Catalyst for ring-opening polymerization of cyclic ester and preparation method thereof
A ring-opening polymerization, catalyst technology, applied in the field of cyclic ester ring-opening polymerization catalyst and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
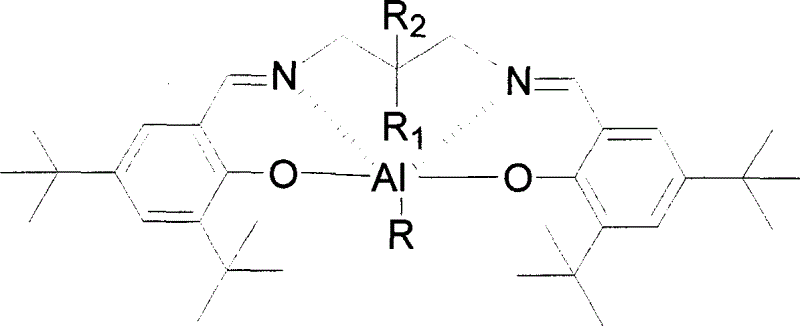
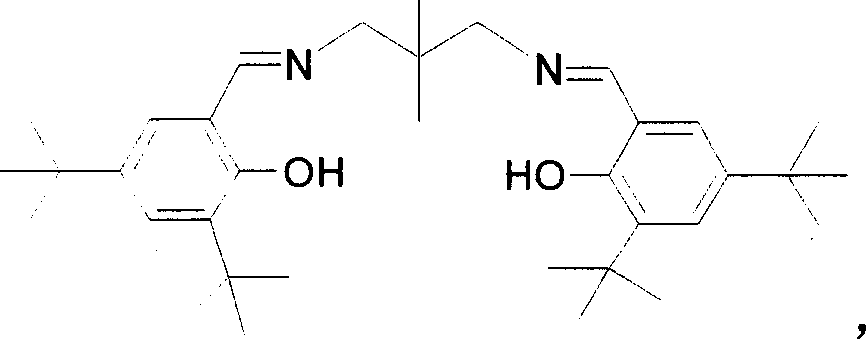
[0027] Embodiment 1: Catalyst Salmpen ( t Synthesis of Bu)AlEt,
[0028] Under the protection condition of stirring and inert gas, put 4ml of Schiff's base Salmpen ( t Bu)H 2 Toluene solution (1mol / L) and 4ml of AlEt 3 Toluene solution (1mol / L) is mixed, temperature control 80 ℃, stirs 48 hours, then cools down, vacuumizes and removes volatile matter, obtains product Salmpen ( t Bu)AlEt 2.24g, yield 95.1%. Elemental analysis results,
[0029] Calculated: C, 75.47; H, 9.76; N, 4.76; Found: C, 75.52; H, 9.59; N, 4.88.
Embodiment 2
[0030] Embodiment 2: Catalyst Salmpen ( t Bu)AlO i Synthesis of Pr
[0031] In addition to the AlEt in Example 1 3 Change aluminum isopropoxide into, and temperature of reaction changes outside 120 ℃, and other steps are identical with example 1, obtain product Salmpen ( t Bu)AlO i Pr 2.31g, yield 93.3%. Elemental analysis results, calculated value: C, 73.75; H, 9.61; N, 4.53; found value: C, 73.98; H, 9.87; N, 4.16.
Embodiment 3
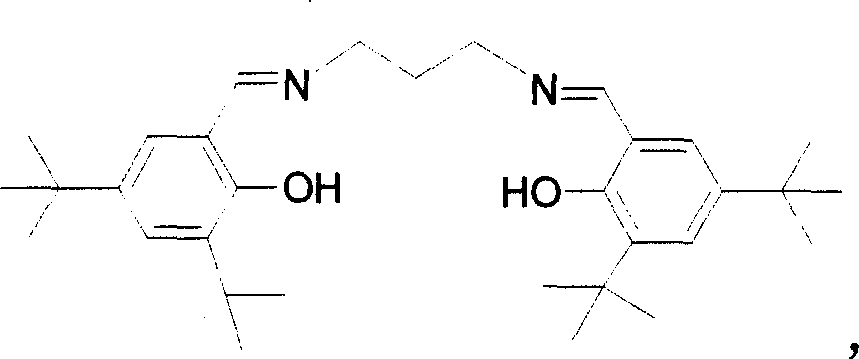
[0032] Embodiment 3: Catalyst Salepen ( t Synthesis of Bu)AlEt
[0033] In addition to changing the Schiff base in Example 1 into Salepen ( t Bu)H 2 , the temperature of reaction is changed to 50 ℃, and other steps are identical with example 1, obtain product Salepen ( t Bu)AlEt 2.38g, yield 96.5%. Elemental analysis results, calculated value: C, 75.93; H, 9.97; N, 4.54; found value: C, 75.97; H, 10.21; N, 4.66.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com