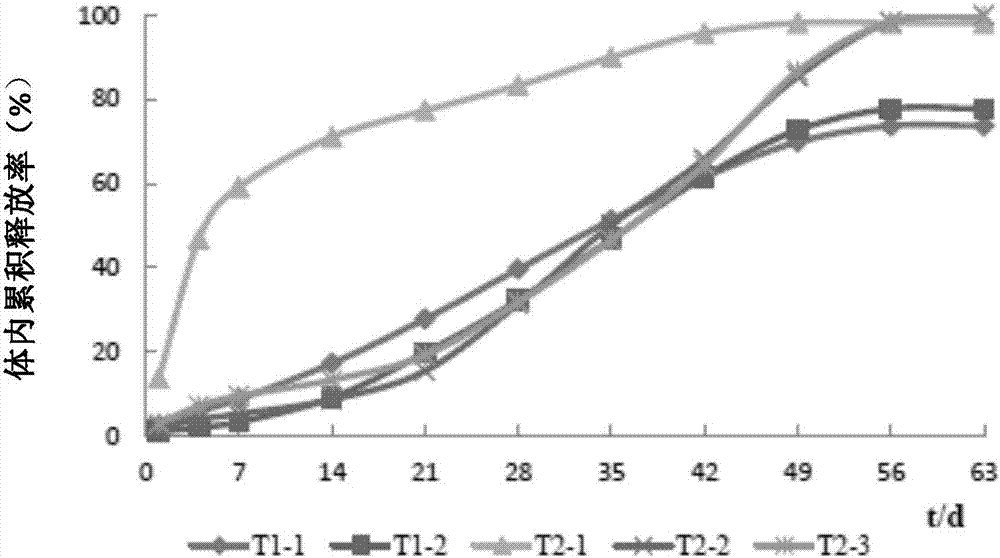
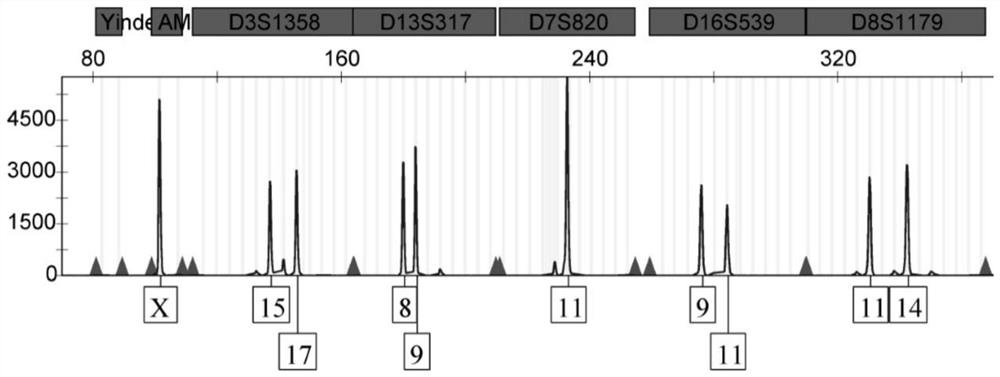
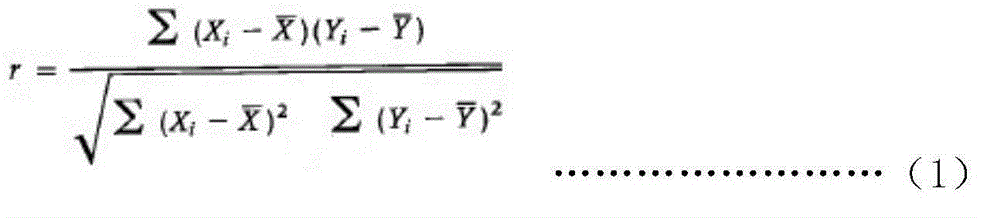Patents
Literature
71results about How to "Meet the needs of clinical applications" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
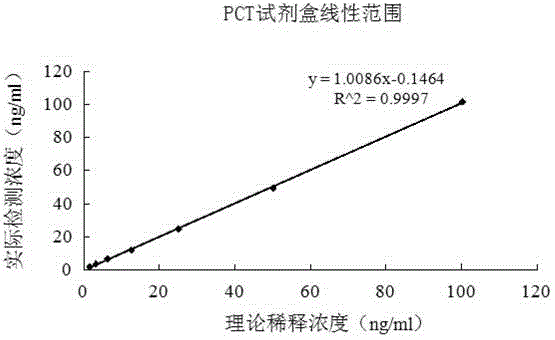
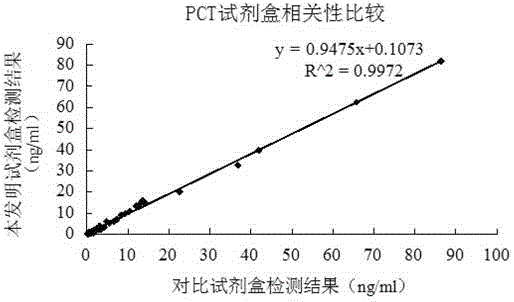
Latex enhanced turbidimetric immunoassay kit of quantitatively detecting procalcitonin PCT
ActiveCN102759631AHigh detection sensitivityMeet the needs of clinical applicationsMaterial analysis by observing effect on chemical indicatorBiotin-streptavidin complexTurbidimetry
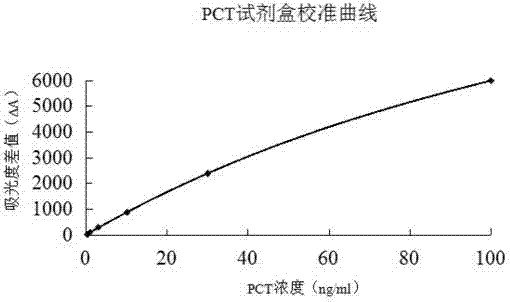
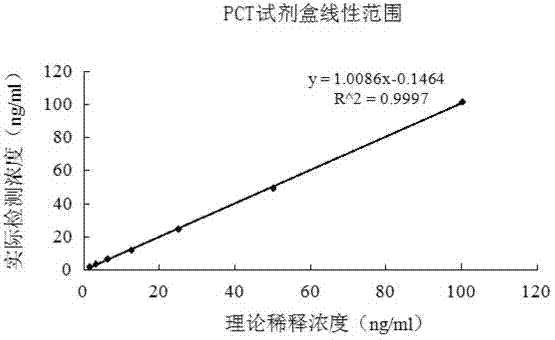
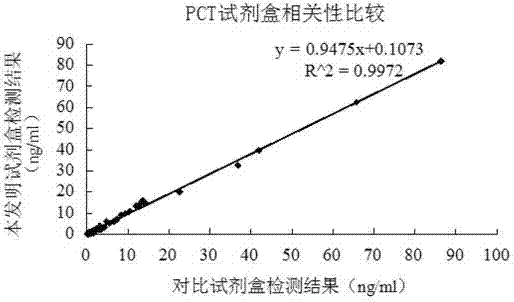
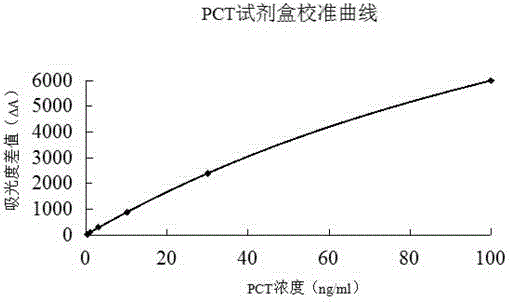
The invention relates to a latex enhanced turbidimetric immunoassay kit of quantitatively detecting procalcitonin PCT. The kit comprises an R1 reagent, an R2 reagent and a calibrator, wherein the R1 reagent comprises a protecting agent, a reaction enhancing agent, a preservative and buffer solution; the R2 reagent comprises a protecting agent, a preservative, buffer solution and anti-human PCT antibody coated sensitization polystyrene latex particles; the calibrator comprises a protecting agent, a preservative, buffer solution and PCT recombinant protein; the human PCT antibody in the R2 reagent is linked with polystyrene latex particles through streptavidin-biotin; and the particle diameter of the latex particles in the R2 reagent is 40-500 nm. The kit can be used on a biochemical analyzer and a scatter turbidimetry analyzer for quantitatively detecting the PCT content in human blood. The invention provides the PCT detection kit which has the advantages of convenience, quickness, high sensitivity, strong specificity and accurate quantification; and the kit has high instrument compatibility, is low in detection cost and meets the requirements on PCT turbidimetric products in clinical use.
Owner:NANJING NORMAN BIOLOGICAL TECH
Mussel mucoprotein gel for repairing and reliving itching and preparation method of mussel mucoprotein gel
InactiveCN104645313ANo stimulationGood bioadhesionPeptide/protein ingredientsAerosol deliveryBiocompatibility TestingWound surface
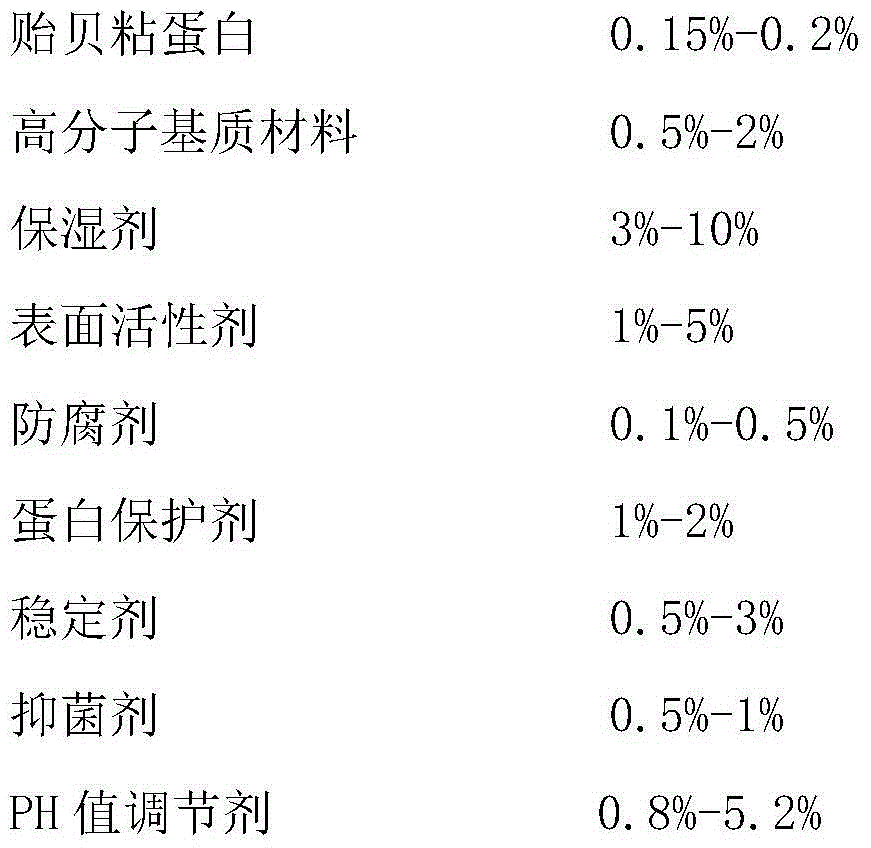
The invention discloses a mussel mucoprotein gel for repairing and reliving itching. The mussel mucoprotein gel is prepared from the following components in percentage by weight: 0.15%-0.2% of mussel mucoprotein, 0.5%-2% of a macromolecule host material, 3%-10% of a humectant, 1%-5% of a surfactant, 0.1%-0.5% of a preservative, 1%-2% of a protein protectant, 0.5%-3% of a stabilizer, 0.5%-1% of a bacteriostatic agent, 0.8%-5.2% of a pH modifier and the balance of purified water. The mussel mucoprotein gel has good biological adhesion, is rapidly and effectively adhered to a wound surface, is capable of rapidly reliving itching and easing pain, and has the advantages of being low in immunogenicity, good in biocompatibility with a human body, convenient to use, free of thrill to skin, safe, nontoxic and free of bad reaction.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Method for preparing polyvinylidene fluoride affinity membrane using amino acid as ligand
InactiveCN101596422AImprove adsorption efficiencyStable physical and chemical propertiesSemi-permeable membranesHaemofiltrationPolyvinylidene difluorideBlood plasma
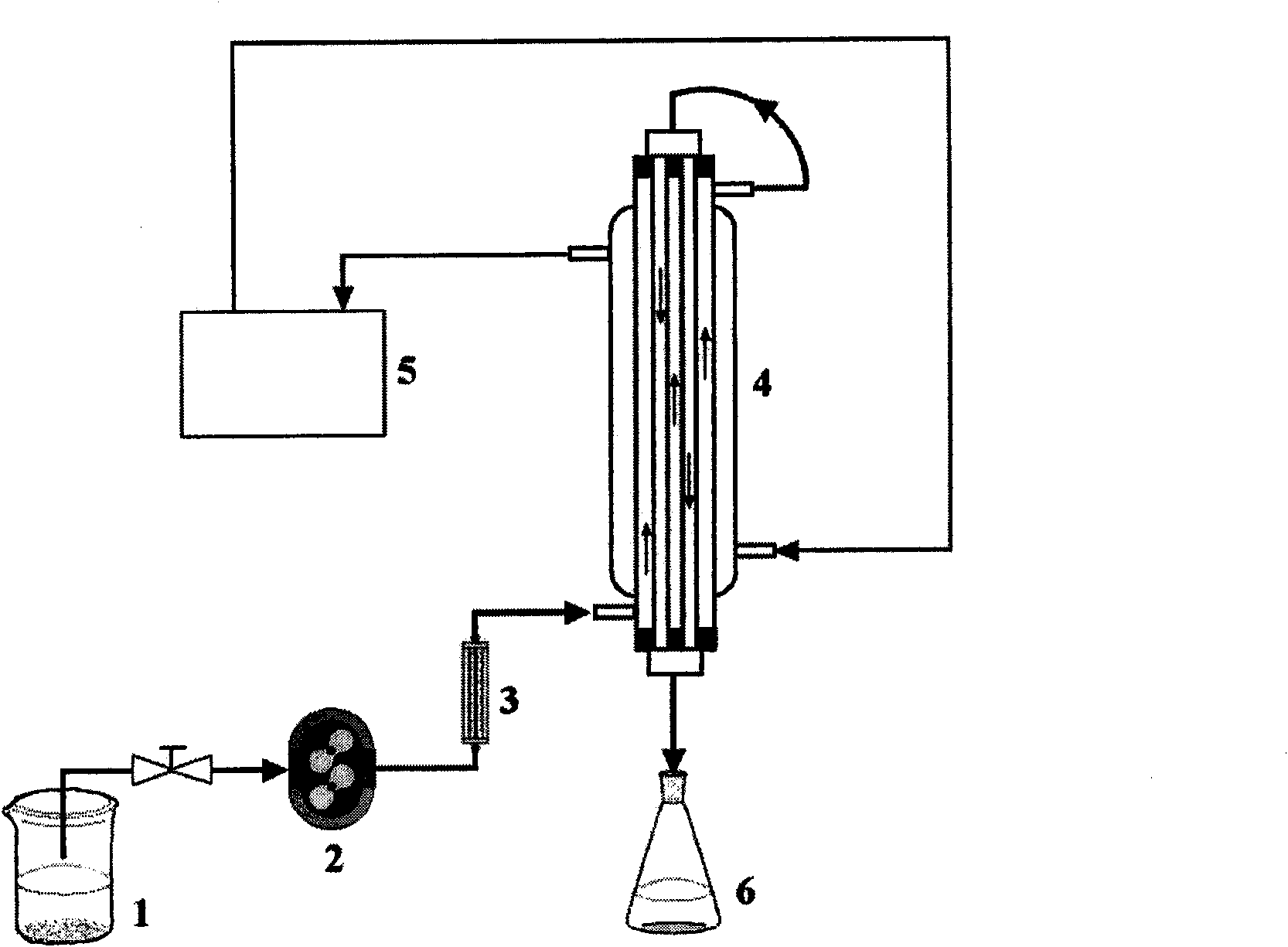
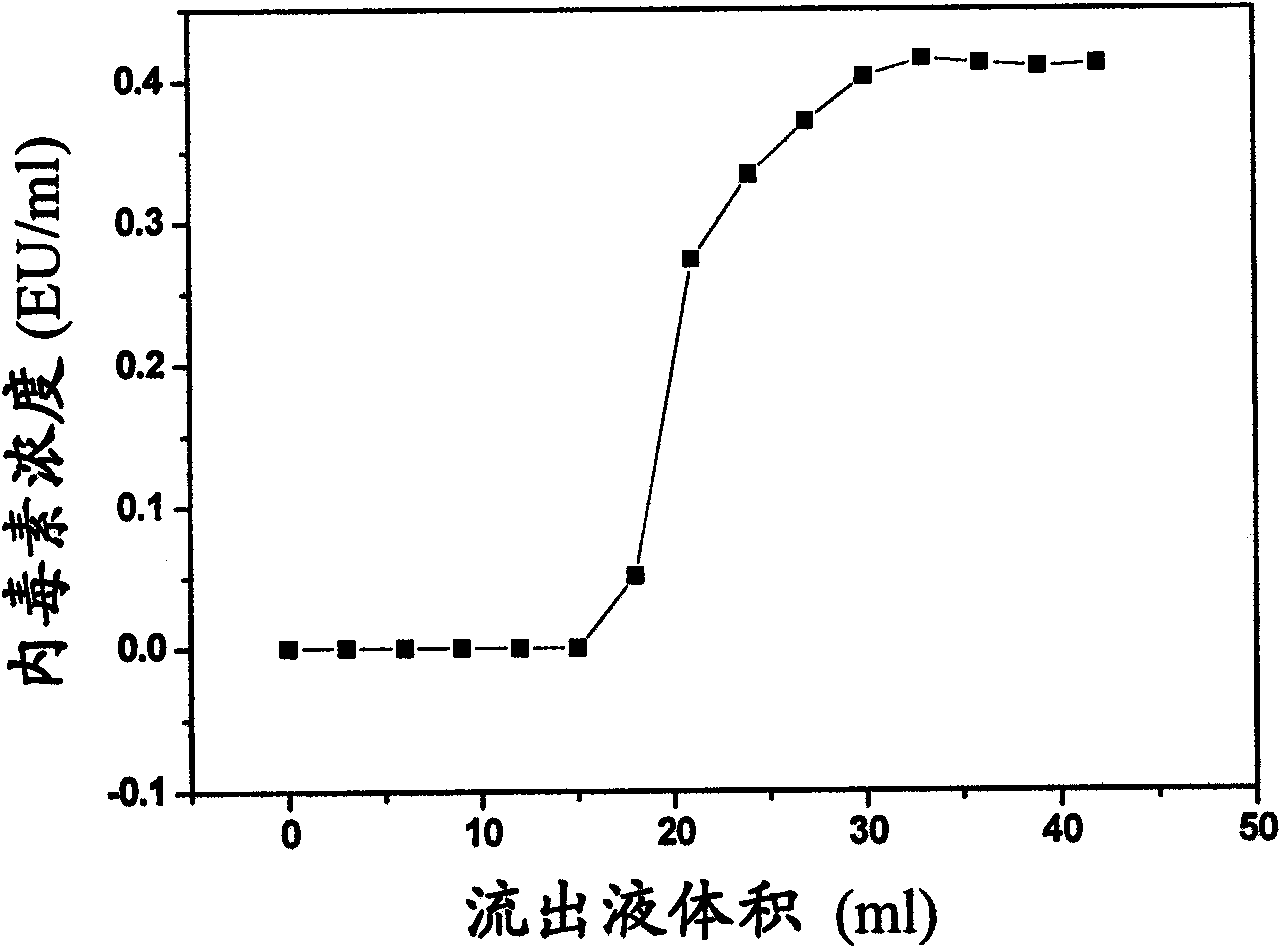
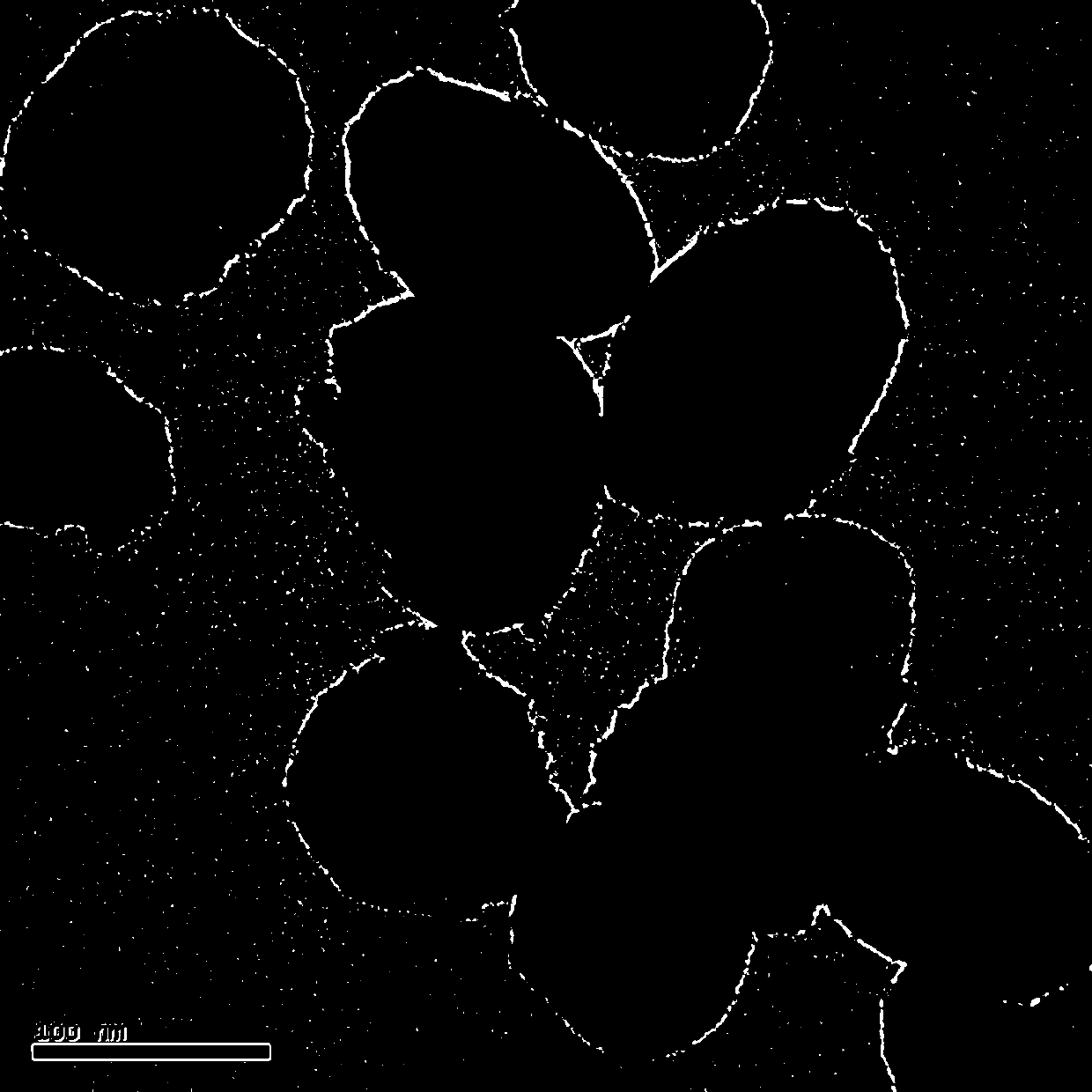
The invention discloses a method for preparing a polyvinylidene fluoride affinity membrane using amino acid as a ligand. The affinity membrane is prepared by hydrophilically modifying a polyvinylidene fluoride hollow fiber membrane and grafting the amino acid ligand. The grafting amount of the amino acid is 150 to 250mg / g for each membrane. The invention also discloses application of the affinity membrane in removing endotoxin in blood plasma, which removes the endotoxin in the blood plasma by a dynamic absorption means. The polyvinylidene fluoride affinity membrane prepared by the method has stable performance, good bio-compatibility and high endotoxin removing efficiency, and can be used for whole blood perfusion as well as blood plasma perfusion.
Owner:ZHEJIANG UNIV
Dual-mode fluorescent/magnetic resonance imaging contrast agent based on mesoporous silica and preparation thereof
InactiveCN103845741AHigh biosecurityGood physical and chemical stabilityNMR/MRI constrast preparationsDual modeFluorescence
The invention relates to a dual-mode fluorescent / magnetic resonance imaging contrast agent based on mesoporous silica and a preparation method thereof. The preparation method comprises the following steps: dissolving CTAB, and adding NaOH; dropwise adding TEOS; stirring, aging, washing, drying and roasting; dispersing a product in a toluene solution, adding APTES, refluxing, washing, dispersing through water to obtain a solution a, adding a chelating agent in DMSO, dissolving, separately adding NHS and EDC, stirring, adding the solution a, adding a gadolinium salt, filtering, washing, and dispersing with water to obtain a solution b; adding fluorescent molecules containing carboxyl in another DMSO, separately adding NHS and EDC, stirring, adding the solution b, stirring, filtering, washing, drying to obtain the dual-mode fluorescent / magnetic resonance imaging contrast agent based on mesoporous silica. The difunctional contrast agent has the advantages of small particle size, stable performance, good biocompatibility and strong fluorescence and magnetic resonance signals.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Method for preparing nano-silver-based dual-curing dental antibacterial adhesive
InactiveCN104546508AEasy to prepareStrong maneuverabilityAntibacterial agentsImpression capsNano sio2Surface modification
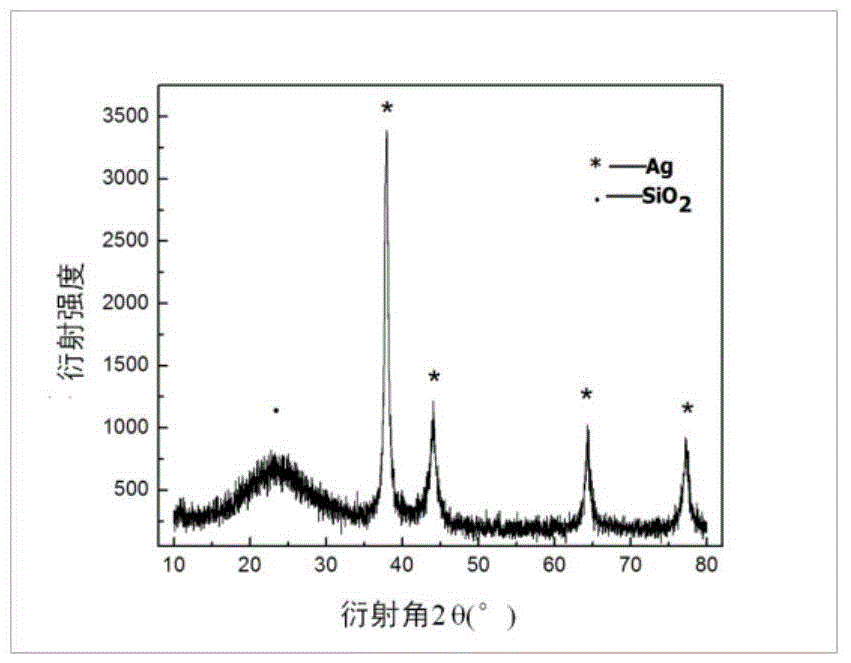
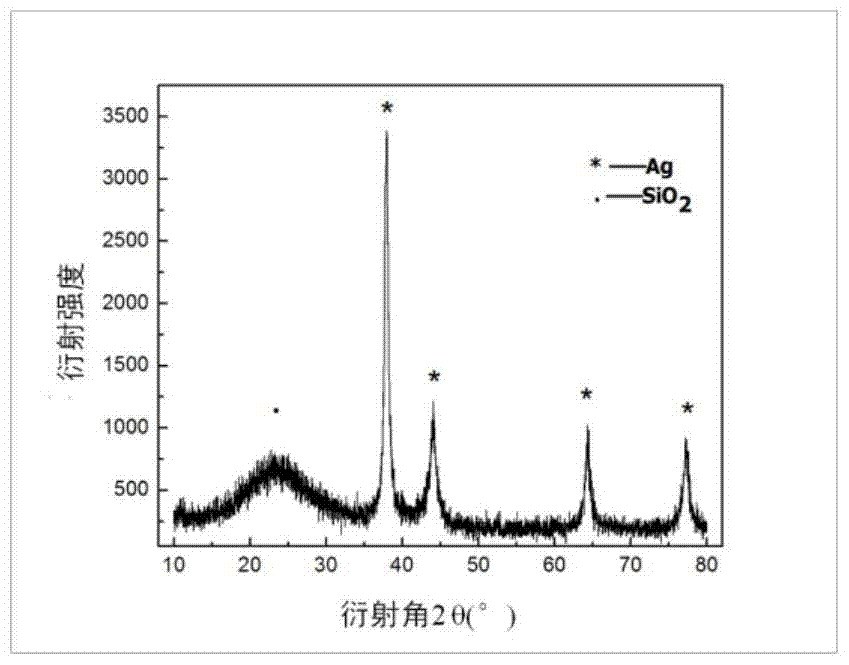
The invention provides a method for preparing a nano-silver-based dual-curing dental antibacterial adhesive. The method comprises the following specific steps: (1) preparing monodisperse spherical nano SiO2 particles by using a Stober method; (2) generating nano silver particles with small particle sizes on the surfaces of the nano SiO2 particles by utilizing a reduction reaction of an ammonia silver nitrate solution, namely preparing a nanoscale silver-loaded inorganic antibacterial agent; (3) performing proper surface modification on the previous silver-loaded inorganic antibacterial agent; and (4) preparing the optical and thermal dual-curing dental adhesive, and adding the inorganic antibacterial agent. The dental adhesive prepared by the invention has the multiple advantages of simple and feasible preparation process, product system stability, high operability, excellent antibacterial performance, durability and the like and has high clinical application potential.
Owner:SHANGHAI JIAO TONG UNIV +1
Lactobacillus gasseri strain and uses thereof
ActiveCN103911306AAchieve antagonismHigh lactic acid production capacityAntibacterial agentsAntimycoticsDrugExternal genitalia
The present invention provides a strain, which is a Lactobacillus gasseri strain and is characterized in that the preservation number is CGMCC No.6362. The present invention further provides uses of the strain in preparation of drugs for prevention and / or treatment of vaginitis, preparation of vagina care products, and preparation of external genitalia sanitary products. With the technical scheme, antagonism effects of the Lactobacillus gasseri strain on multiple pathogenic bacteria can be achieved. In addition, the strain has high adhesion to vaginal epithelial cells. The strain can well meet practical clinical application requirements.
Owner:XINLIFEI BIOMEDICAL TECH TIANJIN +1
Preparation method of ultrasound and magnetic resonance two-mode contrast medium having lymph targeting
InactiveCN104524601AThe technical preparation method is simpleGood stabilityEchographic/ultrasound-imaging preparationsNMR/MRI constrast preparationsSodium hyaluronateContrast medium
The invention discloses a preparation method of an ultrasound and magnetic resonance two-mode contrast medium having lymph targeting. The preparation method comprises the following steps: adding a surfactant to an alkaline solution, stirring, adding a silicon source to the solution after the surfactant is dissolved completely, continuously stirring, adding a magnetic compound to generate a sol mixture, stirring, washing and carrying out centrifugal drying to obtain a powdery product; carrying out high-temperature calcination on the product to obtain powdery nanometer mesoporous silica particles without the surfactant; dispersing the particles in an organic solvent, adding a silane coupling agent, stirring, washing with ethyl alcohol and water, and carrying out centrifugal drying to obtain a powdery product; dispersing the powder in deionized water, adding a cross-linking agent and sodium hyaluronate, stirring, washing with water and carrying out centrifugal drying to obtain nano-particles; and dispersing the particles in deionized water, dropwise adding fluorocarbon, stirring, washing with water and drying to obtain the contrast medium. By utilizing the preparation method, integration of double imaging modes can be realized, and two imaging manners can be developed.
Owner:SHANGHAI JIAO TONG UNIV +1
Lactobacillus gasseri strain and its uses
ActiveCN103911309AAchieve antagonismHigh lactic acid production capacityAntibacterial agentsAntimycoticsVaginal epithelial cellMicrobiology
The invention provides a strain. The strain is a Lactobacillus gasseri strain, and the preservation number of the strain is CGMCC No.6361. The invention also provides a use of the strain in the preparation of colpitis prevention and / or treatment drugs, a use of the strain in the preparation of vagina nursing articles, and a use of the strain in the preparation of external genitals health articles. By adopting the above technical scheme, the strain can effectively realize an antagonistic effect on a plurality of pathogenic bacteria. The strain has a high adherence ability to vaginal epithelial cell. The strain can well meet practical clinic application demands.
Owner:XINLIFEI BIOMEDICAL TECH TIANJIN +1
Preparation method of emulsion for magnetic resonance imaging of lung
InactiveCN104056285AImprove stabilityStabilize the airwayEmulsion deliveryIn-vivo testing preparationsPolyethylene glycolEthanolamine synthesis
The invention provides a preparation method of an emulsion for magnetic resonance imaging of lung. The preparation method of the emulsion for magnetic resonance imaging of the lung comprises the following specific steps: (1) adding a certain amount of magnevist solution into water, stirring and adding dipalmitoyl phosphatidyl choline, mPEG (polyethylene glycol)-DSPE (distearoyl phosphatidyl ethanolamine), phospholipid, surfactant, co-surfactant and stabilizer; (2) magnetically stirring at the room temperature till no obvious blocky substance is produced to obtain an emulsion; (3) adding pulmonary alveoli spreading agent into the emulsion and further stirring to obtain a mixed solution; (4) intermittently stirring the mixed solution by using a homogenizer, then standing, centrifuging and removing sediments to obtain an emulsion containing magnevist. The prepared emulsion for magnetic resonance imaging of lung is capable of entering the body through a pulmonary drug delivery way, avoiding a large quantity of inconveniences brought by parenteral drug delivery and meeting the requirements on a variety of clinical applications.
Owner:SHANGHAI JIAO TONG UNIV +1
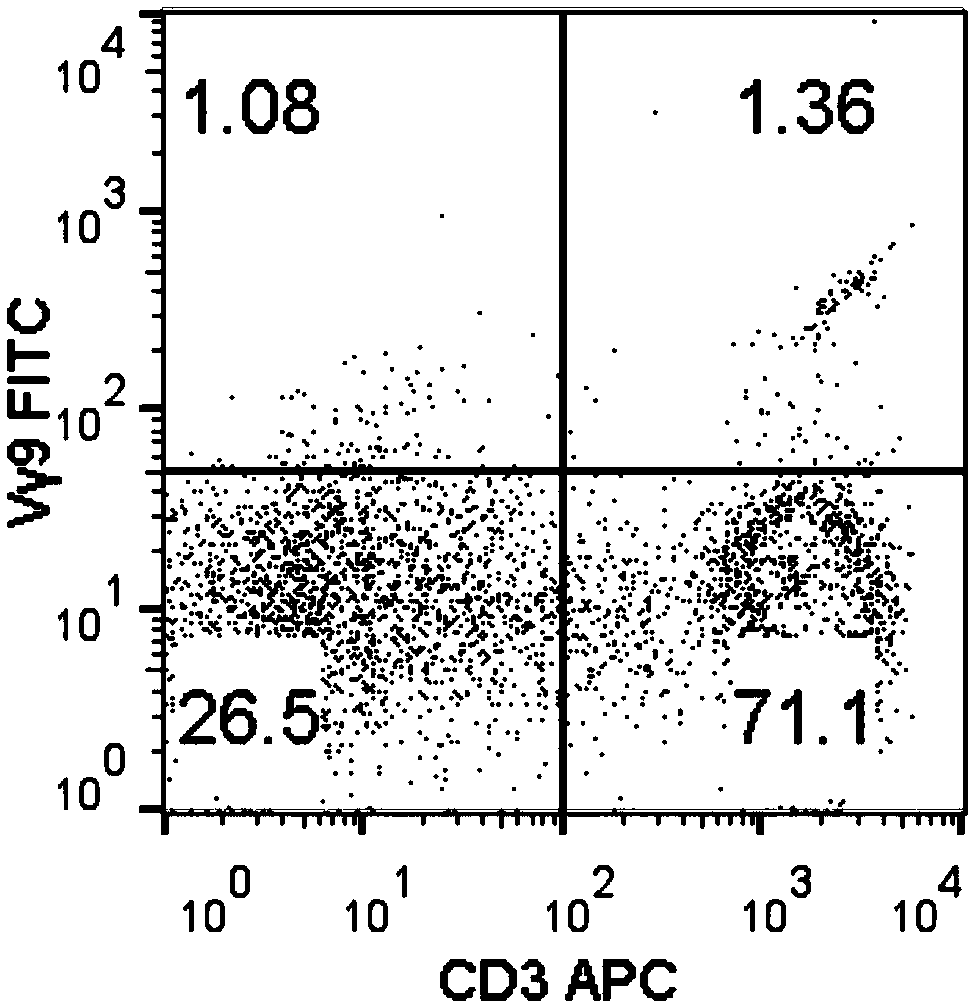
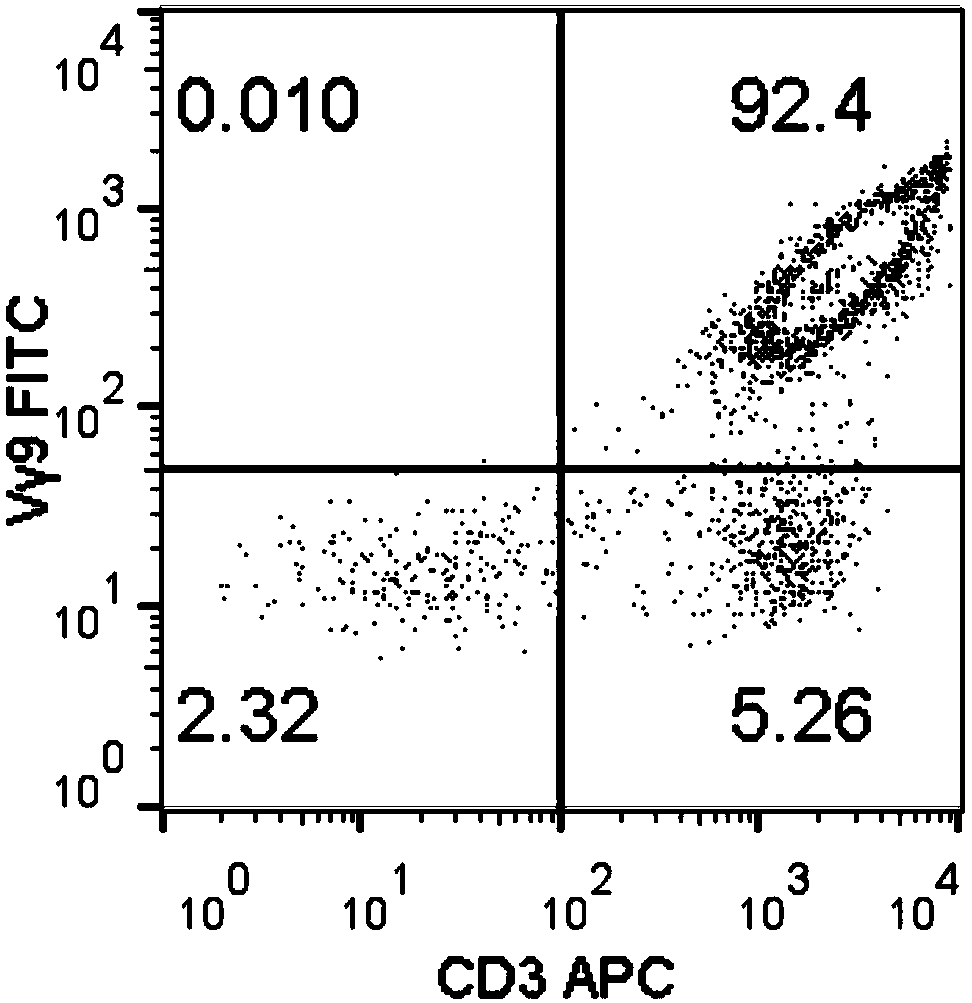
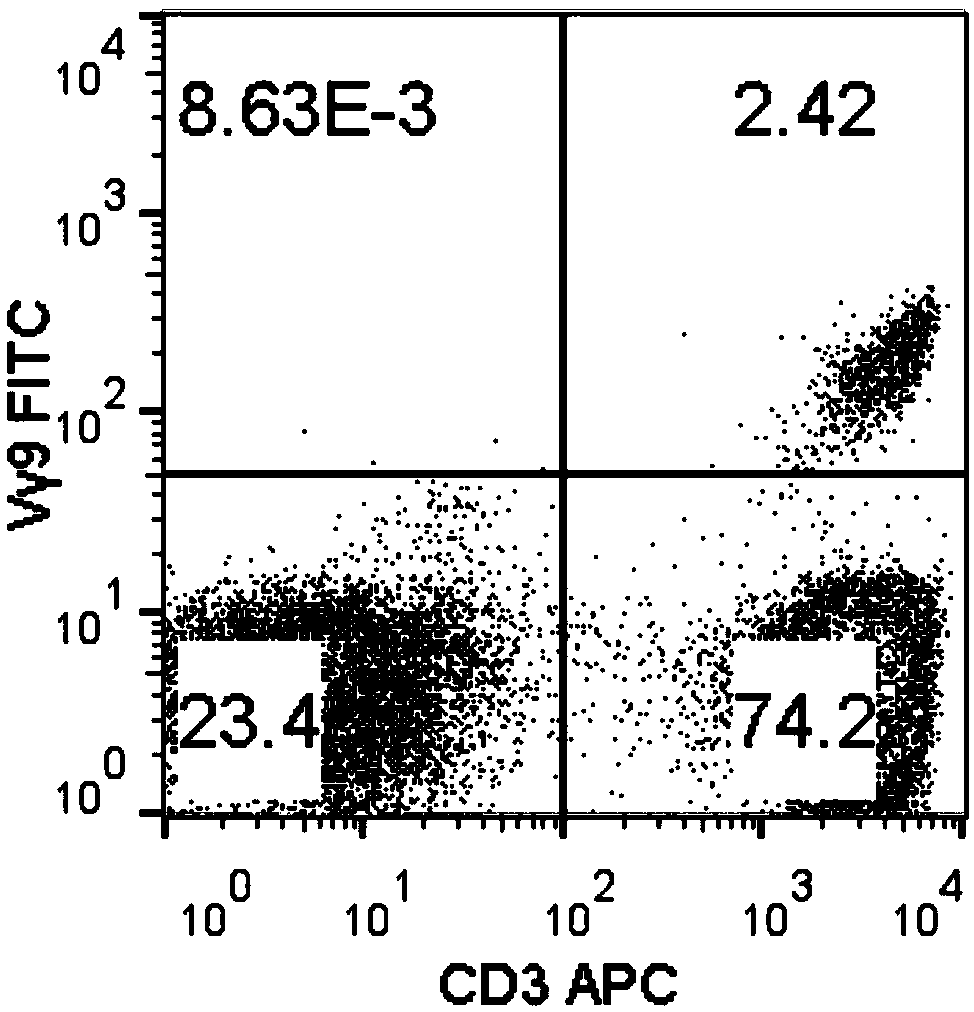
Method for amplifying killing activity gamma-delta T cell by induction in vitro
ActiveCN108949685AGood antitumor activityA large amountCulture processBlood/immune system cellsSerum free mediaLymphocyte
The invention relates to a method for amplifying a gamma-delta T cell by induction in vitro, and belongs to the technical field of biology. The method comprises the following steps: acquiring peripheral blood mononuclear cells (PBMCs) through a lymphocyte separation medium; performing stimulation culturing for 3 days by utilizing a serum-free medium containing zoledronic acid, IL-2, IL-7 and IL-15; performing stimulation amplifying culturing with a serum-free medium containing IL-2, IL-7 and IL-15; and adding nicotinamide when culturing is performed for 7-9 days to improve the anti-tumor activity of the gamma-delta T cells. According to the method, the PBMCs do not needed to be purified, and the gamma-delta T cells having the purity of 90 percent or more can be obtained when culturing is performed for 14 days; and the anti-tumor activity of the gamma-delta T cells can be remarkably improved by adding the nicotinamide. The gamma-delta T cells can be amplified for 1500 times or more, which can meet the requirement for the number of gamma-delta T cells in clinical application. The method is simple in operation steps and strong in operability, and has a good popularization value.
Owner:吉林省吉恩致合生物治疗技术有限公司
Multifunctional probe and preparation method and application thereof
InactiveCN105396133AGood dispersionEasy to makeEnergy modified materialsIn-vivo testing preparationsDispersityChemistry
The invention discloses a multifunctional probe and a preparation method and application thereof. The nano diagnosis and treatment probe with the two functions of magnetic resonance imaging and thermal therapy is formed in the mode that nano gold bars are formed by adding surfactant, chloroauric acid, a reduction agent and silver nitrate, target macromolecules are inoculated, and gadolinium ions are adsorbed. The preparation method is simple, the probe is good in dispersity in an aqueous solution, good in stability and capable of meeting requirements of clinical application and combining radiography and thermal therapy, and the dual functions of lymphoma diagnosis and treatment are achieved.
Owner:SHANGHAI JIAO TONG UNIV +1
Preparation method for porous calcium phosphate stent
ActiveCN108638494AAvoid problems such as shrinkage and deformationReduce consumptionAdditive manufacturing apparatusPharmaceutical delivery mechanismPhosphoric acidSlurry
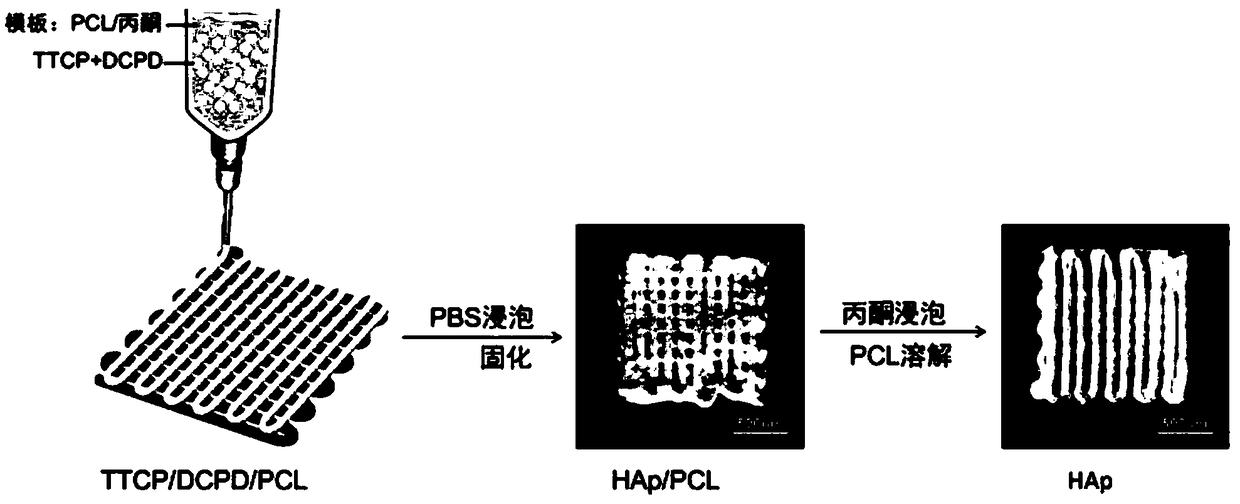
The invention provides a preparation method for a porous calcium phosphate stent. The preparation method comprises the following steps: with polycaprolactone (PCL) as a printing template, dissolving polycaprolactone in acetone so as to obtain a polycaprolactone solution, and uniformly mixing a mixture of tetracalcium phosphate (TTCP) and calcium hydrogen phosphate (DCPD) with the above polycaprolactone solution to prepare printing slurry; subjecting the slurry to 3D printing to print a green stent body with a certain shape and of a certain structure, and placing the stent in a low-temperaturehydration environment for a reaction so as to form a hydroxyapatite HAp porous stent, thereby avoiding dimensional shrinkage deformation caused by high-temperature sintering and reducing energy loss;and finally, putting the porous stent in acetone to dissolve and remove the stent template PCL so as to obtain the individually-controllable porous calcium phosphate stent with HAp as a main componentand with certain osteoinductivity and mechanical strength.
Owner:SICHUAN UNIV
High-purity minicircle DNA (deoxyribonucleic acid) and preparation method and application thereof
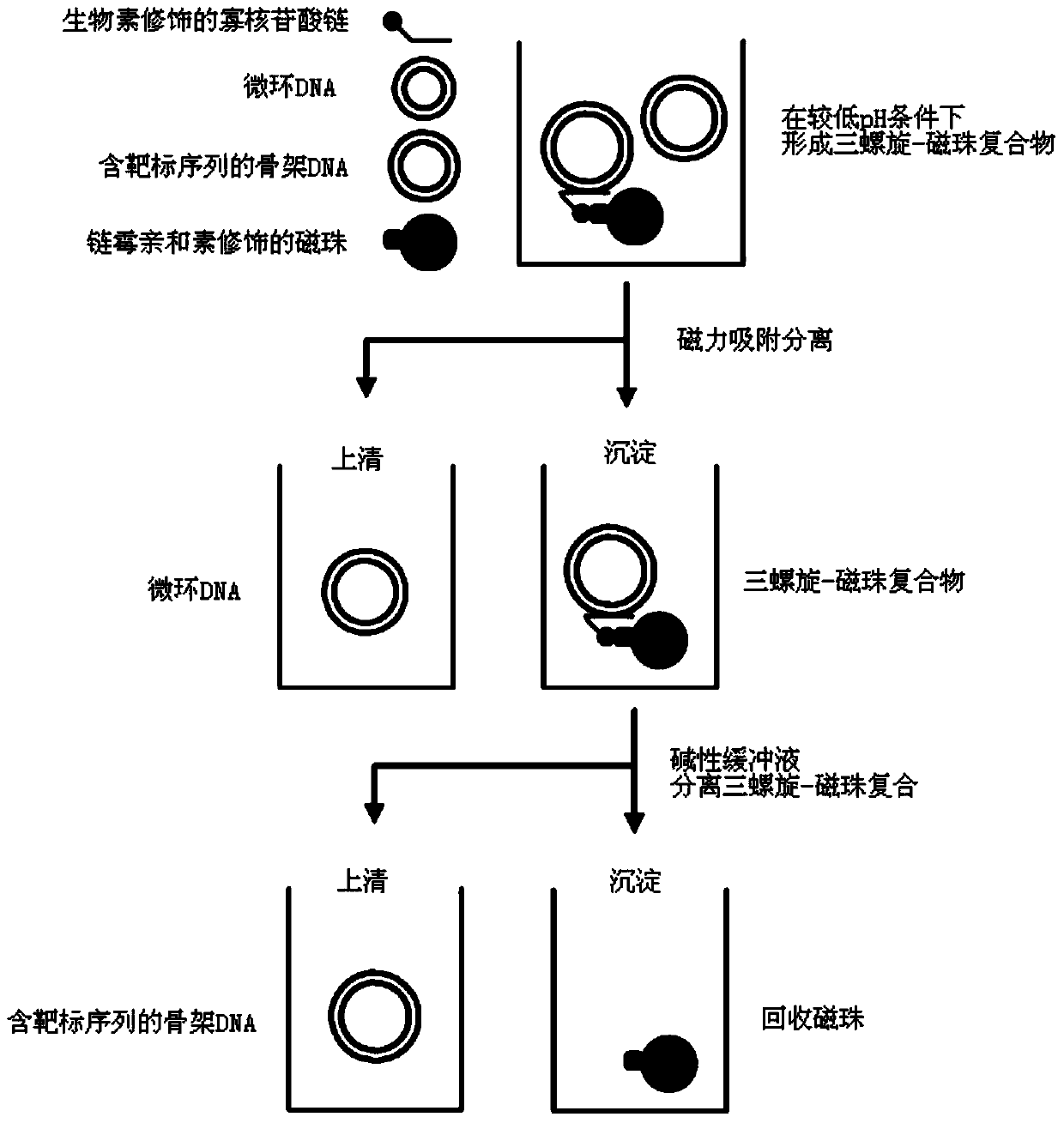
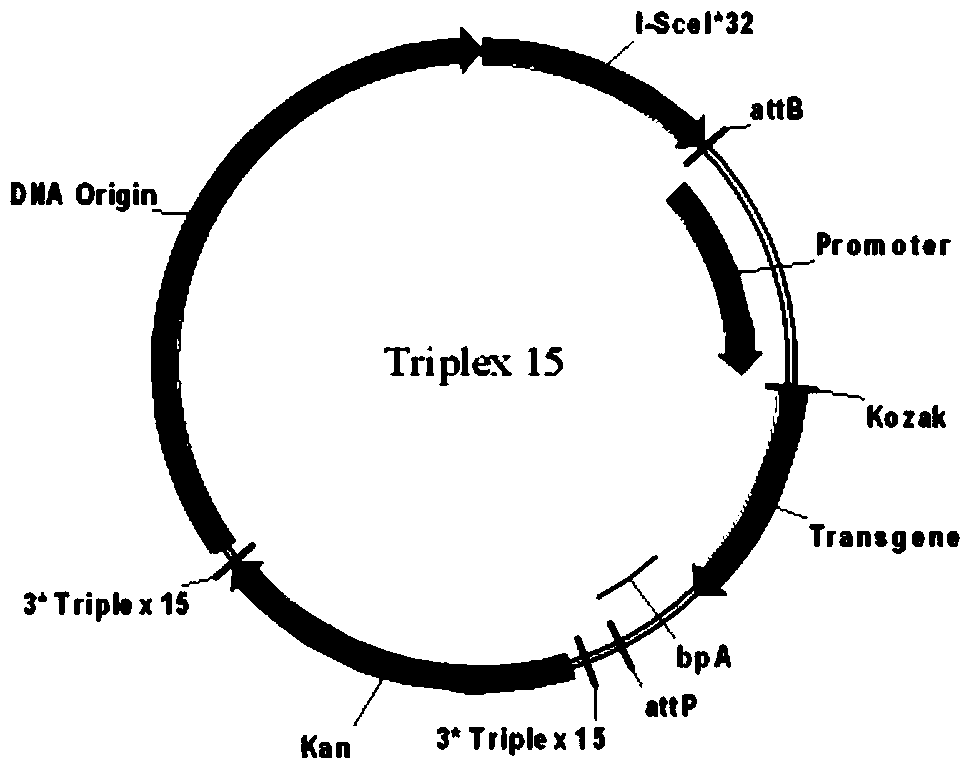
ActiveCN105316314AHigh purityMeet the purification requirementsMicrobiological testing/measurementGenetic material ingredientsPurification methodsLysis
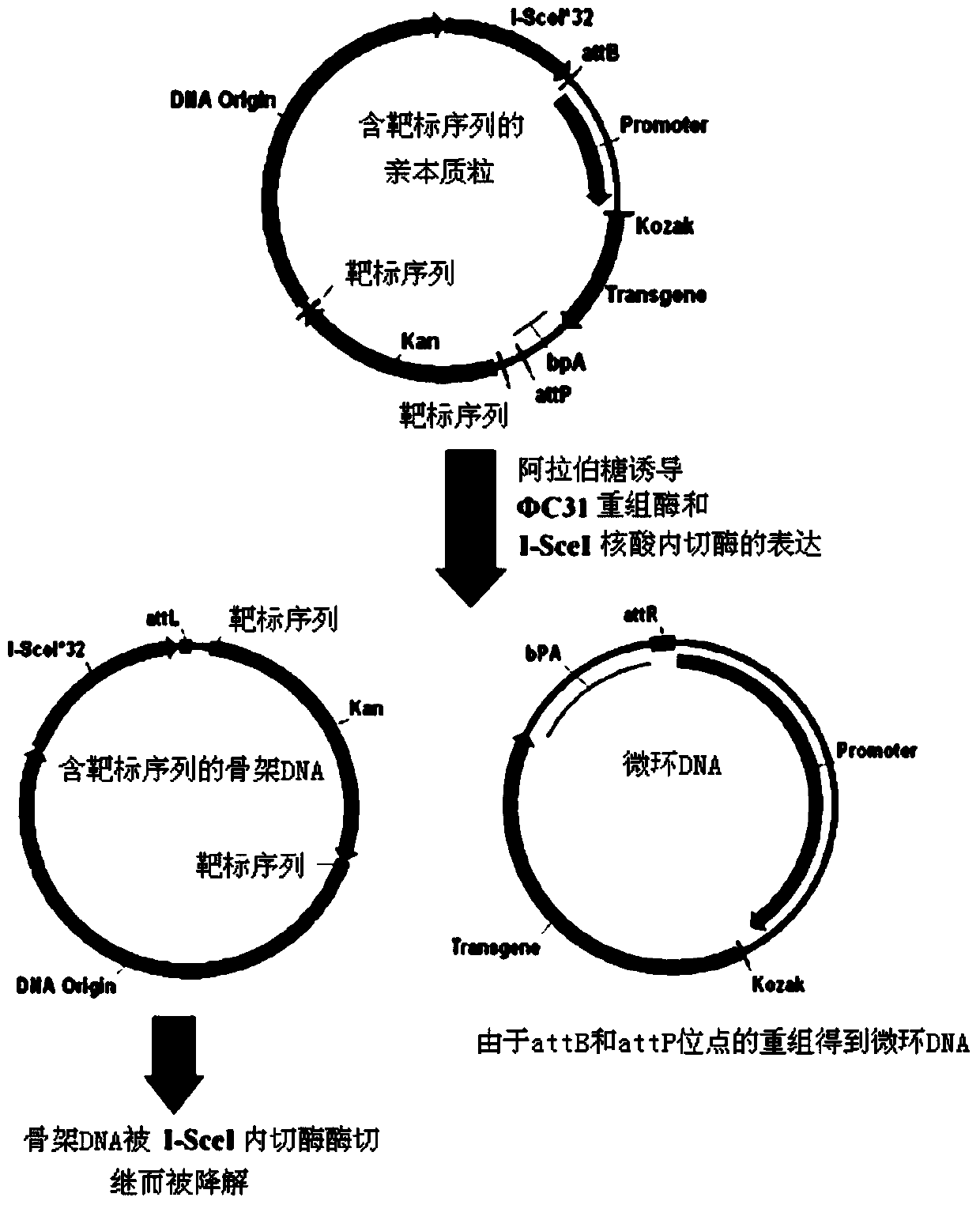
The invention provides a high-purity minicircle DNA (deoxyribonucleic acid) and a preparation method and application thereof. The preparation method includes the steps: 1) providing a parental plasmid containing a target sequence, wherein the parental plasmid has a specific recombination site, a nucleotide sequence of a skeleton DNA and a nucleotide sequence of the minicircle DNA; 2) transferring the parental plasmid to a host cell, inducing the parental plasmid to generate the minicircle DNA and the skeleton DNA containing the target sequence under the action of site-specific recombination; 3) subjecting the host cell to lysis, and subjecting plasmids to prepurification to obtain mixed plasmids including the minicircle DNA, the parental plasmid containing the target sequence and / or the skeleton DNA containing the target sequence; 4) removing the parental plasmid containing the target sequence and / or the skeleton DNA containing the target sequence from the mixed plasmids according to a tri-spiral purification method to obtain the high-purity minicircle DNA.
Owner:SYNO MINICIRCLE BIOTECH CO LTD
Porous metal bone implant material and its preparation method and application
InactiveCN107185039AAntagonize pathological effectsPromote bone regenerationAdditive manufacturing apparatusTissue regenerationImplant materialFreeze dry
The invention discloses a porous metal bone implant material and its preparation method and application and belongs to the technical field of biomedical materials. The porous metal bone implant material comprises a porous metal material matrix and a silk fibroin material filling the pores of the porous metal material matrix. The porous metal material matrix is prepared from metal raw material powder through 3D printing. The silk fibroin material is loaded with a bioactive drug. The preparation method comprises 1) preparing a customized 3D printed porous metal material matrix according to demands, 2) preparing a silk fibroin solution and loading the silk fibroin with a bioactive drug, and 3) filling pores of the porous metal material matrix with the silk fibroin with the bioactive drug, and carrying out freeze-drying to obtain the porous metal bone implant material. The porous metal bone implant material is precisely controlled in structure, can be customized, can effectively improve osseointegration of a metal bone implant in diabetic patients and can reduce the loosening rate of the implant.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Bone marrow cell classification method and system, computer equipment and storage medium
PendingCN114580501ARealize detectionAchieve reductionCharacter and pattern recognitionNeural architecturesMicroscopic imageData set
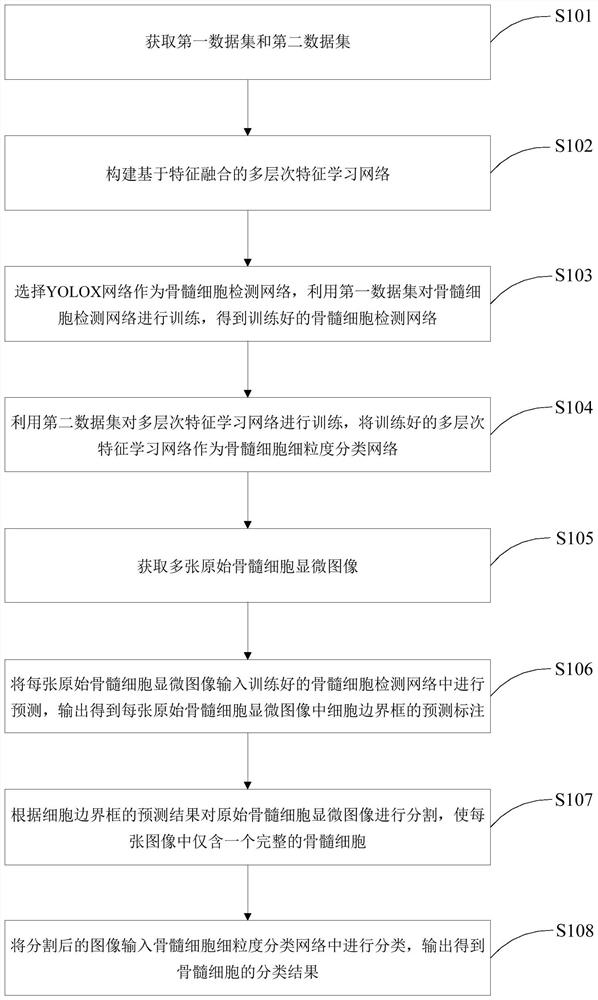
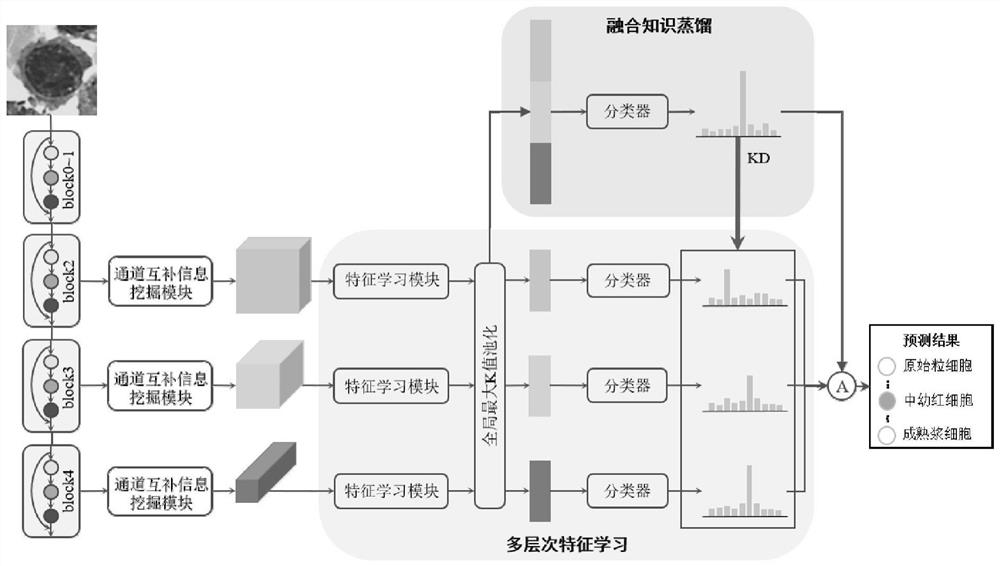
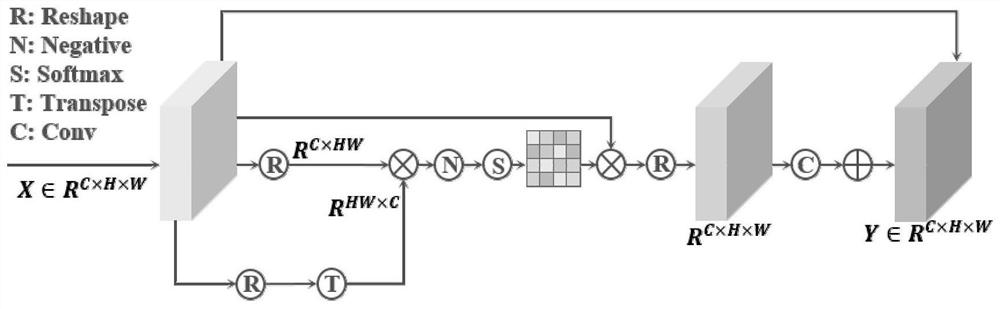
The invention discloses a bone marrow cell classification method and system, computer equipment and a storage medium. The method comprises the following steps: acquiring a first data set and a second data set; constructing a bone marrow cell fine-grained classification network; acquiring a plurality of original bone marrow cell microscopic images; inputting each original bone marrow cell microscopic image into the trained bone marrow cell detection network for prediction, and outputting a prediction mark of a cell bounding box in each original bone marrow cell microscopic image; segmenting the original bone marrow cell microscopic image according to the prediction result of the cell bounding box, so that each image only contains one complete bone marrow cell; and inputting the segmented image into a bone marrow cell fine-grained classification network for classification, and outputting to obtain a classification result of the bone marrow cells. According to the invention, through learning of multi-layer features in the deep network and introduction of a knowledge distillation mechanism, fusion information is introduced into a single-layer feature classifier, and classification precision of bone marrow cells is improved.
Owner:SOUTH CHINA UNIV OF TECH
Contrast agent having two functions of up-conversion luminescence and magnetic resonance imaging, and method for preparing same
InactiveCN102940893AHigh biosecurityLow toxicityNMR/MRI constrast preparationsFluorescenceUpconversion luminescence
The invention relates to a contrast agent having two functions of up-conversion luminescence and magnetic resonance imaging, and a method for preparing the same. The contrast agent is obtained by modifying a gadolinium chelating agent on the surfaces of carbon nanospheres through taking up-conversion luminescent material carbon nanospheres as cores and using a coupling agent, wherein the mol ratio of the carbon nanospheres to the coupling agent to the gadolinium chelating agent is 1: 1: 1 to10: 1: 1. The dual-function contrast agent is small in grain size, high in crystallinity, even in dispersion, stable in performance, and strong in both fluorescence and magnetic resonance signal. The product obtained is capable of meeting the requirements of clinical application.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
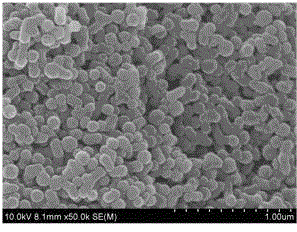
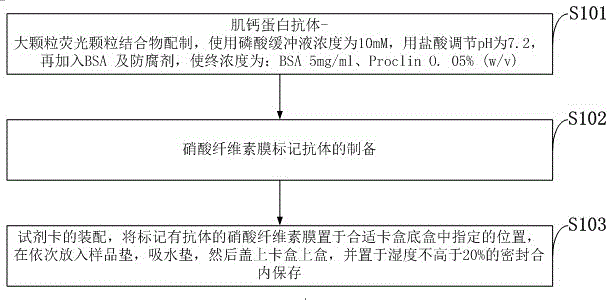
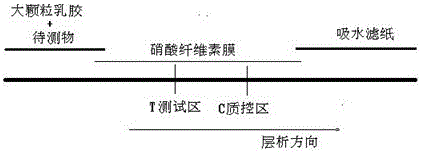
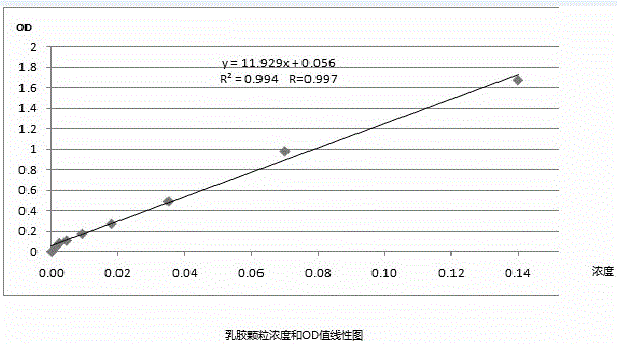
Immunofluorescence kit for quantitatively detecting content of troponin I and preparation method
InactiveCN105759050AHigh detection sensitivityMeet the needs of clinical applicationsBiological material analysisBiological testingImmunofluorescenceLarge particle
The invention discloses an immunofluorescence kit for quantitatively detecting the content of troponin I and a preparation method. The preparation method comprises the following steps: preparing a troponin antibody-large-particle fluorescent particle conjugate; preparing a nitrocellulose membrane labeled antibody; assembling a reagent card; preparing a TNI calibration product; establishing a standard curve; calculating the content of the troponin in a sample to be detected. The immunofluorescence kit for quantitatively detecting the content of the troponin I comprises a nitrocellulose membrane, a reagent and the calibration product. The lowest detection limit of the kit can reach 0.1ng / ml and the requirement of clinical application is met; the fastest time of a process from sample acquisition to detection result presentation only needs 10 to 15 minutes so that more expensive time for patients is provided, and illness states of the patients can be dynamically detected. The immunofluorescence kit for quantitatively detecting the content of the troponin I has good result relevance, no remarkable difference and an accurate and reliable detection result, and can be used for clinically replacing imported products; the detection cost is greatly reduced.
Owner:苏州联辰生物技术有限公司
Preparation, product and application of sandwich type degradable stent for alveolar bone repair
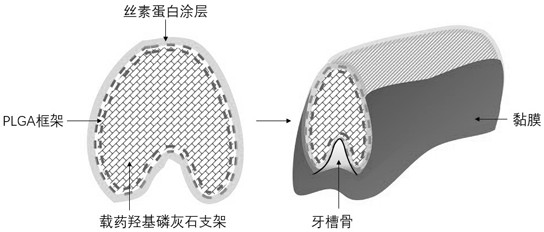
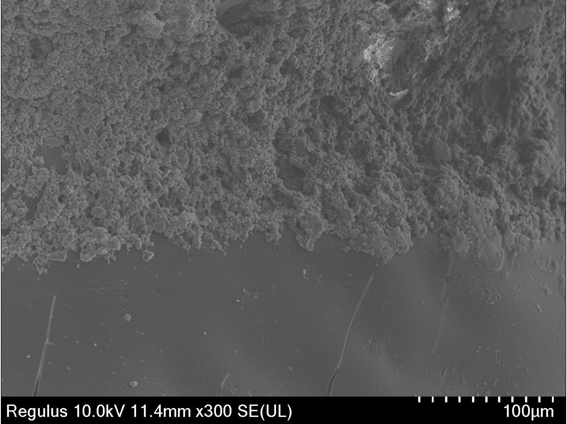
PendingCN112604029AImprove compatibilityOvercoming extramucosal exposureAdditive manufacturing apparatusTissue regenerationBiomedical engineeringBone grafting
The invention relates to a preparation method of a sandwich type degradable stent for alveolar bone repair as well as a product and application thereof. The preparation method comprises the following steps: preparing a self-curing hydroxyapatite and PLGA double-layer stent through double-nozzle 3D printing; and afterwards, performing amination of the surface of the polyester, and chemically fixing the silk fibroin coating loaded with the endothelial growth promoting medicine through an EDC / NHS coupling reaction. The hydroxyapatite stent matched with bone trauma is prepared, the bioactive coating modified PLGA provides a matched base for external fixation of bone wounds, the hydroxyapatite stent is used in cooperation with bone glue, bone nails and suture lines to achieve the fixing effect, and accurate bone grafting is achieved. All the materials are biodegradable, the degradation rate of the materials is suitable for growth of new bones, the silk fibroin coating loaded with VEGF improves the compatibility of PLGA and mucous membranes, the defects that a traditional titanium mesh stent is exposed outside the mucous membranes in advance and cannot be degraded in vivo are overcome, and the requirements of clinical application are met.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Preparation method of lymphatic targeted molybdenum sulfide nanosheet and product thereof
InactiveCN109481679ABig spaceFacilitate strippingEnergy modified materialsInorganic non-active ingredientsOrganic solventNanoparticle
The invention relates to a preparation method of a lymphatic targeted molybdenum sulfide nanosheet and a product thereof. The molybdenum sulfide is synthesized by means of a hydrothermal synthetic method, and sulfide nanoparticles are embedded at the same time, so that the spaces among layers are enlarged, and peeling is facilitated further. By taking an organic solvent as a reaction solvent, molybdenum sulfide is peeled by a liquid phase to prepare the two-dimensional molybdenum sulfide nanosheet. Finally, modified by hyaluronic acid, the lymphatic targeted two-dimensional molybdenum sulfidenanosheet is prepared. The invention relates to the novel method of preparing molybdenum sulfide. The method, by being applied to treating cancers clinically, can achieve targeted photothermal therapyof tumor cells and prevent normal cells from being killed.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Preparation method of detection probe for alpha-synuclein accumulation as well as product and application of detection probe
InactiveCN109001452ASmall particle sizeImprove targetingMaterial analysisSynthesis methodsSilicon dioxide
The invention relates to a preparation method of a detection probe for alpha-synuclein accumulation as well as a product and application of the detection probe. According to the preparation method, magnetic ferroferric oxide nanoparticles (Fe3O4 NPs) are used as carriers, and tetraethyl orthosilicate (TEOS) and 3-aminopropyltrimethoxysilane (APTES) are used for aminated modification of the surfaceof Fe3O4 NPs; further, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-hydroxysuccinimide (NHS) are used as coupling agents for binding alpha-synuclein monoclonal antibodies; the Fe3O4 NPscarriers are coated with silica shell by utilizing the autocatalytic activity of Fe3O4 NPs, and then the alpha-synuclein monoclonal antibodies are bound to synthesize the detection probe which targets a Parkinson's disease, and is capable of fixed-point clearing intracellular ROS, and delaying the further accumulation of alpha-synuclein. The detection probe combines targeting, imaging and treatment into one, and is simple in synthesis method; the raw materials used are high in biosafety, relatively good in catalytic activity and good in physical stability.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
A latex-enhanced immune turbidimetric kit for quantitative detection of procalcitonin pct
ActiveCN102759631BHigh detection sensitivityMeet the needs of clinical applicationsMaterial analysis by observing effect on chemical indicatorLatex particlePolystyrene
The invention relates to a latex-enhanced immune turbidimetric kit for quantitatively detecting procalcitonin PCT. The kit includes R1 reagent: containing a protective agent, a reaction enhancer, a preservative and a buffer; R2 reagent: containing a protective agent, a preservative reagent, buffer and sensitized polystyrene latex particles coated with anti-human PCT antibody; and calibrator: containing protective agent, preservative, buffer and PCT recombinant protein; The ethylene latex particles are linked by streptavidin-biotin; the particle size of the latex particles in the R2 reagent is 40-500nm. The kit can be used for quantitative detection of PCT content in human blood on a biochemical analyzer and a scattering turbidimetric analyzer. The invention provides a convenient and fast PCT detection kit with high sensitivity, strong specificity and accurate quantification, which has strong instrument compatibility and low detection cost, and makes up for the clinical demand for PCT turbidimetric products.
Owner:NANJING NORMAN BIOLOGICAL TECH
Preparation method of a dual-cure dental antibacterial adhesive based on nano-silver
InactiveCN104546508BEasy to prepareEasy to operateAntibacterial agentsImpression capsProduct systemNano sio2
The present invention provides a kind of preparation method of double solidification dental antibacterial adhesive based on nanometer silver, its concrete steps: (1) Stober method prepares monodisperse spherical nanometer SiO2 particle; (2) utilizes the reduction of ammoniacal silver nitrate solution reaction, on the surface of nano-SiO2 spheres to generate nano-silver particles with a smaller particle size, that is, to prepare nano-scale silver-loaded inorganic antibacterial agents; (3) properly modify the surface of the above-mentioned silver-loaded inorganic antibacterial agents; (4) prepare light and heat A dual-cure dental adhesive with the addition of the above-mentioned inorganic antimicrobial agent. The dental adhesive prepared by the invention has multiple advantages such as simple and easy preparation process, stable product system, strong operability, excellent and long-lasting antibacterial performance, and has the potential of clinical application.
Owner:SHANGHAI JIAO TONG UNIV +1
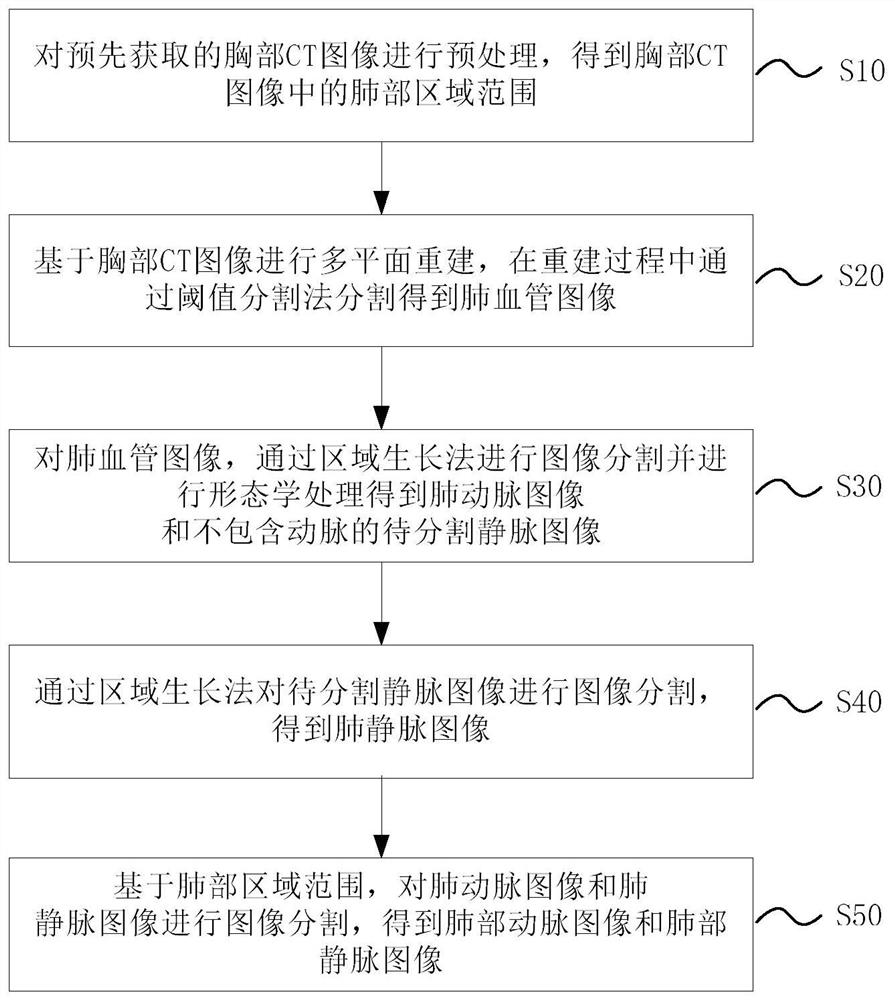
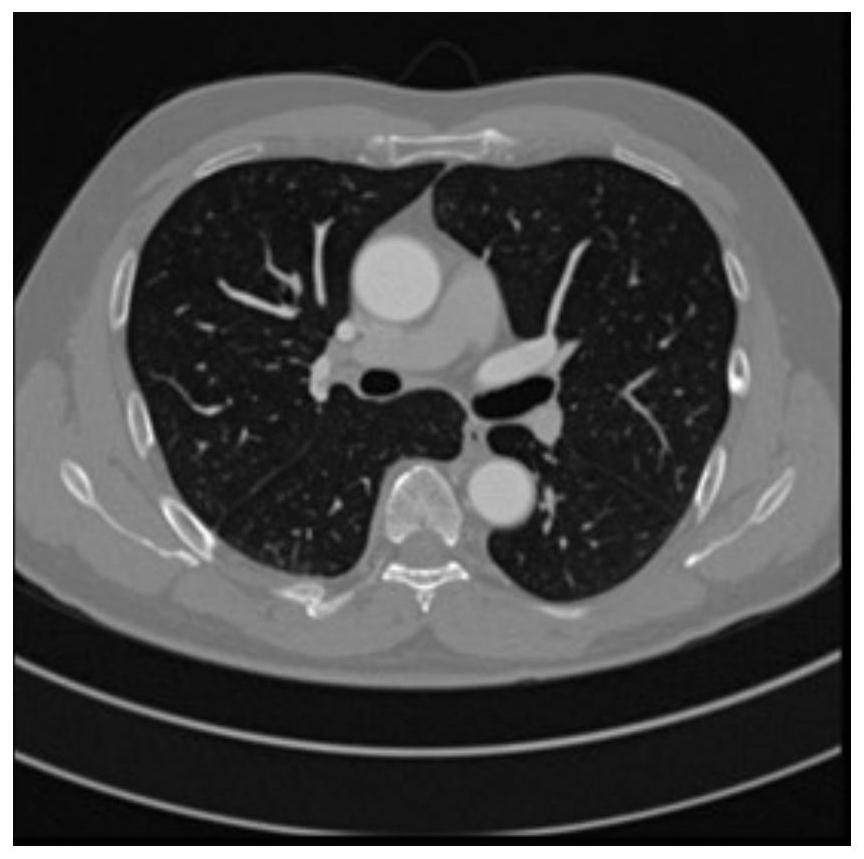
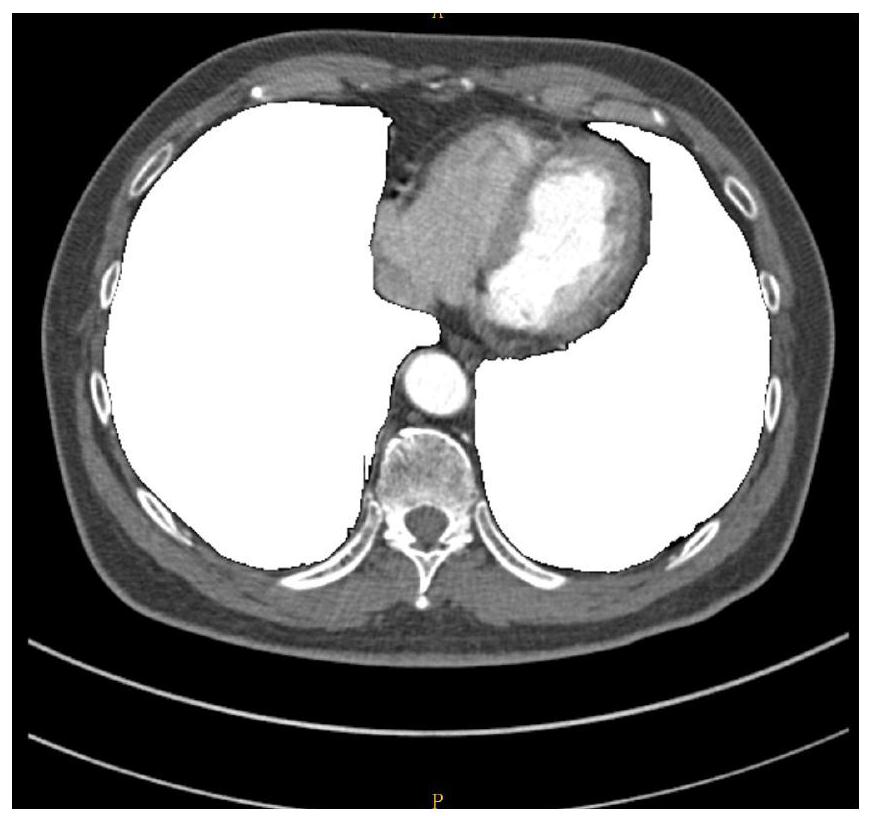
Lung artery and vein segmentation method and device for CT image, medium and equipment
PendingCN113409328AImprove accuracyMeet the needs of clinical applicationsImage enhancementImage analysisImage segmentationLung region
The embodiment of the invention relates to a lung artery and vein segmentation method and device for a CT image, a medium and electronic equipment, and the method comprises the steps: carrying out the preprocessing of a chest CT image obtained in advance, and obtaining a lung region range in the chest CT image; performing multi-plane reconstruction based on the chest CT image, and performing segmentation through a threshold segmentation method in the reconstruction process to obtain a pulmonary blood vessel image; carrying out image segmentation on the pulmonary blood vessel image through a region growing method to obtain a pulmonary artery image and a to-be-segmented vein image which does not contain arteries; performing image segmentation on the vein image to be segmented through a region growing method to obtain a pulmonary vein image; and based on the lung region range, carrying out image segmentation on the pulmonary artery image and the pulmonary vein image to obtain the pulmonary artery image and the pulmonary vein image. According to the lung artery and vein segmentation method, the segmentation result is high in accuracy, and the clinical application requirement can be met.
Owner:NORTHEASTERN UNIV
Method for improving adipogenic differentiation efficiency of mesenchymal stem cells
ActiveCN106350484AImprove the efficiency of adipogenic differentiationHigh biosecuritySkeletal/connective tissue cellsCell culture supports/coatingPolystyreneCytotoxicity
The invention relates to a method for improving the adipogenic differentiation efficiency of mesenchymal stem cells. The method comprises the following steps: performing extraction and primary culture on MSC, performing subculture on MSC, and performing adipogenic differentiation characterization on MSC. According to the method, adipose cells of different quantities are obtained by controlling the replacement quantity of a culture solution and a cell culture period during subculture, so that extremely high MSC adipogenic differentiation efficiency can be still obtained on the surface of a polystyrene cell culture substrate under the condition that an inducible factor is not contained. The method has the characteristics of simple system, absence of extra biological factors, cytotoxicity avoidance, high repeatability and the like. The method is capable of meeting the in-vitro stem cell culture requirements and clinical application requirements.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Thymopentin sustained release microsphere and its preparation method and application
InactiveCN107198765AHigh drug loadingEfficient releasePeptide/protein ingredientsDigestive systemPorosityMedicine
The invention discloses a thymopentin sustained release microsphere and its preparation method and application. The thymopentin sustained release microsphere comprises thymopentin and biodegradable pharmaceutical polymers; the drug loading capacity and porosity of the sustained release microsphere have specific scale. Through controlling the porosity and the drug loading capacity in the scale of the invention, the long-cycle release of the drug can be realized and the internal drug release characteristics of the thymopentin sustained release microsphere can be controlled; thus the thymopentin sustained release microsphere capable of meeting various clinical application demands is prepared.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Fast propagation method for cultivating salt-tolerant and low temperature-tolerant periwinkle strain
InactiveCN102613088AExtended growing seasonLarge biomassPlant tissue cultureHorticulture methodsBiomassEuryhaline
The invention relates to a fast propagation method for cultivating a salt-tolerant and low temperature-tolerant periwinkle strain. The fast propagation method disclosed by the invention is based on preparation of a periwinkle growth culture medium, induction of the growth culture medium and screening of the culture medium in a salt-tolerant and low temperature-tolerant manner, wild periwinkle blades are taken as original materials for inducing an aseptic seedling, and a salt-tolerant and low temperature-tolerant periwinkle strain cultivation system is established by fast propagation and multi-generation induction acclimatization of the aseptic seedling, wherein the alkaloid content of the salt-tolerant and low temperature-tolerant periwinkle strain is higher than that of wild periwinkle by about 20%. The fast propagation method disclosed by the invention has the advantages that, (1) the cultivation process is controllable completely and can not affected by a natural environment; (2) the salt-tolerant and low temperature-tolerant periwinkle can grow normally on a saline-alkali mudflat; and (3) the growth period of the periwinkle in the Yangtze River valley is prolonged and the biomass of the periwinkle is increased.
Owner:上海安体康生植物化学有限公司
Method for obtaining maternal mesenchymal stem cells from placenta
PendingCN111793598AIncrease the number ofHigh purityCell dissociation methodsSkeletal/connective tissue cellsEnzyme digestionMesenchymal stem cell
The invention discloses a method for obtaining maternal mesenchymal stem cells from placenta. The method for obtaining the maternal mesenchymal stem cells from the placenta comprises the following steps: obtaining placenta tissues from a position, which are within 5cm away from the radius of an umbilical cord and are 0.5 cm away from a fetal surface, of the placenta; and carrying out enzyme digestion treatment on placenta tissues by adopting an enzyme digestion solution, and then carrying out cell culture on a product of the enzyme digestion treatment to obtain the maternal mesenchymal stem cells. According to the method disclosed by the invention, the technical problem of separating the maternal mesenchymal stem cells from the placenta is solved, the prepared maternal mesenchymal stem cells are large in quantity and high in purity, neonatal cells are not easy to mix, and the clinical application requirements can be met; the method provided by the invention solves the problems that thequantity of the maternal mesenchymal stem cells obtained from the decidua basalis is small and the decidua basalis is easy to lose, and provides a new scheme and way for obtaining the maternal mesenchymal stem cells from the placenta.
Owner:深圳华大基因细胞科技有限责任公司
Preparation of polyester/periodic mesoporous bone-filling composite material with fluorescently-labeled degradation rate, product and application
ActiveCN109568675AImprove luminous efficiency and stabilityImprove dispersion uniformityTissue regenerationProsthesisPoly l lactic acidNanoparticle
The invention relates to preparation of polyester / periodic mesoporous bone-filling composite material with a fluorescently-labeled degradation rate, a product and application. The preparation comprises the steps of firstly, synthesizing rare-earth periodic mesoporous nanoparticles with fluorescence performance, then, subjecting the rare-earth periodic mesoporous nanoparticles to chemical modification by taking poly-L-lactic acid with low molecular weight as a modifier, and finally, adding the modified nanoparticles into a polyester matrix by a solution blending method. The invention further provides the product obtained by the method and application of the product in bone restoration as a composite bone filling material. The fluorescent mesoporous material obtained by the method provided by the invention is uniformly dispersed in an organic matrix, mechanical properties of a polyester are improved greatly, and the degradation behavior of the composite bone filling material can be monitored in real time. The preparation process is simple, and the composite bone filling material can serve as a bone filling material of load bearing bone parts with relatively high performance index requirements. The obtained composite material can meet the requirements of clinical application.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Quantitative detection kit for human Dickkopf-1 protein (DKK-1)
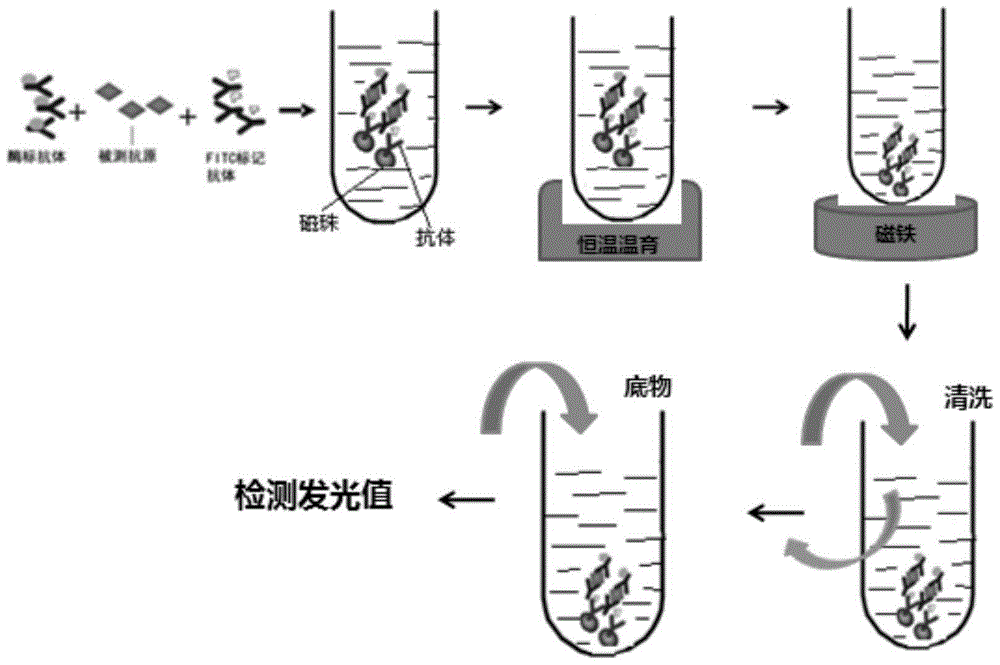
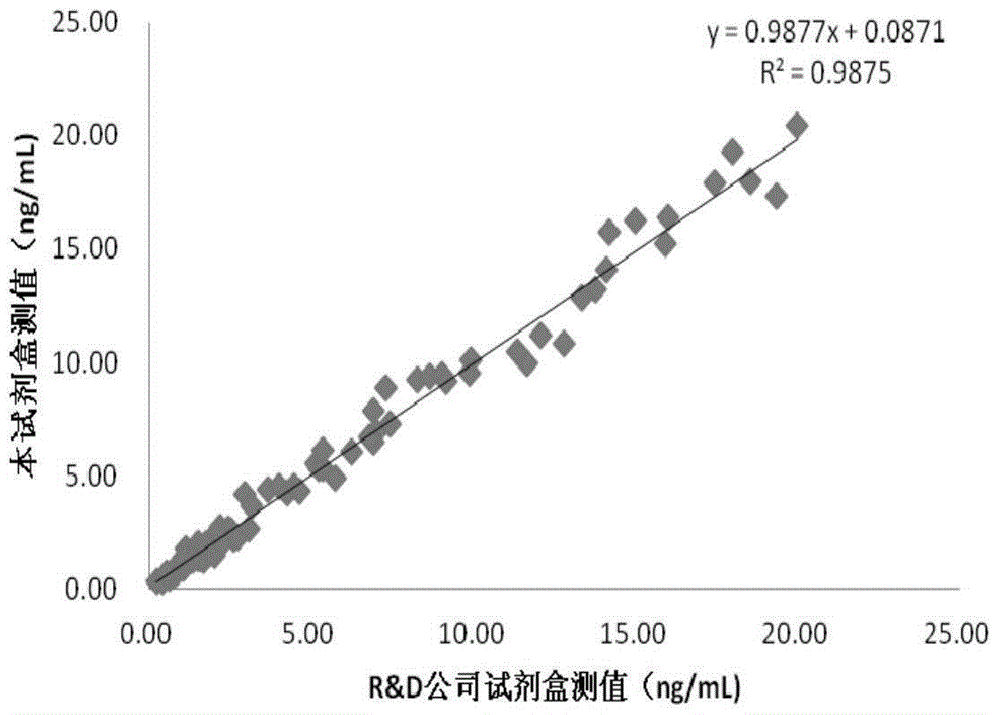
InactiveCN105203773AWide detection rangeHigh sensitivityBiological testingFluorescein isothiocyanateLuminescence
The invention provides a quantitative detection kit for human Dickkopf-1 protein (DKK-1). The quantitative detection kit comprises a calibrator, a quality control product, a DKK-1 monoclonal antibody labeled by fluorescein isothiocyanate and DKK-1 monoclonal antibody labeled by alkaline phosphatase solution, a magnetic separation reagent, cleaning liquid and a substrate solution. An adopted detection method combines magnetic particle separation chemiluminescence immune detection, enzyme labeling technology, magnetic separation technology and chemiluminescence technology, and is convenient and simple to operate, mild in reaction condition, stable in luminescence value and little in influence by outside conditions. Compared with existing detection methods, the quantitative detection kit has the advantages of simple sample treatment process, low detection cost, quickness in detection, accurate testing result and high repeatability.
Owner:CHENGDU CAPITALBIODX MEDICAL TECH CO LTD +1
No-mark surgery registration method used for optical surgery navigation system
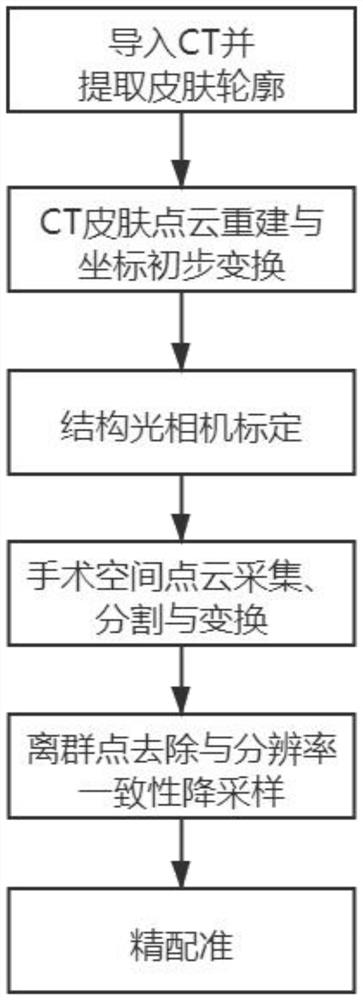
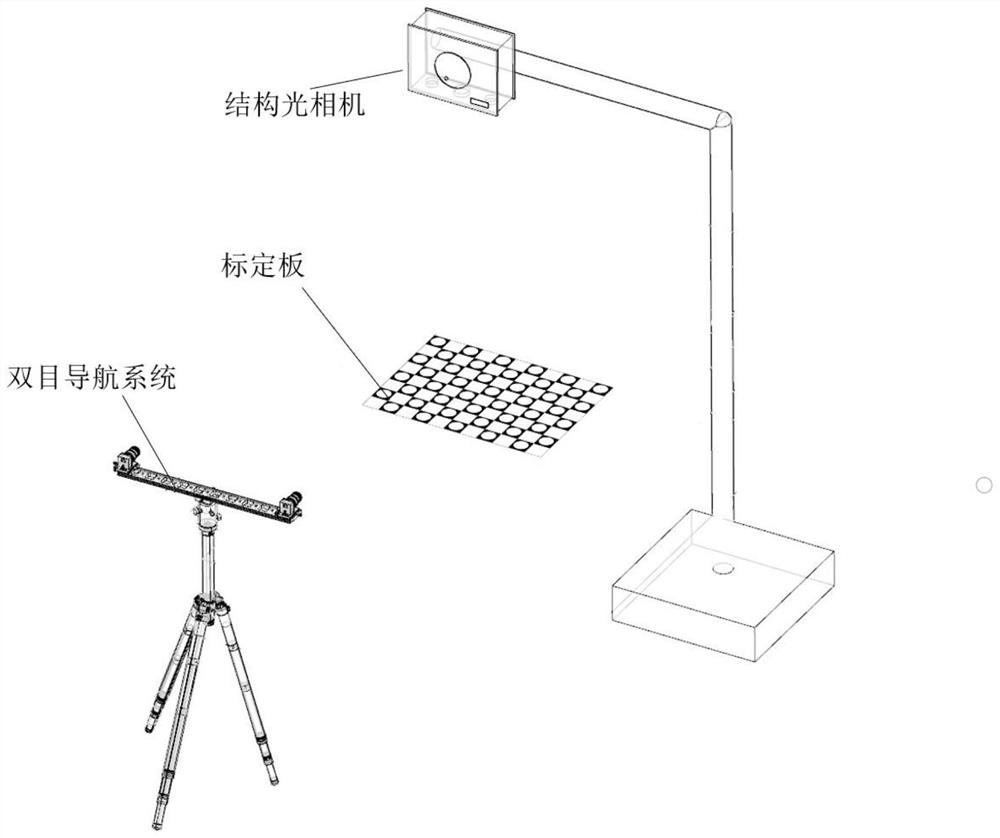
PendingCN113274130AFully automated surgical registrationMeet the needs of clinical applicationsSurgical navigation systemsPoint cloudImage resolution
The invention discloses a no-mark surgery registration method used for an optical surgery navigation system. The no-mark surgery registration method used for the optical surgery navigation system comprises the following steps of: S1: importing the CT sequence of a focus position, processing a CT image, and extracting an upper surface skin outline; S2: reconstructing a two-dimensional skin outline into a three-dimensional point set, and carrying out preliminary coordinate transformation on the CT coordinate system of the point set; S3: calibrating a structured light camera to the coordinate system of a surgical space; S4: utilizing the structured light camera to collect the point cloud of the surgical space, and finishing segmentation and transformation of the point cloud; S5: removing outliers in the surgical space, and adopting resolution ratio consistency to carry out downsampling to obtain a simplified image and the simplified point cloud of the surgical space; and S6: carrying out registration on the point cloud of a CT image space to the surgical space to finish surgery registration. A surgery registration process disclosed by the invention does not need human intervention, and enough surgery navigation accuracy can be guaranteed while surgery registration operation complexity is lowered and operation time is shortened.
Owner:SHANGHAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com