Nifedipine controlled release capsule and preparation method thereof
A technology for nifedipine and capsules, applied in the field of nifedipine controlled-release capsules and its preparation, which can solve problems such as the complex preparation process of osmotic pump preparations, and achieve the effects of avoiding blood drug concentration fluctuations, easy industrialization, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
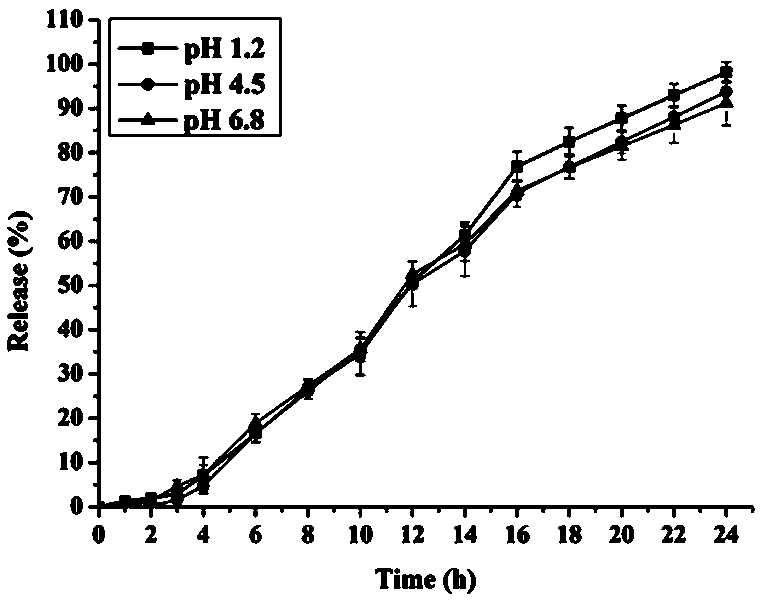
Embodiment 1
[0037] The prescription is shown in Table 1 (1000 capsules):
[0038] Table 1:
[0039]
[0040] Preparation process: 1. Preparation of capsules: After heating and dissolving polyethylene glycol 6000 at 60-80°C, add nifedipine and mix evenly, and cool to obtain nifedipine solid dispersion; grind the yellow solid dispersion and pass through 100 mesh Sieve; mix the solid dispersion powder, polyoxyethylene, citric acid, talcum powder and magnesium stearate, and put it in a hard capsule shell; 2. Coating operation: weigh the prescribed amount of cellulose acetate and poloxa M188 was dissolved in acetone solution. After the dissolution was completed, the above-mentioned nifedipine capsules were placed in a high-efficiency coating pot, and the coating liquid was sprayed onto the surface of the nifedipine capsules. The coating pot was set at a blast volume of 18 (m 3 / h), atomization pressure 1.6±0.2bar, spray speed 20±1g / min, air inlet temperature 45±2°C, material temperature 28...
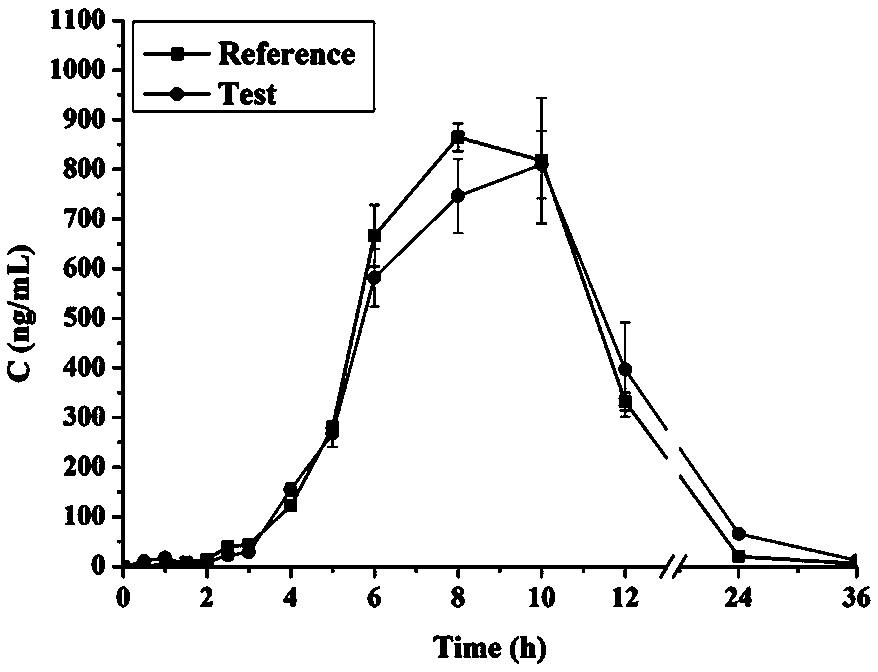
Embodiment 2
[0043] Example 2 Pharmacokinetic study of nifedipine microporous osmotic pump controlled-release capsules
[0044] Six rabbits were randomly divided into two groups, and the rabbits were fasted (freely drinking water) for 12 h before administration. The self-made microporous osmotic pump capsules (prescription in Example 1) and the commercially available nifedipine controlled-release tablets (Baixintong, 30 mg / tablet) were respectively placed deep in the oral cavity of rabbits and swallowed whole. At 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 24 and 36 h after administration, 0.5 mL blood was collected from the ear vein, and immediately transferred into heparin-treated Centrifuge at 4000 rpm for 10 min. Plasma was separated and stored in a -20°C refrigerator until use.
[0045] Precisely measure 200 μL of plasma sample, place in a 5 mL centrifuge tube, add 60 μL of ammonia water, vortex for 2 min, add 1.6 mL of anhydrous ether, vortex for 10 min, and centrifuge at 4000 r...
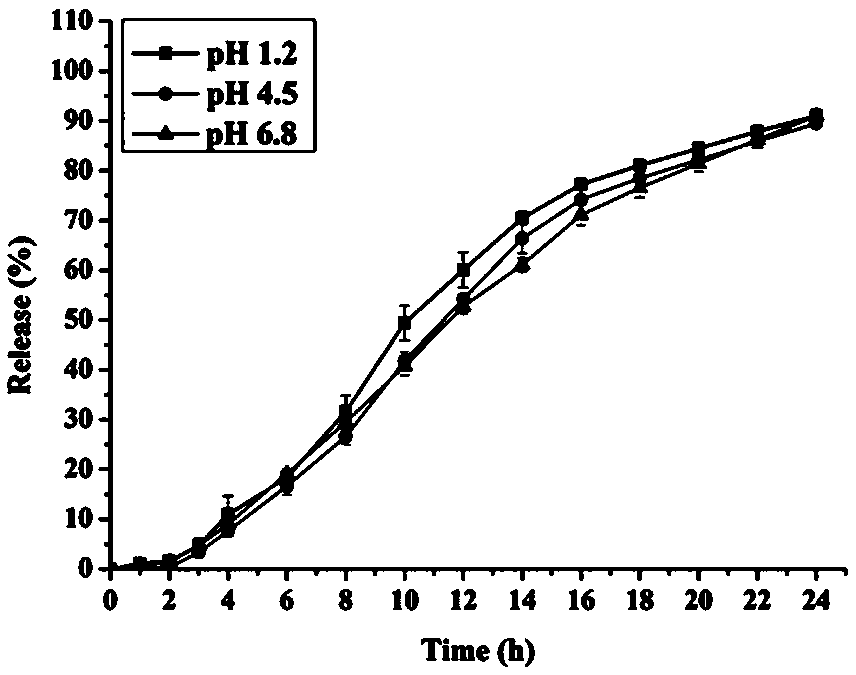
Embodiment 3
[0049] The prescription of embodiment 3 is shown in Table 2 (1000 grains):
[0050] Table 2:
[0051]
[0052] Preparation process: 1. Preparation of capsules: After heating and dissolving polyethylene glycol 6000 at 60-80°C, add nifedipine and mix evenly, and cool to obtain nifedipine solid dispersion; grind the yellow solid dispersion and pass through 100 mesh Sieve; mix the solid dispersion powder, polyoxyethylene, talcum powder and magnesium stearate, and put them in the hard capsule shell; 2, coating operation: take the prescribed amount of cellulose acetate, poloxamer 188 and dissolve in In the acetone solution, after the dissolution is completed, the above-mentioned nifedipine capsules are placed in a high-efficiency coating pan, and the coating liquid is sprayed onto the surface of the nifedipine capsules, and the coating pan setting condition is that the blast volume is 18 (m 3 / h), atomization pressure 1.6±0.2bar, spray speed 20±1g / min, air inlet temperature 45±2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com