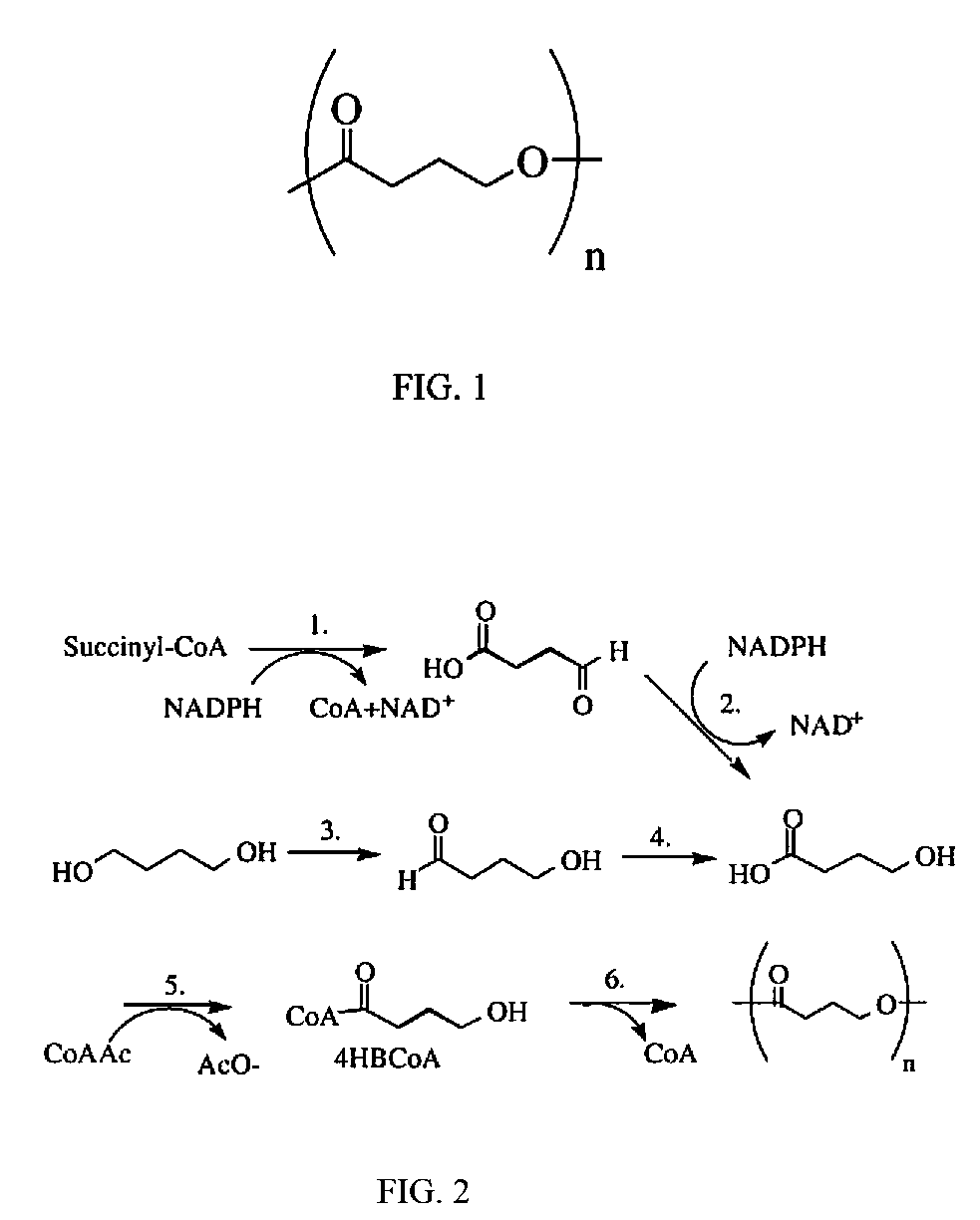
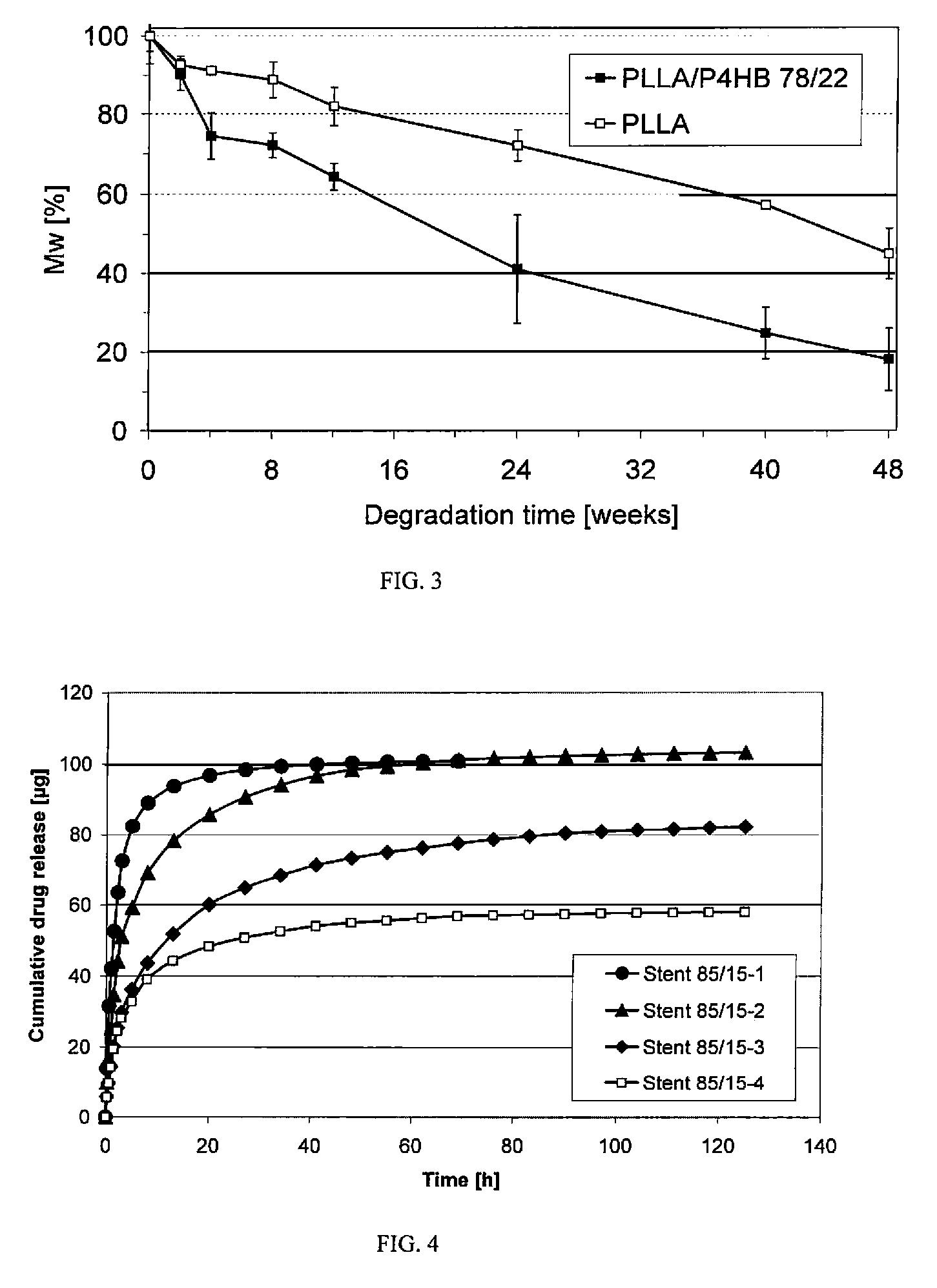
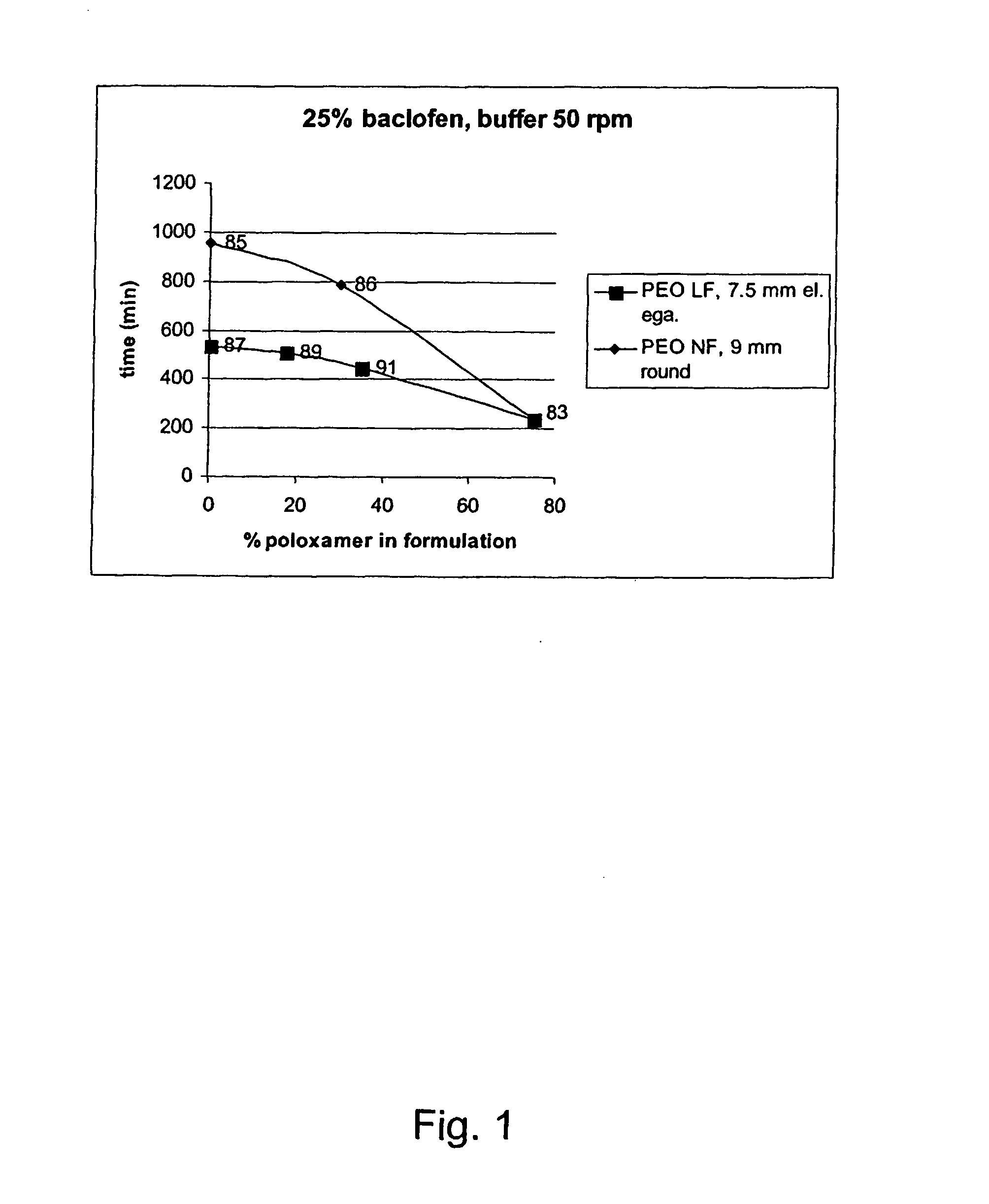
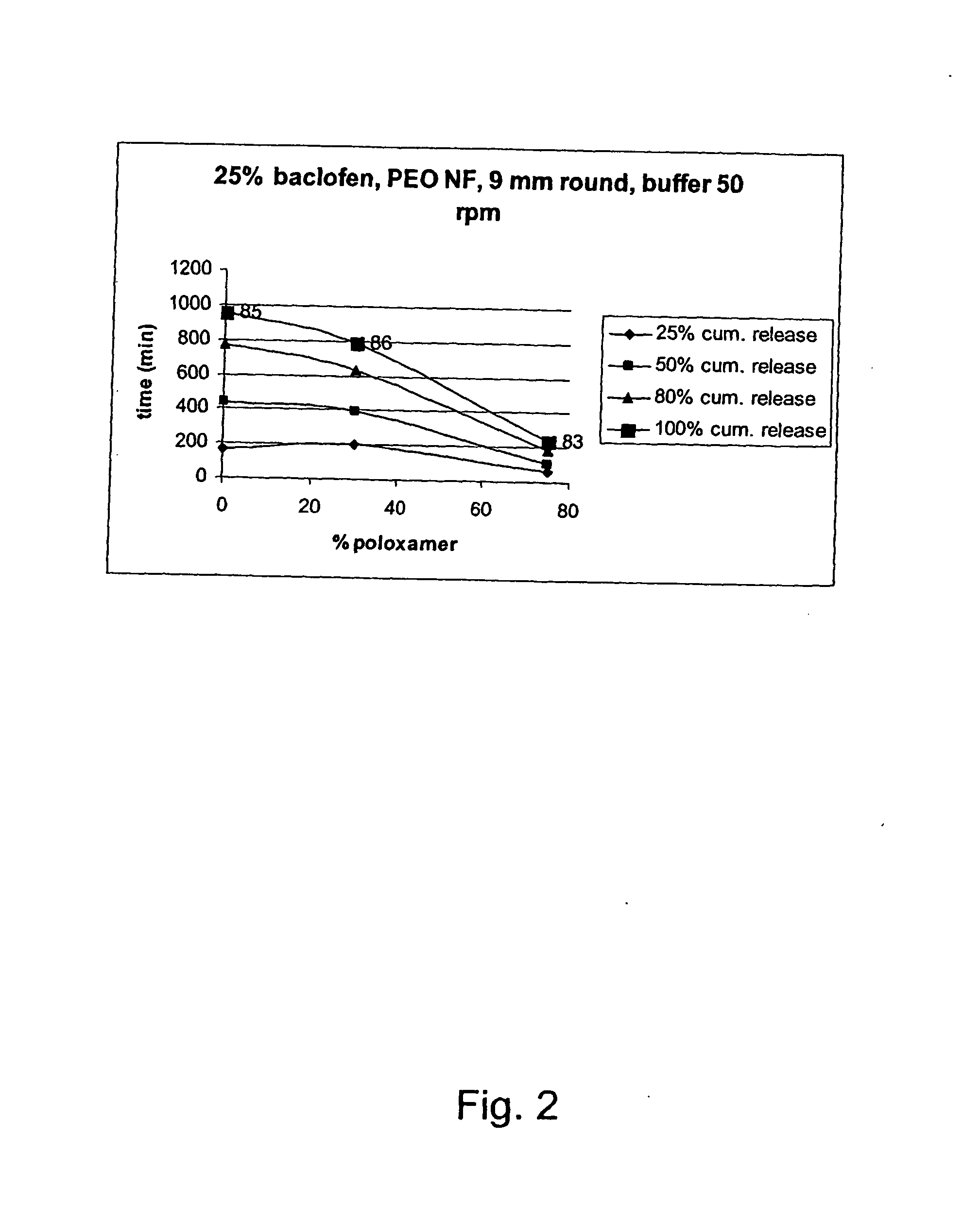
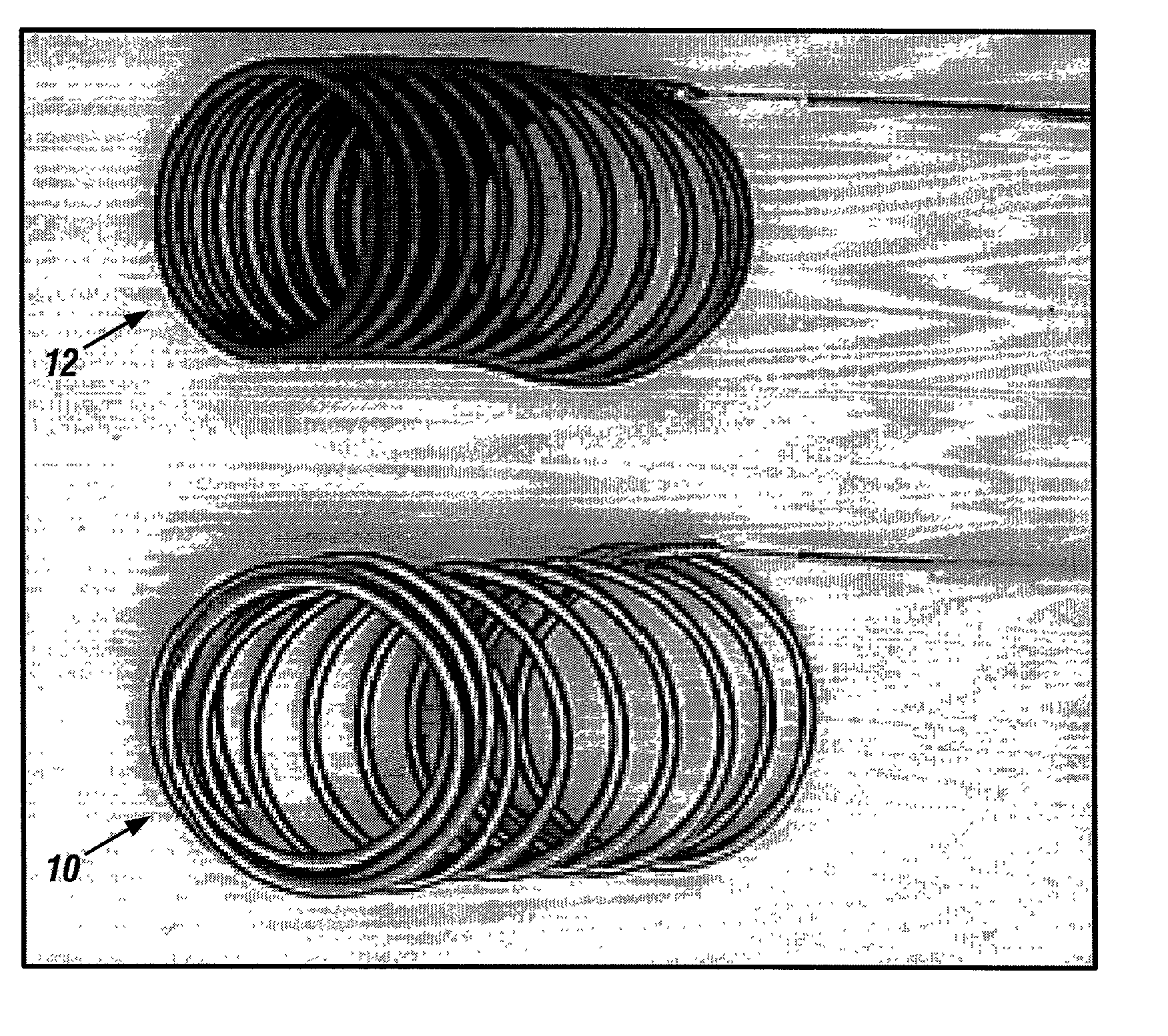
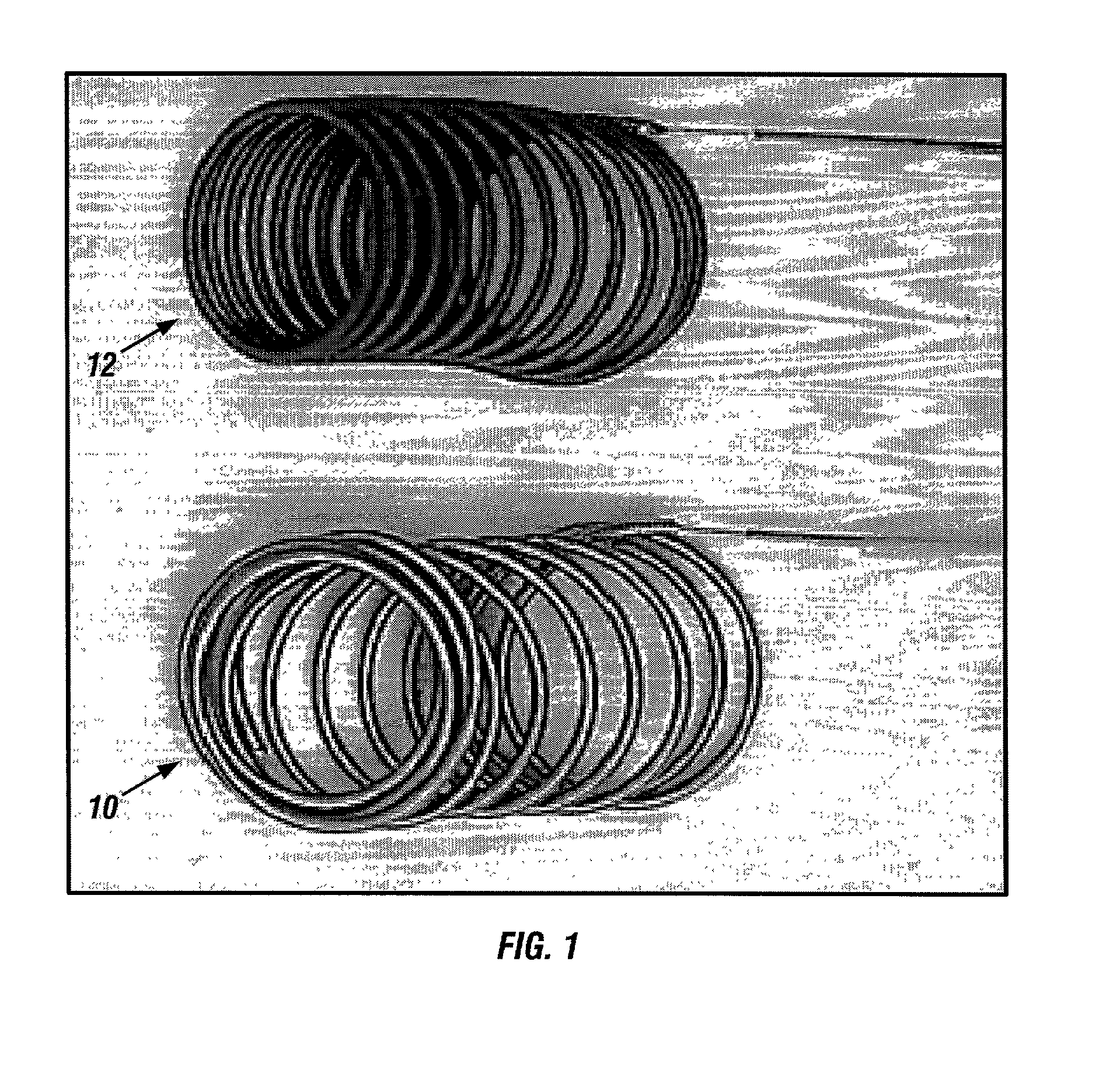
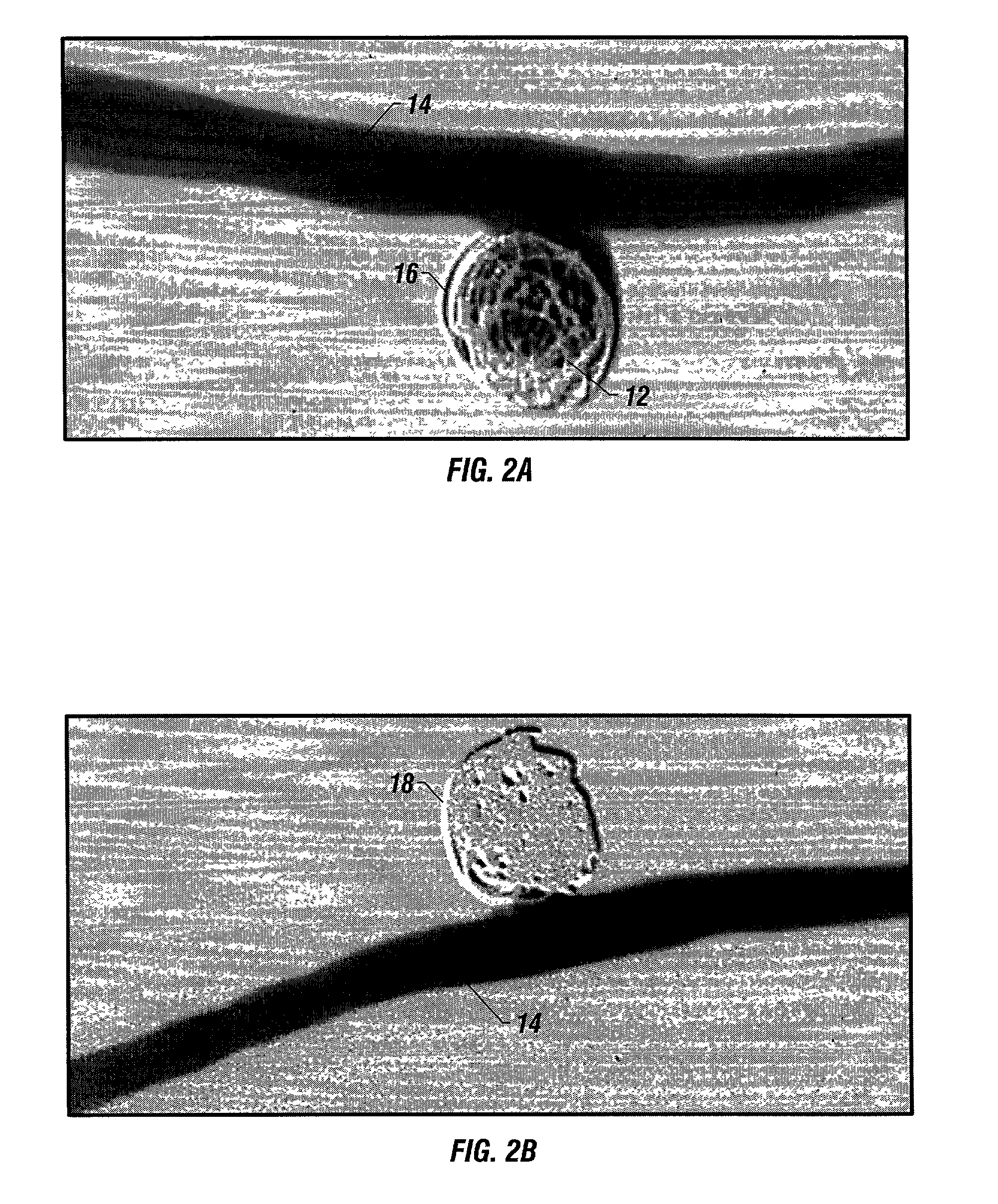
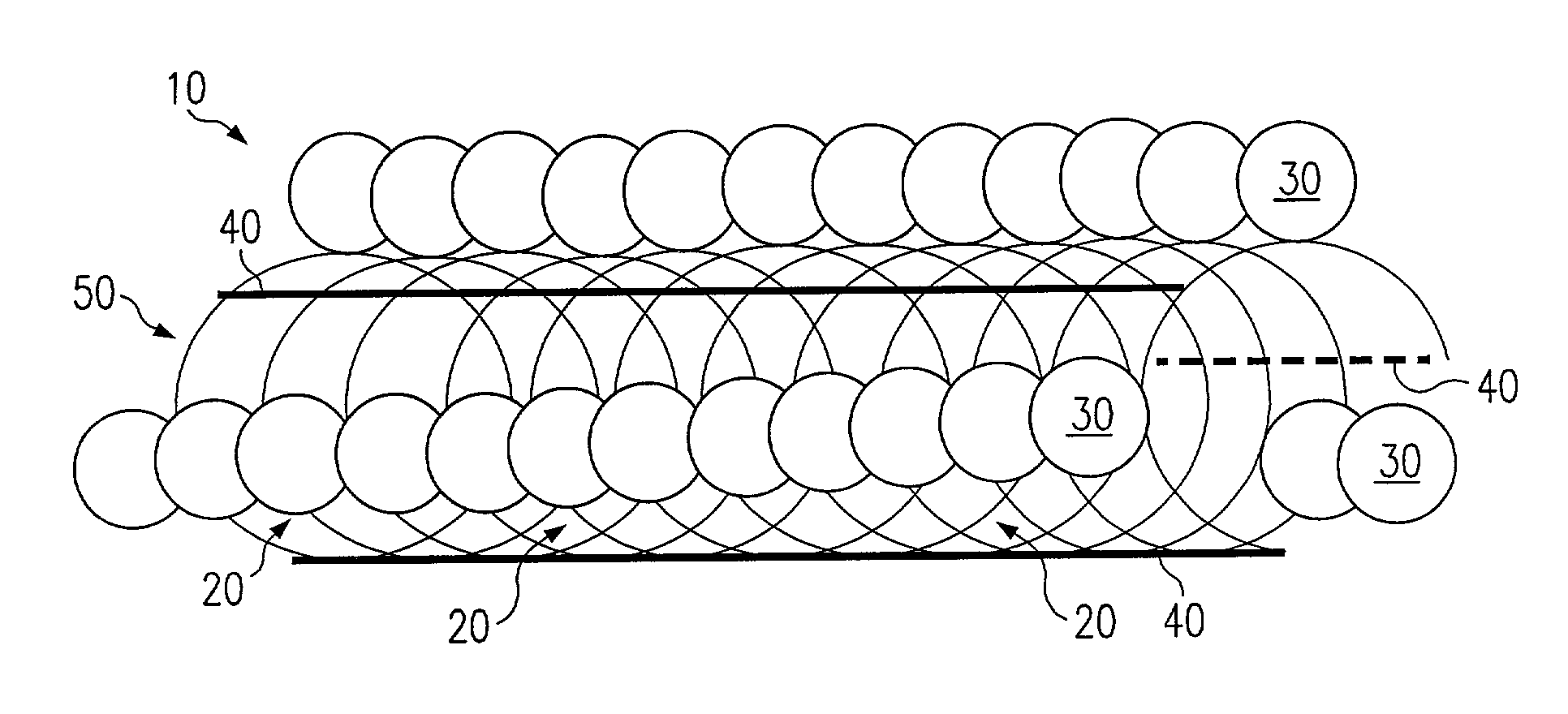
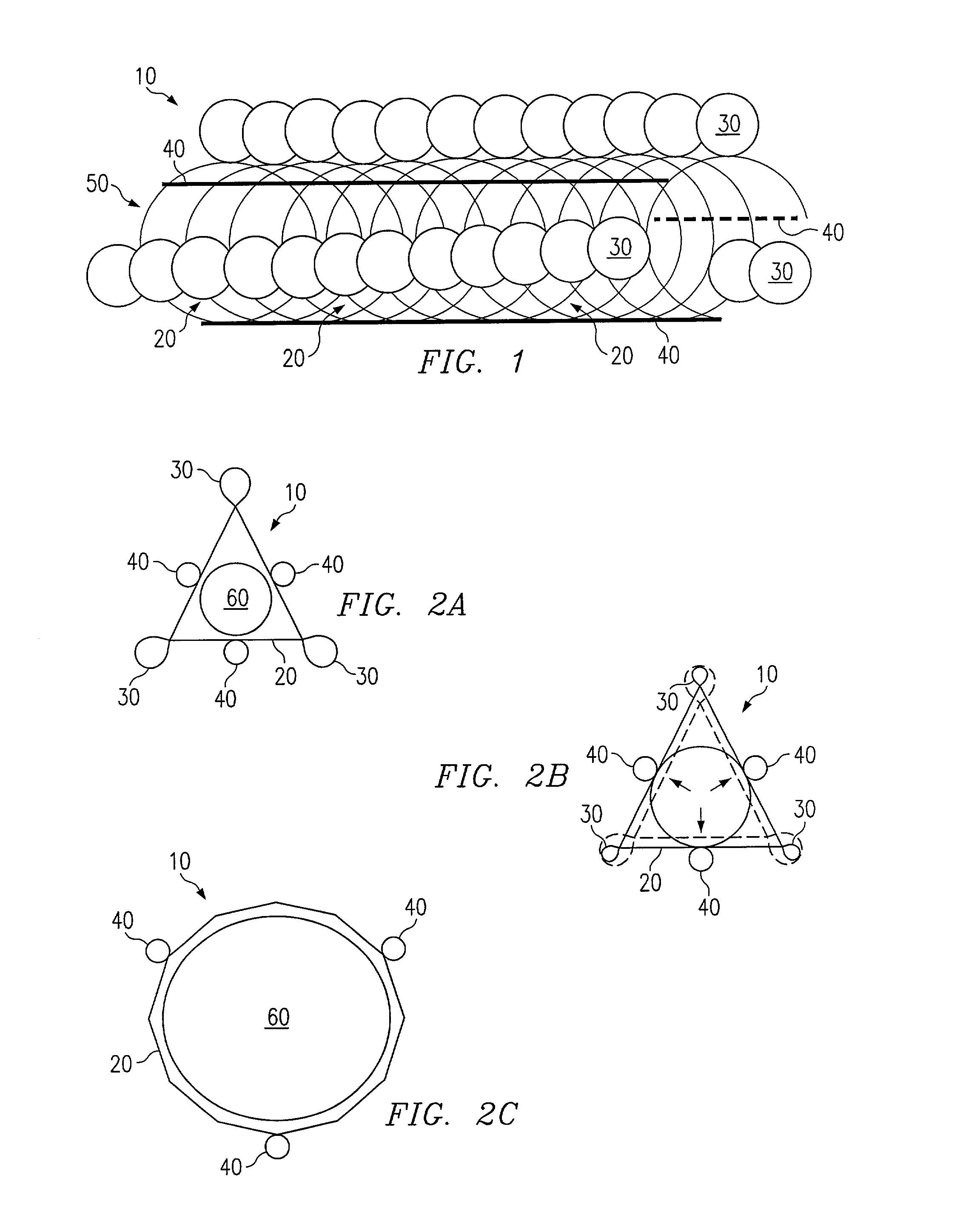
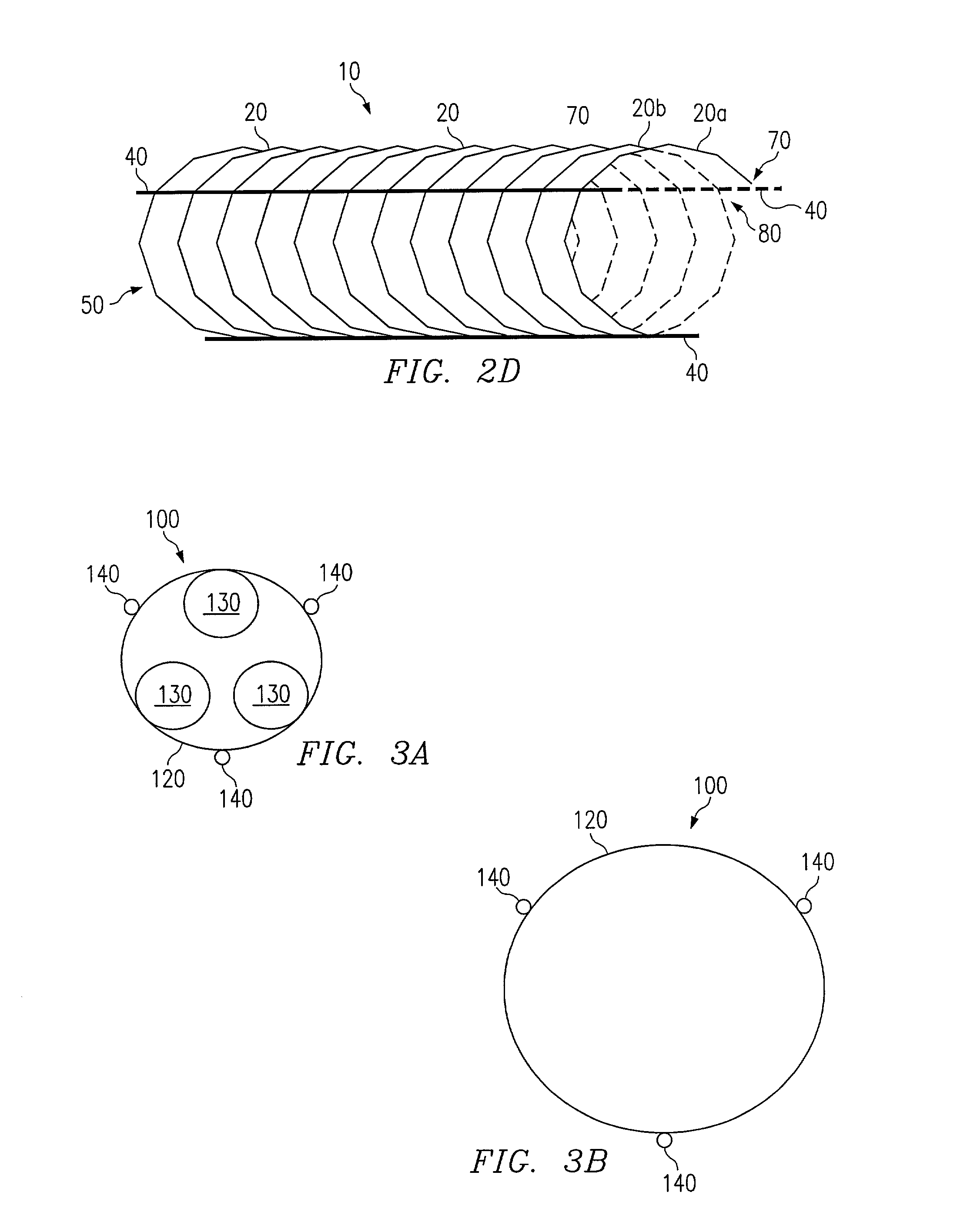
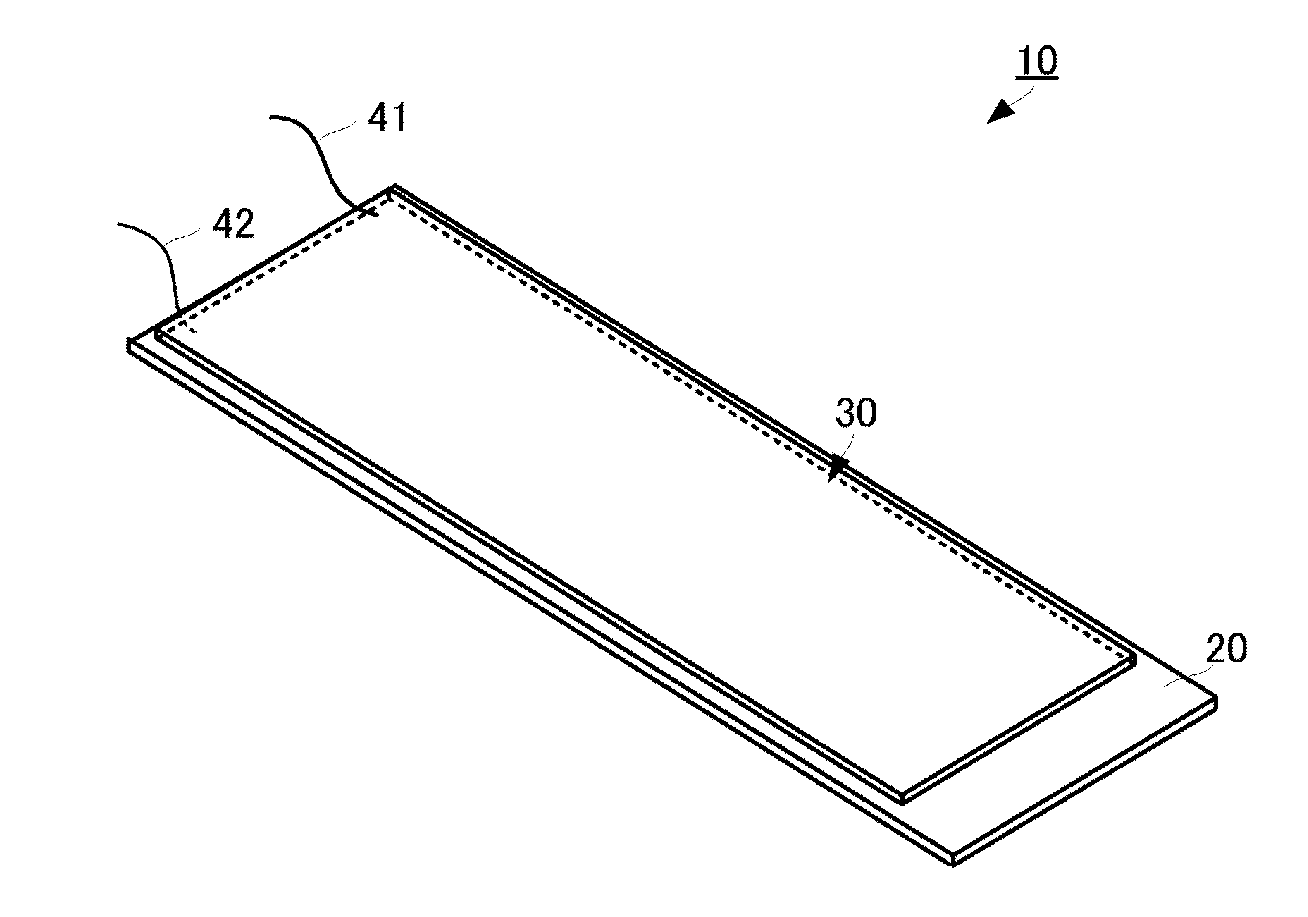
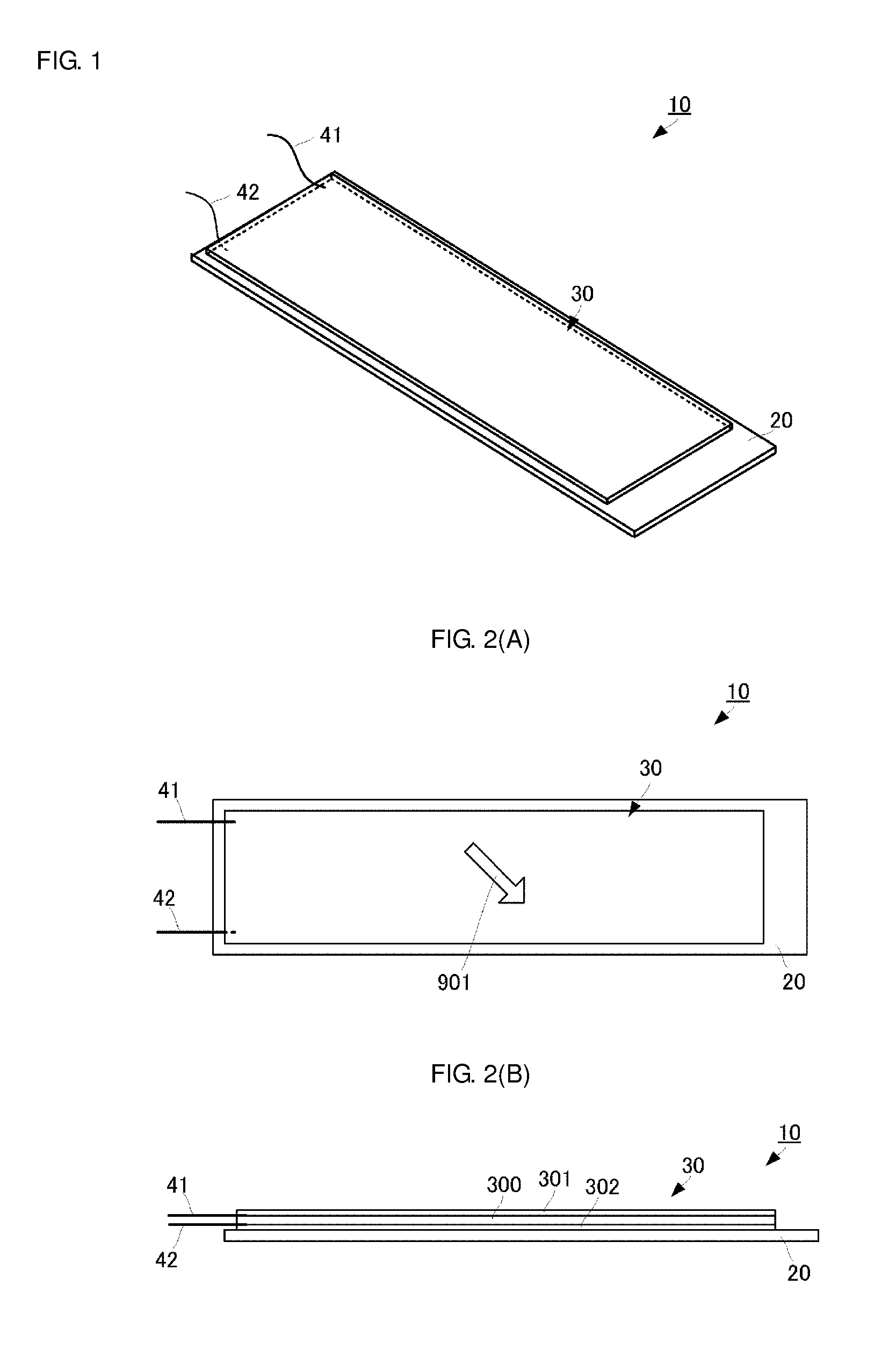
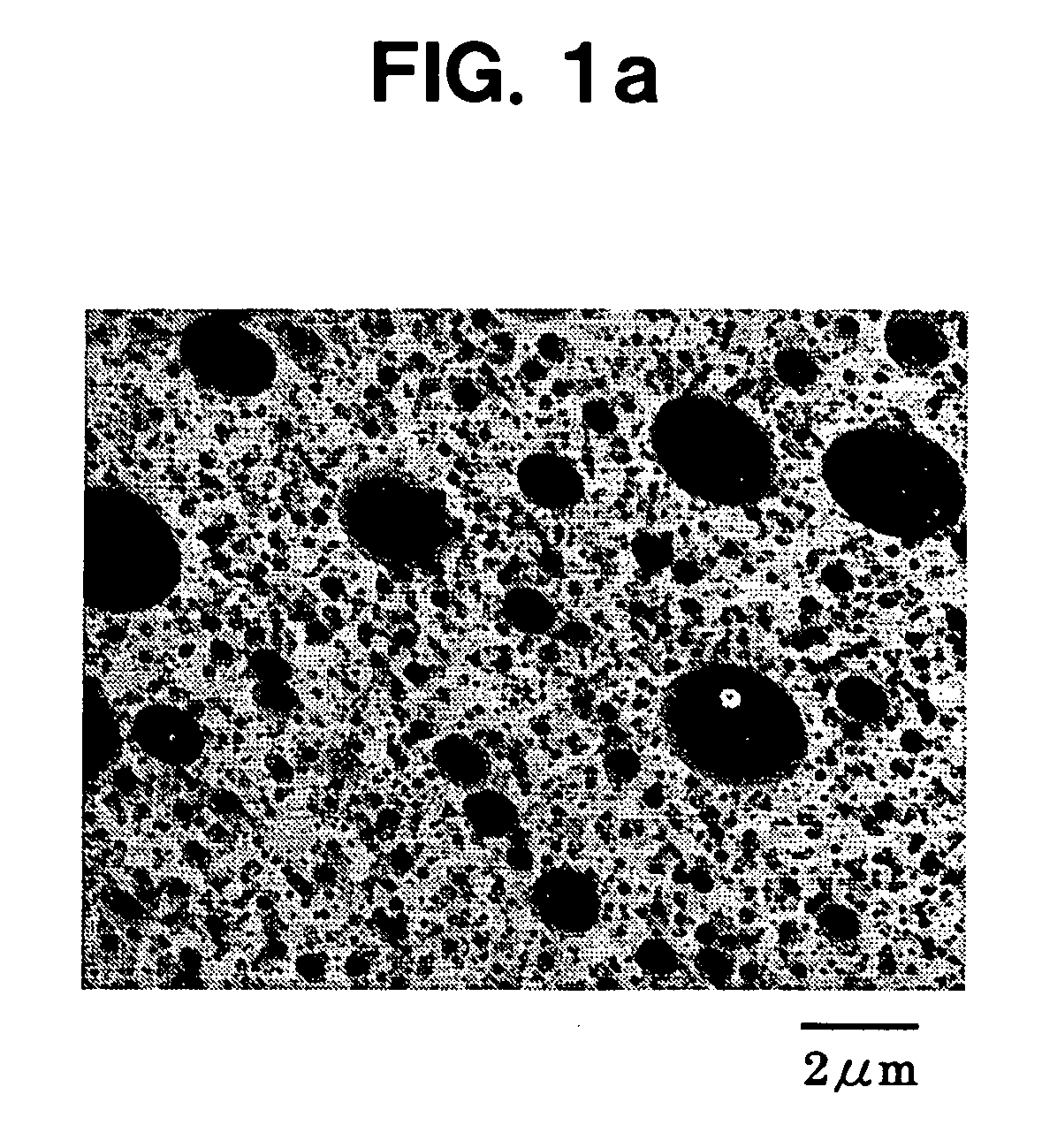
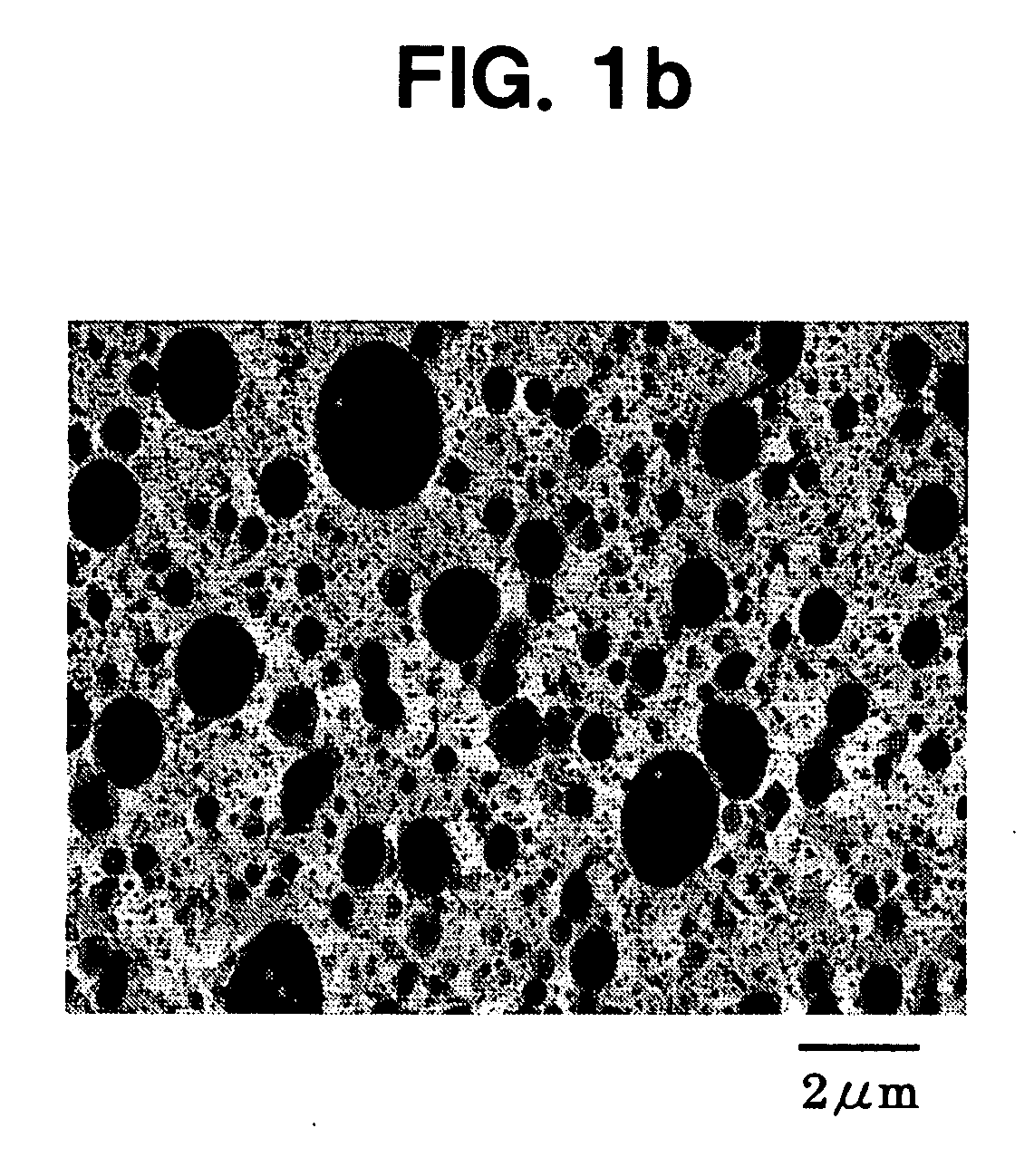
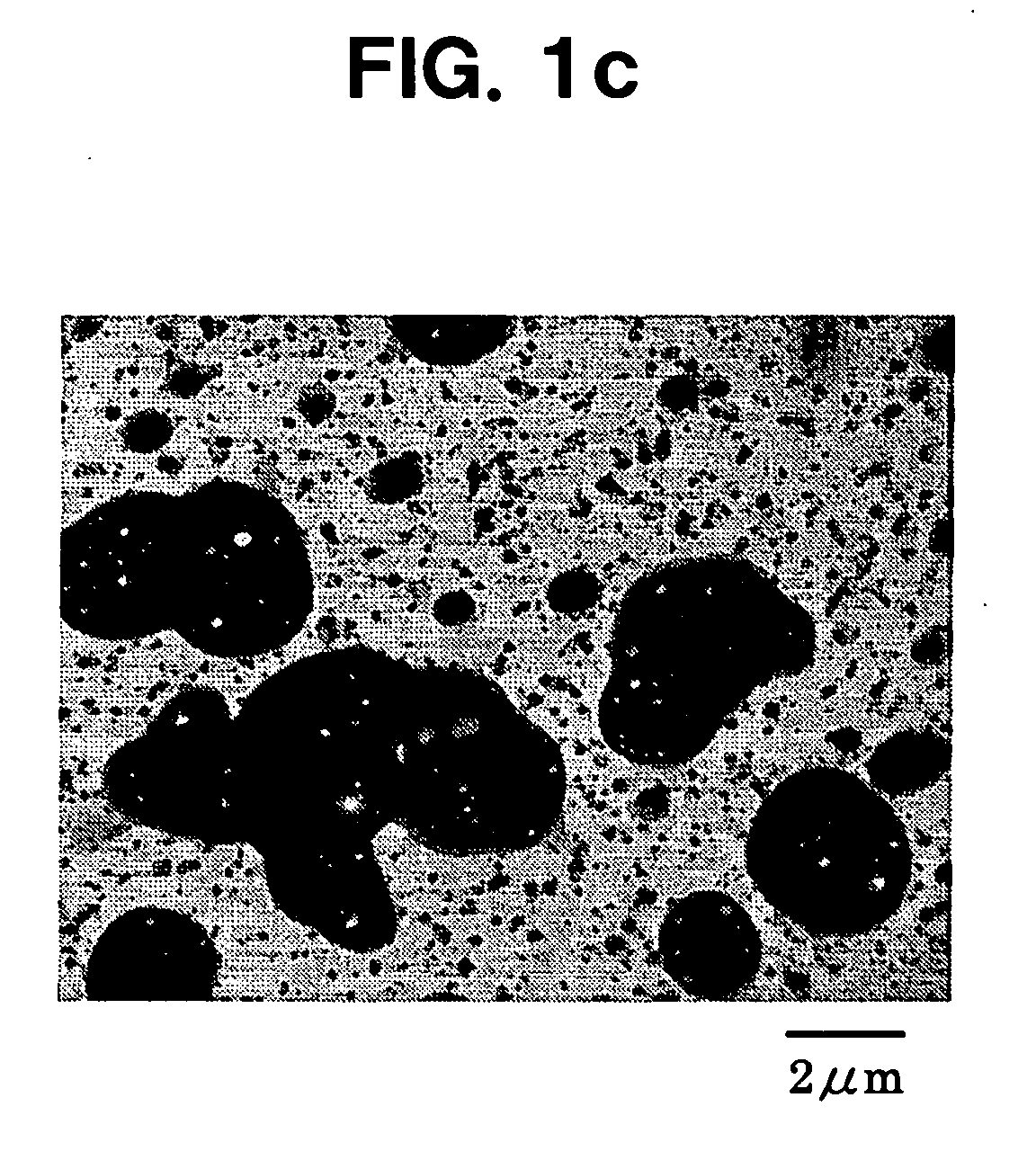
Patents
Literature
1266 results about "Poly l lactic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Introduction. Poly-l-lactic acid (PLLA) is a synthetic polymer derived from lactic acid that is both biocompatible and biodegradable. The purpose of this chapter is to discuss current techniques used with PLLA to effectively and safely address changes observed with facial aging. Proper patient selection and assessment,...
Soluble deverting agents
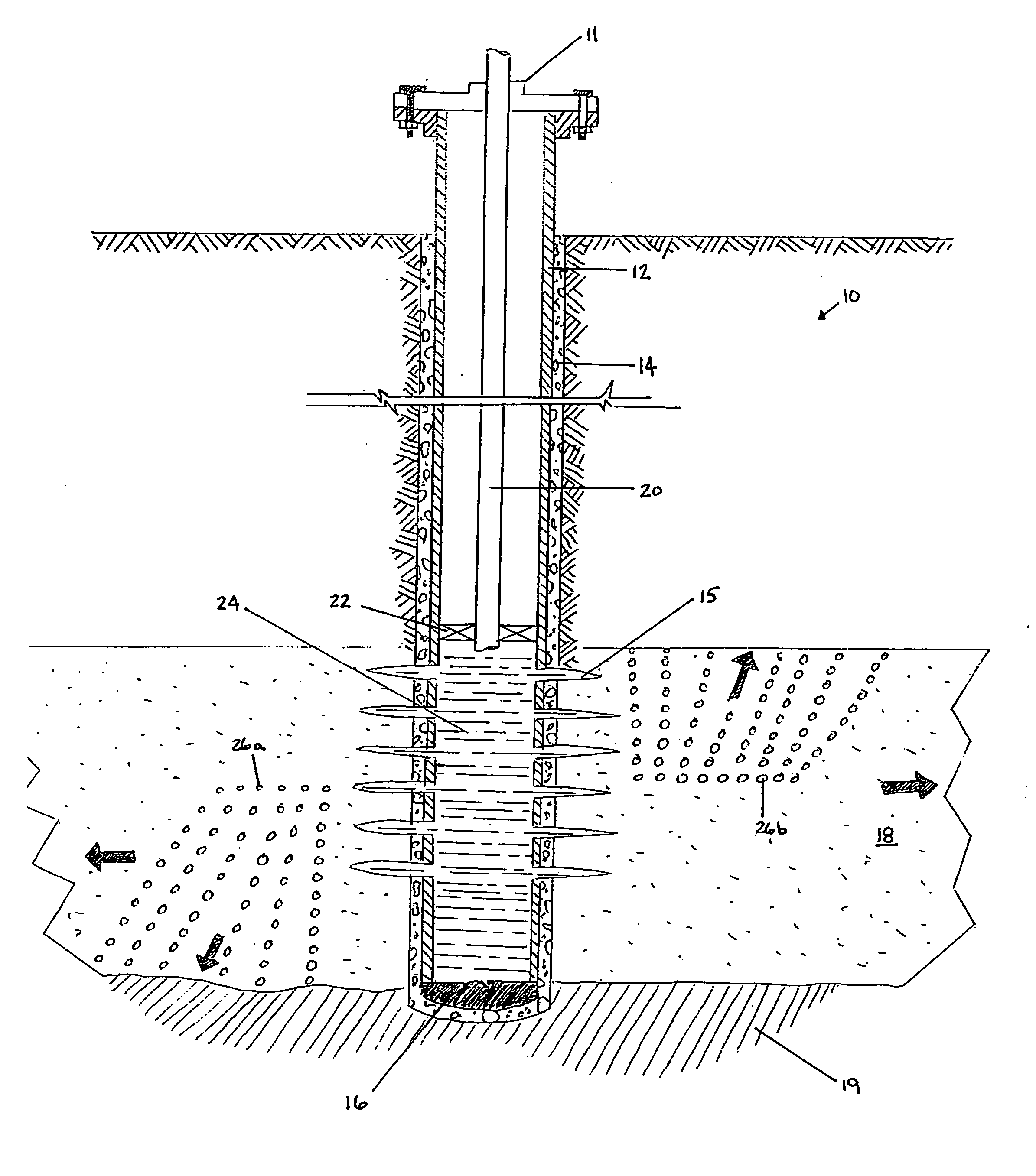
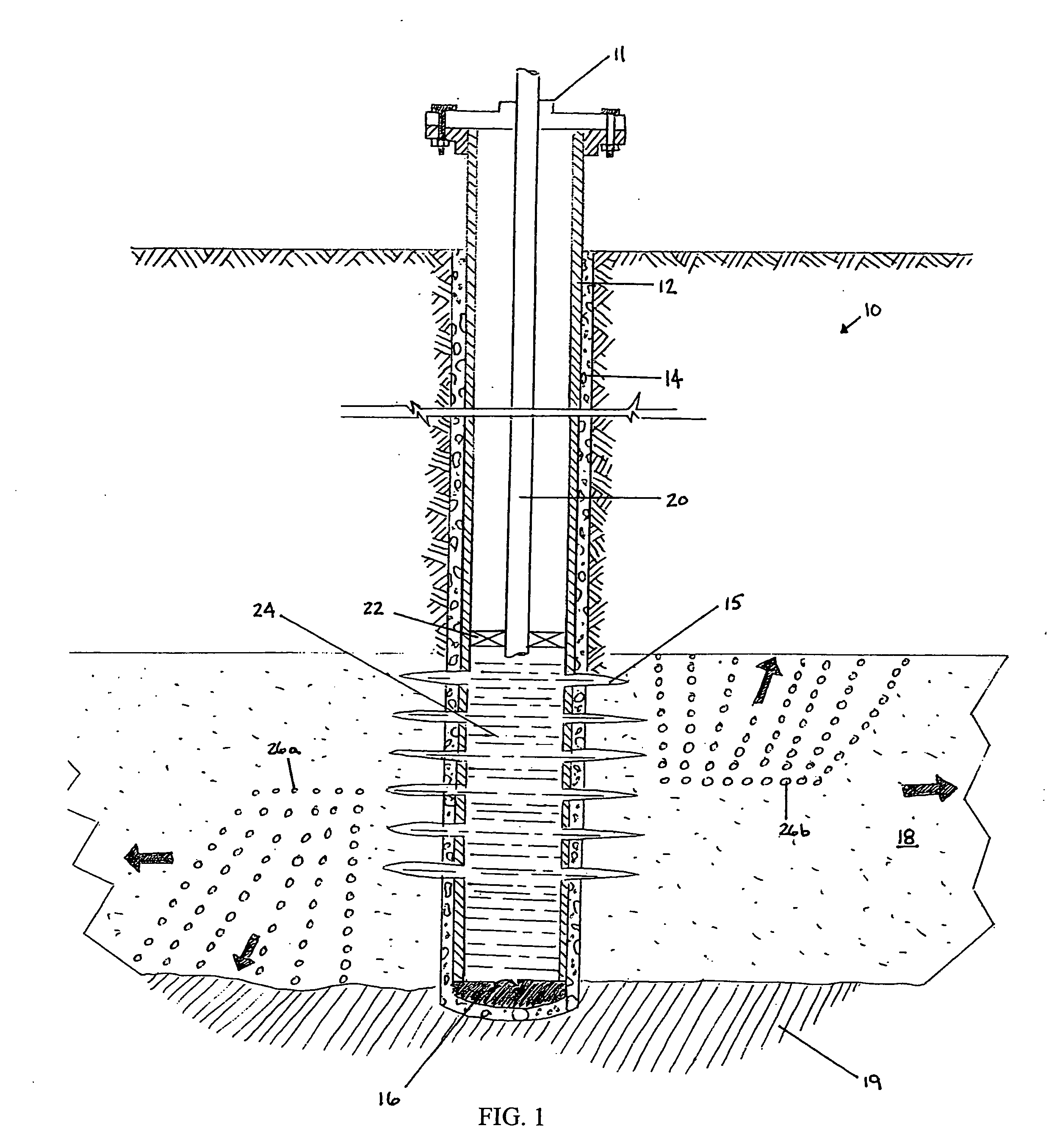
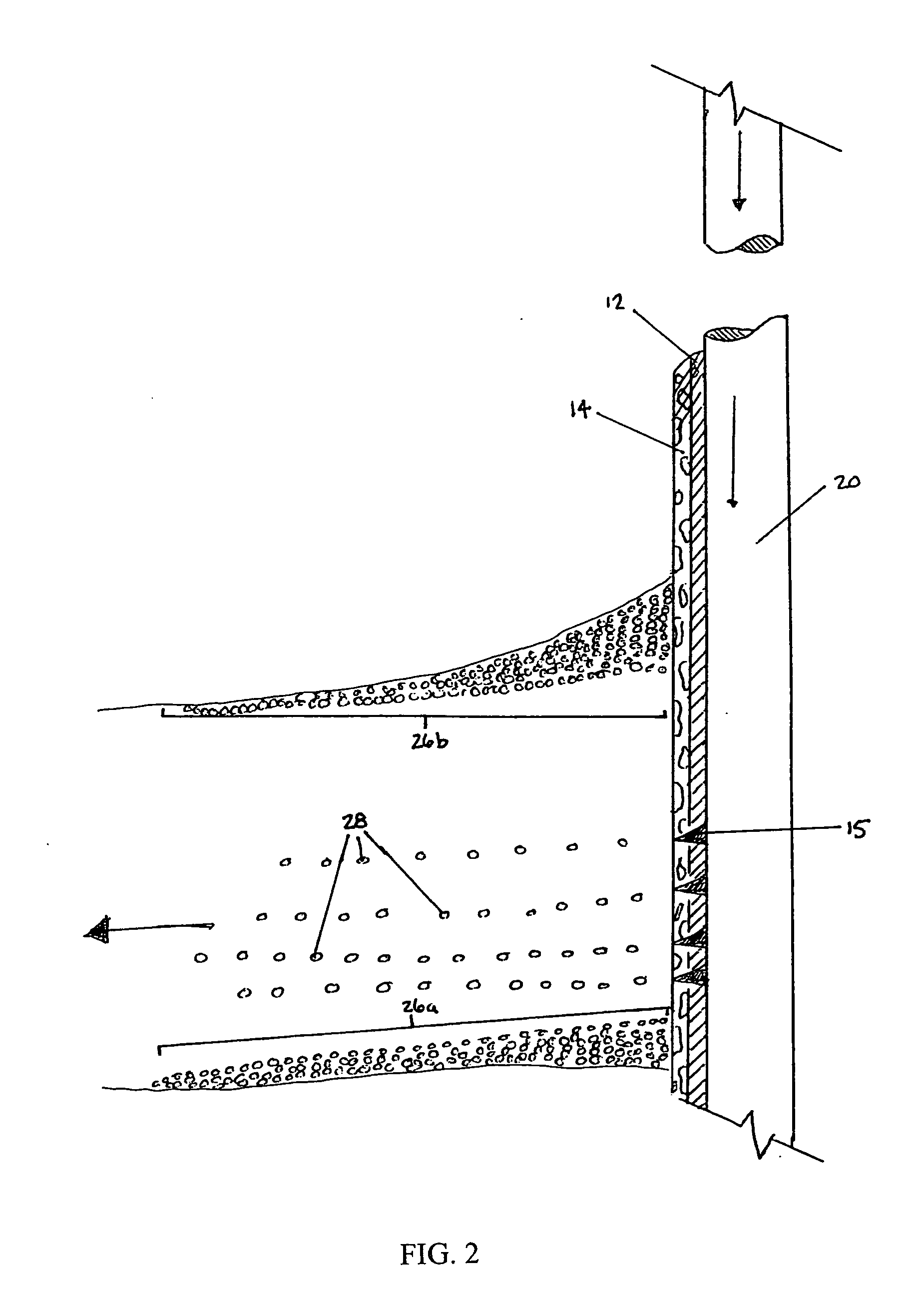
Methods and compositions for stimulating single and multiple intervals in subterranean wells by diverting well treatment fluids into a particular direction or into multiple intervals using water soluble coated diverting agents are described. The water soluble coating of the diverting material is preferably a collagen, poly(alkylene) oxide, poly(lactic acid), polyvinylacetate, polyvinylalcohol, polyvinylacetate / polyvinylalcohol polymer or a mixture thereof applied as a coating on any number of proppants. The method allows for the diverting of the flow of fluids in a downhole formation during a well treatment, such as during a fracturing process. Following completion of a treatment such as a hydraulic stimulation, the soluble diverting agent can be dissolved and removed by the water component of the well production.
Owner:FAIRMOUNT SANTROL
Bioabsorbable polymeric implants and a method of using the same to create occlusions
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly˜glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
Polymeric, degradable drug-eluting stents and coatings
ActiveUS7618448B2Improve performanceImprove propertiesSuture equipmentsPharmaceutical containersAbsorbable polymersEthylene Homopolymers
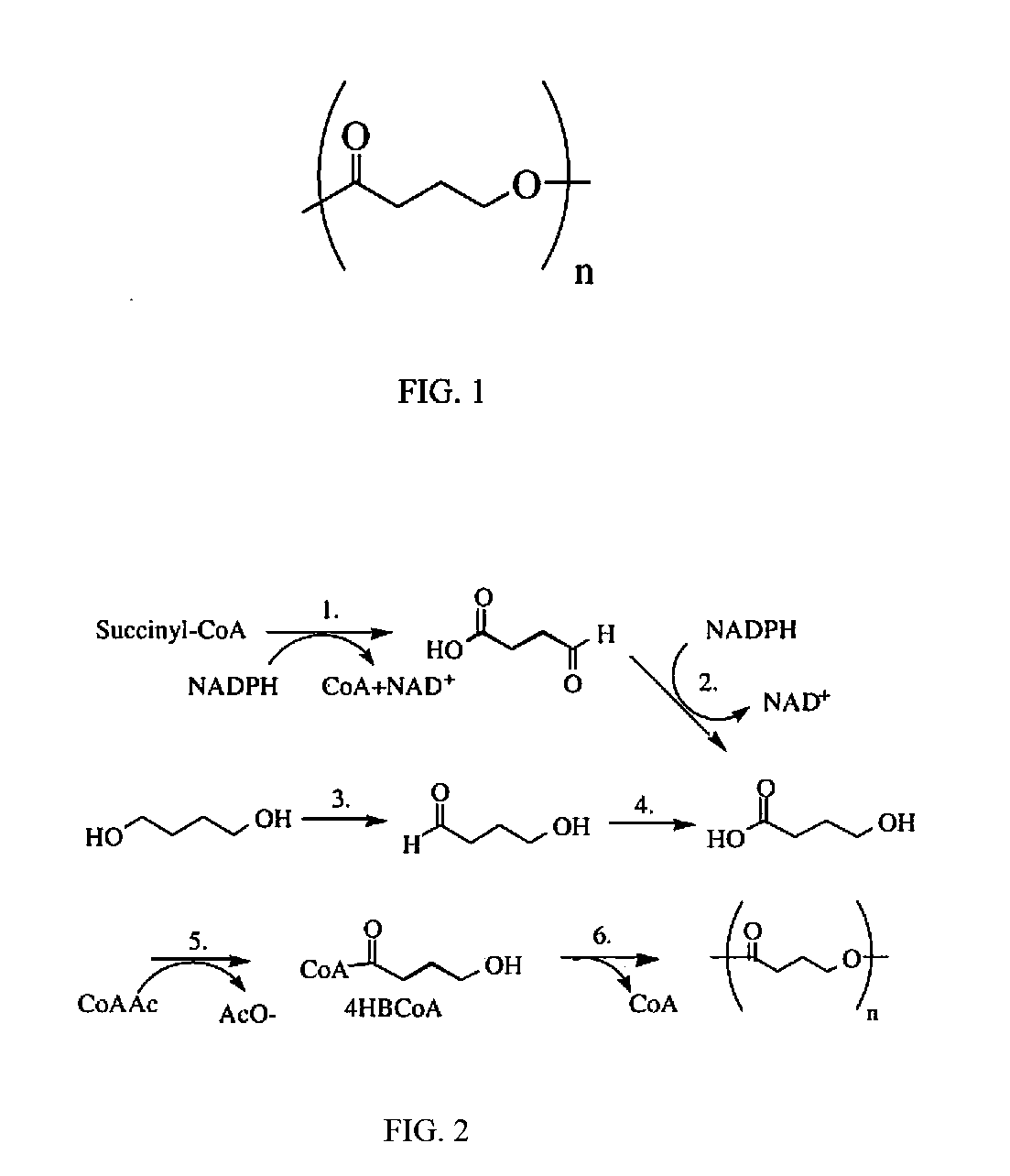
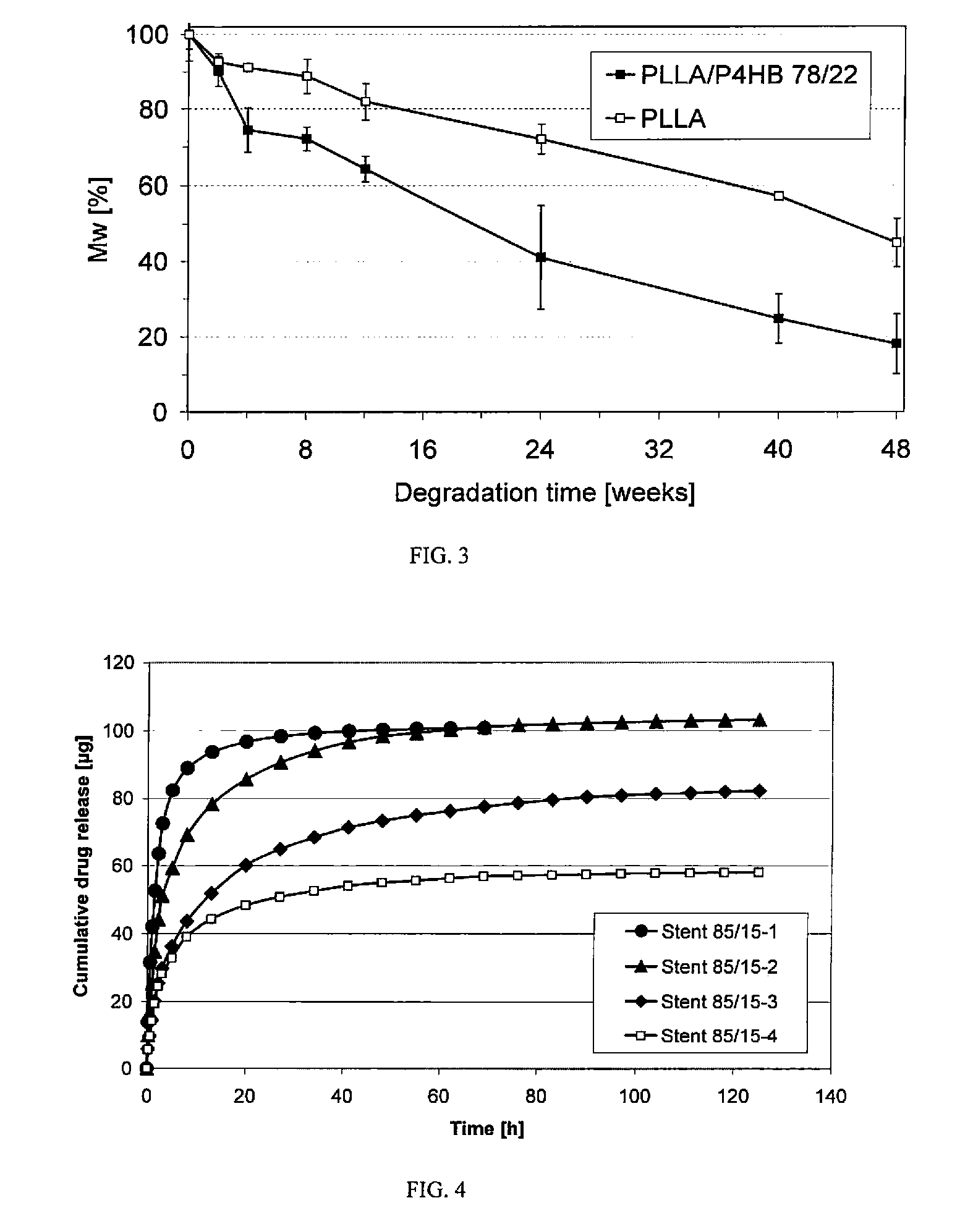
Absorbable stents and absorbable stent coatings have been developed with improved properties. These devices preferably comprise biocompatible copolymers or homopolymers of 4-hydroxybutyrate, and optionally poly-L-lactic acid and other absorbable polymers and additives. Compositions of these materials can be used to make absorbable stents that provide advantageous radial strengths, resistance to recoil and creep, can be plastically expanded on a balloon catheter, and can be deployed rapidly in vivo. Stent coatings derived from these materials provide biocompatible, uniform coatings that are ductile, and can be expanded without the coating cracking and / or delaminating and can be used as a coating matrix for drug incorporation.
Owner:TEPHA INC
Matrix compositions for controlled delivery of drug substances
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
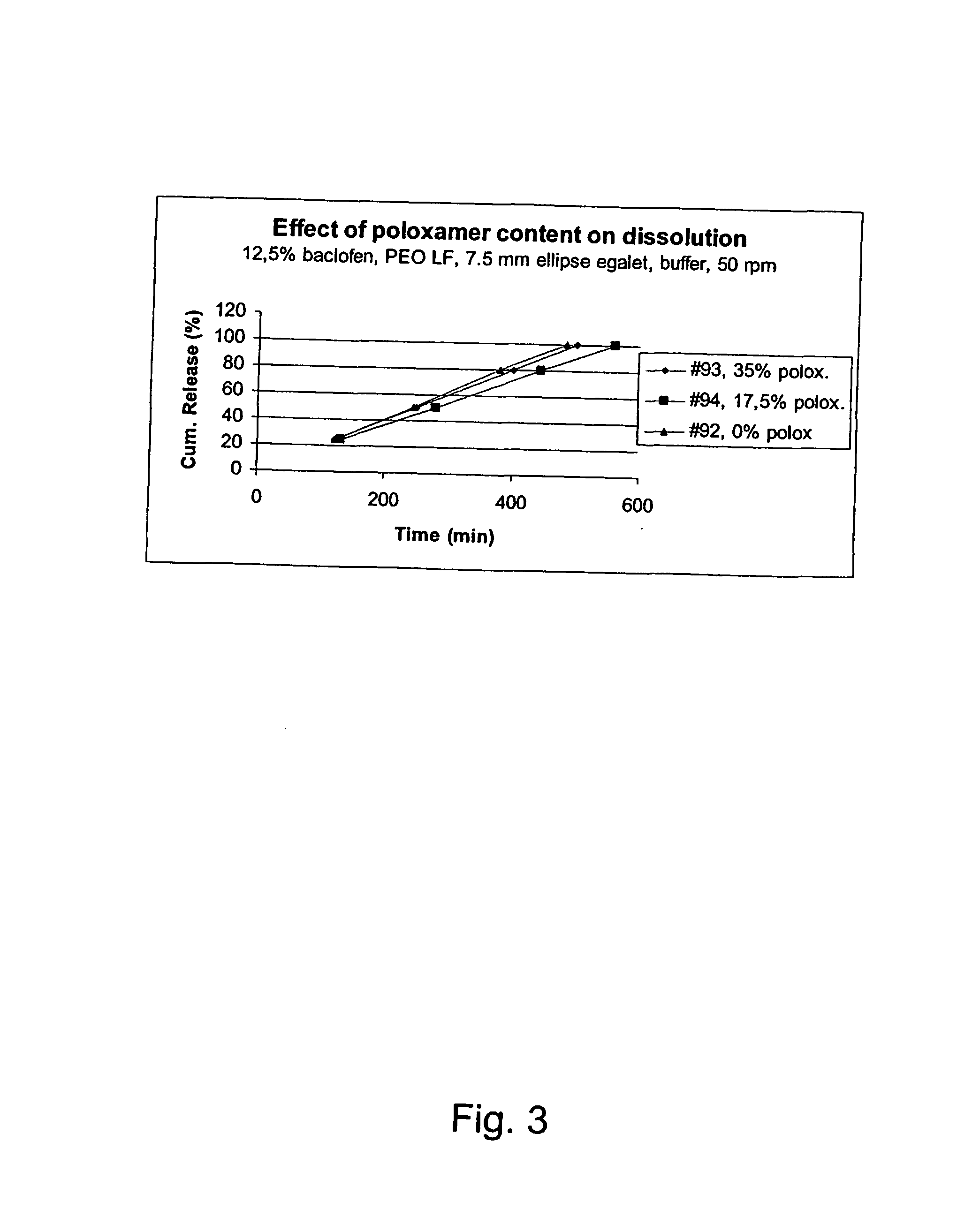
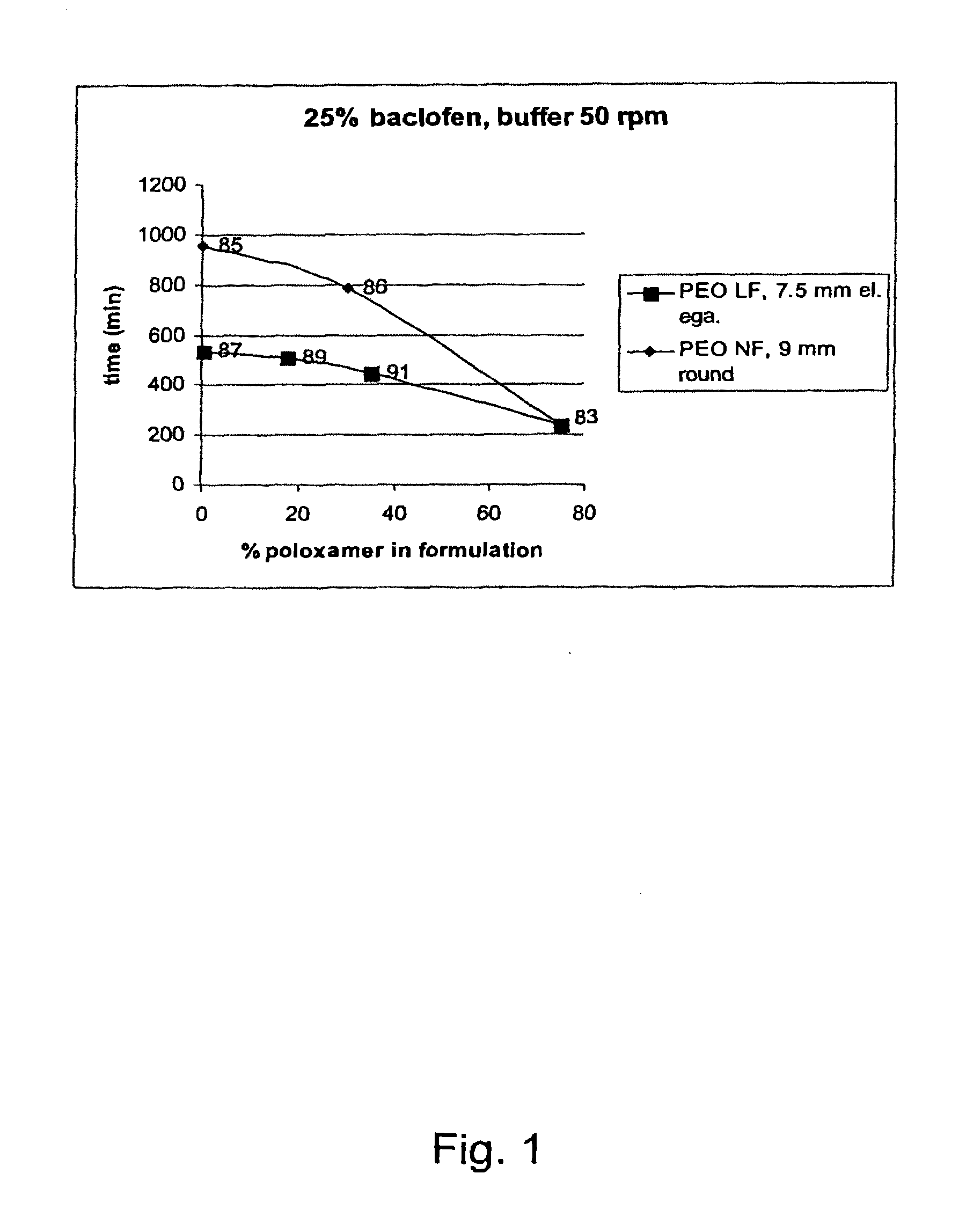
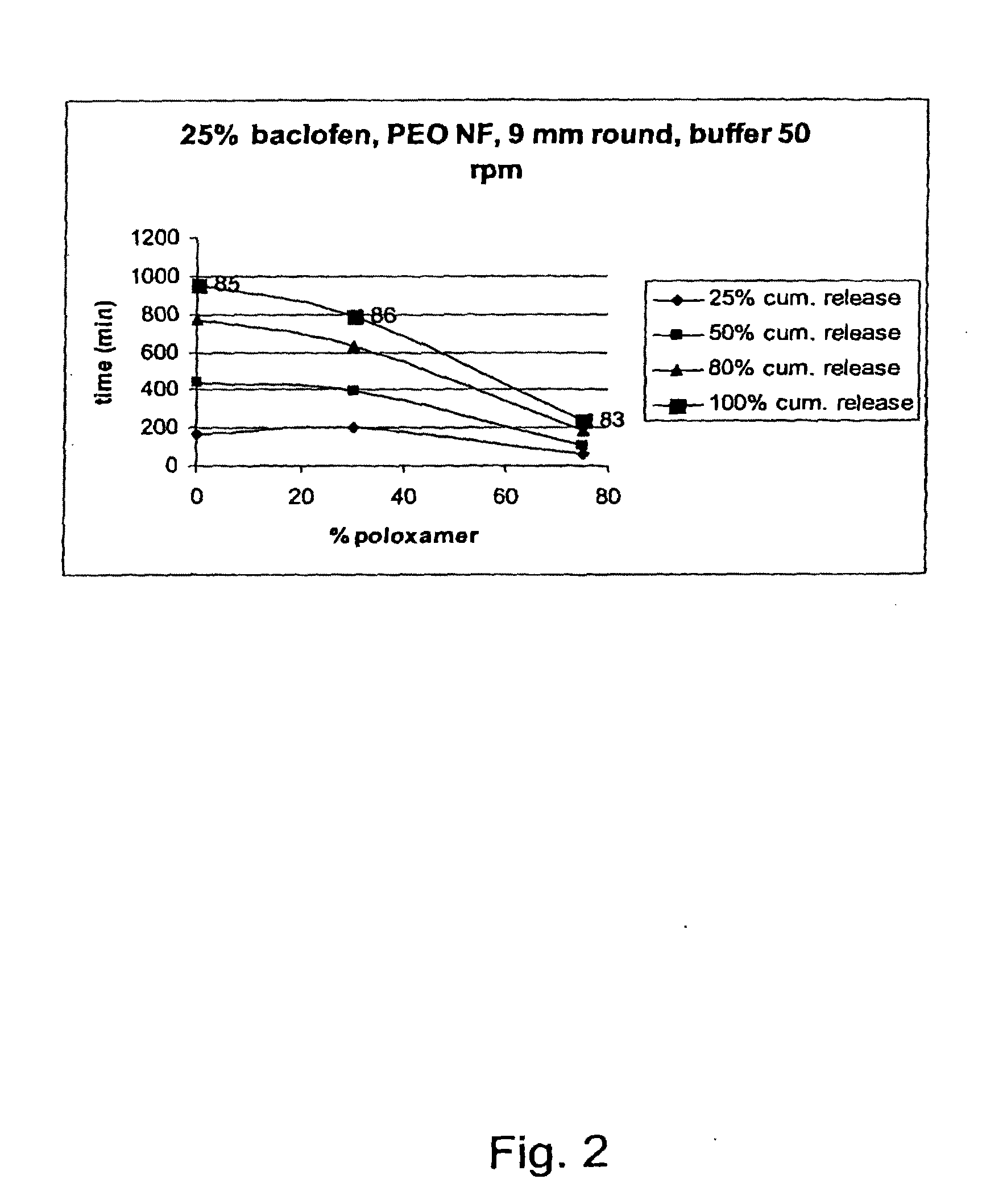
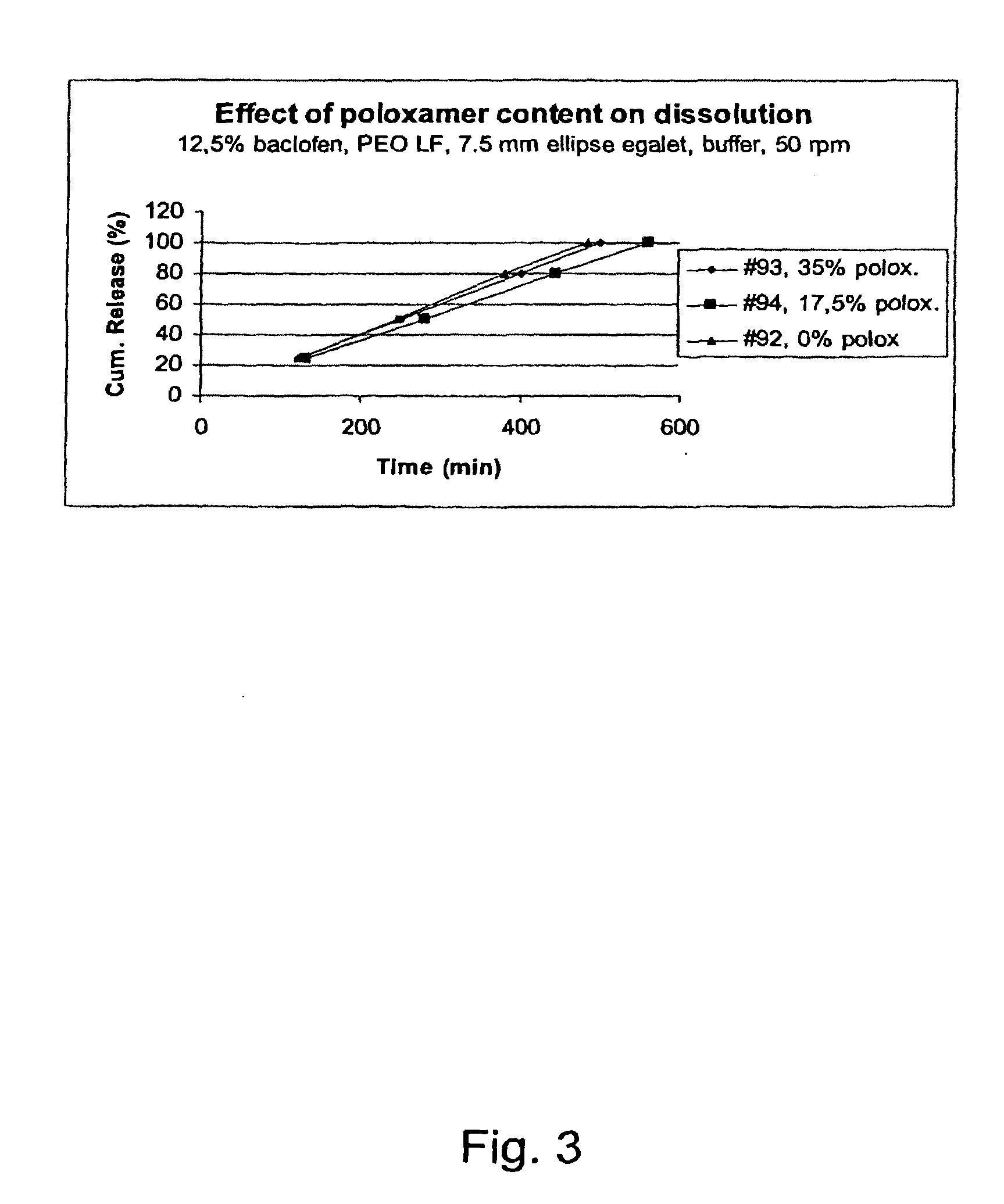
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
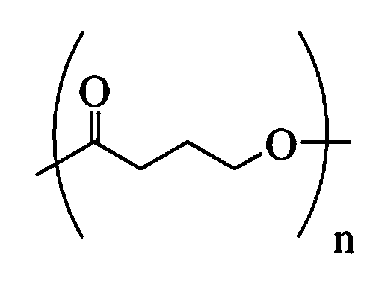
Toughened poly(lactic acid) compositions
Toughened poly(lactic acid) resin compositions comprising poly(lactic acid) and an impact modifier comprising an ethylene copolymer made from monomers (a) ethylene; (b) one or more olefins of the formula CH2═C(R1)CO2R2, where R1 is hydrogen or an alkyl group with 2-8 carbon atoms and R2 is an alkyl group with 1-8 carbon atoms, such as methyl, ethyl, or butyl; and (c) one or more olefins of the formula CH2═C(R3)CO2R4, where R3 is hydrogen or an alkyl group with 1-6 carbon atoms, such as methyl, and R4 is glycidyl. The ethylene copolymer may further be made from carbon monoxide monomers. The compositions may further comprise one or more ethylene / acrylate and / or ethylene / vinyl ester polymers, ionomers, and cationic grafting agents.
Owner:PERFORMANCE MATERIALS NA INC
Medical device with coating that promotes endothelial cell adherence
InactiveUS20050043787A1Prevent restenosisPreventing other thromboembolic complicationMaterial nanotechnologyPeptide/protein ingredientsAntigenPolyethylene glycol
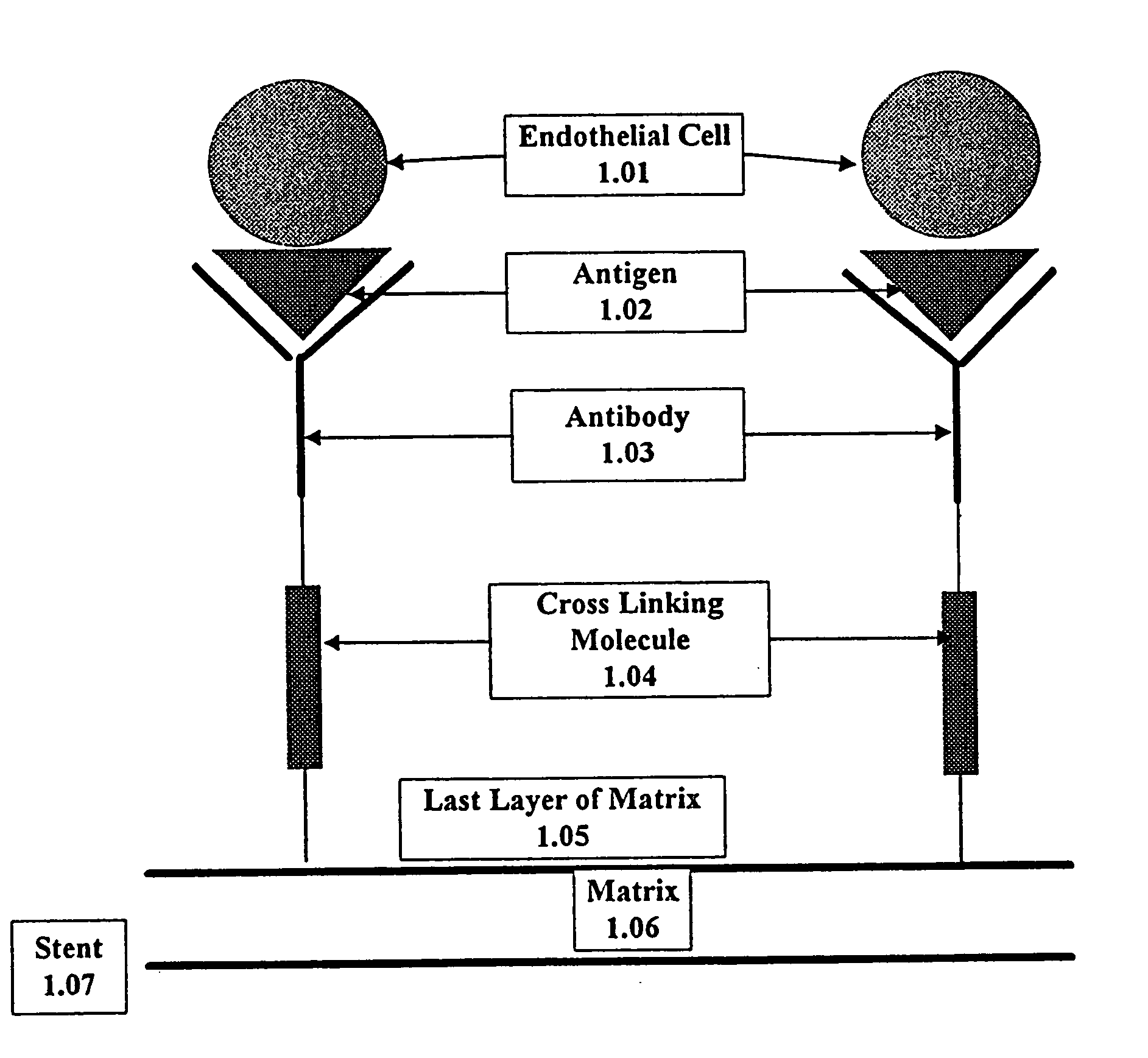
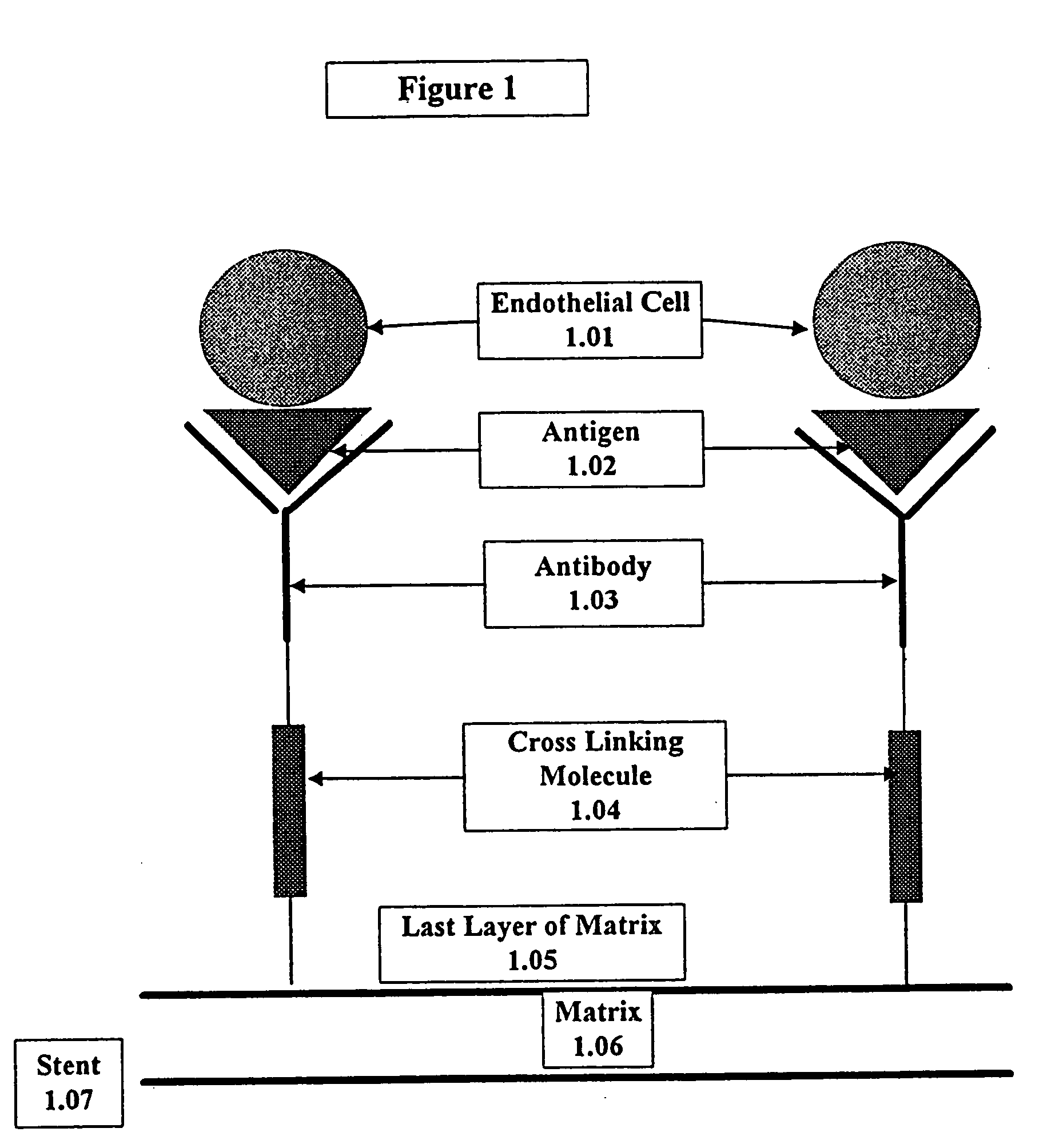
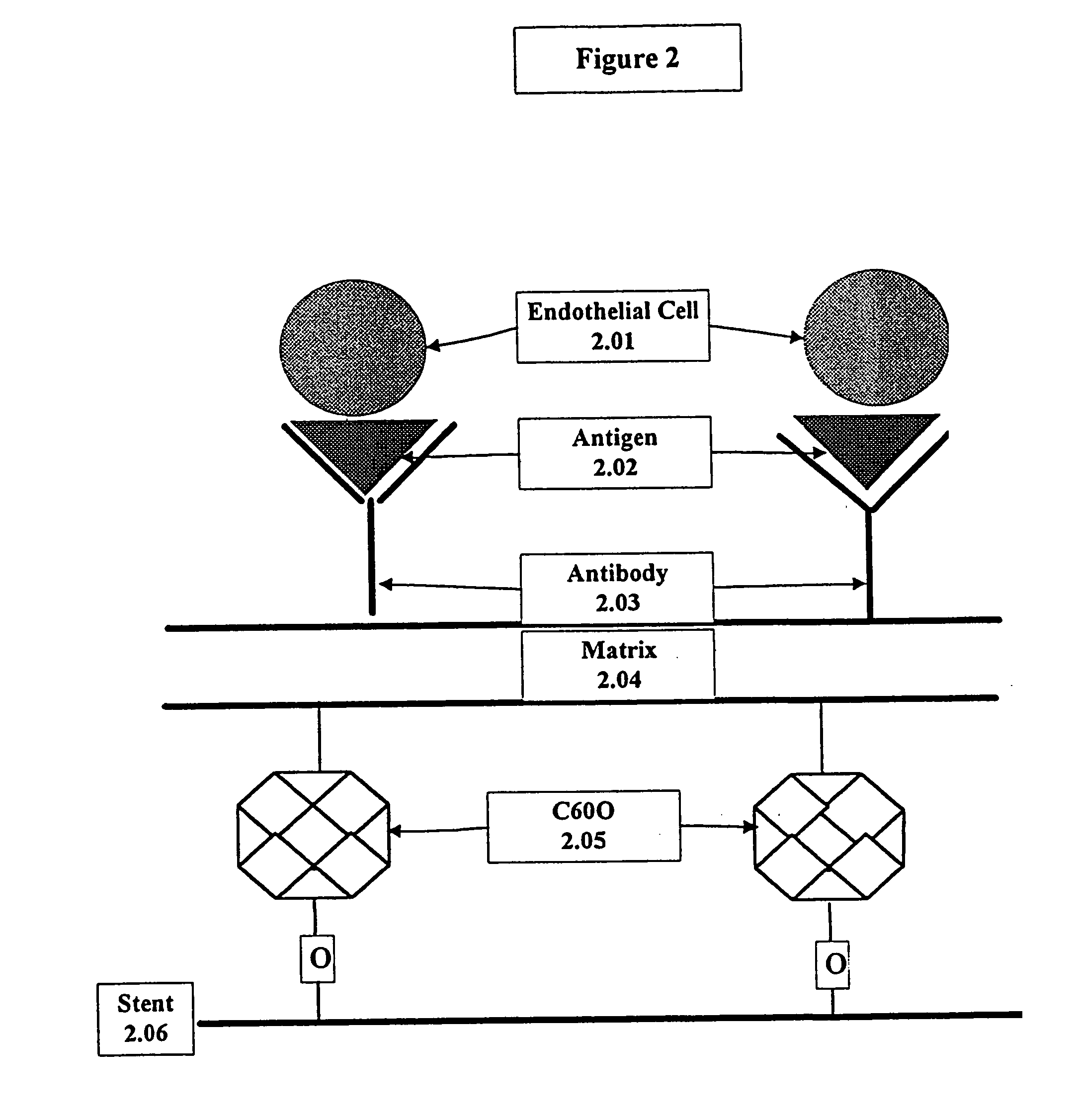
This invention provides compositions and methods for producing a medical device coated with a matrix and an antibody which reacts with an endothelial cell antigen. The matrix coating the medical device may be composed of synthetic material, such as polyurethane, poly-L-lactic acid, cellulose ester or polyethylene glycol. In another embodiment, the matrix is composed of naturally occurring materials, such as collagen, fibrin, elastin, amorphous carbon. In a third embodiment, the matrix may be composed of fullerenes. The fullerenes range from about C60 to about C100. The medical device may be a stent or a synthetic graft. The antibodies promote adherence of endothelial cells on the medical device. The antibodies may be mixed with the matrix or covalently tethered through a linker molecule to the matrix. Following adherence to the medical device, the endothelial cells differentiate and proliferate on the medical device. The antibodies may be different types of monoclonal antibodies. Methods of preparing such composition and methods of treating a mammal with atherosclerosis or other types of vessel obstruction are disclosed. By facilitating adherence of endothelial cells to the surface of the medical device, the methods and compositions of this invention will decrease the incidence of restenosis as well as other thromboembolic complications resulting from implantation of medical devices.
Owner:ORBUSNEICH MEDICAL PTE LTD
Toughened polyoxymethylene-poly(lactic acid) compositions
Toughened polyoxymethylene-poly(lactic acid) resin compositions comprising polyoxymethylene, poly(lactic acid), and an impact modifier comprising an ethylene copolymer impact modifier made from monomers (a) ethylene; (b) one or more olefins of the formula CH2═C(R1)CO2R2, where R1 is hydrogen or an alkyl group with 2-8 carbon atoms and R2 is an alkyl group with 1-8 carbon atoms, such as methyl, ethyl, or butyl; and (c) one or more olefins of the formula CH2═C(R3)CO2R4, where R3 is hydrogen or an alkyl group with 1-6 carbon atoms, such as methyl, and R4 is glycidyl. The ethylene copolymer impact modifier may further be made from carbon monoxide monomers. The compositions may further comprise one or more ethylene / acrylate and / or ethylene / vinyl ester polymers, ionomers, and cationic grafting agents.
Owner:DUPONT POLYMERS INC
Toughened poly(lactic acid) compositions
Toughened poly(lactic acid) resin compositions comprising poly(lactic acid) and an impact modifier comprising an ethylene copolymer made from monomers (a) ethylene; (b) one or more olefins of the formula CH2═C(R1)CO2R2, where R1 is hydrogen or an alkyl group with 2-8 carbon atoms and R2 is an alkyl group with 1-8 carbon atoms, such as methyl, ethyl, or butyl; and (c) one or more olefins of the formula CH2═C(R3)CO2R4, where R3 is hydrogen or an alkyl group with 1-6 carbon atoms, such as methyl, and R4 is glycidyl. The ethylene copolymer may further be made from carbon monoxide monomers. The compositions may further comprise one or more ethylene / acrylate and / or ethylene / vinyl ester polymers, ionomers, and cationic grafting agents.
Owner:PERFORMANCE MATERIALS NA INC
Bioabsorbable polymeric implants and a method of using the same to create occlusions
InactiveUS20020040239A1Peptide/protein ingredientsPharmaceutical containersPoly-L-lactideVascular compartment
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
Expandable biodegradable polymeric stents for combined mechanical support and pharmacological or radiation therapy
InactiveUS7128755B2Avoid residual stressAbsorb in timeStentsBlood vesselsAngioplasty balloonRadiation therapy
An expandable biodegradable polymeric stent is fabricated with biodegradable polymer fibers (Poly-L-lactic acid, PLLA) in a coil shape that is constructed with both central and external or internal peripheral lobes. It is delivered and expanded using a conventional angioplasty balloon system. The disclosed stent can serve as a temporary scaffold for coronary vessels after PTCA or for peripheral endovascular stenting, or it can provide mechanical palliation for strictures of ductile organs (trachea, esophagus, bile and pancreatic ducts, ureter etc.). The disclosed stent also serves as a unique device for specific local drug delivery. Therapeutic agents (chemical compounds, protein enzyme and DNA sequences) and cells can be loaded into the stent and gradually released to target tissues. Local radiation therapy can also be delivered by a specially adapted stent.
Owner:DUNING
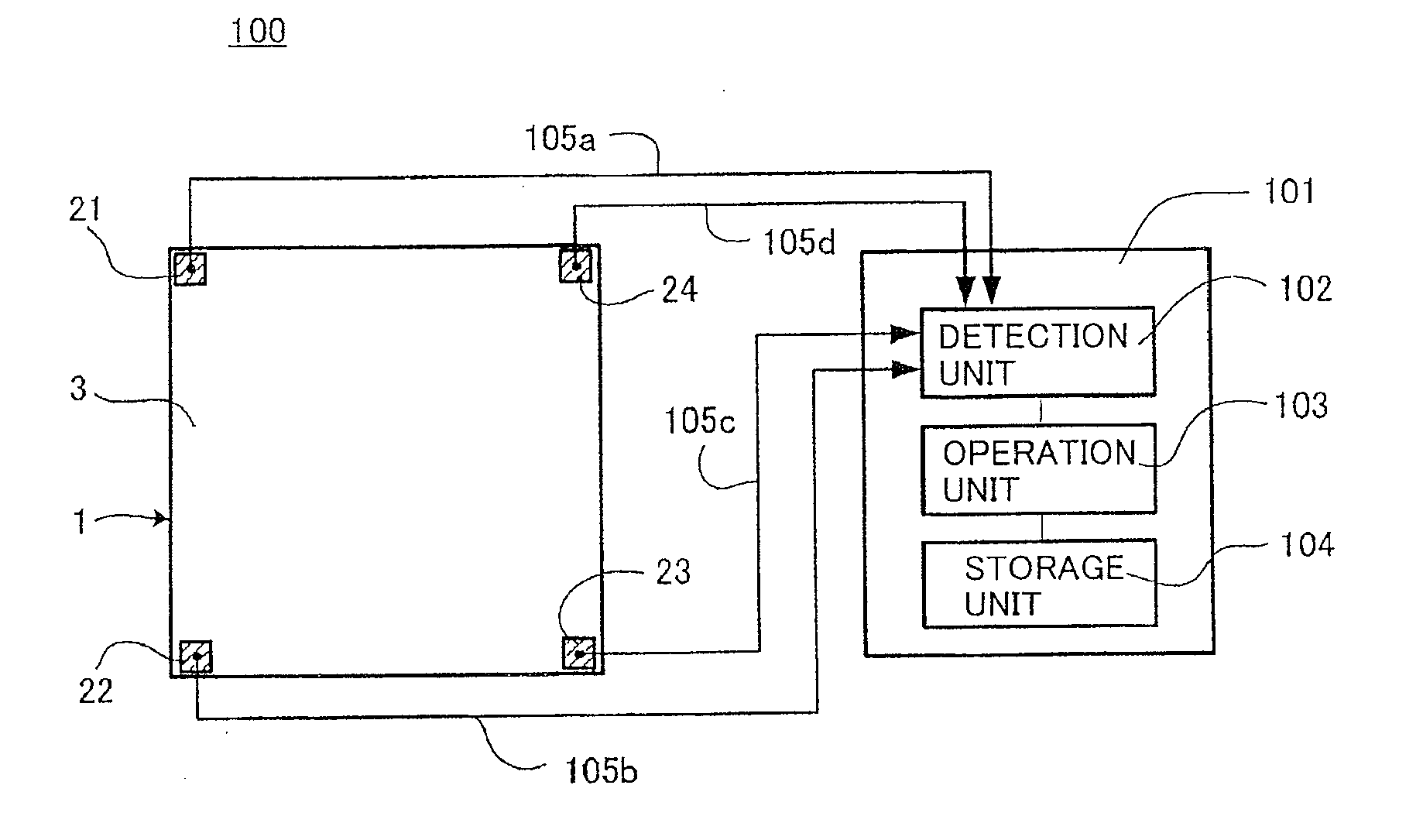
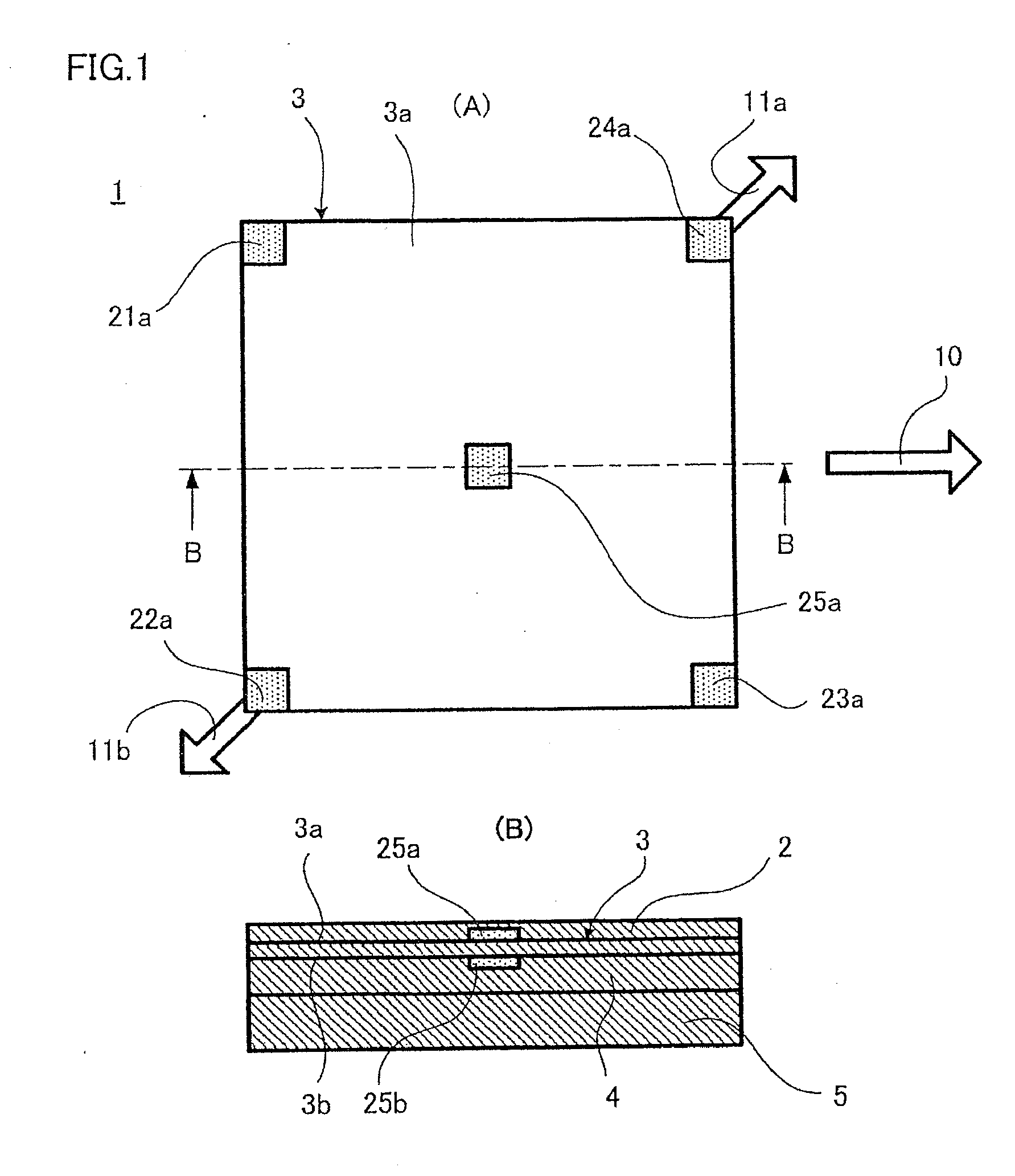
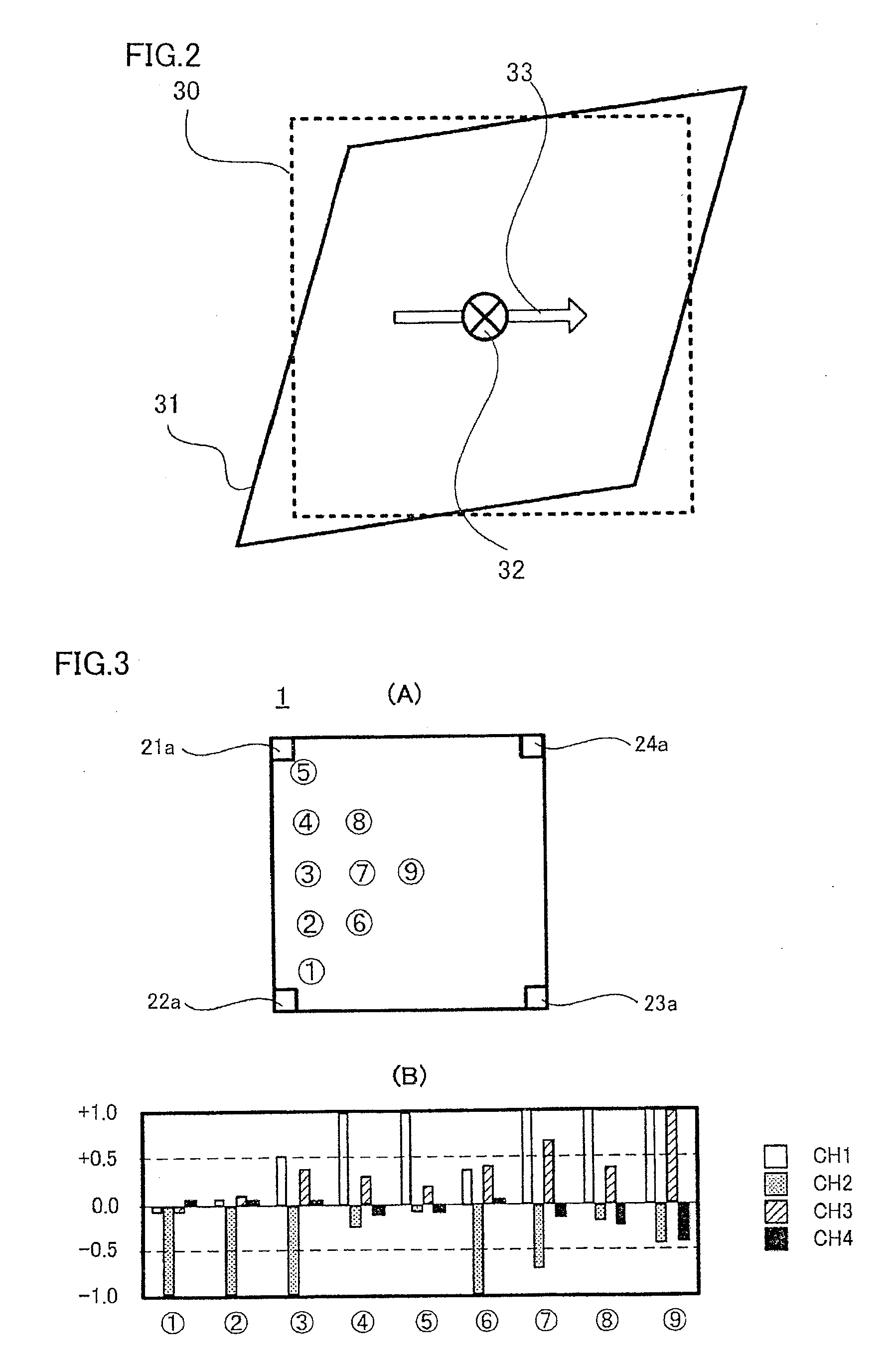
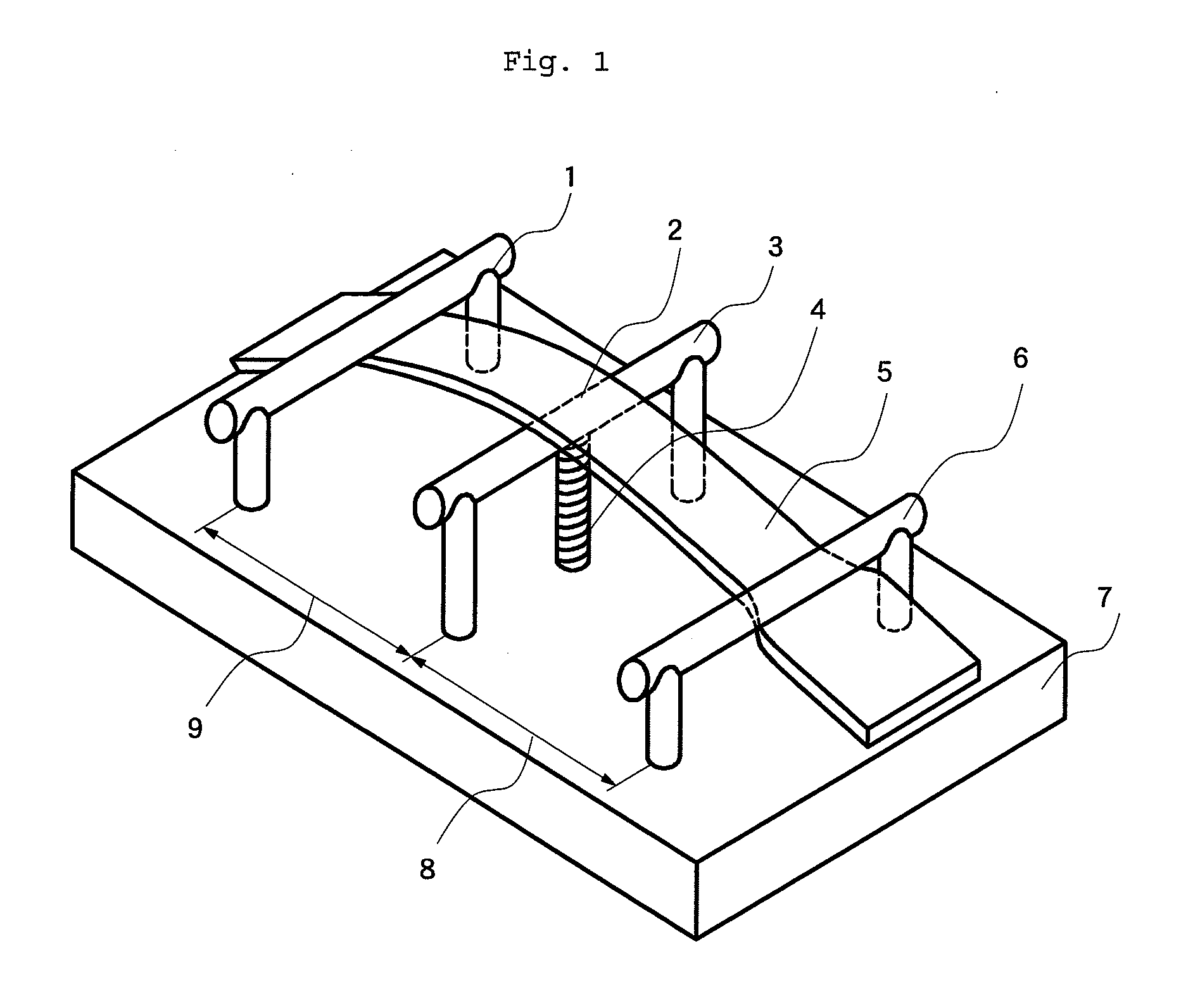
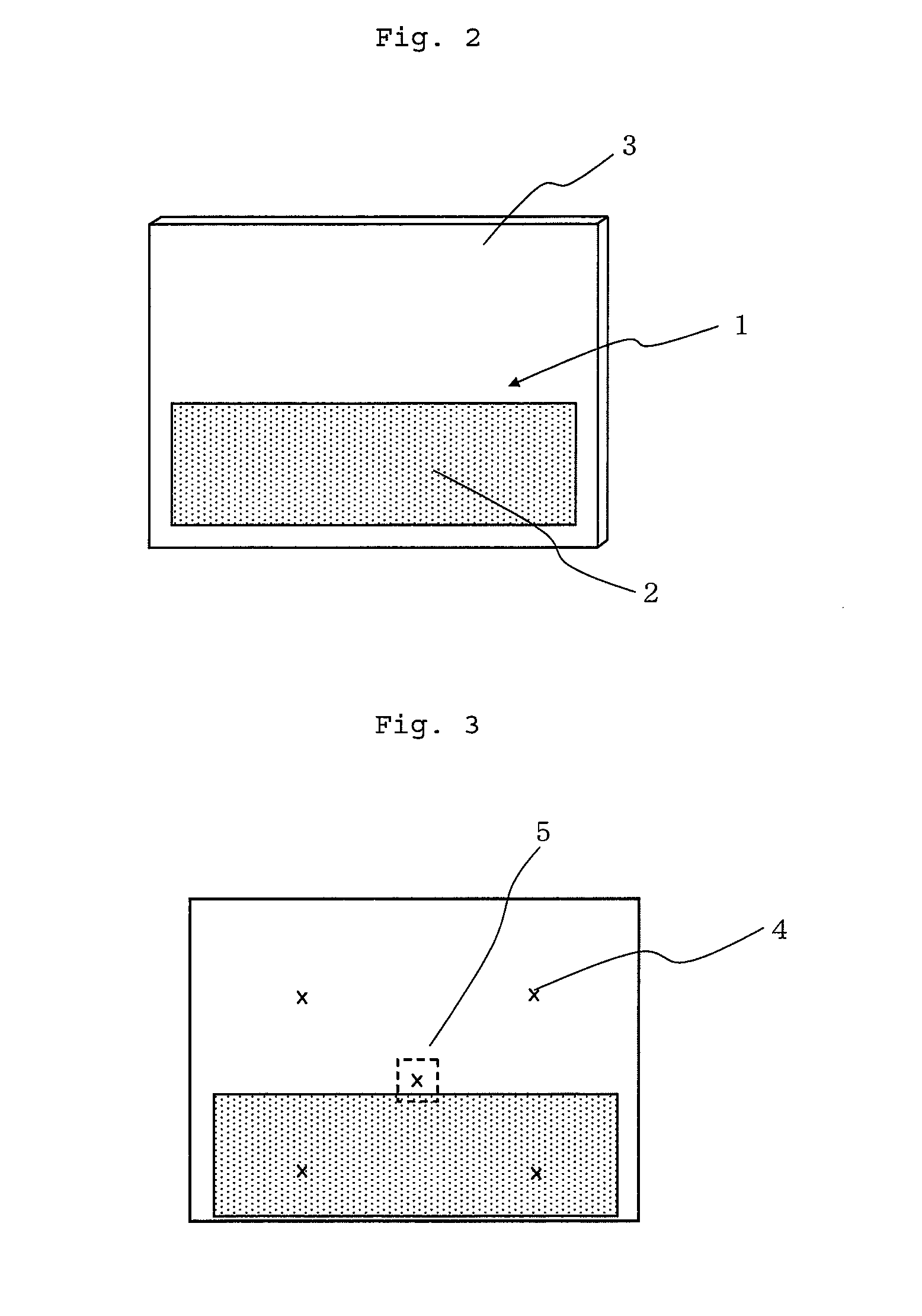
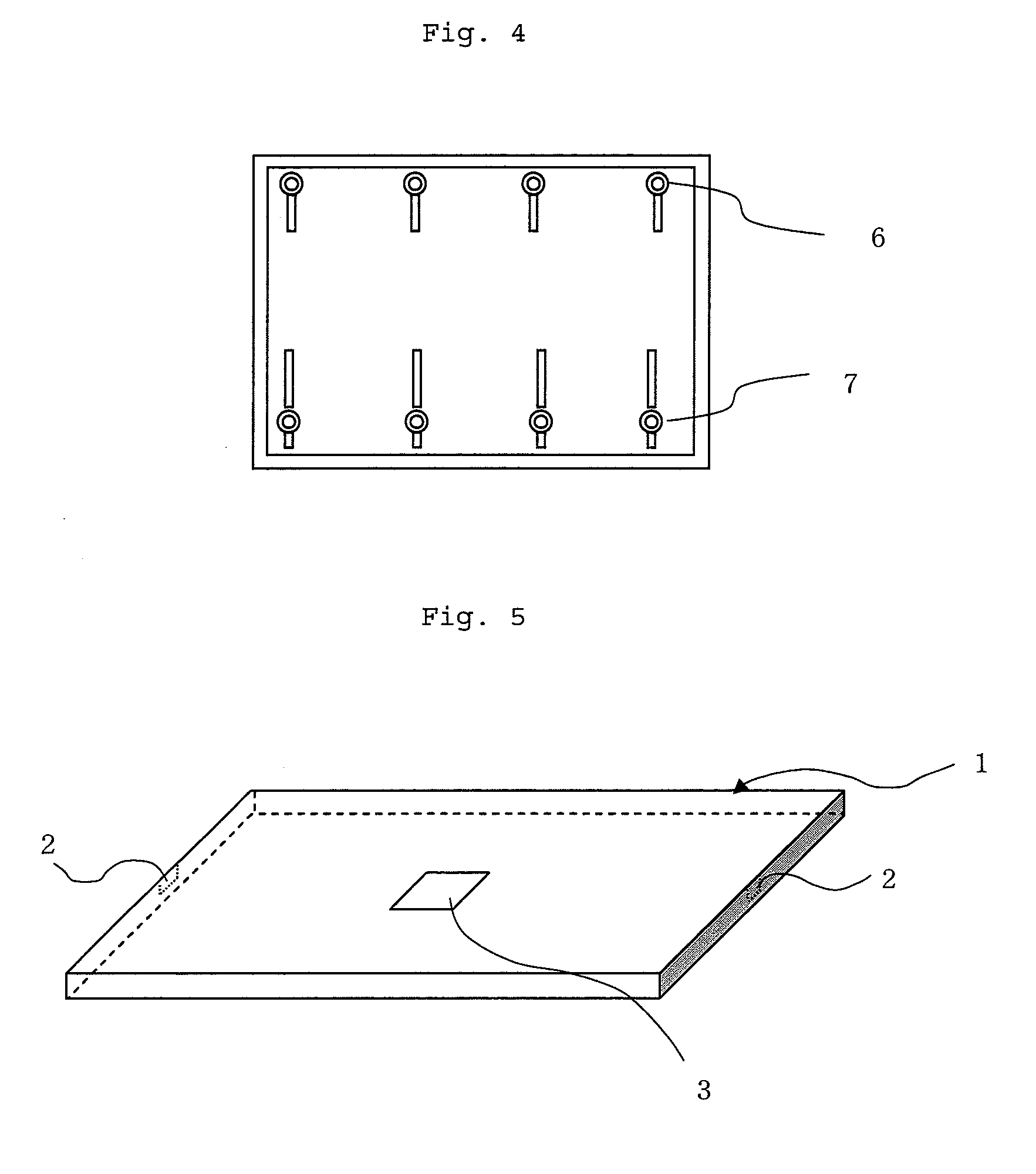
Displacement Sensor, Displacement Detecting Device, and Operation Device
ActiveUS20140049137A1Simple and thin structureEfficient and accuratePiezoelectric/electrostriction/magnetostriction machinesForce measurement using piezo-electric devicesEngineeringPoly l lactic acid
A displacement sensor having a rectangular shaped elastic member. A piezoelectric element is attached to a first main face of the elastic member. The piezoelectric element has a rectangular-shaped piezoelectric sheet and electrodes on both main faces of the piezoelectric sheet. The piezoelectric sheet is made of poly-L-lactic acid and is at least uniaxially-stretched. The piezoelectric element is attached so that the uniaxial-stretching direction of the piezoelectric sheet is 45° relative to a long-side direction of the elastic member. When the elastic member is bent along the long-side direction, the piezoelectric sheet is stretched along the long-side direction, and the piezoelectric element generates voltage of predetermined level.
Owner:MURATA MFG CO LTD
Marker or filler forming fluid
ActiveUS7877133B2Easy to detectEasy to distinguishCosmetic preparationsImpression capsWater insolubleGlycolic acid
Owner:SENORX
Touch panel, and touch-type input apparatus and control method therefor
ActiveUS20130057499A1Determination moreReliable determinationCathode-ray tube indicatorsInput/output processes for data processingEngineeringPoly l lactic acid
A touch panel capable of detecting a pen and a finger, capable of corresponding to multi-touch, capable of detecting pressing force, and capable of reducing the use amount of a transparent electrode as much as possible. The touch panel has a piezoelectric sheet of poly-L-lactic acid having a predetermined stretching axial direction, electrodes that are opposed to each other and formed on the piezoelectric sheet do not cover the entire surface of the piezoelectric sheet and are formed so that they are discretely distributed in plural positions. The piezoelectric sheet is brought into the condition that tension is imparted in directions not coincident with the stretching axial direction.
Owner:MURATA MFG CO LTD
Polymeric, Degradable Drug-Eluting Stents and Coatings
ActiveUS20070185561A1Improve radial strengthRisk minimizationSuture equipmentsPharmaceutical containersAbsorbable polymersEthylene Homopolymers
Absorbable stents and absorbable stent coatings have been developed with improved properties. These devices preferably comprise biocompatible copolymers or homopolymers of 4-hydroxybutyrate, and optionally poly-L-lactic acid and other absorbable polymers and additives. Compositions of these materials can be used to make absorbable stents that provide advantageous radial strengths, resistance to recoil and creep, can be plastically expanded on a balloon catheter, and can be deployed rapidly in vivo. Stent coatings derived from these materials provide biocompatible, uniform coatings that are ductile, and can be expanded without the coating cracking and / or delaminating and can be used as a coating matrix for drug incorporation.
Owner:TEPHA INC
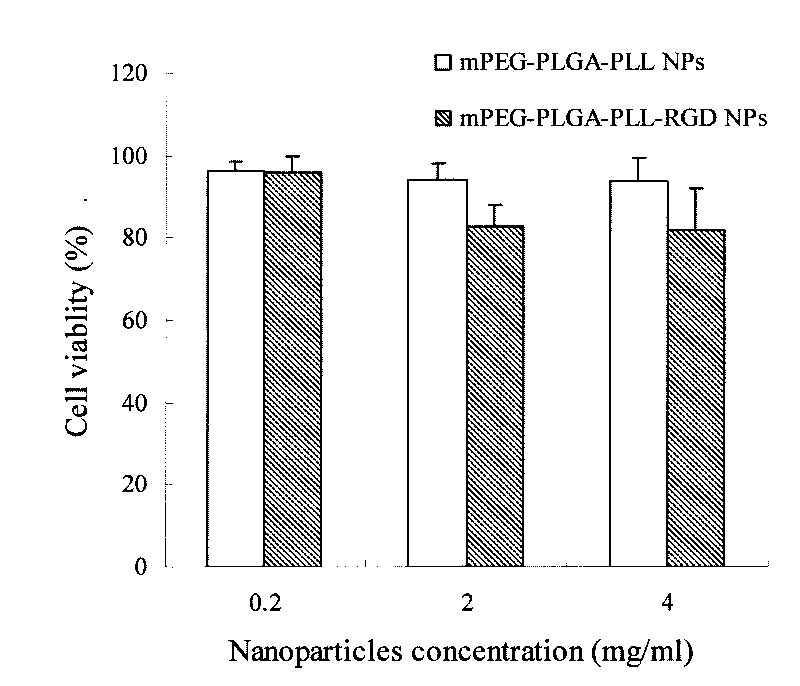
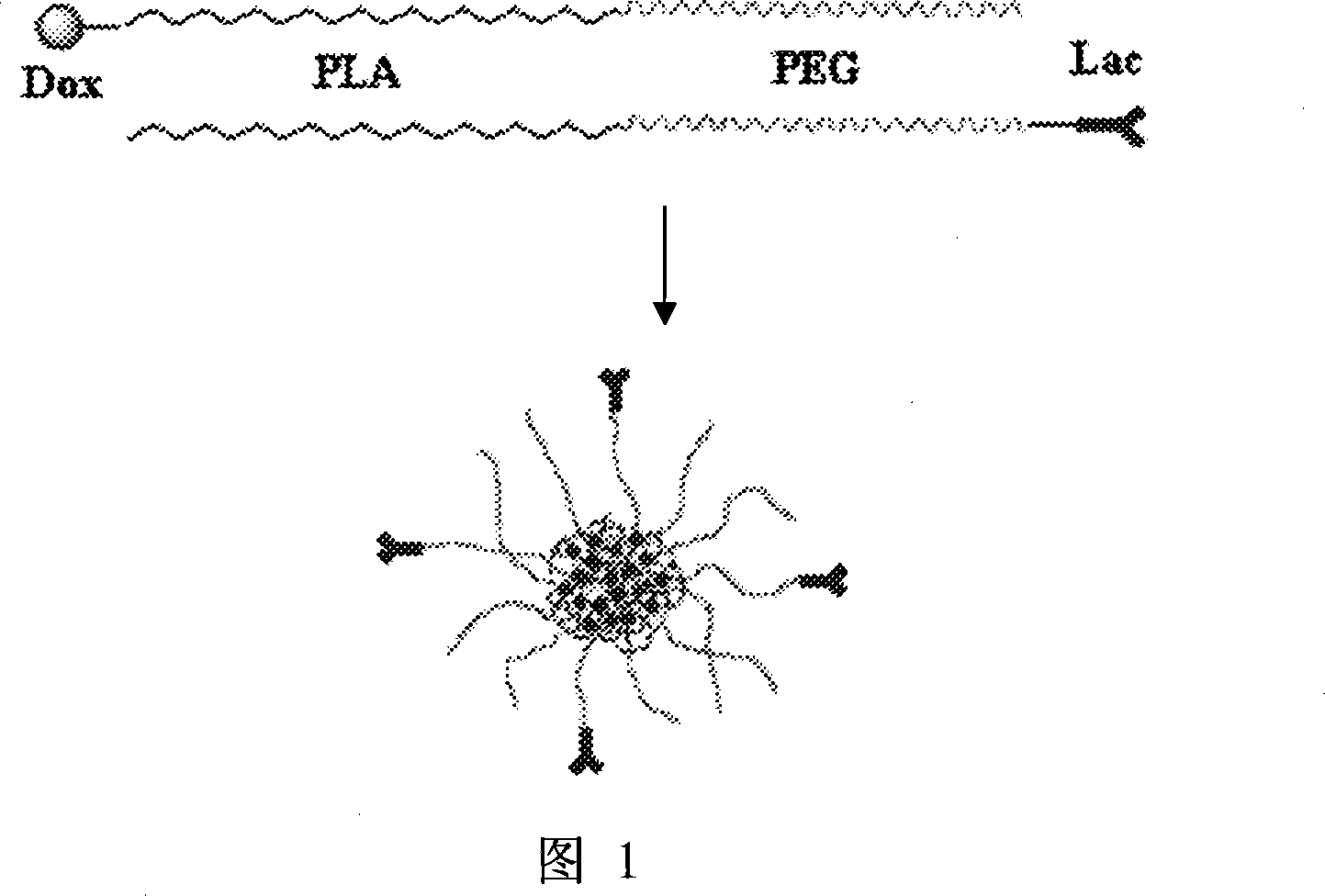
Polyethylene glycol-poly(lactic-co-glycolic acid)-polylysine nano-delivery system, preparation method and application thereof
ActiveCN101732723AEffective targeted deliveryPowder deliveryGenetic material ingredientsTumor targetingPolyethylene glycol
The invention belongs to the nanotechnical field, and discloses preparation of a methoxy polyethylene glycol-poly(lactic-co-glycolic acid)-polylysine(mPEG-PLGA-PLL) cationic polymer nano-drug delivery system and application thereof. The nano-drug delivery system can have multi-functional characteristics such as tumor targeting, reversing drug resistance and medical diagnosis functions through modification, and can be used for supporting organic medicaments, water-soluble medicaments, non-water-soluble medicaments, or developers for diagnosis. The preparation method is simple and convenient, is suitable for mass production, and is particularly suitable for the preparation of targeting drug delivery systems.
Owner:森心(上海)科技有限公司
Novel biodegradable bone plates and bonding systems
ActiveUS20080234754A1Early resorptionMonocarboxylic acid ester polymer adhesivesSurgical adhesivesHot-melt adhesiveBiomedical engineering
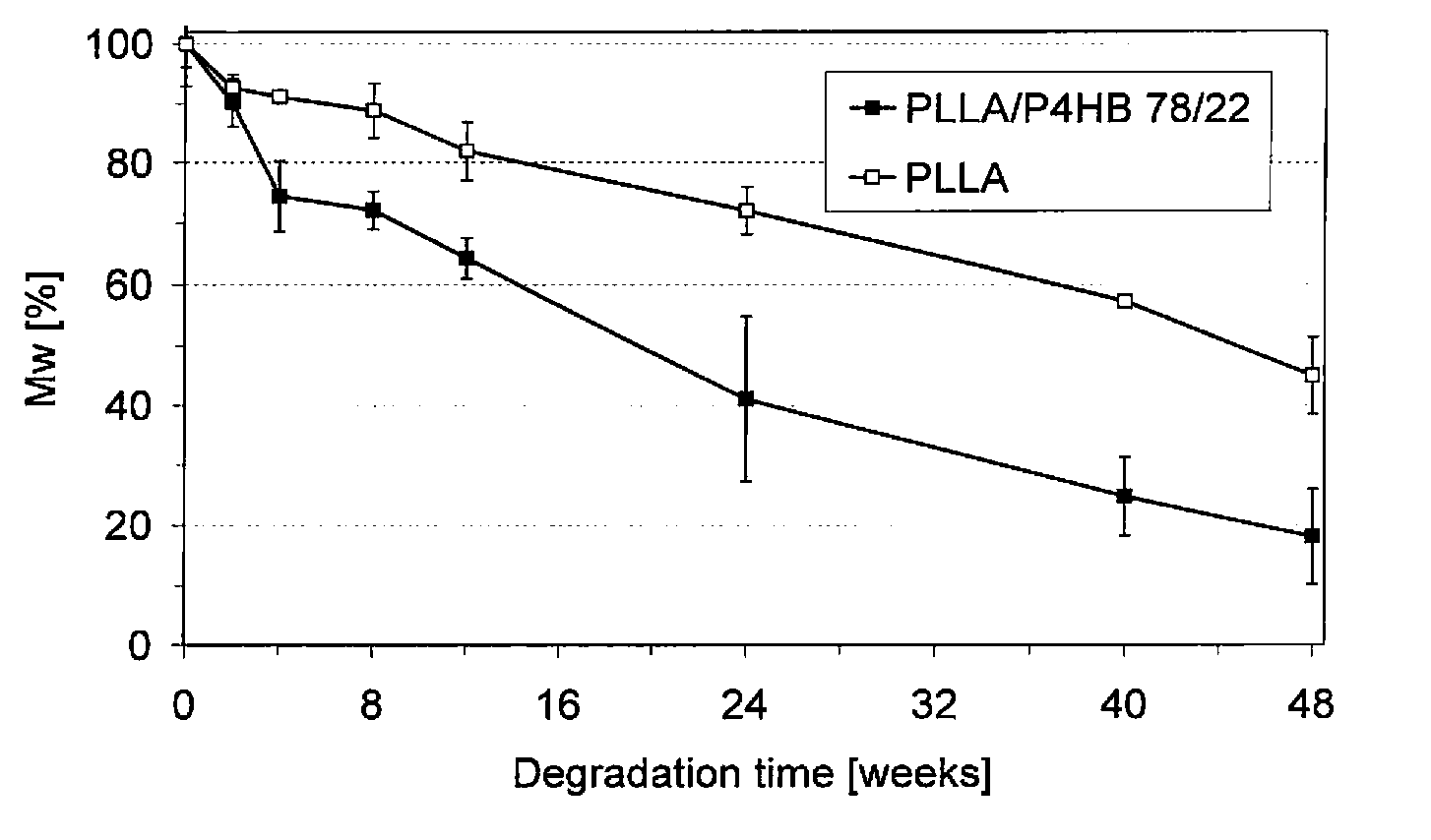
The invention relates to novel internal fixation devices, such as bone plates, generally and novel craniomaxillofacial bone plates more specifically and systems for bonding the same. More specifically, the invention relates to bone plates made of a polymer blend of (poly)lactic acid and Ecoflex as well as a novel hot-melt adhesive polymer blend of the same material.
Owner:NOVAPLAST CORP +1
Environmental raw material capable of providing impact resistance, method of producing the same, and moldings
InactiveUS20060263394A1Envelopes/bags making machinerySynthetic resin layered productsPolylactic acidImpact resistance
A resin composition containing a crystal structure of poly(lactic acid), which is obtained by melt-kneading 30 to 99 mass % of poly(lactic acid) and 70 to 1 mass % of a copolymer having a functional group reactive with the poly(lactic acid); a method of producing the same; and moldings.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Touch panel, and touch-type input apparatus and control method therefor
ActiveUS9110532B2Reduce usageSolve the real problemCathode-ray tube indicatorsInput/output processes for data processingEngineeringPoly l lactic acid
A touch panel capable of detecting a pen and a finger, capable of corresponding to multi-touch, capable of detecting pressing force, and capable of reducing the use amount of a transparent electrode as much as possible. The touch panel has a piezoelectric sheet of poly-L-lactic acid having a predetermined stretching axial direction, electrodes that are opposed to each other and formed on the piezoelectric sheet do not cover the entire surface of the piezoelectric sheet and are formed so that they are discretely distributed in plural positions. The piezoelectric sheet is brought into the condition that tension is imparted in directions not coincident with the stretching axial direction.
Owner:MURATA MFG CO LTD
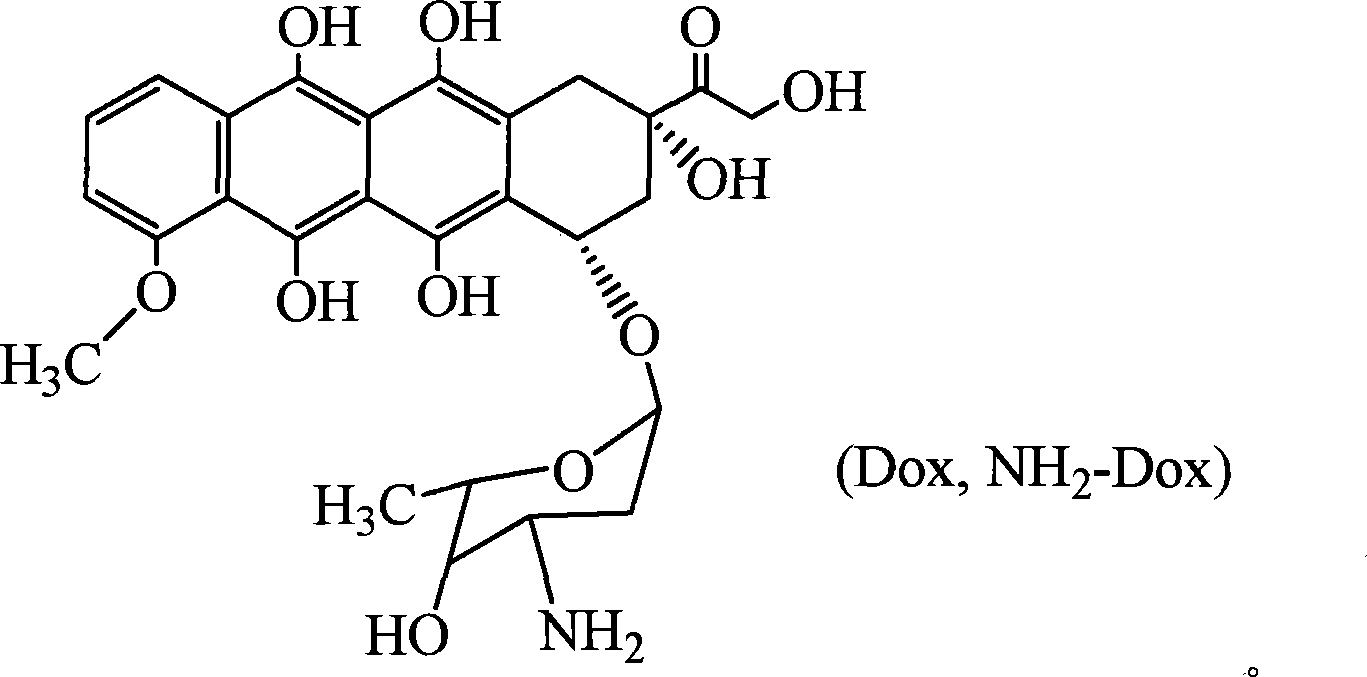
High molecule bonding adriamycin medicine, nano capsule and preparation thereof
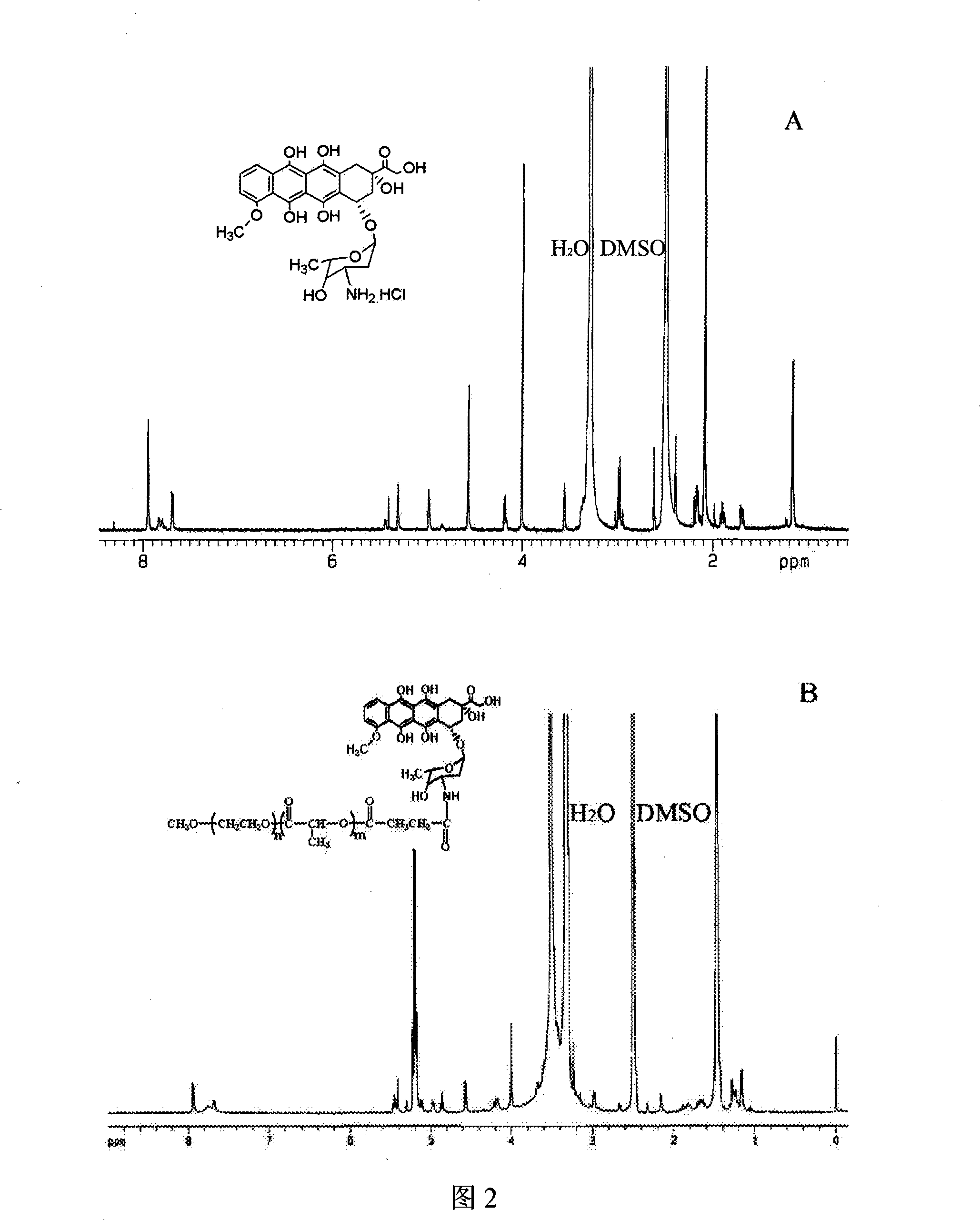
InactiveCN101234204AReduce manufacturing costPlay a role in isolationOrganic active ingredientsPharmaceutical non-active ingredientsPolymer scienceEnd-group
The invention provides a macromolecule bonding adriamycin medicine, a nanometer capsule and a preparation method thereof. The bonding medicine is bonded by a block copolymer of poly ethylene glycol-polylactic acid and adriamycin. Firstly, the block copolymer of poly ethylene glycol-polylactic acid is acquired by ring-opening polymerization of aliphatic cyclic esters with poly ethylene glycol, solvent and catalyst being in existence; then a carboxyl end group is transformed by a hydroxyl-terminated; and then with a condensing agent being in existence, the carboxyl end group carries out amidation reaction with the adriamycin; thus acquiring the macromolecule adriamycin bonding medicine. The bonding medicine has amphiphilic property, thus being able to self assembly to prepare the nanometer capsule with a core-shell structure in the water solution. The adriamycin is wrapped inside the capsule to play a role of isolation and protection, which overcomes the deficiencies of short circulation time in vivo, large dosage and severe allergic reaction existing in the current adriamycin preparation. In addition, the nanometer particles are expected to congregate in the blood circulation through the so-called 'enhanced infiltration and retention effect', so as to improve the targeting action of the adriamycin on the tumor location.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
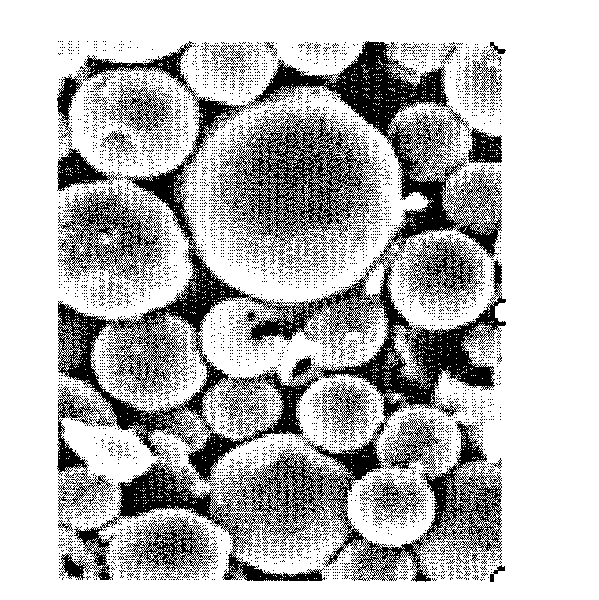
PLA/PLGA shell-core microballoons prepared by oil in water-solid in oil method, and preparation method thereof
InactiveCN101461786ASmooth and rounded surfaceDisadvantages of Avoiding PollutionPharmaceutical non-active ingredientsGranular deliveryControlled releaseAcetic acid
The invention relates to a PLA / PLGA shell-core microsphere prepared by solid-in-oil-in-water in the technical field of pharmacy and a preparation method thereof. The microsphere comprises 0.01 to 50 percent of medicine, 20 to 99.99 percent of polylactic acid and / or polylactic acid-glycolic acid, or / and 0 to 30 percent of pharmaceutical excipient (weight percentage). The method comprises the steps of: adding medicine particles into a PLA and / or PLGA organic solution to be emulsified, then selecting a hydrophilic organic solvent to re-emulsify to form unhardened balls, finally hardening in another large oil phase, removing the organic solvent and collecting micropheres. The method overcomes the disadvantages of low envelope rate of the prior W / O and W / O / W, serious burst release of S / O / O, and environmental pollution, controls the grain diameter of the microsphere according to the need, does not pollute the environment, and can be applied to the preparation of slow release or controlled release microspheres of various medicines and adjuvants of vaccines.
Owner:SHANGHAI JIAO TONG UNIV
Resin composition, molded article, and production methods thereof
InactiveUS20100227963A1Big burden to solveImprove hydrolysis resistanceHollow wall articlesPolymer chemistryPolylactic acid
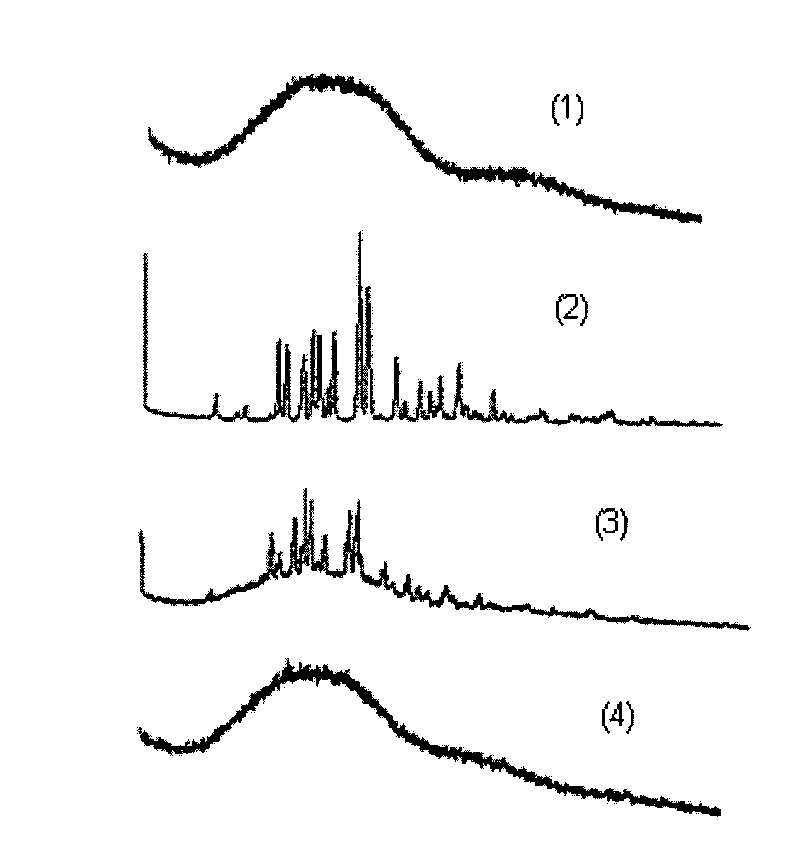
There are provided a resin composition comprising a polylactic acid which (i) comprises a poly-L-lactic acid (component B-1) and a poly-D-lactic acid (component B-4), (ii) has a weight ratio of the component B-1 to the component B-4 (component B-1 / component B-4) of 10 / 90 to 90 / 10, and (iii) shows a proportion of melt peaks at 195° C. or higher to all melt peaks in a temperature rising process in measurement by a differential scanning calorimeter (DSC) of at least 20%; a molded article of the resin composition; and methods for producing the resin composition and the molded article.
Owner:TEIJIN CHEM LTD +1
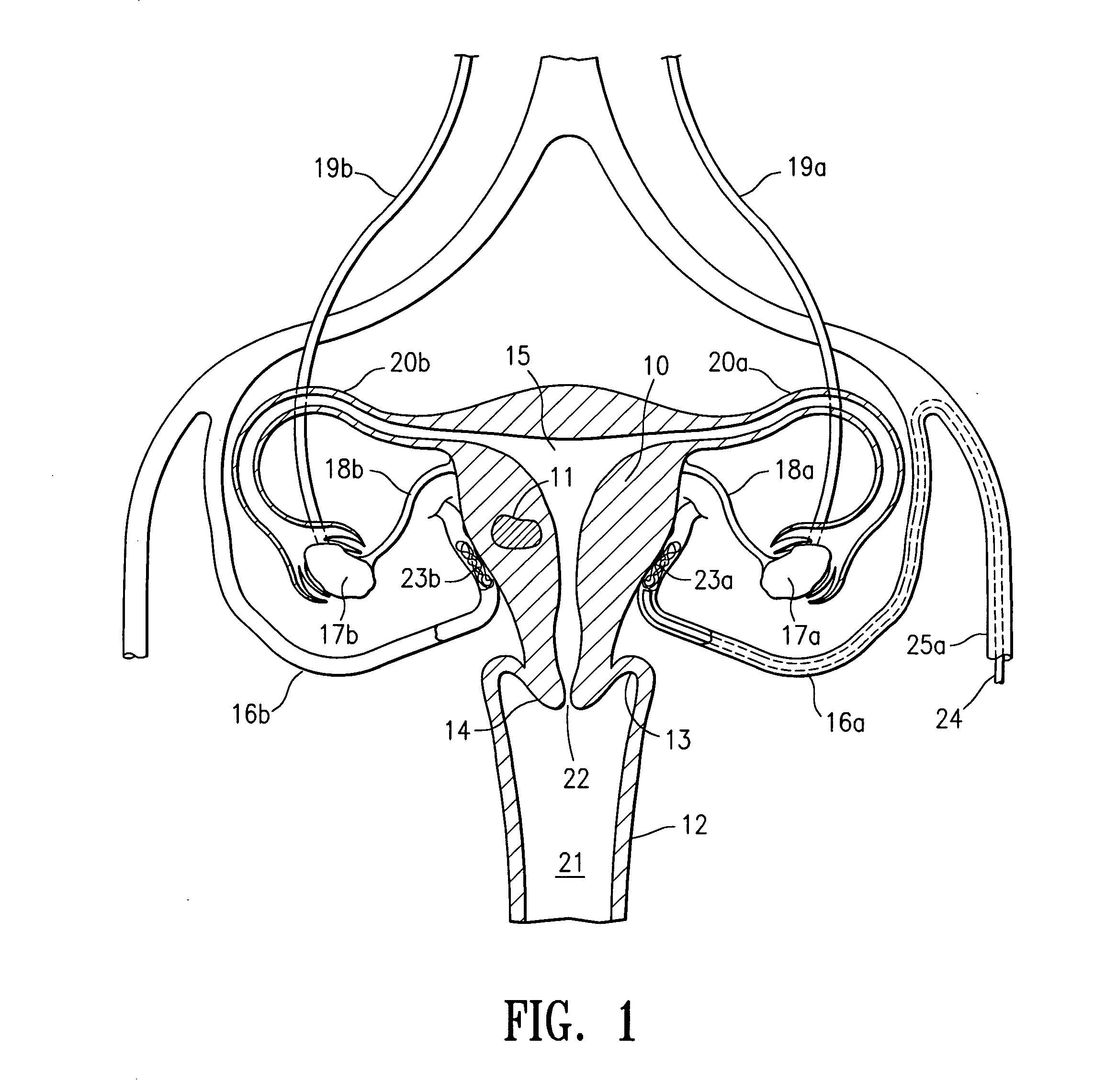
Embolic occlusion of uterine arteries
InactiveUS20040202694A1Decreased blood flowSlow and stop flowSurgical adhesivesFemale contraceptivesWater basedUterine Disorder
A treatment procedure is disclosed which involves the short term, non-permanent occlusion of the patient's blood vessels by depositing a bioabsorbable embolic mass within the patient's blood vessel. The procedure is particularly suitable for treating uterine disorders by occluding a patient's uterine arteries. A therapeutically effective time period for occlusion of a uterine artery is from about 0.5 to about 48 hours, preferably about 1 to about 24 hours, with occlusion times of about 1 to about 8 hours being suitable in many instances. The embolic mass may bioabsorbable particulate with minimum transverse dimensions of about 100 to about 2000 micrometers, preferably about 300 to about 1000 micrometers. The particulate may be a polymeric material formed of polylactic acid, polyglycolic acid or copolymers thereof, or a swellable copolymer of lactic acid and polyethylene glycol. The embolic material may be delivered to an intracorporeal site as a biocompatible solution containing a solute which is relatively insoluble in a water based fluid and a solvent which is relatively soluble in the water based fluid, where the solute forms the embolic mass which occludes or partially occludes a body lumen or fills or partially fills a body cavity.
Owner:VASCULAR CONTROL SYST
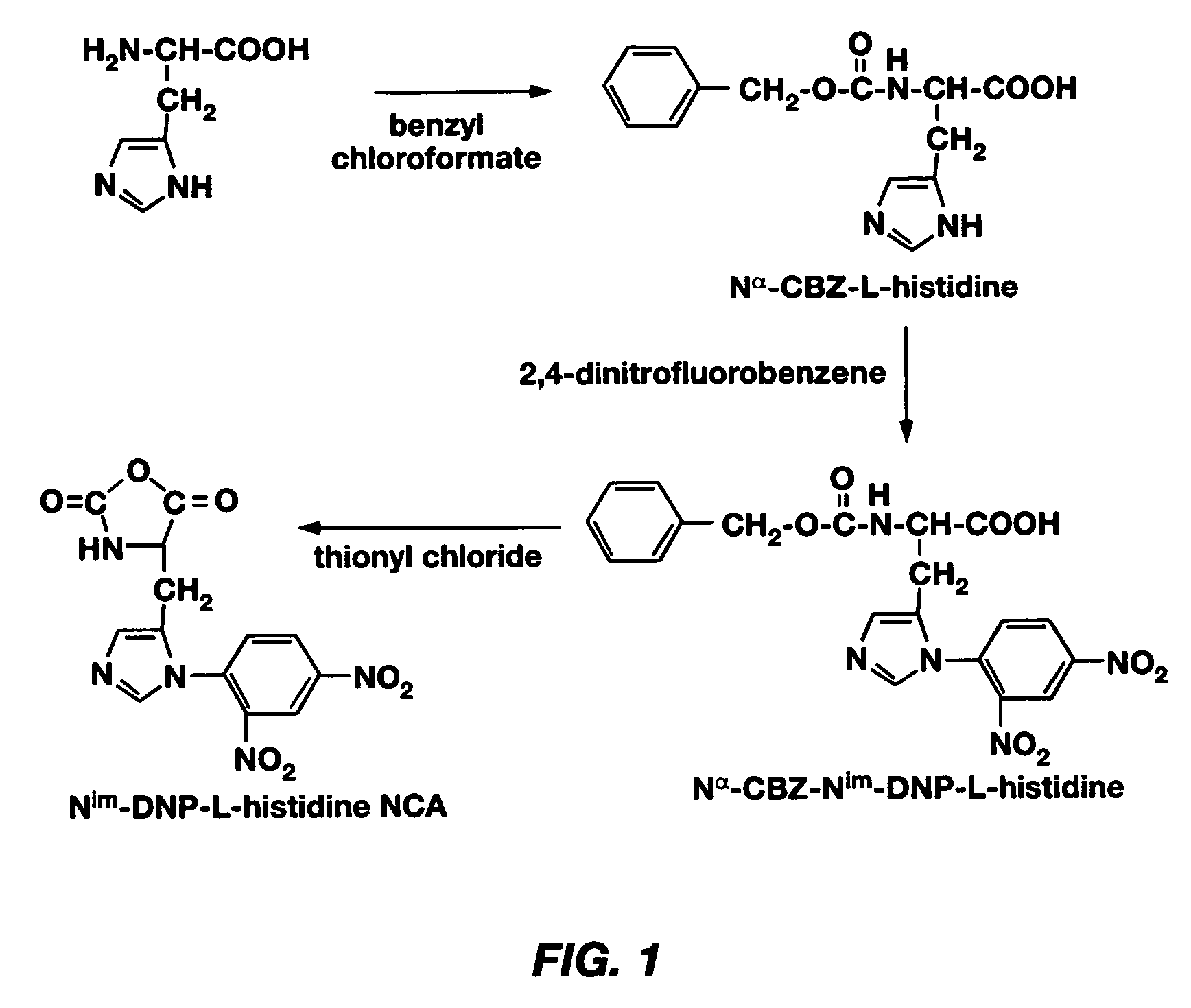
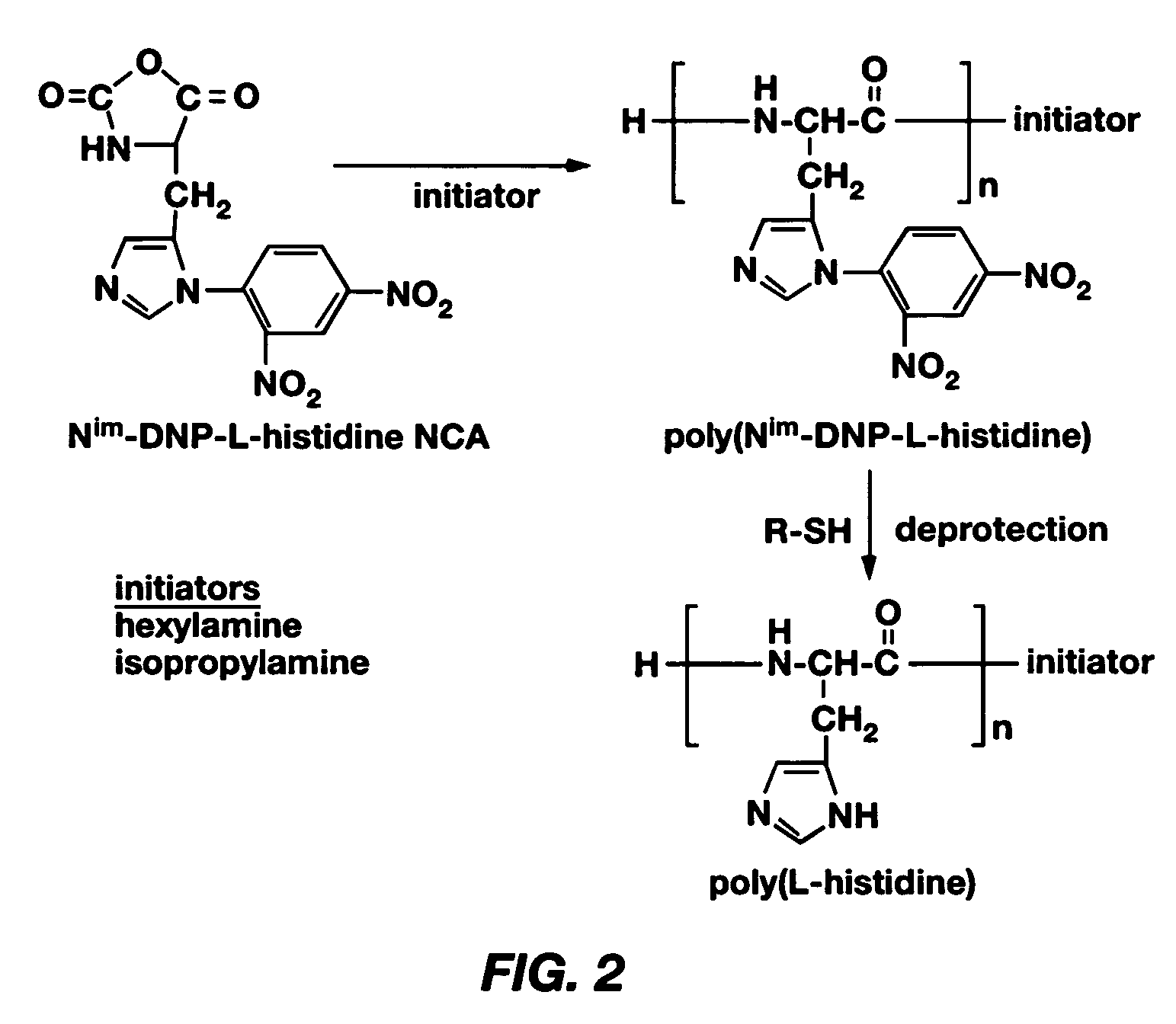
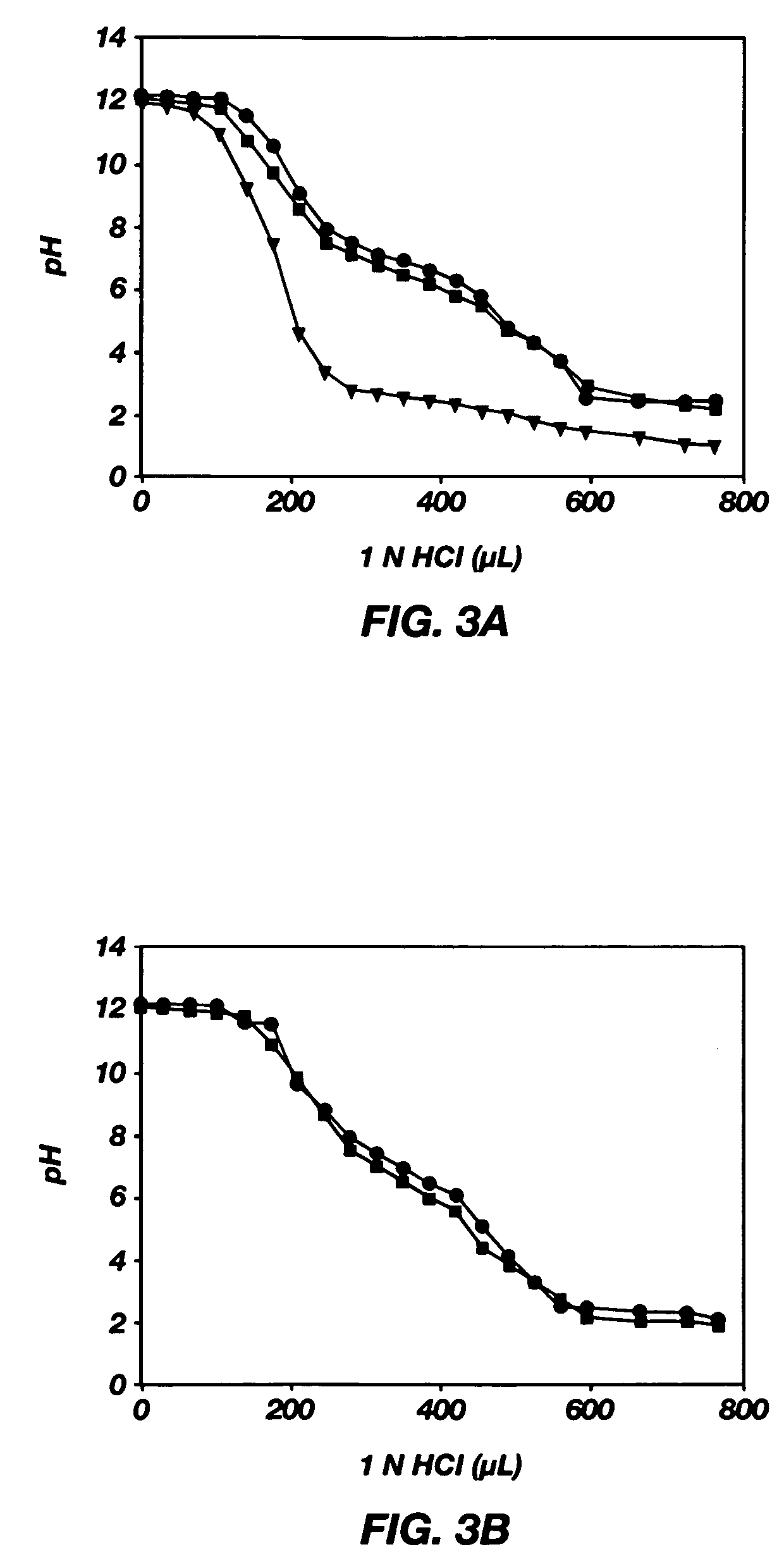
PH-sensitive polymeric micelles for drug delivery
Mixed micelles containing poly(L-histidine)-poly(ethylene glycol) block copolymer and poly(L-lactic acid)-poly(ethylene glycol) block copolymer are a pH-sensitive drug carrier that release the drug in an acidic microenvironment, but not in the blood. Since the microenvironment of solid tumors is acidic, these mixed micelles are useful for treating cancer, including those cancers exhibiting multidrug resistance. Targeting ligands, such as folate, can also be attached to the mixed micelles for enhancing drug delivery into cells. Methods of making poly(L-histidine), synthetic intermediates, and block copolymers are also described.
Owner:UNIV OF UTAH RES FOUND
Matrix compositions for controlled delivery of drug substances
InactiveUS20100166866A1Improve solubilityImprove oral bioavailabilityPowder deliveryBiocidePolyethylene oxidePEG-PLGA-PEG
Owner:EGALET LTD
Catalyst for ring-opening polymerization of cyclic ester and preparation method thereof
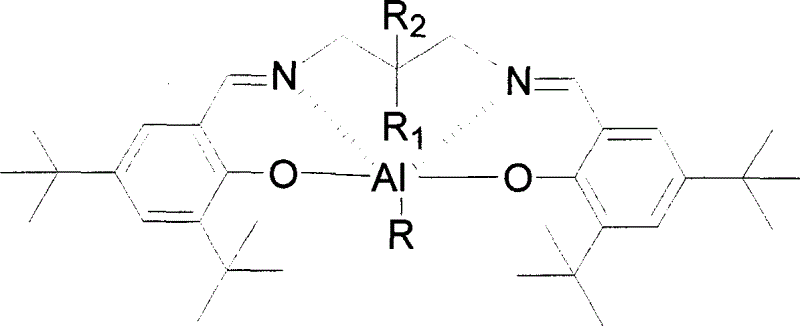
The invention relates to a category of cyclic ester open loop polymerization catalyst and method for preparation, it also relates to the process for polymerizing racemic lactide by using these catalysts, wherein the catalysts are the product of the reaction between Schiff's base and aluminium triethide or aluminium isopropoxide, they exhibit stereoselectivity to the polymerization of racemic lactide, thus crystallinity polylactic acid can be obtained through polymerizing racemic lactide.
Owner:长春宸泰科技有限公司
High molecule adriamycin bonding medicine nano capsule with targeting function and preparation thereof
InactiveCN101234205AReduce manufacturing costReduce doseOrganic active ingredientsPharmaceutical non-active ingredientsPolymer sciencePolyethylene glycol
The invention provides a nanometer capsule of a macromolecule adriamycin bonding medicine with a targeting function and a preparation method thereof. The nanometer capsule is mixed assembled with two block copolymers of poly ethylene glycol-polylactic acid, wherein the polylactic acid chain end of one block copolymer is connected with adriamycin, with a molar ratio of 98-70 percent in the nanometer capsule, while the poly ethylene glycol chain end of the other block copolymer is connected with lactose, with a molar ratio of 2-30 percent in the nanometer capsule. The adriamycin connected with the polylactic acid chain end is located in the capsule inner core, which is double protected by polylactic acid and poly ethylene glycol and has sustained release function; while the lactose connected with the poly ethylene glycol chain end is located in the outer layer of the capsule, which has targeting function and leads the nanometer capsule preferentially enter the cells carrying the lactose receptors.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
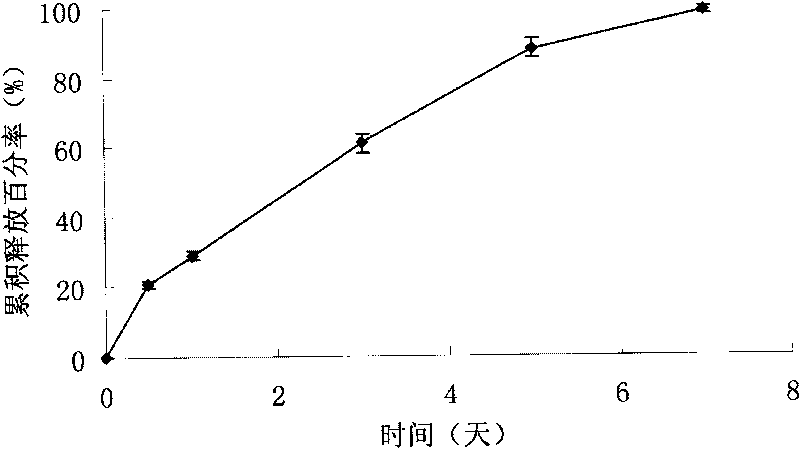
Sustained intraocular delivery of drugs from biodegradable polymeric microparticles
ActiveUS20100261646A1Lower eye pressureSustained releaseHormone peptidesBiocideMicrosphereActive agent
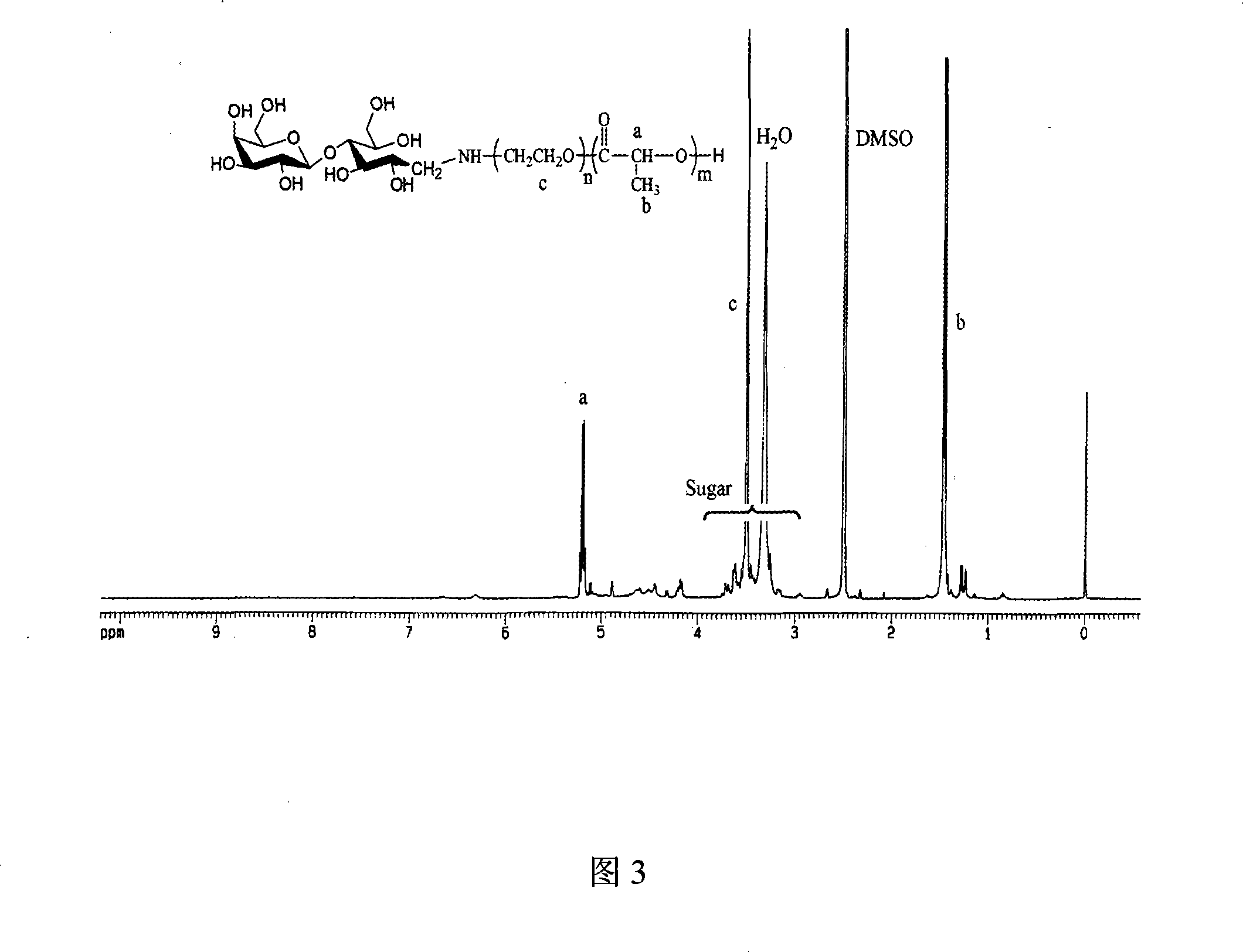
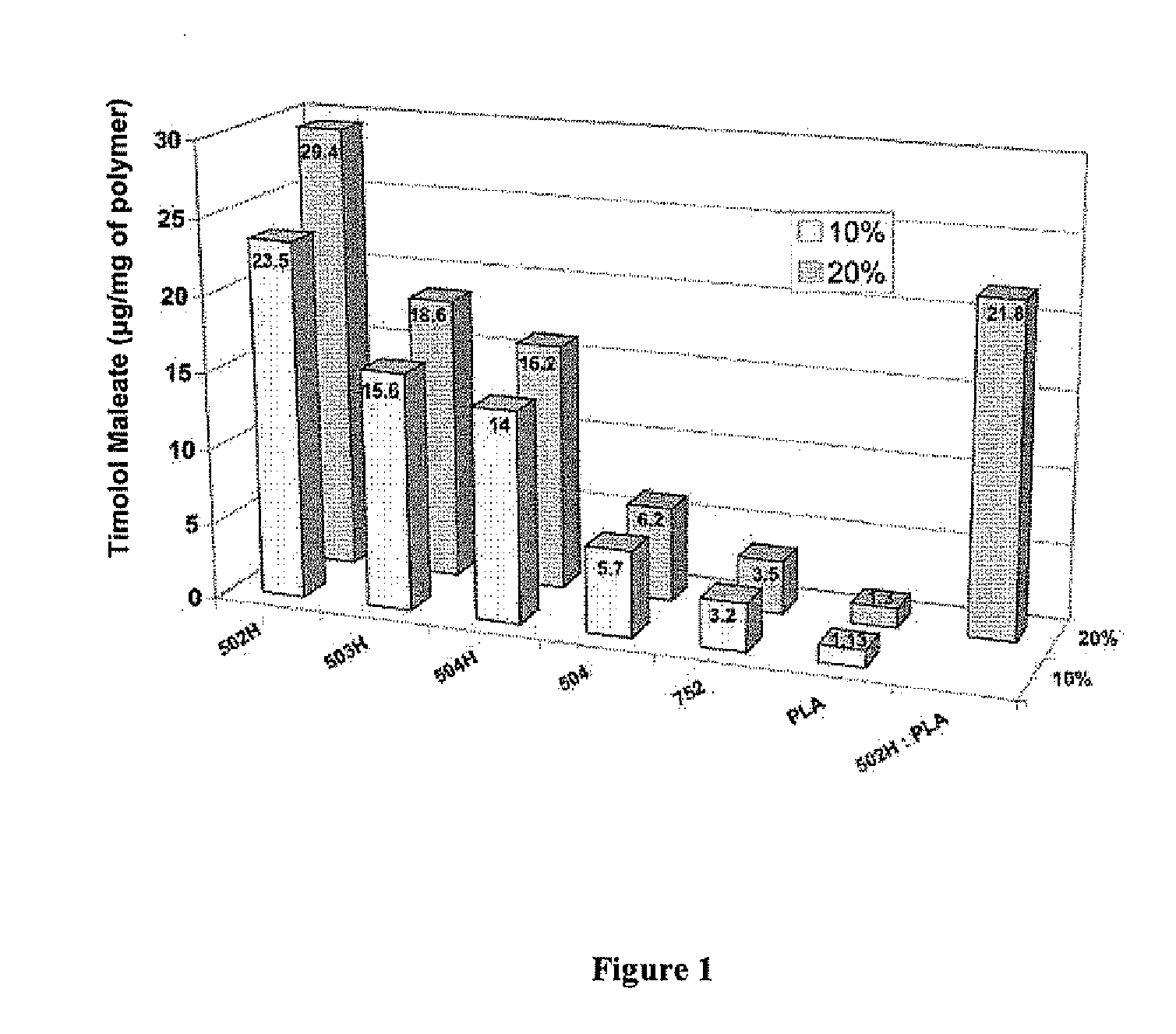
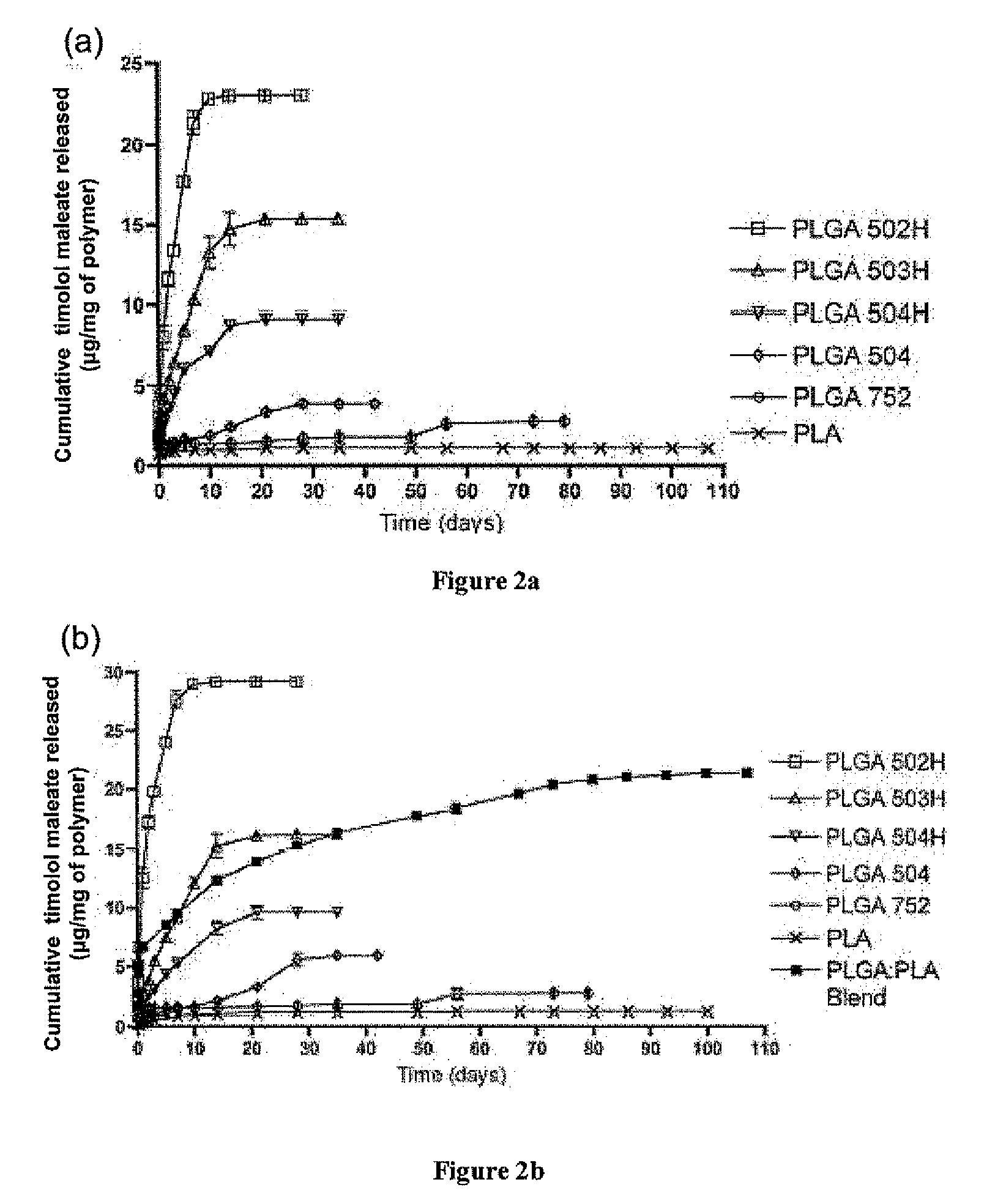
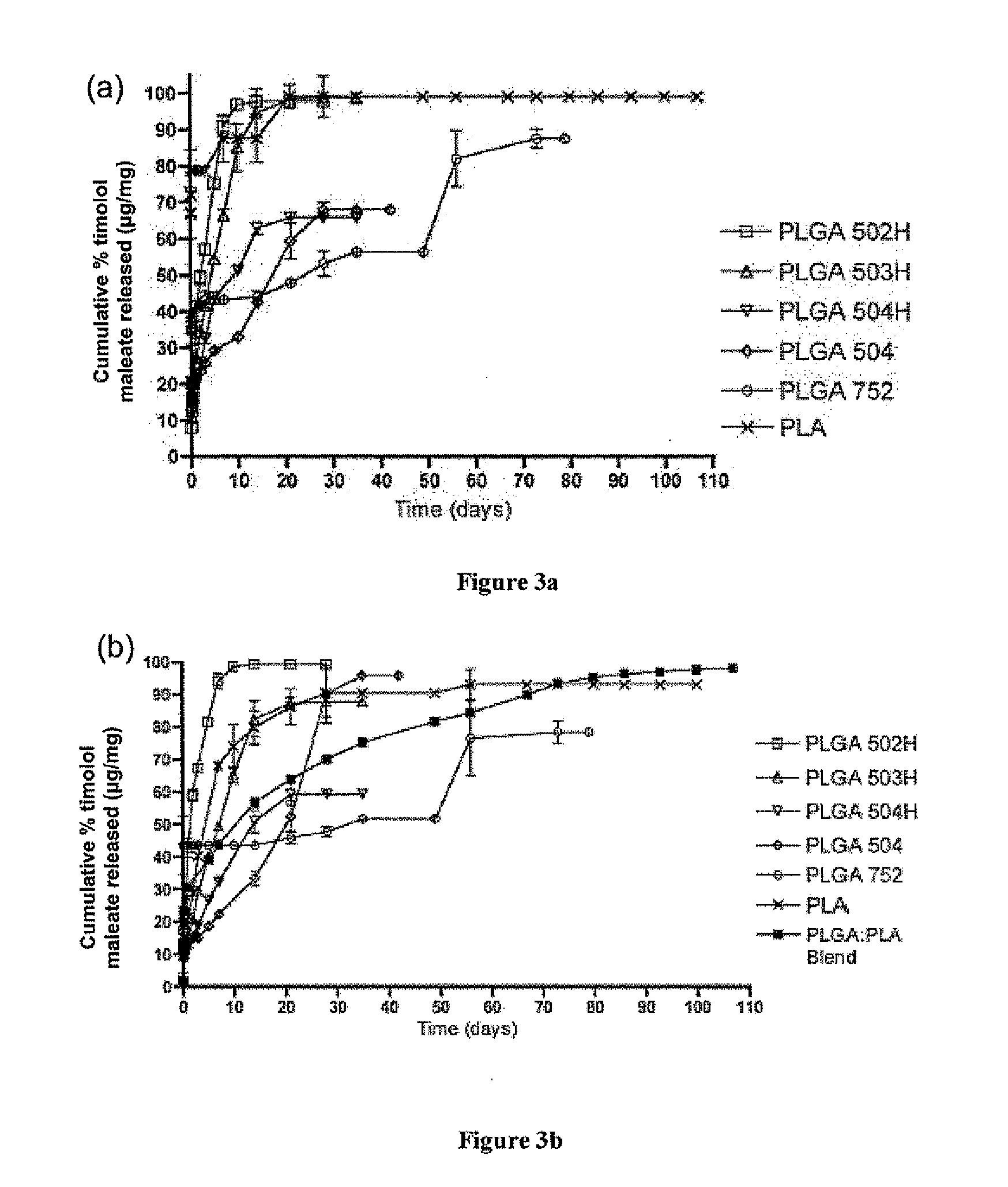
Biodegradable polymeric microparticle compositions containing one or more active agents, especially those useful for treating or preventing or one or more diseases or disorders of the eye, and methods of making and using thereof, are described. The microsphere compositions release an effective amount of the one or more active agents for a period greater than 14 days in vivo, preferably greater than 60 days in vivo, more preferably up to 73 days in vivo, more preferably greater than 90 days in vivo, even more preferably over 100 days in vivo, and most preferably greater than 107 days in vivo. In a preferred embodiment, the microparticle compositions contain one or more active agents useful for managing elevated intraocular pressure (TOP) in the eye. In one embodiment, the microspheres are formed from polylactide-co-glycolide (“PLGA”); in another embodiment, the microspheres are formed from a blend PLGA and poly lactic acid (“PLA”). Relatively hydrophilic, and preferably carboxylated, polymeric materials such as PLGA are used for a drug such as timolol maleate, which is relatively water soluble, to increase drug loading. Higher molecular weight polymers, as well as the ratio of LA (which has a longer degradation time, up to one to two years) to GA (which has a short degradation time, as short as a few days to a week), are used to provide release over a longer period of time. The combination of drug loading and release rate, as well as the minimization of initial burst release, result in prolonged release of a higher amount of drug.
Owner:YALE UNIV +1
Rivastigmine slow-release microspheres and preparation method thereof
InactiveCN101708164AHigh drug loadingHigh encapsulation efficiencyNervous disorderPharmaceutical non-active ingredientsMicrosphereRivastigmine
The invention discloses slow-release microspheres coated with rivastigmine tartrate / rivastigmine for PLGA / PLA injection and a preparation method thereof. The slow-release microspheres mainly comprise rivastigmine tartrate / rivastigmine and PLGA and PLA serving as biodegradable polymer medicinal materials, and can be prepared by adopting a W1 / O / W2 composite emulsified solvent volatilization method, an O / W emulsified solvent volatilization method, an O1 / O2 emulsion drying method and a spray drying method. The grain diameter of the microspheres is less than 100 microns. The slow-release microspheres have high medicament loading amount, have no obvious burst release effect, can be continuously released for one week, one mouth or three mouths, are used for treating senile diseases such as Alzheimer's disease, Parkinson disease and the like, and can prolong the acting time of the medicament, reduce the application times and greatly improve the administration compliance of patients.
Owner:SUZHOU UNIV
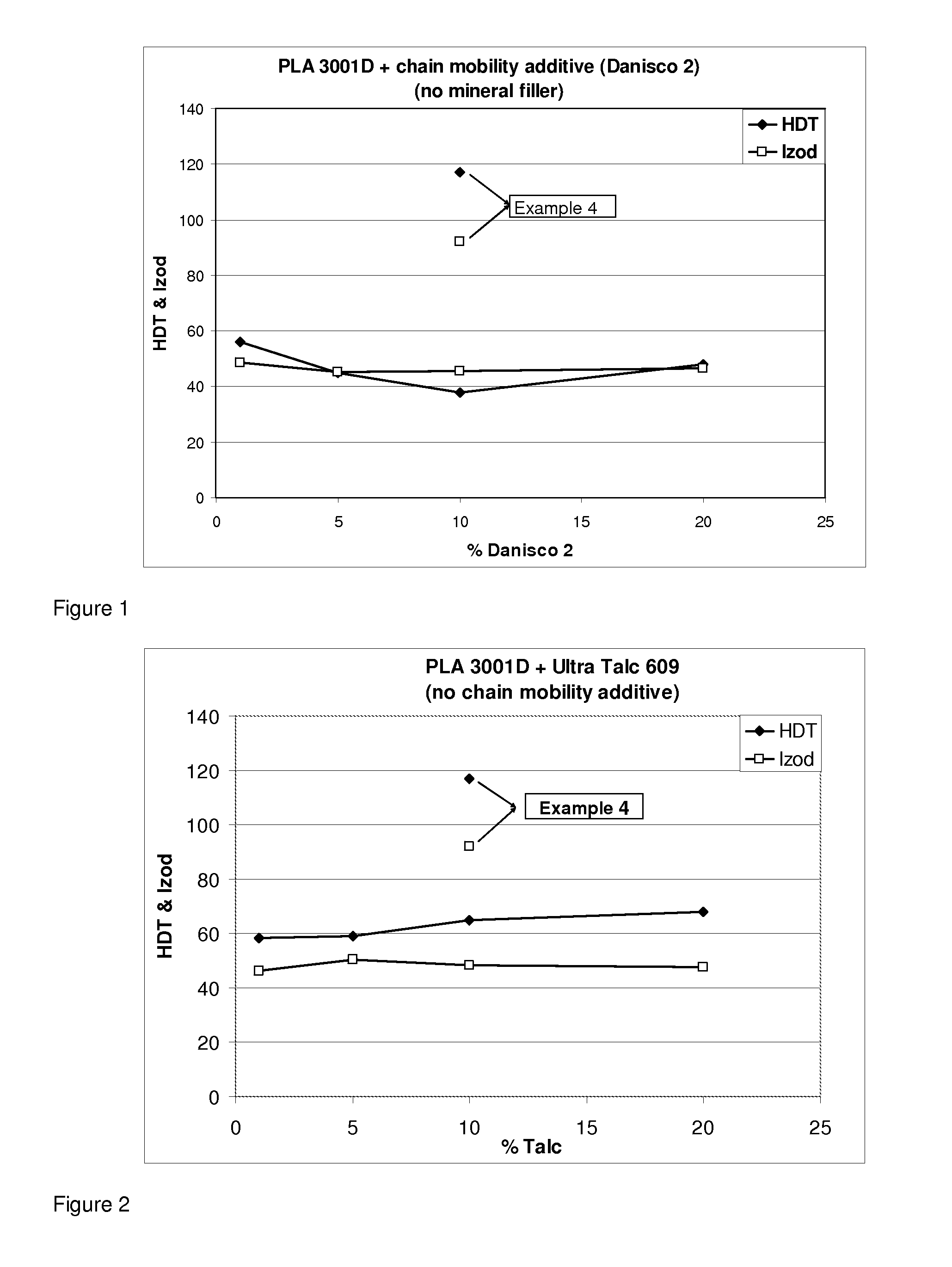
Poly (lactic-acid) resin compositions
InactiveUS20120184672A1Improve impact resistanceMixingCeramic shaping apparatusHeat deflection temperaturePolylactic acid
There is provided a polylactic acid resin composition having elevated impact resistance and / or elevated heat deflection temperature, the composition comprising polylactic acid as a major phase. There is also provided an article made of this composition and a masterbatch for producing this composition through dilution with polylactic acid. Finally, there is also provided a process for making such a composition, the process comprising the step of compounding together the following ingredients: polylactic acid, a mineral filler, a chain mobility additive, optionally an impact modifier, and optionally a chain extender, thereby producing the resin composition.
Owner:TEKNOR APEX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com