Three-stage pulsed release controlled release tablet and preparation method thereof
A controlled-release tablet and pulse technology, which is applied in the field of metoprolol pulse osmotic pump controlled-release tablets and its preparation, can solve the problems of reducing curative effect, easy to develop tolerance, and inability to provide blood drug concentration, etc., to achieve optimal curative effect, The effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Prescription: (1000 tablets)
[0059] Drug-containing layer:
[0060]
[0061] Boost layer:
[0062]
[0063] Lag layer:
[0064]
[0065] Controlled Release Coating:
[0066]
[0067]
[0068] Preparation:
[0069] (1) Pretreatment Pass each component of the raw material through an 80-mesh sieve for subsequent use;
[0070] (2) Preparation of drug-containing layer granules According to the prescription of Example 1, the components of the drug-containing layer (except magnesium stearate) are uniformly mixed by equal addition method, added to a fluidized bed, and sprayed into 95% ethanol aqueous solution to prepare granules; after fully drying, pass through a 20-mesh sieve, granulate, then add magnesium stearate and mix evenly, as drug-containing layer granules, set aside;
[0071] (3) Preparation of booster layer granules According to the prescription in Example 1, mix the components of the booster layer (except magnesium stearate) according to the p...
Embodiment 2
[0079] Prescription: (1000 tablets)
[0080] Drug-containing layer:
[0081]
[0082] Boost layer:
[0083]
[0084] Lag layer:
[0085]
[0086] Controlled Release Coating:
[0087]
[0088] Preparation method: the preparation method is the same as that in Example 1, except that the time-lag coating layer and the controlled-release coating layer are formulated according to Example 2.
[0089] Release measurement method: with embodiment 1.
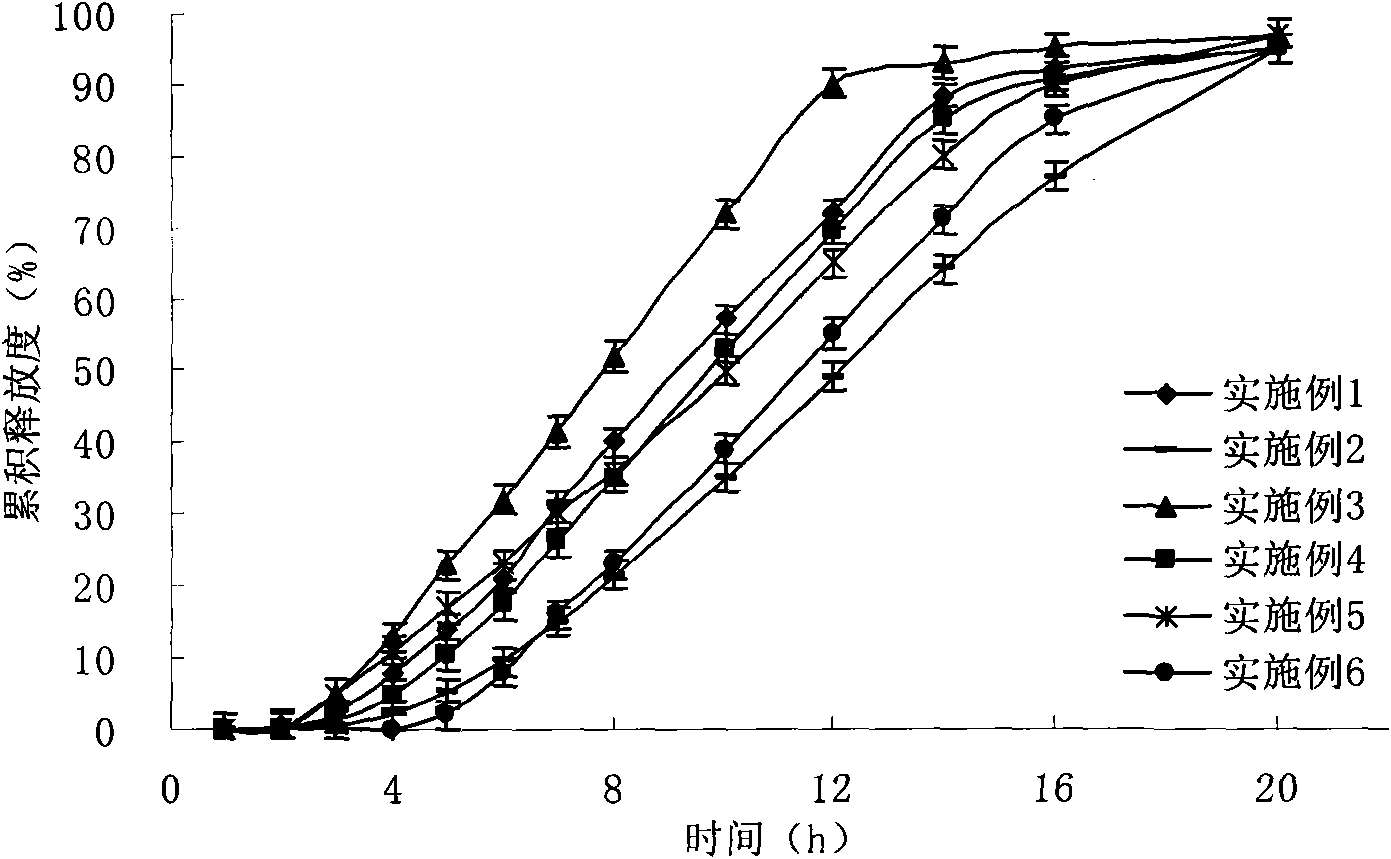
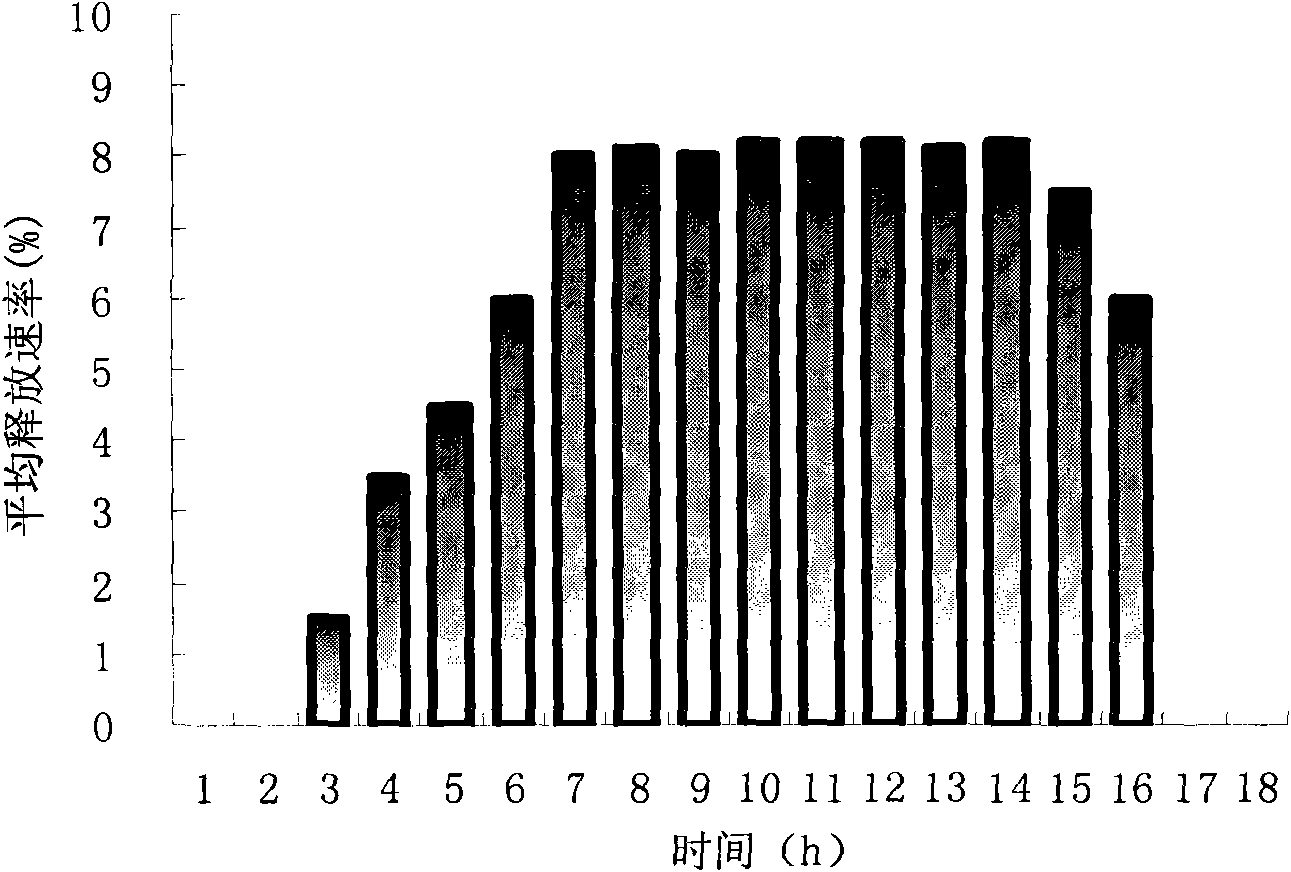
[0090] see results figure 1 , the drug release has a time lag of about 4 hours, the incremental drug release time is about 3 hours, and the constant rate drug release lasts for 9 hours.
Embodiment 3
[0092] Prescription: (1000 tablets)
[0093] Drug-containing layer:
[0094]
[0095] Boost layer:
[0096]
[0097] Lag layer:
[0098]
[0099] Controlled Release Coating:
[0100] Preparation method: the preparation method is the same as that in Example 1 except that the composition of the prescription is different.
[0101] Release measurement method: with embodiment 1.
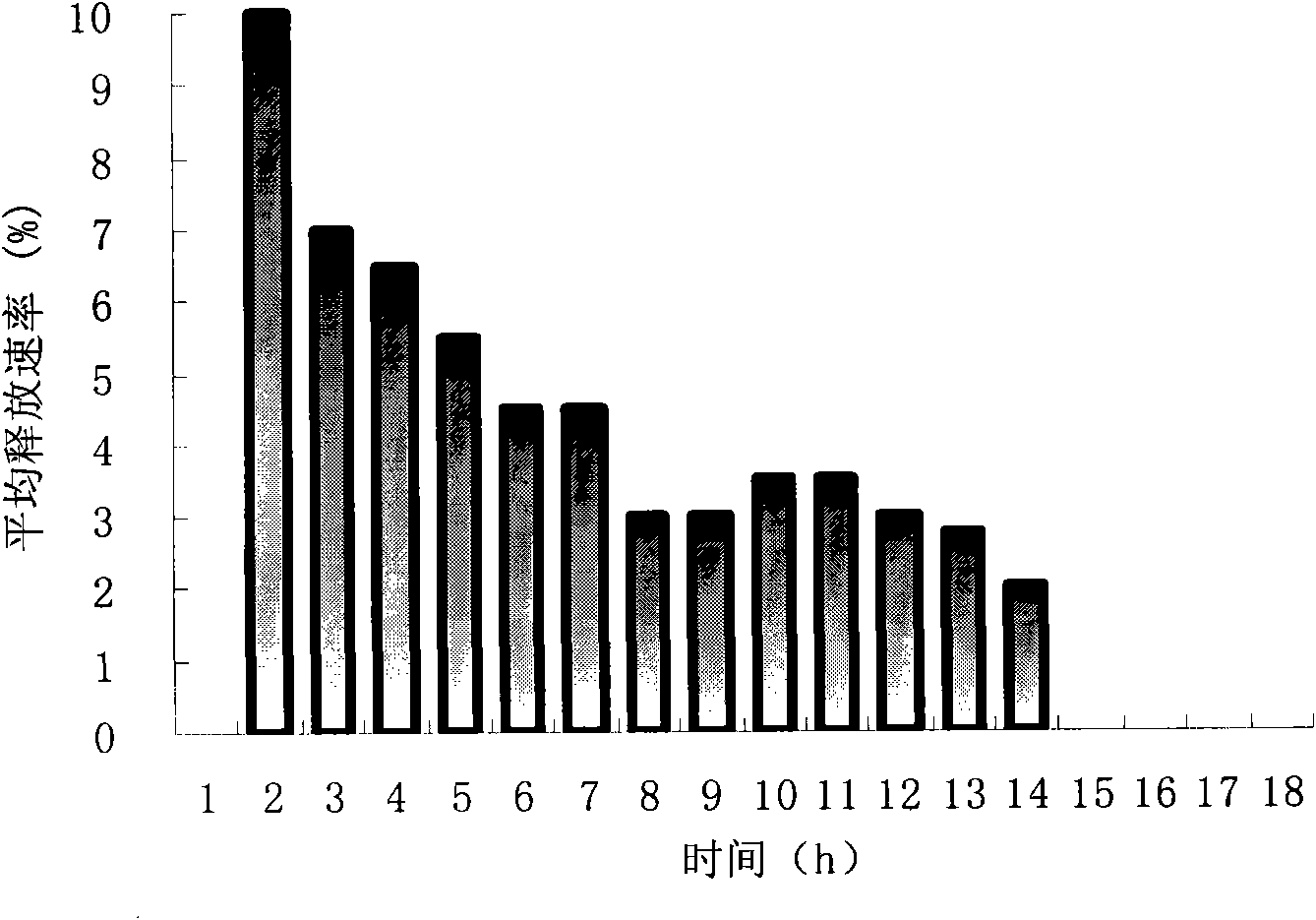
[0102] see results figure 1 , the drug release has a time lag of 2 hours, the incremental drug release time is 2 hours, and the constant rate drug release duration is 8 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com