Polyethylene glycol-poly(lactic-co-glycolic acid)-polylysine nano-delivery system, preparation method and application thereof
A technology of polylactic acid glycolic acid and glycolic acid, applied in emulsion delivery, other methods of inserting foreign genetic materials, capsule delivery, etc., can solve the problem of rapid emergence of tumor cell drug resistance, lack of selectivity of chemotherapy drugs, and limitations of chemotherapy drugs in clinical practice. treatment effects etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The present invention is further described below with embodiment, but content of the present invention is not limited. Embodiment 1, preparation of polyethylene glycol monomethyl ether-polylactic acid glycolic acid-polylysine (mPEG-PLGA-PLL) (1) polyethylene glycol monomethyl ether-polylactic acid glycolic acid (mPEG-PLGA) Preparation: heat-resistant glass tube is heated and dried in vacuum, lactide / glycolide raw material with a certain molar mass ratio (ratio of 8:2, 7:3, 6:4, 5:5) is added, and the proportion of total raw material is added Add PEG with a mass percentage of 1% to 20% and a molecular weight range of 350 to 5000, then add a catalyst, pass nitrogen, heat to dissolve and vacuumize, cool and solidify, vacuumize for 2 hours, seal the tube, and react at 120-150°C for 8-50h. (2) Preparation of polyethylene glycol monomethyl ether-polylactic acid glycolic acid-tert-butoxycarbonyl (mPEG-PLGA-Boc(Z)): dissolve a certain amount of mPEG-PLGA in a dry organic solven...
Embodiment 2
[0046] Example 2, preparation of mPEG-PLGA-PLL nanoparticles loaded with mitoxantrone hydrochloride drug
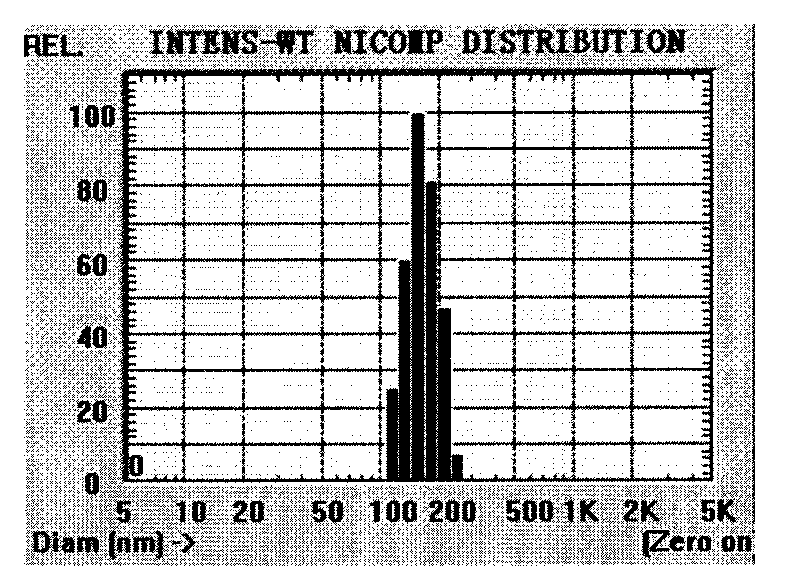
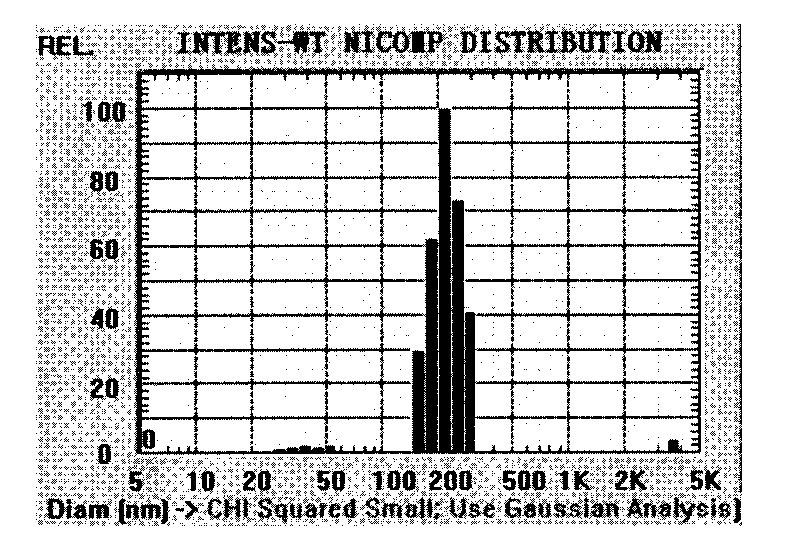
[0047] Prepared by emulsification evaporation method, take the material mPEG-PLGA-PLL and dissolve it in dichloromethane or a mixed solvent of dichloromethane and acetone, add the aqueous solution of mitoxantrone hydrochloride, after ultrasonic emulsification, then add it into the aqueous solution containing F68, again ultrasound. Then stir at room temperature for 0.5-5h to remove the organic phase to obtain nanoparticle suspension. The particle size of the nanoparticles prepared above is controlled at 10-1000nm.
[0048] It is prepared by thin film emulsification method. The material mPEG-PLGA-PLL and mitoxantrone hydrochloride are dissolved in acetone solvent, and rotated to evaporate to form a film. Then add a certain amount of aqueous solution and stir at room temperature for 0.5-6h to obtain a nanoparticle suspension. . The particle size of the nanoparticles prepa...
Embodiment 3
[0050] Prepared by interfacial precipitation method, the material mPEG-PLGA-PLL and mitoxantrone hydrochloride were dissolved in acetone solvent, and at a certain stirring speed, the above solution was injected into a certain concentration and volume of polyvinyl alcohol (PVA) solution, pressurized The acetone is removed by volatilization, and the nanoparticle suspension is obtained. The particle size of the nanoparticles prepared above is controlled at 10-1000nm. Embodiment 3, the preparation of the mPEG-PLGA-PLL nanoparticle of carrying gene
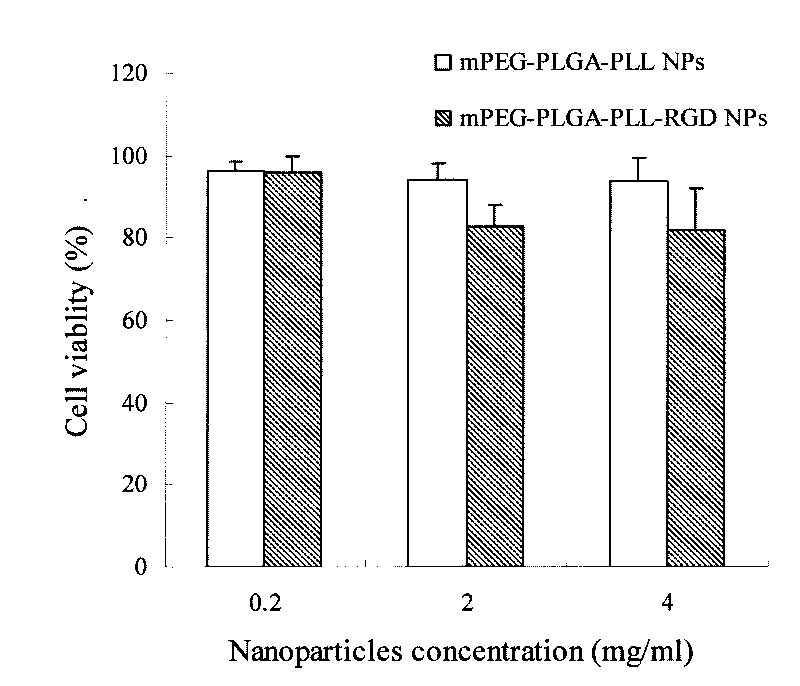
[0051] Preparation by emulsification evaporation method: take the material mPEG-PLGA-PLL and dissolve it in dichloromethane or a mixed solvent of dichloromethane and acetone, add it into an aqueous solution containing F68 and sonicate. Then stir at room temperature for 0.5-5h to remove the organic phase to obtain mPEG-PLGA-PLL nanoparticle solution. An appropriate amount of mPEG-PLGA-PLL nanoparticle solution was added dropwise to an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com