A medicine core composition of controlled release drug delivery and controlled release preparation as well as its preparing method
一种组合物、药芯的技术,应用在医药配方、药物输送、非有效成分的医用配制品等方向,能够解决吸水速度和水合速度慢、有机溶剂残留量高、药物释放时滞长等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] prescription:
[0049] (1) Drug-containing layer (per tablet):
[0050] Nifedipine 33mg
[0051] Povidone (Plasdone K-90D) 30mg
[0052] Copovidone (Plasdone S630) 91mg
[0053] Magnesium Stearate 1.5mg
[0054] Micronized silica gel 0.5mg
[0055] (2) Booster layer (per piece):
[0056] Sodium starch glycolate 37mg
[0057] Hypromellose (K15M) 30mg
[0058] Carbomer (971PNF) 8mg
[0060] Copovidone (Plasdone S630) 15mg
[0061] Red Iron Oxide 1.1mg
[0062] Magnesium stearate 0.6mg
[0063] Micronized silica gel 0.4mg
[0064] (3) Composition of semi-permeable membrane coating solution (for every 1000 tablets)
[0065] Cellulose acetate 59.5g
[0066] Diethyl phthalate 3g
[0067] Acetone 1500ml
[0068] Single tablet weight gain 38mg
[0069] (4) Composition of moisture-proof coating solution:
[0070] Color blue pink (CM-0317)
[0071] Preparation Process:
[0072] 1. Preparation of drug-containing layer particles:
...
Embodiment 2
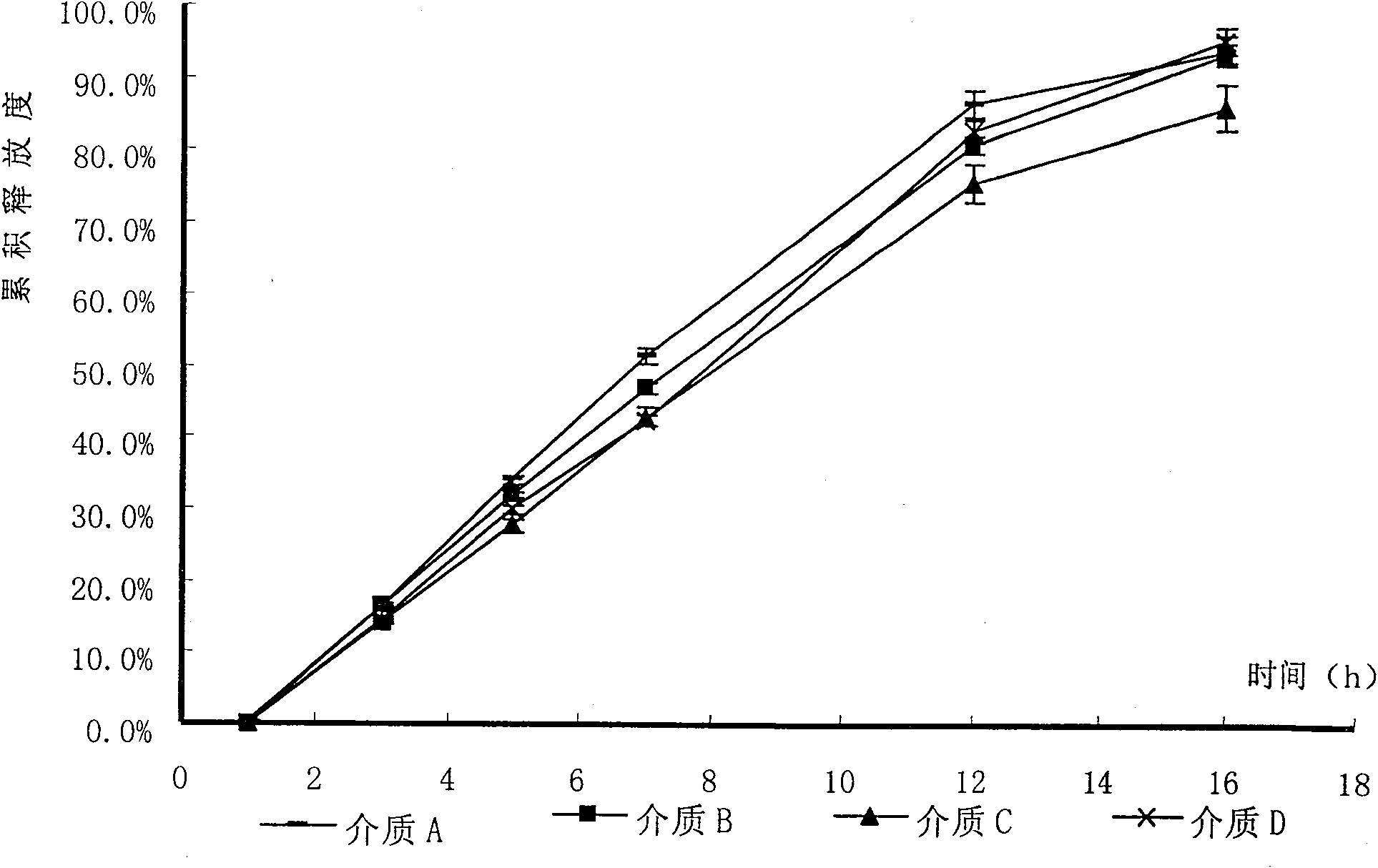
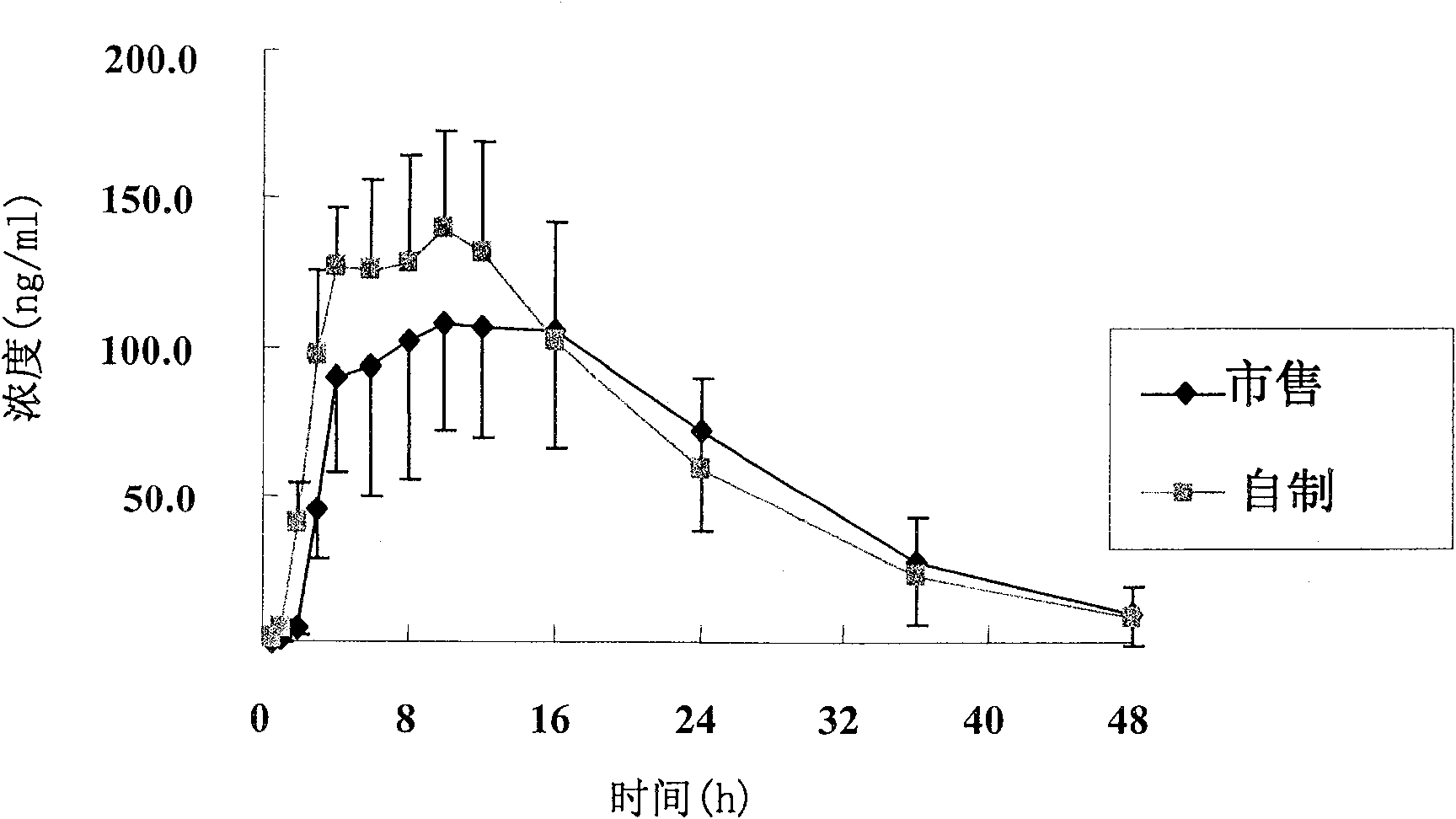
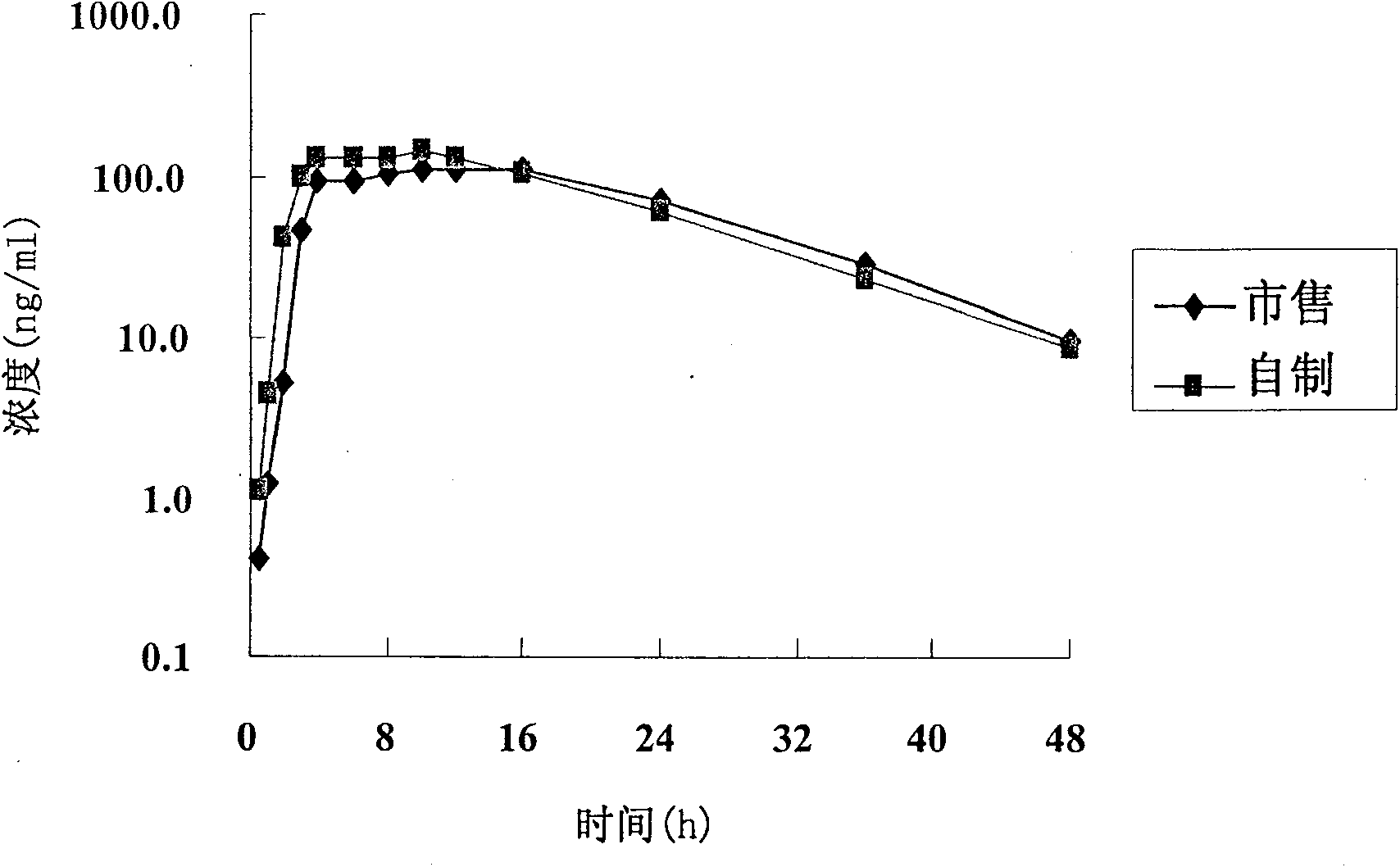
[0085] Under the conditions of dissolution media of different pH, the tablet prepared in Example 1 and the commercially available product (trade name Baixintong, produced by Bayer, Germany) were respectively tested for release rate, wherein, according to the usual practice, the dosage was 110% , for example, the theoretical value is 30 mg, and the actual dosage is 33 mg.
[0086] The results are shown in Table 1.
[0087] (1) 1% hydrochloric acid solution (pH1.2) of sodium lauryl sulfate;
[0088] (2) acetic acid-sodium acetate buffer (pH4.5) of 1% sodium lauryl sulfate;
[0089] (3) Phosphate-citrate buffer solution (pH 6.8) of 1% sodium lauryl sulfate.
[0090] Table 1 The release rate of self-made tablets and commercial products
[0091]
[0092] The test results show that the release rate of the sample prepared in Example 1 and the commercially available product all meet the standard requirements. Compared with the commercially available product, the self-made sample...
Embodiment 3
[0094] The prescription is as follows:
[0095] (1) Drug-containing layer (per tablet):
[0096] Nifedipine 33mg
[0097] Povidone (Plasdone K-90D) 30mg
[0098] Copovidone (Plasdone S630) 91mg
[0099] Magnesium Stearate 1.5mg
[0100] Micronized silica gel 0.5mg
[0101] (2) Booster layer (per piece):
[0102]Low-substituted hydroxypropyl cellulose 150mg
[0103] Hypromellose (K15M) 30mg
[0104] Carbomer (971PNF) 10mg
[0106] Copovidone (Plasdone S630) 30mg
[0107] Red Iron Oxide 1.1mg
[0108] Magnesium stearate 0.6mg
[0109] Micronized silica gel 0.4mg
[0110] (3) Composition of semi-permeable membrane coating solution (for every 1000 tablets)
[0111] Cellulose acetate 59.5g
[0112] Diethyl phthalate 3g
[0113] Acetone 1500ml
[0114] Single tablet weight gain 38mg
[0115] (4) Composition of moisture-proof coating solution:
[0116] Color blue pink (CM-0317) Appropriate amount
[0117] The preparation process is the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com