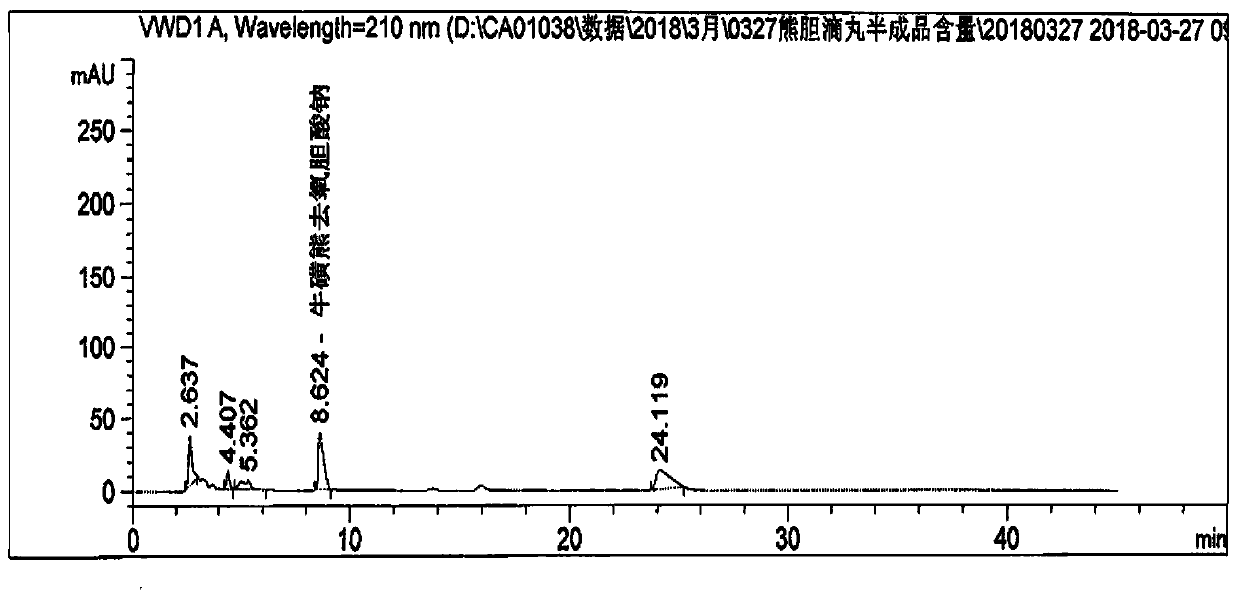
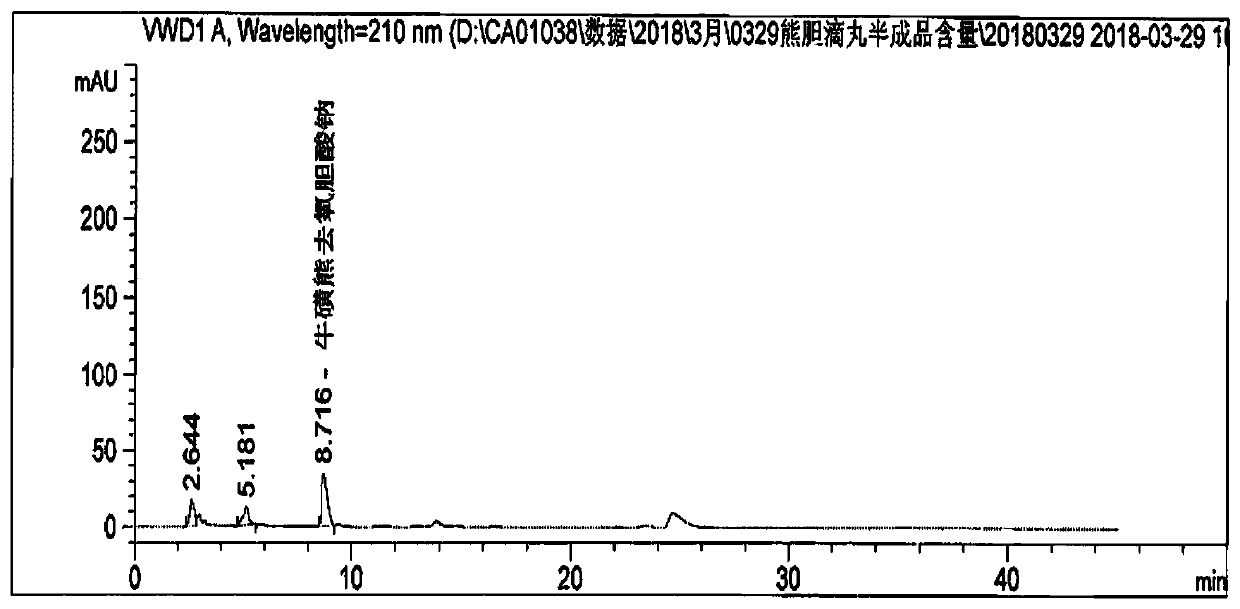
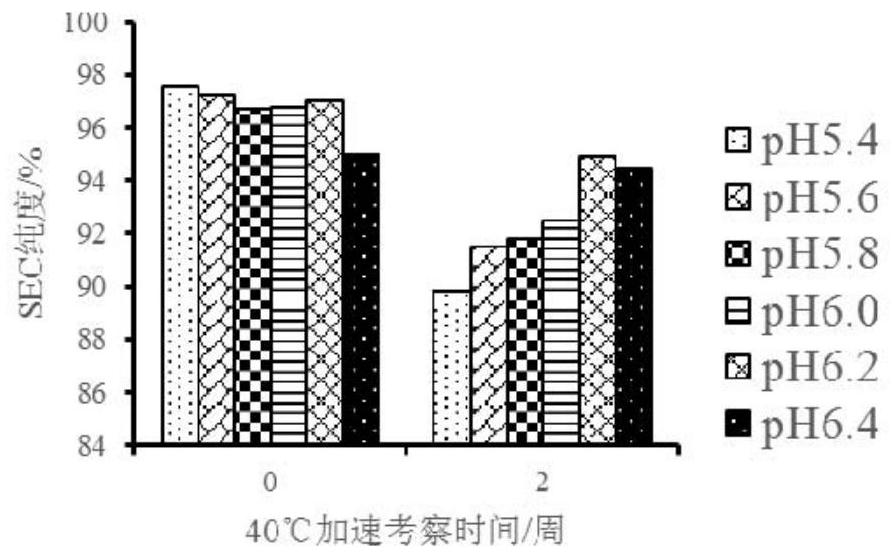
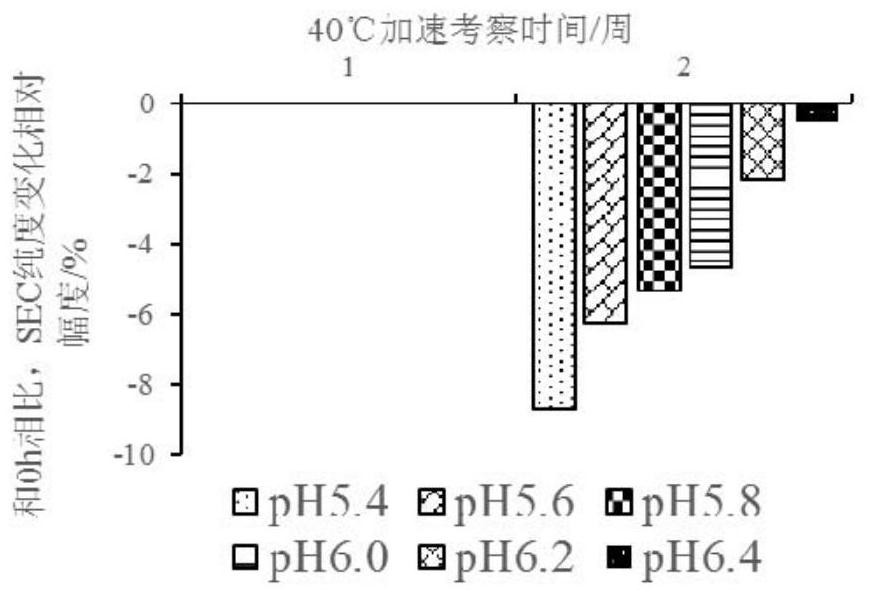
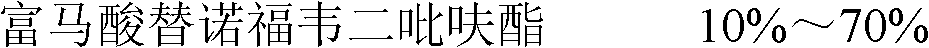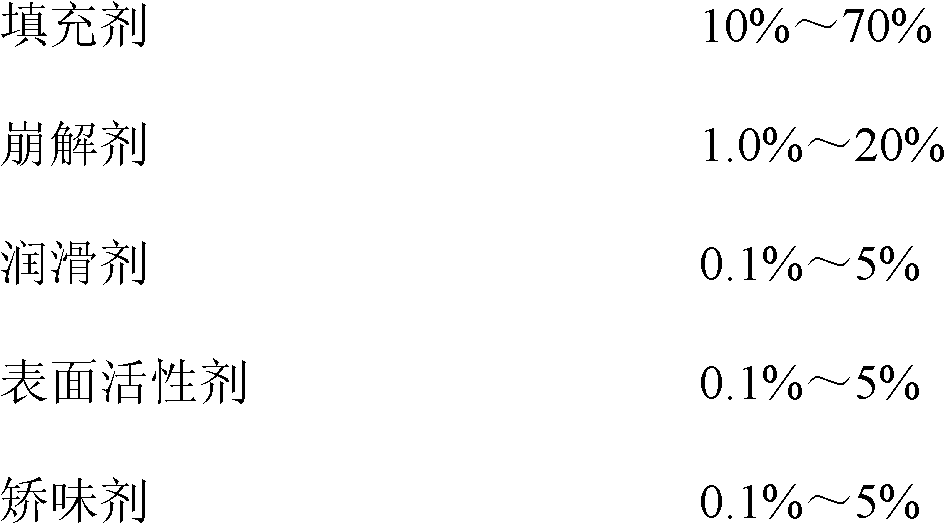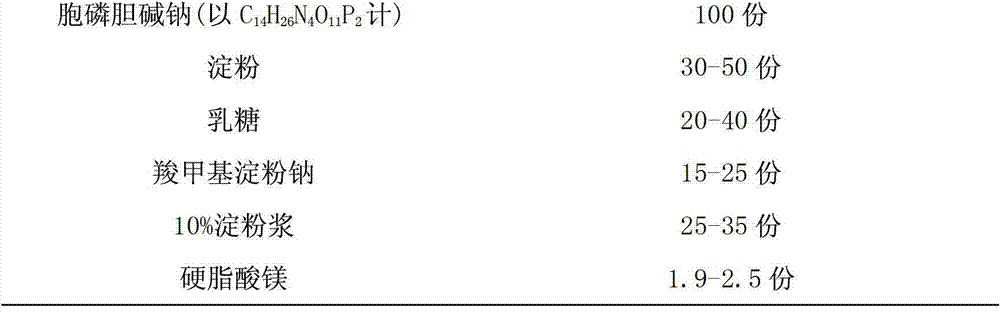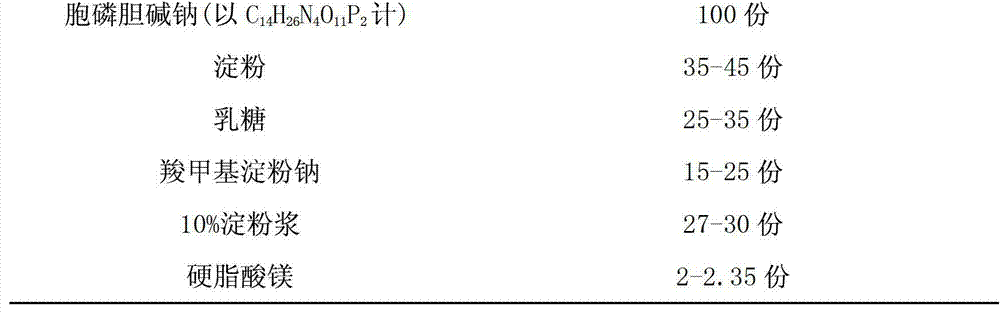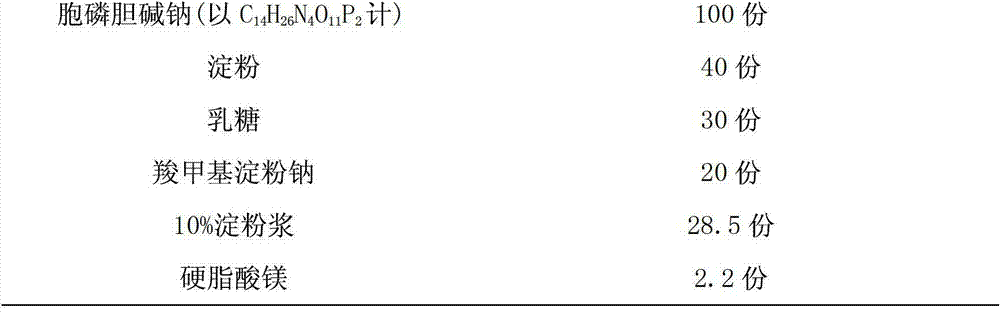Patents
Literature
90results about How to "Meet medication needs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
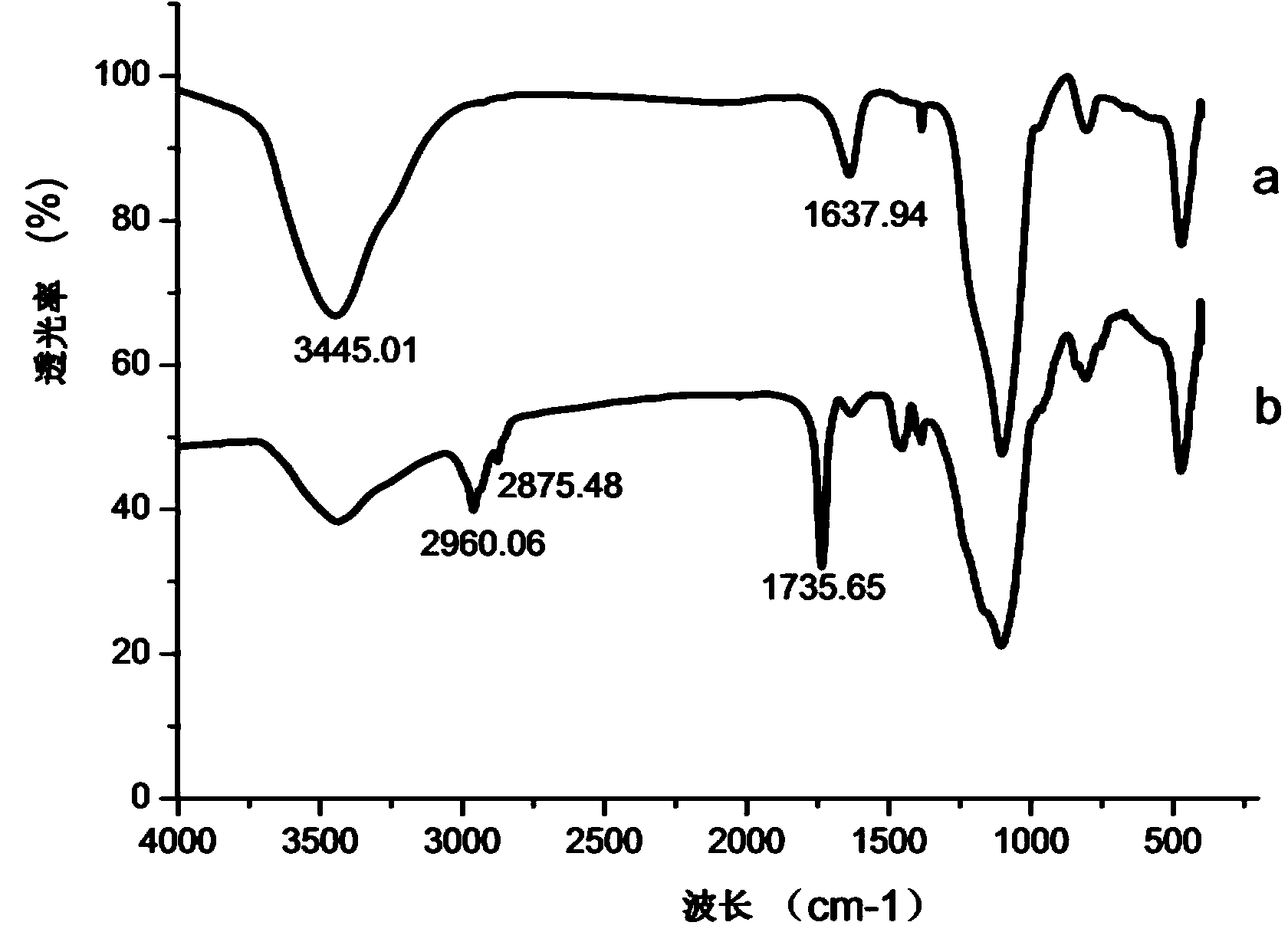
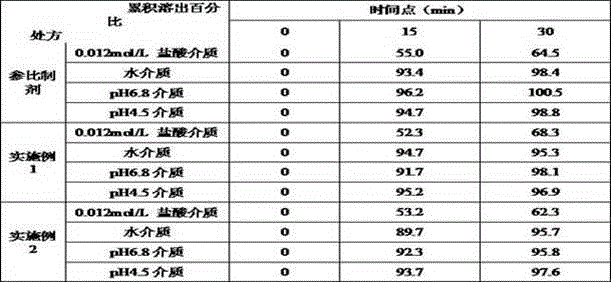
Mussel mucoprotein gel for repairing and reliving itching and preparation method of mussel mucoprotein gel
InactiveCN104645313ANo stimulationGood bioadhesionPeptide/protein ingredientsAerosol deliveryBiocompatibility TestingWound surface
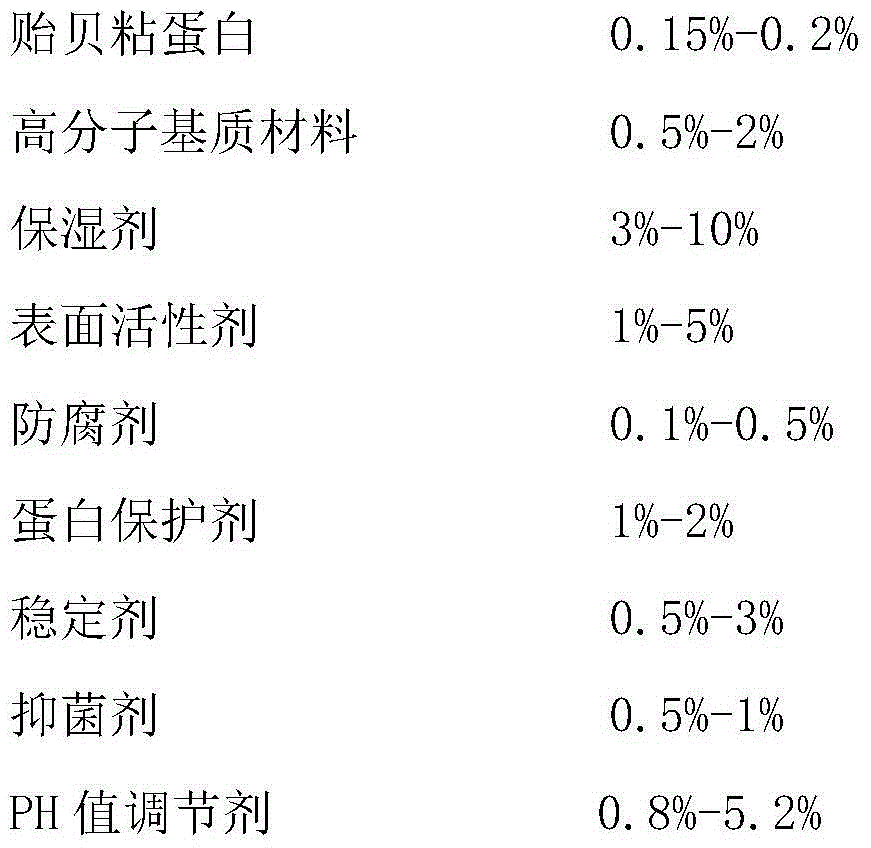
The invention discloses a mussel mucoprotein gel for repairing and reliving itching. The mussel mucoprotein gel is prepared from the following components in percentage by weight: 0.15%-0.2% of mussel mucoprotein, 0.5%-2% of a macromolecule host material, 3%-10% of a humectant, 1%-5% of a surfactant, 0.1%-0.5% of a preservative, 1%-2% of a protein protectant, 0.5%-3% of a stabilizer, 0.5%-1% of a bacteriostatic agent, 0.8%-5.2% of a pH modifier and the balance of purified water. The mussel mucoprotein gel has good biological adhesion, is rapidly and effectively adhered to a wound surface, is capable of rapidly reliving itching and easing pain, and has the advantages of being low in immunogenicity, good in biocompatibility with a human body, convenient to use, free of thrill to skin, safe, nontoxic and free of bad reaction.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Effervescence dispersible tablet
InactiveCN101138554AEvenly dispersedUniform disintegrationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsEffervescent tabletOral medication
The present invention relates to a new preparation of a Chinese medicine. The present invention particularly relates to a novel preparation of a drug with the effervescent tablet property and the dispersing agent property. In the effervescent dispersing tablet of the present invention, the weight ratio of each components are as following, which comprises 5 percentage to 60 percentage of effervescent agent, 3 percentage to 30 percentage of effervescing agent, 3 percentage to 30 percentage of disintegrant, 3 percentage to 30 percentage of excipient of the hydrophilic medicine, 1 percentage to 5 percentage of correctant. The effervescent dispersing tablet of the present invention is a novel preparation of the Chinese medicine, which has the effervescent tablet property and the dispersing agent property. The present medicine is a tablet, which can produce the gas in the water, which can disaggregate quickly and disperse evenly. The present invention is a novel preparation of the medicine, which can generate the gas; as a result the medicine can disaggregate more quickly. The drug loading dosage of the agent is large, which is beneficial for improving the dispersion uniformity, the dissolution and the bioavailability of the medicine. The present invention has the characteristics of convenience for oral administration and small dose. The present invention can sufficiently display the drug efficacy in order to meet the drug requirement of the patients.
Owner:YUNNAN BAIYAO GROUP
Tenofovir disoproxil fumarate dispersible tablets and preparation method thereof
ActiveCN102198110AModerate hardnessSmall weight differenceOrganic active ingredientsAntiviralsDissolutionLubricant
The invention discloses tenofovir disoproxil fumarate dispersible tablets and a preparation method thereof. The tenofovir disoproxil fumarate dispersible tablets are prepared from tenofovir disoproxil fumarate as an effective pharmaceutical ingredient and pharmaceutically acceptable auxiliary ingredients, wherein the pharmaceutically acceptable auxiliary ingredients comprise a filler, a disintegrant, a lubricant, a surfactant and a flavoring agent. The tenofovir disoproxil fumarate dispersible tablets prepared by the invention have appropriate hardness, small weight difference, bright and clean tablet surfaces and good taste, fully meet the requirements on the disintegration time and dispersion uniformity of dispersible tablets, and notably improve the pharmaceutical bioavailability, and in addition, the tenofovir disoproxil fumarate dispersible tablets have quick dissolution rate, the dissolution percentage of the product is about 80-90% in 2 minutes, and the product is almost completely dissolved in 5 minutes.
Owner:杭州康本医药科技有限公司 +2
Citicoline sodium tablet and preparation method thereof
ActiveCN103191079AImprove securityEnsure safetyOrganic active ingredientsNervous disorderCiticoline sodiumSlurry
The invention provides a citicoline sodium tablet and a preparation method thereof. The citicoline sodium tablet provided by the invention contains citicoline sodium, starch, lactose, sodium carboxymethyl starch, 10% starch slurry and magnesium stearate. The prescription of a coating solution includes stomach-soluble coating powder and ethanol. The preparation method comprises the steps of: (1) preparation of an uncoated tablet: uniformly mixing main drugs with the lactose, grinding the mixture to pass through a 80-mesh sieve, respectively adding the starch and the sodium carboxymethyl starch and carrying out uniform mixing, passing through the 80-mesh sieve twice, adding the 10% starch slurry, adequately and uniformly mixing to prepare soft materials, carrying out pelletizing by utilizing a 20-mesh sieve, drying at 60 DEG C, adding the magnesium stearate, and carrying out uniform mixing, tabletting and testing; and (2) coating. The citicoline sodium tablet has the advantages of good stability, low cost, high active ingredient content and the like.
Owner:JINAN LIMIN PHARMA
Culture method for ramulus uncariae cum uncis
PendingCN107873516ATechnical stabilityImprove induction efficiencyPlant cultivationCultivating equipmentsHypocotylBud
The invention discloses a culture method for ramulus uncariae cum uncis. The culture method comprises the following steps: firstly, culturing a ramulus uncariae cum uncis seed in a seed culture mediumfor 20-25d to obtain a sterile seedling hypocotyl; secondly, cutting a hypocotyl explant having the length of 0.4-0.8cm and containing no cotyledonary nodes from the sterile seedling hypocotyl, horizontally placing the hypocotyl explant in a first callus culture medium for dark culture for 20-25d, and then, transferring the hypocotyl explant into a second callus culture medium for dark culture toobtain a fresh callus; thirdly, transferring the fresh callus into a differential culture medium for differential culture to obtain a cluster bud; and fourthly, transplanting the cluster bud into a strong seedling culture medium for strong seedling culture, cutting off a basal part of a stem when the length of a single seedling is 3-4cm, transferring the seedling into a rooting culture medium forrooting culture, and growing a root with the length of 1.5-2.0cm to obtain a ramulus uncariae cum uncis seedling. The ramulus uncariae cum uncis obtained by using the culture method is purely natural, high in yield and high in medicinal component content.
Owner:GUANGXI BOTANICAL GARDEN OF MEDICINAL PLANTS
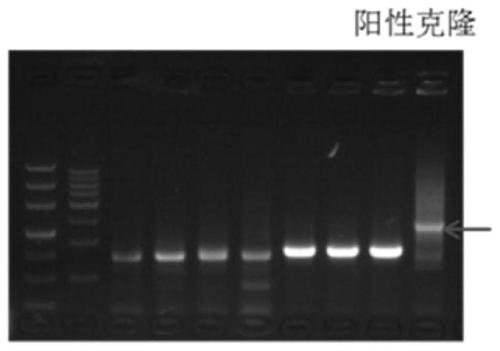
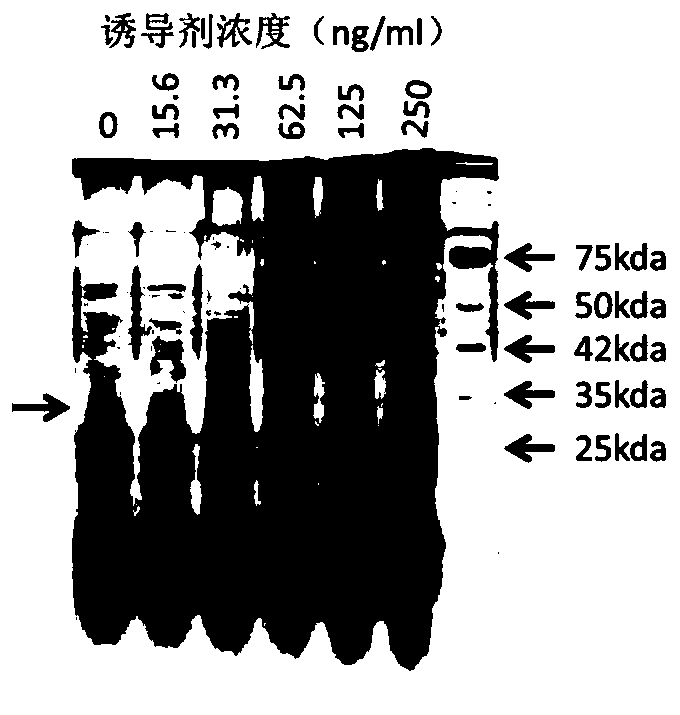
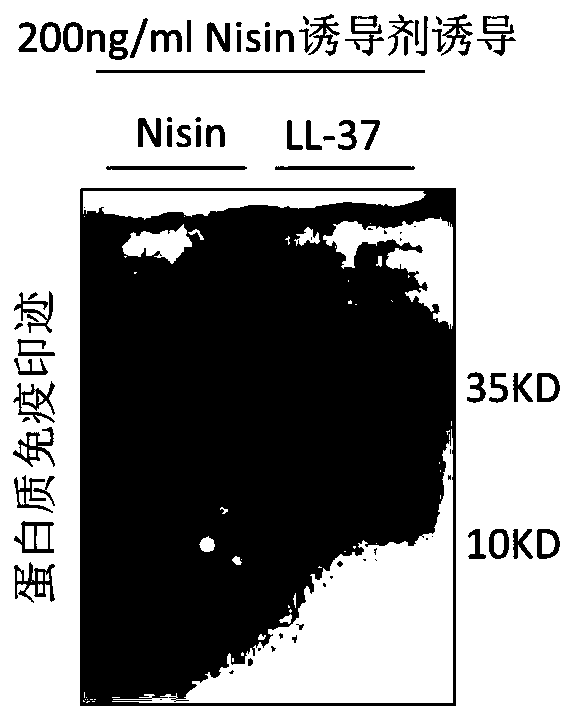
Recombinant expression vector for expressing LL-37 polypeptide, recombinant lactococcus lactis, antiviral drug, construction method and application
ActiveCN111500615AAvoid cumbersomenessAvoid efficiencyAntibacterial agentsPolypeptide with localisation/targeting motifStaphylococcus lactisNucleotide
The invention provides a recombinant expression vector for expressing LL-37 polypeptide, recombinant lactococcus lactis, an antiviral drug, a construction method and application, and belongs to the technical field of gene engineering. A skeleton plasmid for constructing the recombinant expression vector is PNZ8149; a fusion gene is inserted into the recombinant expression vector; the nucleotide sequence of the fusion gene is shown as SEQ ID NO: 1. The recombinant expression vector disclosed by the invention can be efficiently expressed in lactococcus lactis, and the LL-37 obtained by expression can effectively inhibit SARS coronavirus and SARS-CoV2 coronavirus. The invention also provides recombinant lactococcus lactis containing the recombinant expression vector in the scheme. According to the recombinant lactococcus lactis disclosed by the invention, an antiviral polypeptide drug LL-37 can be input into a human body in an oral form, the antiviral effect is good, and the production cost is low.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Eye gellan gum in-situ gel made of bendazac lysine and preparing method of eye gellan gum in-situ gel
ActiveCN105106107AReduce releaseIncrease concentrationOrganic active ingredientsSenses disorderGellan gumBacteriostatic agent
The invention discloses eye gellan gum in-situ gel made of bendazac lysine. The eye gellan gum in-situ gel made of bendazac lysine is prepared from 0.4%-1% of bendazac lysine, 0.2%-0.6% of gellan gum, 2%-8% of osmotic pressure modifier, 0.05%-0.2% of pH modifier, 0.05%-0.4% of bacteriostatic agent, 0%-0.2% of thickening agent and the balance purified water, wherein the pH modifier is tromethamine. The eye gellan gum in-situ gel made of bendazac lysine is good in stasis capability on the cornea, convenient to apply and good in application prospect. The invention further discloses a preparing method of the eye gellan gum in-situ gel made of bendazac lysine. The method is easy and convenient to implement, easy to control and suitable for industrial production.
Owner:BEIJING GUGE MEDICINE DEV CO LTD
Pharmaceutical composition containing interferon and application
InactiveCN103341160AReduce dependenceEliminate side effectsPeptide/protein ingredientsAerosol deliveryPatient complianceInterferon alpha
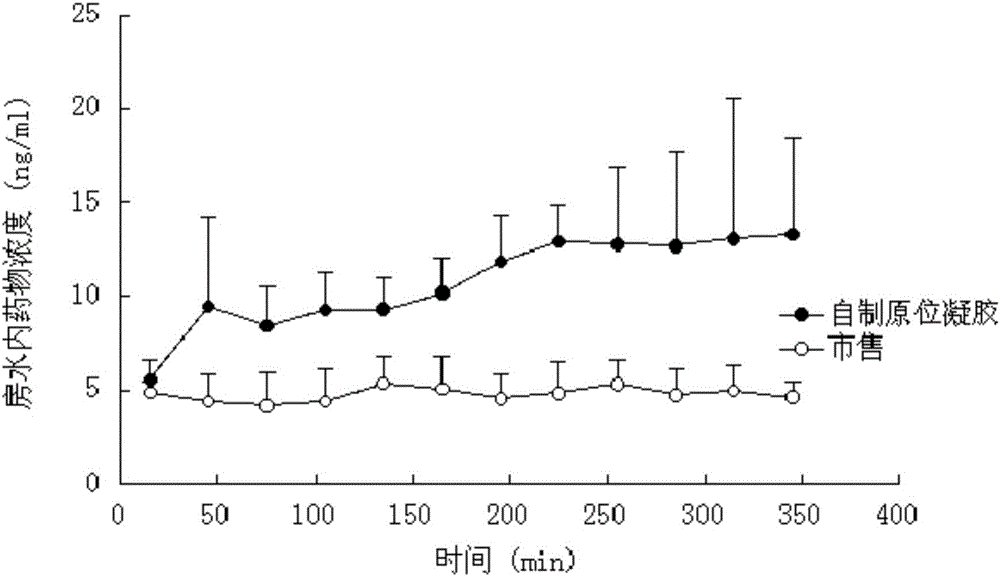
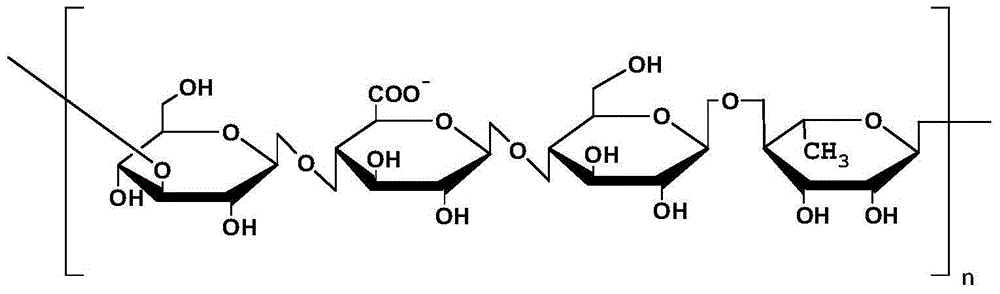
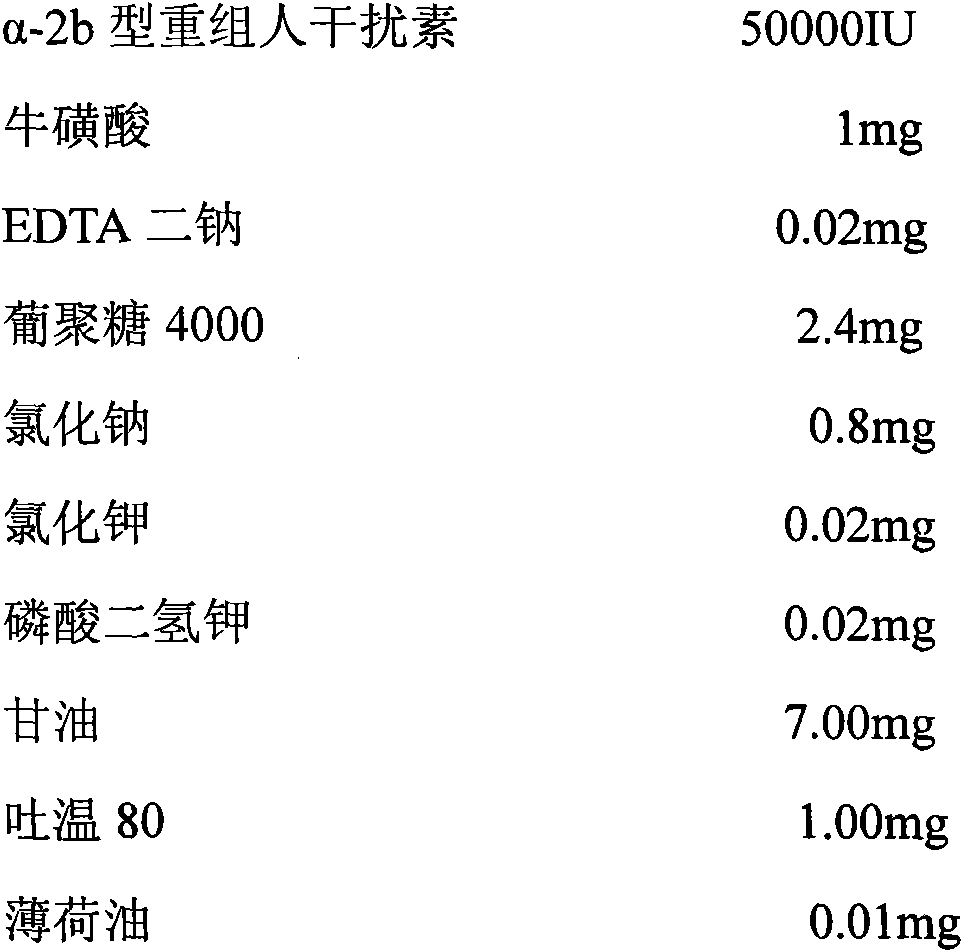
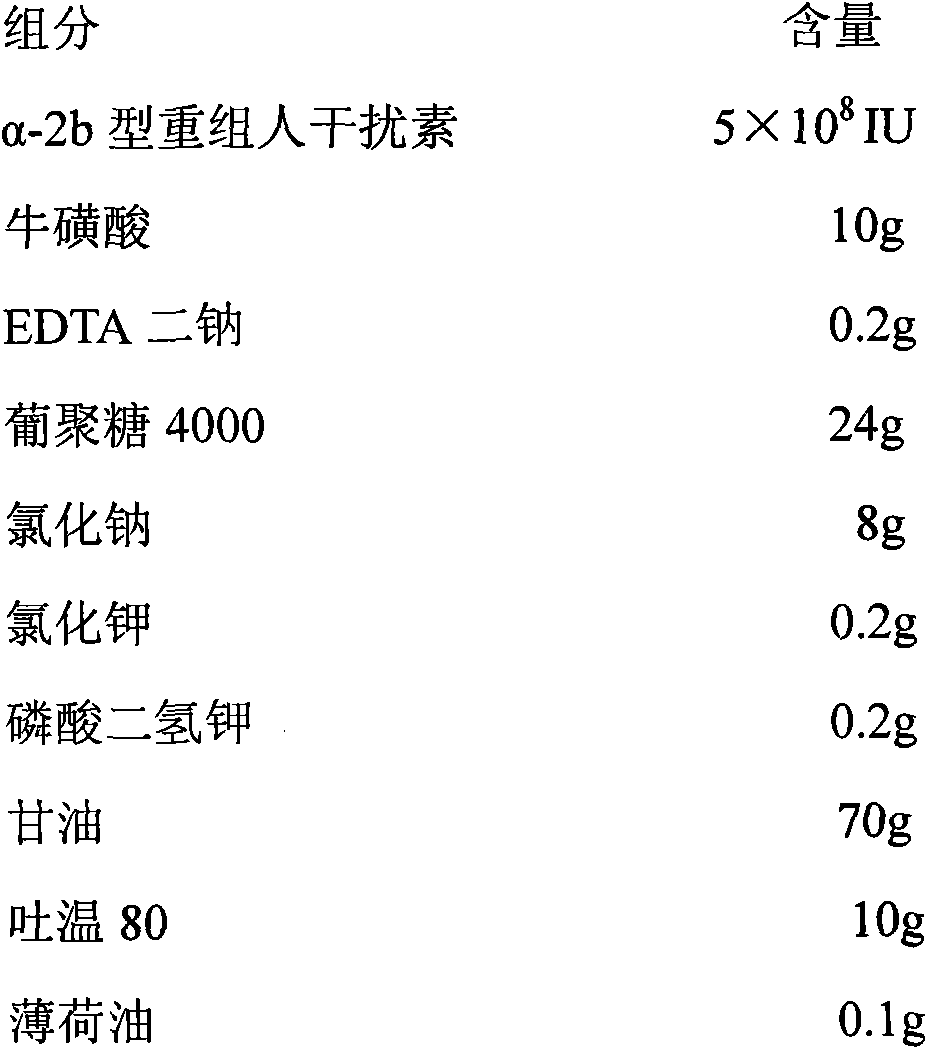
The invention discloses a pharmaceutical composition containing interferon and an application. Pharmacologically active ingredients of the pharmaceutical composition are the interferon and taurine, wherein an activity unit content of the interferon is 10 to 1x10<6> IU / mL, and a content of the taurine is 0.05-0.5 mg / ml. The pharmaceutical composition containing the interferon provided by the invention has effects of resisting activity of vesicular stomatitis virus and murine encephalomyocarditis virus, and protecting cells. Especially, each of nasal spray agent provided by the invention only has 50000 IU of alpha-2b recombinant human interferon and 1 mg of the taurine, but has a specific anti-viral activity of 9.15x10<5> to 2.8x10<6> IU, and shows substantial synergistic effect, thereby being significant for improving patient compliance and reducing medication costs, and meeting clinical needs of interferon medications.
Owner:澳蒲生物科技(上海)有限公司 +1
Nifedipine sustained release tablet and preparation method thereof
ActiveCN103349651AProlong the action timeImprove bioavailabilityAntibacterial agentsOrganic active ingredientsNifedipineProlonged-release tablet
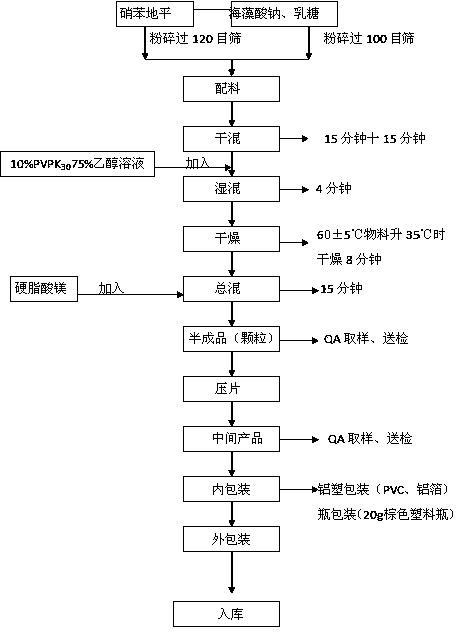
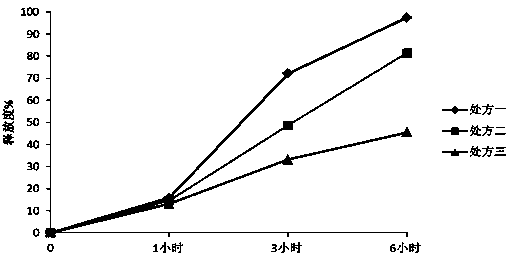
The invention provides a nifedipine sustained release tablet and a preparation method thereof. The nifedipine sustained release tablet is prepared from nifedipine, sodium alginate, lactose, a PVP-K30 ethanol solution, magnesium stearate and calcium gluconate. The preparation method comprises the following steps: preparing raw materials, preparing the PVP-K30 ethanol solution, thoroughly mixing the raw materials according to the prescribed amount, then adding an appropriate amount of PVP-K30 ethanol solution, and performing mixed granulation; after drying, adding a right amount of magnesium stearate, mixing totally, and then performing tabletting and packaging. The nifedipine sustained release tablet provided by the invention has the advantages of good safety, dissolution curve and stability, low cost and the like.
Owner:DEZHOU BOCHENG PHARMA
Notoginseng vulnerary extract and method for making same and application
InactiveCN101081254AFully exert the medicinal effectIncrease useHydroxy compound active ingredientsAntipyreticTopical preparationTraditional medicine
The present invention is Notoginseng Vulnerary extract and its preparation process and application. All the ingredients of Notoginseng Vulnerary except borneol are extracted to obtain the effective components, and the extracted effective components together with borneol are prepared into different dosage forms for both orally taking and external applying. The present invention expands the application range of Notoginseng Vulnerary.
Owner:YUNNAN PHYTOPHARML
Nanoparticle and polymer composite particle and application thereof
ActiveCN103665697AEasy to makeGood dispersionPharmaceutical non-active ingredientsEmulsion deliveryPolymer scienceIn situ polymerization
The invention relates to a nanoparticle and polymer composite particle and an application thereof in the field of biomedicines. The nanoparticle and polymer composite particle is prepared from an organic monomer and inorganic nanoparticles through in-situ polymerization. In addition, the invention relates to an application of the nanoparticle and polymer composite particle as a coating or stable dispersion, and also relates to a composite emulsion system comprising a waterborne continuous phase and an oil phase, and the nanoparticle and polymer composite particle is obtained through mixing an aqueous dispersion of the composite particle with the oil phase and dispersing, wherein the volume of the oil phase is 1 / 100-100 times that of the aqueous dispersion. The invention also relates to a medicine-carrying composite emulsion system. The nanoparticle and polymer composite particle provided by the invention is favorable in dispersibility and stability in a water solution and wide in application in the field of biomedicines.
Owner:SHANGHAI JIAO TONG UNIV
Preparation of composite motidine dispersant pills
InactiveCN1456160AMeet medication needsDisintegrates quicklyOrganic active ingredientsDigestive systemCross-linkAdhesive
A dispersing tablet of famotidine features that on the basis of its chewing tablet, the special additives are used for it, such as excellent disintegrant (cross-linked povidone, sodium laurylsulfate, or microcrystal cellulose) and high-performance adhesive (povidone K30) are used in it. Its advantages are high disintegrating speed, high curative effect and high safety.
Owner:金日制药(中国)有限公司
Planting method for tetrastigma hemsleyanum Diels et Gilg
InactiveCN104871806ARealize artificial plantingMeet medication needsPlant cultivationCultivating equipmentsSocial benefitsPest control
The invention discloses a planting method for tetrastigma hemsleyanum Diels et Gilg. The planting method mainly comprises the following steps of seedling selection, seedling culture, field management, pest and disease control, timely harvesting and the like. Rattan seedlings need to be treated through rooting hormone before seedling culture and insertion implantation, so the survival rate of the rattan seedlings can reach 90% or more. By the adoption of the method, the tetrastigma hemsleyanum Diels et Gilg can be produced on a large scale, so rich tetrastigma hemsleyanum Diels et Gilg herbs can be provided for people, and the medicine consumption requirements of people are satisfied. Besides, the planting method has good economic and social benefits.
Owner:GUIZHOU PROVINCE RENHUAI CITY QINGSHANXIUSHUI CHINESE HERBAL MEDICINES PLANTING PROFESSIONAL COOP
High-efficacy medicine supply method and device, medium, and electronic equipment
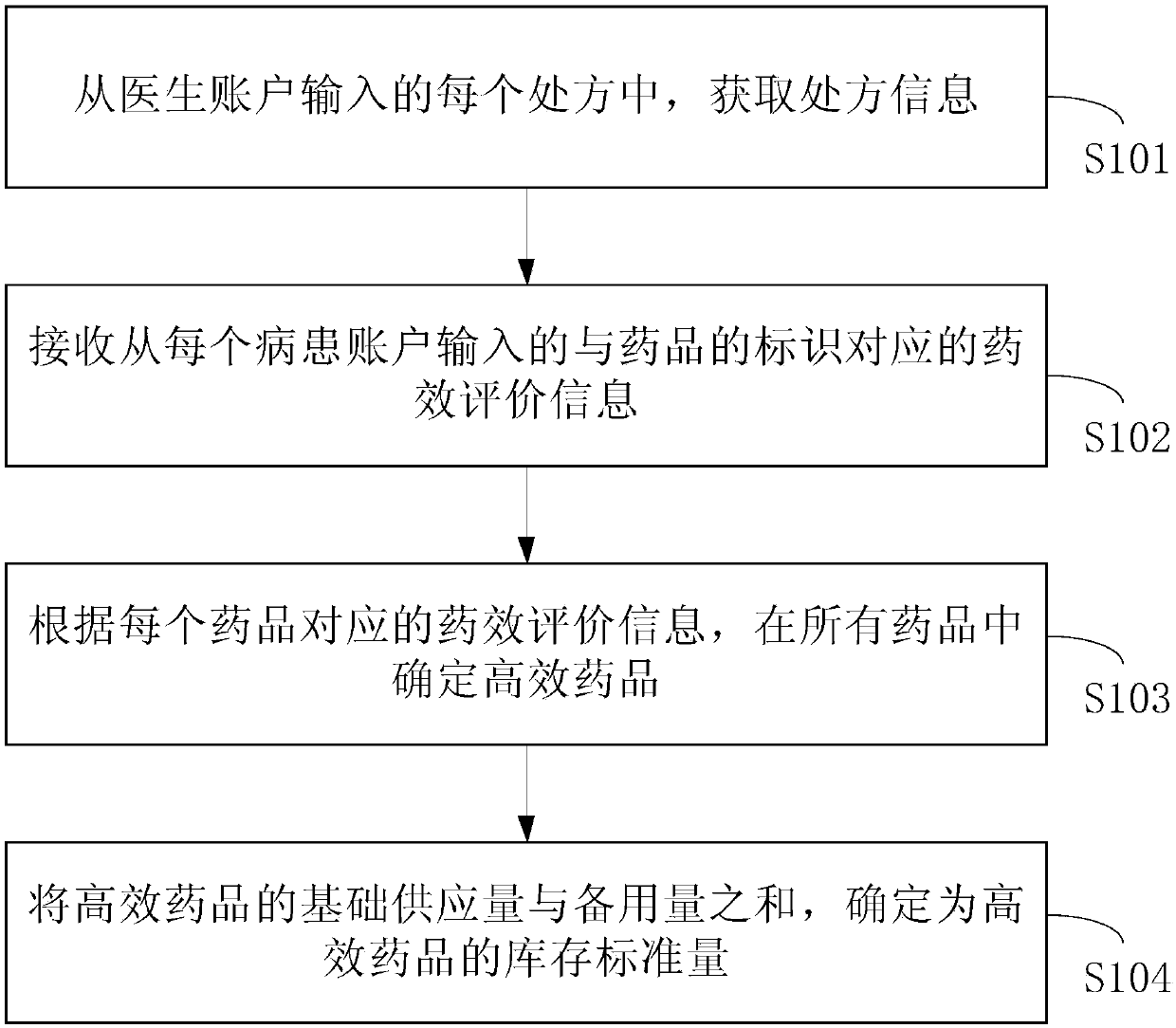
InactiveCN108022160AAlleviate shortagesMeet medication needsHealthcare managementBuying/selling/leasing transactionsDiseaseMedicine
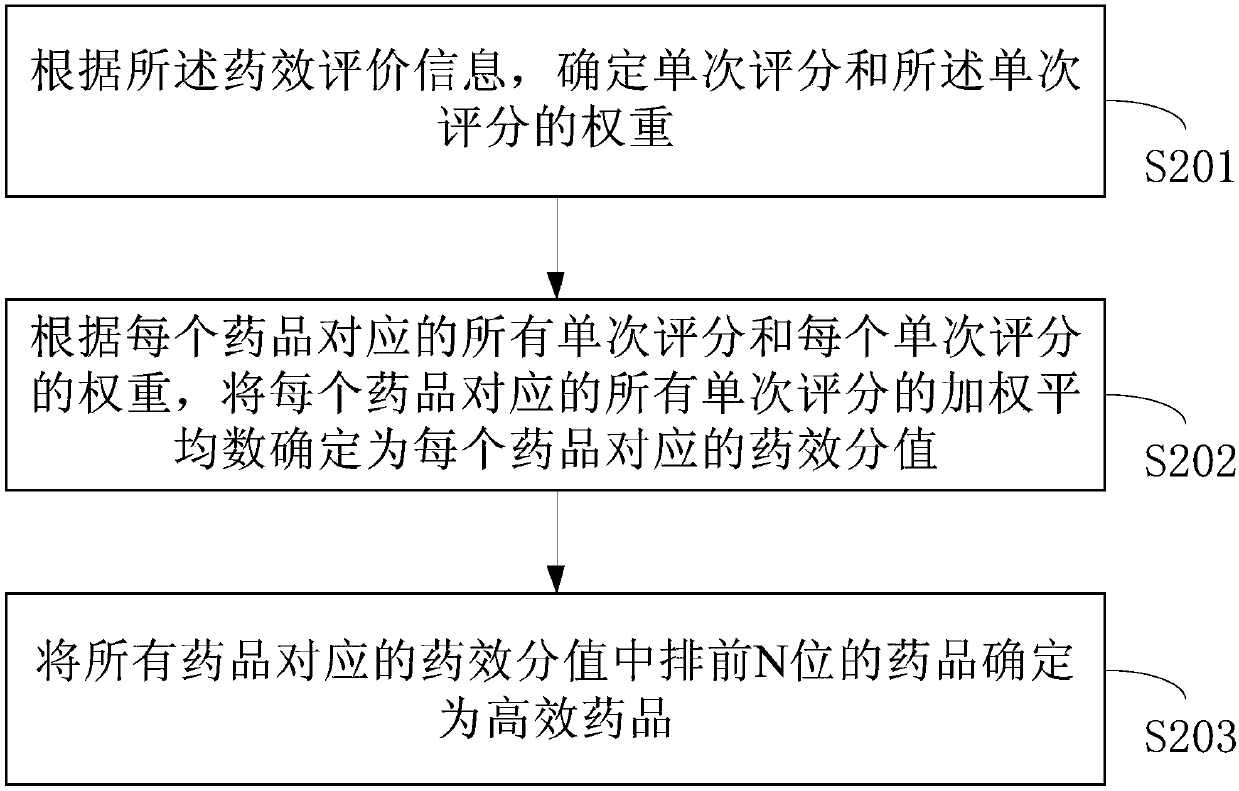
The invention provides a high-efficacy medicine supply method and device, a medium, and electronic equipment. The method comprises the steps: obtaining prescription information from each prescriptioninputted from a doctor's account, wherein the prescription information comprises the labels of medicines in the prescriptions and the account information of patients, so as to build the correspondingrelation between the medicines and the patients; receiving efficacy evaluation information which is inputted from the account of each patient and is corresponding to the labels of the medicines, so asto determine the efficacy of the medicines according to the evaluation of the patients; determining the high-efficacy medicine in all medicines according to the efficacy evaluation information corresponding to each medicine; determining the sum of the basic supply quantity and reserve quantity of the high-efficacy medicine as the inventory standard quantity of the high-efficacy medicine, determining the high-efficacy medicine according to the feedback of the patients, and carrying out the adjustment of the inventory standard quantity of the high-efficacy medicine, so as to enable the inventory of the high-efficacy medicine to meet the demands during the disease outbreak, and reduce the possibility of shortage of the high-efficacy medicine.
Owner:TAIKANG LIFE INSURANCE CO LTD
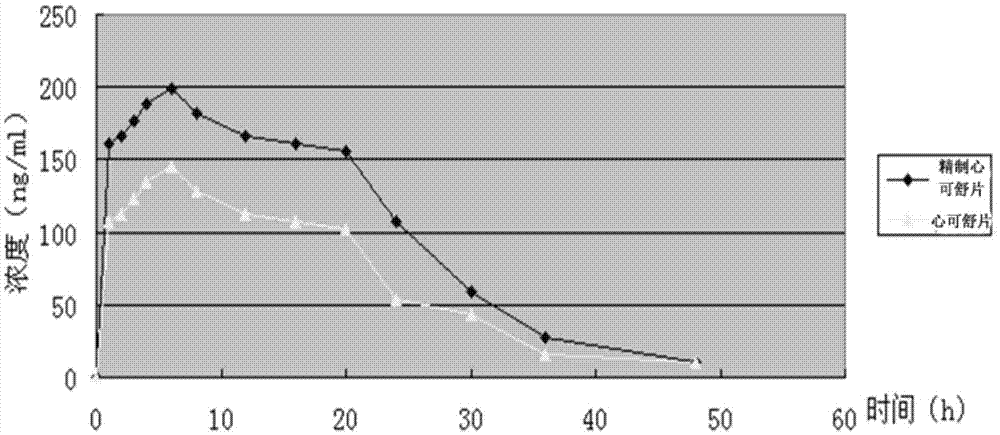
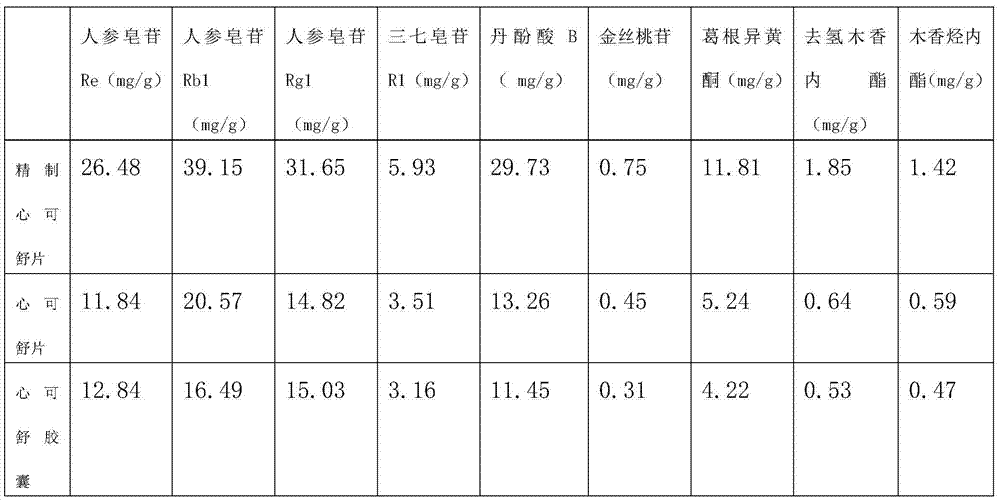
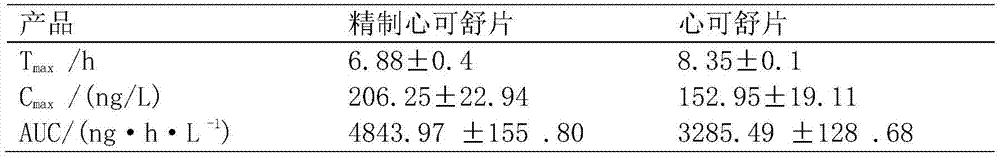
Semi-bionic preparation method for Xinkeshu preparations
InactiveCN105434583ASuitable for absorptionSmall dosePill deliveryCardiovascular disorderHydrogenDistillation
The invention discloses a semi-bionic preparation method for Xinkeshu preparations. The preparation method comprises steps as follows: (1), costus roots are taken, ground, mixed with water, heated and subjected to reflux distillation, preliminary distillate is collected, redistillation is performed, volatile oil is extracted and the distillate is filtered; (2), other raw materials are taken and combined with medicine residues in the step (1), water with the pH (potential of hydrogen) value being 2.0-2.5 is added, and the mixture is heated, extracted and filtered; (3), water with the pH being 6.5-7.0 is added to medicine residues obtained in the step (2), and the mixture is heated, extracted and filtered; (4), water with the pH value being 7.8-8.5 is added to medicine dregs obtained in the step (3), and the mixture is heated, extracted and filtered; (5), filtrate obtained in the steps (2), (3) and (4) is combined and concentrated until an ointment is obtained, the ointment is granulated, dried and mixed with the volatile oil obtained in the step (1), a carrier or an auxiliary material acceptable in pharmaceutics is added, and medicine preparations are prepared. The content of effective ingredients of the Xinkeshu preparations prepared with the method is substantially increased, and the Xinkeshu preparations have higher bioavailability and are better absorbed by human bodies.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Tibetan-medicine three-component Tibet inula soup-granula composition and preparation process thereof
The invention relates to a Tibetan medicine, and particularly relates to a Tibetan-medicine three-component Tibet inula soup-granula composition and a preparation process thereof. The composition disclosed by the invention is prepared from the following raw materials in parts by weight: Ramulus Rubi, Tinospora sinensis and Tibet inula in a proportion of 2:1:1. The preparation process comprises the following steps: 1) carrying out superfine grinding on the Tibet inula; 2) placing the Tinospora sinensis and the Ramulus Rubi in a multifunctional extraction pot to decoct, filtering and concentrating to obtain an aqueous extract, and drying the aqueous extract by using a centrifugal spray drier; 3) combining dry cream powder with micro-powder; and 4) adding ethanol into the obtained powder, preparing into a soft material, granulating the soft material by using a granulator, drying, and carrying out straightening granulation to obtain the composition. According to the composition disclosed by the invention, when the quality of Tibetan medicines is improved, due to the reference and introduction of some new processes, new techniques and new devices, the stability and bioavailability of preparations can be improved, and traditional Tibetan medicine and modern preparation methods are combined, thus a way for the rational development of Tibetan medicines is explored.
Owner:多杰 +3
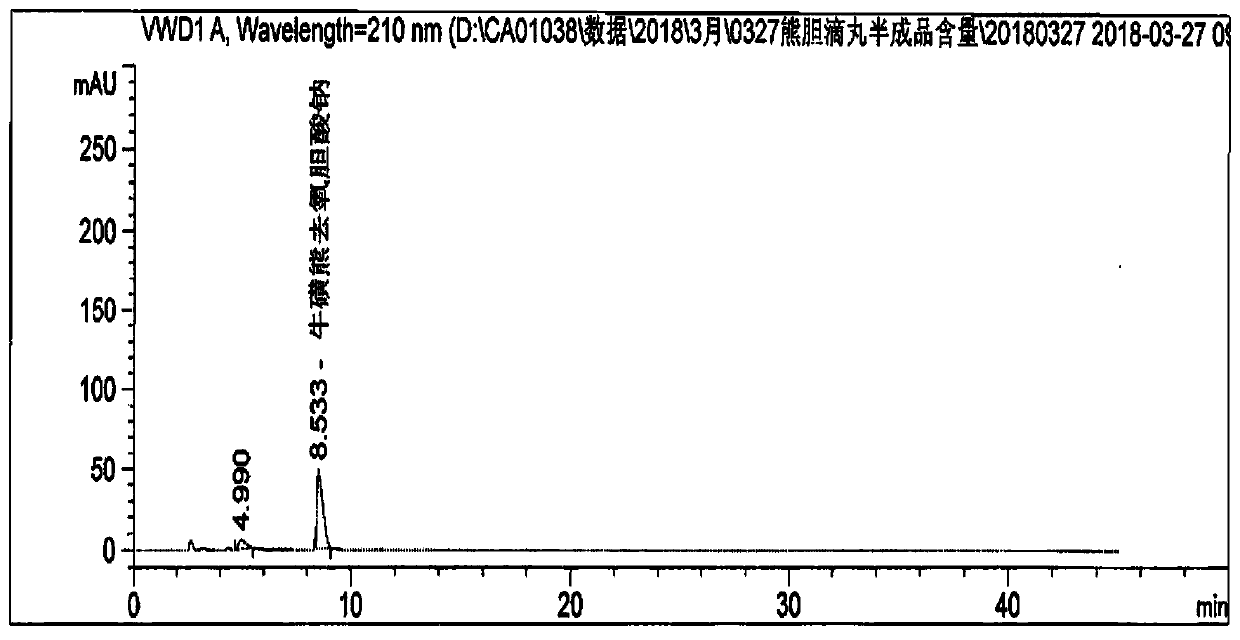
Preparation method of artificial bear gall powder preparation
InactiveCN111789869AMeet needsSolve capacity bottlenecksSenses disorderAntipyreticBiotechnologySucrose
The invention relates to the technical field of medicine production, in particular to a preparation method of an artificial bear gall powder preparation. The preparation method comprises the followingsteps of S1, preparing raw materials: poultry and livestock gall powder, beta cyclodextrin, cane sugar, dextrin, steviosin, menthol, peppermint oil, magnesium stearate, talcum powder, ethanol, starch, licorice root extractum powder, stevioside, PVA-4000, PVA-6000, glycerine, polyvinylpyrrolidone, hydroxypropyl methyl cellulose, hydroxyethyl cellulose, polyethylene glycol, dibutyl phthalate, a coloring agent and silicon dioxide; S2, dissolving the poultry and livestock gall powder with a buffered solution, performing centrifugation under the condition of 11000g, performing repeated filtrationto obtain filter residues and filtrate, and performing freeze drying and preservation on the filter residues; and S3, loading the filtrate in a reactor loaded with 7[alpha]-hydroxysteroid dehydrogenase. According to the preparation method disclosed by the invention, the artificial bear gall powder is designed to be a raw material medicine, and the artificial bear gall powder preparation is developed, so that the problem that natural bear gall raw materials are valuable and scarce is solved, and besides, a new medicine preparation is provided for human.
Owner:SHANGHAI KAIBAO PHARMA
Preparation method of artificial bear bile dripping pills
InactiveCN109864976AMeet production needsHigh content of active ingredientsNervous disorderAntipyreticHeating oilParaffin wax
The invention relates to the technical field of medicines, in particular to a preparation method of artificial bear bile dripping pills. The preparation method comprises the following steps of S1, performing crushing, weighing and blending on raw materials: crushing artificial bear bile powder with a universal crushing machine, performing screening, and performing sealing for standby application;S2, performing compounding and heat preservation: heating oil bath to the temperature of 60-95 DEG C, adding polyethylene glycol 6000 to a blending jar in several times, wherein the addition quantityeach time is 2-20kg, performing heating and stirring for melting, after the polyethylene glycol completely melts, performing stirring, adding the crushed artificial bear bile powder, performing mixingto obtain cream which is dark pale brown to dark brown, and putting the compounded materials to be dripped in the blending jar for heat preservation; and S3, preparing dripping pills: adding 50-300kgof light weight liquid paraffin to a pill dripping machine as a condensing agent, wherein the temperature of oil bath in the pill dripping machine rises to 60-95 DEG C, adding materials to be drippedinto a seasoning jar, and performing dripping with 2.5# dripping heads of 1 row of dripping trays. According to the preparation method disclosed by the invention, the artificial bear bile powder is used as a raw material medicine, an artificial bear bile dripping pill preparation is developed, and the problem that natural bear bile raw materials are valuable and scarce is solved.
Owner:SHANGHAI KAIBAO PHARMA
Liquid composition comprising antibody of human interleukin-4 receptor alpha
ActiveCN111686247AHigh viscosityLow viscosityAntipyreticAnalgesicsHypodermoclysisSubcutaneous injection
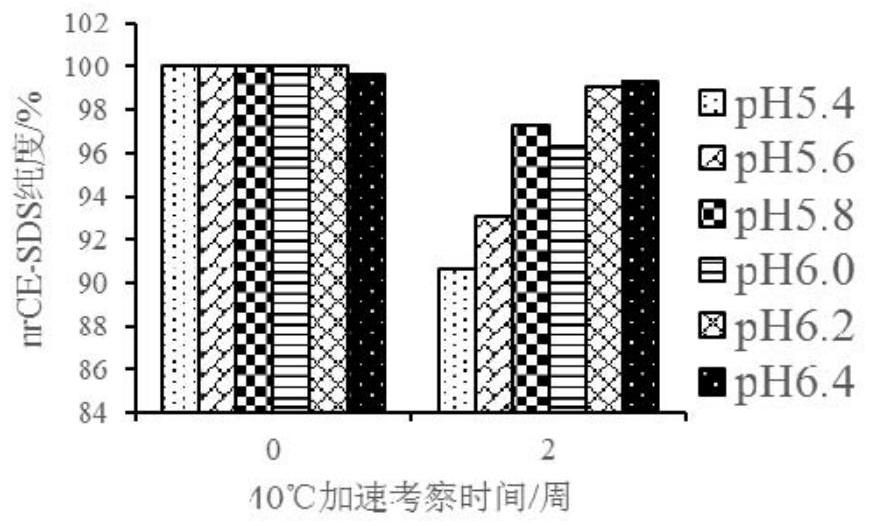
The invention discloses a liquid composition comprising an antibody of human interleukin-4 receptor alpha. The liquid composition comprises the antibody at a concentration of 50-200 mg / ml, a buffer agent, a protective agent and a surfactant, wherein the buffer agent, the protective agent and the surfactant serve as auxiliary materials, and the liquid composition has pH of 5.4 to 6.4. When the pharmaceutical composition is used for subcutaneous injection or intravenous injection, the high-dose antibody can be provided, the administration requirement is met, and the curative effect of a medicineis improved.
Owner:SUZHOU CONNECT BIOPHARMACEUTICALS LTD
Method for preparing FEIBA (factor eight inhibitor bypassing activity) from human plasma Cohn component III serving as raw material
ActiveCN105440127AYield will not changeEfficient use ofThrombomodulinPeptide preparation methodsWhole blood productElution
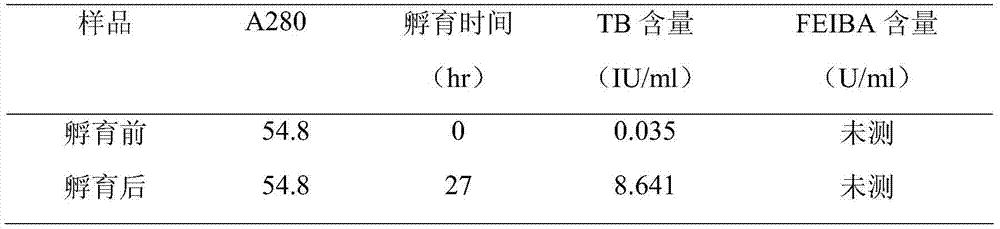
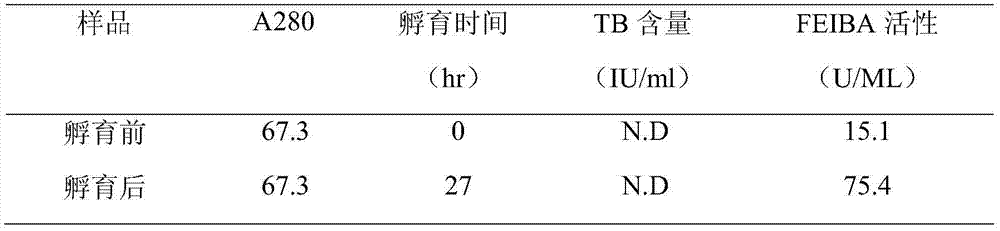
The invention relates to the pharmacy field of blood products, in particular to a method for preparing FEIBA (factor eight inhibitor bypassing activity) from a human plasma Cohn component III serving as a raw material. The method for preparing FEIBA from the human plasma Cohn component III serving as the raw material comprises steps as follows: (1), dissolution of the component III; (2), addition of a precipitator and centrifugalization; (3), inactivation of viruses; (4), gel adsorption; (5), washing and elution of gel; (6), condensation and solution replacement; (7) generation of FEIBA.
Owner:SHANGHAI RAAS BLOOD PRODUCTS CO LTD
Artemether liposome for injection and preparation method and application thereof
ActiveCN107669637ASmall particle sizeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsHigh concentrationEthanol Injection
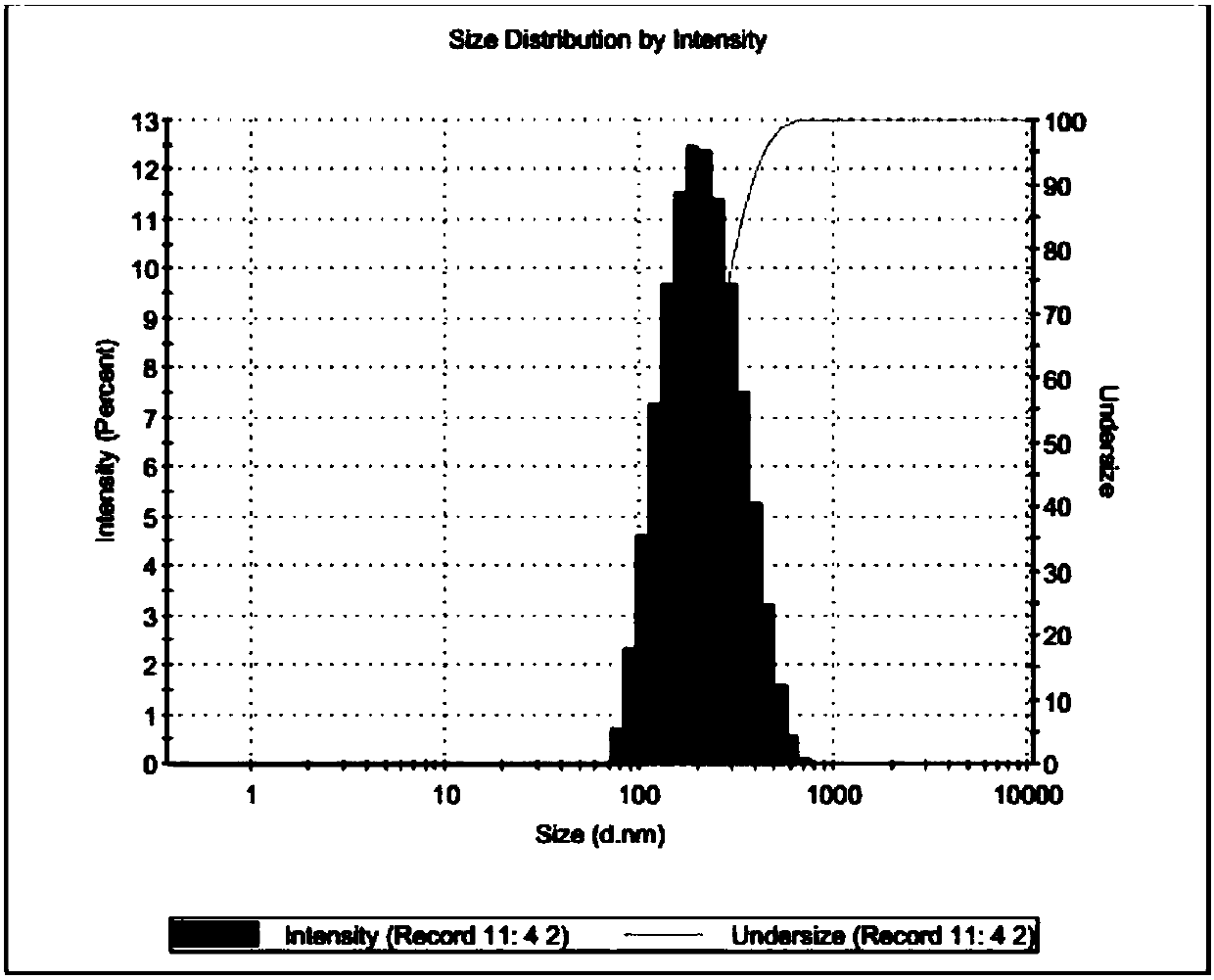
The invention provides an artemether liposome for injection. The artemether liposome is prepared by adopting an ethanol injection method. The method is simple in preparation process and can be appliedto industrial production. According to the invention, by selecting appropriate material components and adopting appropriate preparation processes, the prepared artemether liposome is small in particle size (150-200nm) and uniform in particle size distribution and has the encapsulation efficiency greater than 90%. The artemether liposome is slowly released in vivo to avoid adverse reactions causedby excessively high concentration of drugs at the initial stage of the injection. Furthermore, the inventor finds that the artemether liposome prepared with the method has high stability, high bioavailability, good resolubility and long storage time, and meets needs of drug use.
Owner:SHANDONG UNIV
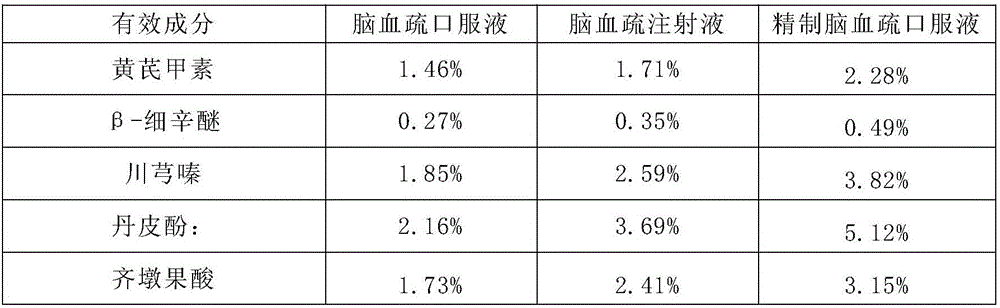
Naoxueshu preparation method
InactiveCN106266343ASuitable for absorptionSmall doseCardiovascular disorderLeech/worm material medical ingredientsSaline waterHas active ingredient
The invention discloses a Naoxueshu preparation method which comprises the following steps: (1) fetching acorus gramineus and cortex moutan, and extracting volatile oil; (2,3,4) fetching radix astragali, achyranthes bidentata, ligusticum wallichii and rheum officinale, grinding and merging with the residue; sequentially adding water with pH value of (1.5-2.5), (6.5-7.0) and (7.8-8.5); and heating and extracting; (5) fetching leech, adding normal saline and soaking; (6,7,8) sequentially adding water with pH value of (1.5-2.5), (6.5-7.0) and (7.8-8.5) into the filter residue, and heating and extracting; (9) merging the filtrate obtained in the steps (5,6,7,8), concentrating and drying to obtain hirudin; and (10) merging the filtrate obtained in the steps (2,3,4), and concentrating to obtain clear paste; adding the volatile oil and hirudin, and mixing uniformly; and adding pharmaceutically acceptable carrier or accessories to obtain a medicinal preparation. The Naoxueshu preparation obtained by the method has the advantages of high content of active ingredients, high bioavailability, better adaptability to human body absorption and the like.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Three-stage pulsed release controlled release tablet and preparation method thereof
ActiveCN101773482AImprove curative effectLittle side effectsOrganic active ingredientsPharmaceutical delivery mechanismControlled Release TabletOsmotic pump
The invention provides a metoprolol pulse osmotic pump controlled release tablet and a preparation method thereof. The controlled release tablet comprises a tablet core, a time lag coating layer and a controlled release coating layer with a release orifice from inside to outside, wherein the tablet core consists of a drug containing layer and a boosting layer, based on the gross weight of the drug containing layer, the drug containing layer comprises 5-65 wt% of metoprolol as active material and 35-95 wt% of pharmaceutically acceptable carrier; based on the gross weight of the boosting layer, the boosting layer comprises 30-85 wt% of swelling agent and 15-70 wt% of osmotic pressure promoting agent; the weight gain of the time lag coating layer is 5-50 wt% of the tablet core; and the weight gain of the controlled release coating layer is 3-30 wt% of the tablet core. The metoprolol pulse osmotic pump controlled release tablet has three-stage release behavior, and is characterized in that the tablet has 2-5 hours time lag at the initial period after being taken orally, increased release is showed at the intermediate period, and release at constant speed is showed at the later period.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Oral tablet of acotiamide hydrochloride trihydrate and preparation method thereof
ActiveCN105769784AReduce volumeEasy to acceptOrganic active ingredientsDigestive systemPatient complianceAcotiamide Hydrochloride
The invention discloses o an oral tablet of acotiamide hydrochloride trihydrate and a preparation method thereof. The acotiamide hydrochloride trihydrate disclosed by the invention adopts a water insoluble filler as the first filler, and aims to still maintain the original particle shape when drug particles contact a dissolution medium and other solutions so as to prevent acotiamide hydrochloride from gathering, thus achieving a good dissolution effect. At the same time, through additional adding of a second filler and a disintegrating agent, the material is ensured with good compressibility and dissolution performance. According to the acotiamide hydrochloride tablet and the preparation method provided by the invention, the operation is simple, the dosage of auxiliary materials is significantly reduced, the tablet weight is small, and while the dissolution rate is guaranteed, the patient compliance is also improved.
Owner:REGENEX PHARMA LTD
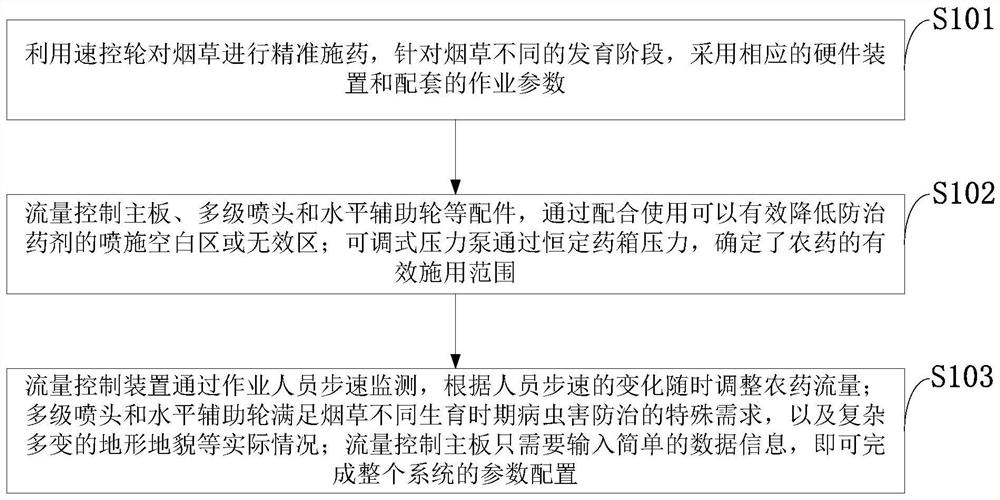
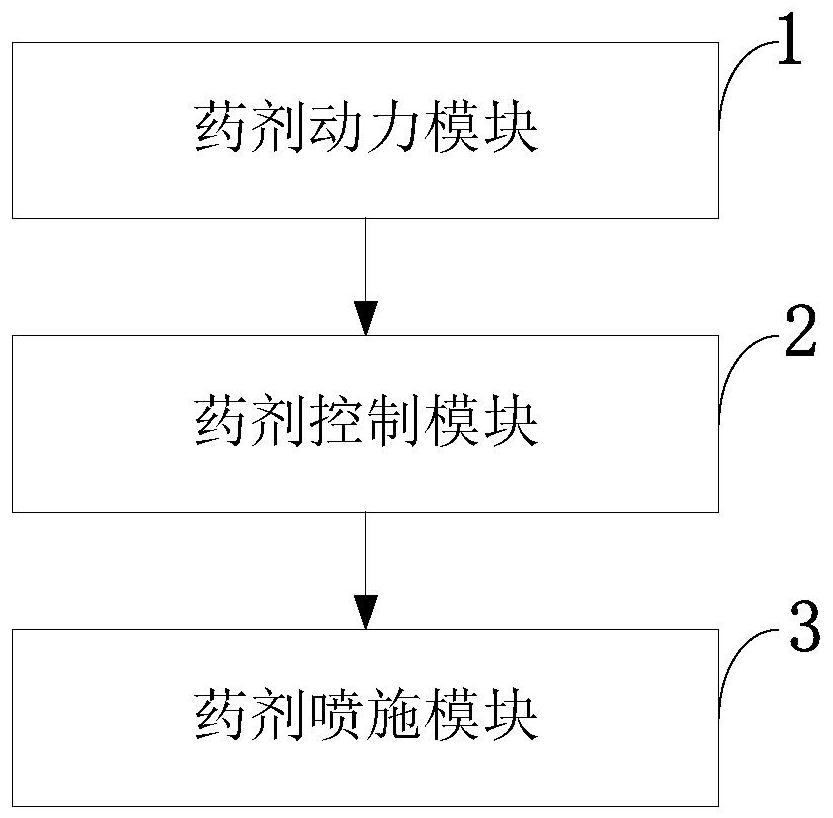
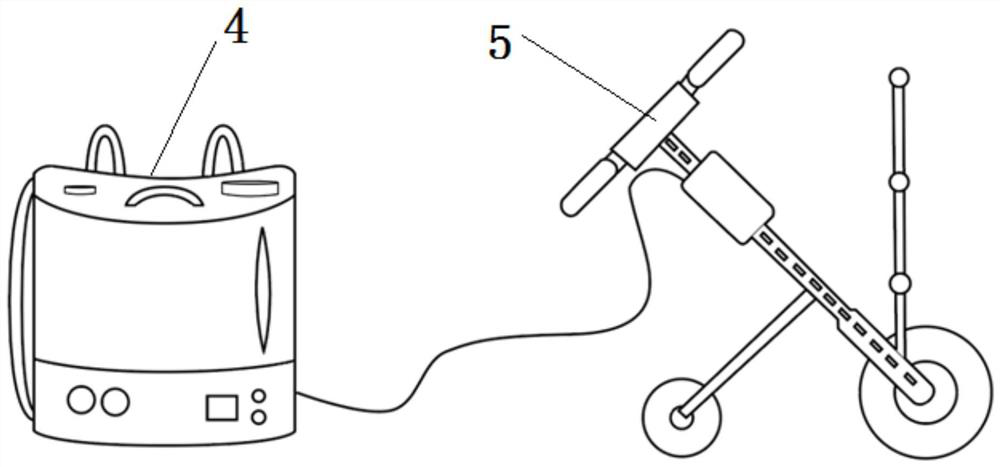
Control method, system and device for accurately applying pesticide to tobaccos and application
PendingCN111758707AImprove automationImprove the level of intelligenceTobacco cultivationPlant protectionAgricultural scienceNicotiana tabacum
The invention belongs to the technical field of tobacco pesticide application control, and discloses a control method, system and device for accurately applying pesticide to tobaccos and application.A speed control wheel is used for accurately applying the pesticide to the tobaccos, and corresponding hardware devices and matched operation parameters are adopted for different development stages ofthe tobaccos; accessories such as a flow control main board, a multi-stage spray head and a horizontal auxiliary wheel are matched for use to reduce a spraying blank area or an invalid area of the prevention and treatment pesticide; an adjustable pressure pump determines the effective application range of the pesticide through the constant pesticide box pressure; the flow control device adjusts the pesticide flow at any time according to the change of the step speed of an operator through the step speed monitoring of the operator; the multi-stage spray head and the horizontal auxiliary wheelmeet the special requirements of pest control in different growth periods of the tobaccos; and the flow control mainboard only needs to input simple data information to complete parameter configuration of the whole system. The requirements of current precise pesticide application technicians, instruments, sites and the like are reduced, and the requirements of different tobacco planting productionareas can be met.
Owner:TOBACCO RES INST CHIN AGRI SCI ACAD
Silky fowl soft capsule and preparation method thereof
InactiveCN104740230ADefinite curative effectAntispasmodic effect is obviousAnthropod material medical ingredientsAntipyreticSalvia miltiorrhizaOyster
The invention relates to a silky fowl soft capsule and a preparation method thereof. The silky fowl soft capsule is prepared by: subjecting, by weight, 50-60 parts of a silky fowl extract, 100-110 parts of deerhorn glue, 50-60 parts of vinegar processed turtle shell, 30-50 parts of calcined oyster, 50-60 parts of Mantis Egg-case, 100-110 parts of ginseng, 20-30 parts of astragalus, 120-130 parts of Chinese angelica, 100-110 parts of Radix Paeoniae Alba, 100-110 parts of vinegar processed cyperus rotundus, 50-60 parts of radix asparagi, 20-30 parts of licorice, 200-220 parts of rehmannia glutinosa, 200-220 parts of Radix Rehmanniae Preparata, 50-60 parts of Ligusticum wallichii, 20-30 parts of Radix Stellariae, 100-110 parts of Salvia Miltiorrhiza, 100-110 parts of Chinese yam, 50-60 parts of fried Euryale ferox, and 30-50 parts of Cornu Cervi Degelatinatum to extraction and adding appropriate auxiliary materials.
Owner:津药达仁堂集团股份有限公司乐仁堂制药厂
Preparation of composite motidine dispersant pills
InactiveCN1188126CMeet medication needsImprove medication flexibilityOrganic active ingredientsDigestive systemCross-linkAdhesive
A dispersing tablet of famotidine features that on the basis of its chewing tablet, the special additives are used for it, such as excellent disintegrant (cross-linked povidone, sodium laurylsulfate, or microcrystal cellulose) and high-performance adhesive (povidone K30) are used in it. Its advantages are high disintegrating speed, high curative effect and high safety.
Owner:金日制药(中国)有限公司
Injection preparation of leaf of nakedflower beautyberry, and preparation method
InactiveCN1872242AAct quicklySignificant effectAntibacterial agentsSenses disorderMedicineCurative effect
Owner:倪友洪
Chinese medicine preparation for treating cardiac and cerebral vascular diseases and its preparing method
InactiveCN1857287AImprove bioavailabilityMeet medication needsOrganic active ingredientsPowder deliveryVascular diseaseFreeze-drying
The present invention discloses a kind of Chinese medicine injection for treating cardiac and cerebral vascular diseases and its preparation process, and the injection and freeze dried powder for injection has dioscin as main component. The present invention also discloses nanometer dioscin preparation prepared through nanometer technology. The present invention has the advantages of fast acting, high bioavailability, fast absorption, etc.
Owner:王衡新
Medicine composition for preventing and treating respiratory system diseases, as well as preparation method and application thereof
InactiveCN105582060ALess irritatingSimple preparation processPill deliveryRespiratory disorderDrugIrritation
The invention provides a medicine composition for preventing and treating respiratory system diseases. The medicine composition is an externally applied preparation prepared from the following raw materials and auxiliaries in parts by weight: 10-50 parts of semen brassicae, 10-50 parts of rhizoma corydalis, 5-40 parts of kansui roots, 5-40 parts of asarum, 50-300 parts of a disintegrating agent, 30-60 parts of adhesive and 2-15 parts of a lubricating agent. The invention further provides a preparation method and application of the medicine composition. The medicine composition is prepared from specific raw materials and auxiliaries in a specific dosage. Compared with a traditional acupoint application preparations, the medicine composition has the advantages of equivalent curative effect and convenience in use, can be used for remarkably reducing the drug irritation for skin and improving the acceptance of drug users, and can meet the medication requirement of patients. The medicine composition is simple in preparation process, low in cost and high in production efficiency, is available for volume production, and can meet the market requirement.
Owner:XIHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com