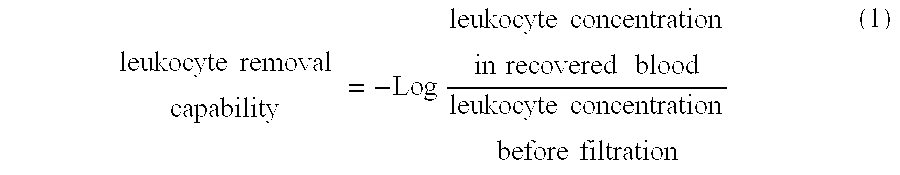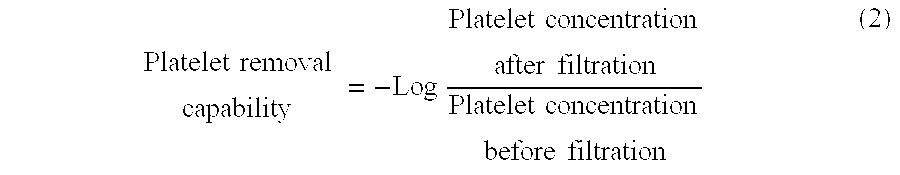Patents
Literature
548 results about "Whole blood product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A blood product is any therapeutic substance prepared from human blood. This includes: whole blood; blood components; and plasma derivatives. Whole blood is not commonly used in transfusion medicine.
Methods for the isolation of nucleic acids and for quantitative DNA extraction and detection for leukocyte evaluation in blood products
InactiveUS6958392B2Sugar derivativesMicrobiological testing/measurementWhole blood productWhite blood cell
A method for isolating nucleic acid which comprises:(a) applying a sample comprising cells containing nucleic acid to a filter, whereby the cells are retained as a retentate and contaminants are removed;(b) lysing the retentate from step (a) while the retentate is retained by the filter to form a cell lysate containing the nucleic acid;(c) filtering the cell lysate with the filter to retain the nucleic acid and remove remaining cell lysate;(d) optionally washing the nucleic acid retained by the filter; and(e) eluting the nucleic acid, wherein the filter composition and dimensions are selected so that the filter is capable of retaining the cells and the nucleic acid.Additionally, there is provided a substrate for lysing cells and purifying nucleic acid having a matrix and a coating and an integrity maintainer for maintaining the purified nucleic acid. Also provided is a method of purifying nucleic acid by applying a nucleic acid sample to a substrate having an anionic detergent affixed to a matrix, the substrate physically capturing the nucleic acid, bonding the nucleic acid to a substrate and generating a signal when the nucleic acid bonds to the substrate indicating the presence of the nucleic acid. A kit for purifying nucleic acid containing a coated matrix and an integrity maintenance provider for preserving the matrix and purifying nucleic acid is also provided. Further, there is provided a method for quantifying DNA, such as double-stranded or genomic DNA, isolated from cells, such as leukocytes to determine the numbers of leukocytes in a sample of leukoreduced blood.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Method and apparatus for blood separations
InactiveUS20060226090A1Reduces direct collectionReduce processing costsWater/sewage treatment by centrifugal separationCentrifugal force sediment separationWhole blood productMedicine
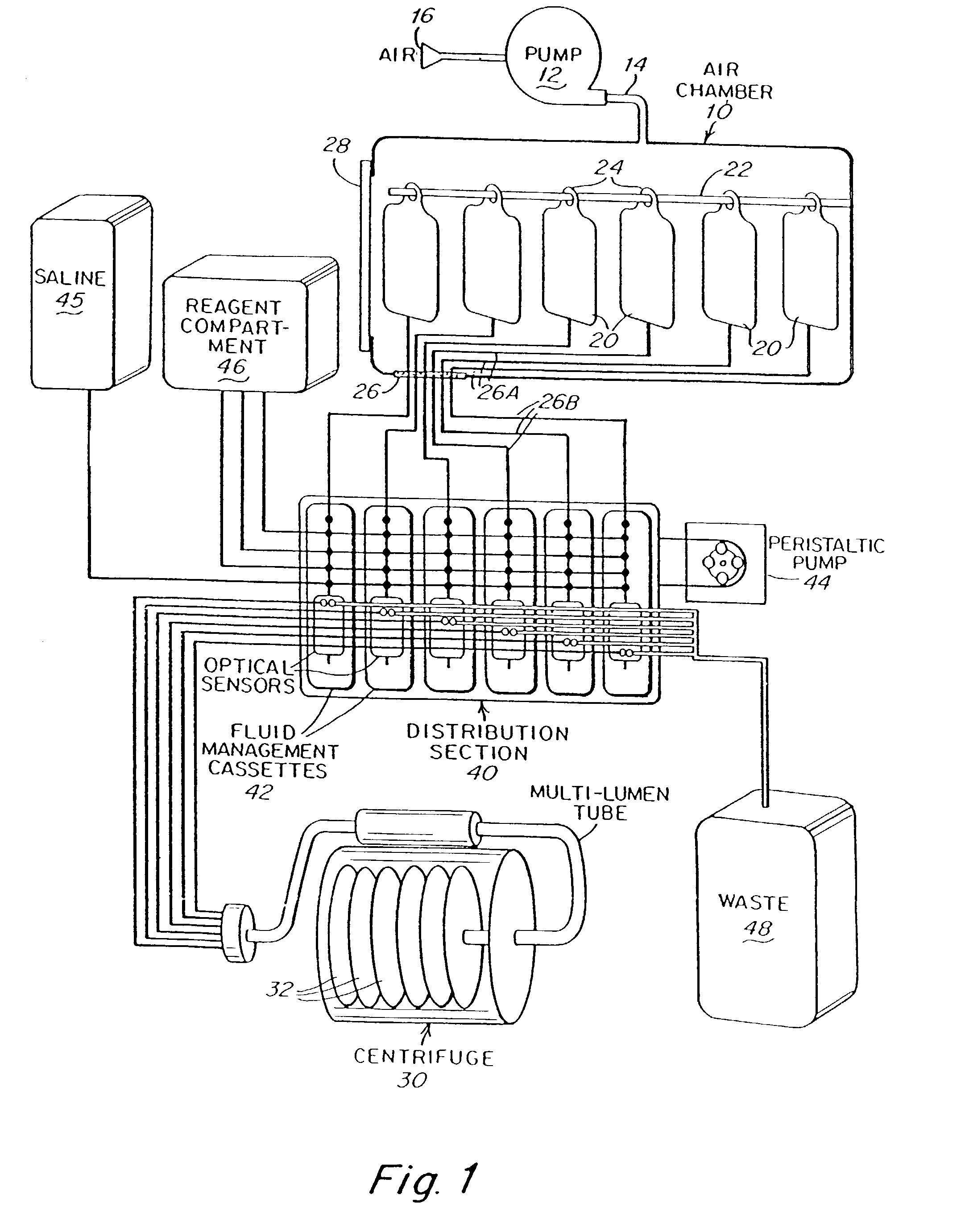
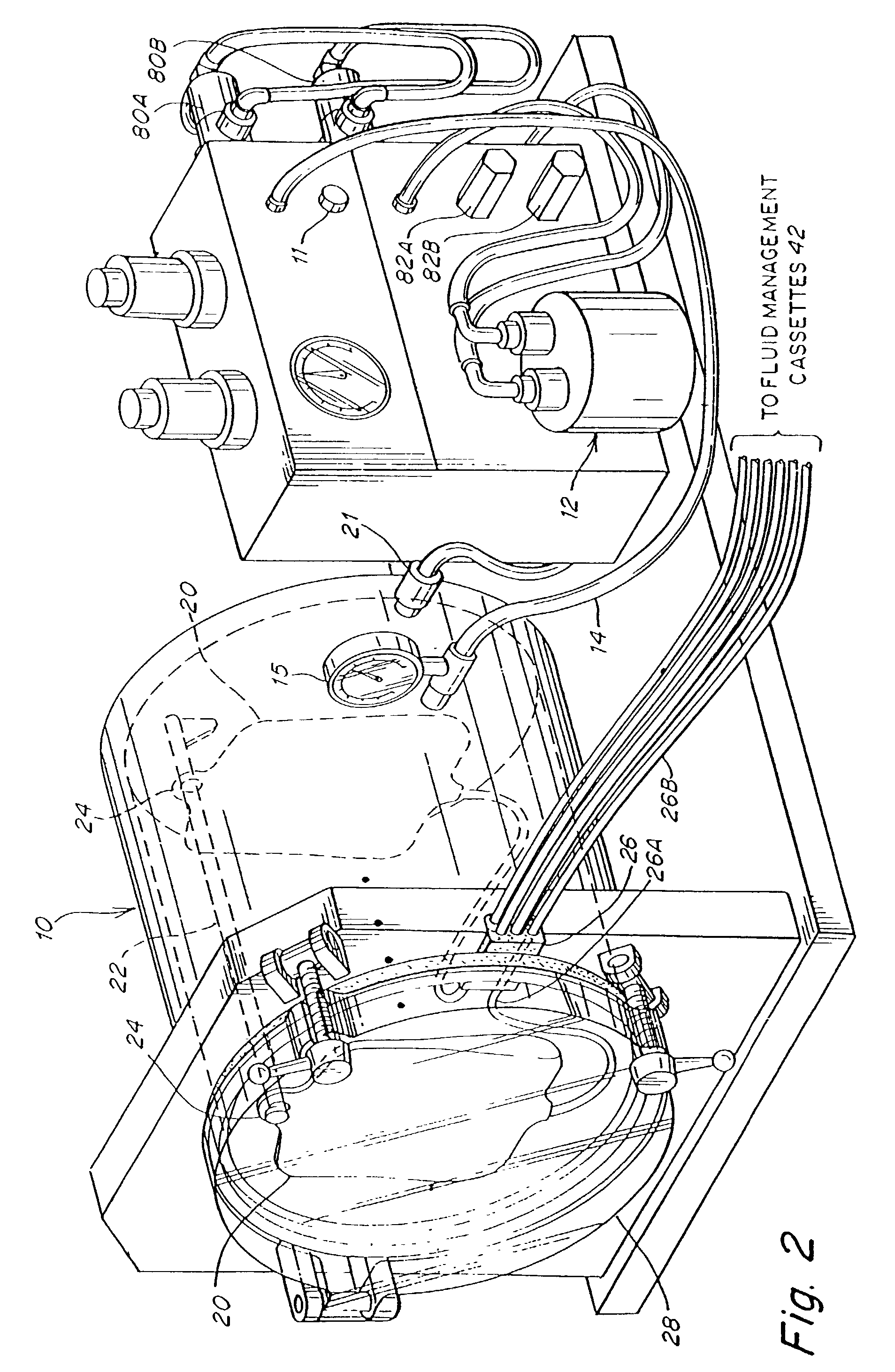
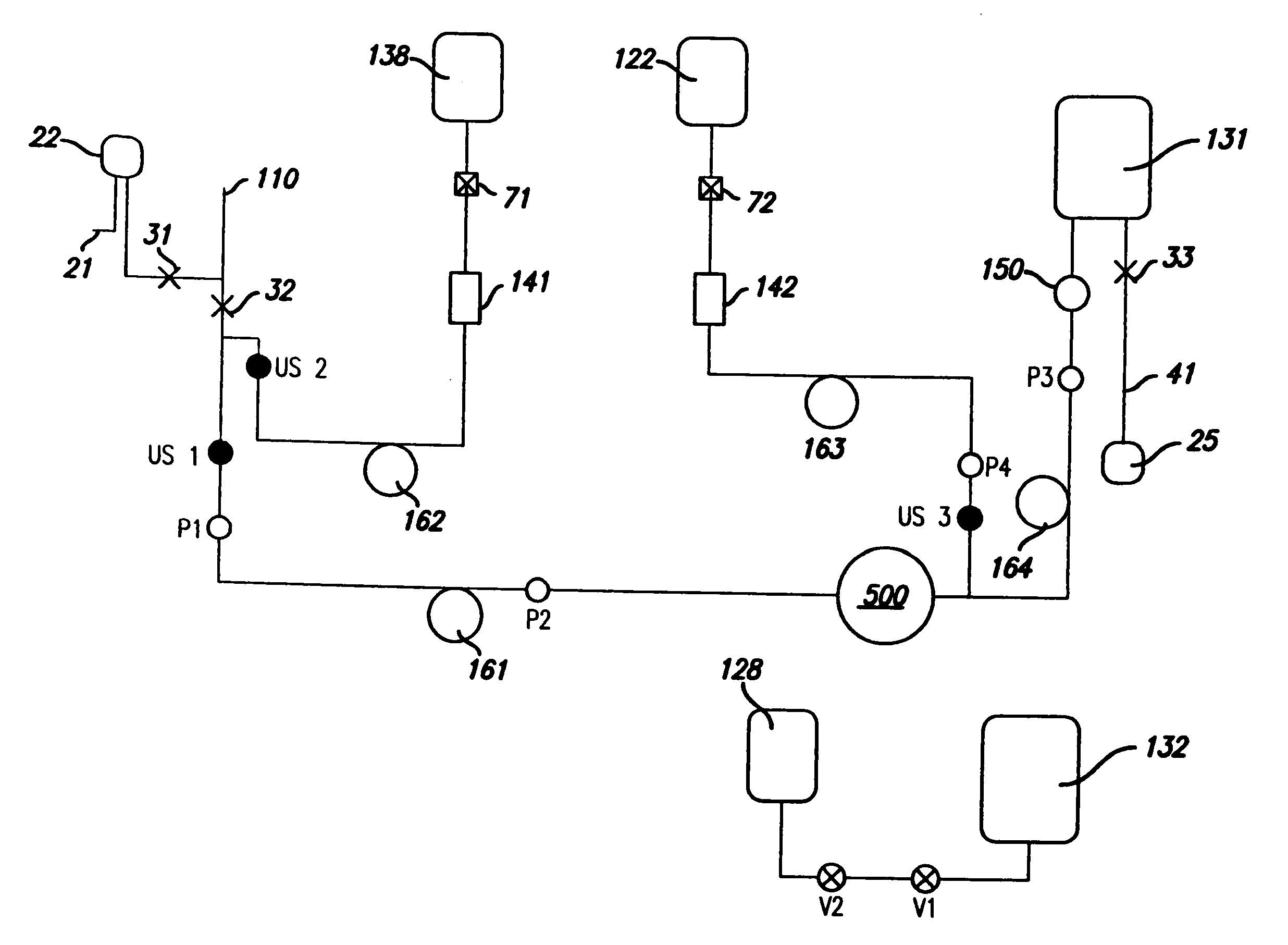
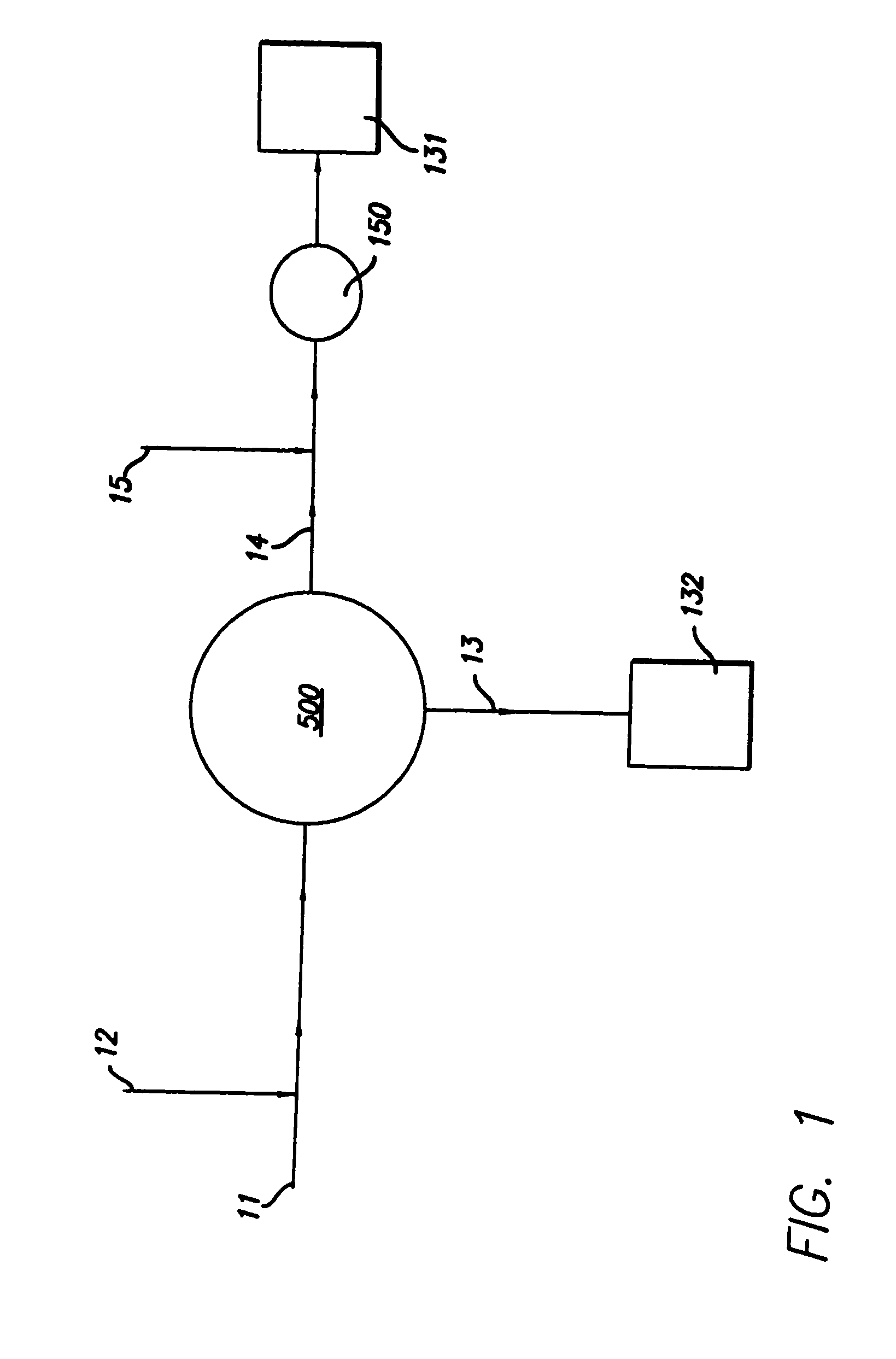
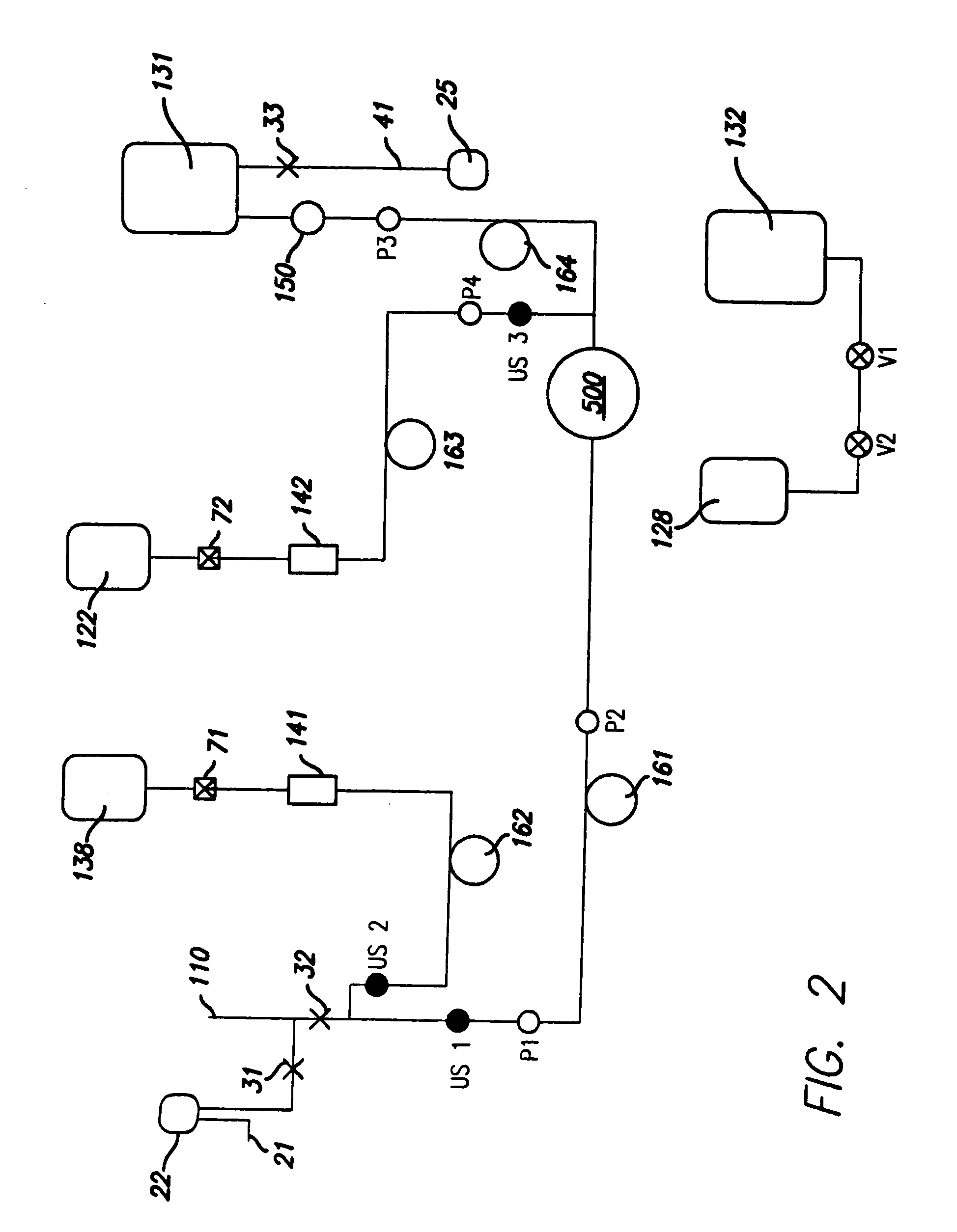
Described herein is a method and apparatus for collecting and separating whole blood into its components, including collecting an amount of plasma and red blood cells. The collection and separation system includes a console and a disposable set. The method may include processing the blood through the centrifuge, collecting plasma, collecting red blood cells and returning red blood cells until a desired plasma volume is reached. Then a final volume of red blood cells and plasma may be collected. The disposable set may include a manifold, a CFC, and various components attached by tubing. These components may include one or more solution bags, blood product bags, bacterial filters, leukofilters, and a donor blood collection tube with access needle.
Owner:TERUMO MEDICAL CORP
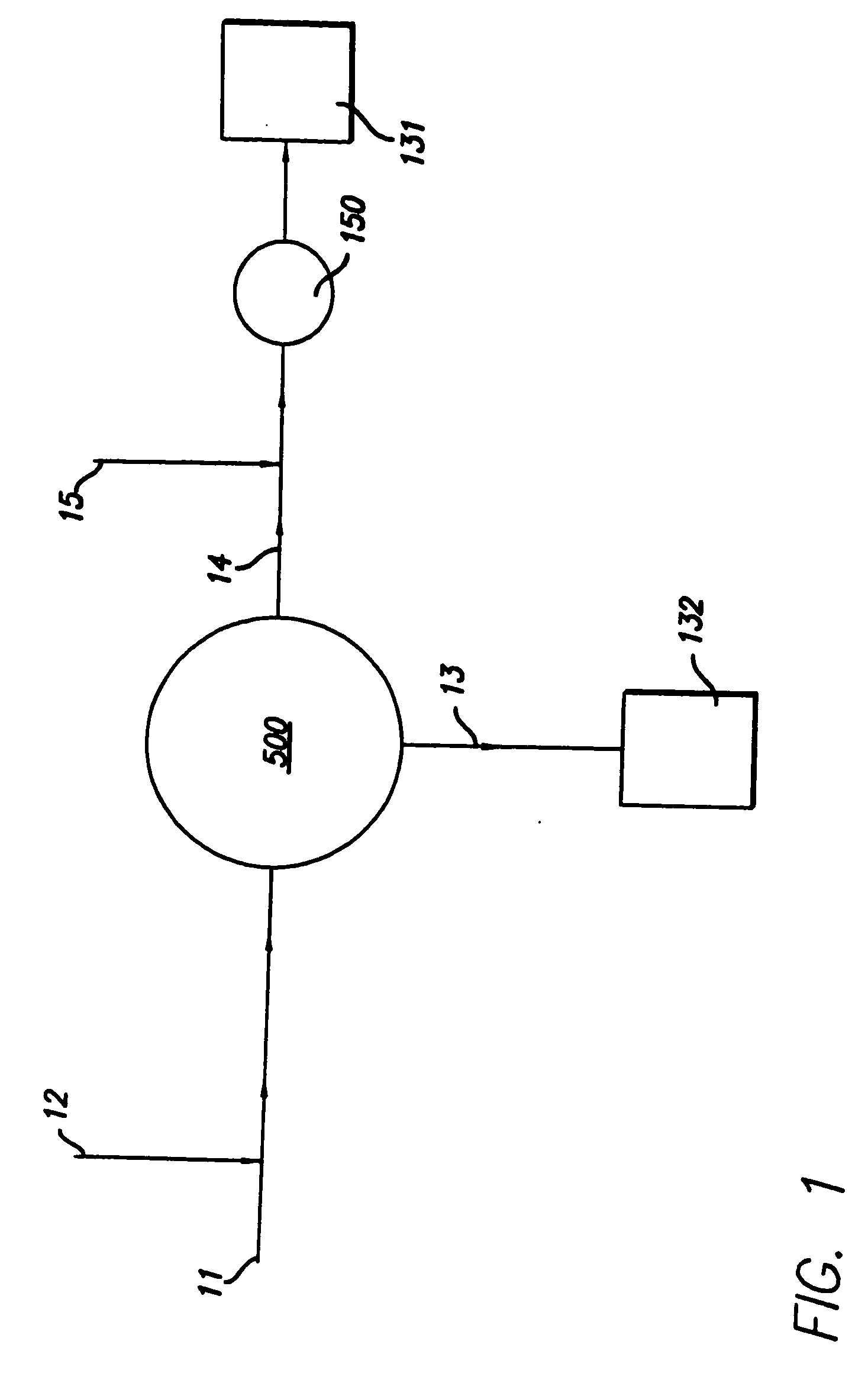
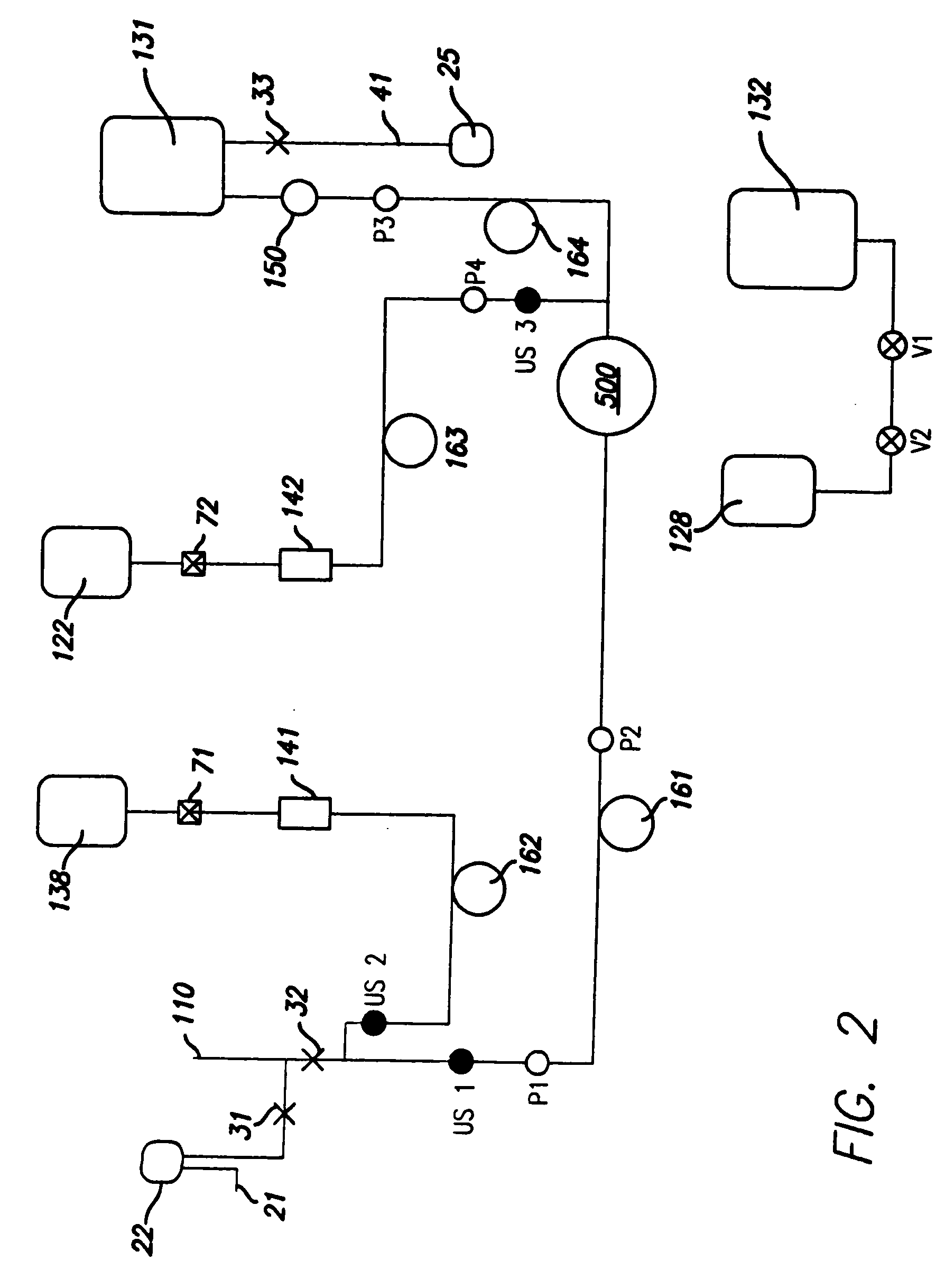
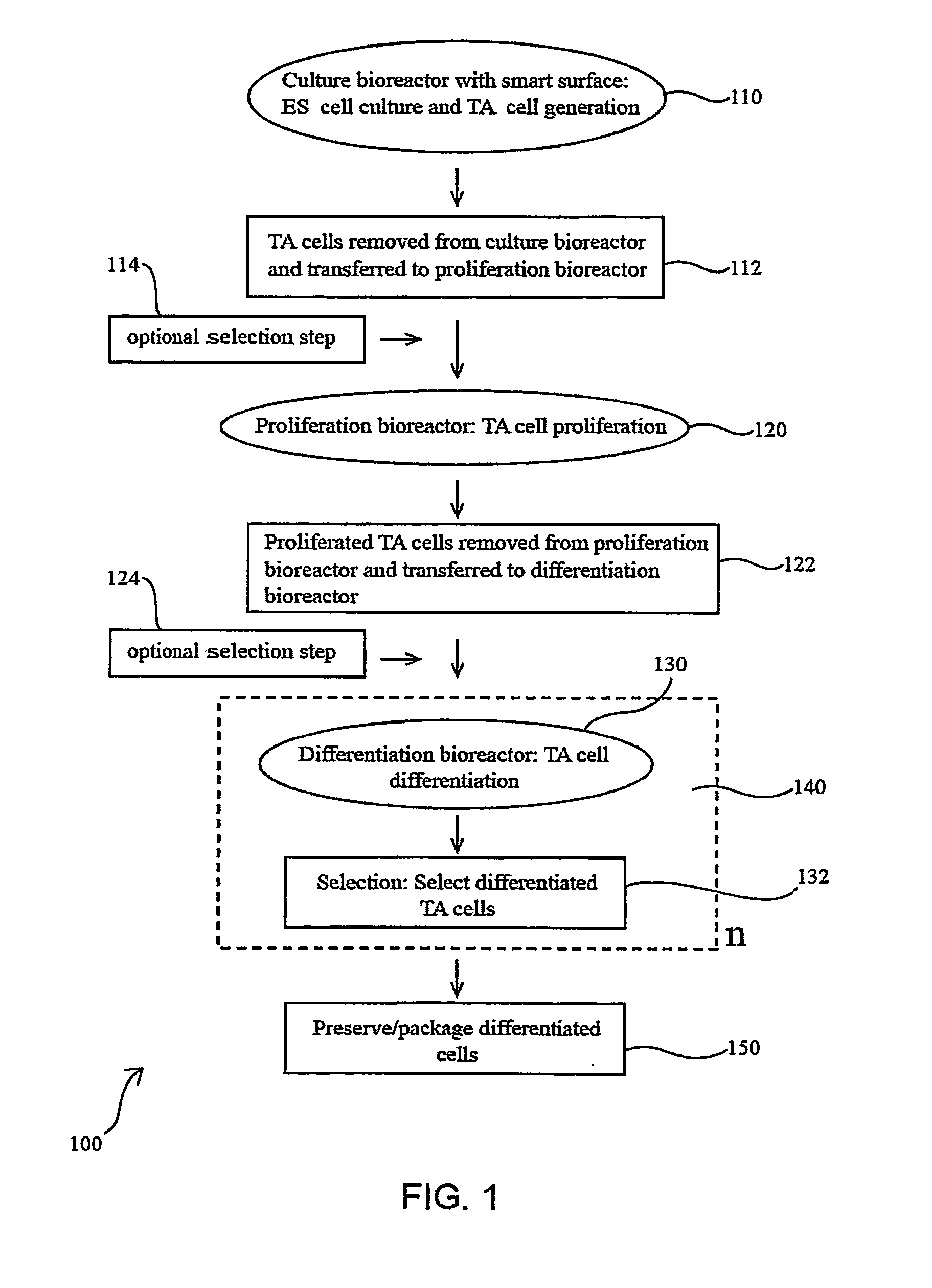
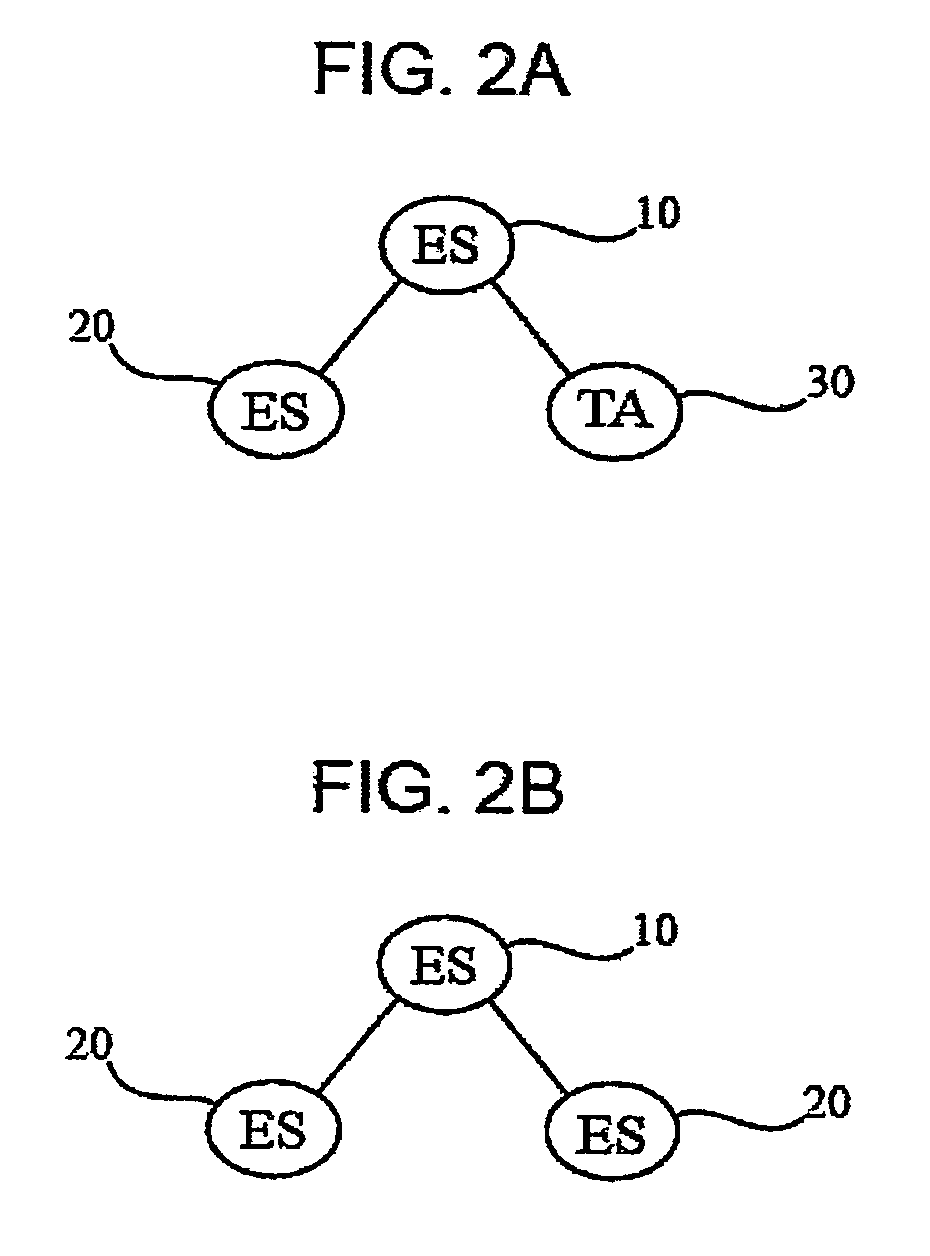
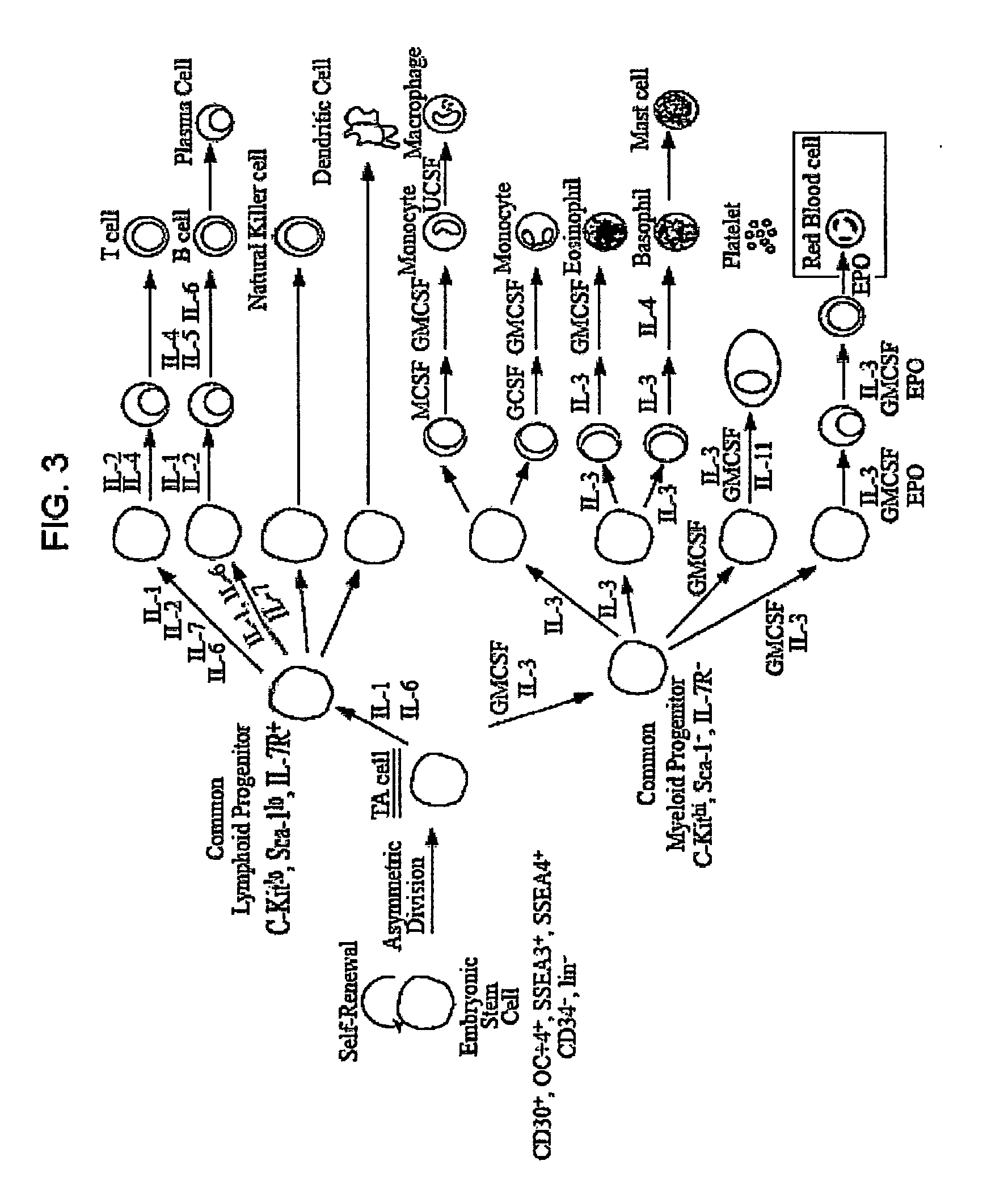
Methods for producing blood products from pluripotent cells in cell culture
The present invention provides methods for in vitro production of clinically useful quantities of differentiated human blood cells. In various embodiments of the present invention, immortal pluripotent cells are used to produce differentiated blood cell populations using a cell production device. In a specific embodiment, the device is a sequential series of bioreactors utilizing growth media containing specific combinations of maintenance-, proliferation- or differentiation-promoting factors that maintain, expand and promote the maturation and differentiation of the desired cell types. The immortal pluripotent cells can optionally be genetically modified so as to remove histcompatibility or blood group antigens.
Owner:AUSTRALIAN STEM CELL CENT
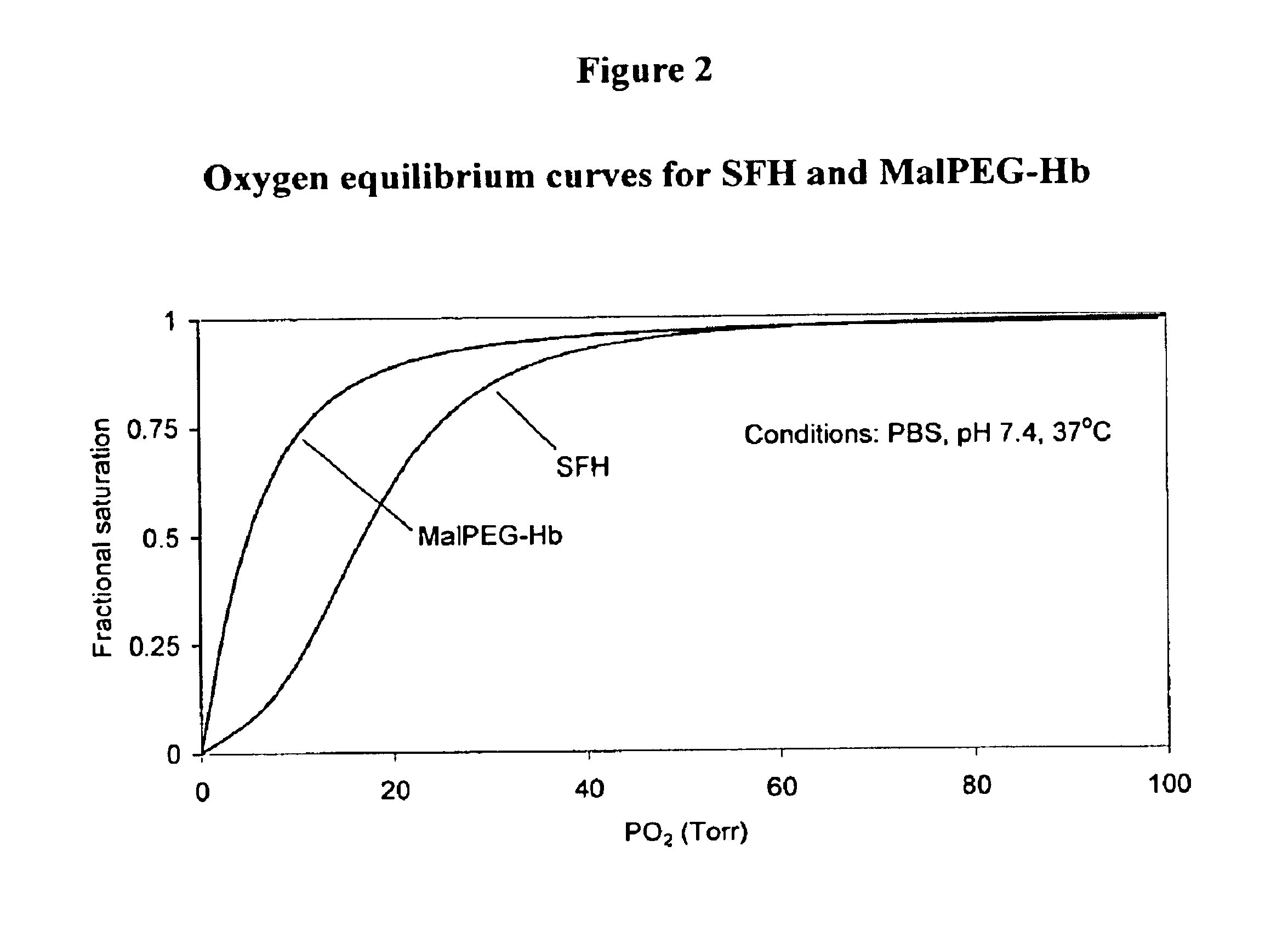
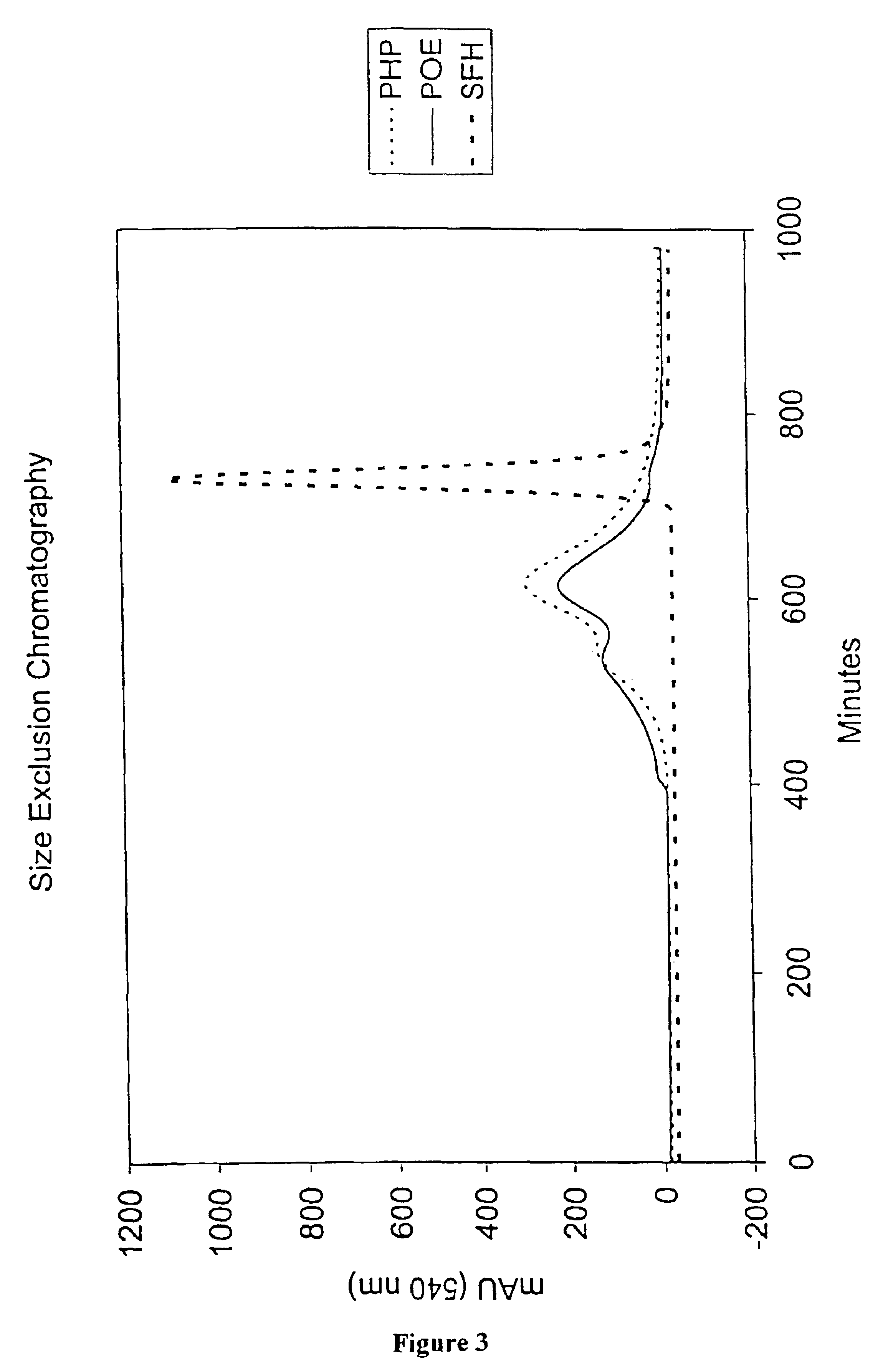
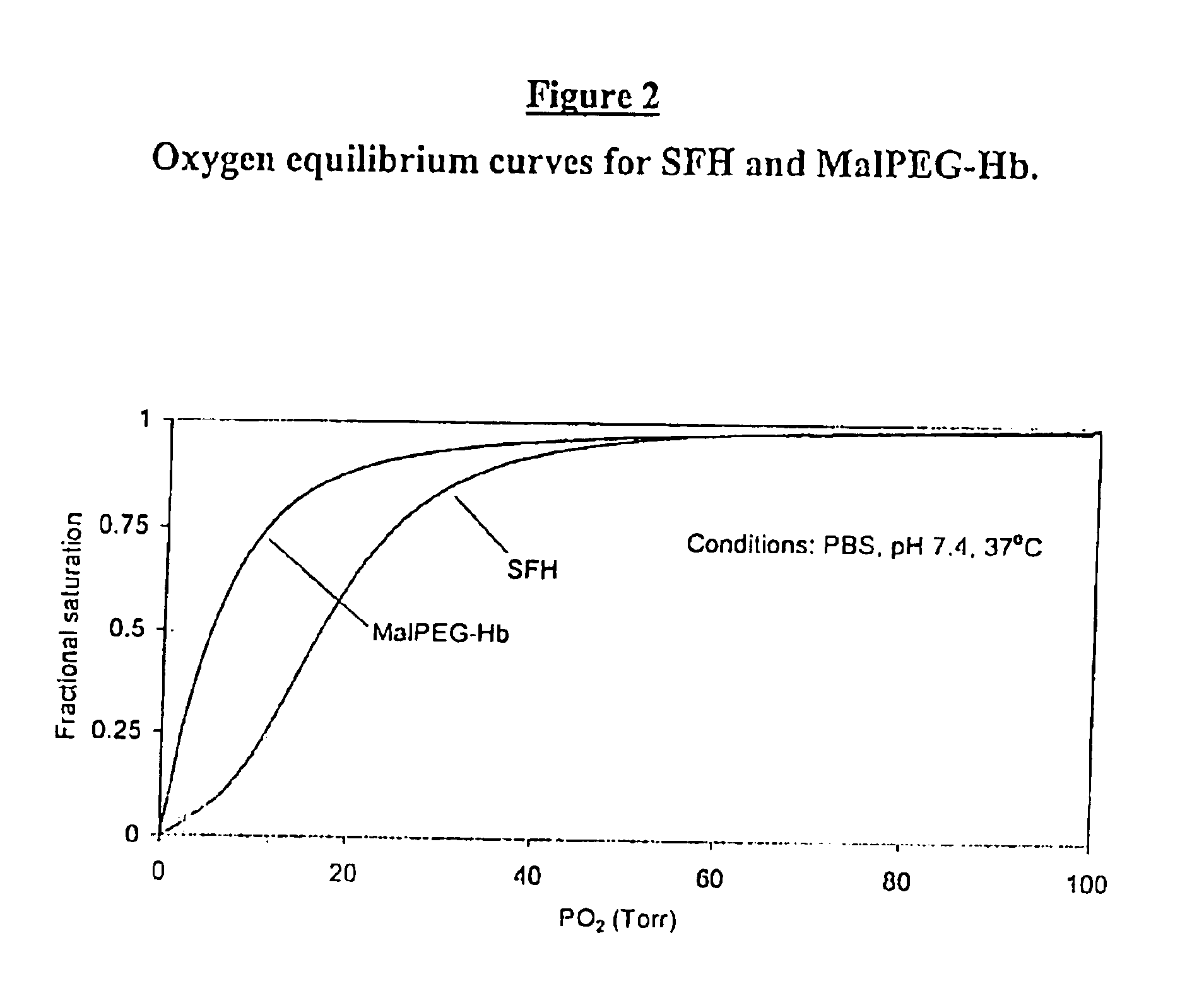
Methods for oxygen transport comprising a high oxygen affinity modified hemoglobin
InactiveUS6844317B2Low toxicityImprove stabilityAntibacterial agentsBiocideWhole blood productOxygenated Hemoglobin
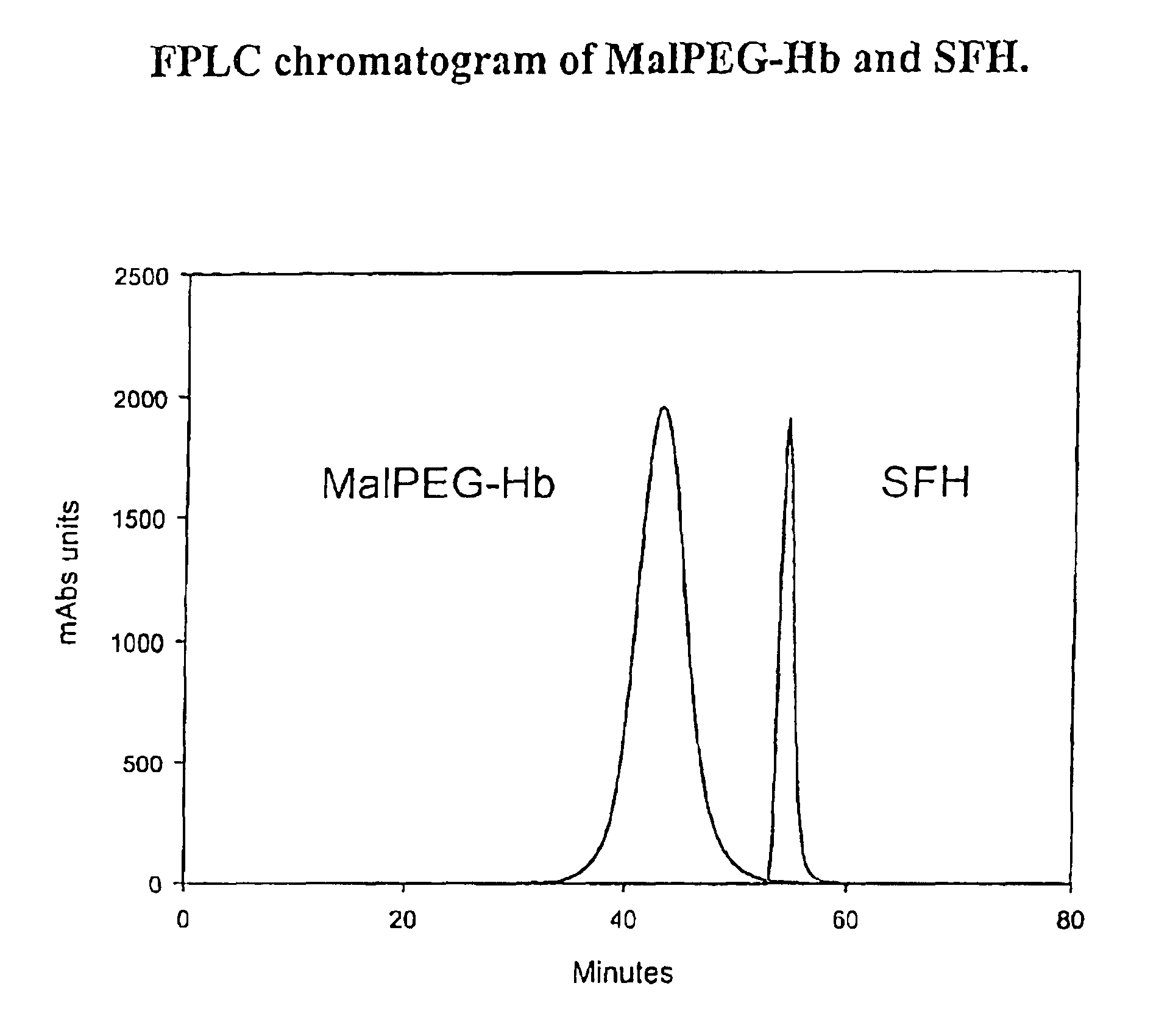
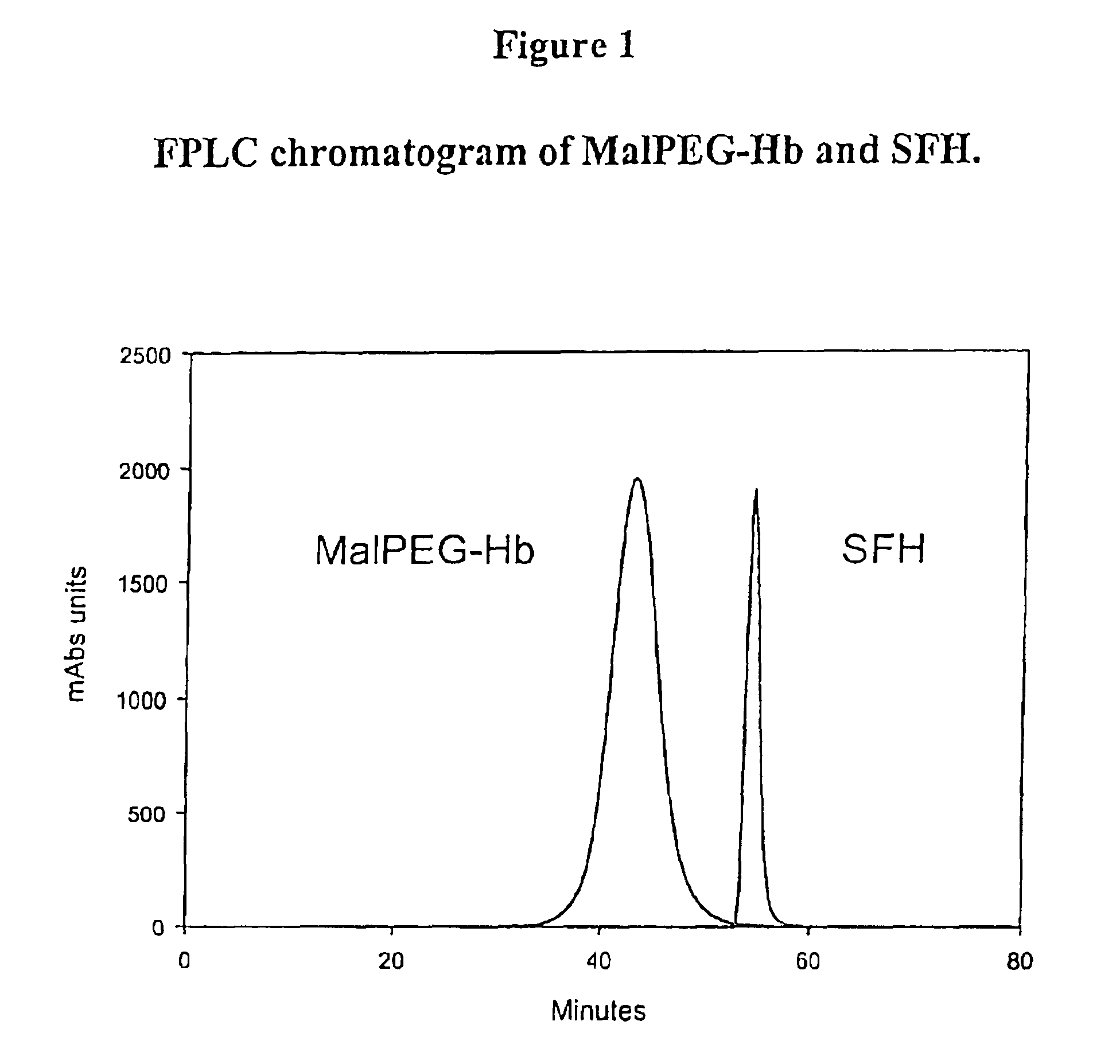
The present invention relates to blood products, and more particularly to compositions comprising a modified oxygenated hemoglobin having a high affinity for oxygen and methods for making such compositions. Such compositions according to the present invention have better stability to autooxidation and superior oxygen carrying characteristics.
Owner:SANGART INC
Method for screening blood using a preservative that may be in a substantially solid state form
InactiveUS20100167271A1Constant cross-sectionBioreactor/fermenter combinationsBiological substance pretreatmentsWhole blood productBlood component
Methods and devices useful for screening a blood product for a transfusion, pursuant to which leukocytes in drawn whole blood are contacted with a formaldehyde releaser screening preservative so that the presence of any residual leukocytes can be screened. A substantially solid state form preservative for one or more blood components (e.g., leukocytes) and use thereof is also described.
Owner:STRECK INC
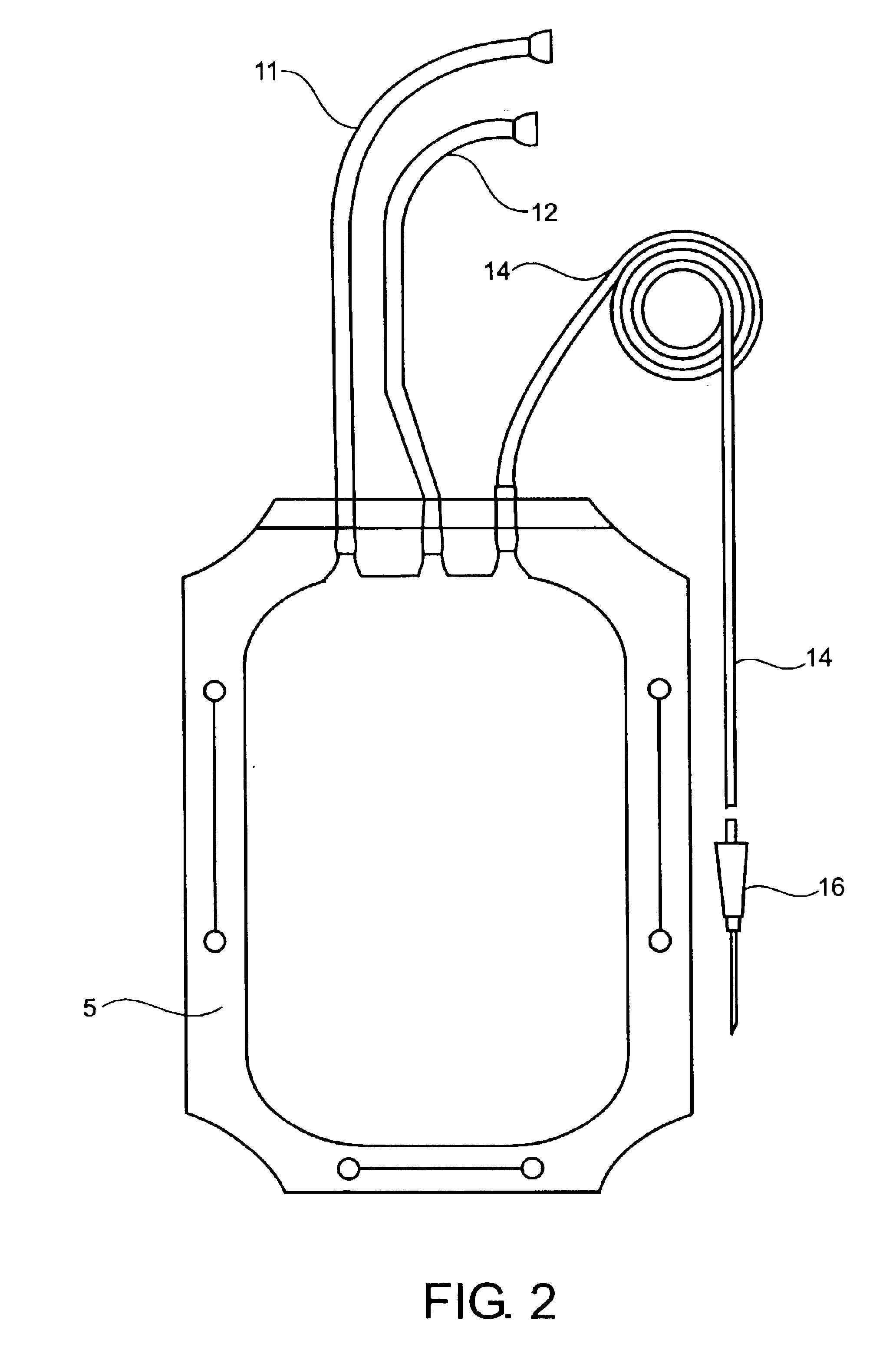
Container for transporting blood and blood products
InactiveUS20060276768A1Small sizeEasy to cleanSurgical furnitureDiagnosticsWhole blood productMedicine
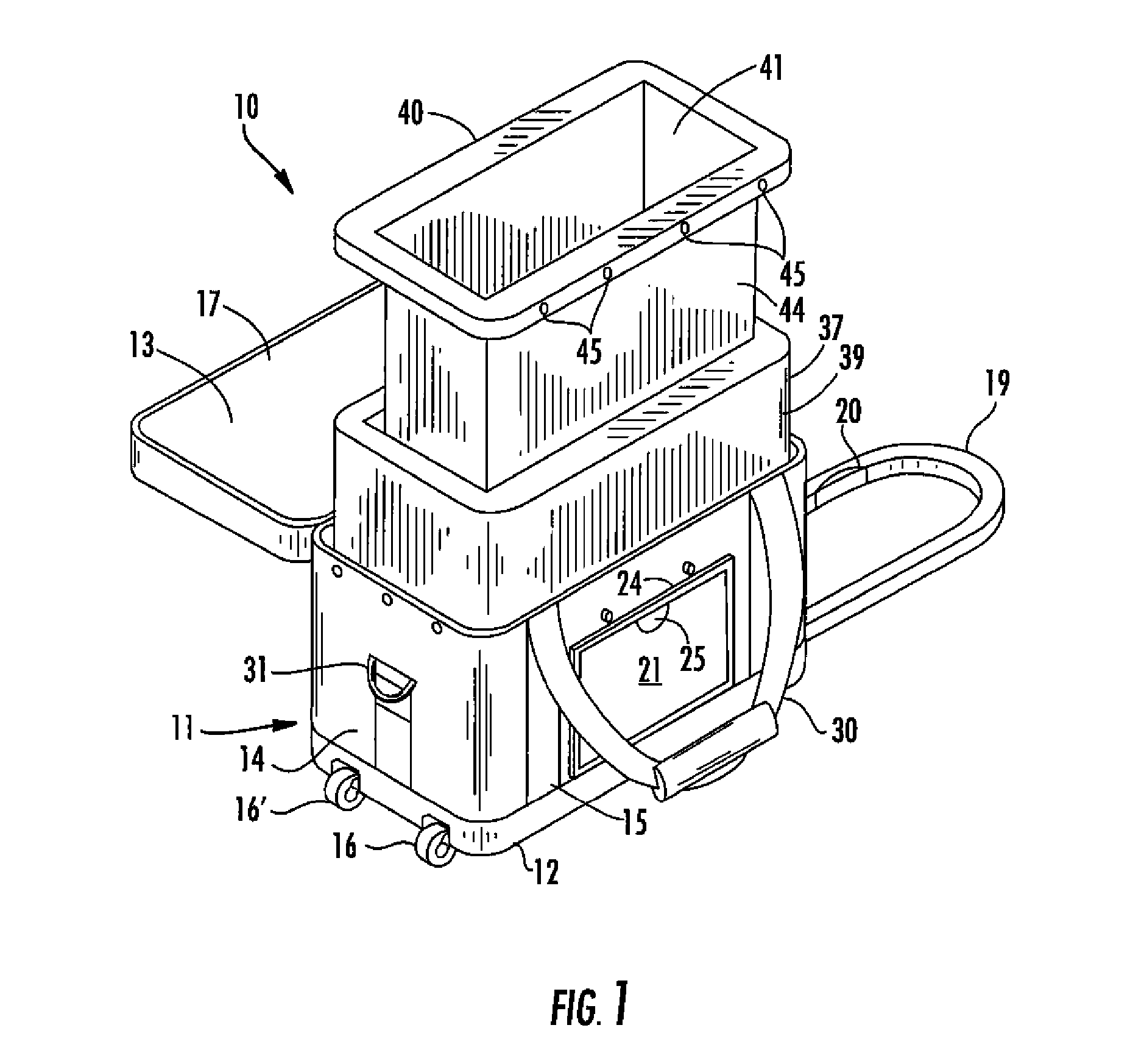
The invention is directed to a reusable container for transporting blood and blood products providing the ability to not only transport, but also to monitor and maintain refrigerated blood or blood products at optimal temperature range for more than twenty-four (24) hours without the use of wet or dry ice, or gel packs.
Owner:INT THERMAL WIZARDS
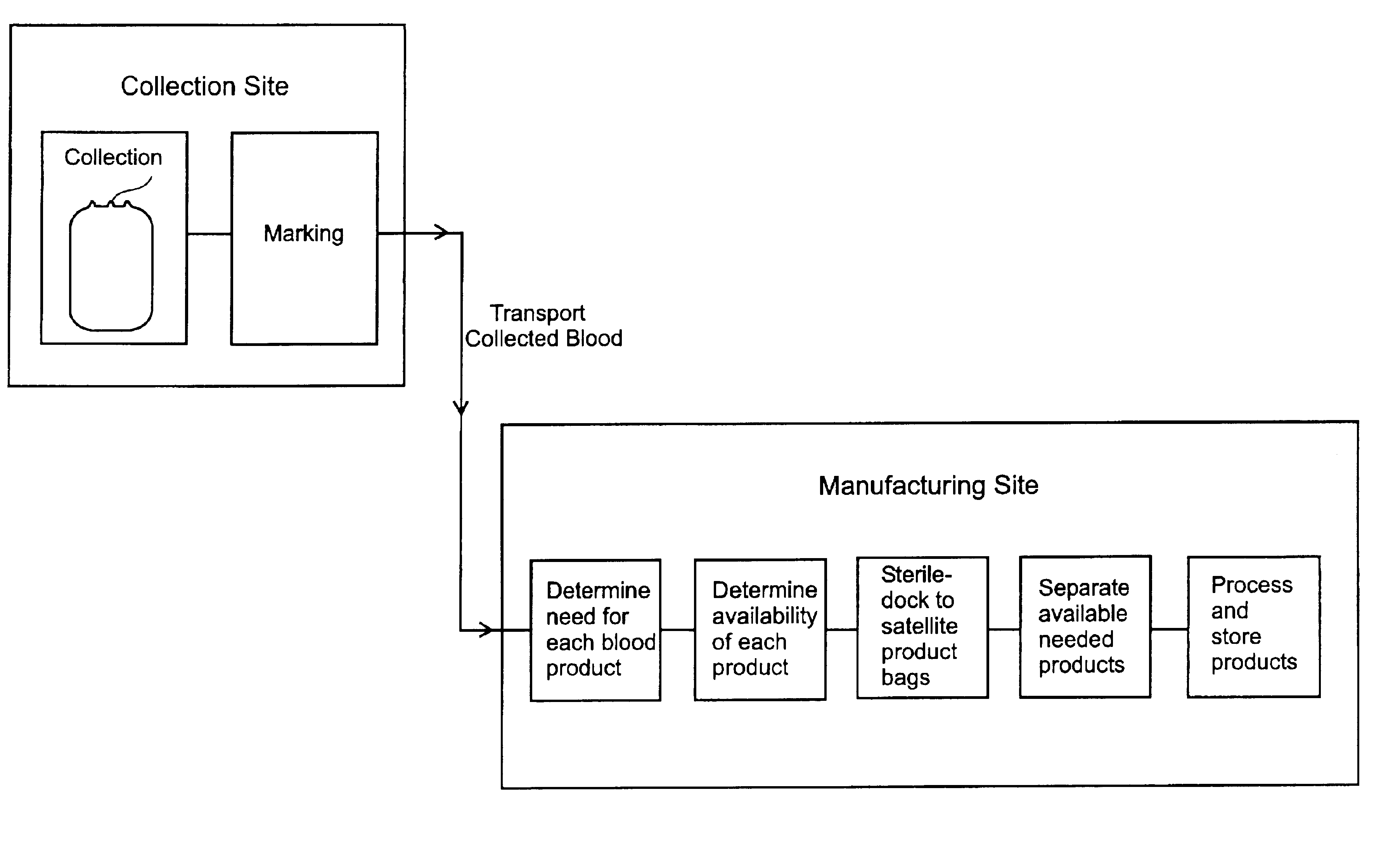
Whole blood collection and processing method
InactiveUS6994790B2Increase speedOther blood circulation devicesDiagnosticsBlood collectionWhole blood product
A method is disclosed for collecting and processing whole blood. Whole blood is collected at several remote donor sites and transported to a central blood-processing center, where information regarding demand for blood products is used to direct processing of whole blood into blood products. Whole blood is initially collected in collection bags unattached to satellite bags instead of commonly used multiple bag sets. When the determination is made at the blood processing center as to which blood products are to be made, the appropriate satellite bags and / or other system components are sterile docked to the blood-collection bag and the whole blood is processed. The use of blood-collection bags initially unattached to satellite bags eliminates waste and simplifies the transportation and processing of whole blood. Also disclosed are blood collection systems that remove leukocytes and collect whole blood into blood-collection bags unattached to satellite bags.
Owner:TERUMO BCT
Method and apparatus for blood separations
InactiveUS20060226089A1Reduce collectionLow costWater/sewage treatment by centrifugal separationRotary centrifugesWhole blood productWhite blood cell
Described herein is a method and apparatus for collecting and separating whole blood into its components, including collecting an amount of leukoreduced red blood cells. The collection and separation system includes a console and a disposable set. The method may include processing the blood through the centrifuge, collecting the leukoreduced red blood cells, and collecting and returning plasma to the source. The disposable set may include a manifold, a CFC, and various components attached by tubing. These components may include one or more solution bags, blood product bags, bacterial filters, leukofilters, and a donor blood collection tube with access needle.
Owner:TERUMO MEDICAL CORP
Valve mechanism for infusion fluid systems
InactiveUS7306736B2Prevent backflowLow costLiquid separation auxillary apparatusSolvent extractionWhole blood productPinch valve
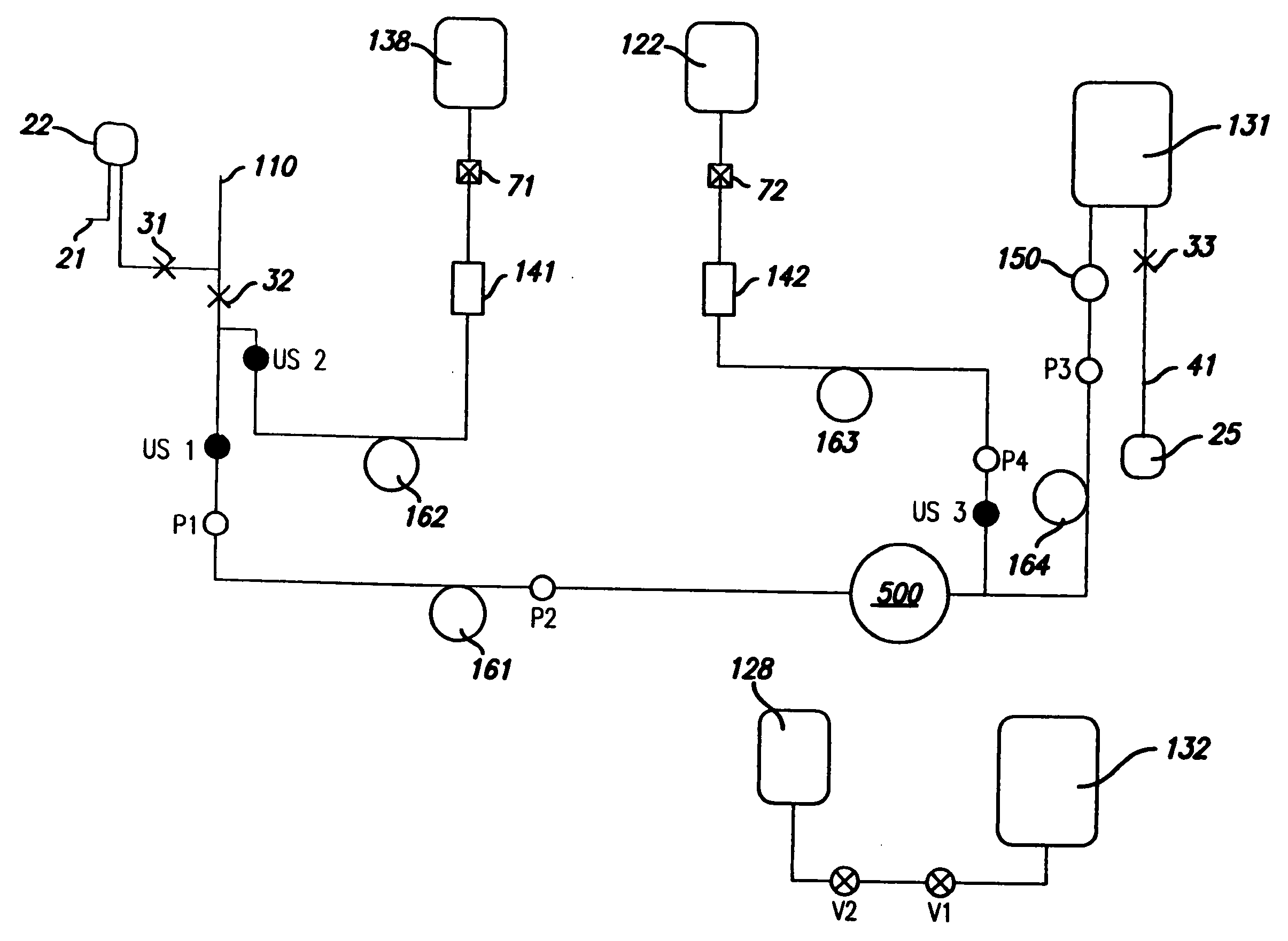
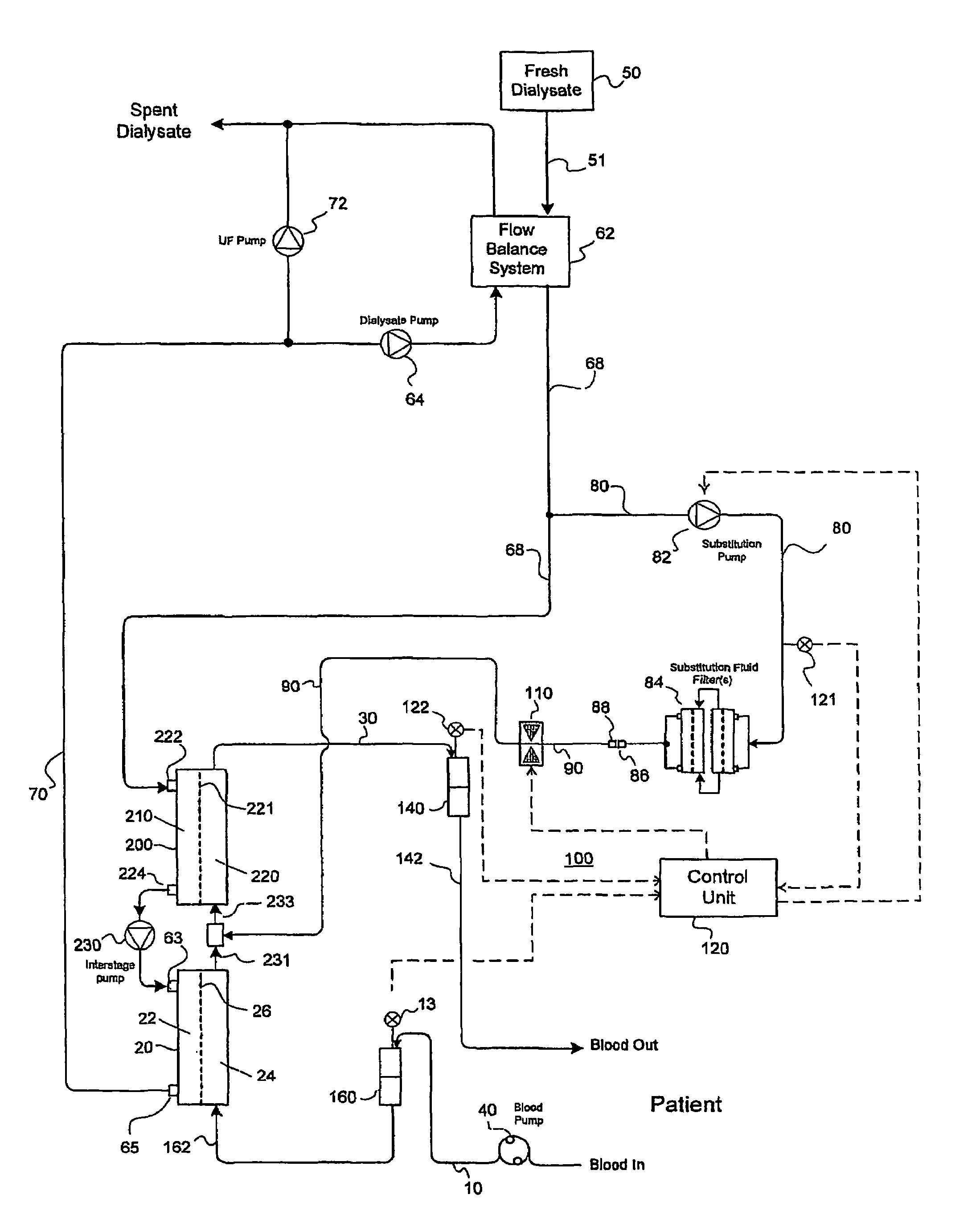
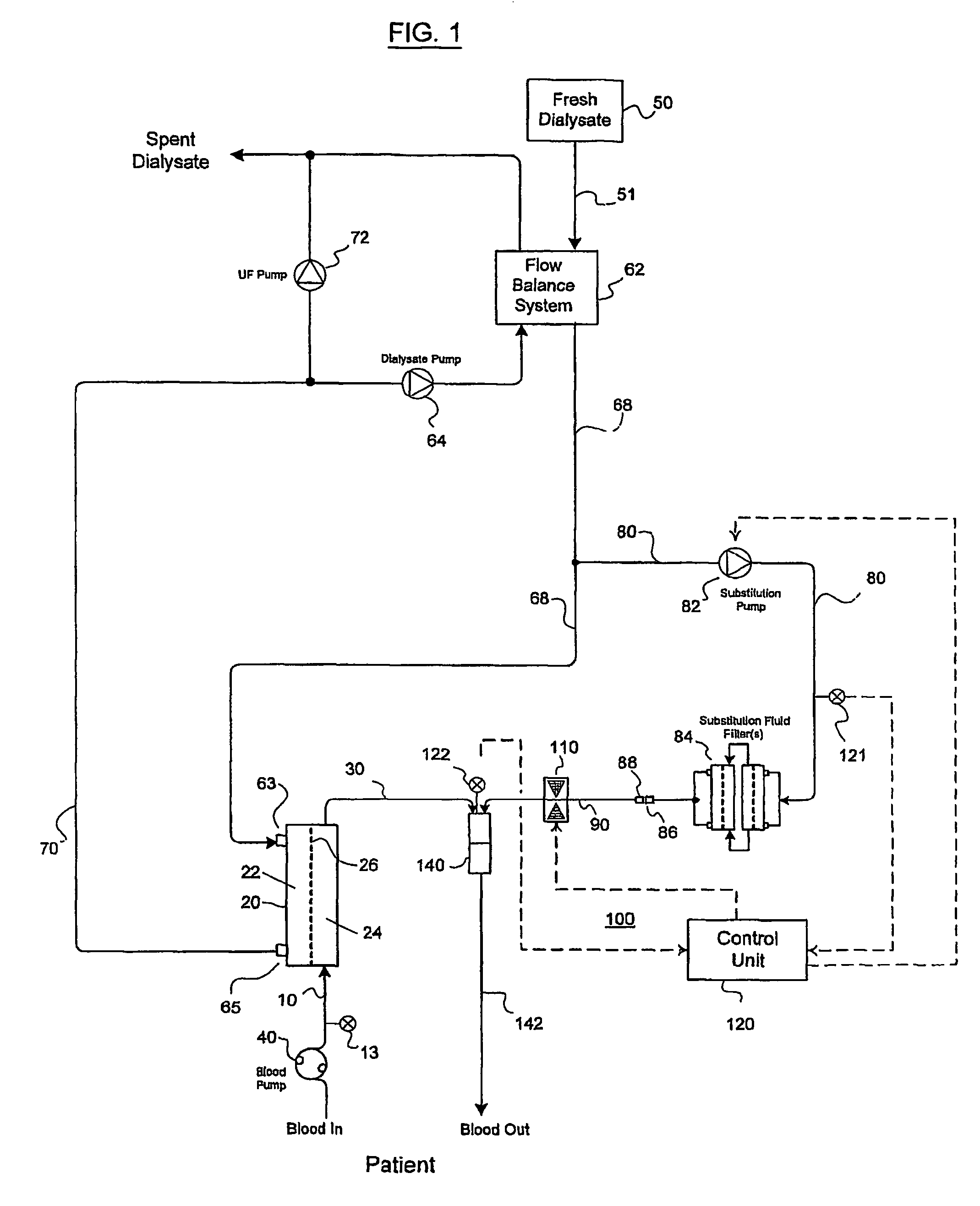
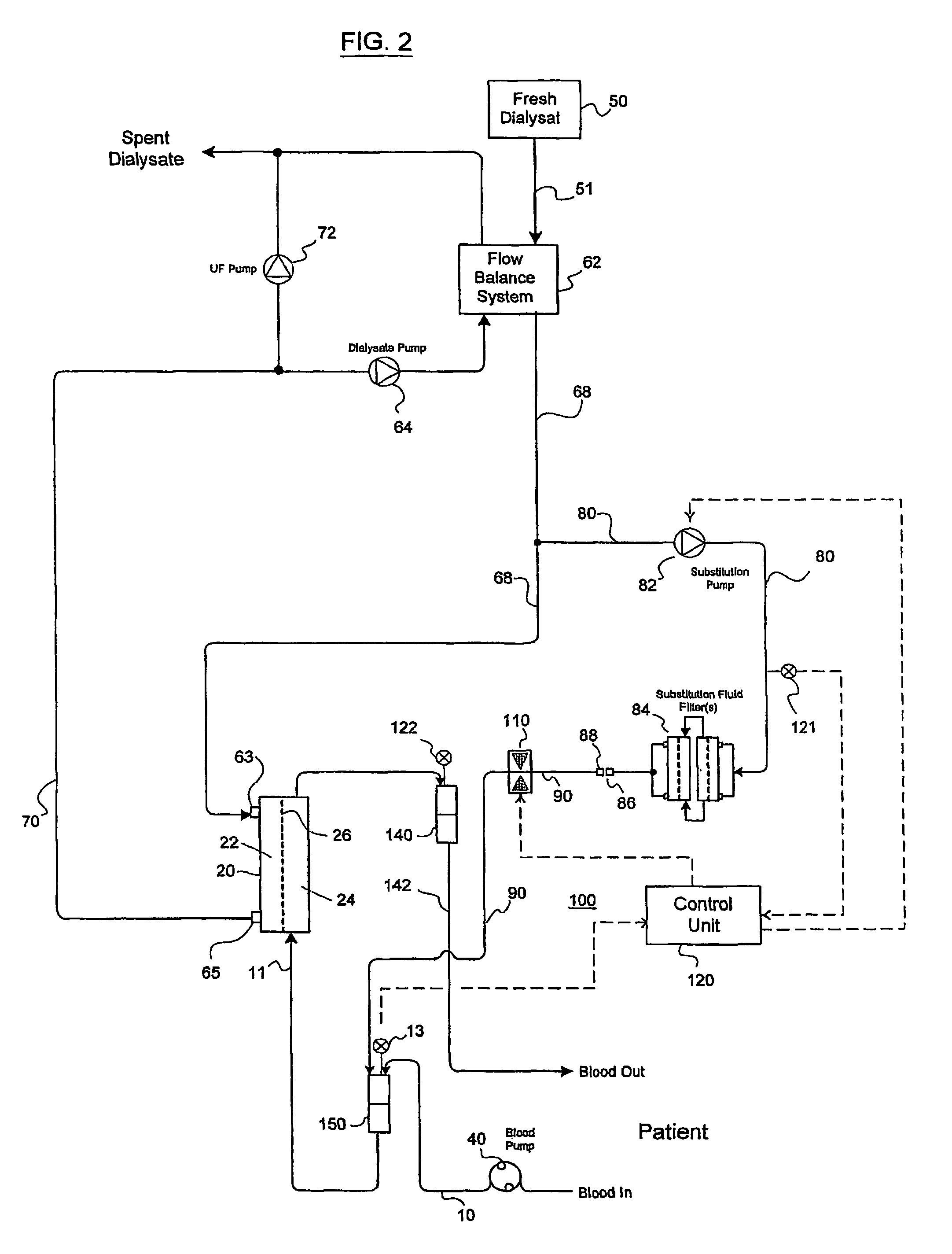
A method and an apparatus are provided for preventing retrograde flow of fluid, e.g., blood products, into a source of sterile substitution fluid (50). The apparatus of the present invention includes a controllable pinch valve member (110) that is placed on a section of a conduit (90) which carries sterile substitution fluid to an extracorporeal circuit (30). In one embodiment, control over the valve member (110) is based on a control unit (120) using fluid pressures that are sensed upstream and downstream of the valve member (110) by upstream sensor (121) and downstream pressure (122) respectively. The valve member (110) is preferably opened only when the upstream pressure is greater than the downstream pressure. This assures that the substitution fluid flows only in a single direction when the pinch valve member (110) completely occludes the conduit (90) when in a closed position. Therefor, blood will not contaminate the sterile fluid by being drawn into the conduit (90) due to pressure differences.
Owner:LAMBDA INVESTORS
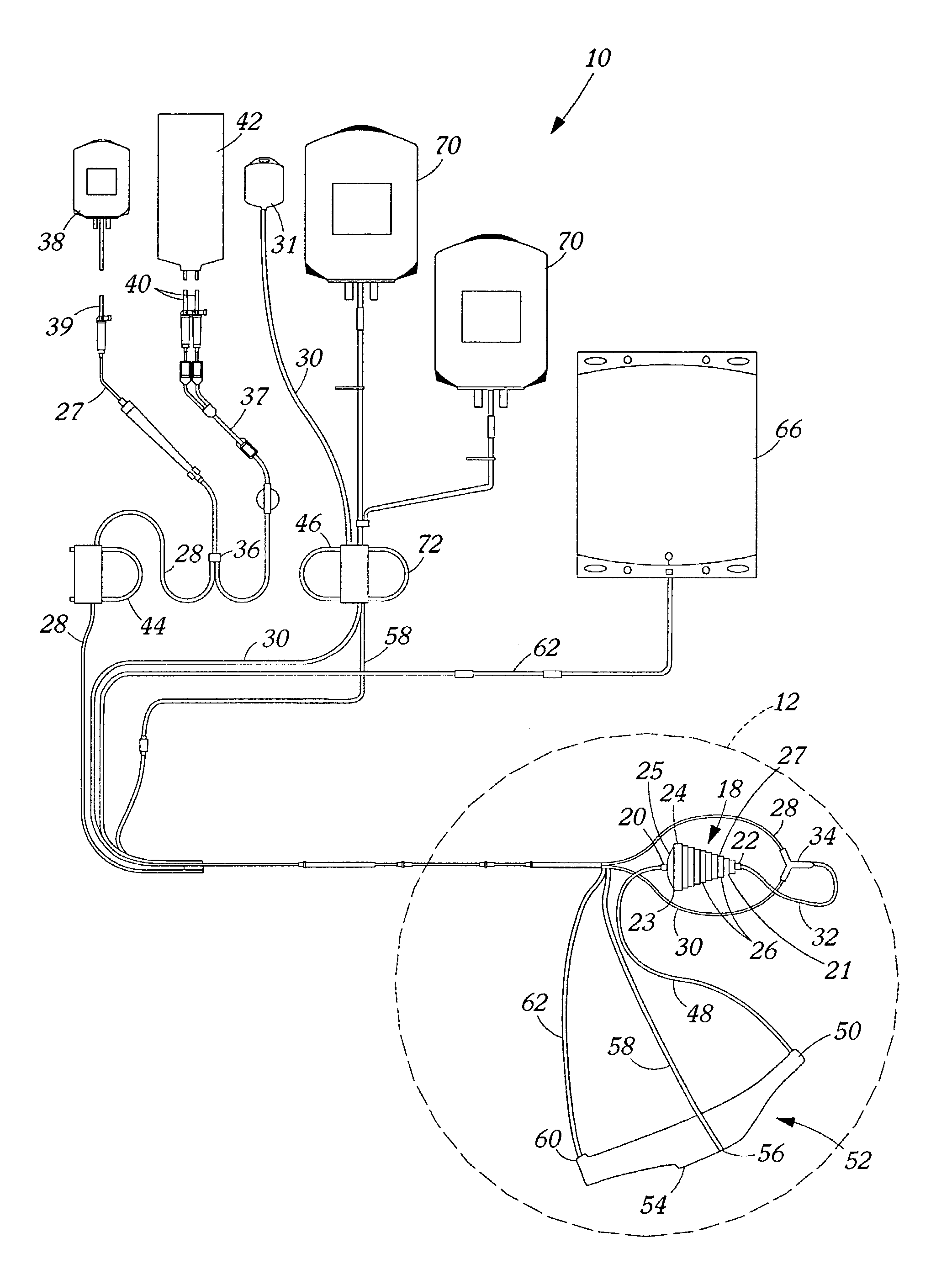
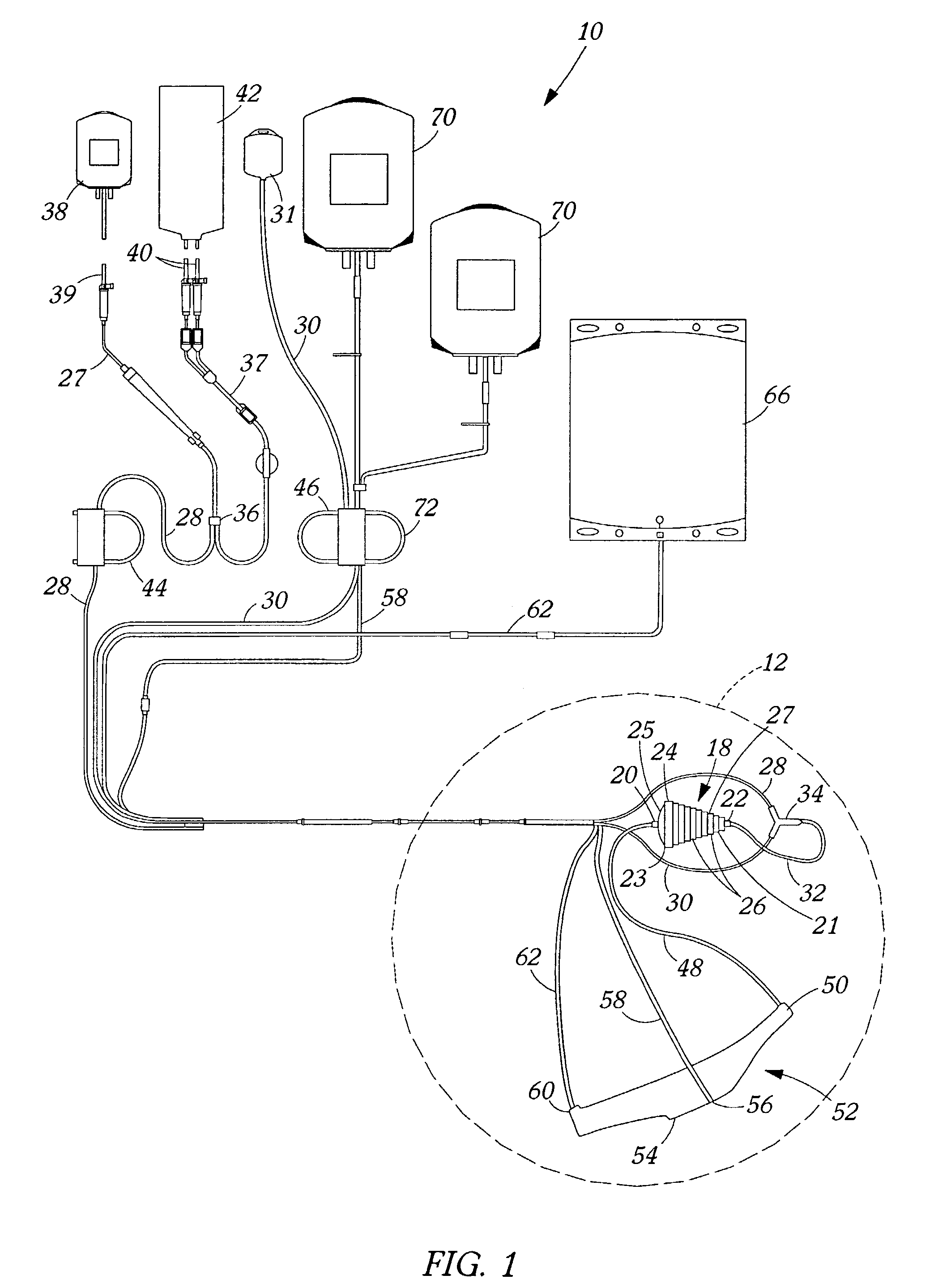
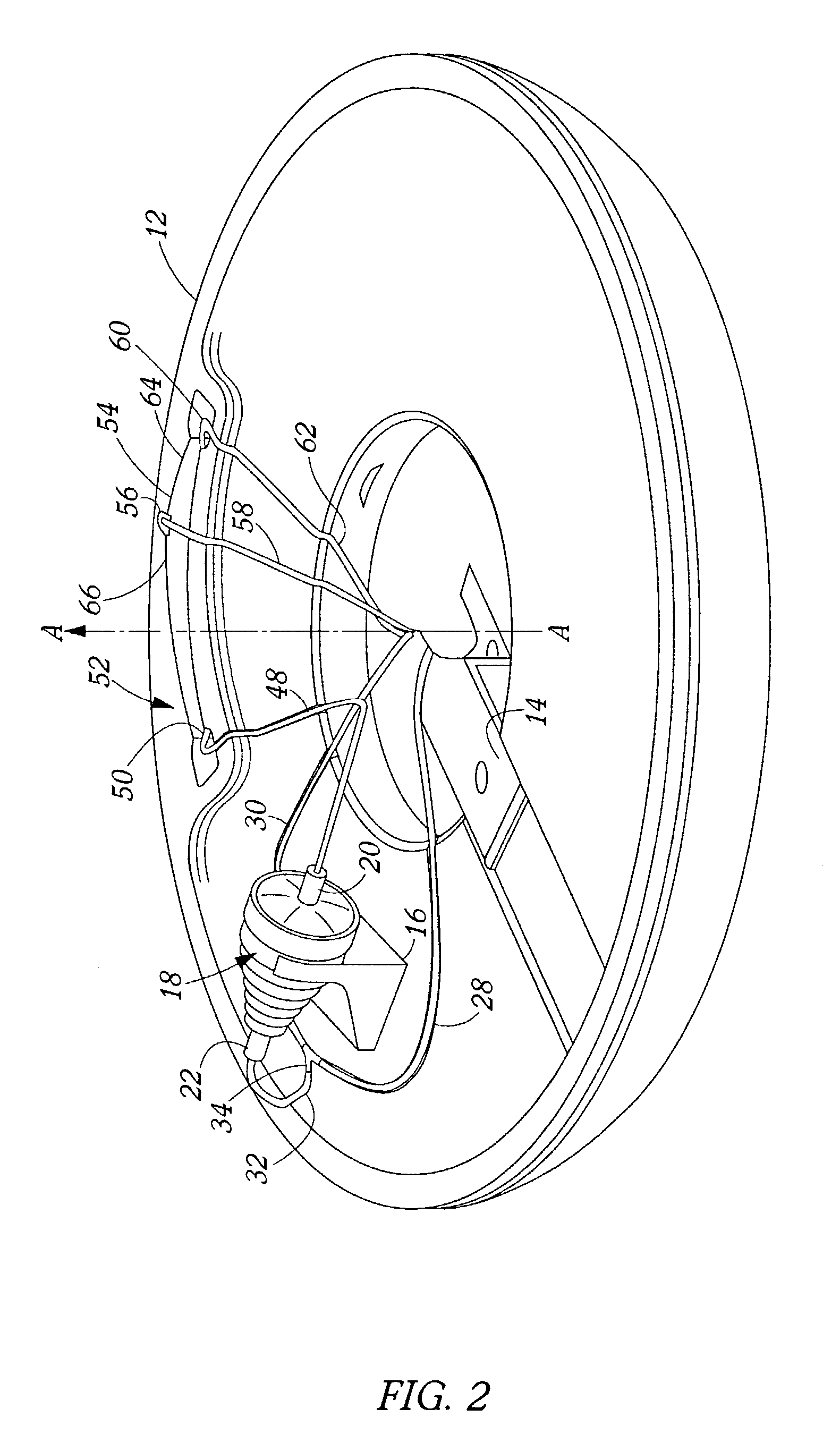
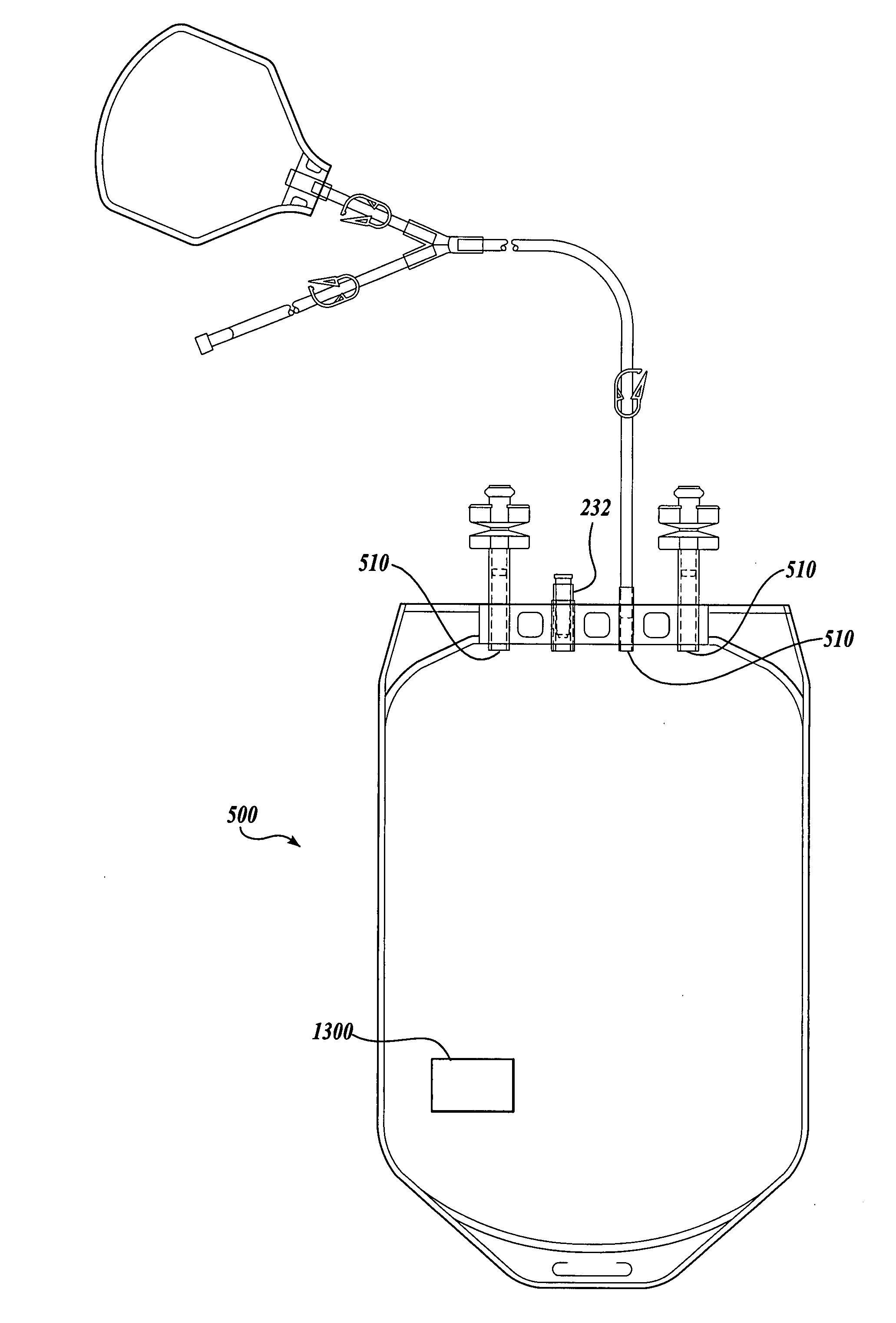
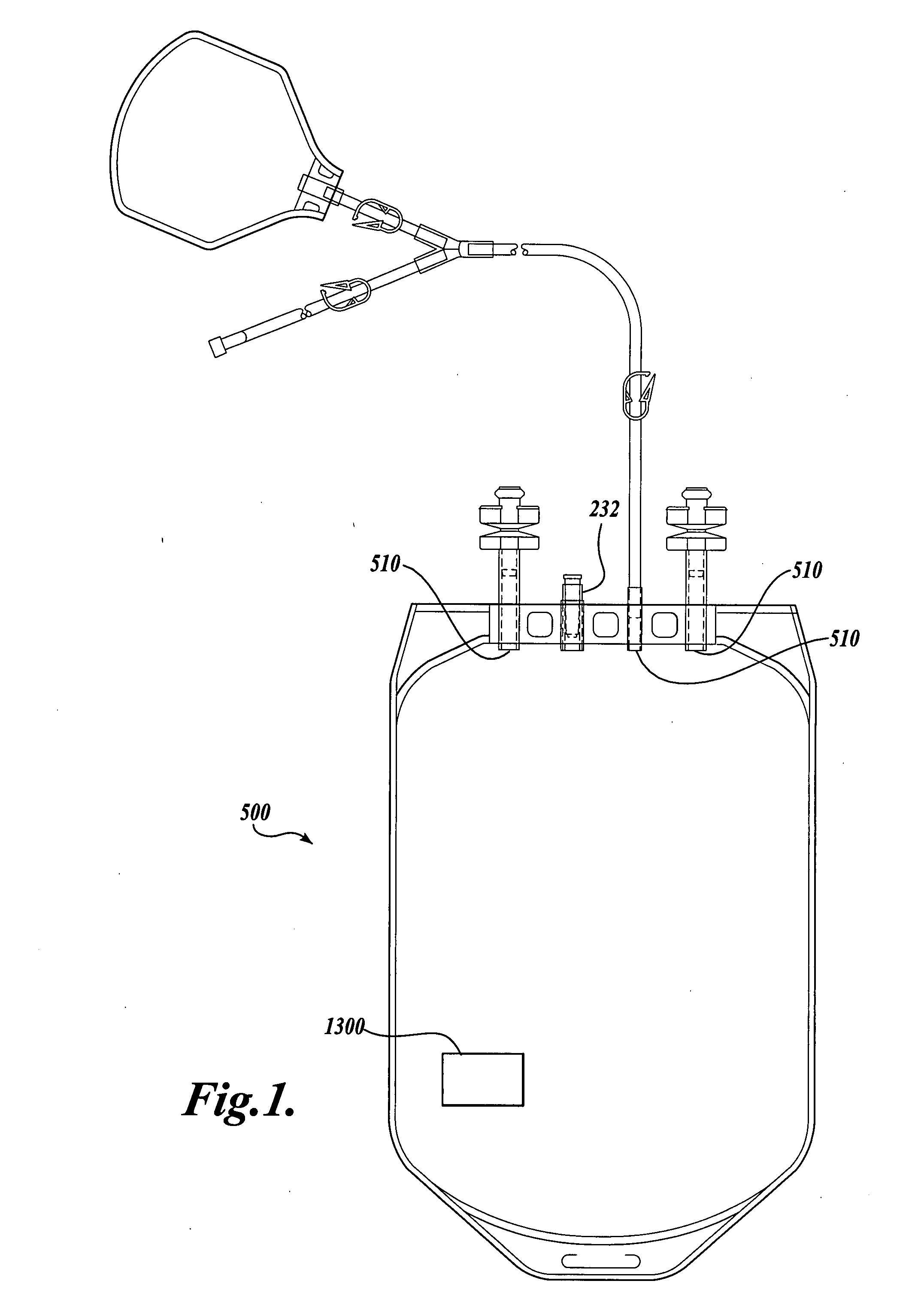
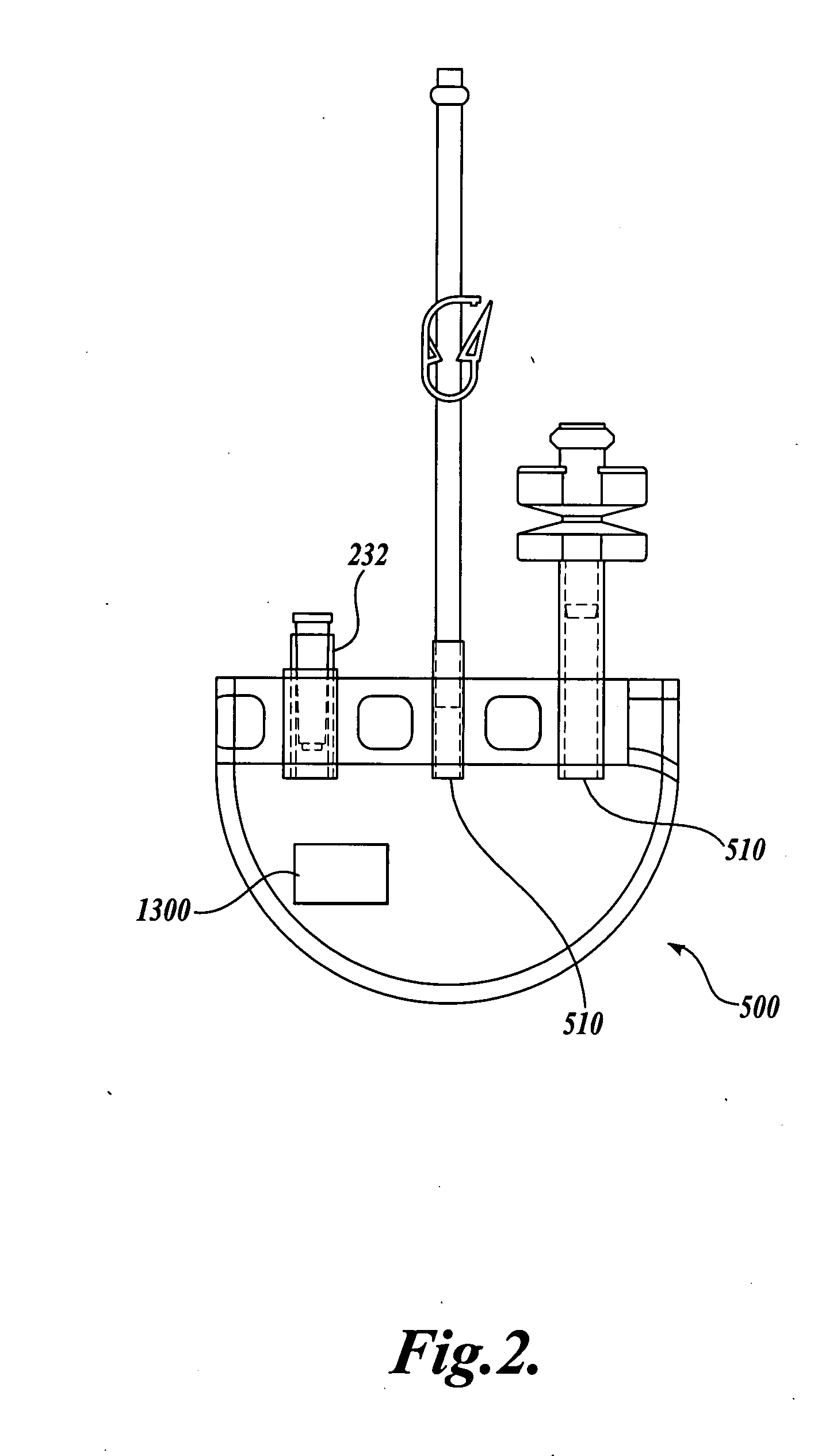
Blood product transfer system
InactiveUS7011742B2Speed up the flowGreat speed and efficiencyOther blood circulation devicesDiagnosticsWhole blood productTransfer system
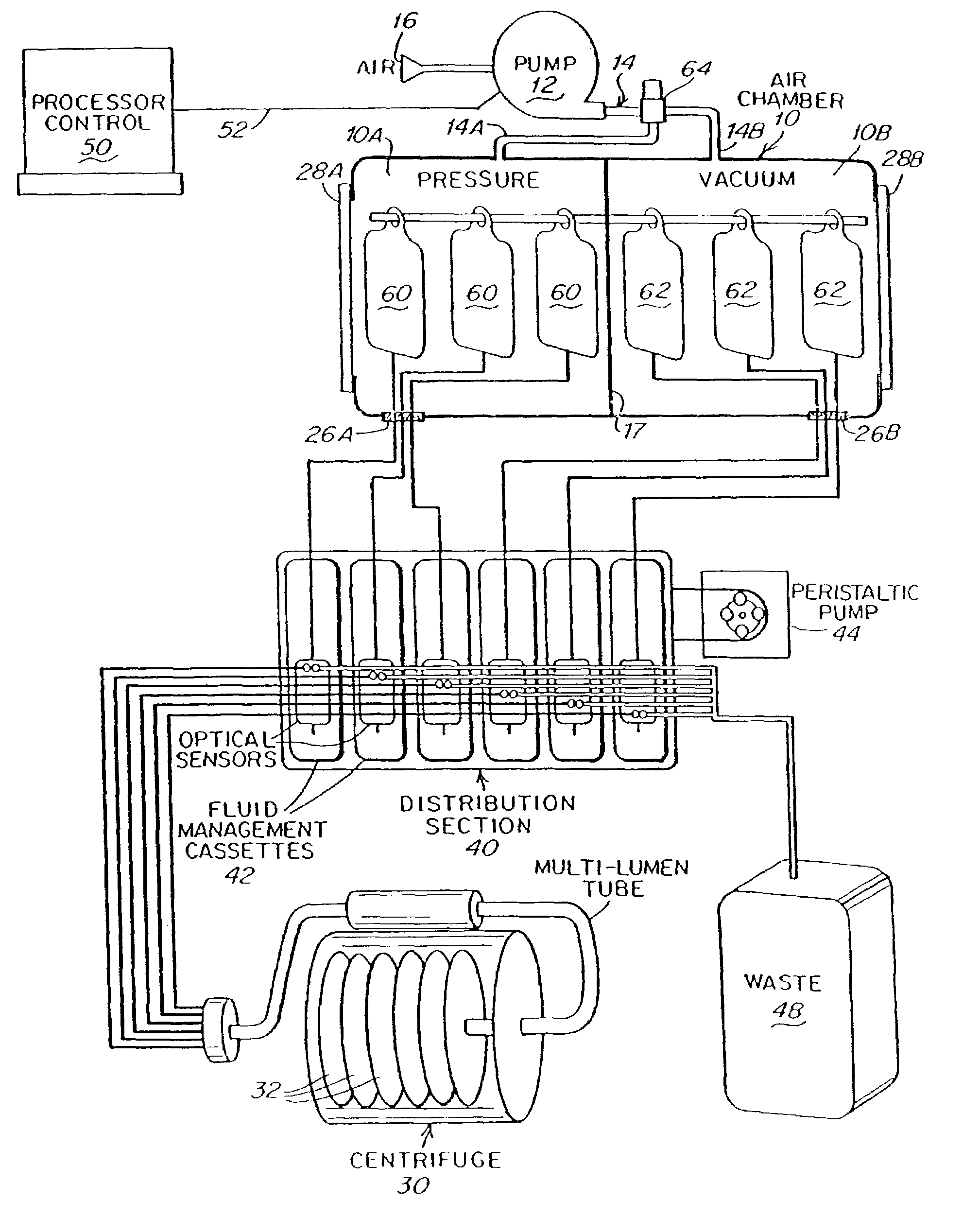
A system for transferring blood product between a blood storage bag and a processing bag. The system includes an airtight containment chamber for supporting therein one or more blood storage bags. A door is provided for access to the chamber and there is included an airtight fixture that allows tubing from the blood storage bag to exit the chamber. A fluid pump is coupled to the containment chamber for establishing either pressure or vacuum within the containment chamber. A controller controls the air pump to, in turn, control the transfer of a blood product.
Owner:VELICO MEDICAL
Compositions for oxygen transport comprising a high oxygen affinity modified hemoglobin
InactiveUS6974795B2Earlier and safer intervention in patient treatmentLow toxicityPeptide/protein ingredientsMammal material medical ingredientsWhole blood productOxygenated Hemoglobin
The present invention relates to blood products, and more particularly to compositions comprising a modified oxygenated hemoglobin having a high affinity for oxygen and methods for making such compositions. Such compositions according to the present invention have better stability to autooxidation and superior oxygen carrying characteristics.
Owner:SANGART INC
Removing compounds from blood products with porous particles immobilized in a matrix
InactiveUS7037642B2Reduce concentrationMaintain biological activityImmobilised enzymesOther blood circulation devicesWhole blood productSorbent
Methods and devices are provided for reducing the concentration of low molecular weight compounds in a biological composition containing cells while substantially maintaining a desired biological activity of the biological composition. The device comprises highly porous adsorbent particles, and the adsorbent particles are immobilized by an inert matrix.
Owner:CERUS CORP
Coating material for leukocyte removal filter and the filter
InactiveUS7721898B2Good removal effectSmall volumeSemi-permeable membranesHaemofiltrationWhole blood productWhite blood cell
It is intended to provide a polymer for coating a leukocyte removal filter material which is excellent in the capability of removing leukocytes. It is further intended to provide a filter whereby both of leukocytes and platelets can be highly efficiently removed from a blood product containing leukocytes and platelets. The above objects can be achieved by using a polymer for coating a leukocyte removal filter material which comprises a unit originating in a hydrophobic polymerizable monomer, a unit originating in a polymerizable monomer containing a basic nitrogen-containing part, and a unit originating in a polymerizable monomer containing a protonic neutral hydrophilic part.
Owner:ASAHI KASEI MEDICAL CO LTD
Absorbing pathogen-inactivating compounds with porous particles immobilized in a matrix
InactiveUS6951713B2Reduce risk of leakageReduce decreaseImmobilised enzymesOther blood circulation devicesWhole blood productSorbent
Methods and devices are provided for reducing the concentration of low molecular weight compounds in a biological composition, while substantially maintaining a desired biological activity of the biological composition. The device comprises highly porous adsorbent particles, and the adsorbent particles are immobilized by an inert matrix. The matrix containing the particles is contained in a housing, and the particles range in diameter from about 1 μm to about 200 μm. The matrix can be fibrous, and the particles can have a surface area greater than 750 m2 / g and a pore diameter between about 25 and 800 Å. The device can be used to adsorb and remove a pathogen-inactivating compound that is a nucleic acid-binding compound such as psoralen, an acridine derivative or a dye from a biological composition such as a blood product.
Owner:CERUS CORP
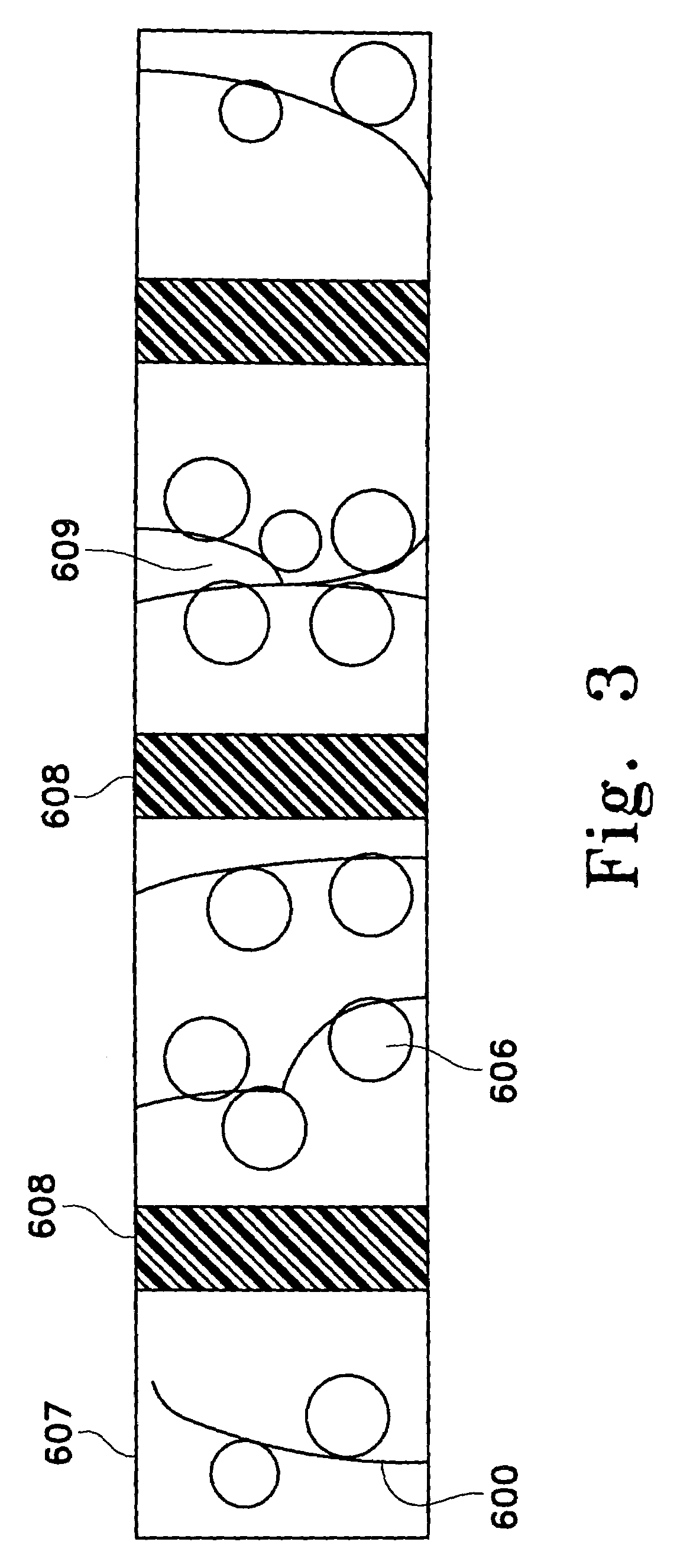
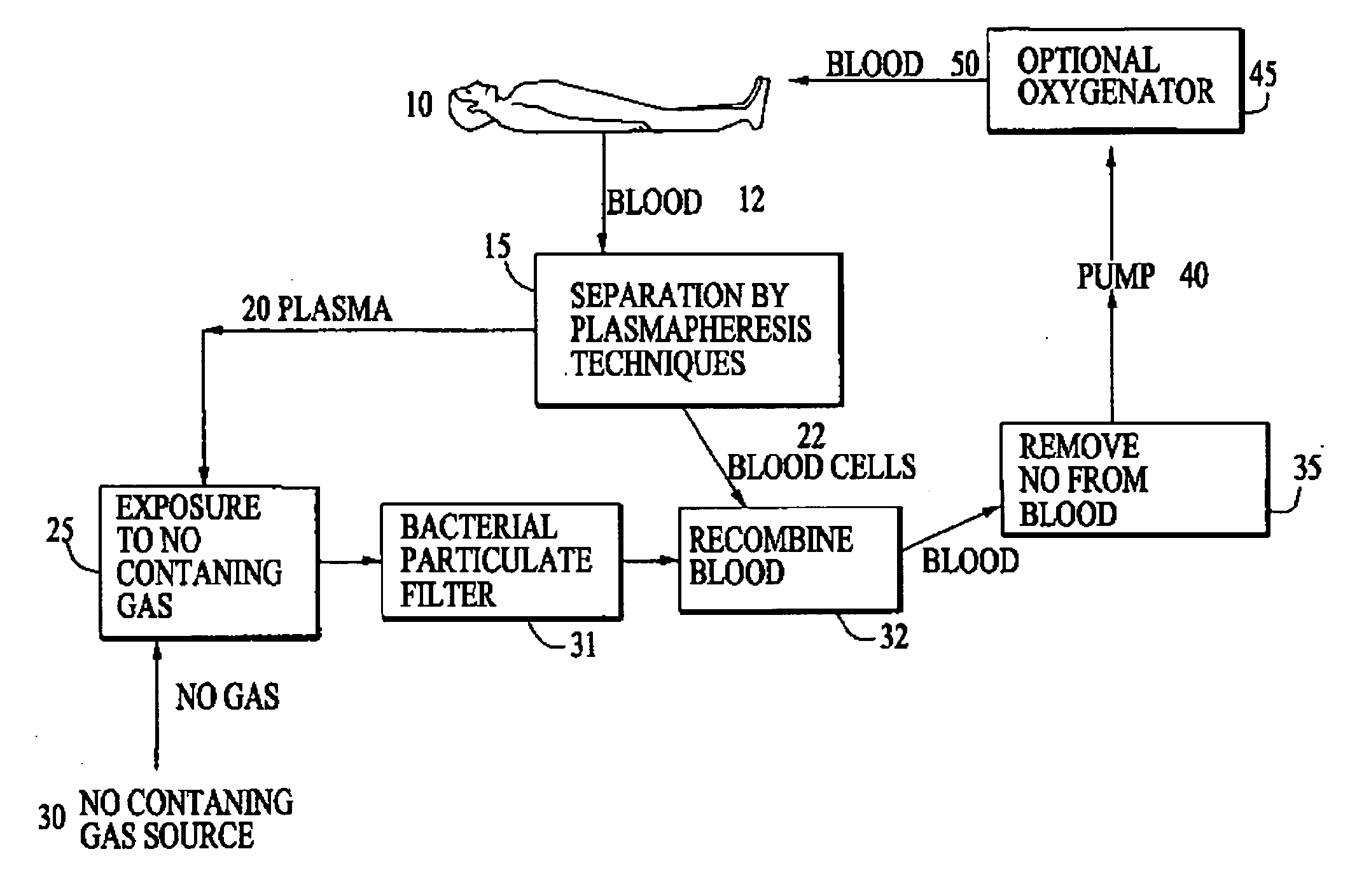
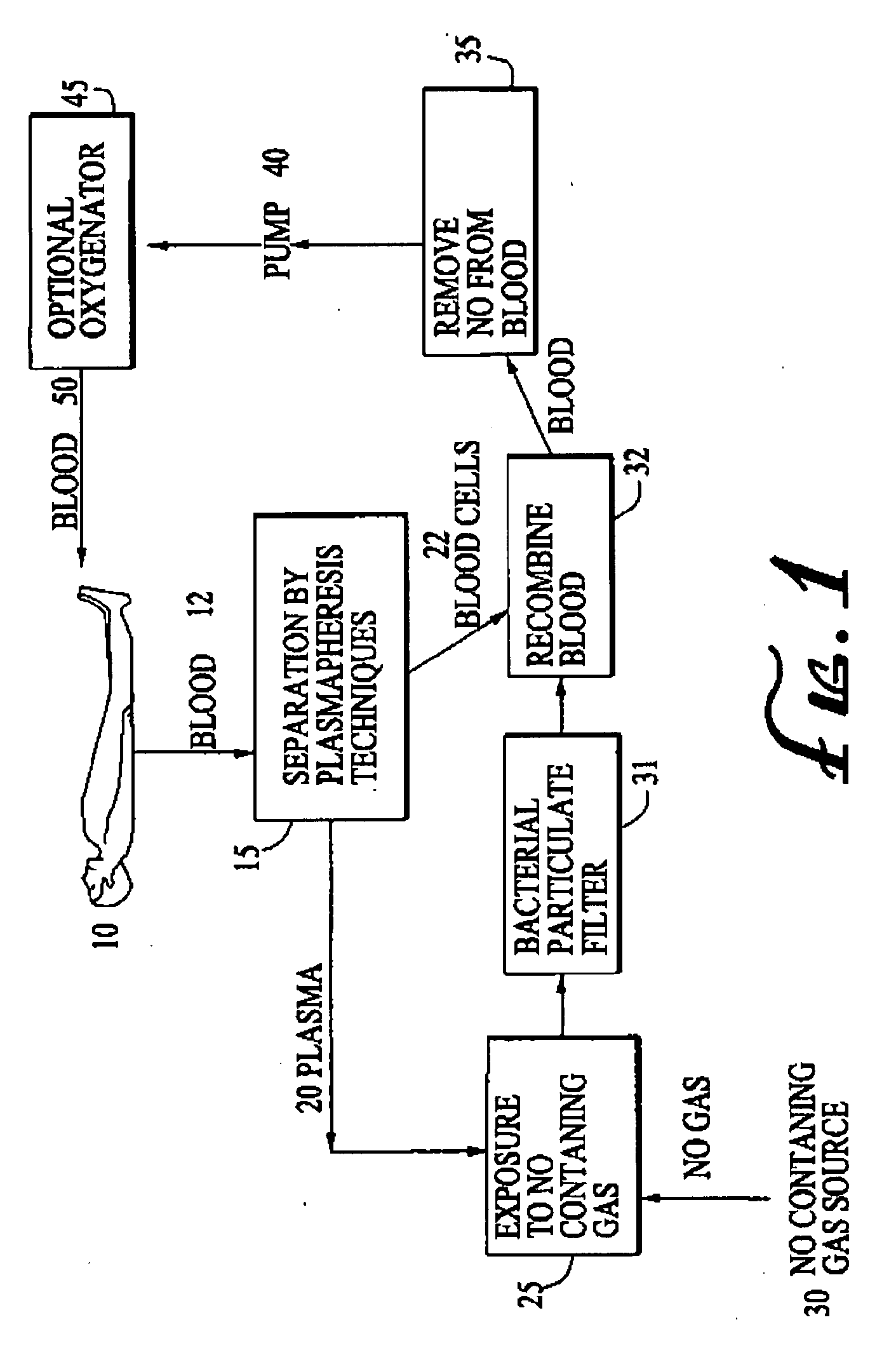
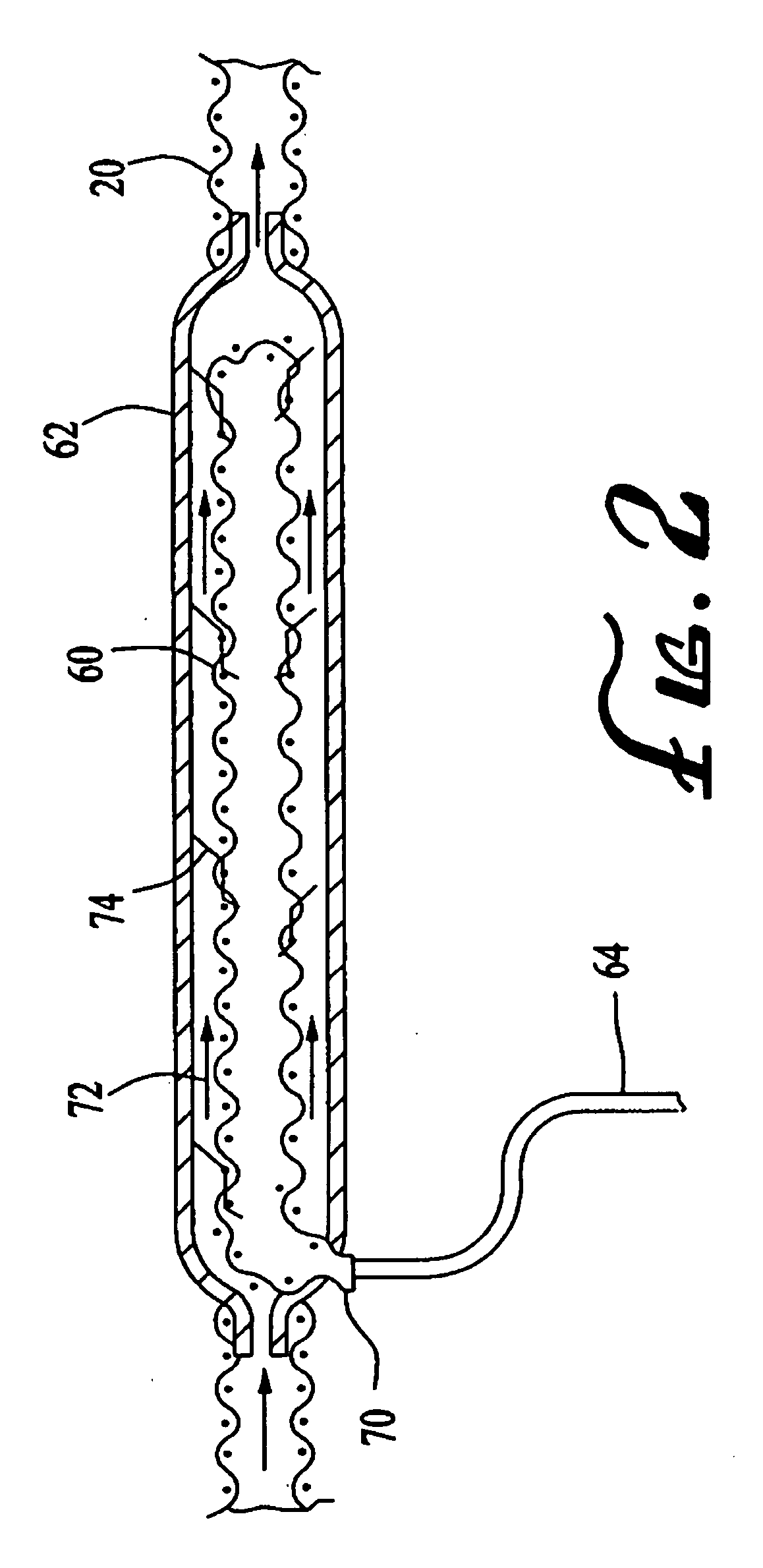
Use of nitric oxide gas to treat blood and blood products
InactiveUS20080160107A1Reduce inflammationReduce vascular resistanceBiocideInorganic active ingredientsWhole blood productProduct gas
The present invention relates to compositions and methods for treatment of blood and blood products using gaseous nitric oxide. The treatment involves the contacting blood or a blood product with gaseous nitric oxide.
Owner:NITRIC BIOTHERAPEUTICS INC +1
Method and apparatus for blood separations
InactiveUS20060226087A1Reduces direct collectionReduce processing costsWater/sewage treatment by centrifugal separationRotary centrifugesWhole blood productMedicine
Described herein is a method and apparatus for collecting and separating whole blood into its components, including collecting an amount of plasma. The collection and separation system includes a console and a disposable set. The method may include processing the blood through the centrifuge, collecting the plasma, and returning red blood cells remaining in the centrifuge to the source. The disposable set may include a manifold, a CFC, and various components attached by tubing. These components may include one or more solution bags, blood product bags, bacterial filters, leukofilters, and a donor blood collection tube with access needle.
Owner:TERUMO MEDICAL CORP
Absorbing pathogen-inactivating compounds with porous particles immobilized in a matrix
InactiveUS20050142542A1Reduce risk of leakageEasy to manufactureImmobilised enzymesOther blood circulation devicesWhole blood productSorbent
Methods and devices are provided for reducing the concentration of low molecular weight compounds in a biological composition, while substantially maintaining a desired biological activity of the biological composition. The device comprises highly porous adsorbent particles, and the adsorbent particles are immobilized by an inert matrix. The matrix containing the particles is contained in a housing, and the particles range in diameter from about 1 μm to about 200 μm. The matrix can be fibrous, and the particles can have a surface area greater than 750 m2 / g and a pore diameter between about 25 and 800 Å. The device can be used to adsorb and remove a pathogen-inactivating compound that is a nucleic acid-binding compound such as psoralen, an acridine derivative or a dye from a biological composition such as a blood product.
Owner:CERUS CORP
Device for treatment of sample of blood products
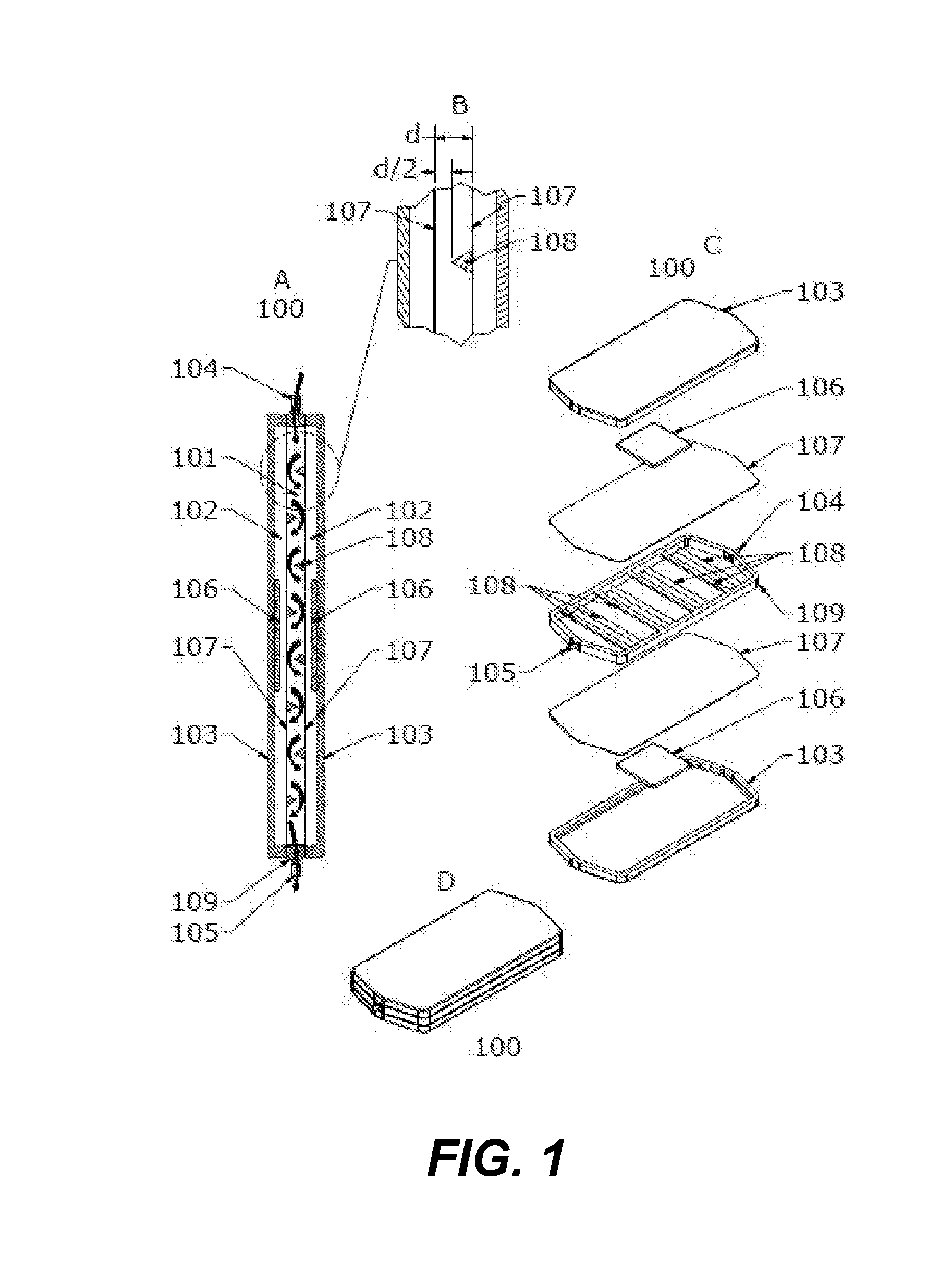
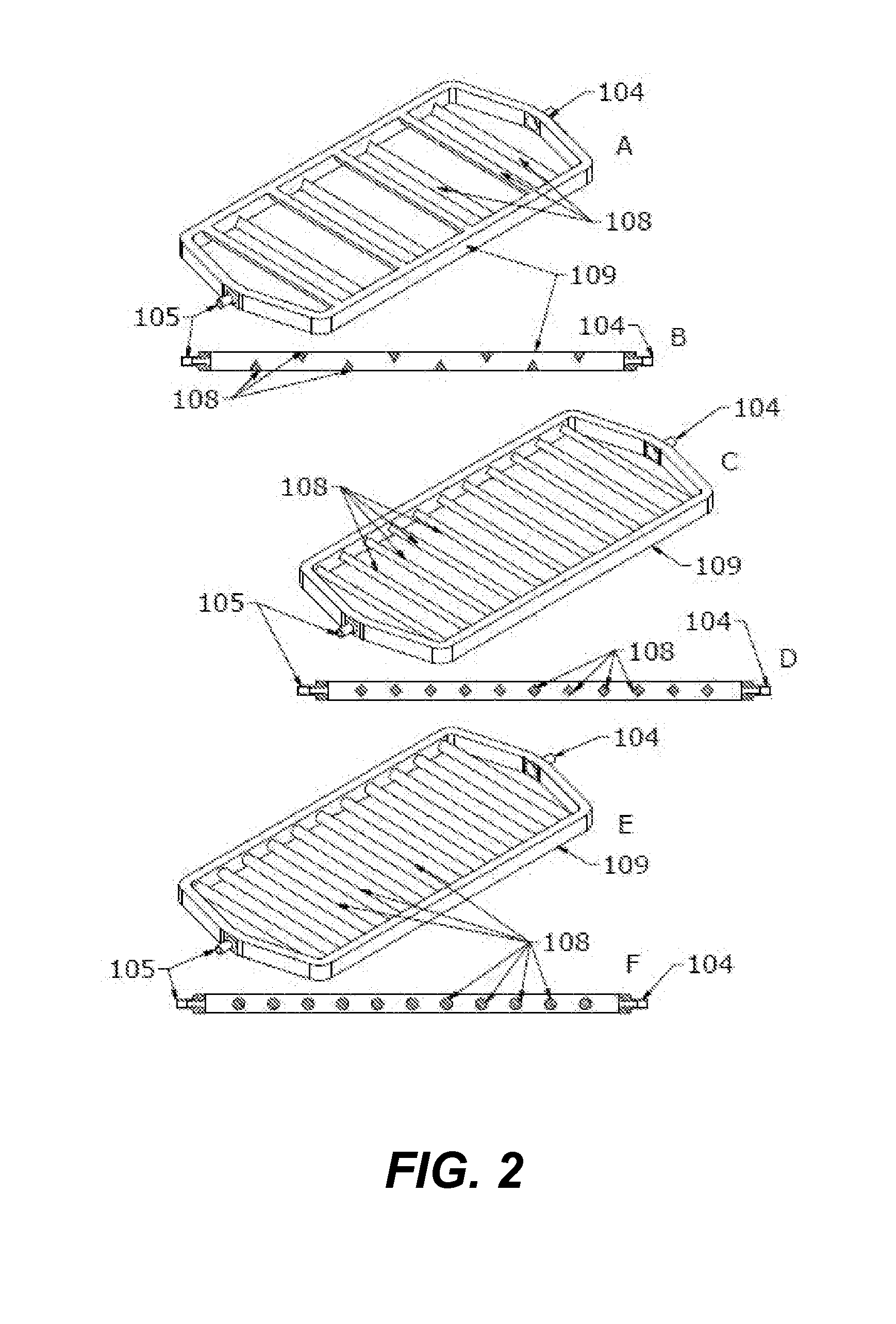
InactiveCN1334453AEasy to put inSmall sizeShaking/oscillating/vibrating mixersSamplingWhole blood productEngineering
This device for processing a blood product sample included in tubes (18) loaded in cassettes (12) and closed with plugs. The moving means 10 is designed to individually move the cassettes on a predetermined path. The agitating means 30 has at least one pickup mechanism (32) that can be started by a driving means (88), and picks up at least one selected tube from the immobile cassette on the path, moves the tube far from the cassette, agitates the tube and returns it to the cassette. The sampling means (34) is designed to take away a predefined sample quantity from the pre- agitated tube returned to the cassette and applicable to a blood analysis.
Owner:ABX SA
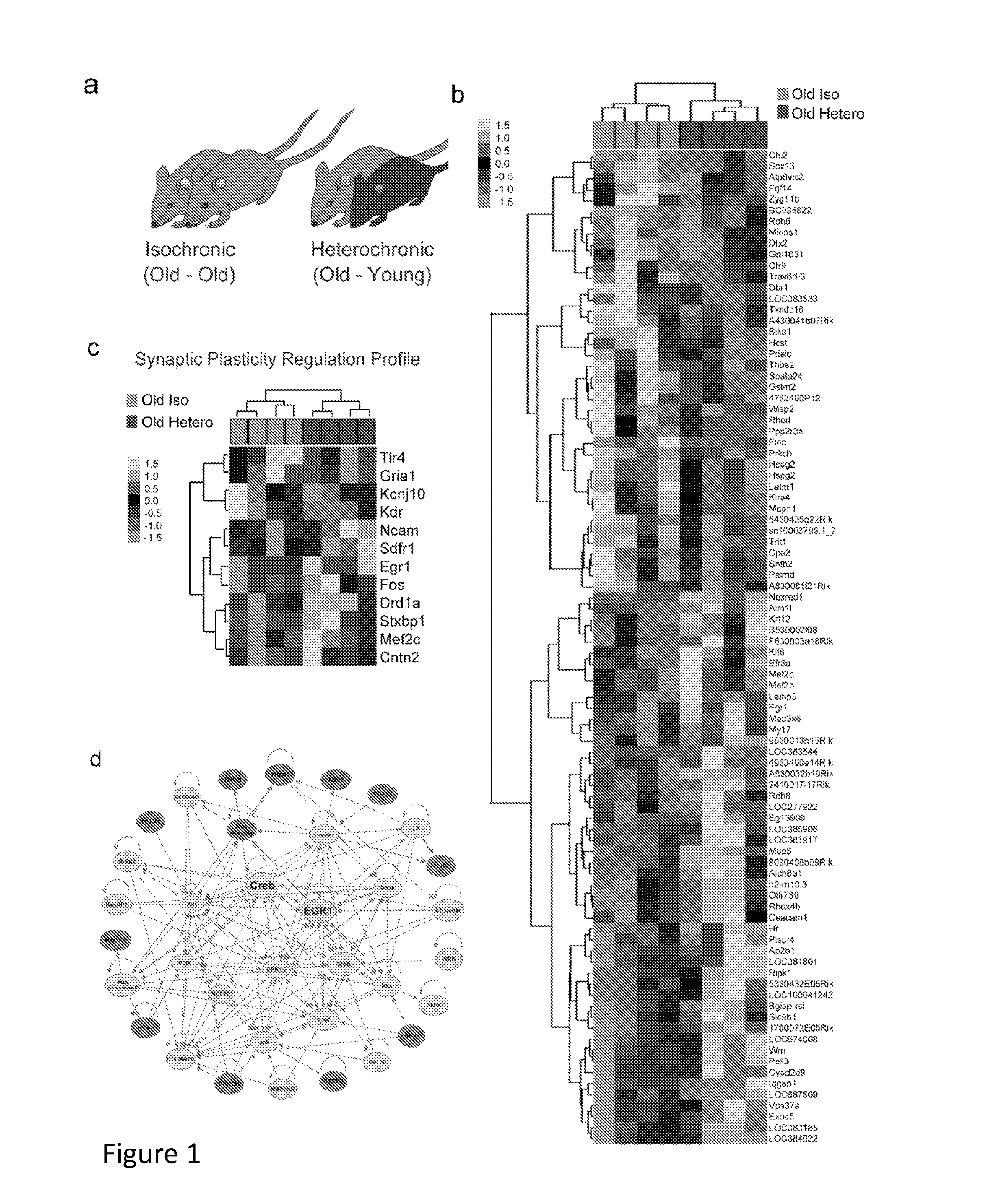
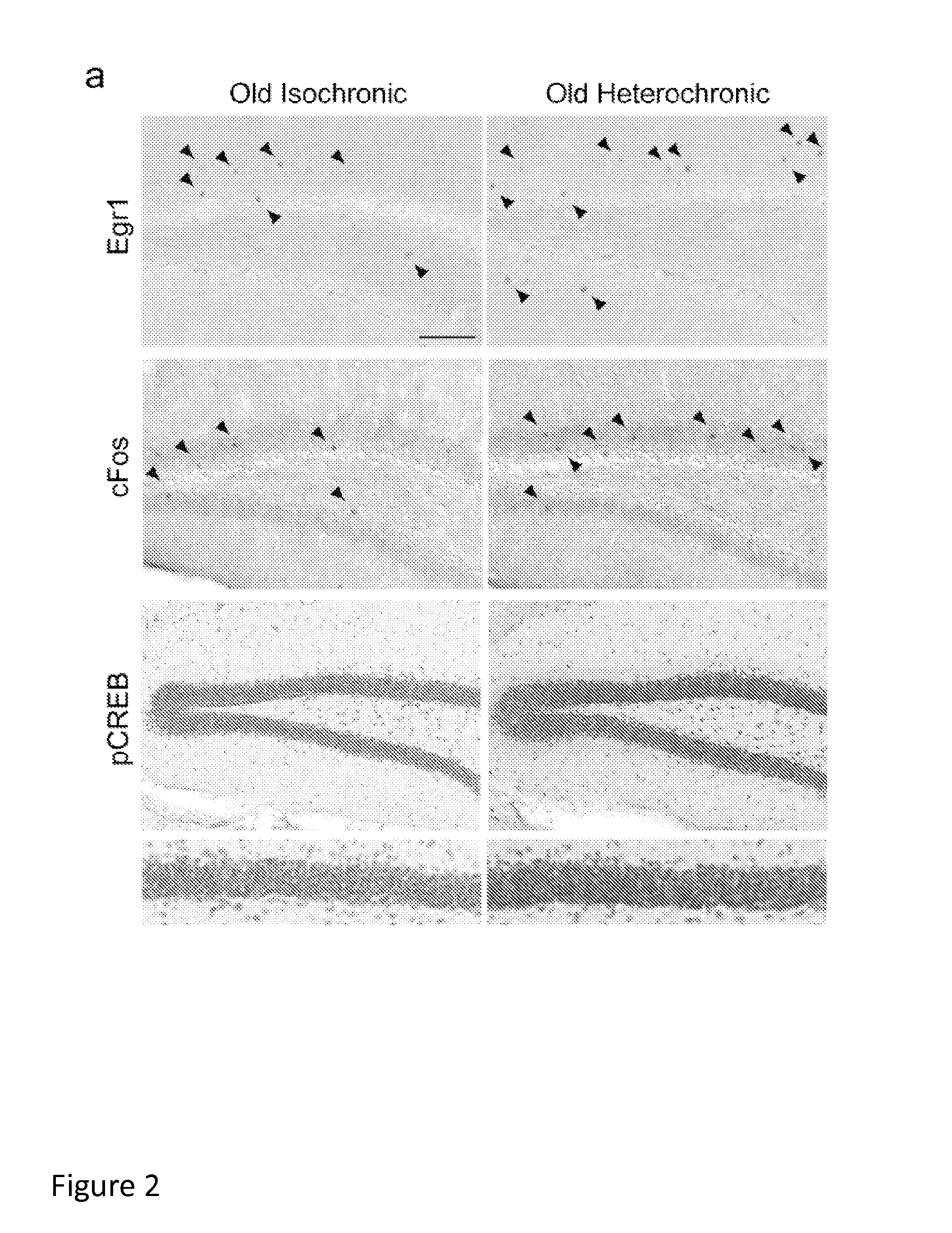
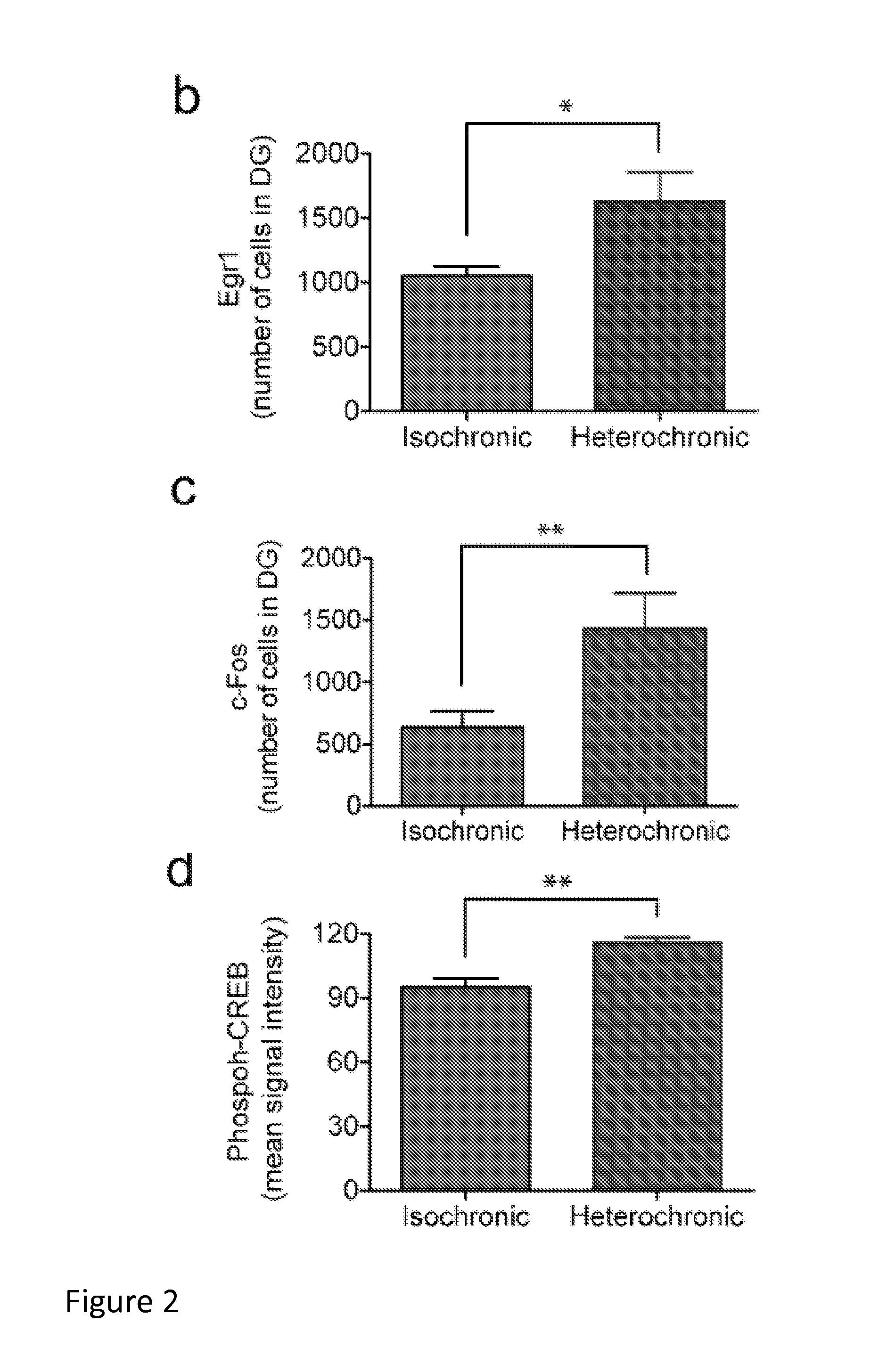
Methods and compositions for treating aging-associated conditions
Methods and compositions are provided for treating a subject for aging-associated conditions, e.g., cognitive impairment conditions. Aspects of the methods include administering a young plasma-comprising blood product to an individual in need thereof, e.g., an individual suffering from or at risk of developing the aging-associated condition, e.g., aging-associated cognitive impairment. Also provided are compositions and kits thereof that find use in practicing methods of the invention.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Methods and apparatus for separation of particles
InactiveUS7201848B2Easily debulkedFacilitate fractionationOther blood circulation devicesRotary centrifugesWhole blood productWhite blood cell
The present invention is directed to a method and system that separates first particles from second particles, or white blood cells from red blood cells, by sedimentation in a fluid chamber with debulking of one of the first or second particles or red blood cells through the inlet of the fluid chamber. The method and system further includes fractionation of the remaining particles or white blood cells into selected subsets. In one embodiment of the instant invention a blood product containing white blood cells is loaded in a separation chamber, a diluting or sedimenting agent is added to encourage rouleaux formation of any red blood cells, the cells are sedimented and the red blood cells are removed.
Owner:TERUMO BCT
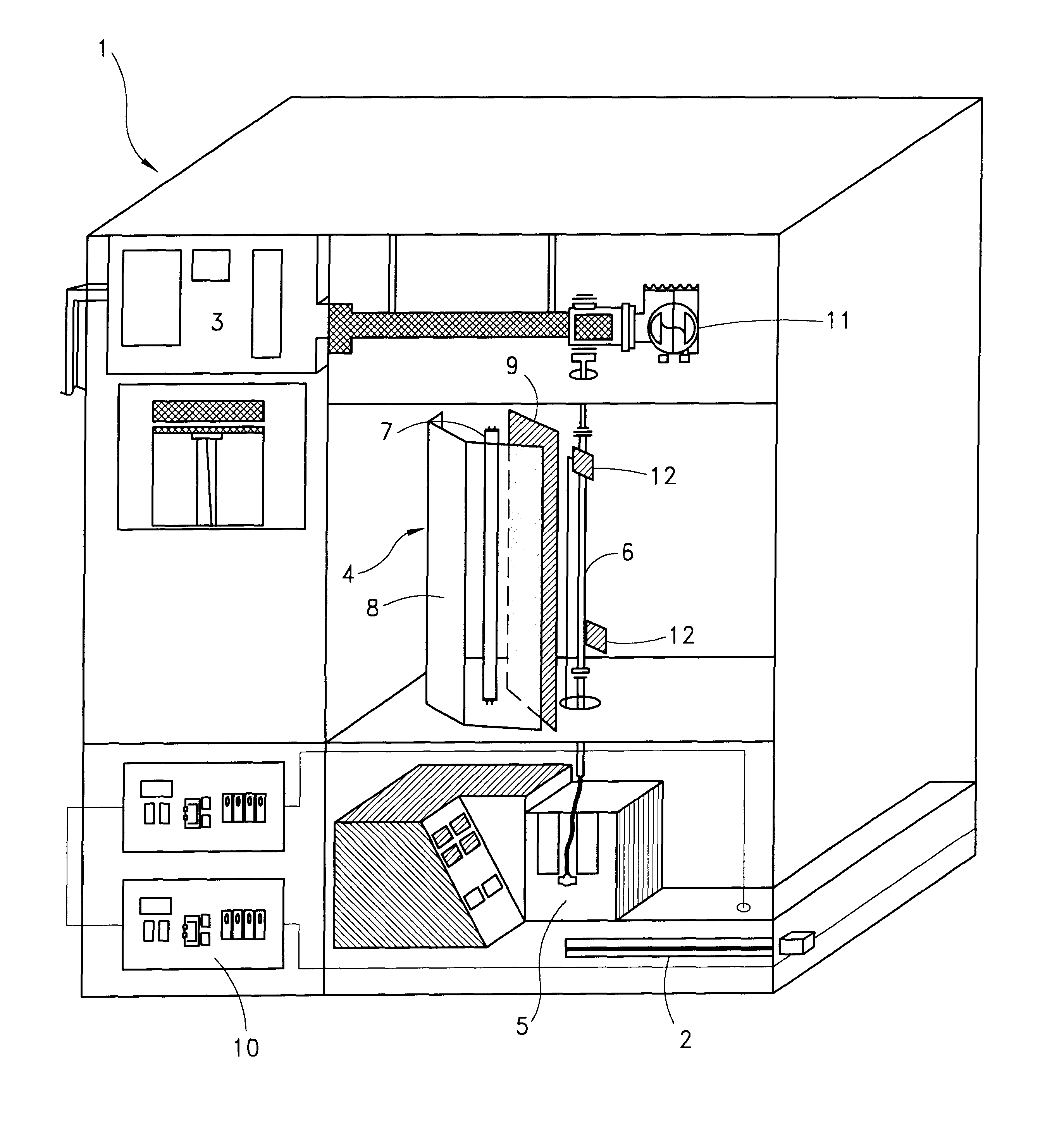
Method and apparatus for inactivating contaminants in blood products
InactiveUS6190608B1Structure is often disruptedAvoid disruptionOther blood circulation devicesMedical devicesPotential toxicityWhole blood product
A method and apparatus for inactivating contaminants, particularly nonenveloped viruses, in blood products without the need for quenchers in the blood products. The blood product is flowed through a flow meter at a controlled flow rate and subjected to type C ultraviolet radiation, where the irradiation dose received by the blood product is less than 640 joules / m2. The blood product does not contain quenchers when being irradiated, and the blood product retains more than 85% of its Factor VIII activity after being subjected to the radiation. The produced blood product is free of viruses and quenchers, avoiding potential toxicity due to the quenchers. The apparatus includes a source of UVC light, a quartz tube containing the blood product while it is exposed to the UVC light, a flow meter for controlling the flow rate of the blood product to be treated, and a pump. The UV light preferably emits light at 254 nm.
Owner:SARTORIUS STEDIM BIOTECH GMBH
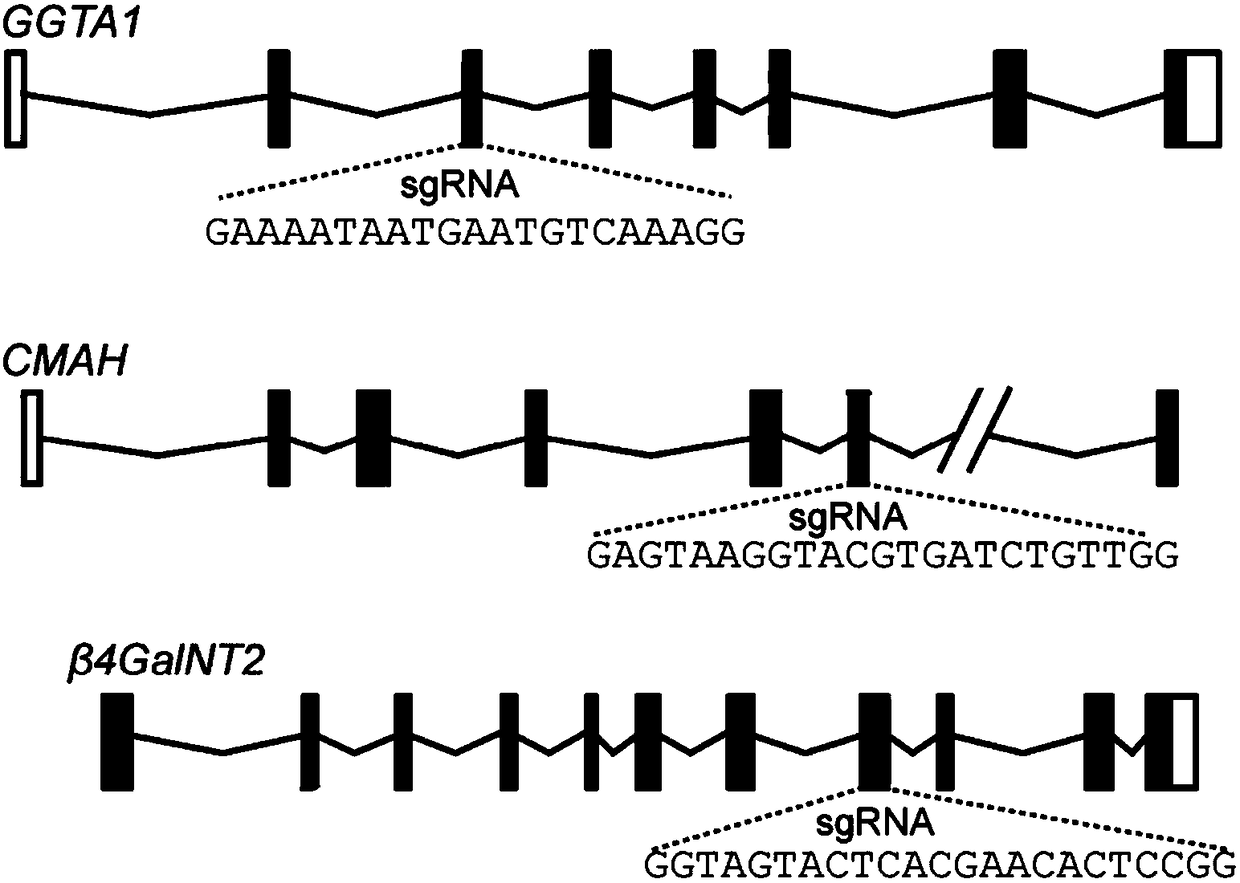
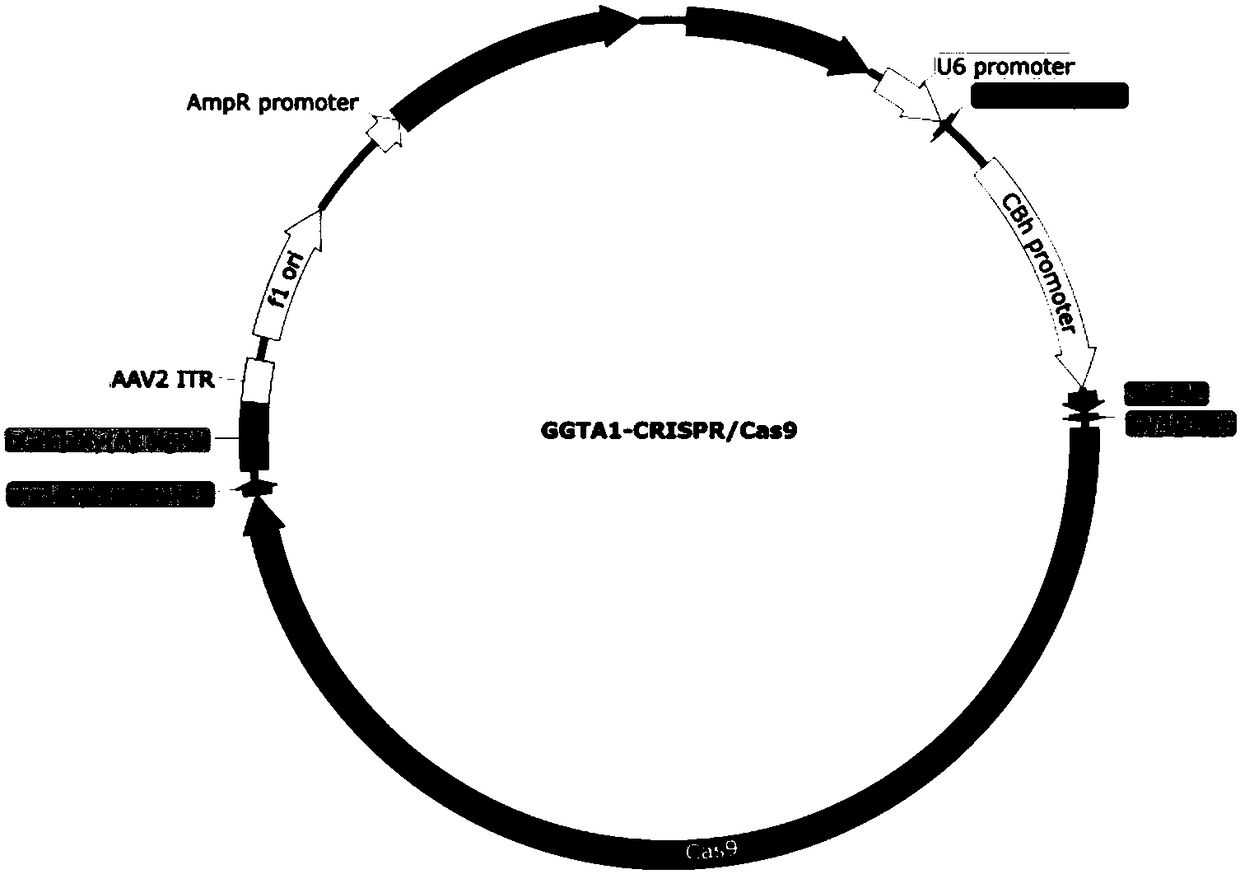
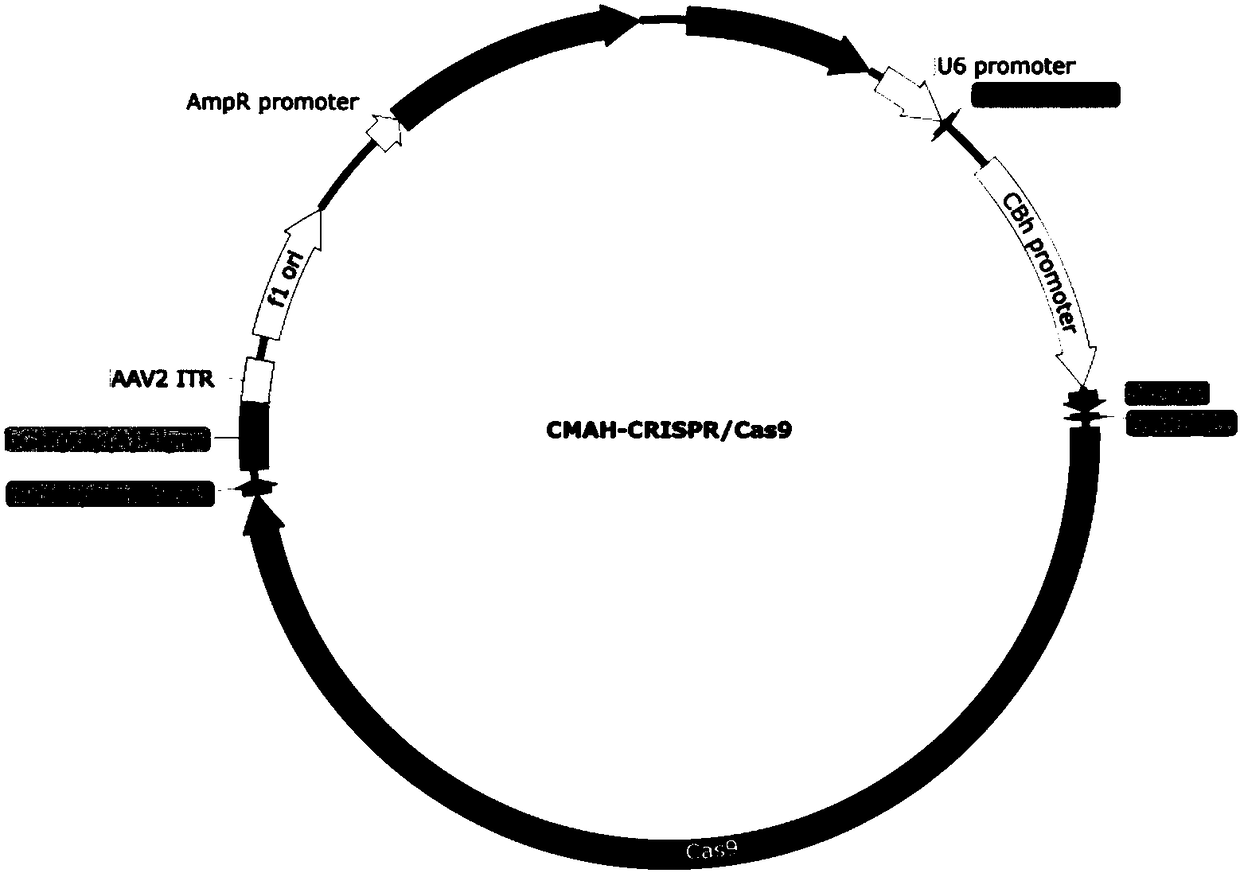
Application of CRISPR/Cas9 carrier combination in preparation of blood product of gene knockout pig
PendingCN108588123AReduce the binding forceSolve the problem of ischemiaHydrolasesStable introduction of DNAWhole blood productRed Cell
The invention discloses the application of a CRISPR / Cas9 carrier combination in preparation of a blood product of a gene knockout pig. The gene knockout pig is a pig without GGTA1 genes, CMAH genes and beta4Ga1NT2 genes. The CRISPR / Cas9 carrier combination comprises a GGTA1-CRISPR / Cas9 carrier, a CMAH-CRISPR / Cas9 carrier and a beta4Ga1NT2-CRISPR / Cas9. By designing a specifically targeted SgRNA sequence, the knockout efficiency of the three genes is respectively 56 percent, 63 percent and 41 percent; after the three genes relating to immunological rejection are knocked out, the gene knockout pig is obtained; the combination between erythrocytes and immune globulin in human serum is obviously reduced; an outstanding effect is achieved to overcome hyperacute immunological rejection; the problem of clinical ischemia is effectively solved; a precious material source is provided for clinical blood transfusion.
Owner:GCREATENE SUZHOU BIOTECH CO LTD
Fluorescent detector systems for the detection of chemical perturbations in sterile storage devices
ActiveUS20070251337A1Analysis using chemical indicatorsChemical analysis using titrationWhole blood productFluorescence
System and method for detecting and measuring chemical perturbations in a sample. The system and method are useful for non-invasive pH monitoring of blood or blood products sealed in storage bags.
Owner:BLOOD CELL STORAGE
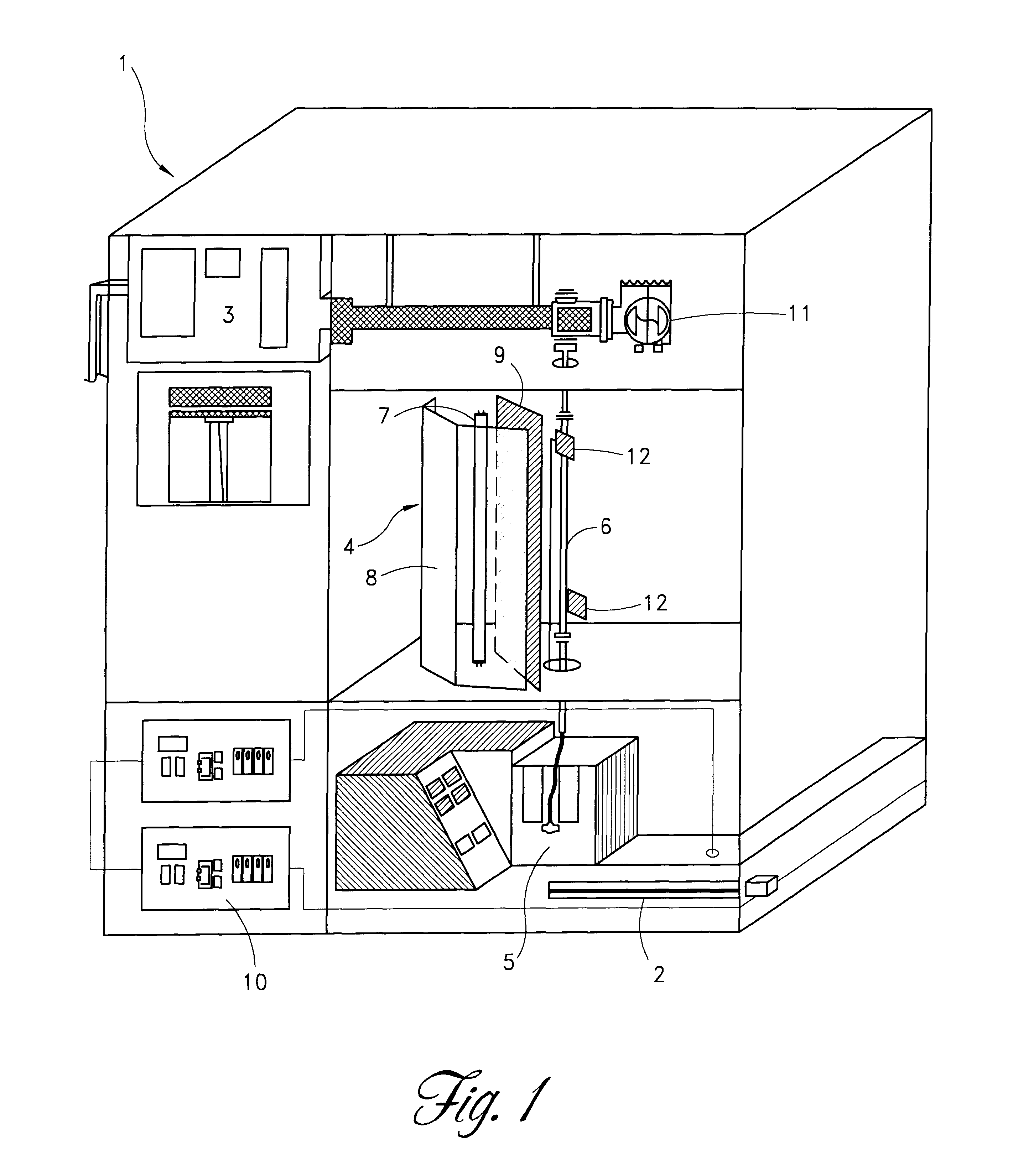
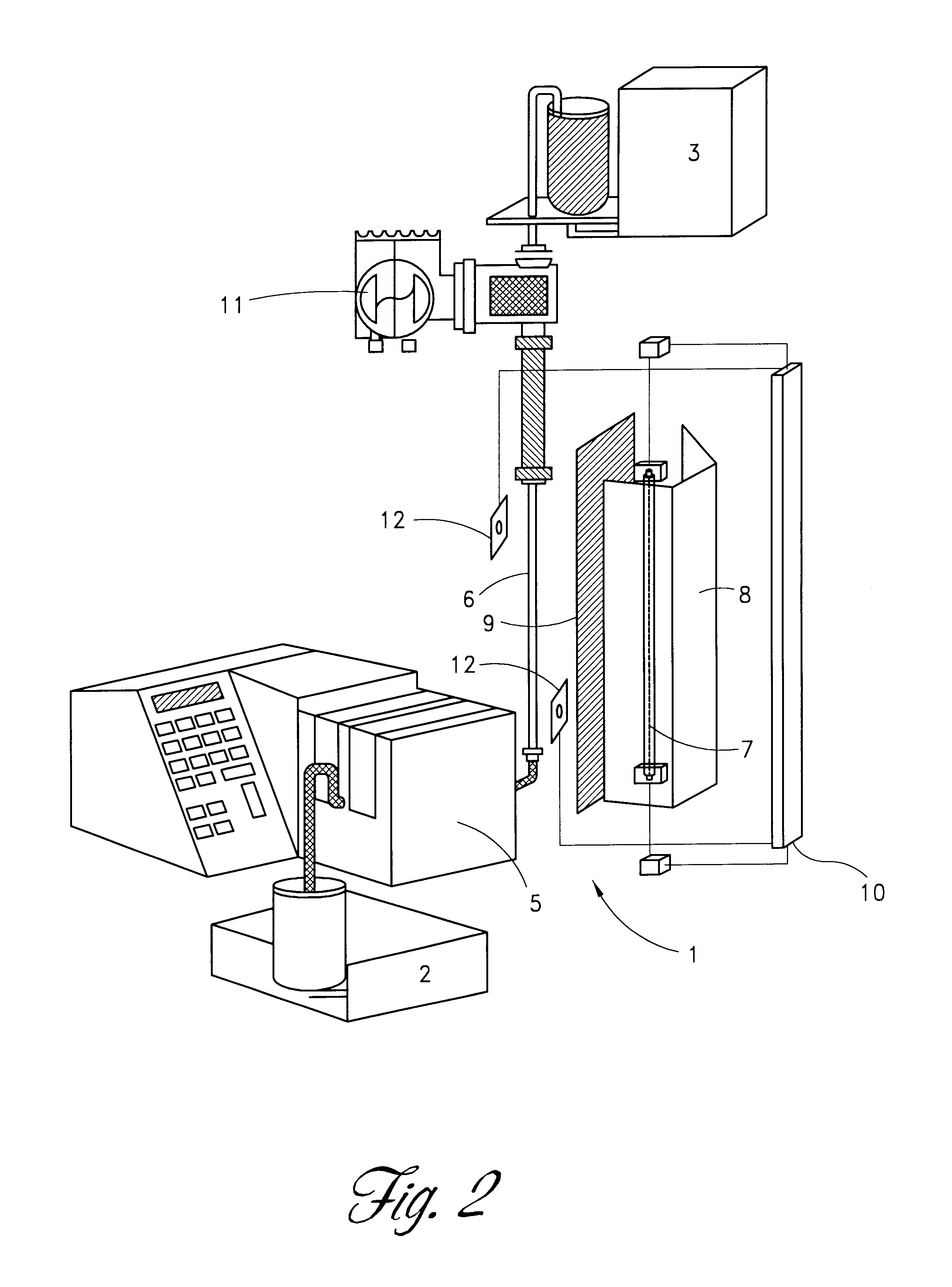
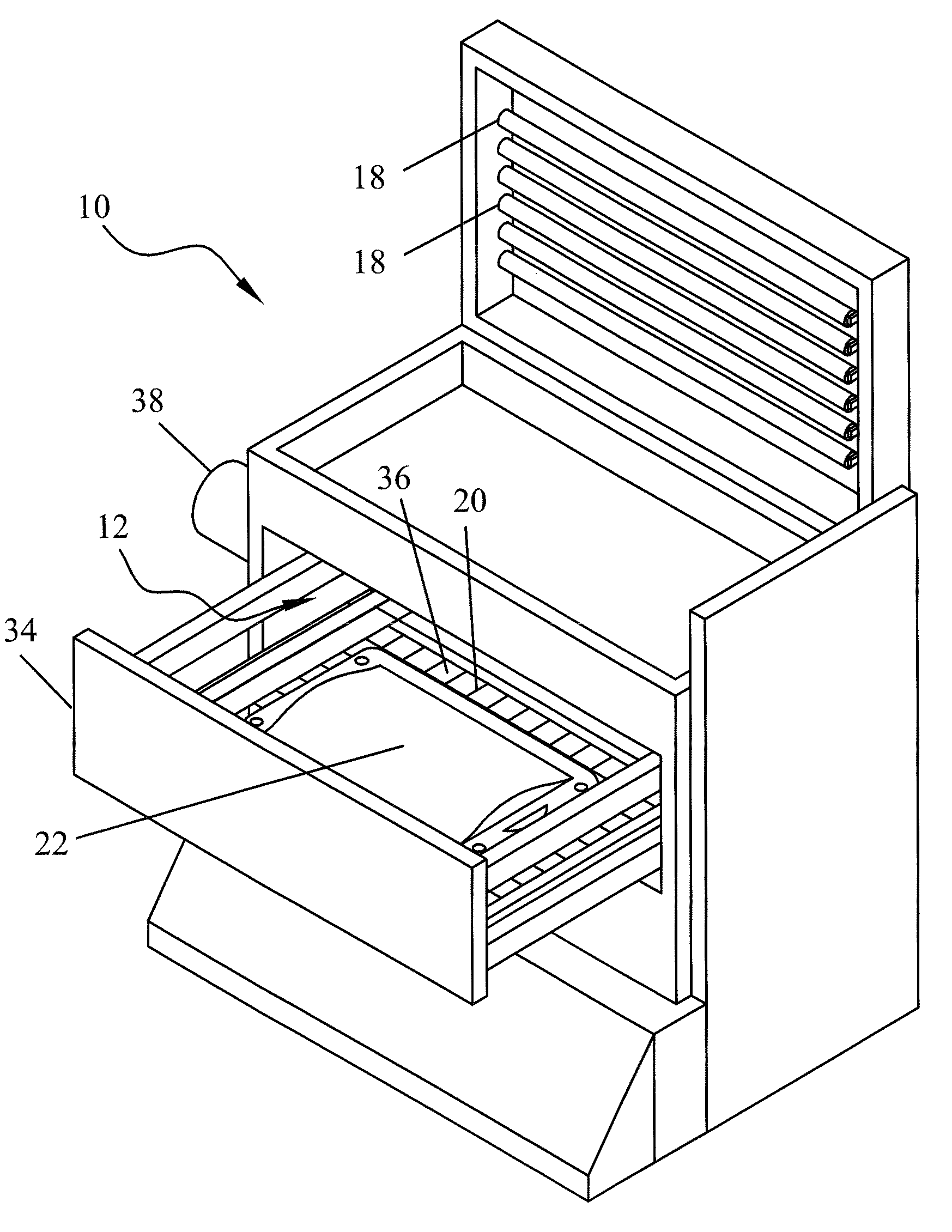
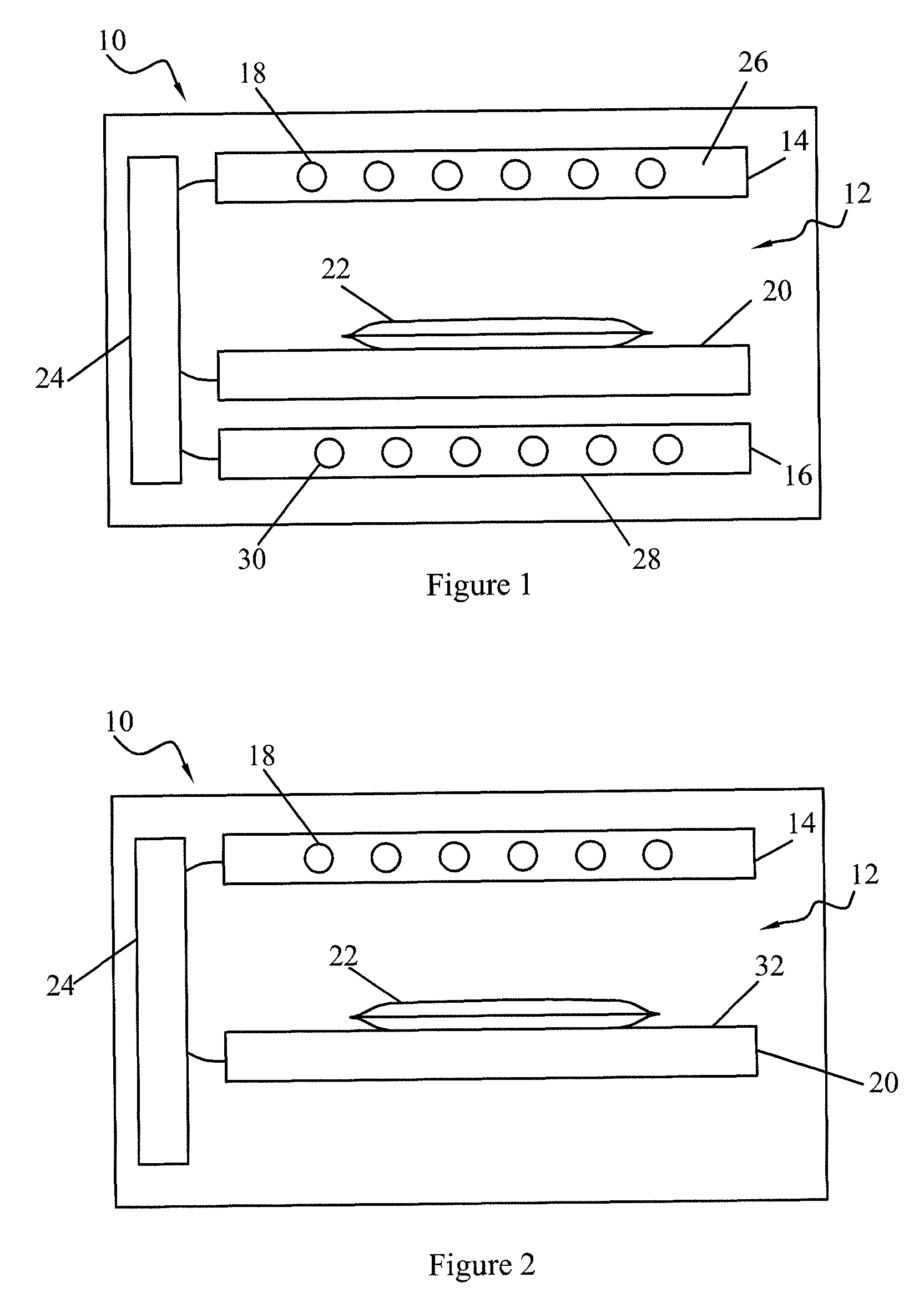
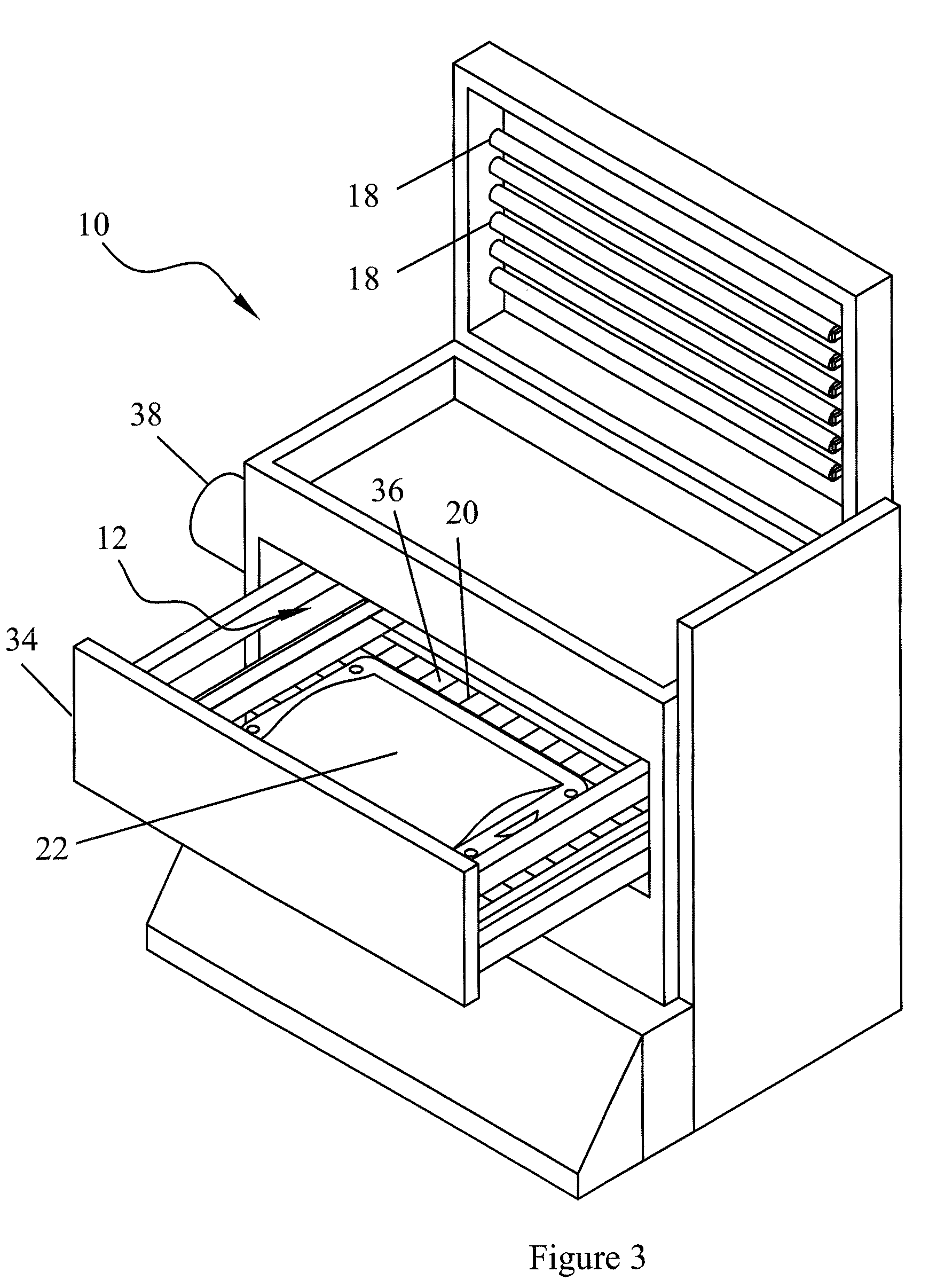
Apparatus for photo reduction of contaminants in blood and blood products with calibration means
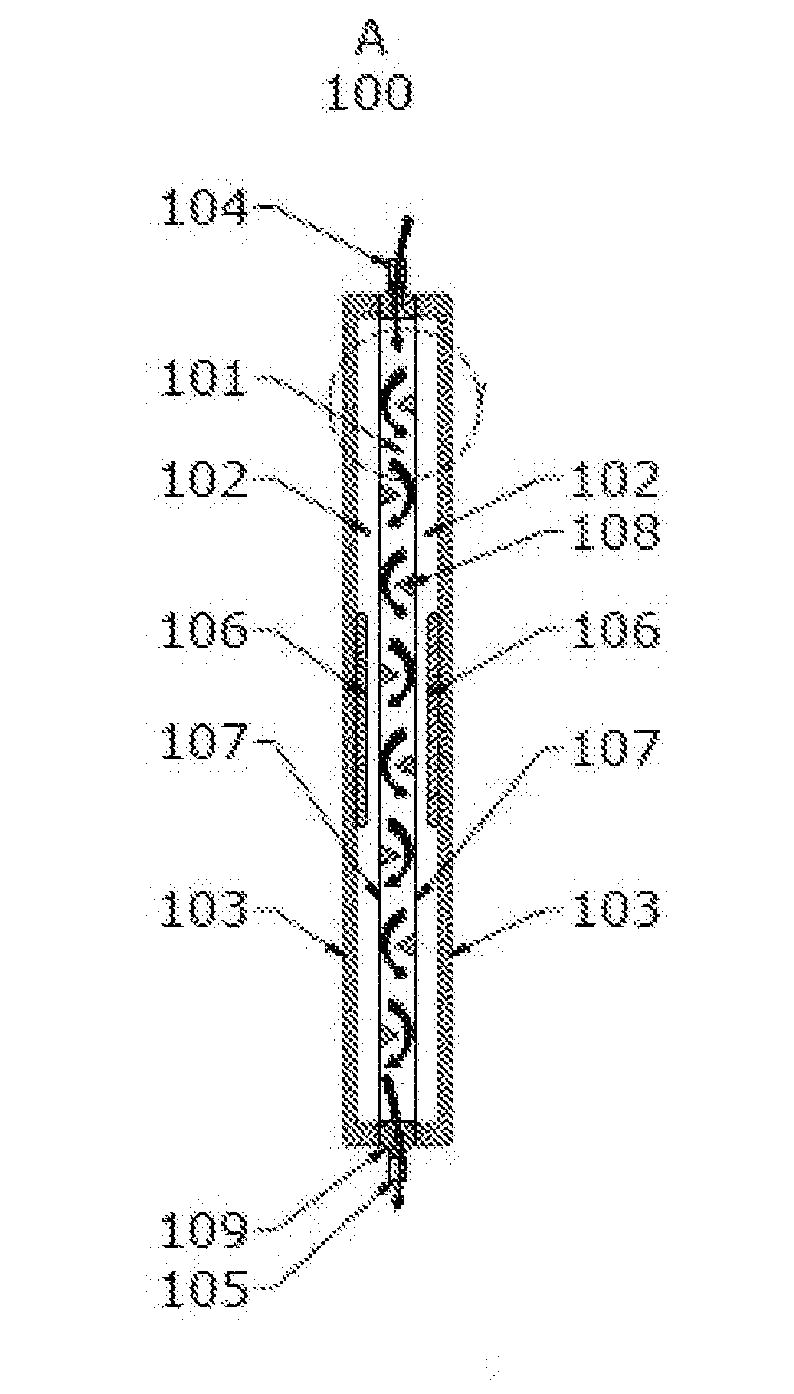
ActiveUS7829867B2Easy to controlMaterial analysis using wave/particle radiationElectric discharge tubesWhole blood productRadiometer
Owner:TERUMO BCT BIOTECH
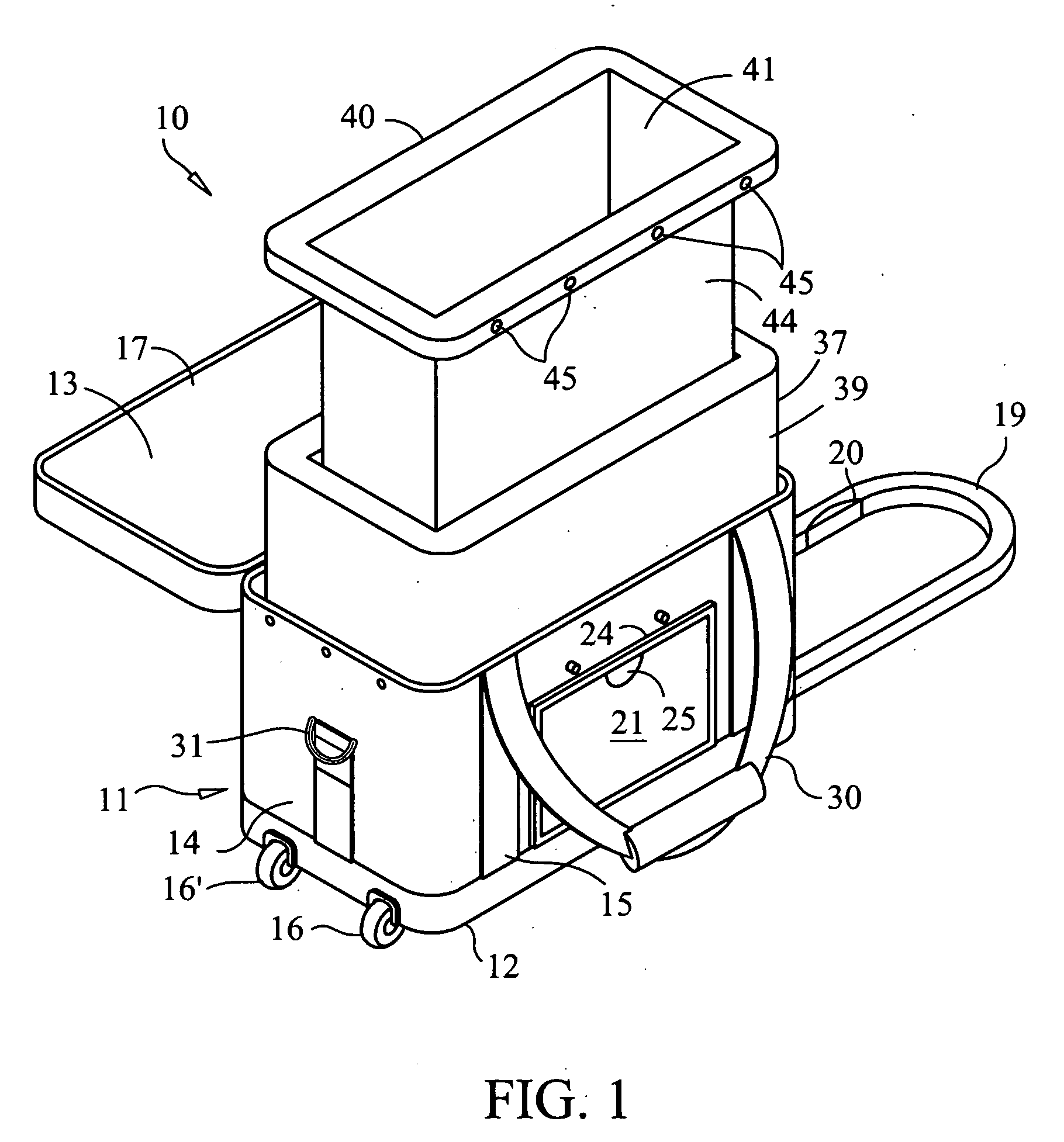
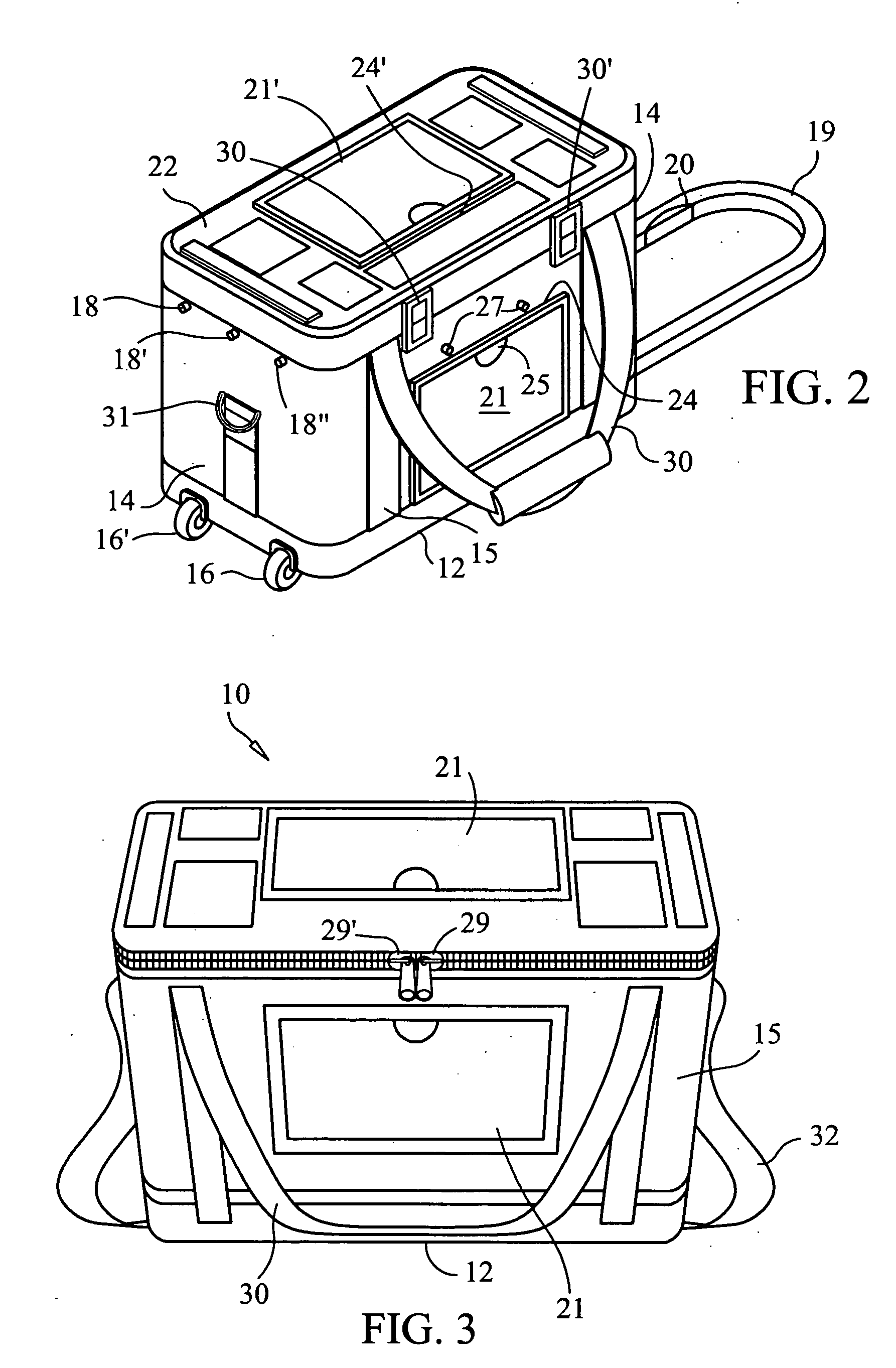
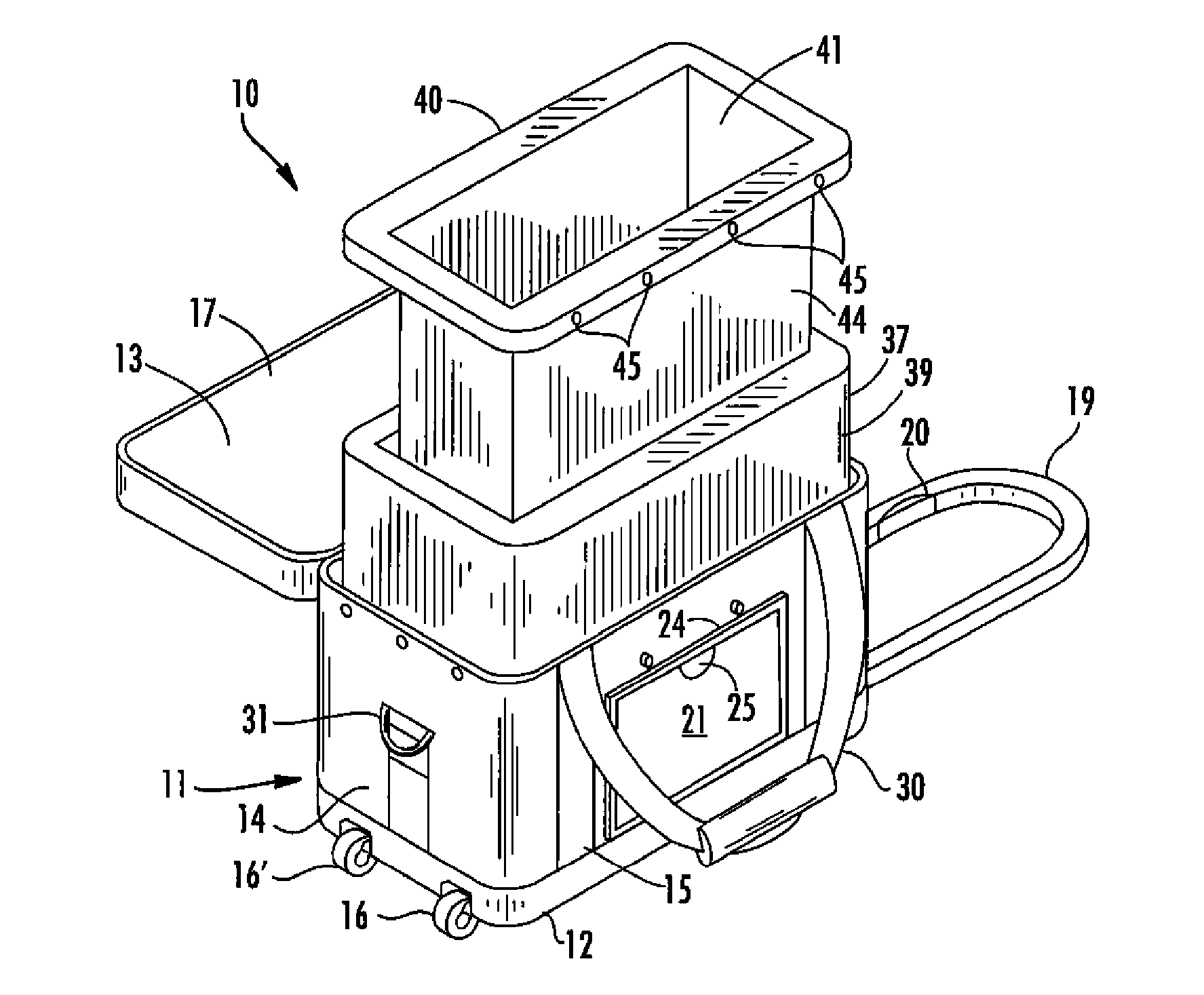
Container for transporting blood and blood products
A reusable container for transporting blood and blood products, which includes an outer case including a lid, and an insulating layer substantially inside the outer case and the lid. A generally rigid water-resistant well has a lip which abuts walls of the outer case so as to substantially seal the insulated layer to the outer case. At least one cooling element is provided, which is capable of maintaining temperature within a prescribed temperature range for predetermined periods of time, the cooling element being disposed within the well and at least partially surrounding a receptacle area. A caddy is located in the receptacle area, at least partially surrounded by the cooling element, and is designed to accommodate at least one unit of blood or blood products. The cooling element may include phase change gel sealed within a plastic surround, and is designed to be separately removable and freezable prior to use.
Owner:INT THERMAL WIZARDS
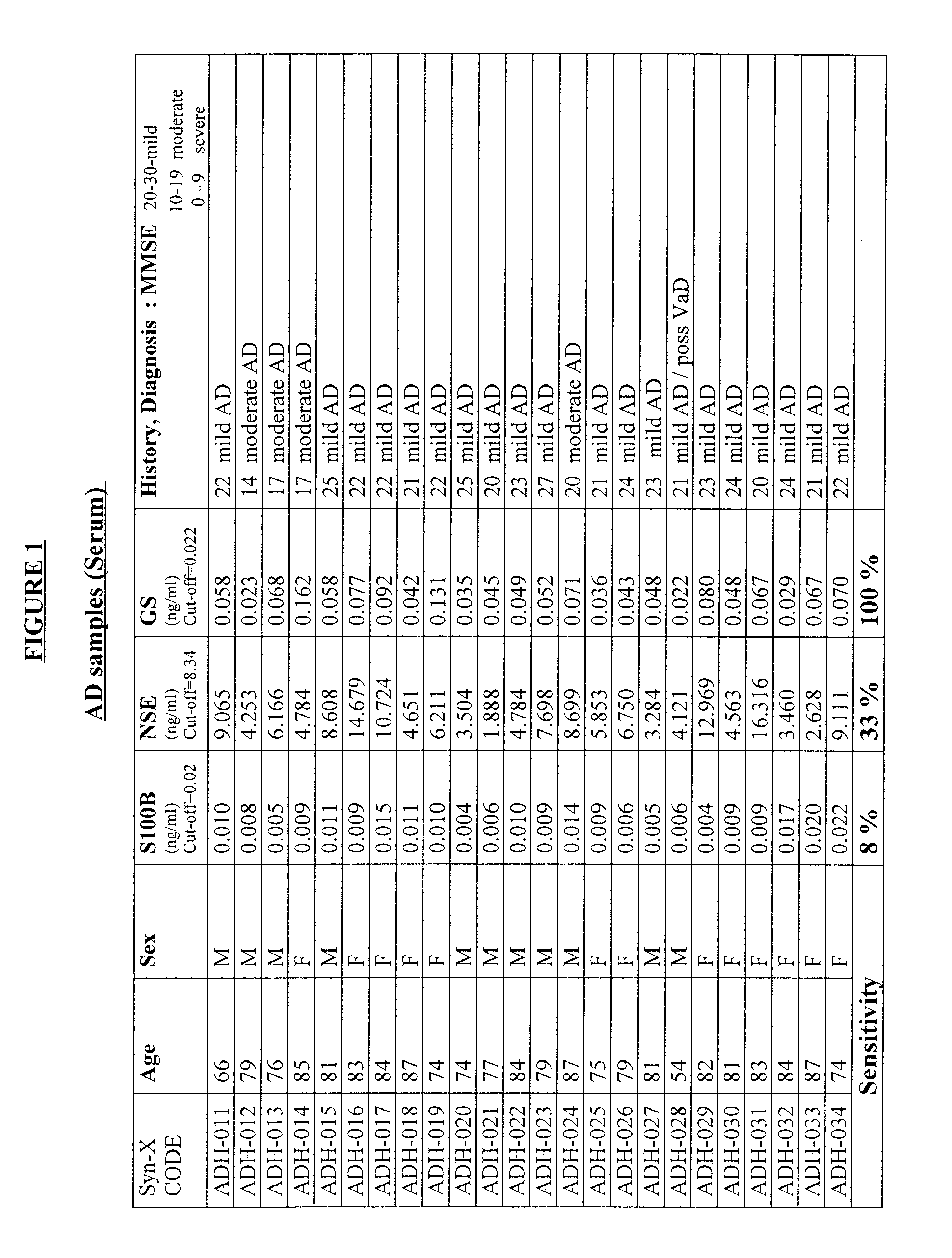
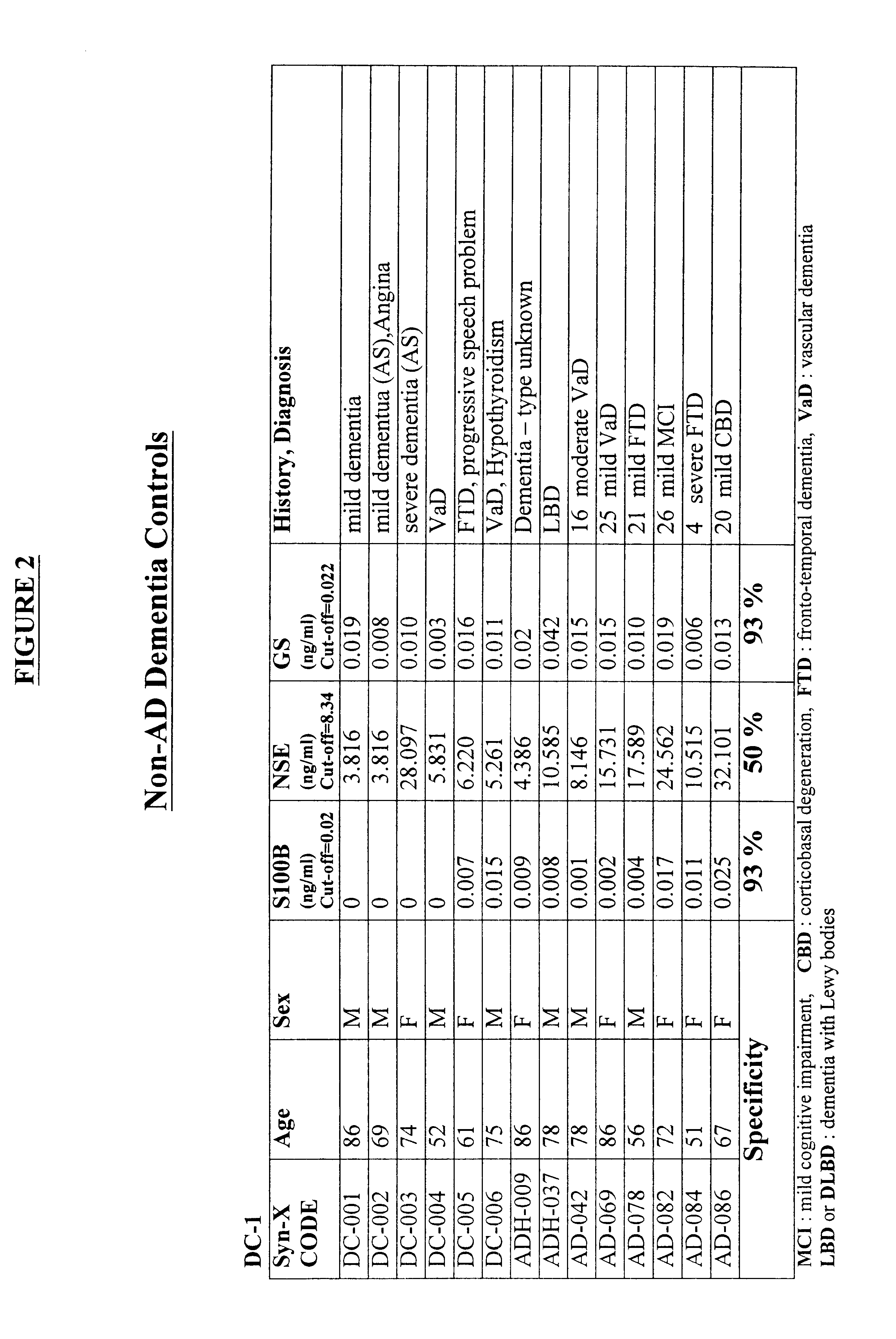
Process for differential diagnosis of Alzheimer's dementia and device therefor
InactiveUS6451547B1Efficient identificationImmunoglobulins against animals/humansDisease diagnosisWhole blood productBiochemical markers
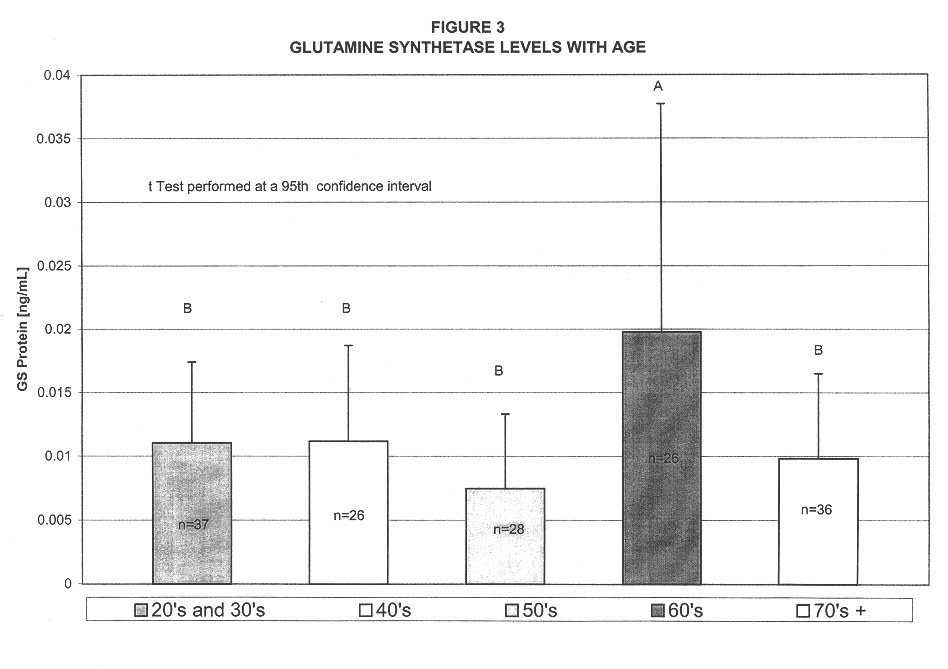
A method for diagnosing Alzheimer's disease(AD) is disclosed. The method involves directly detecting the presence of a biochemical marker, specifically human glutamine synthetase, in bodily fluid, preferably blood or a blood product. The detection is by an immunoassay incorporating an antibody specific to human glutamine synthetase. In addition, a method for distinguishing between AD and non-AD dementia is disclosed.
Owner:NANOGEN INC
Calibrator material for instruments which measure interferents in serum and plasma specimens
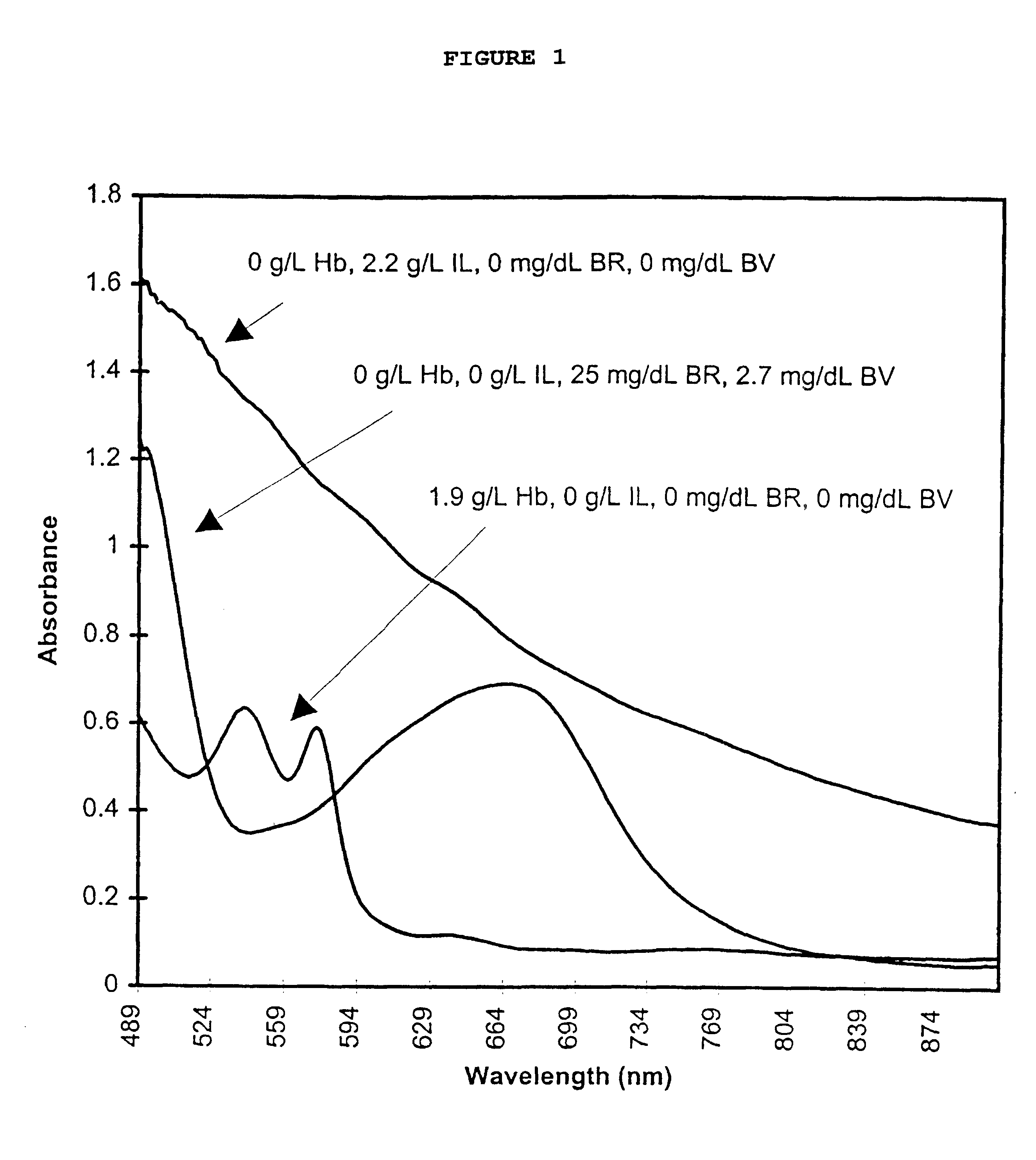
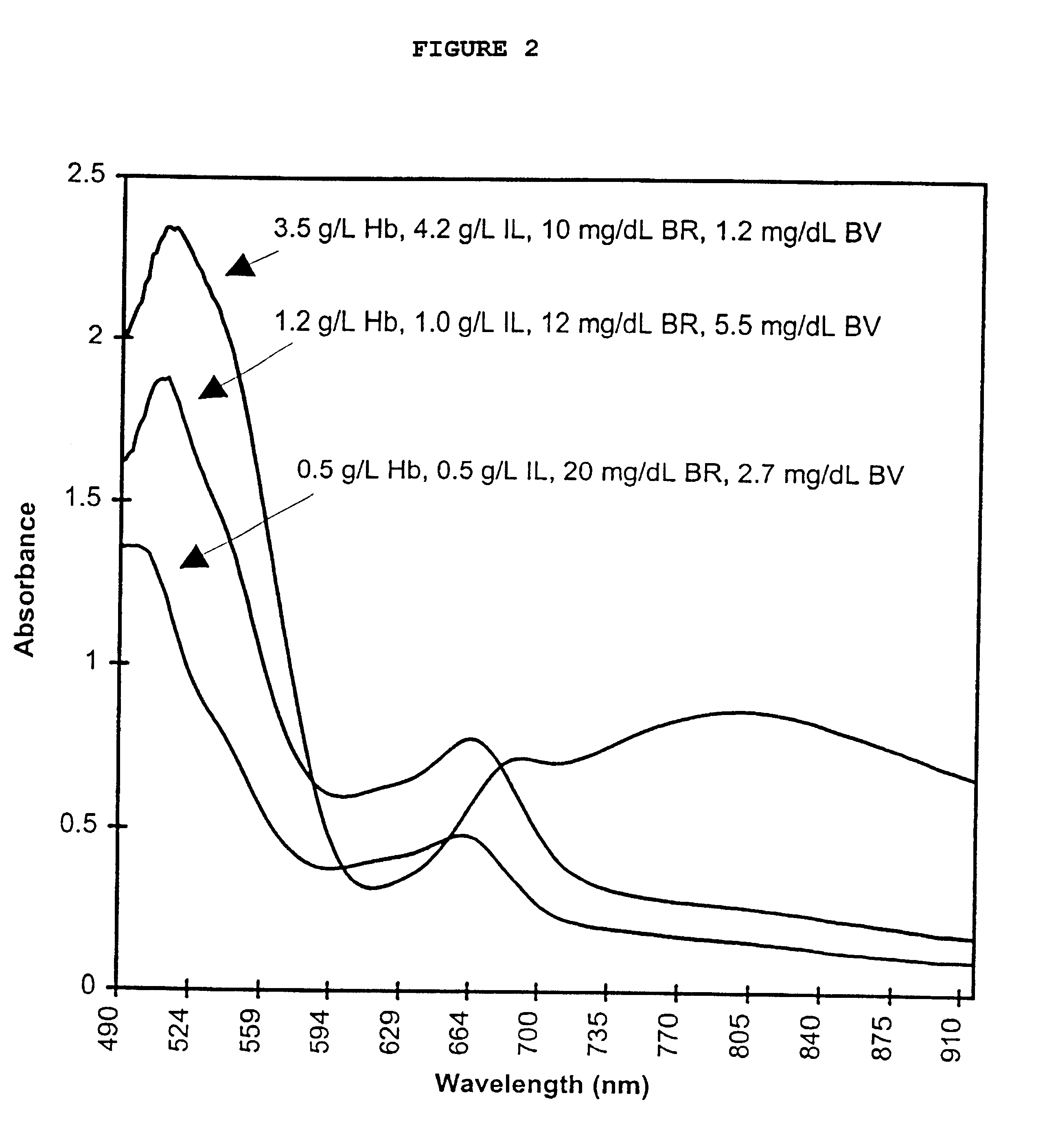
InactiveUS6372503B1Increase absorbanceLeft outBiological testingSpecial data processing applicationsBILIRUBINAEMIAHemolysis
A quality control material is disclosed which is used to monitor the calibration or used for recalibration of instruments used to screen for interferents in serum or plasma specimens. In particular, the quality control material disclosed is used to monitor instrument calibrations or used for recalibration for instruments which assess the amount of hemolysis, turbidity, bilirubinemia and biliverdinemia, either separately, or any two, or any three, or all four simultaneously, in plasma or serum samples. The quality control material does not contain any blood products such as plasma lipids, bile pigments, or hemoglobin, is stable at room temperature, and is ready for use with up to four constituents.
Owner:TYCO HEALTHCARE GRP LP
Gas depletion and gas addition devices for blood treatment
ActiveUS20160007588A1Reduce blood volumeBioreactor/fermenter combinationsGas treatmentBlood treatmentsWhole blood product
Devices for the preparation of depleted blood products that provide for the depletion of oxygen, carbon dioxide, or oxygen and carbon dioxide are described. In addition, devices for the replenishment of oxygen and other gases to an anaerobic blood product are described. Methods and systems incorporating depletion and addition devices are provided to optimize the preparation, storage and transfusion of blood products to a recipient are described.
Owner:HEMANEXT INC
Filter material for removing aggregates and method of filtering blood product
InactiveUS20110031191A1Efficient removalAvoid cloggingSemi-permeable membranesDialysis systemsWhole blood productMedicine
An aggregate-removing filter material efficiently removes aggregates that are contained in a blood product for transfusion and may cause transfusion reactions without clogging, and exhibits excellent quality stability, and a blood product filtration method uses a filter apparatus that includes the aggregate-removing filter material and a leukocyte-removing filter material. The aggregate-removing filter material includes short fibers having a fineness of 0.7 to 4.0 dtex and a fiber length of 1 to 80 mm, and a ground fabric that includes long fibers, a fiber axis of the long fibers being oriented in a planar direction of the ground fabric, the short fibers being entangled with the ground fabric so that the aggregate-removing filter material has a total weight per unit area of 10 to 80 g / m2, and a layer of the short fibers forming a three-dimensional structure.
Owner:ASAHI KASEI MEDICAL CO LTD
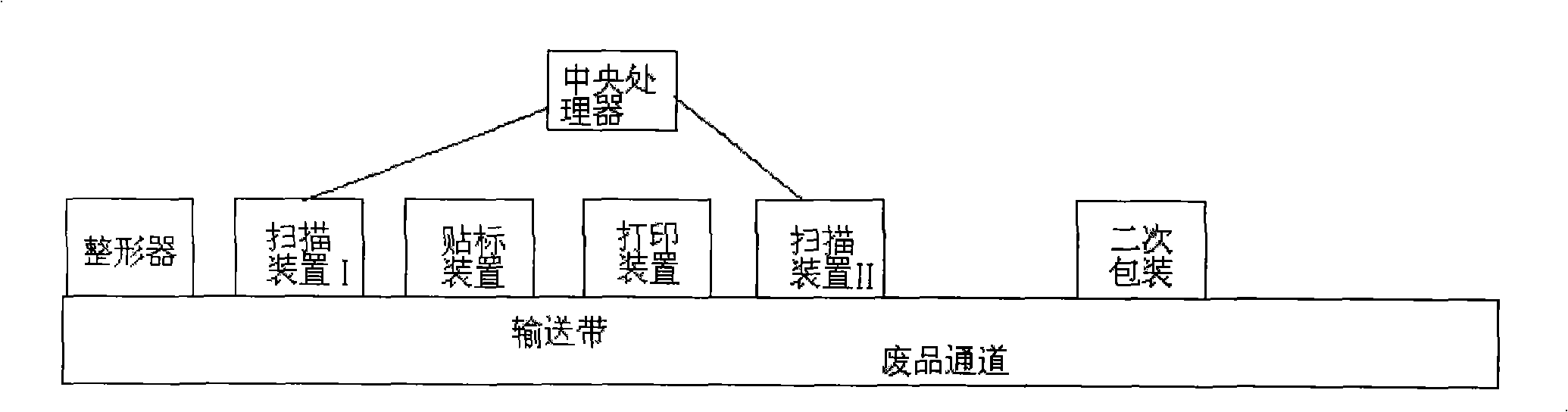
Full-automatic intelligentized blood bar code safety recognition sorting and checking package method and system
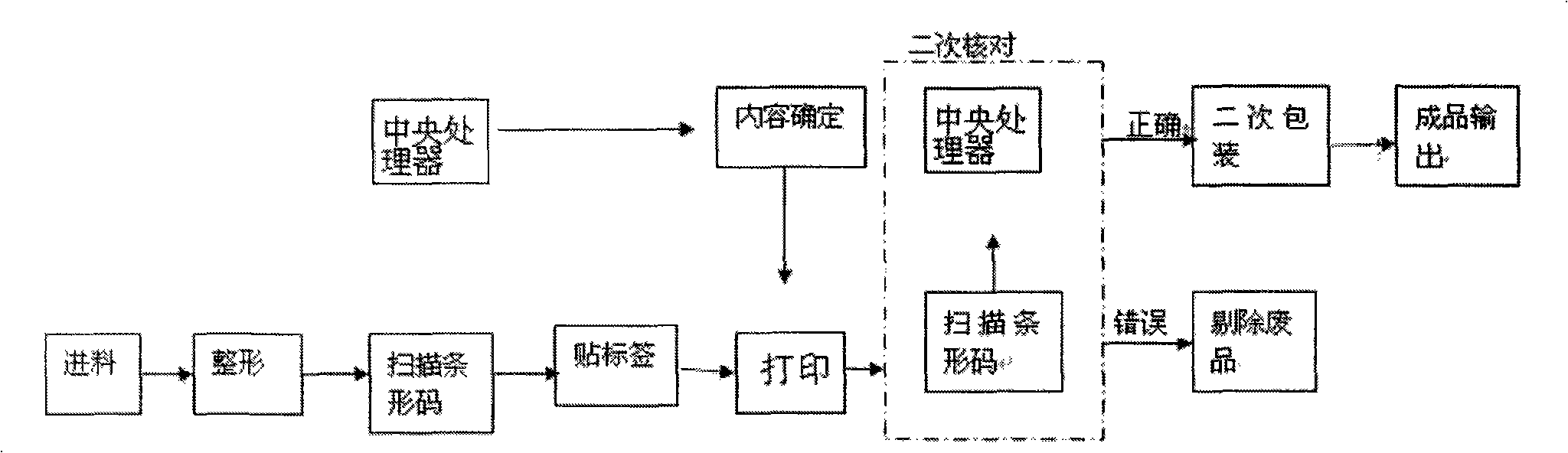
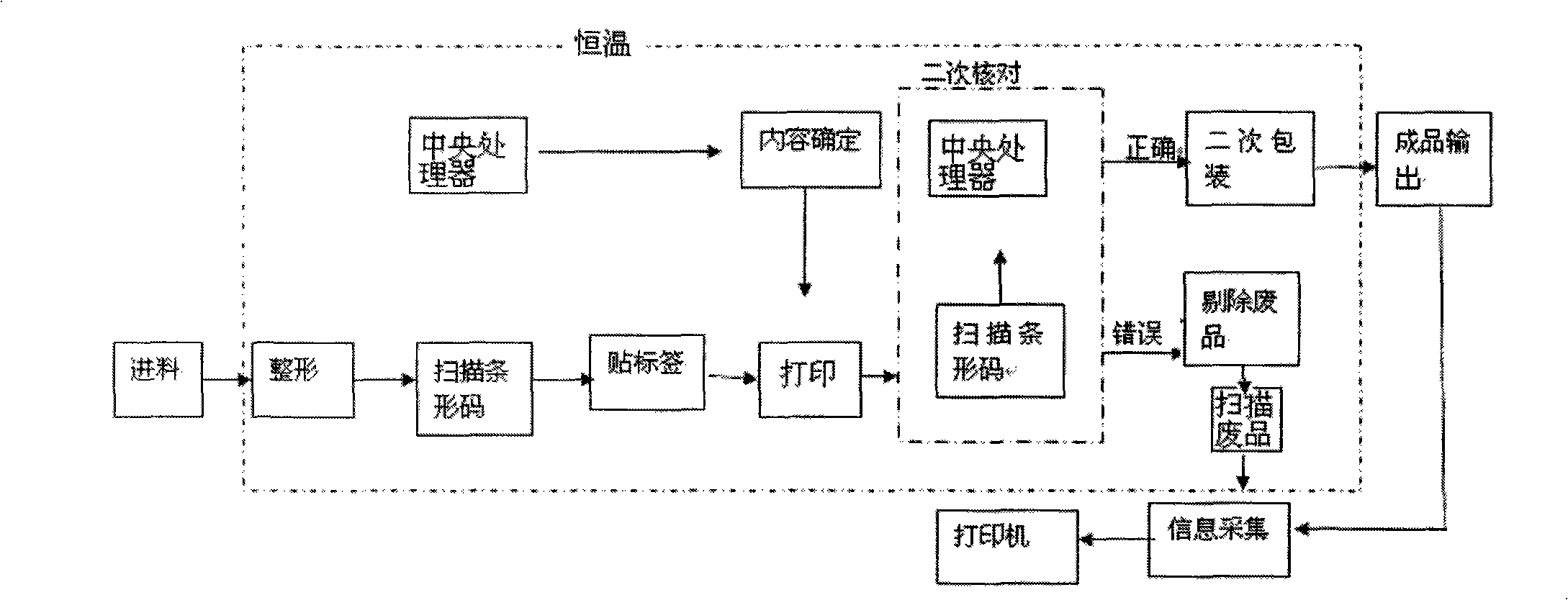
ActiveCN101342955ARealize intelligent identificationProduction Process AutomationLabelling non-rigid containersPackaging automatic controlWhole blood productBlood product
The invention discloses a fully automatic intelligentized blood bar code safety identification, partial checking and packing method and a system thereof. A blood bag is first reshaped and then is scanned; the scanning information is sent to a central processor; after that, the blood bag is labeled and printed and then is scanned again; the central processor checks the bar code of the blood bag again, the blood bags with right information will enter a secondary packing device and the blood bags with wrong information will be sent to a waster passage. The invention realizes intelligentized identification, automatic labeling, automatic classification and packing of blood products and is safe, standard, scientific and reasonable, and the production can be quicker, more accurate and automatic; the invention ensures the quality of the product and eliminates the defects of low efficiency and wrong labels caused in manual processing.
Owner:ZHENGZHOU FEILONG MEDICAL EQUIP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com