Nifedipine sustained release tablet and preparation method thereof
A technology of nifedipine and nifedipine is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of poor stability, strong hygroscopicity, and insufficient calcium hydrogen phosphate release. Good and other problems, to achieve the effect of good release, reduce toxic and side effects, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
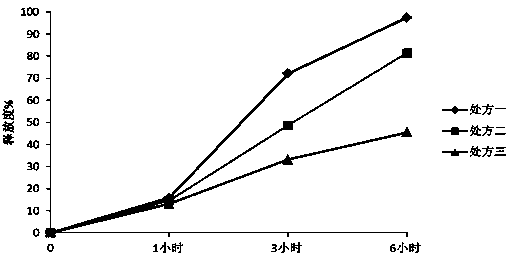
[0071] Example 1 , Nifedipine sustained-release tablets, made of raw and auxiliary materials comprising the following weight ratio:
[0072] Nifedipine 20 parts
[0073] Sodium alginate 30 parts
[0074] Lactose 180 parts
[0075] PVPK30 ethanol solution 36.67 parts
[0076] 1.84 parts of magnesium stearate;
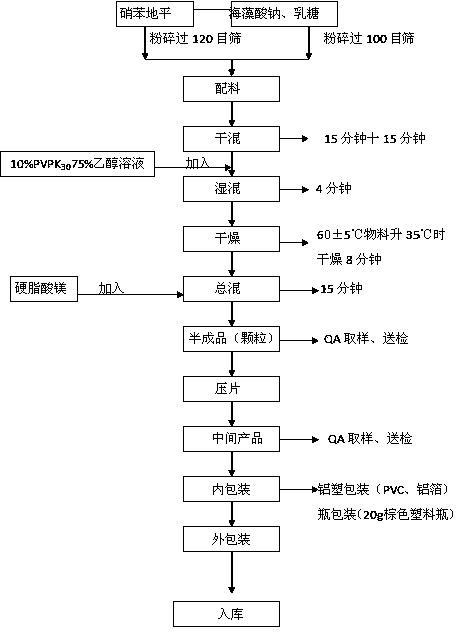
[0077] The preparation process is as follows:
[0078] (1) Ingredients: Take the raw and auxiliary materials of the prescription amount, nifedipine is crushed and passed through a 120-mesh stainless steel sieve; sodium alginate and lactose are passed through a 100-mesh stainless steel sieve;
[0079] (2) Adhesive preparation: put an appropriate amount of 95% ethanol in a clean ingredient barrel, add water to an appropriate amount, then add an appropriate amount of PVPK30 and stir to dissolve it completely, forming a 10% PVP-K30 75% ethanol solution;
[0080] (3) Granulation: Pour part of the raw materials: nifedipine, lactose, sodium alginate into the pot, start t...
Embodiment 2
[0086] Example 2 , Nifedipine sustained-release tablets, made of raw and auxiliary materials comprising the following weight ratio:
[0087] Nifedipine 20 parts
[0088] Sodium alginate 30 parts
[0089] Lactose 180 parts
[0090] Calcium gluconate 5 parts
[0091] PVPK30 ethanol solution 36 parts
[0092] Magnesium stearate 1.84 parts
[0093] The preparation process is the same as in Example 1.
[0094]
Embodiment 3
[0095] Example 3 , Nifedipine sustained-release tablets, made of raw and auxiliary materials comprising the following weight ratio:
[0096] Nifedipine 10 parts
[0097] Sodium alginate 30 parts
[0098] Lactose 150 parts
[0099] PVPK30 ethanol solution 30 parts
[0100] 1 part magnesium stearate
[0101] The preparation process is the same as in Example 1.
[0102]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com