Quality evaluation and control method of florfenicol sustained-release particles
A technology of sustained-release granules and florfenicol, applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and measuring devices, etc. Problems such as high peak concentration, to achieve the effect of ensuring consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The in vitro dissolution limit determination of embodiment 1 Florfenicol sustained-release granules
[0055] 1. Test method
[0056] 1. Composition and preparation of Florfenicol sustained-release granules
[0057] The composition of the florfenicol sustained-release granules includes florfenicol raw material drug and auxiliary materials, and the auxiliary materials include monoglyceride, polyethylene glycol 4000 (PEG-4000) and starch.
[0058] In parts by weight, the present invention has prepared 9 groups of 10% Florfenicol sustained-release granules according to the formula in Table 1:
[0059] Table 1. Composition of different formulations of Florfenicol sustained-release granules
[0060]
[0061] Preparation method: Heat the monoglycerides of the above formula to 80°C in proportion and keep it warm for 5 minutes, then add florfenicol raw material, stir for 15 minutes with a JJ-1 precision small electric mixer at 100 rpm, and then After adding polyethylene gl...
Embodiment 2
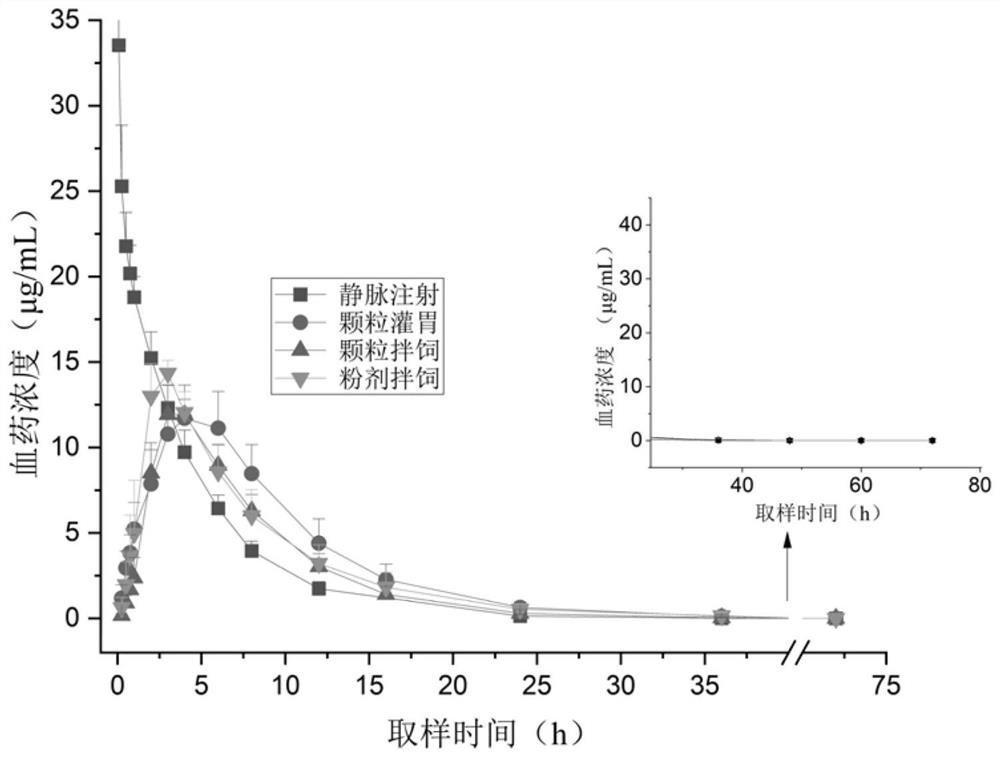
[0078] Example 2 Pharmacokinetic experiments of florfenicol sustained-release granules in pigs and correlation verification analysis in vivo and in vitro
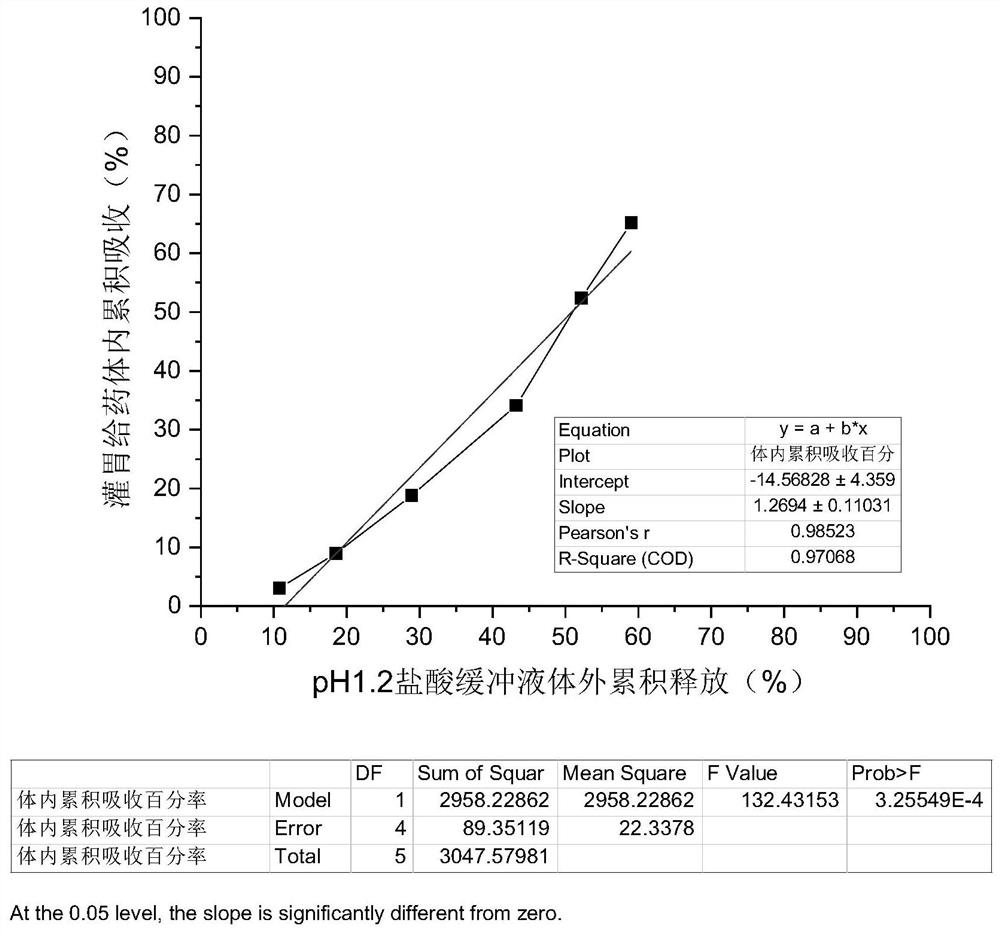
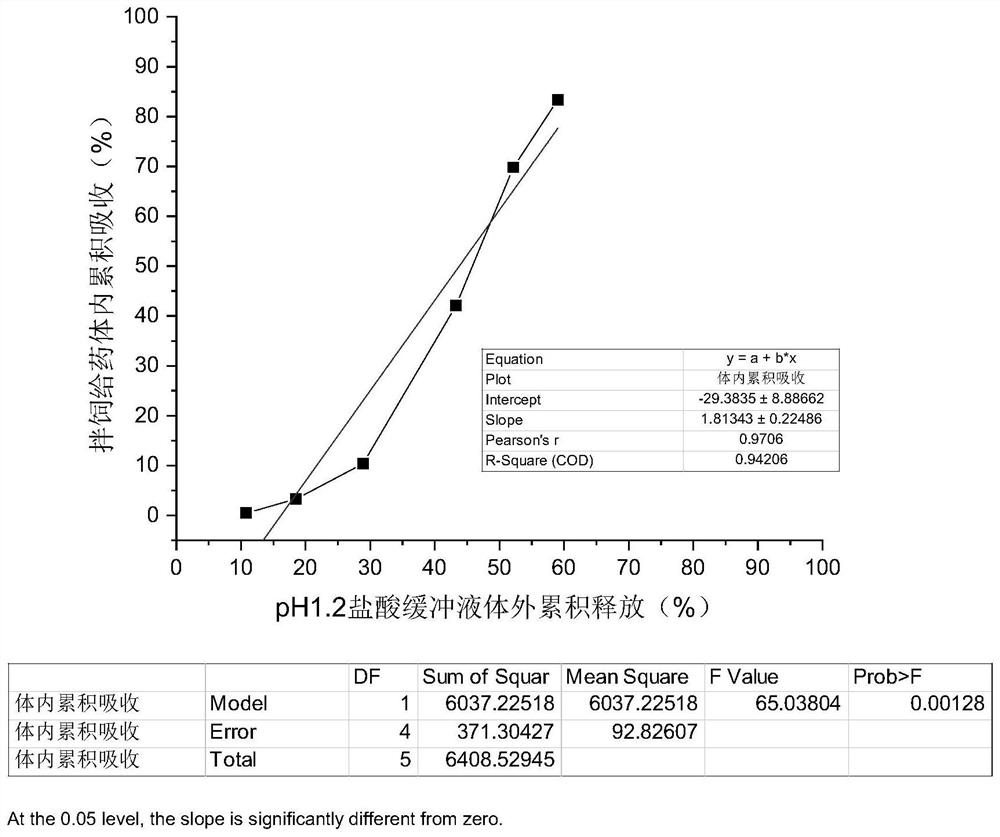
[0079] In order to verify the rationality and accuracy of the dissolution limit of the florfenicol sustained-release granules measured by the method in Example 1, the present invention carries out pharmacokinetic experiments in animals on the florfenicol sustained-release granules, and conducts in vivo and in vitro correlations. A study of gender analysis.
[0080] 1. Test method
[0081] 1. Pharmacokinetic experiment of florfenicol sustained-release granules in pigs
[0082] Get 5% florfenicol injection (5.0g florfenicol crude drug+dimethylpyrrolidone 40mL+10% propylene glycol and settle to 100mL), 10% florfenicol powder (Guangdong Foshan Zhengdian Biotechnology Co., Ltd. ), the 10% Florfenicol sustained-release granule of group 2 in the embodiment 1, with 32 landrace × large white binary mixed pigs, are randomly, averag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com