A explosive core composition of controlled release administer drug and controlled release preparation as well as its preparing method
A composition and drug core technology, which is applied in pharmaceutical formulations, drug delivery, medical preparations with inactive ingredients, etc., can solve the problem that PEO is prone to sticking, PEO does not have thermal stability, preparation quality and adverse effects on release, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] prescription:
[0049] (1) Drug-containing layer (per tablet):
[0050] Nifedipine 33mg
[0051] Povidone (Plasdone K-90D) 30mg
[0052] Copovidone (Plasdone S630) 91mg
[0053] Magnesium Stearate 1.5mg
[0054] Micronized silica gel 0.5mg
[0055] (2) Booster layer (per piece):
[0056] Sodium starch glycolate 37mg
[0057] Hypromellose (K15M) 30mg
[0058] Carbomer (971PNF) 8mg
[0060] Copovidone (Plasdone S630) 15mg
[0061] Red Iron Oxide 1.1mg
[0062] Magnesium stearate 0.6mg
[0063] Micronized silica gel 0.4mg
[0064] (3) Composition of semi-permeable membrane coating solution (for every 1000 tablets)
[0065] Cellulose acetate 59.5g
[0066] Diethyl phthalate 3g
[0067] Acetone 1500ml
[0068]
[0069] Single tablet weight gain 38mg
[0070] (4) Composition of moisture-proof coating solution:
[0071] Color blue pink (CM-0317)
[0072] Preparation Process:
[0073] 1...
Embodiment 2
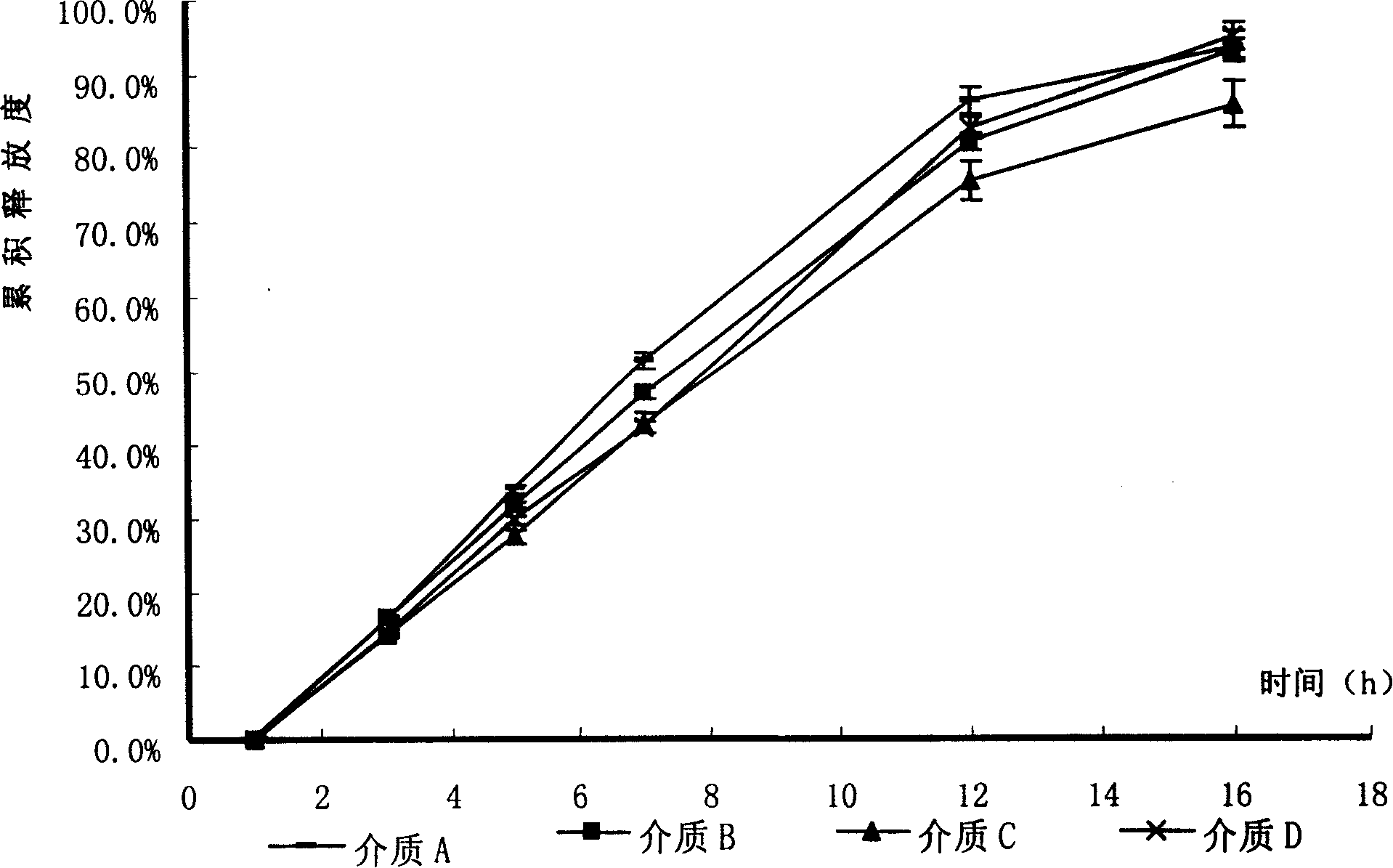
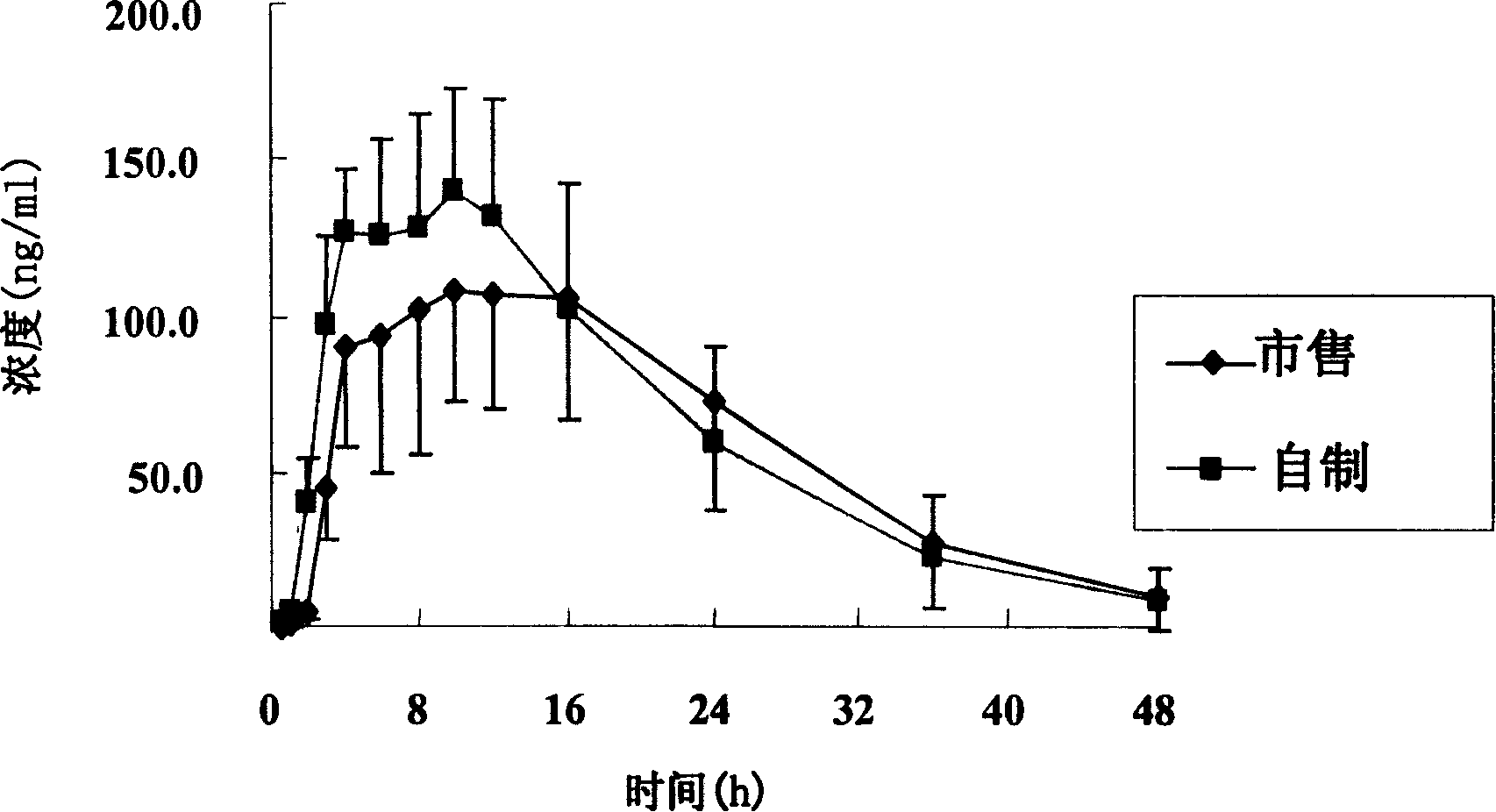
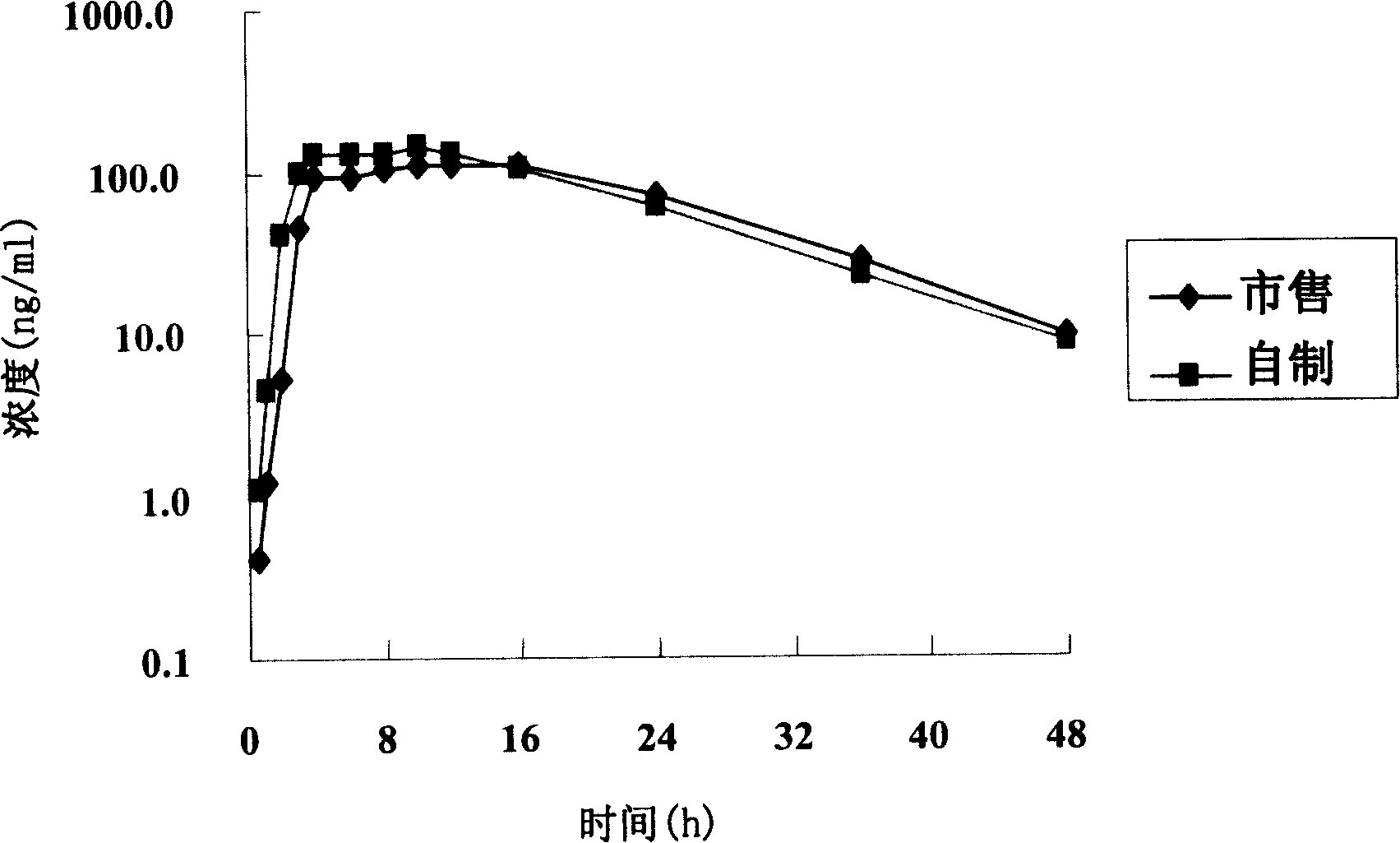
[0086] Under the conditions of dissolution media of different pH, the tablet prepared in Example 1 and the commercially available product (trade name Baixintong, produced by Bayer, Germany) were respectively tested for release rate, wherein, according to the usual practice, the dosage was 110% , for example, the theoretical value is 30 mg, and the actual dosage is 33 mg.
[0087] The results are shown in Table 1.
[0088] (1) 1% hydrochloric acid solution (pH1.2) of sodium lauryl sulfate;
[0089] (2) acetic acid-sodium acetate buffer (pH4.5) of 1% sodium lauryl sulfate;
[0090] (3) Phosphate-citrate buffer solution (pH 6.8) of 1% sodium lauryl sulfate.
[0091] Table 1 The release degree of self-made tablets and commercially available products
[0092]
[0093] The test results show that the release rate of the sample prepared in Example 1 and the commercially available product all meet the standard requirements. Compared with the commercially available product, the se...
Embodiment 3
[0095] The prescription is as follows:
[0096] (1) Drug-containing layer (per tablet):
[0097] Nifedipine 33mg
[0098] Povidone (Plasdone K-90D) 30mg
[0099] Copovidone (Plasdone S630) 91mg
[0100] Magnesium Stearate 1.5mg
[0101] Micronized silica gel 0.5mg
[0102] (2) Booster layer (per piece):
[0103] Low-substituted hydroxypropyl cellulose 150mg
[0104] Hypromellose (K15M) 30mg
[0105] Carbomer (971PNF) 10mg
[0107] Copovidone (Plasdone S630) 30mg
[0108] Red Iron Oxide 1.1mg
[0109] Magnesium stearate 0.6mg
[0110] Micronized silica gel 0.4mg
[0111] (3) Composition of semi-permeable membrane coating solution (for every 1000 tablets)
[0112] Cellulose acetate 59.5g
[0113] Diethyl phthalate 3g
[0114] Acetone 1500ml
[0115] Single tablet weight gain 38mg
[0116] (4) Composition of moisture-proof coating solution:
[0117] Color blue pink (CM-0317) Appropriate amount
[0118] The preparation process is the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com