Metolazone slow-release capsule and method for preparing same
A technology of metolazone and sustained-release capsules, applied in the field of medicine, to achieve the effects of reducing drug resistance, reducing fluctuations and eliminating peak concentrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
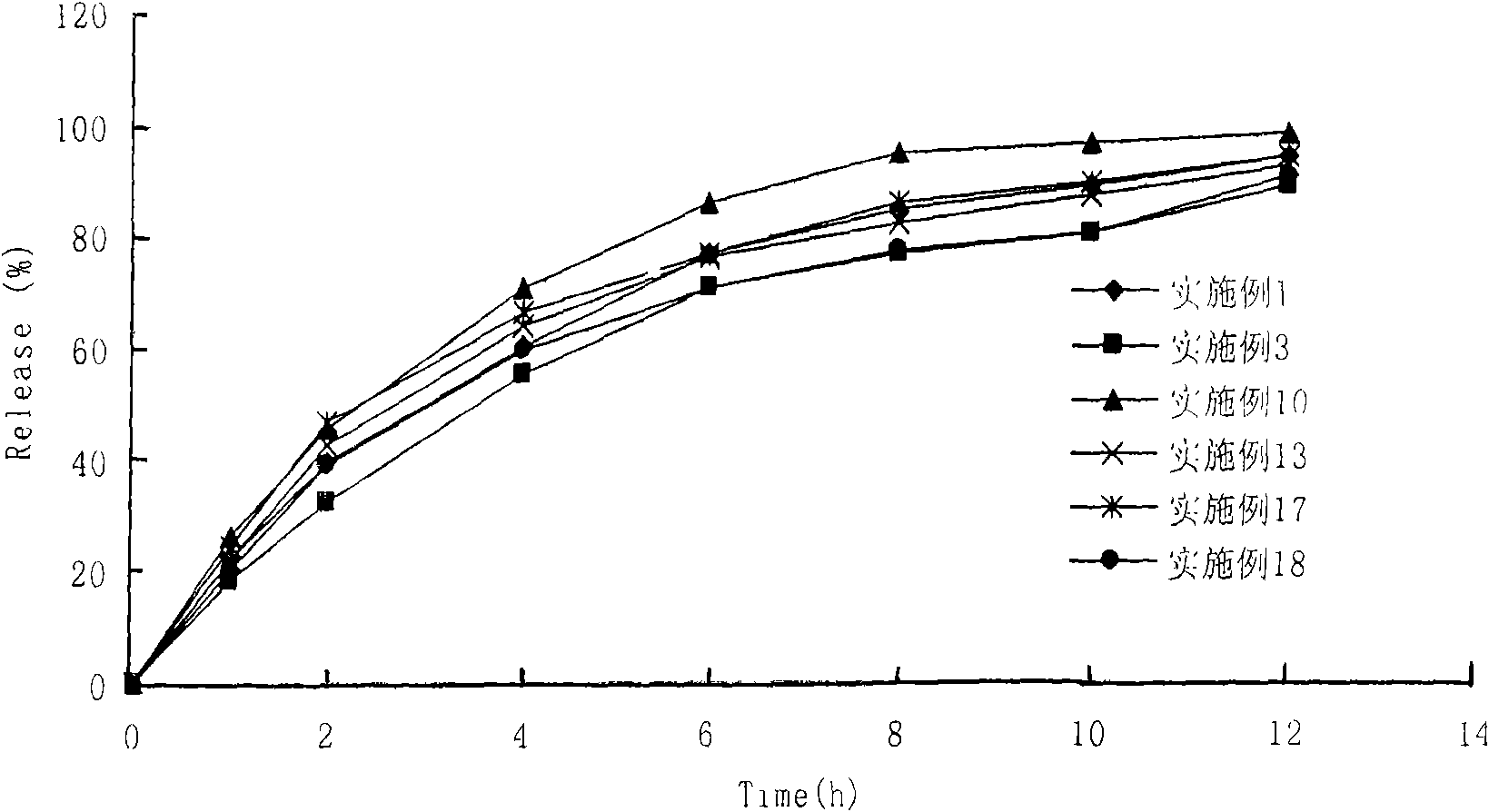
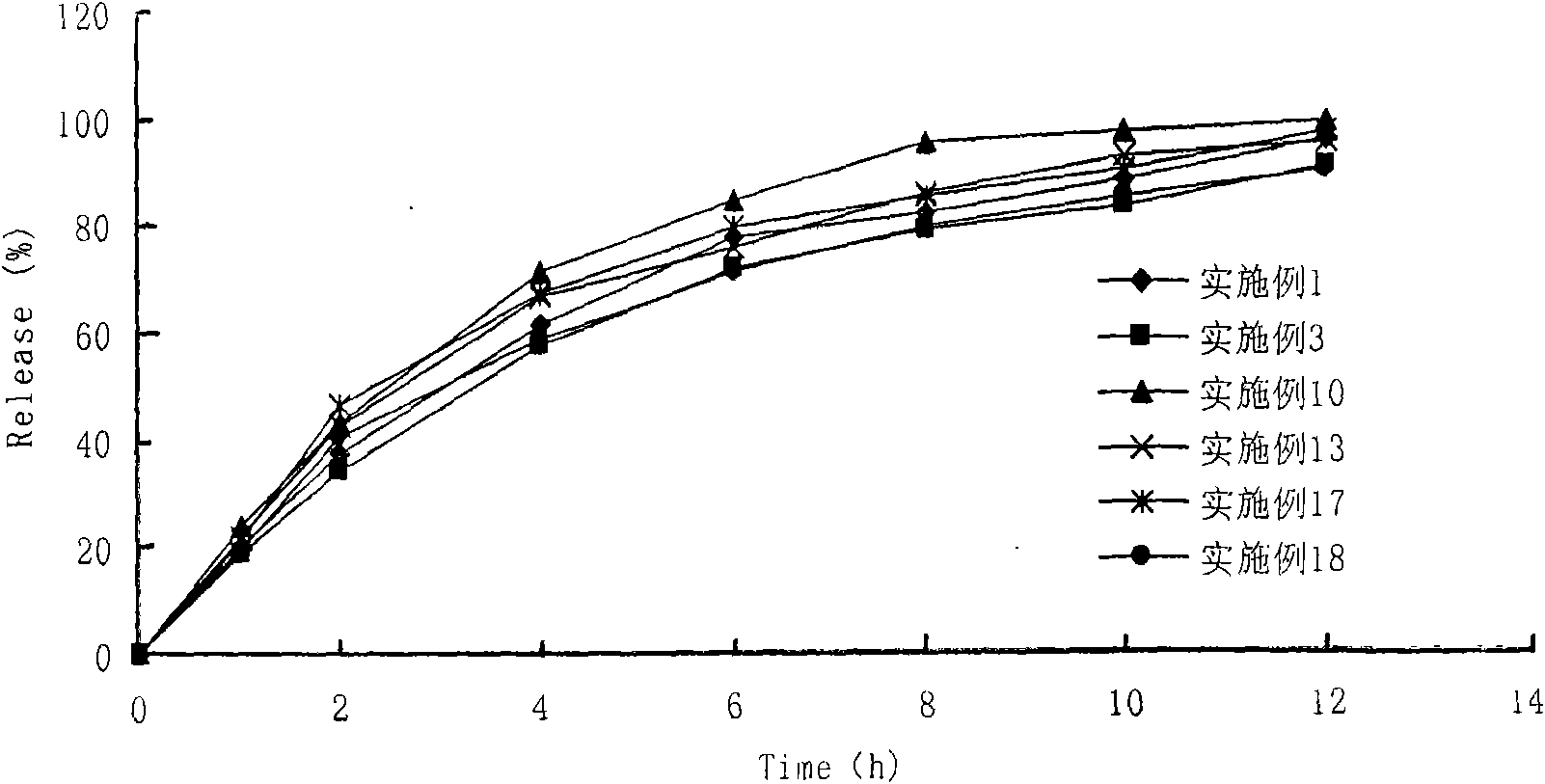
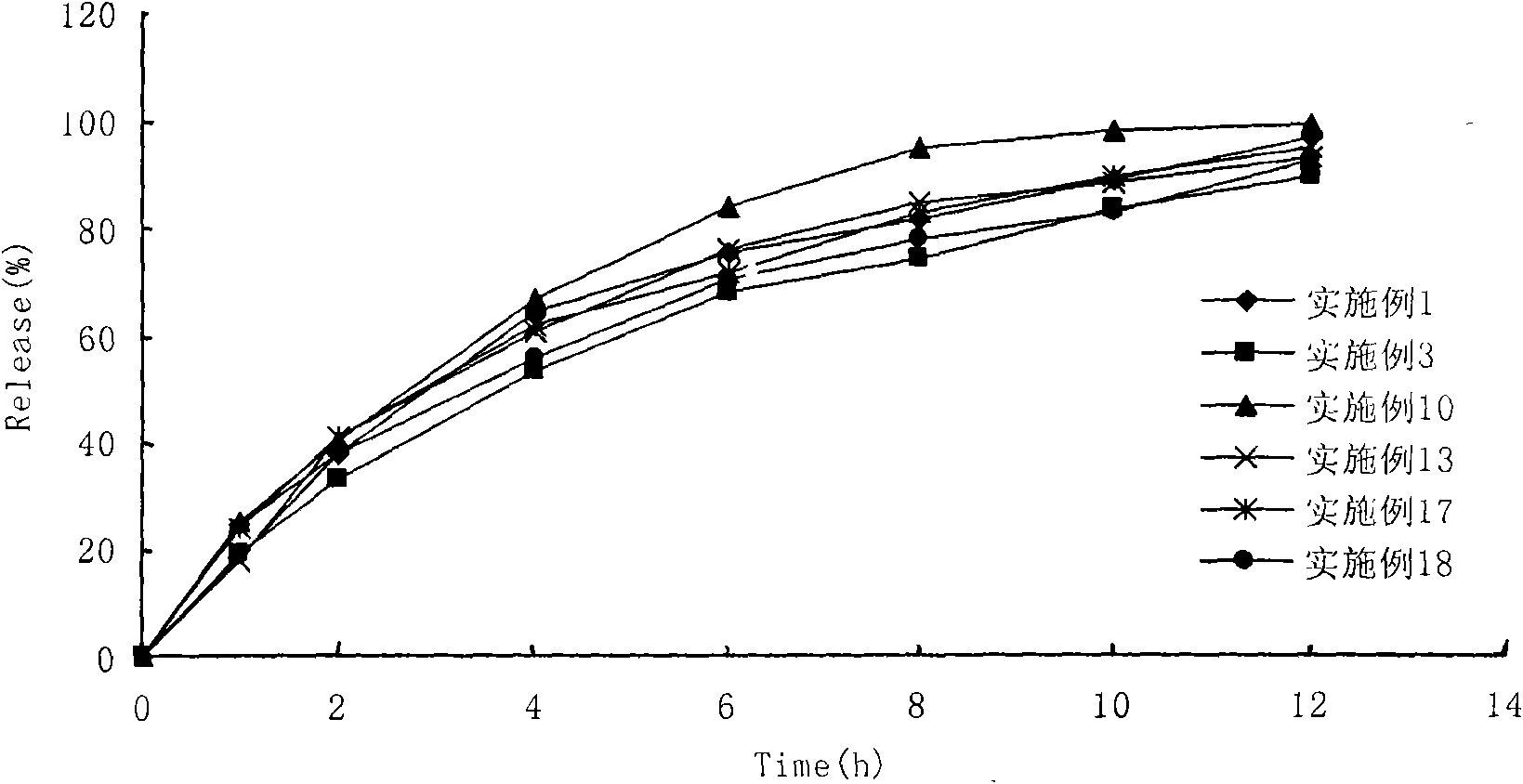
Image
Examples
Embodiment 1
[0056] Component weight / 1000 capsules
[0057] Metolazone 10g
[0058] Hydroxypropyl Methyl Cellulose 65g
[0059] Lactose 60g
[0060] Microcrystalline Cellulose 60g
[0062] 90% ethanol appropriate amount
[0063] Preparation method: respectively pulverize the raw materials and auxiliary materials of the prescription and pass through a 100-mesh sieve for later use; mix metolazone, hydroxypropyl methylcellulose, microcrystalline cellulose and lactose through a 100-mesh sieve, and mix with an appropriate amount of 90% ethanol To make soft material, granulate with 20 mesh sieve, dry the wet granules at 60±5°C, granulate with 20 mesh sieve; add magnesium stearate, mix well, pack into capsules.
Embodiment 2
[0065] Component weight / 1000 capsules
[0066] Metolazone 10g
[0067] Polyoxyethylene 100g
[0068] Microcrystalline Cellulose 85g
[0070] 90% ethanol appropriate amount
[0071] Preparation method: crush the raw materials and auxiliary materials of the prescription separately and pass through a 100-mesh sieve for later use; mix metolazone, polyoxyethylene, and microcrystalline cellulose through a 100-mesh sieve, use an appropriate amount of 90% ethanol to make a soft material, and pass through a 20-mesh sieve Granulate, dry the wet granules at 60±5°C, and sieve through a 20-mesh sieve; add magnesium stearate, mix well, and pack into capsules.
Embodiment 3
[0073] Component weight / 1000 capsules
[0074] Metolazone 10g
[0075] Sodium Alginate 90g
[0076] Lactose 50g
[0077] Microcrystalline Cellulose 45g
[0079] 90% ethanol appropriate amount
[0080] Preparation method: crush the raw materials and auxiliary materials of the prescription respectively and pass through a 100-mesh sieve for later use; weigh the prescribed amount of metolazone, sodium alginate, microcrystalline cellulose and lactose, pass through a 100-mesh sieve and mix evenly, and use an appropriate amount of 90% ethanol To make soft material, granulate with 20 mesh sieve, dry the wet granules at 60±5°C, granulate with 20 mesh sieve; add magnesium stearate, mix well, pack into capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| osmolarity | aaaaa | aaaaa |
| osmolarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com