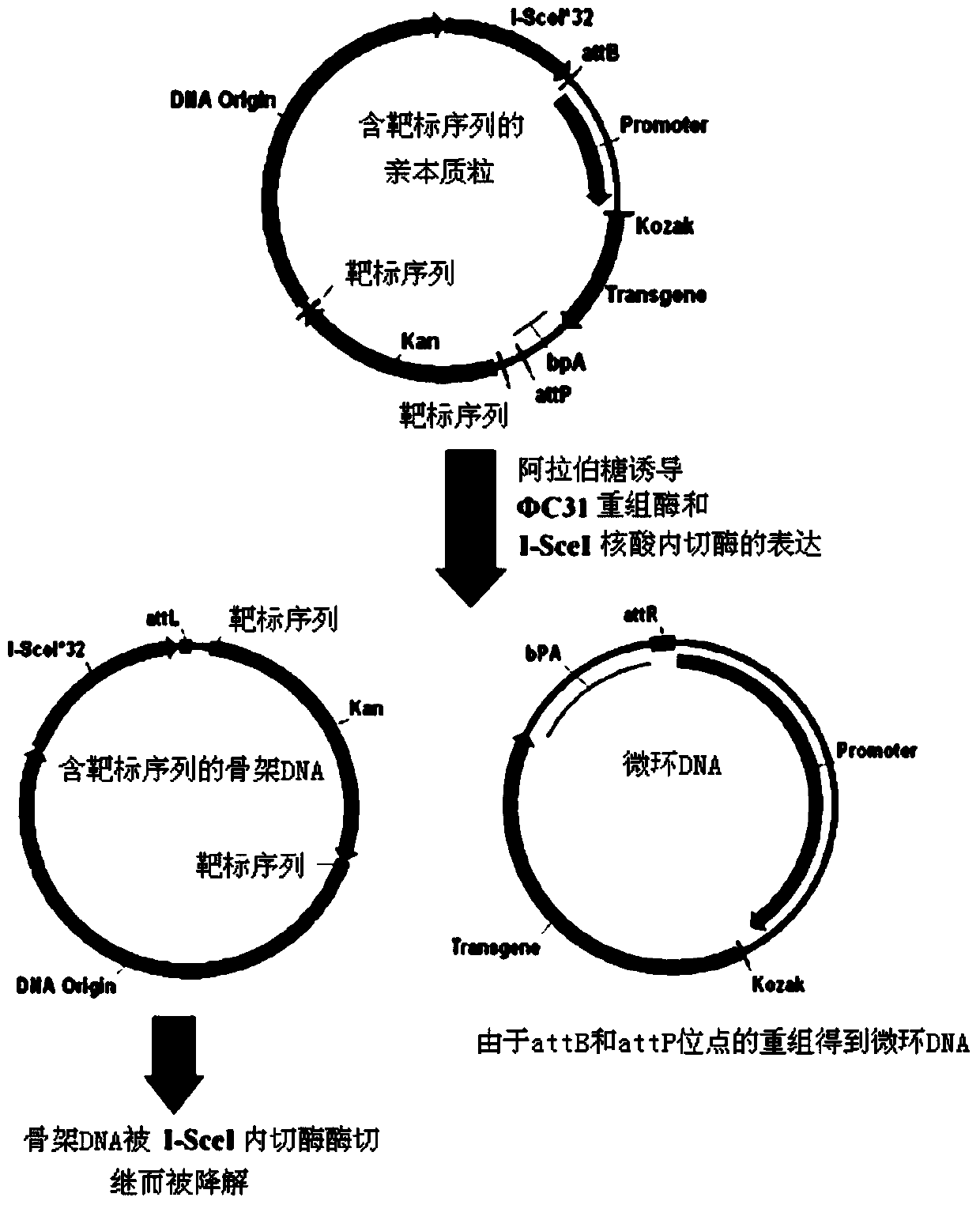
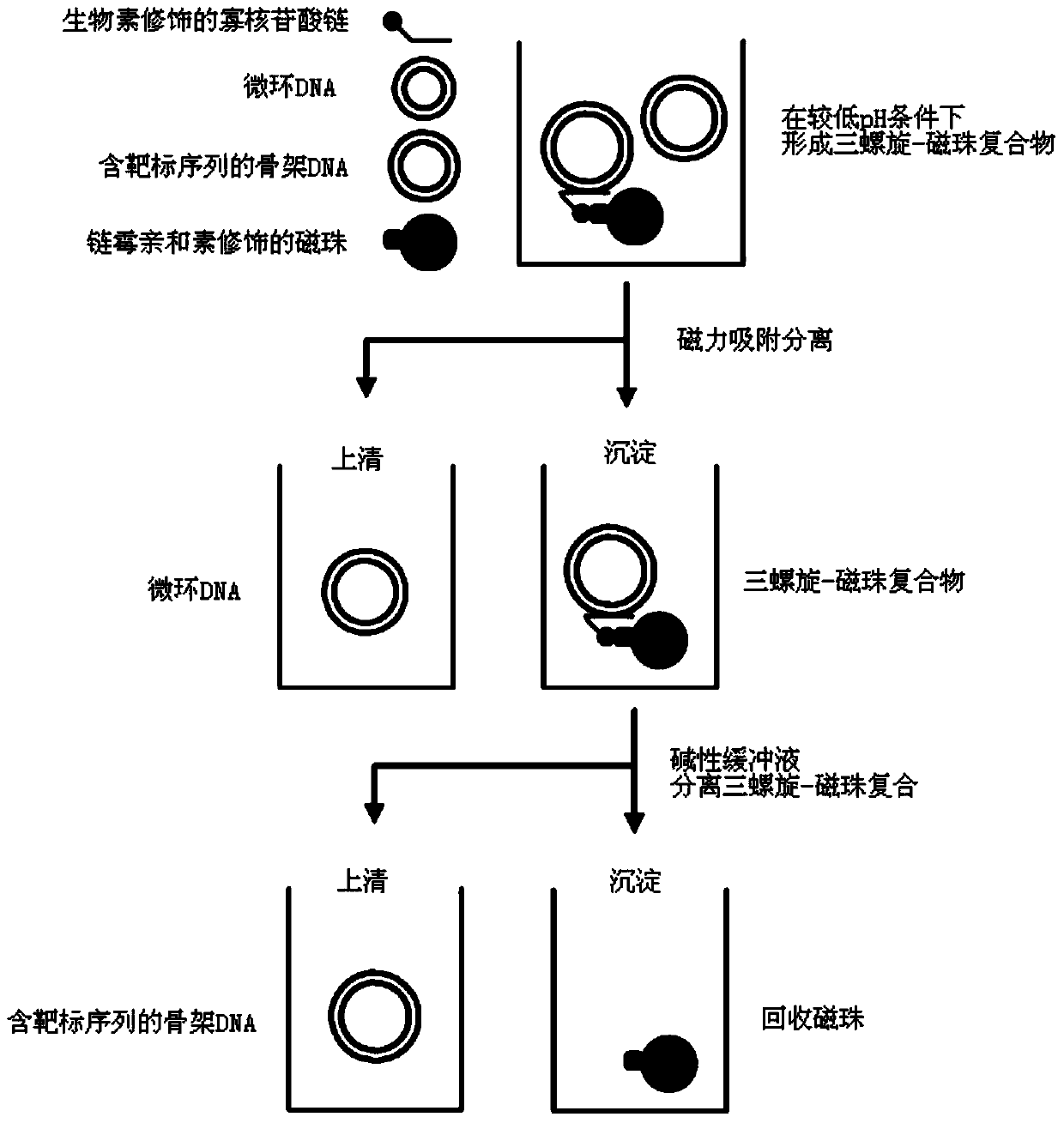
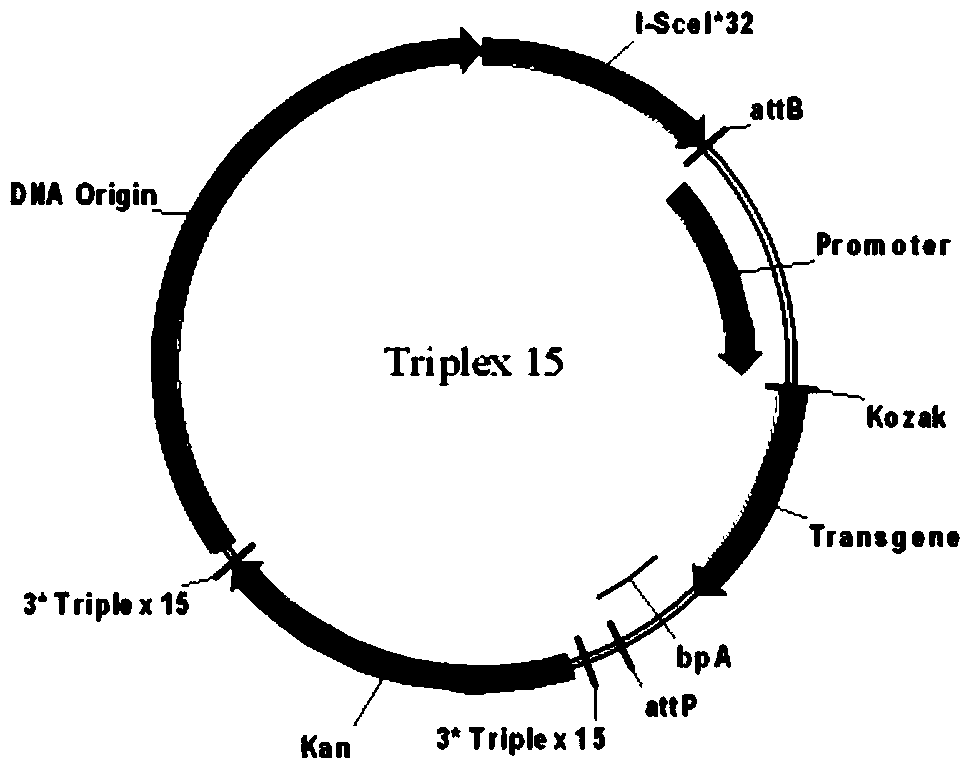
High-purity minicircle DNA (deoxyribonucleic acid) and preparation method and application thereof
A high-purity, micro-circular technology, applied in DNA preparation, recombinant DNA technology, biochemical equipment and methods, etc., can solve problems such as hidden dangers of clinical application of micro-circular DNA, and achieve the effect of improving safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0629] The construction of parental plasmids ATriplex15DsRed, Triplex15DsRed and ATriplex15DsRed, 2TTTriplex21DsRed, Triplex21DsRed with the target sequence includes the following steps:
[0630] a. Using the plasmid ZY781.CMV.DsRed.bpA as a template (the preparation method of ZY781.CMV.DsRed.bpA is: insert the CMV promoter at the SpeI and EcoRI sites of the pMC.BESPX plasmid, insert the DsRed gene at the EcoRI and SalI sites , PspOMI and ScaI sites were inserted into SV40bpA to obtain the ZY781.CMV.DsRed.bpA plasmid, wherein the gene accession numbers of the CMV promoter, DsRed gene and SV40bpA were respectively BD015377.1, FJ226077.1 and NC_001669.1) , using the following 5 pairs of primers to carry out PCR: the first pair of primers: ATriplex15-F (SEQIDNO:41) and ATriplex-R (SEQIDNO:44); the second pair of primers: Triplex15-F (SEQIDNO:21) and Triplex15-R (SEQIDNO:22); The third pair of primers: ATriplex21-F (SEQIDNO:42) and Triplex-R (SEQIDNO:44); The fourth pair of primer...
Embodiment 2
[0638] Construct parental plasmid ZY781-triplex15, ZY781-triplex21 with target sequence, comprising the following steps:
[0639] The plasmid ZY781.bpAp (the PspOMI and ScaI sites of MC.BESPX were inserted into the SV40bpA fragment to obtain the plasmid ZY781.bpAp, and the gene accession number of the SV40bpA is NC_001669.1) was used as a template; PCR was performed with the following 2 pairs of primers:
[0640] The first pair of primers: Triplex15-F (SEQ ID NO:21) and Triplex15-R (SEQ ID NO:22);
[0641] The second pair of primers: Triplex21-F (SEQ ID NO:23) and Triplex21-R (SEQ ID NO:24);
[0642] The PCR products were recovered by gel respectively, and the kan fragments with 6 Triplex15 target sequences were obtained respectively. The arrangement of the 6 Triplex15 target sequences was as follows: there were 3 tandem repeated Triplex15 target sequences in the upper and lower reaches of the Kan gene; 4 Triplex21 The kan fragment of the target sequence, wherein the four Tri...
Embodiment 3
[0645] Construction of parental plasmids Triplex21CMV.bpA, Triplex21RSV.bpA, Triplex21Ubc.bpA and Triplex21ApoE.bpA with target sequences comprises the following steps:
[0646] Using primers described in Table 1 to CMV-F (SEQIDNO:25) and CMV-R (SEQIDNO:26), RSV-F (SEQIDNO:27) and RSV-R (SEQIDNO:28), Ubc-F (SEQIDNO:28) respectively : 29) and Ubc-R (SEQIDNO: 30), ApoE-F (SEQIDNO: 31) and ApoE-R (SEQIDNO: 32) carry out PCR, amplify CMV, RSV, Ubc, ApoE promoter respectively, described PCR amplifies The genebank accession numbers of the added template genes are: CMV promoter (genebank accession number is BD015377.1), RSV promoter (genebank accession number is M77786.1), Ubc promoter (genebank accession number is NG_027722.2), ApoE promoter (genebank accession number is D38257.1); each PCR product is connected to ZY781-triplex21 through the corresponding restriction sites through conventional cloning steps, and after identification by restriction restriction and sequencing, the clo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com