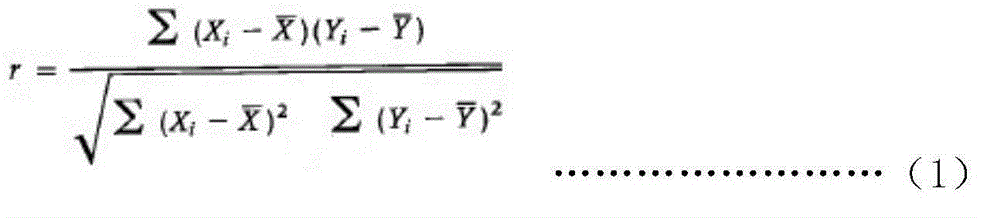Quantitative detection kit for human Dickkopf-1 protein (DKK-1)
A detection kit, DKK-1 technology, applied in biological testing, measuring devices, material inspection products, etc., can solve the problems of inability to meet clinical application requirements, complicated operation steps, long reaction time, etc., to meet clinical application requirements, Simple sample processing and stable luminous value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1, human Dickkopf-1 protein (DKK-1) quantitative detection kit components and methods thereof
[0081] 1. Preparation of Human Dickkopf-1 Protein (DKK-1) Quantitative Detection Kit
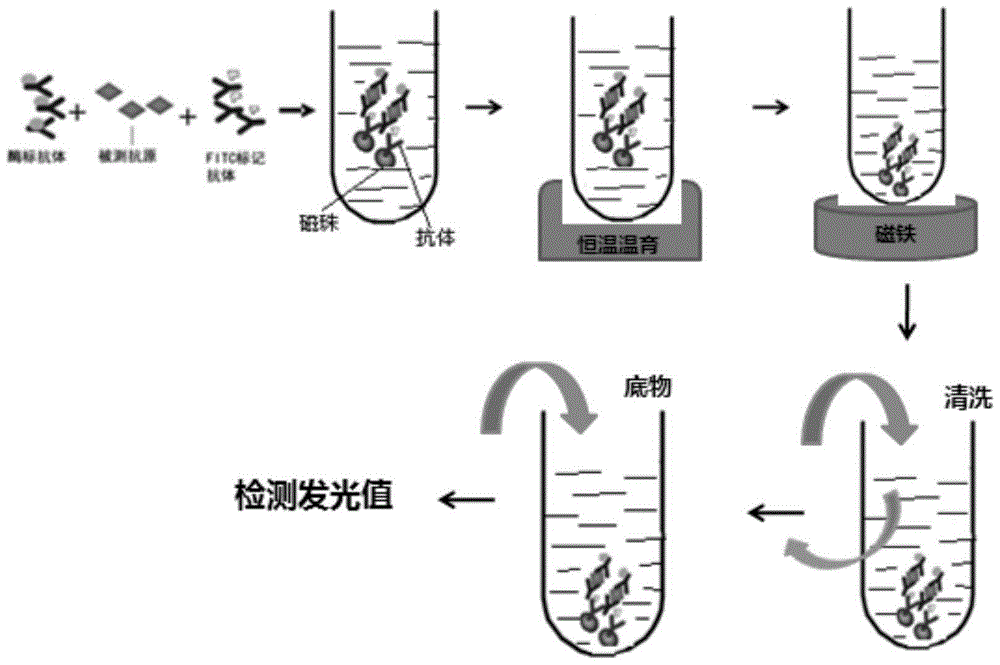
[0082] The following 6 components are individually packaged and then packaged as a kit as a whole, which is the human Dickkopf-1 protein (DKK-1) quantitative detection kit: 1. Calibrators (containing a series of concentrations of DKK-1, used to establish standard curve); 2. Quality control product (containing a certain concentration of DKK-1); 3. Reagent A (containing a certain concentration of fluorescein isothiocyanate-labeled DKK-1 monoclonal antibody and containing a certain concentration of alkaline phosphatase Labeled DKK-1 monoclonal antibody solution); 4. Magnetic separation reagent (magnetic particle suspension combined with anti-fluorescein isothiocyanate antibody); 5. Cleaning solution (for preparing magnetic bead cleaning solution); 6 , Substrate solution. 1, 2, 3, ...
Embodiment 2
[0153] Embodiment 2, kit performance evaluation
[0154] According to the characteristics of in vitro diagnostic reagents, the linear range, minimum detection limit, accuracy and precision of the kit prepared in Example 1 were tested according to the usual practice. The specific operation steps are as follows:
[0155] 1. Reagent preparation:
[0156] Before the experiment, take out the reagent A, calibrator, magnetic separation reagent, substrate solution, cleaning solution, and quality control in the kit of Example 1, and equilibrate to room temperature.
[0157] 2. Instrument preparation:
[0158] This kit uses ChemLite from Boao Bio Group Co., Ltd. TM 1200 automatic chemiluminescent immunoassay analyzer.
[0159] details as follows:
[0160] Add calibrators, samples or quality control substances into the sample cup. The sample volume for a single tube reaction is 30 μL. Calculate the volume in the sample cup based on the number of repeated tubes. The minimum sample vo...
Embodiment 3
[0206] Embodiment 3, the kit provided by the present invention compares with foreign kits
[0207] 1. The test kit provided by the present invention is compared with foreign kit performance indicators as follows:
[0208] Detect same sample with existing test kit, compare as follows with the test result of test kit of the present invention:
[0209] Table 5 Performance Index Comparison Results
[0210]
[0211]
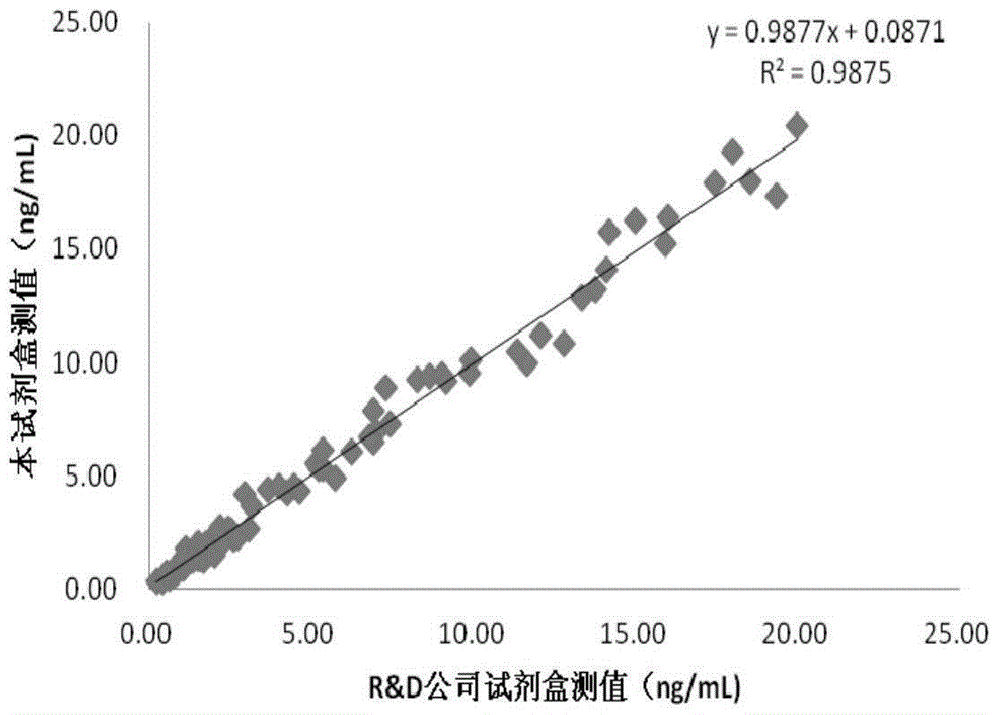
[0212] 2. The test kit provided by the present invention is compared with foreign kits for clinical sample measurement values
[0213] QuantikineELISAHumanDKK-1 assay kit (for research) produced by the kit provided by the invention and R&DSystems company is respectively detected simultaneously to 100 parts of human serum samples (patients are informed), and the results are shown in figure 2 The serum DKK-1 concentration measured by the kit provided by the present invention is the ordinate, and the result measured by the QuantikineELISAHumanDKK-1 kit produced ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com