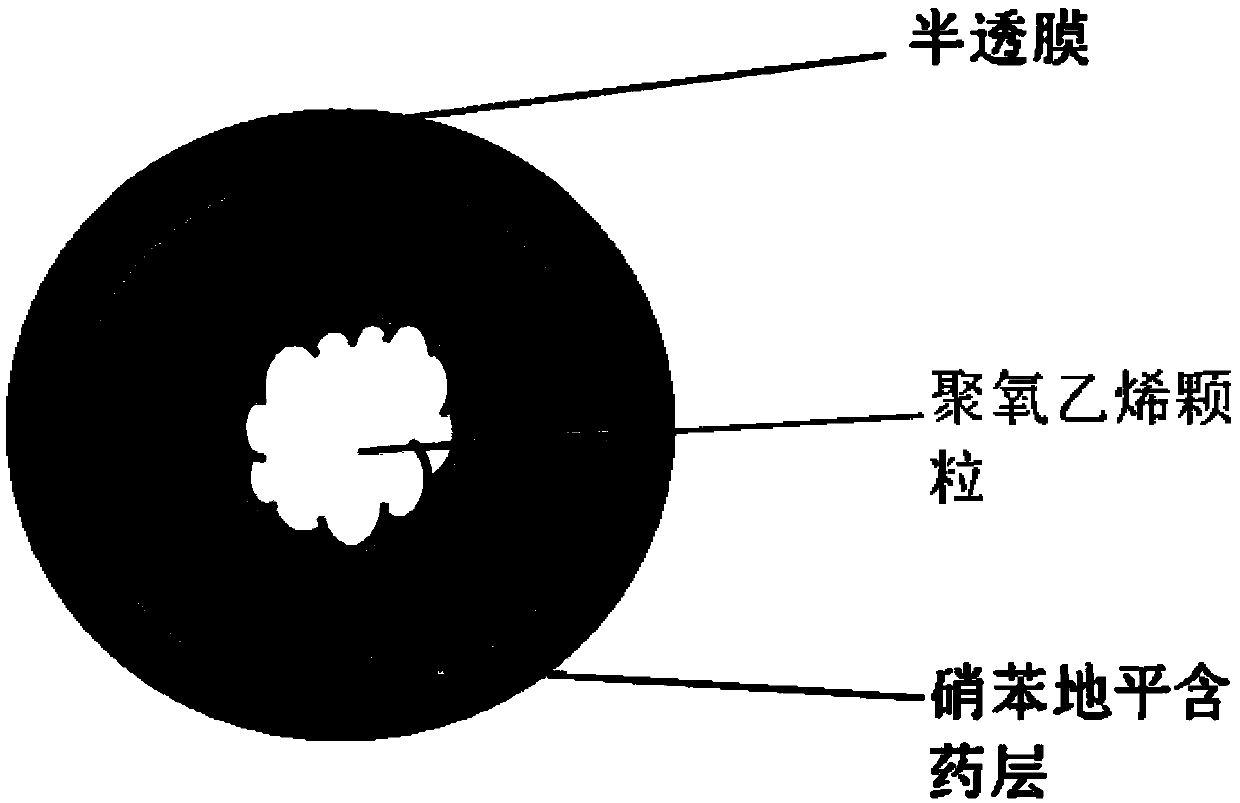
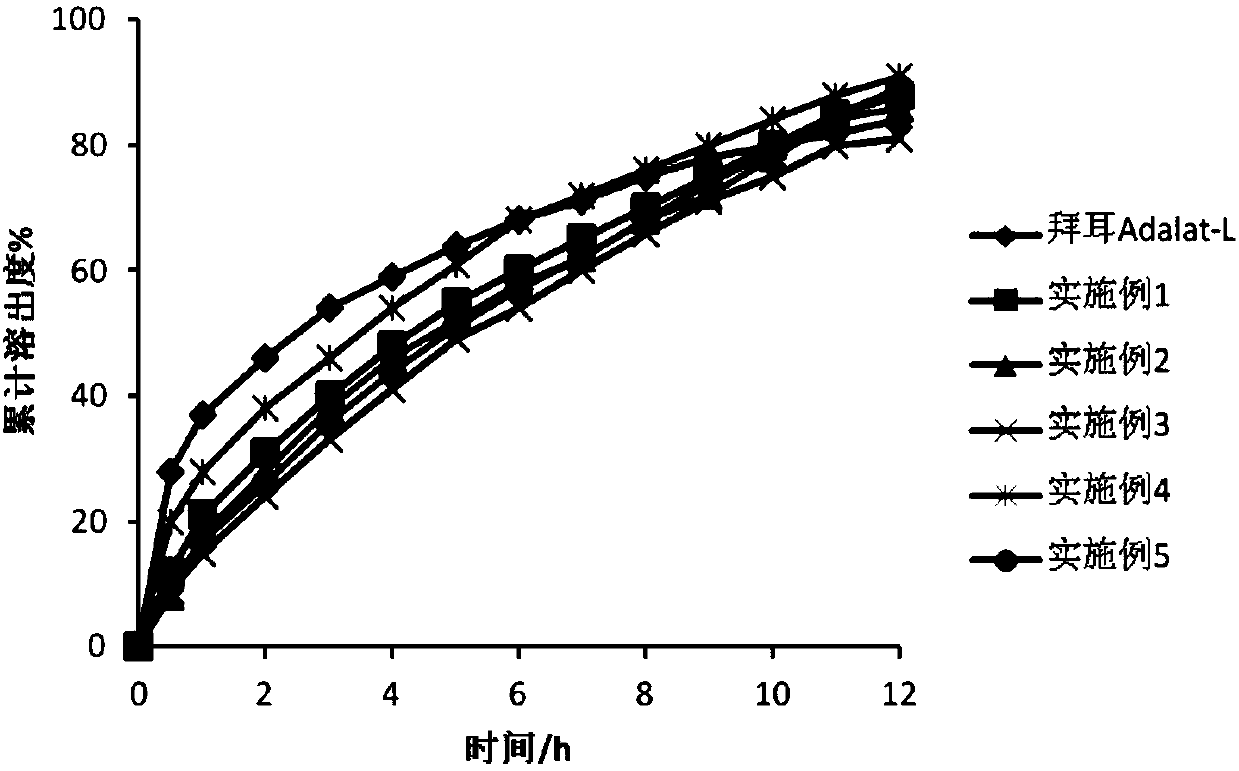
Nifedipine micro-porous osmotic pump particle and preparation method thereof
A nifedipine and osmotic pump technology, applied in the field of medicine, can solve the problem that the sustained release time can only reach more than 6 hours, and achieve the effects of avoiding the fluctuation of blood drug concentration, reducing the number of times of taking the medicine, and improving the correlation between the body and the body.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
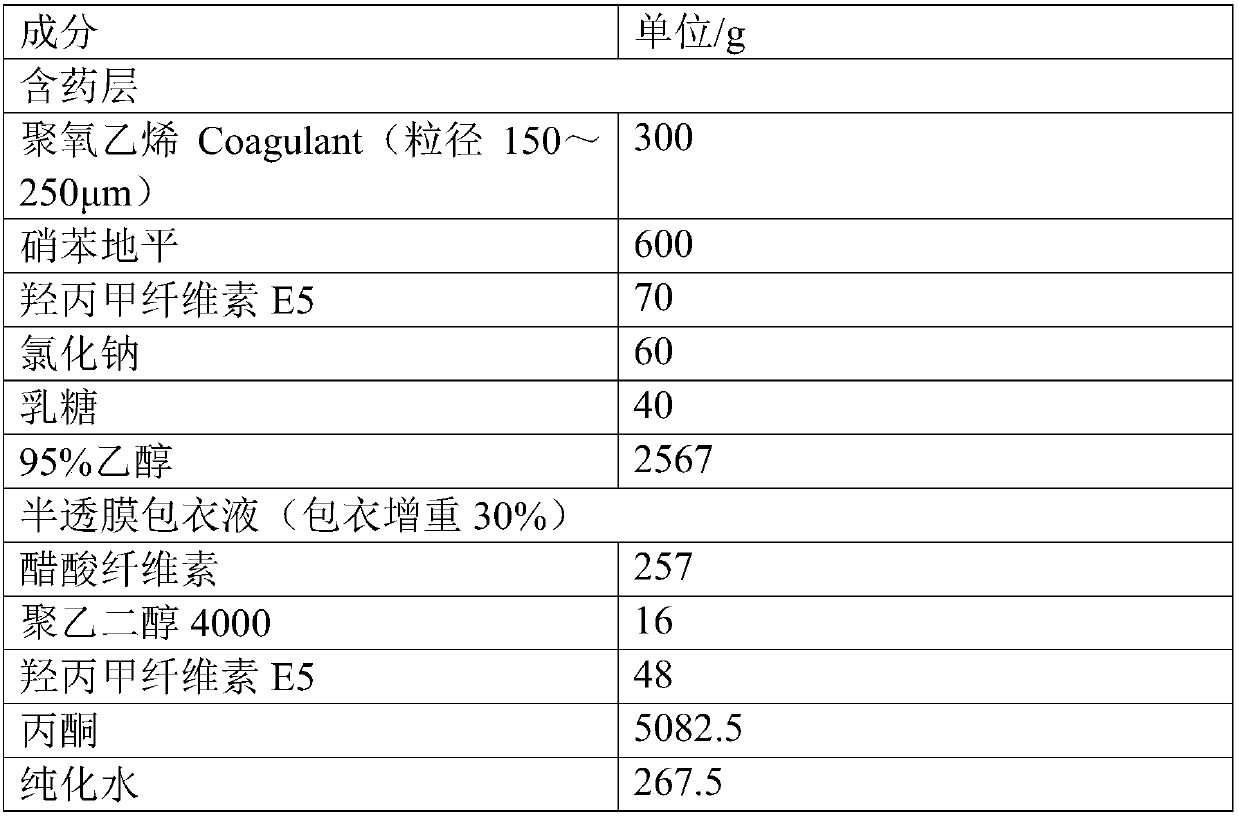
[0033] prescription
[0034]
[0035] Preparation Process:
[0036] 1. Preparation of pills containing:
[0037] Screen 300g of polyoxyethylene coagulant particles with a particle size of 150~250μm, and place them in a DPP-II fluidized bed granulating and coating machine; weigh the prescribed amount of nifedipine, hypromellose E5, sodium chloride, and lactose , Dispersed in 95% ethanol, evenly dispersed. Turn on the fluidized bed blast volume 15±2 (m 3 / h) / kg, atomizing pressure 1.5±0.2bar, spraying speed 15±1g / min, inlet air temperature 50±2℃, material temperature 30±2℃, after spraying, continue drying in the fluidized bed , Get nifedipine-containing pills.
[0038] 2. Semi-permeable membrane coating
[0039] Weigh the prescribed amount of cellulose acetate, polyethylene glycol 4000, and hypromellose E5 and dissolve them in acetone-water solution. After the dissolution is complete, place the above-mentioned nifedipine-containing pills in the DPP-II fluidized bed granulation package ...
Embodiment 2
[0043] prescription
[0044]
[0045] Preparation Process:
[0046] 1. Preparation of pills containing:
[0047] Screen 300g of polyoxyethylene 303 particles with a particle size of 150-250μm, and place them in a DPP-II fluidized bed granulating and coating machine; weigh the prescription amount of nifedipine, hydroxypropyl cellulose LF, potassium chloride, and mannitol , Dispersed in 95% ethanol, evenly dispersed. Turn on the fluidized bed blast volume 15±2 (m 3 / h) / kg, atomizing pressure 1.5±0.2bar, spraying speed 14±1g / min, inlet air temperature 50±2℃, material temperature 30±2℃, after spraying, continue drying in fluidized bed , Get nifedipine-containing pills.
[0048] 2. Semi-permeable membrane coating
[0049] Weigh the prescribed amount of cellulose acetate, triethyl citrate, and hydroxypropyl cellulose LF and dissolve them in an acetone-water solution. After the dissolution is complete, place the nifedipine-containing pills in the DPP-II fluidized bed granulation bag In the...
Embodiment 3
[0053] prescription
[0054]
[0055] Preparation Process:
[0056] 1. Preparation of pills containing:
[0057] Screen 300g of polyoxyethylene 303 particles with a particle size of 150-250μm, and place them in a DPP-II fluidized bed granulating and coating machine; weigh the prescription amount of nifedipine, hypromellose E5, sodium chloride, and lactose, Disperse in 95% ethanol, evenly dispersed. Turn on the fluidized bed blast volume 16±2(m 3 / h) / kg, atomizing pressure 1.6±0.2bar, spraying speed 18±1g / min, inlet air temperature 50±2℃, material temperature 30±2℃, after spraying, continue drying in the fluidized bed , Get nifedipine-containing pills.
[0058] 2. Semi-permeable membrane coating
[0059] Weigh the prescription amount of ethyl cellulose, polyethylene glycol, and hypromellose E5 and dissolve it in acetone-water solution. After the dissolution is completed, place the nifedipine-containing pills in the DPP-II fluidized bed granulation bag In the clothes machine, spray th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com