Oral tablet of acotiamide hydrochloride trihydrate and preparation method thereof
A technology of acotiamide hydrochloride trihydrate and oral tablet, which is applied in the field of oral tablet of acotiamide hydrochloride trihydrate and its preparation, can solve the problem of too large and too heavy tablet, enlargement of preparation shape, Swallowing problems and other problems, to achieve the effect of shrinking tablet size, reducing the amount of excipients, and reducing product costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
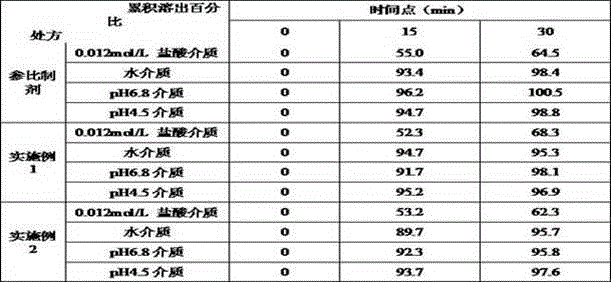
[0027] prescription:
[0028] Material composition Prescription ratio (g) Acotiamide Hydrochloride Trihydrate 50 starch 35 starch 9 Croscarmellose Sodium 3 Magnesium stearate 3 Production volume 500 pieces
[0029] Preparation method: 1) Weigh each material separately; 2) Fully mix acotiamide hydrochloride trihydrate and 35 g of starch, and add appropriate amount of water to granulate after mixing; 3) Dry in a wet granule drying box until the moisture content of the granules is not more than 3.0 %, drying temperature is 45°C; 4) The dry granules are passed through a 20-mesh sieve for granulation; 5) The obtained granules are mixed with 9 g of starch, croscarmellose sodium and magnesium stearate; 6) According to the theoretical tablet weight (200mg ) tablet.
Embodiment 2
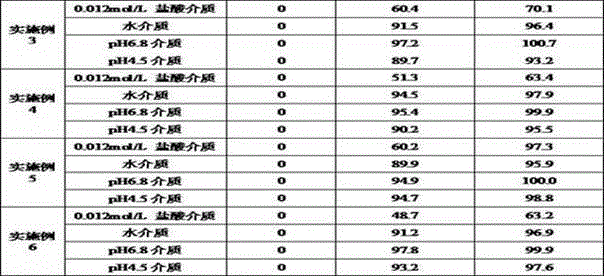
[0031] prescription:
[0032] Material composition Prescription ratio (g) Acotiamide Hydrochloride Trihydrate 75 pregelatinized starch 10 microcrystalline cellulose 8.5 sodium starch glycolate 5 Micropowder silica gel 1.5 25% ethanol aqueous solution Appropriate amount Production volume 750 tablets
[0033] Preparation method: 1) Weigh each material separately; 2) Fully mix acotiamide hydrochloride trihydrate and pregelatinized starch, add an appropriate amount of 25% ethanol aqueous solution to granulate after mixing; 3) Dry the wet granules in a drying oven until The moisture content of the granules is not more than 4.0%, and the drying temperature is 50°C; 4) The dry granules are sieved through a 40-mesh sieve; 5) The obtained granules are mixed with microcrystalline cellulose, sodium starch glycolate and micropowder silica gel; 6) According to the theoretical tablet weight ( 133.3mg) into tablets.
Embodiment 3
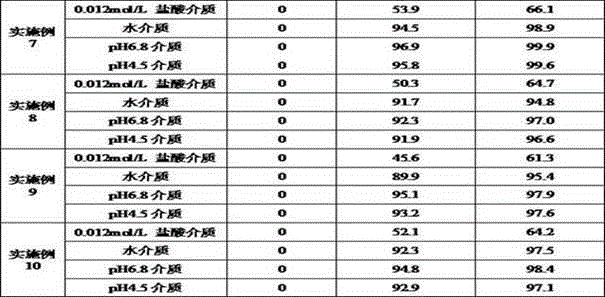
[0035] prescription:
[0036] Material composition Prescription ratio (g) Acotiamide Hydrochloride Trihydrate 60 microcrystalline cellulose 30.3 pregelatinized starch 5 Crospovidone 1.7 talcum powder 3 10% ethanol aqueous solution Appropriate amount Production volume 600 pieces
[0037] Preparation method: 1) Weigh each material separately; 2) Fully mix acotiamide hydrochloride trihydrate and microcrystalline cellulose, add an appropriate amount of 10% ethanol aqueous solution to granulate after mixing; 3) Dry the wet granules in a drying oven until The moisture content of the granules is not more than 4.0%, and the drying temperature is 40°C; 4) The dry granules are sized; 5) The obtained granules are mixed with pregelatinized starch, crospovidone and talc; 6) According to the theoretical tablet weight (166.7mg) Tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com