Angelica oil transdermal patch and preparation method thereof
A technology of transdermal patch and angelica oil, applied in the field of medicine, can solve problems such as adverse effects, and achieve the effects of reducing individual differences, reducing toxic and side effects, and being convenient to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
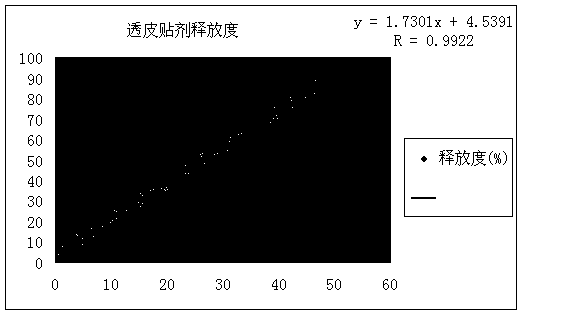
Examples
Embodiment 1
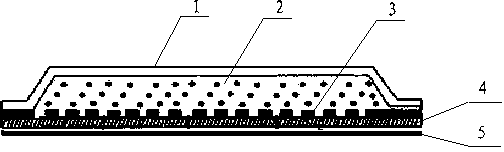
[0026] Example 1: (1) Heat, melt and mix PEG400 4g and PEG4000 5g evenly. (2) Take 4g of angelica oil, heat and mix it with the matrix evenly to make angelica oil ointment. (3) Spread the ointment on the backing film (thickness 0.3mm, area 4cm x6cm or 6cm x8cm), cover the EVA controlled release film (thickness 50μm), and seal it with thermoplastic around it. (4) The pressure-sensitive adhesive is hot-melt coated on the release layer, and the adhesive surface is covered outside the release-controlling film. (5) Sealed in an aluminum-plastic composite film bag and stored in a cool, dark place.
Embodiment 2
[0027] Example 2: (1) Heat, melt and mix PEG400 4g and PEG4000 5g evenly. (2) Take 4g of angelica oil, heat and mix it with the matrix evenly to make angelica oil ointment. (3) Spread the ointment on the backing film (thickness 0.5mm, area 4cm x6cm or 6cm x8cm), cover the EVA controlled release film (thickness 50μm), and seal it with thermoplastic around it. (4) Coat the PEG matrix (thickness 0.2mm) on the outside of the controlled-release membrane, then cover with an anti-adhesive layer, and attach a medical adhesive sticker. (5) Sealed in an aluminum-plastic composite film bag and stored in a cool, dark place.
Embodiment 3
[0028]Example 3: (1) PEG400 40g and PEG4000 50g were heated, melted and mixed uniformly. (2) Take 40g of angelica oil, heat and mix with the matrix evenly to make angelica oil ointment. (3) Spread the ointment on the backing film (thickness 0.4mm, area 4cm x6cm or 6cm x8cm), cover with EVA controlled-release film (thickness 50μm), and seal it with thermoplastic around it. (4) Coat the PEG matrix (thickness 0.2mm) on the outside of the controlled-release membrane, then cover with an anti-adhesive layer, and attach a medical adhesive sticker. (5) Sealed in an aluminum-plastic composite film bag and stored in a cool, dark place.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com