Rivastigmine slow-release microspheres and preparation method thereof
A technology of slow-release microspheres and rivastigmine bitartrate, which is applied in pharmaceutical formulations, medical preparations of non-active ingredients, nervous system diseases, etc., and can solve problems such as tartrate/rivastigmine bitartrate preparations that have not yet been found. Achieve the effects of sustained slow release, high drug loading, and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
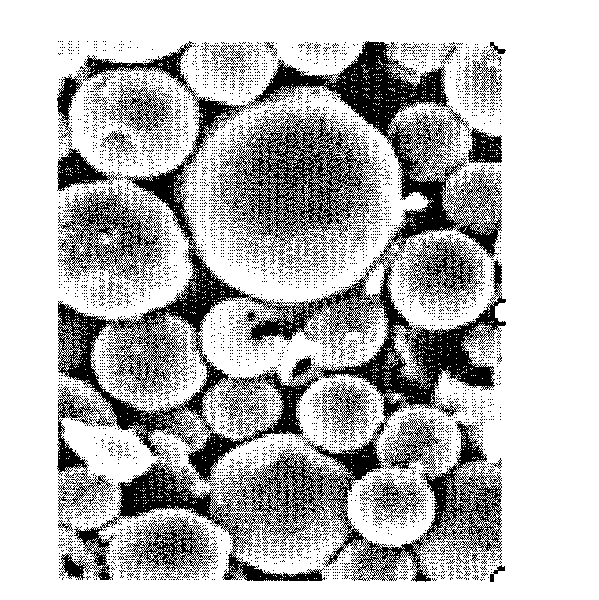
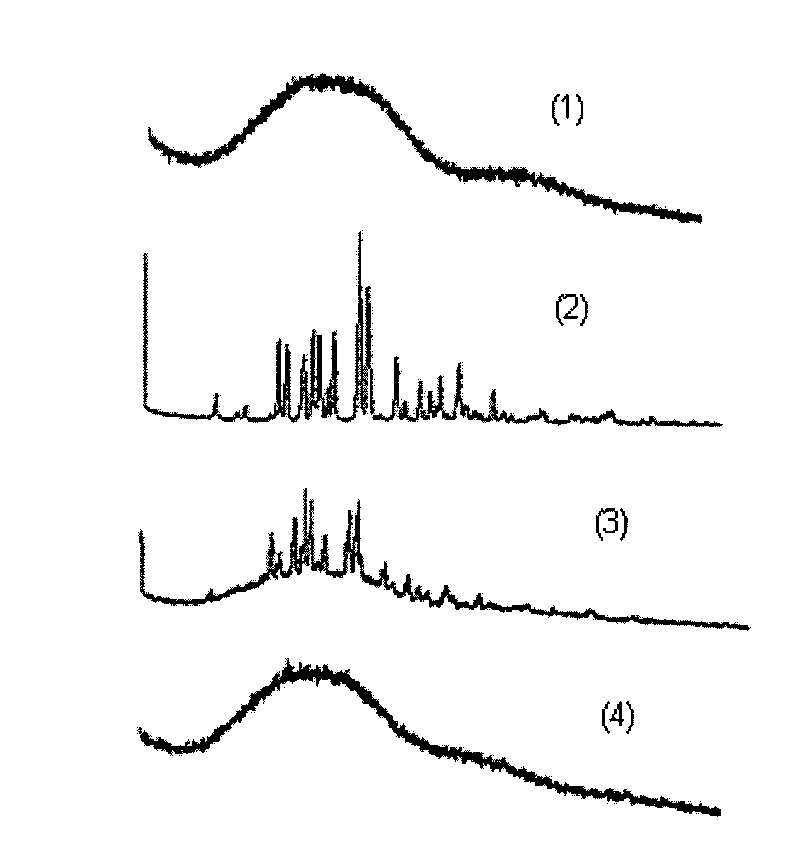
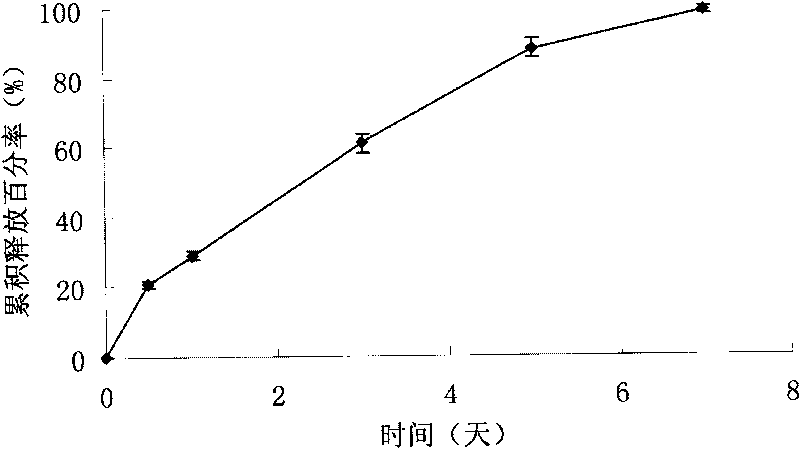
Image
Examples
Embodiment 1
[0042] Example 1: W 1 / O / W 2 Preparation of sustained-release microspheres of rivastigmine tartrate by double emulsification-solvent evaporation
[0043] Dissolve 25mg rivastigmine tartrate and 50mg poloxamer 188 in 0.2mL water as the inner aqueous phase; dissolve 100mg PLGA (RG502H, PLA:PGA=50:50, Mw=20000) in 2.0mL ethyl acetate , forming the oil phase. Add the inner water phase to the oil phase, ultrasonically emulsify the probe, work at a frequency of 200 watts, work for 1 second, rest for 1 second, and get W 1 / O colostrum. Quickly pour the colostrum into 40mL of rapidly stirred 0.1% PVA aqueous solution (adjust the pH to 9.0 with glycine-sodium hydroxide, which contains 1% sodium chloride), stir at 7,000 rpm, and emulsify for 2 minutes at room temperature Continue stirring at a speed of 500 rpm for 6 hours to evaporate the organic solvent, collect the solidified microspheres by centrifugation, wash with distilled water three times, and freeze-dry to obtain the micros...
Embodiment 2
[0044] Embodiment 2: O / W emulsification-solvent volatilization method prepares rivastigmine tartrate sustained-release microspheres
[0045] Dissolve 25 mg of rivastigmine tartrate and 100 mg of PLGA (RG502H, PLA:PGA=50:50, Mw=20000) in a mixed solvent consisting of 2 mL of ethyl acetate and 0.2 mL of propylene glycol to form an oil phase. Pour the oil phase into rapidly stirred 40mL 0.1% PVA aqueous solution (adjust pH 9.0 with glycine-sodium hydroxide, which contains 1% sodium chloride), stir at 7,000 rpm, emulsify for 2 minutes, at room temperature Continue stirring at a speed of 500 rpm for 6 hours to evaporate the organic solvent, collect the solidified microspheres by centrifugation, wash with distilled water three times, and freeze-dry to obtain the microspheres.
Embodiment 3
[0046] Example 3: O 1 / O 2 Preparation of sustained-release microspheres of rivastigmine tartrate by emulsification-in-liquid drying method
[0047] Dissolve 20mg of rivastigmine tartrate and 200mg of PLGA (RG502H, PLA:PGA=50:50, Mw=20000) in 2mL of a mixed solvent composed of dichloromethane and acetonitrile (volume ratio 3:2), and ultrasonically vibrate to make it Dissolve to form the internal oil phase; inject the internal oil phase into 40mL liquid paraffin containing 0.2% Span 80, stir at 800 rpm for 15 minutes to emulsify, continue stirring at 500 rpm for 6 hours at room temperature to evaporate the organic solvent, filter , followed by washing the microspheres with n-hexane and water, redissolving in a small amount of water, and freeze-drying to obtain the microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com