Liquid composition comprising antibody of human interleukin-4 receptor alpha
A technology of liquid composition and human interleukin, which is applied in the direction of antibodies, drug combinations, antibody medical components, etc., can solve the problems of not being able to pass through the filter membrane, increase the side effects of drugs, and large deviations in dosage, so as to achieve easy production and storage, and improve Effect of drug efficacy, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1 pH value and buffer
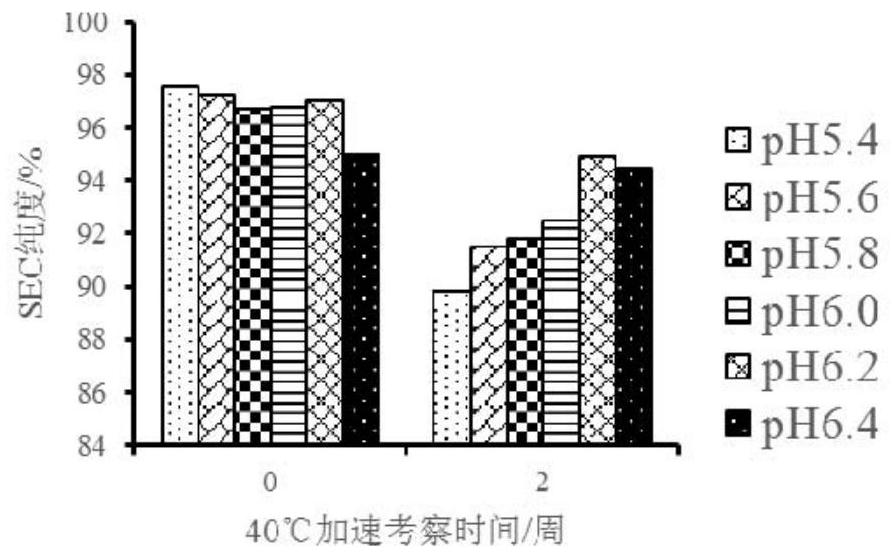
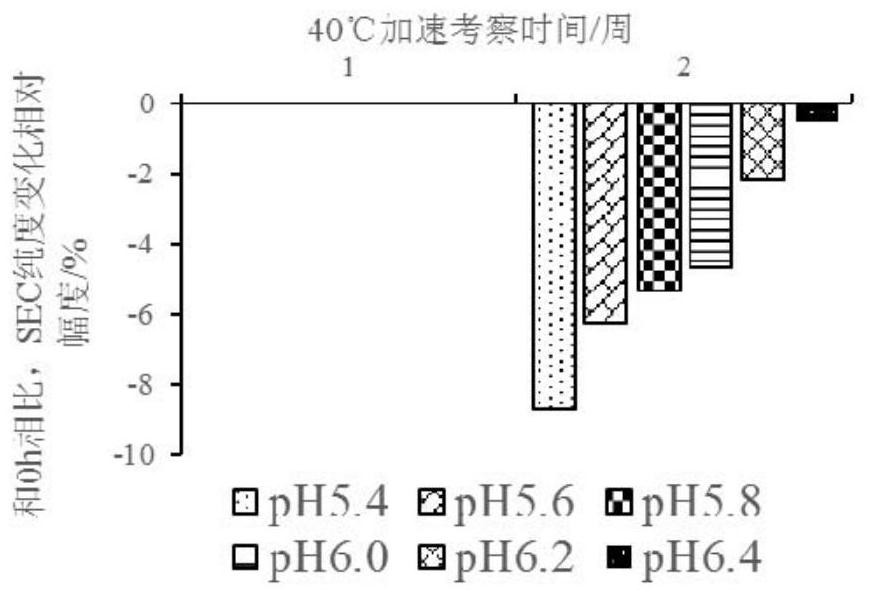
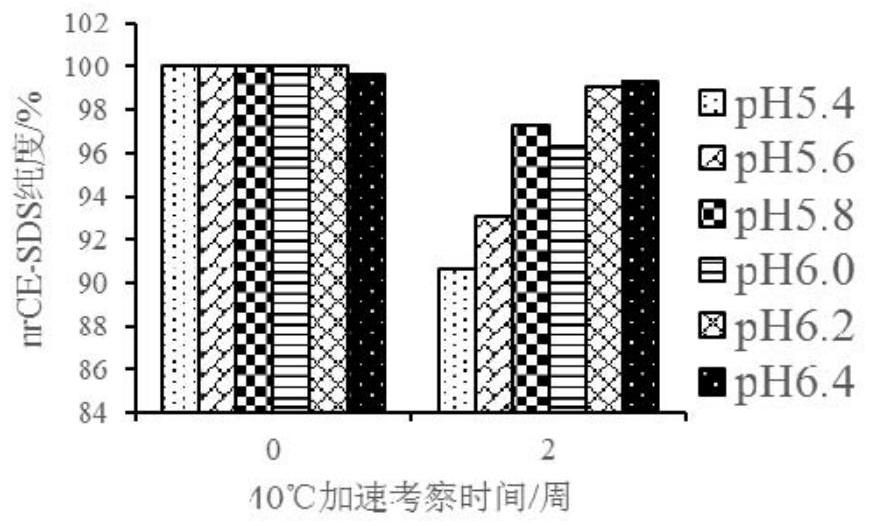
[0106] The pH range and buffers of CBP-201 formulations were studied. Choose pH 5.4, 5.6, 5.8, 6.0, 6.2, 6.4, a total of 6 points, choose sodium acetate, histidine hydrochloride, sodium dihydrogen phosphate as buffer, and add sodium chloride. The following protein solutions containing 133.6mg / ml CBP-201 were subjected to accelerated experiments at 40°C to determine the appropriate pH range and buffer (see Table 1).
[0107] Table 1 pH value and buffer
[0108] serial number protein solution pH Preparation volume A 10mM NaAc 150mM NaCl 5.4 1L B 10mM NaAc 150mM NaCl 5.6 1L C 10mM His-HCl 150mM NaCl 5.8 3L D 10mM His-HCl 150mM NaCl 6.5 1L E 10mM NaH2PO4 150mM NaCl 6.4 1L
[0109] Appearance, protein concentration, SEC purity, CE-SDS purity, charge heterogeneity, DSC, and viscosity items were detected through accelerated experiments at 40°C.
[0110] Appearance inspection results...
Embodiment 2
[0120] Example 2 Protective agent
[0121] Suitable protectants for CBP-201 formulations were investigated. At pH 6.0, trehalose, sucrose, mannitol, proline, arginine hydrochloride, glycine, and sodium chloride were added, and the sodium chloride group was used as a control. The following protein solutions containing 133.6 mg / ml CBP-201 were subjected to accelerated experiments at 40°C to determine suitable protective agents (see Table 2).
[0122] Table 2 Protective agent
[0123]
[0124] Through accelerated experiments at 40°C, the appearance, protein concentration, SEC purity, CE-SDS purity, charge heterogeneity, viscosity, and DSC items were detected.
[0125] Appearance inspection results: all protein solutions were whitish in appearance.
[0126] Protein concentration test results: the protein concentrations were all within the range of 133.6±5% mg / ml.
[0127] In addition, after accelerating 2W at 40°C for protein solutions under different protective agent con...
Embodiment 3
[0134] Example 3 Viscosity in buffer only
[0135] Investigate the change of viscosity with concentration of CBP-201 in the case of only buffer (pH6.0 10mmol / L His-HCl) without protective agent, so as to judge the effect of each protective agent on reducing the viscosity of the preparation; challenge the highest concentrated concentration , and detect the viscosity to provide a basis for the selection of protein concentration in the preparation. The buffer solution in this embodiment does not contain NaCl, which is compared with the previous experiment containing NaCl.
[0136] Dialyze the protein with dialysis buffer (pH6.0 10mmol / L His-HCl), and concentrate the dialyzed protein to 71.23mg / ml, 89.04mg / ml, 106.85mg / ml, 133.56mg / ml, >151.37mg / ml (Protein concentration detection), and then filtered. Carry out appearance and viscosity inspection. The experimental results are shown in Table 3. Figure 18 .
[0137] Table 3 The protein solution appearance and viscosity test ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com