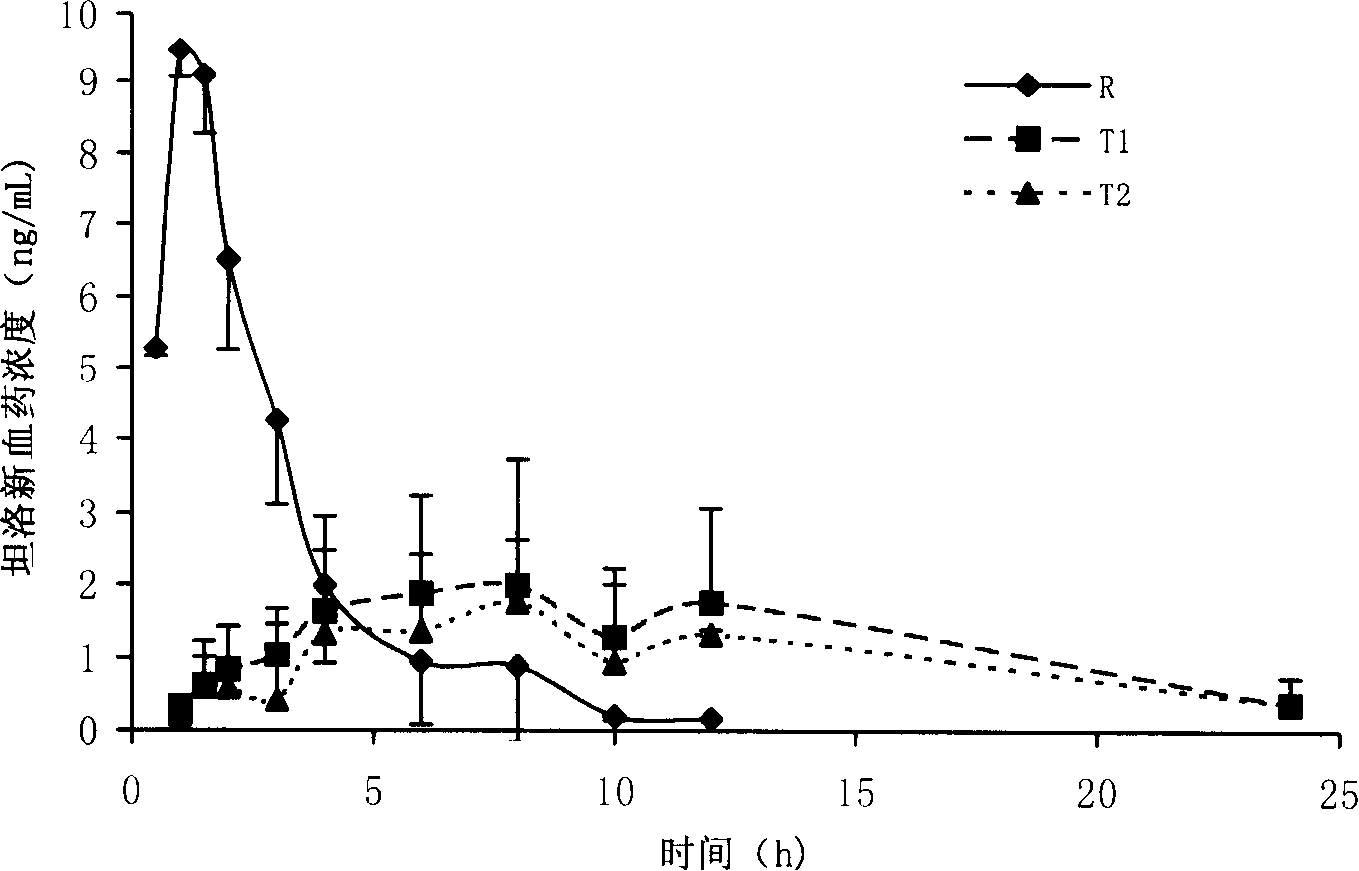
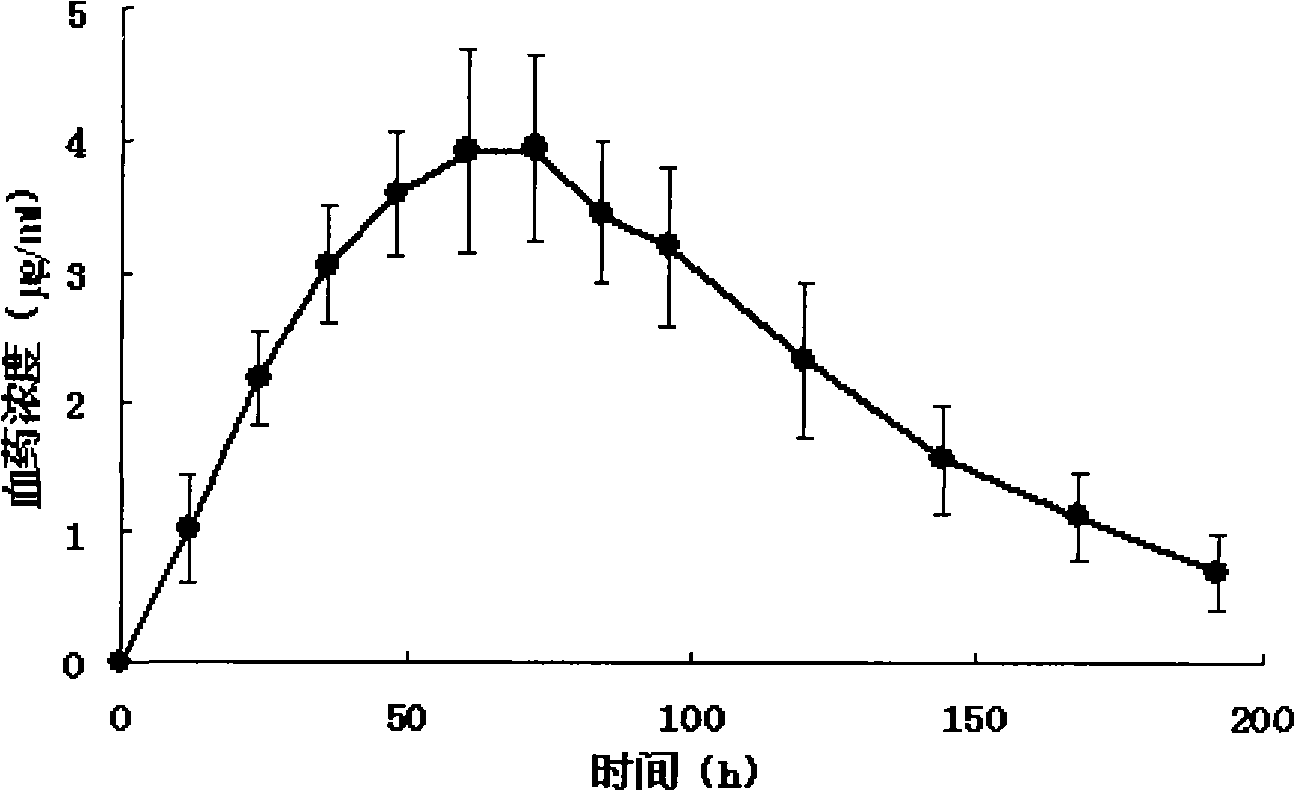
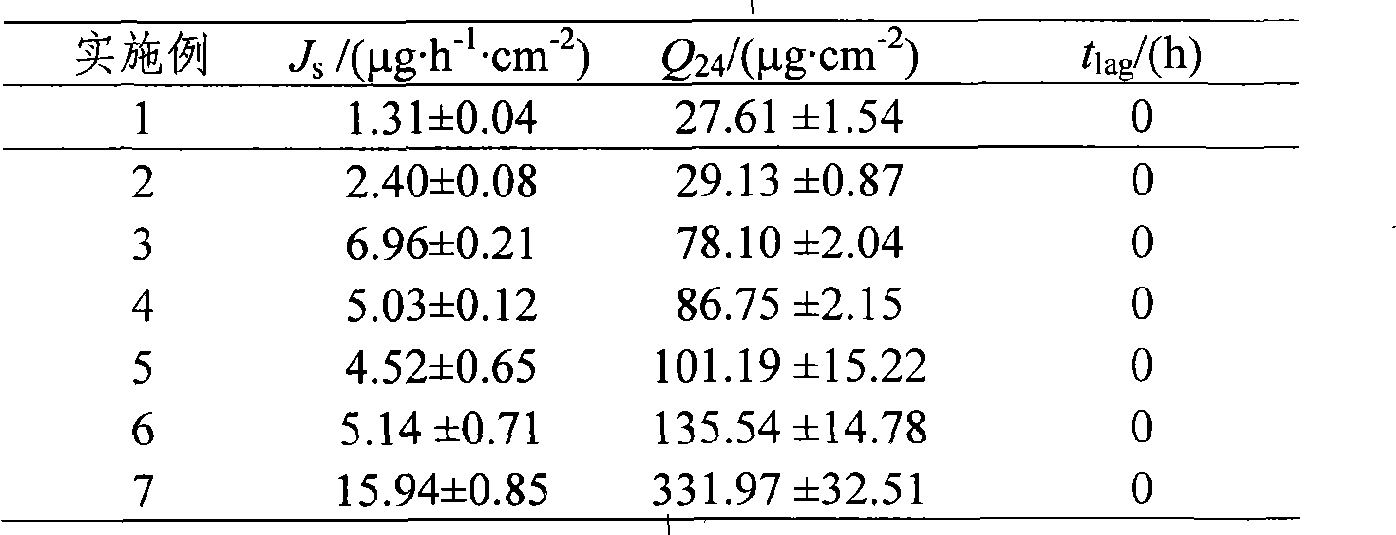
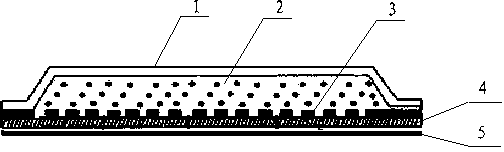
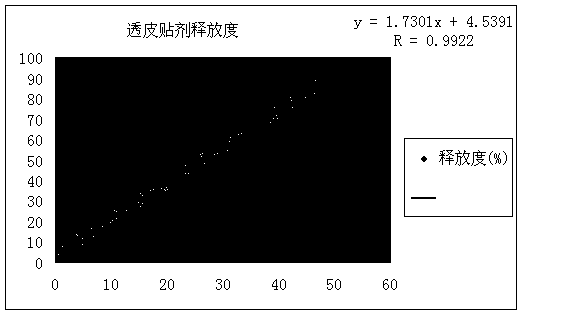
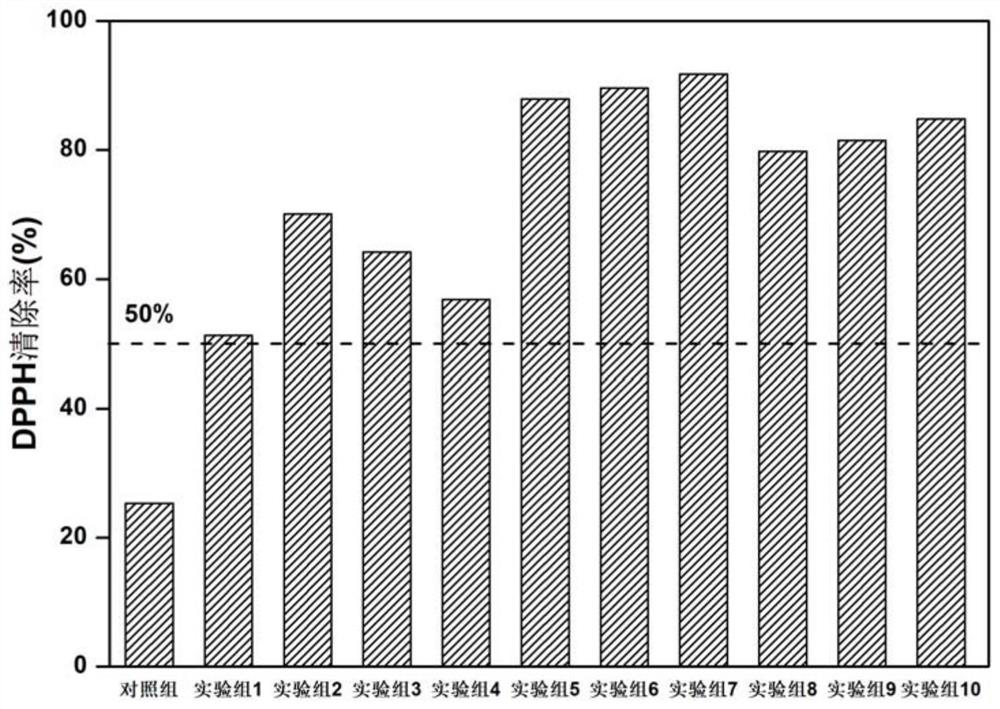
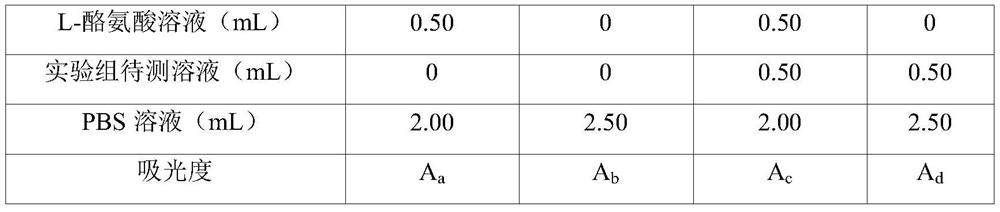
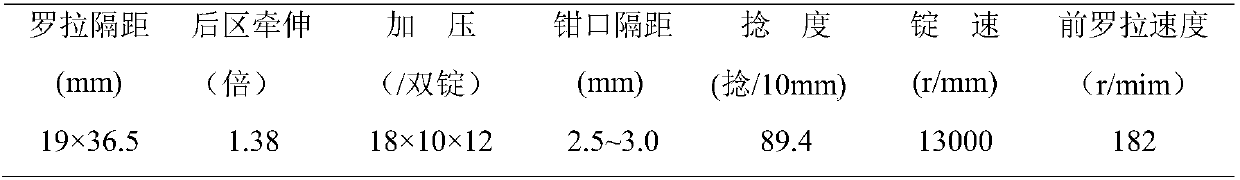
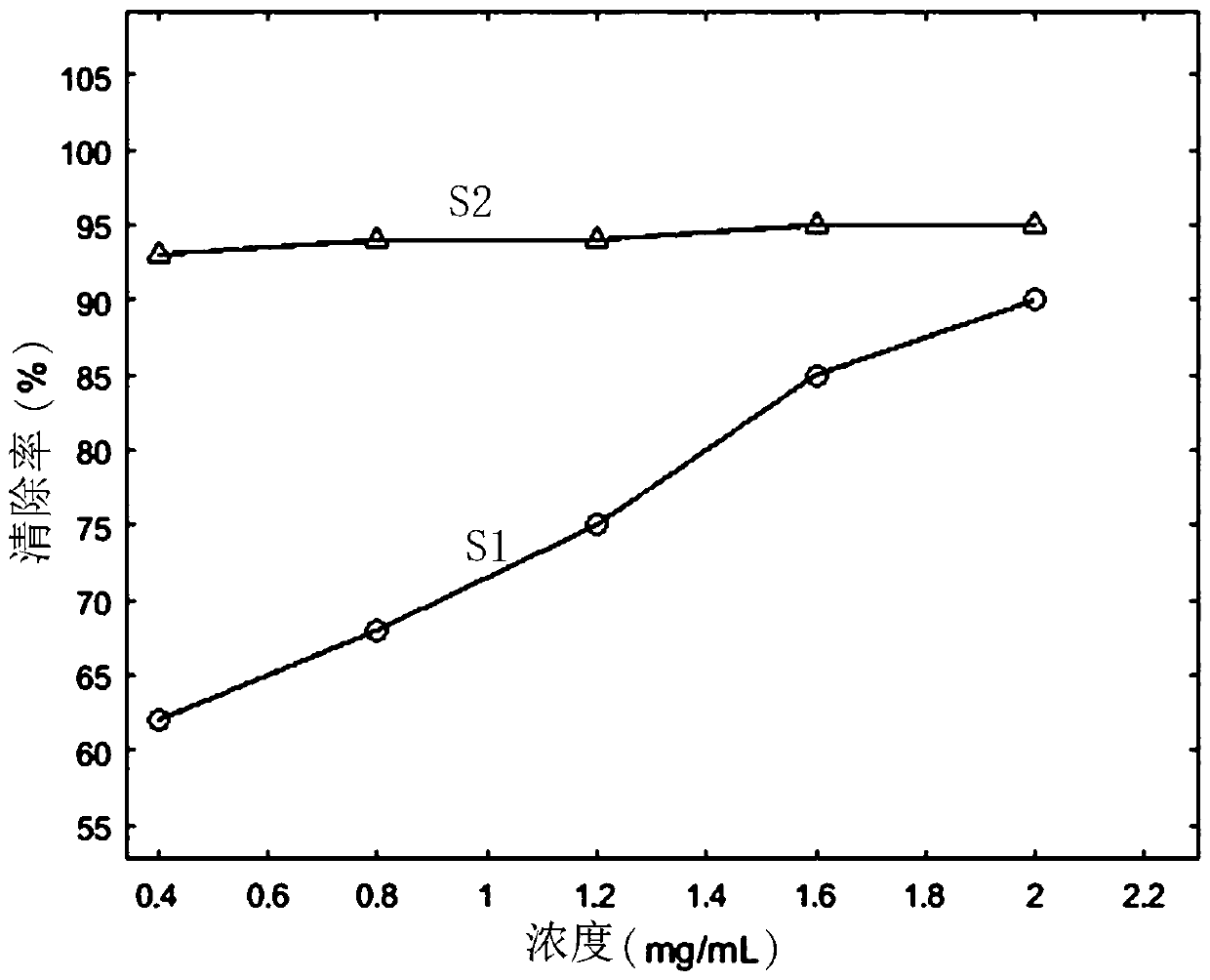
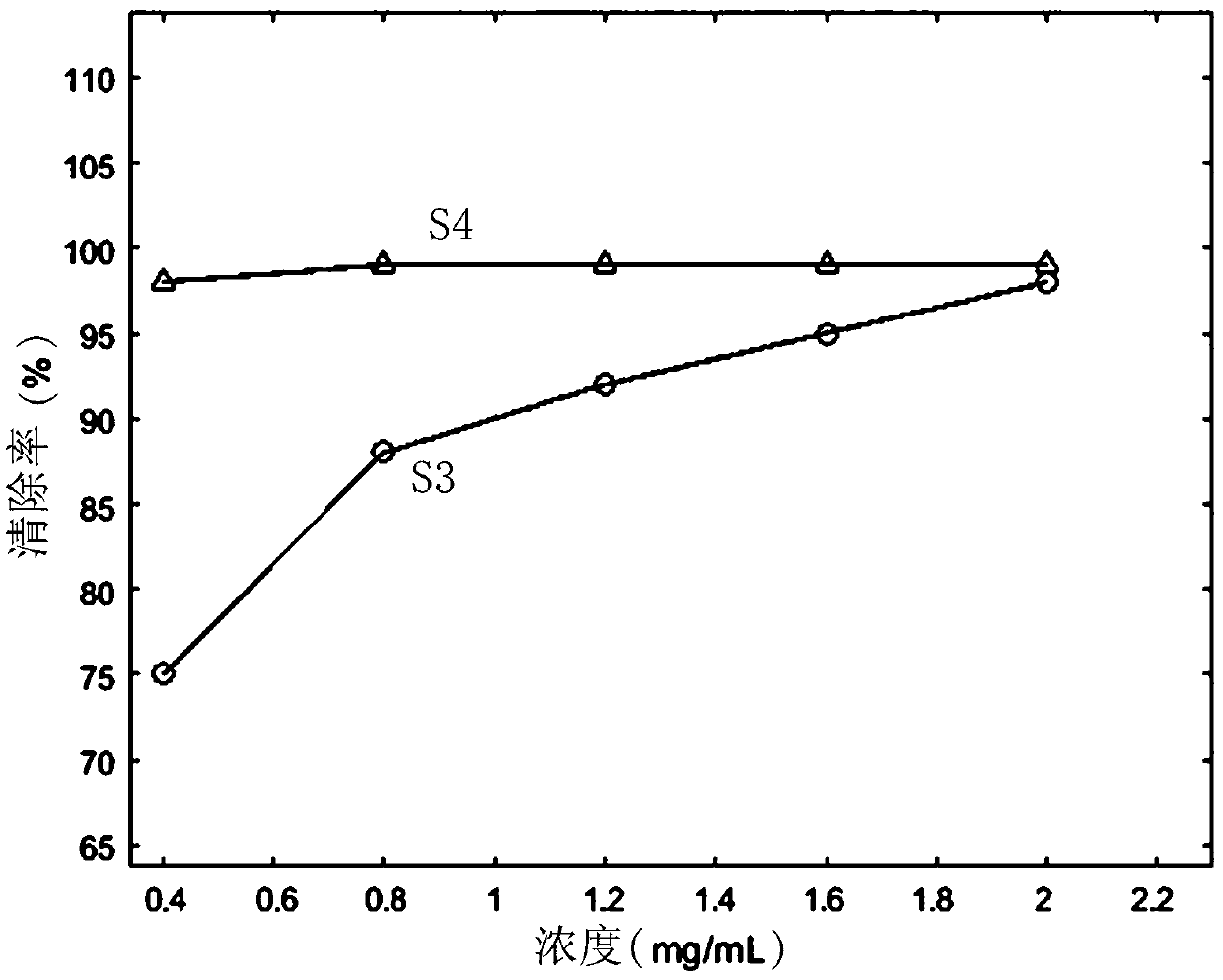
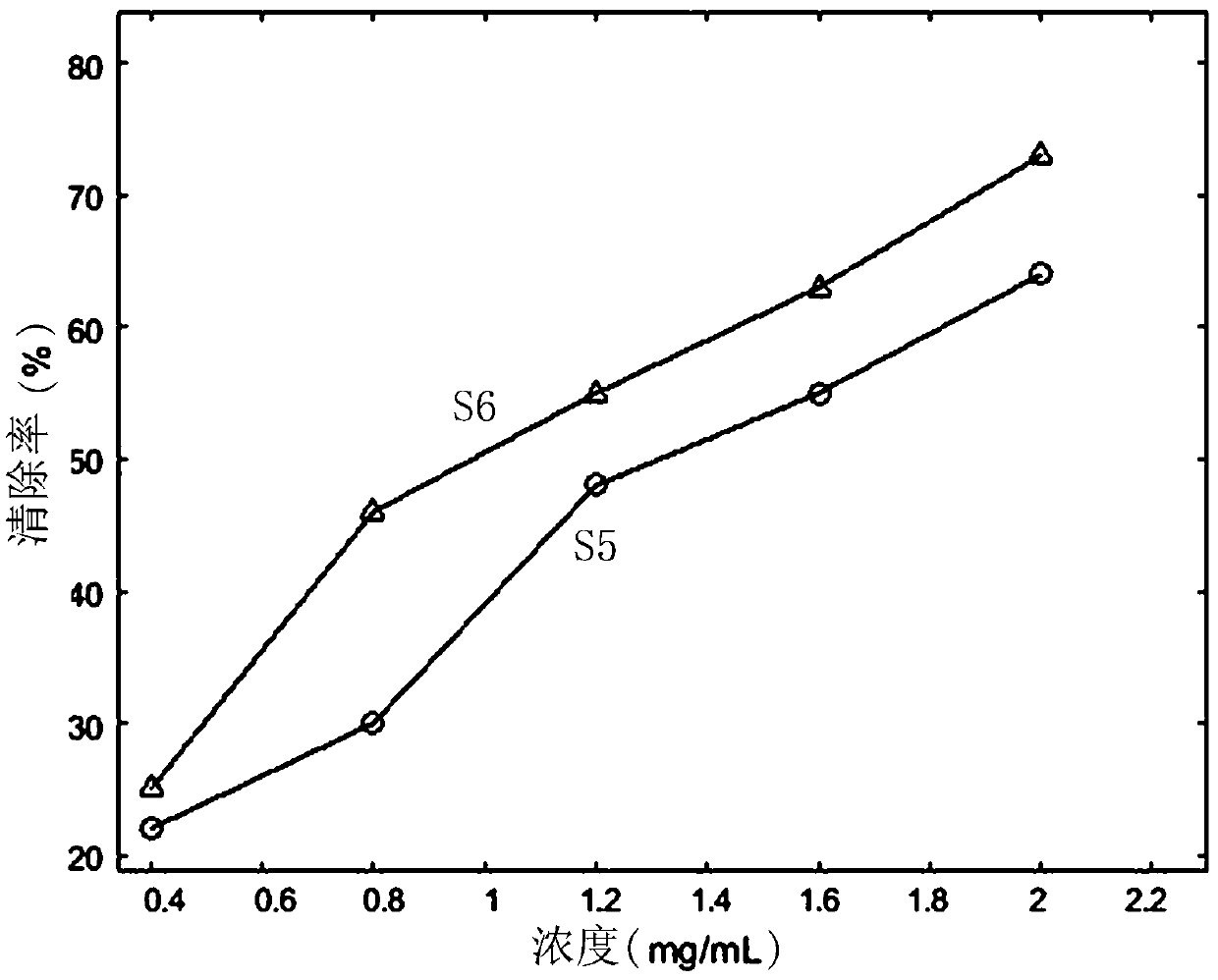
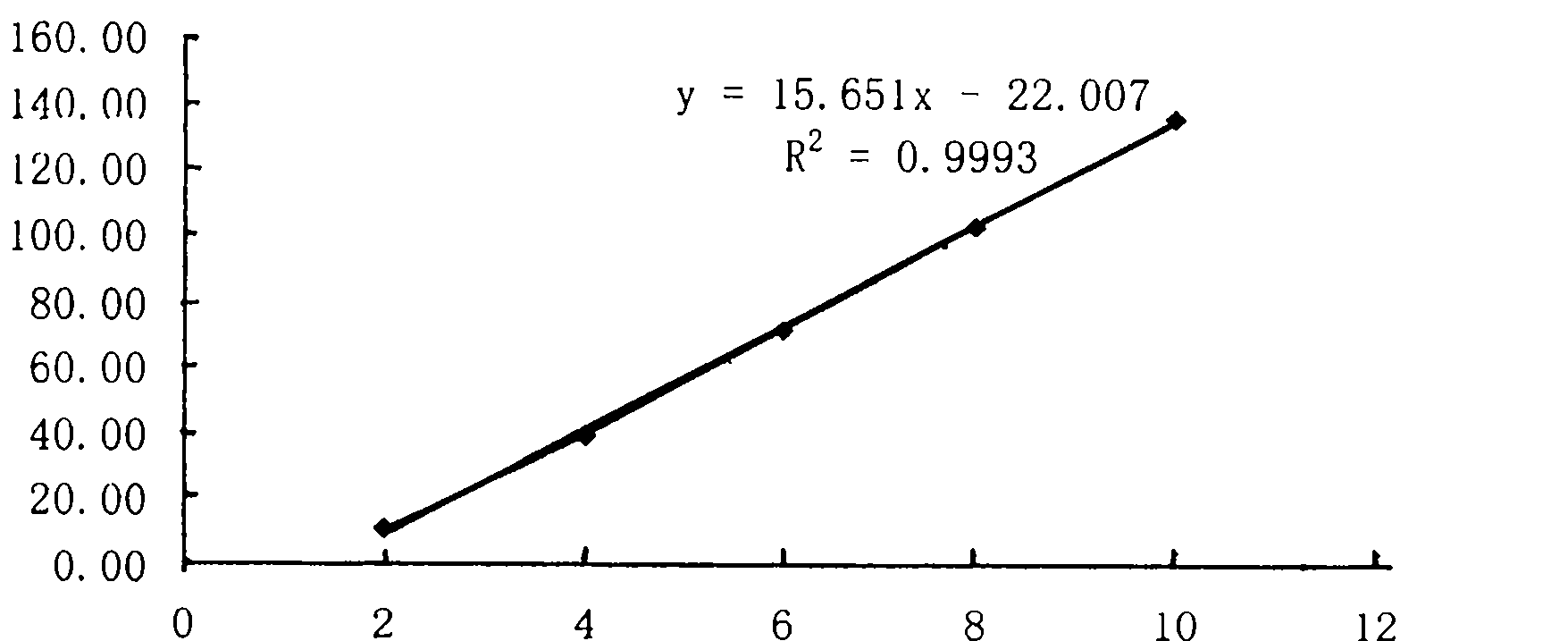Patents
Literature
48results about How to "Long-lasting and stable effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transdermal plaster of aryl propionic non-steroid antiphlogistic
InactiveCN1387842AImprove adhesionImprove stabilityOrganic active ingredientsAntipyreticTransdermal patchWhole body
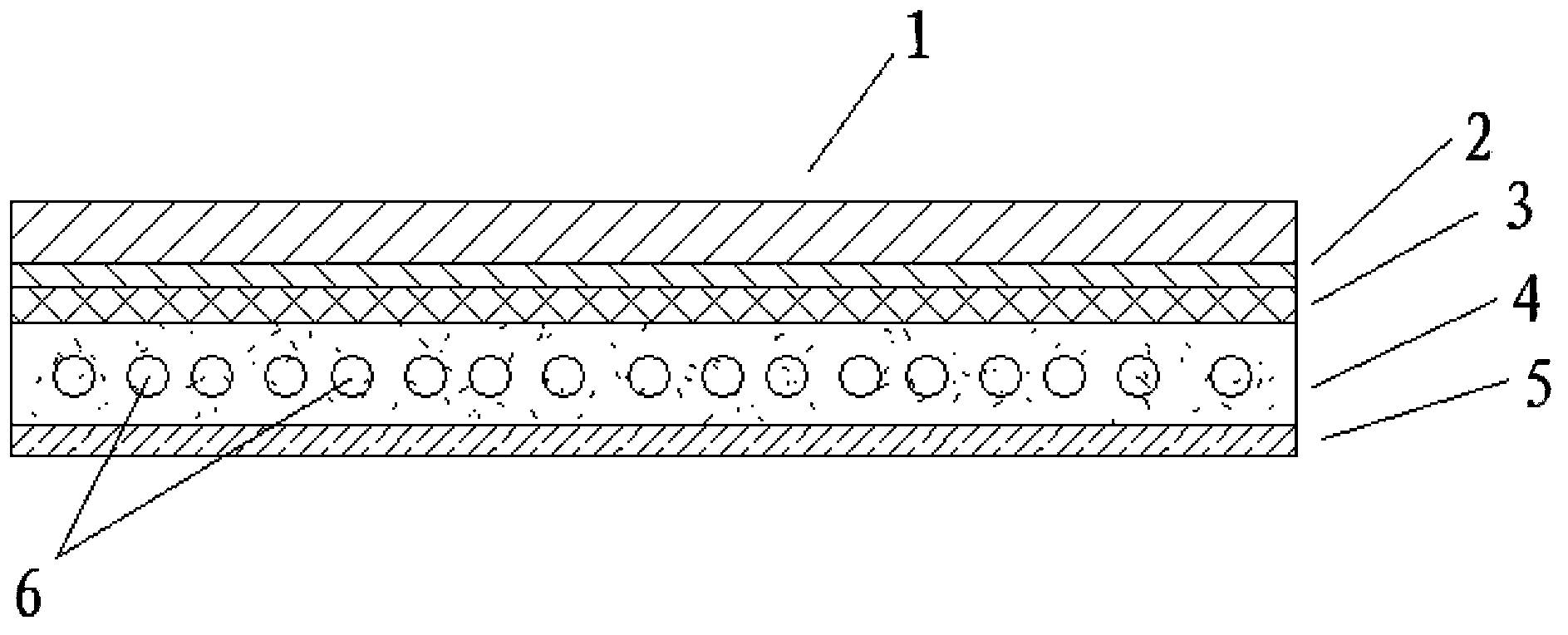
The present invention relates to medicine technology and is especially one kind of new preparation form. The transdermal plaster is noe kind of antiphlogistic containing Flubiprofen, Ketoprofen, Ibuprofen, Rosorolfen, Naproxan and other aryl propionic non-steroid. It has three parts including non-sticking layer, medicine layer and lining layer. The medicine layer incldues medicine dispersed in matrix, and the matrix consists of non-polar polymer and plasticizer and may contains tackifier, transdermal promoter and oxidant. It has accurate admistration amount, no stimulation to gastrointestinaltract, relatively higher local medicine density in the affected part and controllable medicine release.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
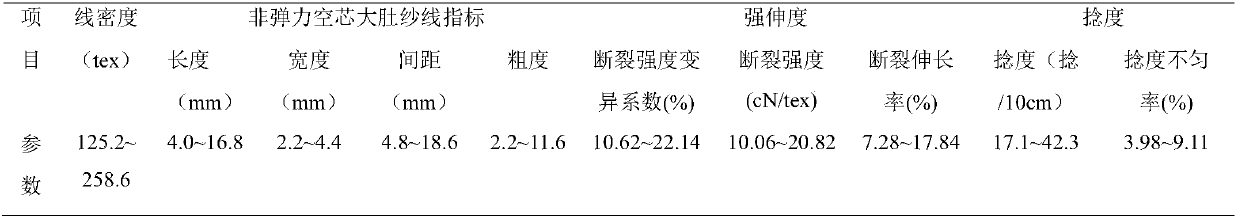
Comfortable skin-care warming health-care hollow porous yarn, and preparation method and application thereof
The invention provides a preparation method and application of a comfortable skin-care warming health-care hollow porous yarn. The comfortable skin-care warming health-care hollow porous yarn is formed by firstly blending and then processing such six hollow fibers (one or more fibers) as fine staple cotton fibers, seashell fibers (or Amicor antibiotic fibers or Cleancool fibers), water-soluble polyvinyl short fibers (or water-soluble polyvinyl filaments), kawo kawo fibers ( or Viloft fibers or Prolivon fibers or antibiotic hollow polyester fibers or Porel hollow fibers or Poral hollow fibers or micropore polyester fibers) and the like. The product has a body temperature adjustment effect, keeps a human body to always feel comfortable, is good in stiffness, feels soft and smooth, is good in drapability, unique in appearance and good in wrinkle resistance, enables a wearer to feel comfortable and is fluffy in texture, thereby being suitable for production of high-grade knitting machine woven fabrics, cold-proof underwear, bedding articles and the like.
Owner:ZHONGYUAN ENGINEERING COLLEGE
Moisture-absorption and sweat-discharge fiber spunlaced nonwoven fabric and preparation method thereof
InactiveCN107419438AImprove performanceLong-lasting and stable effectNon-woven fabricsPolyesterViscose
The invention relates to an antibacterial health-care spunlaced faric, in particular to a preparation method and application of a moisture-absorption and sweat-discharge fiber spunlaced nonwoven fabric. The moisture-absorption and sweat-discharge fiber spunlaced nonwoven fabric is prepared from, by weight, 10-70% of moisture-absorption and sweat-discharge fiber (including Coolmax, Coolplus, Cooldry and Topocool), 0-50% of pearl fiber, 0-50% of mint fiber, 0-60% of antibacterial viscose fiber (or viscose fiber) and 0-60% of antibacterial polyester fiber or polyester fiber. The moisture-absorption and sweat-discharge fiber spunlaced nonwoven fabric has the advantages that the developed moisture-absorption and sweat-discharge fiber spunlaced nonwoven face towel base fabric has the effects of disinfection, cleaning, maintenance, moisturizing nourishing and the like and also has the effects of corrosion prevention, inflammation diminishing, hygiene, health care and the like.
Owner:ZHONGYUAN ENGINEERING COLLEGE
Mint fiber spunlace non-woven mask substrate and preparation method thereof
The invention relates to an antibacterial healthcare spunlace fabric, in particular to a preparation method and application of a mint fiber spunlace non-woven mask substrate. The mint fiber spunlace non-woven mask substrate is prepared from, by weight, 10-100% of mint fiber, 0-90% of copper ammonia fiber, 0-90% of pearl fiber, 0-90% of viscose and 0-90% of polyester fiber. The mint fiber spunlacenon-woven mask substrate has the advantages that the developed mint fiber spunlace non-woven mask substrate is soft in touch and good in adsorption, has the water wetting and lightsome skin feeling and has the efficient anti-aging, anti-radiation, anti-bacterial and anti-oxidative effects, and the mask substrate has the functions of preserving moisture, activating cells, supplying oxygen and the like, and achieves the nourishing, nutritional and healthcare effects on the skin.
Owner:ZHONGYUAN ENGINEERING COLLEGE
Sarcandra glabra fiber spunlaced nonwoven mask base cloth and preparing method thereof
The invention relates to anti-microbial and health-care spunlaced cloth, in particular to a preparing method and application of sarcandra glabra fiber spunlaced nonwoven mask base cloth. The sarcandra glabra fiber spunlaced nonwoven mask base cloth is prepared from, by weight, 10-100% of sarcandra glabra fiber, 0-90% of viscose and 0-80% of polyester fiber. The sarcandra glabra fiber spunlaced nonwoven mask base cloth has the advantages of having health care functions of resisting and inhibiting bacteria, cleansing the skin and strengthening the constitution, protecting and nourishing the skin, moisturizing the skin and the like, being skin friendly, high in hygiene, capable of effectively promoting metabolism, preventing skin ageing, and having a great protective effect on the human body; meanwhile, the nonwoven mask base cloth is good in hygroscopicity, wet permeability and breathability, and has the functions of preserving moisture, activating cells, supplying oxygen and the like, and has the effects of conducting nourishing, supplementing and health care on the skin and the like.
Owner:ZHONGYUAN ENGINEERING COLLEGE
Silk-ramie fiber spunlace non-woven facial mask base fabric and preparation method thereof
The invention relates to an antibacterial health care spunlace fabric, in particular to a preparation method and application of a silk-ramie fiber spunlace non-woven facial mask base fabric. The silk-ramie fiber spunlace non-woven facial mask base fabric is prepared from the following raw materials including, by weight, 10-100% of silk-ramie fibers, 0-50% of real silk fibers, 0-50% of natural silk fibers, 0-90% of viscose fibers, and 0-70% of polyester fibers. The advantages of the silk-ramie fiber spunlace non-woven facial mask base fabric are that the silk-ramie fiber spunlace non-woven facial mask base fabric can clean skins, retain moisture, and protect skins, is high in sterilizing rate, is nonirritating, has a moisture preservation, cell activating, and oxygen supplying function, and can nourish and care skins.
Owner:ZHONGYUAN ENGINEERING COLLEGE
Sun block containing natural plant sun composition and preparation method thereof
InactiveCN101342131AWith protectionMoisturizingCosmetic preparationsToilet preparationsBiotechnologyGlycerol
The invention discloses sunscreen lotion containing natural plant sunscreen components and a preparation method thereof, which comprises A component, B component and de-ionized water; wherein, the A component comprises 5 percent to 10 percent of whiteruss, 3 percent to 8 percent of octodecyl alcohol, 3.5 percent to 6 percent of stearic acid, 0.6 percent to 1.2 percent of sodium lauryl sulfate and 0.1 percent to 0.2 percent of nipagin methyl ester; the B component comprises 6 percent to 9 percent of glycerin, 1 percent to 3 percent of Chinese traditional medicine ingredients, 0.3 percent to 0.5 percent of essence and 0.1 percent to 0.2 percent of nipagine propyl ester; the Chinese traditional medicine ingredients per gram is composed of 0.15 gram to 0.30 gram of flos sophora, 0.50 gram to 0.75 gram of honeysuckle and 0.10 gram to 0.20 gram of peony. When the sunscreen cream is prepared, the Chinese traditional medicine is firstly prepared. Secondly, the A and the B components are respectively heated to about 80 DEG C to be melted, the A component is added into the B component along with stirring, and then the de-ionized water is added into the mixture. The fast stirring can not stop until the mixture is emulsified completely, and then the mixture is cooled to obtain the sunscreen lotion containing natural plant sunscreen components. The invention has broad-spectrum function to UV-A and UV-B rays and compensates the deficiency of a single component, and has favorable effect on absorbing ultraviolet radiation.
Owner:NANJING UNIV OF INFORMATION SCI & TECH
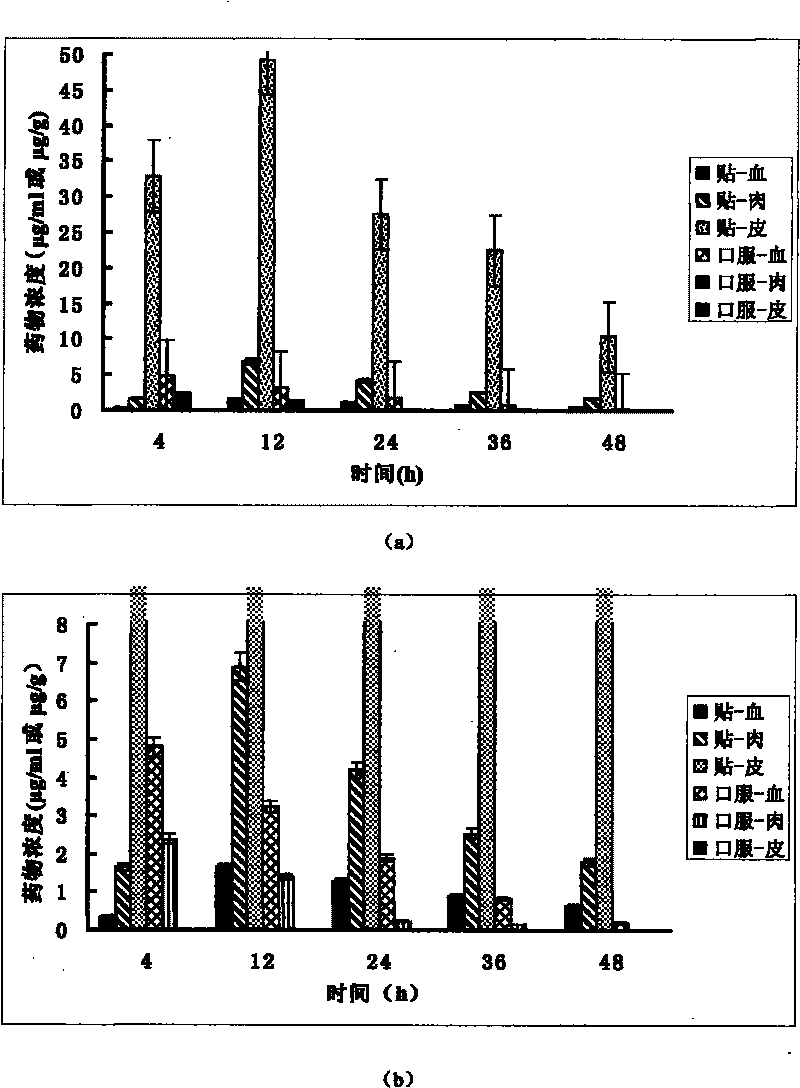
Slow-release patch with daphne giraldii bark extract, and preparation method thereof
InactiveCN101518599AImprove adhesionImprove flexibilityAntipyreticAnalgesicsAntioxidantTherapeutic effect
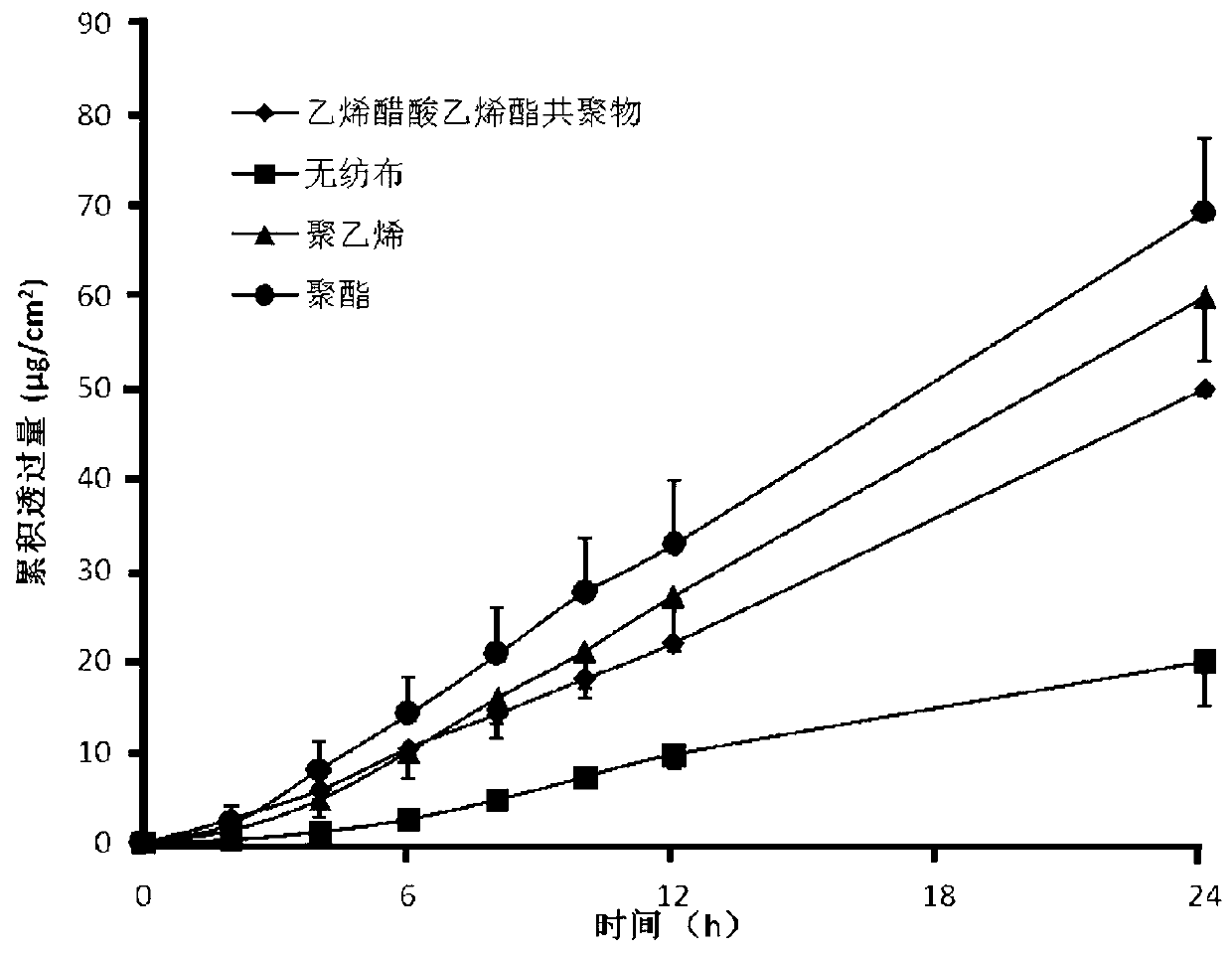
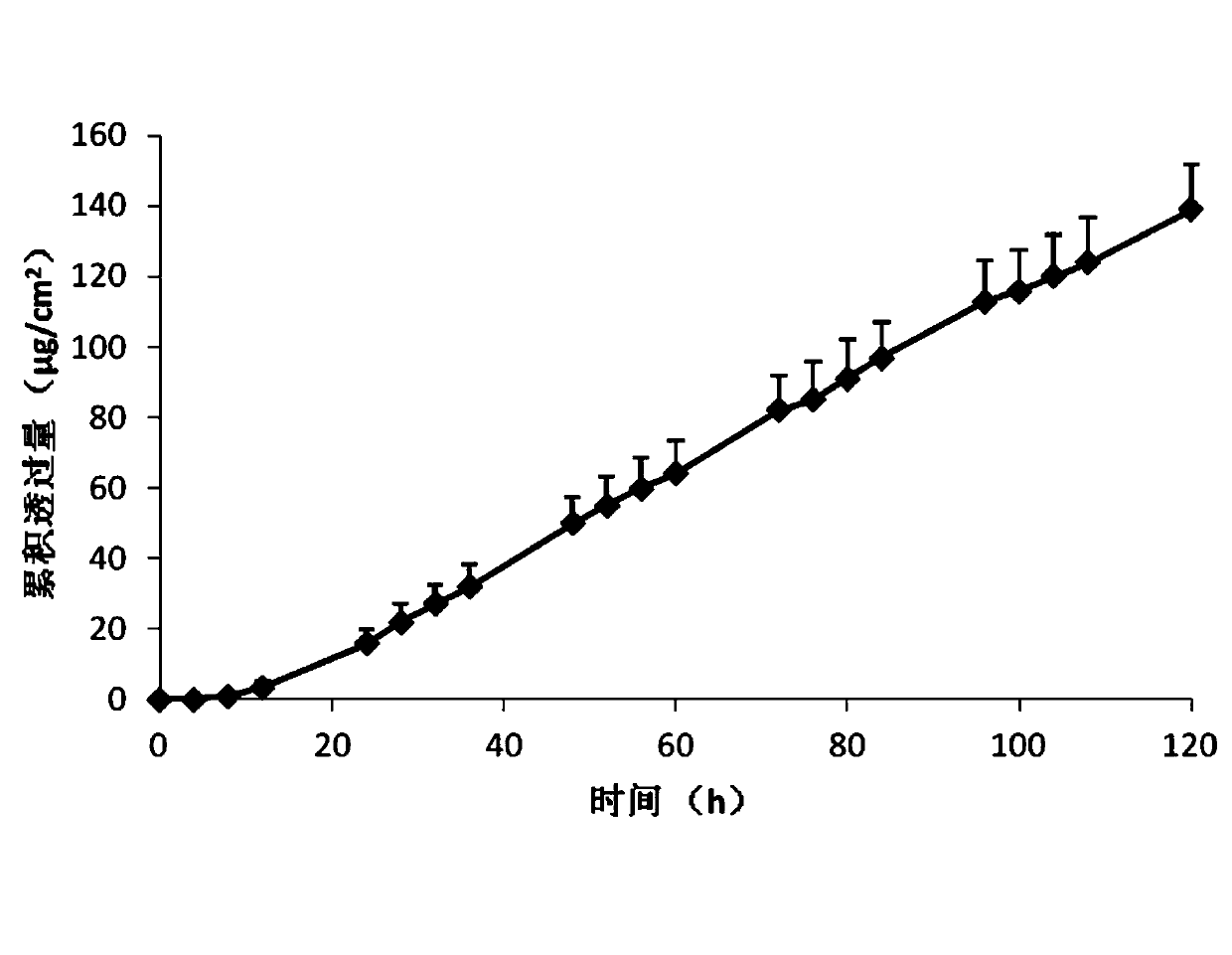
The invention belongs to the technical field of medicament, and discloses a percutaneous slow-release patch with a daphne giraldii bark extract, and a reparation method thereof. The patch with the daphne giraldii bark extract comprises a back lining layer, a drug reservoir and an antisticking layer, wherein the drug reservoir consists of the daphne giraldii bark extract, pressure sensitive adhesive and a percutaneous absorption accelerator; plasticizer, tackifier and antioxidant and the like can be added if needed; the selected pressure sensitive adhesive is silicone pressure sensitive adhesive, isobutylene pressure sensitive adhesive or acrylate pressure sensitive adhesive, and can select one or more compounds therein. The percutaneous patch has the advantages that the percutaneous patch can prevent oral administration from causing liver first-pass effect and irritating gastrointestinal tracts, overcomes deficiency that ointment, plaster, liniment, cream and other preparations are uncertain in dosage, easy to pollute clothes and the like, is constant in drug-releasing speed, ensures lasting steady therapeutic effect, and is convenient to use, good in flexibility and suitable in adhesion. The 24-hour accumulated permeation amount of daphnetin in a daphne giraldii bark patch prepared by the method is obviously higher than that of the commercially available daphne giraldii bark plaster.
Owner:SHENYANG PHARMA UNIVERSITY
Tamsulosin hydrochloride double-layer osmotic pump controlled-releasing tablet and preparation method thereof
InactiveCN101167701AStable concentrationRelease constant speed evenlyInorganic non-active ingredientsPharmaceutical delivery mechanismExcipientMoisture
The invention provides double-layer osmotic pump tablets of tamsulosin ehydrochloride and a process for preparation. The medicament contains tamsulosin ehydrochloride and acceptable medical polymeric excipient, and is characterized in that the invention has excellent zero-level controlled releasing, pH level of environment, movements of the stomach and intestine, and food, has little effect on releasing action and food, and has no effect on the internal pharmacokinetics parameter. According to the percentage by weight, the preparation contains tamsulosin ehydrochloride 0-2%, excipient in pastille layer with the function of controlled-releasing 30-70% excipient in boosting layer with the function of controlled releasing 30-70%, and the rest percentage of other excipient. The of process for preparation the double layer permeable pump controlled-release tablets of tamsulosin ehydrochloride comprises (1) the preparation of pastille layer, (2) the preparation of boosting layer, (3) the compressing of the two layers, (4) the coating of the double layer tablets, (5) the perforating of the coated tablets, (6) the packing of moisture proof cost. The invention is clinically used for the treatment of paruria symptom like frequent micturition, diuresis at night, dysuria caused by prostatic hyperplasia.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Comfortable skin-protection health-care parallel spinning wrap soft yarn and preparing method thereof
The invention relates to health-care yarn, in particular to comfortable skin-protection health-care parallel spinning wrap soft yarn and a preparing method and application thereof. The comfortable skin-protection health-care parallel spinning wrap soft yarn is novel composite yarn and is mainly provided with unidirectional wrap composite soft yarn and bidirectional cross wrap composite soft yarn and comprises core yarn, outer wrap yarn and rough yarn, wherein water-soluble polyvinyl alcohol staple fiber filament is adopted by the outer wrap yarn; polymer filament is adopted by the core yarn; the rough yarn is one or more of fine staple cotton fiber and original-color or color pearl fiber. The health-care parallel spinning wrap soft yarn is novel composite yarn and has the advantages of being bright in dyed color, rich in color, high in color fastness, tender in luster, good in moisture absorption and the like, fabric processed through the health-care parallel spinning wrap soft yarn has the performance of being soft in texture, excellent in elasticity, high in water absorption, large in water storage capability, good in moisture absorption and rapid moisture-permeability, good in breathability, good in moisture permeability, good in comfort, good in heat retention and the like.
Owner:ZHONGYUAN ENGINEERING COLLEGE
Multifunctional health-care porous elastic big-belly yarn and preparation method thereof
The invention relates to a preparation method and application of a multifunctional health-care porous elastic big-belly yarn. The multifunctional health-care porous elastic big-belly yarn is prepared from two core yarns, two fixed yarns and a decorative yarn, wherein the fixed yarns are core-spun yarns obtained by carrying out blended processing on silver fibers, copper fibers, soft silk fibers and DOW-XLA elastic fibers; the core yarns are blended hollow yarns obtained by carrying out blended processing on Porel fibers, hollow polyester fibers and provilion; the decorative yarn is a blended rough yarn obtained by carrying out blended processing on nettle fibers, apocynum fibers and alpaca wool fibers. The multifunctional health-care porous elastic big-belly yarn not only has a special form, but also is rich in yarn colors, multiple in shapes and ever-changing; products developed by using the multifunctional health-care porous elastic big-belly yarns are natural and environment-friendly, have the health care functions of resisting bacteria and eliminating inflammation, protecting skin and keeping fitness, absorbing moisture and releasing sweat, deodorizing and resisting aging, and the like, are good in comfortableness, and do not cause adverse effects when being contacted with a human body; the multifunctional health-care porous elastic big-belly yarn is mainly used for various textiles such as yarn-dyed female lines, knitted fabrics, tweed, coarse worsted fabrics, decorative articles, hand knitted fabrics and shawls.
Owner:ZHONGYUAN ENGINEERING COLLEGE
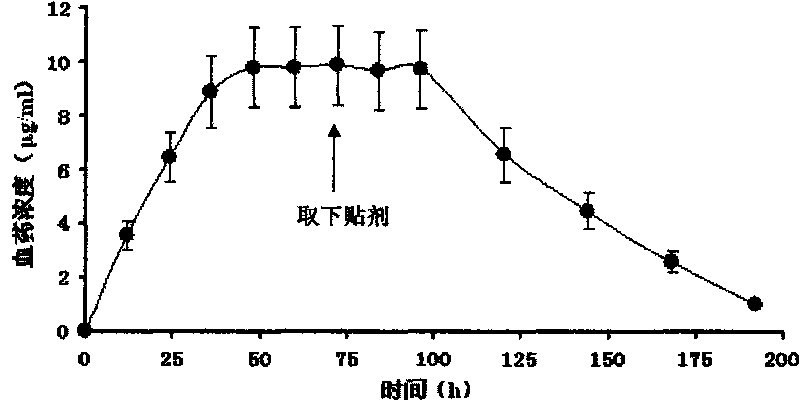
Long-acting donepezil percutaneous absorption sticking agent
InactiveCN102895217AIncreased transdermal penetrationLong-lasting and stable effectNervous disorderPharmaceutical non-active ingredientsPolyesterPercutaneous absorption
The invention belongs to the technical field of medicines, and particularly provides a long-acting donepezil percutaneous absorption sticking agent. The long-acting donepezil percutaneous absorption patch agent consists of a back lining layer, a drug-containing pressure-sensitive glue layer and an anti-sticking layer, wherein the long-acting donepezil percutaneous absorption sticking agent comprises 5-15 parts of main drug donepezil, 70-95 parts of pressure-sensitive glue and 0.5-15 parts of percutaneous absorption accelerant. The long-acting donepezil percutaneous absorption sticking agent is characterized in that the ingredients of a film selected by the back lining layer comprises polyethylene, polyester and ethylvinylacetate. The donepezil percutaneous absorption sticking agent provided by the invention is stable and durable in curative effect, and each sticking agent has 5 days of effective function. The long-acting donepezil percutaneous absorption sticking agent is characterized in that the back lining layer capable of providing maximum drug-permeable quantity is adopted. The long-acting donepezil percutaneous absorption sticking agent provided by the invention is good in percutaneous permeability, durable and stable in curative effect, and good in skin feeling.
Owner:SHENYANG PHARMA UNIVERSITY
Amlodipine controlled release patch and preparing method thereof
InactiveCN101229143ALong-lasting and stable effectEasy to useOrganic active ingredientsSheet deliveryPressure sensitiveDrug reservoir
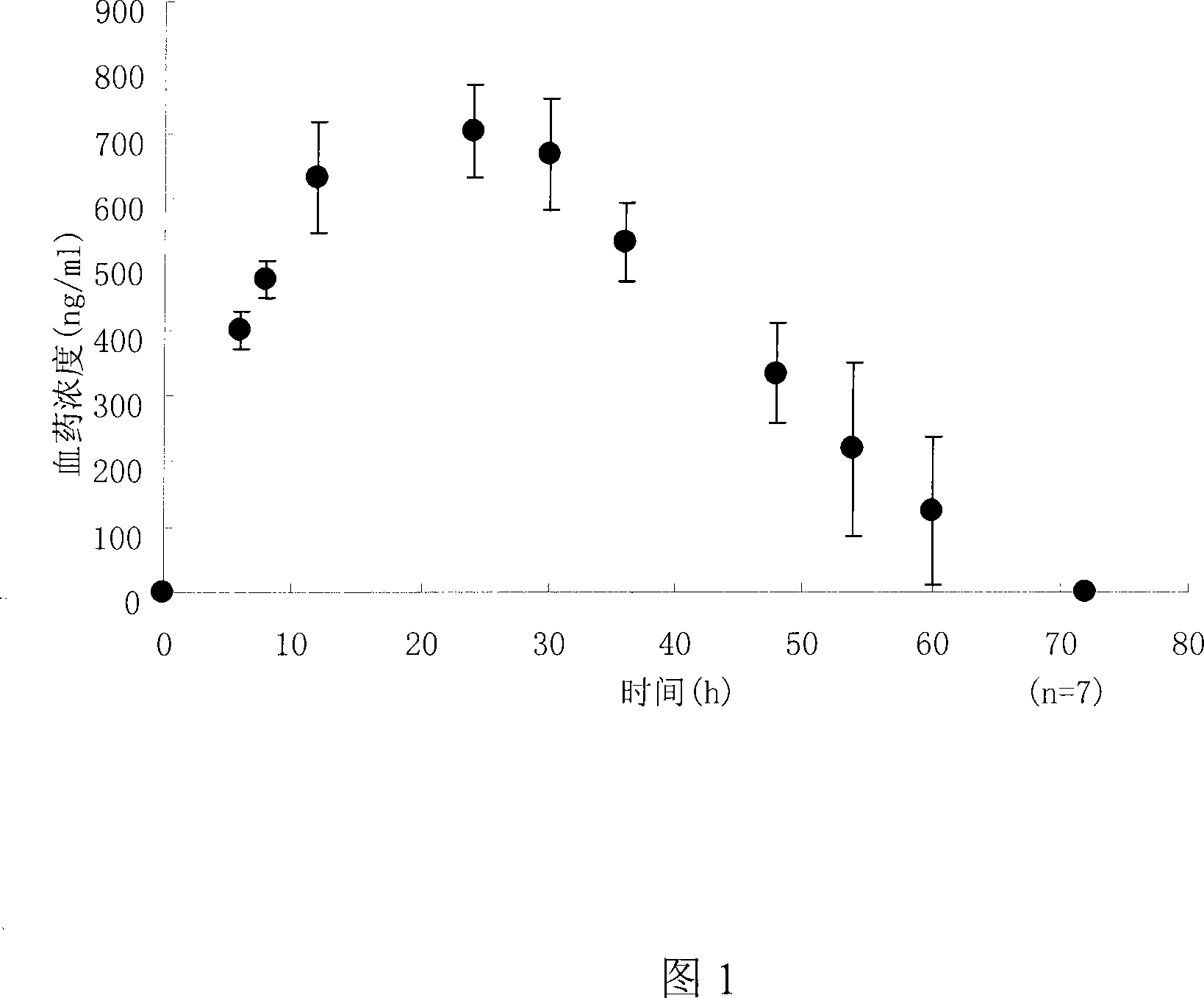
The invention belongs to medical technique field and discloses an amlodipine controlled-release patch and a preparation method. The invention comprises a backing layer, a drug reservoir and an anti-sticking layer. The drug reservoir consists of amlodipine which is the main medicine materials, pressure sensitive adhesive and composite and percutaneous absorption enhancer; wherein, the dosage of amlodipine accounts for 0.5 to 45 wt percent of the weight of drug reservoir, the dosage of pressure sensitive adhesive accounts for 10 to 90 wt percent of the weight of drug reservoir and the dosage of the composite and percutaneous absorption enhancer accounts for 1 to 45 wt percent of the weight of drug reservoir. The invention also can be added with plasticizer, tackifier and chemical inhibitor, etc. an amlodipine controlled-release patch is a novel drug delivery system which can avoid the gastrointestinal stimulation of oral dosage and release drug slowly to relieve the side effects of the drug, and releases drug continuously for 72 hours, thereby having persistent and steady efficacy. When drug delivery needs to be interrupted, people just need to take off the patch, thereby being convenient. The invention also has the advantages of good flexibility and appropriate adhesiveness.
Owner:沈阳药大制剂新技术有限公司
Health-care nourishing tea capable of relieving blood pressure, blood fat and blood sugar
The invention discloses health-care nourishing tea capable of relieving blood pressure, blood fat and blood sugar, belonging to the field of health-care nourishing tea products. The health-care nourishing tea is prepared from China Northeast black fungus, China Northeast wild acanthopanax seeds, cassia seeds, China Northeast corn stigma, eucommia, uncaria and apocynum. The health-care nourishing tea is prepared by the steps as follows: drying China Northeast black fungus and China Northeast wild acanthopanax seeds in high temperature; airing cassia seeds, China Northeast corn stigma, eucommia, uncaria and apocynum; and after the above treatment, mixing the materials by weight ratio. The health-care nourishing tea is simple in the preparation technology and utilizes the efficacies of China Northeast black fungus, China Northeast wild acanthopanax seeds, cassia seeds, China Northeast corn stigma, eucommia, uncaria and apocynum as traditional Chinese medicines, thus having excellent curing and regulating effects to groups suffering from hypertension, hyperlipaemia, hyperviscosity, hyperglycemia, arteriosclerosis and heart cerebrovascular diseases; and the health-care nourishing tea is low in cost, infused with boiled water, convenient to drink and suitable to sub-health groups.
Owner:肖立权
Escitalopram percutaneous patch and preparation method thereof
ActiveCN104840973AEffective regulation of transdermal penetration rateSimple technologyOrganic active ingredientsNervous disorderOrganic acidEscitalopram
The invention belongs to the technical field of medicine and relates to an escitalopram percutaneous patch and a preparation method thereof. The escitalopram percutaneous patch is composed of a backing layer, a medicine-carrying pressure-sensitive adhesive layer and an anti-sticking layer, the medicine-carrying pressure-sensitive adhesive layer comprises escitalopram free alkali or organic acid ion pair compound thereof, pressure-sensitive adhesive and percutaneous absorption promoter, the escitalopram free alkali or organic acid ion pair compound thereof accounts for 2.0-20wt% of total weight of the medicine-carrying pressure-sensitive adhesive layer, pressure-sensitive adhesive accounts for 77-97wt% of the total weight, the percutaneous absorption promoter accounts for 0-6.8wt% of the total weight, and in the escitalopram organic acid ion pair compound, a ratio of escitalopram free alkali and different organic acids is 0.5:1-2:1. Compared with an escitalopram organic acid ion pair compound percutaneous absorption patch and an escitalopram free alkali percutaneous absorption patch, the escitalopram percutaneous patch has the advantages that medicine release up to seven days and similar to constant rate can be provided, so that medication compliance of a patient is improved greatly.
Owner:SHENYANG PHARMA UNIVERSITY
Metolazone slow-release capsule and method for preparing same
InactiveCN101584683ALong-lasting and stable effectReduce adverse reactionsOrganic active ingredientsPharmaceutical delivery mechanismSide effectActive component
The invention belongs to the technical field of medicaments, and discloses a metolazone slow-release capsule and a method for preparing the same. The preparation comprises an active component of metolazone and a pharmaceutically acceptable carrier component by weight percentage: 1 to 30 percent of metolazone, 10 to 70 percent of auxiliary material with slow-release effect, and the balance of other auxiliary materials. The method comprises the following steps that: (1) matters in a capsule are matrix type particles or pills; (2) the medicine-containing pill is prepared first, and then a slow-release preparation is prepared; and (3) the capsule is the coated slow-release preparation. The metolazone slow-release capsule can ensure that the main medicinal component of metolazone is continuously and regularly released when the metolazone slow-release capsule is orally taken; moreover, the metolazone slow-release capsule has the characteristics of convenient drug administration, persistent curative effect, stable curative effect, little side effect and the like.
Owner:SHENYANG PHARMA UNIVERSITY
Double-thick double-wire six-into-one antivirus and antibacterial compound yarn and preparing method thereof
The invention relates to healthcare yarn, in particular to a producing method and application of double-thick double-wire six-into-one anti-ultraviolet antivirus and antibacterial comfortable skincarehealthcare compound yarn. The blended yarn is prepared from, by weight, 15-85% of long staples, 15-85% of Outlast air conditioning fiber, 15-75% of white bamboo charcoal fiber and 25-85% of moistureabsorption and sweat releasing Topocool fiber. The compound functional knitted yarn is good in moisture absorption property and breathability, good in dyeing property, elasticity and plump performance, soft in hand feel, bright in color, rich in color and luster, free of adverse reaction when making contact with a human body, not prone to worm damage or corrosion and unique in appearance, has healthcare functions of bacterial resistance, inflammation elimination, skincare and bodybuilding, skin beautifying, skin moisturizing and good skincare property, and is good in draping, excellent in comfort, good in wear resistance, natural in color and luster and wide in application range.
Owner:ZHONGYUAN ENGINEERING COLLEGE
Radix isatidis antiviral fiber spunlace non-woven wet wipe base cloth and preparation method thereof
InactiveCN108950869AImprove performanceLong-lasting and stable effectCosmetic preparationsToilet preparationsPolyesterWet wipe
The invention relates to a Radix isatidis antiviral fiber spunlace non-woven wet wipe base cloth and a preparation method thereof, wherein the base cloth is made of the following raw material of weight ratio: the weight content of Radix isatidis antiviral fiber is 10-100%, the weight content of soybean protein fiber is 0-90%, the weight content of chitin fiber is 0-70%, the weight content of chitosan fiber is 0-70%, the weight content of antibacterial viscose fiber (or viscose fiber) is 0-70%, and the weight content of antibacterial polyester fiber (or polyester fiber) is 0-70%. The developedRadix isatidis antiviral fiber spunlace non-woven wet wipe base cloth not only has the characteristics of cleaning, nourishing, disinfecting and moisturizing and the like, but also has the functions of refreshing, sanitary, maintenance, nursing, quick and health care and the like.
Owner:ZHONGYUAN ENGINEERING COLLEGE
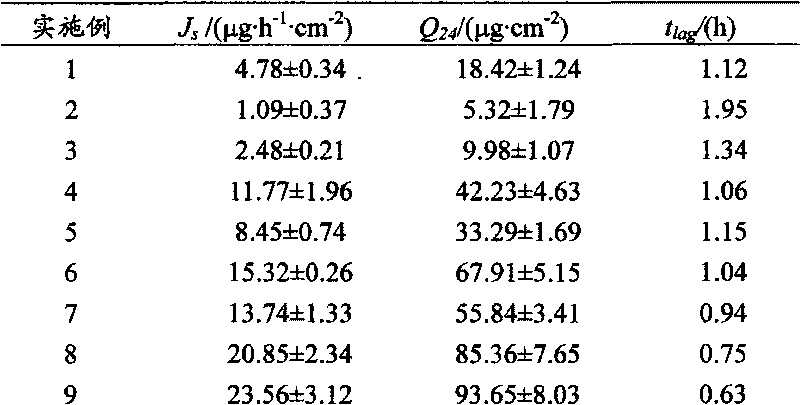
Electret nanoparticle cutaneous penetration system
ActiveCN103505806APromote circulationIncrease valueUltrasound therapyElectrotherapyControl releaseNanoparticle
The invention relates to the technical field of medicines, in particular to a medication system. An electret nanoparticle cutaneous penetration system comprises an isolated layer, wherein a drug-contained pressure-sensitive adhesive layer is arranged on the inner side of the isolated layer, an electret layer made of electret materials is arranged on the outer side of the isolated layer, and a controlled release film layer is arranged on the inner side of the drug-contained pressure-sensitive adhesive layer; the electret layer, the isolated layer, the drug-contained pressure-sensitive adhesive layer and the controlled release film layer are arranged in sequence from outside to inside to form a depot patch; tourmaline powder particles are distributed in the drug-contained pressure-sensitive adhesive layer. According to the electret nanoparticle cutaneous penetration system, the tourmaline powder particles are mixed into the drug-contained pressure-sensitive adhesive layer, so that the electret nanoparticle cutaneous penetration system has the advantages of promoting blood circulation of a human body, enhancing the vitality of cells, improving an electret electric field, replenishing mineral substance for the human body and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
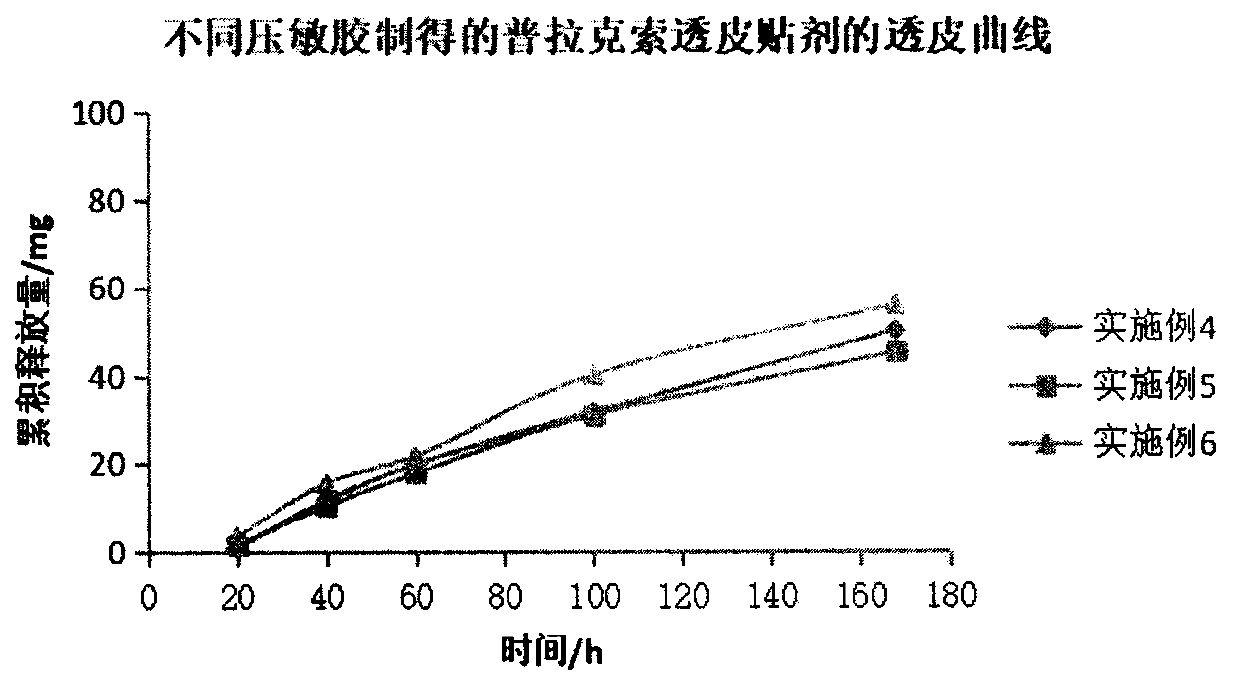
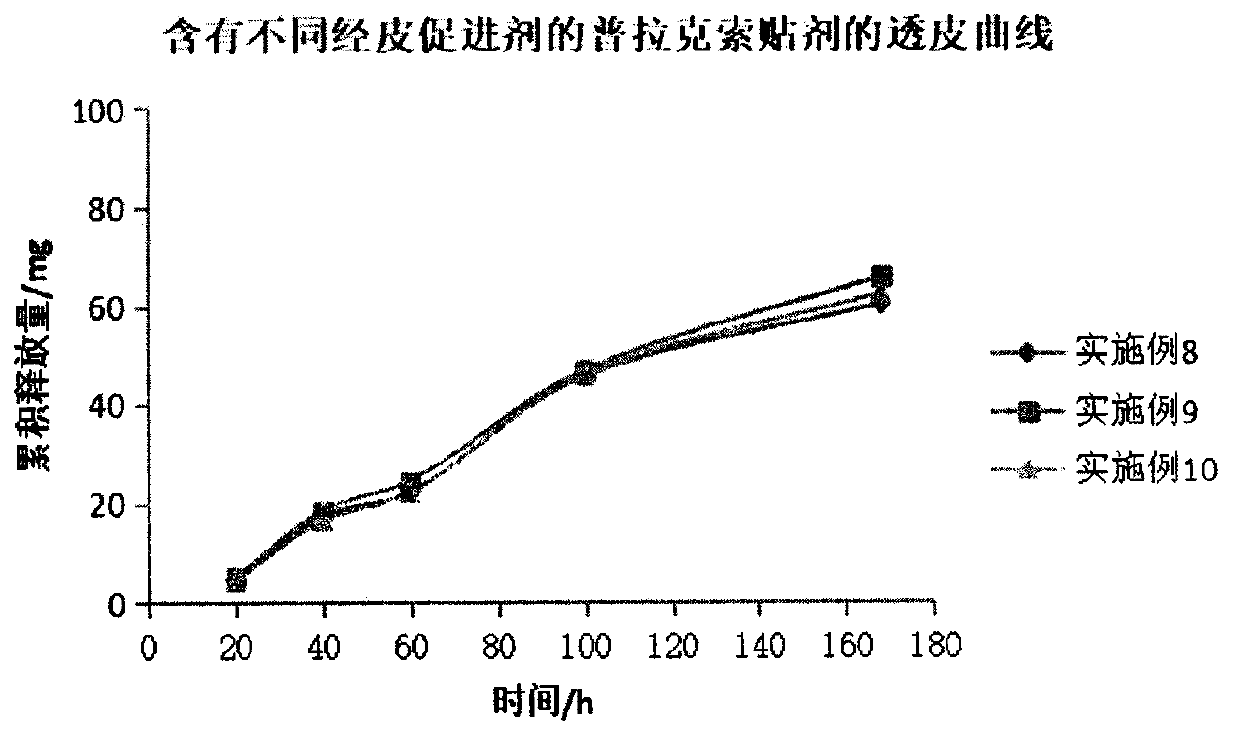
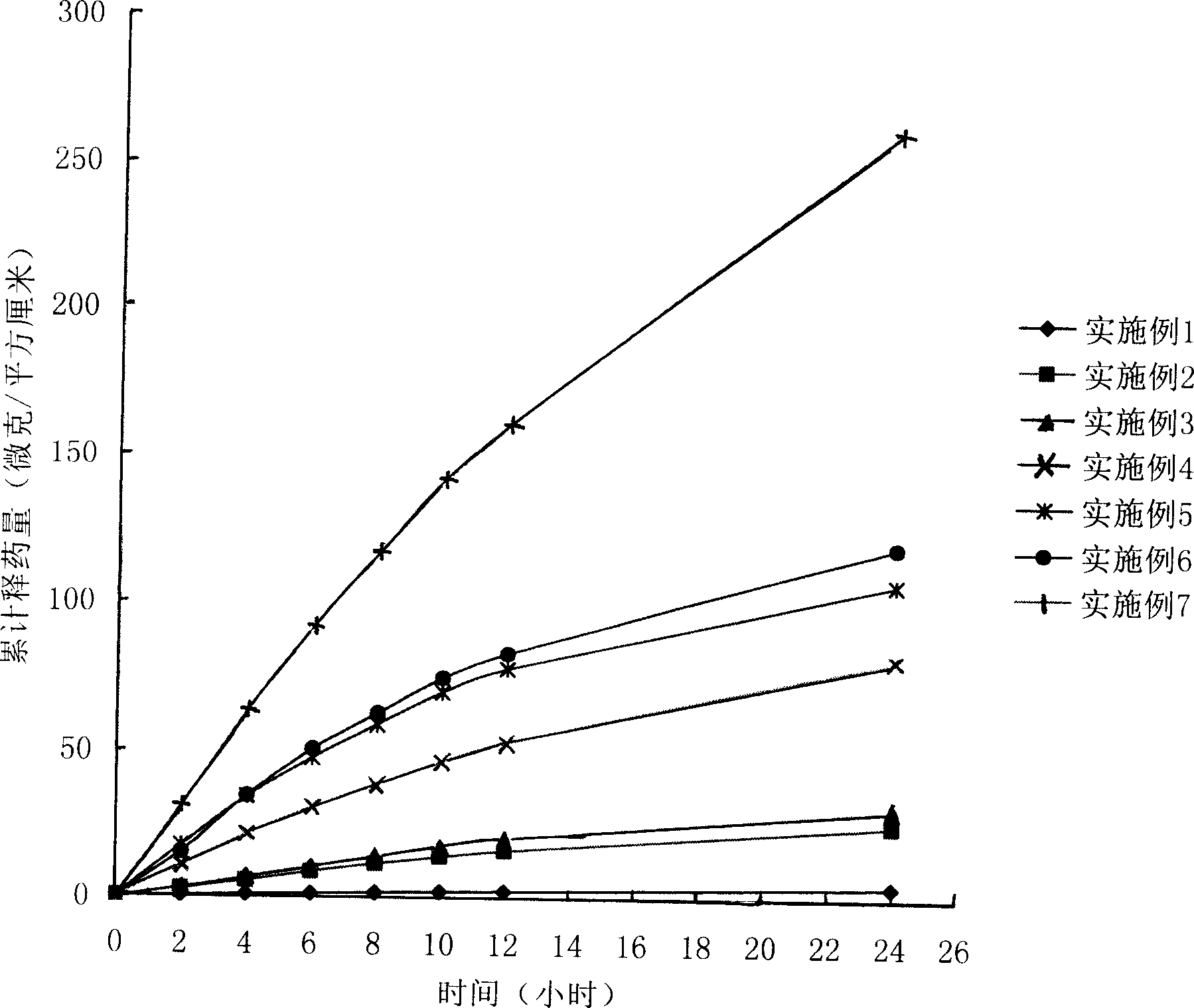
Pramipexole transdermal patch and preparing method thereof
PendingCN109999012AImprove adhesionImprove flexibilityOrganic active ingredientsNervous disorderTransdermal patchSide effect
The invention belongs to the technical field of medicine, and discloses a pramipexole transdermal patch and a preparing method thereof. The pramipexole transdermal patch comprises a back lining, a medicine storage library and a binding-prevention layer, wherein the medicine storage library comprises 1-20 wt% of a main medicine pramipexole, 50-90 wt% of a pressure-sensitive adhesive and 0.5-10 wt%of a transdermal absorption accelerator. An inert filler, a tackifier, an antioxidant and the like can also be added, and the dosages of the inert filler, the tackifier, the antioxidant and the like are 0.1-10 wt% separately. The pramipexole transdermal patch is a novel medicine delivery system, the situation that the medicine delivery adaptability of an injection is poor can be avoided, the irritation of the oral dosage form to the gastrointestinal tract can also be avoided, the drug is released slowly, thus the toxic and side effects of the medicine are alleviated, and the curative effect islasting and steady; if medicine delivery needs to be interrupted, what is only needed is to uncover the patch, and thus the patch is convenient to use. The pramipexole transdermal patch also has theadvantages of being good in adhesiveness and flexibility, causing no contamination and the like.
Owner:大道隆达(北京)医药科技发展有限公司
Indapamide percutaneous controlled releast plaster and its preparation method
InactiveCN1823765AImprove adhesionImprove flexibilityOrganic active ingredientsSheet deliveryPlasticizerCurative effect
A release-controllable percutaneous paster of indopamide for stable releasing within 24 hr is composed of substrate, layer, medicine bearing layer, and antisticking layer. Said medicine bearing layer contains indapamide, pressure sensitive adhesive, composite percutaneous absorption promoter, plasticizer, tackiness agent and antioxidant. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
Sun cream containing natural sun-prevention component and preparation method thereof
InactiveCN101406432AWith protectionHas repairCosmetic preparationsToilet preparationsAdditive ingredientPhosphate
The invention discloses sunblocking cream containing natural sunblocking ingredients and a preparation method thereof. The sunblocking cream comprises a component A, a component B and deionized water, wherein the component A consists of 8 to 12 percent of caprylic acid / caprin, 2 to 4 percent of stearic acid, 3 to 6 percent of hexadeca octadecanol, 2.5 to 6 percent of isopropyl palmitate, 1 to 1.6 percent of gyceryl monostearate, and 0.1 percent of propylparaben; the component B consists of 5 to 10 percent of natural plant ingredients, 5 to 8 percent of glycerol, 2 to 2.4 percent of cetanol ether phosphate sylvite, and 0.1 percent of methyl hydroxybenzoate; the balance being the deionized water; and 1 gram of the natural plant ingredients consist of 0.40 to 0.60 gram of grape leaf, 0.25 to 0.50 gram of sweet osmanthus and 0.10 to 0.15 gram of green tea. During the preparation, the natural plant ingredients are prepared firstly. The component A and the component B are respectively heated up to about 80 DEG C so as to be dissolved, and are stirred and mixed evenly; the deionized water is added into the component A and the component B; and the solution is stirred rapidly for complete emulsification and then is cooled down to obtain the sunblocking cream. The sunblocking cream has the advantages that the sunblocking cream has stronger absorption property for medium-wave and long-wave ultraviolet rays, and can effectively prevent the radiation of the ultraviolet rays on skin without generating any adverse reactions such as stimulus and irritability and so on.
Owner:NANJING UNIV OF INFORMATION SCI & TECH
Microcapsule Chinese herbal medicine composition capable of inhibiting bacteria, resisting inflammation and promoting blood circulation, and preparation method for microcapsule Chinese herbal medicine composition
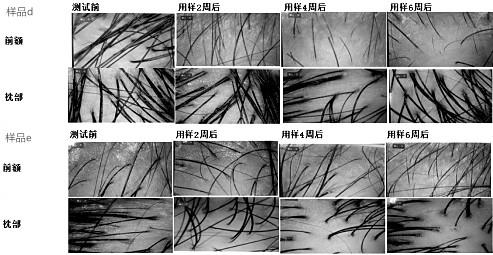
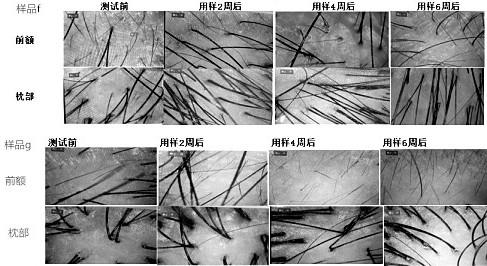
ActiveCN111329981AAntibacterial and anti-inflammatoryPromote circulationPowder deliveryAntipyreticBiotechnologyBULK ACTIVE INGREDIENT
The invention belongs to the daily chemical field. A microcapsule Chinese herbal medicine composition capable of inhibiting bacteria, resisting inflammation and promoting blood circulation is preparedfrom a microcapsule inclusion and following ingredients in parts by weight: polygoni multiflori radix, cacumen platycladi, Zingiber officinale Rosc. roots, radix angelicae sinensis, Eclipta prostrata(L.) L., Piper nigrum L seeds, capsicum annuum fructus and yeast extracts. The composition has the efficacy of resisting inflammation, inhibiting bacteria, promoting blood circulation, preventing hair loss, restoring hair and nourishing hair. According to the preparation method disclosed by the invention, various Chinese herbal medicines are mixed and extracted so as to prevent from importing excessive impurities, the high purity of the active ingredients of an extracting solution is guaranteed, and the microcapsule Chinese herbal medicine composition is moderate and inirritative. The microcapsule inclusion is adopted to carry out inclusion protection on a traditional Chinese medicine compound extracting solution and the yeast extracts, and the stability and the high activity of effectiveingredients are improved. During utilization, the microcapsule Chinese herbal medicine composition exhibits better pertinence, a higher medicine effect and an obvious effect, and a treatment period is effectively shortened.
Owner:OPAL COSMETICS HUIZHOU
Diclofenac salt pleximetric paste and its production
InactiveCN1895242BImprove flexibilityNo pollutionOrganic active ingredientsAntipyreticTransdermal patchAcrylic resin
A transdermal paster of diclofenac salt is composed of substrate layer, medicinal layer and antisticking layer. Said medicine layer consisting of diclofenac salt, matrix (acrylic resin and pressure sensitive adhesive No.4), transdermal osmosis promoter and plasticizer. Said diclofenac salt is prepared through respectively dissolving diclofenac, diethylamine, diethanolamine, triethanolamine and pyrrolidine in acetone, mixing and ultrasonic stirring.
Owner:SHENYANG PHARMA UNIVERSITY
Anastrozole controlled release patch and preparation method thereof
InactiveCN101513397AImprove adhesionImprove flexibilityOrganic active ingredientsPharmaceutical non-active ingredientsSide effectTackifier
The invention belongs to the field of pharmaceutical technology, disclosing an anastrozole controlled release patch and a preparation method thereof. The invention comprises a back lining layer, a medicine reservoir and an anti-sticking layer, wherein, the medicine reservoir comprises remedium cardinale anastrozole, pressure sensitive adhesive and sorbefacient by skin, wherein, the anastrozole accounts for 0.5-30wt%, the pressure sensitive adhesive accounts for 70-90wt% and the sorbefacient by skin accounts for 0.5-50wt%; inert filler, plasticizer, tackifier, chemical inhibitor and the like can also be added to the list. The anastrozole, the pressure sensitive adhesive and the sorbefacient by skin are fully mixed and transferred to be coated on the anti-sticking layer for being dried at the temperature of 40-80 DEG C, and then the mixture are compounded by using PVC or lining materials without woven fabrics and made into the patch of different sizes and specifications by die cutting, thus obtaining the finished product. The patch can not only avoid stimulus to the gastrointestinal tract by oral medication but also reduce the toxic side effect of the medicine by slow release; the release lasts for at least 7 days, thus ensuring lasting and stable therapeutical efficiency; if medicine administration is desired to be halted, removing the patch can achieve the purpose; the patch features convenient use; in addition, the patch is flexible and appropriately adhesive.
Owner:SHENYANG PHARMA UNIVERSITY
Angelica oil transdermal patch and preparation method thereof
InactiveCN103976983ALong-lasting and stable effectSmall individual differencesPharmaceutical non-active ingredientsSexual disorderDrugAdministration time
The invention discloses an angelica oil transdermal patch and a preparation method thereof, which provides a modern TTS (transdermal thrapeutic system) traditional Chinese medicinal preparation to broad women suffering from dysmenorrhea. The patch is characterized in that: angelica oil serving as a raw material and polyethylene glycol serving as a matrix are encapsulated between a lining membrane and a controlled-release membrane to prepare the membrane-controlled transdermal patch for treatment of dysmenorrhea. The patch can release drugs at a constant speed, so that the curative effect is durable and smooth, the acting time of the drug can be prolonged, the number of administration times can be reduced, the compliance of patients can be improved, and thus use of the angelica oil transdermal patch can be stopped at any time. The angelica oil transdermal patch represents the effective, safe, convenient and humanized administration therapeutic characteristics.
Owner:HUBEI HONGTUDI MODERN CHINESE MEDICINE
Composition with skin-whitening, speckle-fading and antioxidant effects as well as preparation method and application of composition
ActiveCN111991337AFunction increaseImprove synergistic stabilityCosmetic preparationsToilet preparationsMirabilis jalapaPhospholipin
The invention discloses a composition with skin-whitening, speckle-fading and antioxidant effects as well as a preparation method and application of the composition. The composition contains the following raw material components in percentage by weight: 0.1%-10.0% of a mirabilis jalapa cell inclusion, 0.01%-1.0% of a centella leaf extract, 0.01%-5.0% of an apple cell extract, 0.01%-2% of a white rose cell active matter and 0.1%-1% of an emulsifying agent. The mirabilis jalapa cell inclusion consists of a mirabilis jalapa cell active matter, nonapeptide-1 permeating the mirabilis jalapa cell active matter and an ascorbic acid glucoside. The outer surface of the mirabilis jalapa cell active matter is coated with double phospholipid layers. The composition has the skin-whitening, speckle-fading and antioxidant effect and further has the characteristic of stable and durable functions.
Owner:湖北省麦诗特生物科技有限公司
Non-elasticity hollow core big-belly yarn and preparation method thereof
The invention provides a non-elasticity hollow core big-belly yarn. The big-belly yarn comprises two core yarns, two fixed yarns and one decorative yarn, wherein the core yarns are hollow core yarns processed by water-soluble vinylon filaments, coffee fibers and seashell fibers in a blended mode; the fixed yarns are non-elasticity binder yarns processed by PTT fiber filaments, silk and linen fibers and coconut carbon fibers in a blended mode; the decorative yarn is a blended rough yarn processed by tapa fibers, nettle fibers and yakwool fibers in a blended mode. Multiple-component fiber raw materials are fed, in this way, the multifunctional multi-form multi-color composite fashion yarn can be produced, and the product variety and design can be enriched. At the present, novel weaving raw materials are rich in type and dazzling; through appropriate compatibility and application of the raw materials, kinds of product forms can be obtained, functions are complementary for kinds of fibers,and new varieties high in technical content and additional value can be developed.
Owner:ZHONGYUAN ENGINEERING COLLEGE
Letrozole targeted slow-release transdermal patch and preparation method thereof
InactiveCN101716163AIncrease drug concentrationGood curative effectOrganic active ingredientsSexual disorderTransdermal patchTreatment effect
The invention belongs to the technical field of medicine, and discloses a letrozole targeted slow-release transdermal patch and a preparation method thereof. The patch is a patch containing a novel sorbefacient, and can maintain the constant speed to release the medicament, thereby ensuring that the therapeutic effect is durably steady, prolonging the action time of the medicament, reducing administration times, and improving the compliance of patients. The patch is attached to the breast, so the medicament can enter mammary tissues through the skin, which remarkably improves the medicament concentration of letrozole in the ammary tissues and achieves better treatment effect. The patch and the preparation method have the advantages of simple preparation method, convenient use, good flexibility of the patch, and suitable adhesivity.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of gardenia volatile component, skin cream and preparation method of skin cream
InactiveCN109602671AReduce lossesFast extractionCosmetic preparationsToilet preparationsBetaineWater vapor
The invention discloses a preparation method of a gardenia volatile component, a skin cream containing the gardenia volatile component, and a preparation method of the skin cream. The skin cream divides the raw materials into two kinds, namely an aqueous phase and an oil phase; the water phase includes cetostearyl alcohol, allantoin, betaine, xanthan gum, and deionized water; the oil phase includes squalane, vitamin E, stearic acid, glycerin, jojoba oil, and phenoxyethanol; and a volatile component containing safranal extracted from gardenia fruits is added into the skin cream. The invention adopts a flash loss extraction auxiliary steam distillation method to obtain the volatile oil with yield of 3.8%; and the skin cream uses a pure natural extract containing the antioxidant component separated from the gardenia, and fully retains the effective components contained in plant materials; and the skin cream contains antioxidant free radicals and is harmless to the body.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com