Bicyclol double-layer osmotic pump control-released tablet and preparation method thereof
An osmotic pump controlled release and bicyclol technology, which is applied in osmotic transportation, coating, antiviral agents, etc., can solve the problems affecting clinical treatment effects, short half-life of bicyclol, large individual differences, etc., to reduce blood drug concentration difference, improve clinical efficacy, enhance the effect of absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
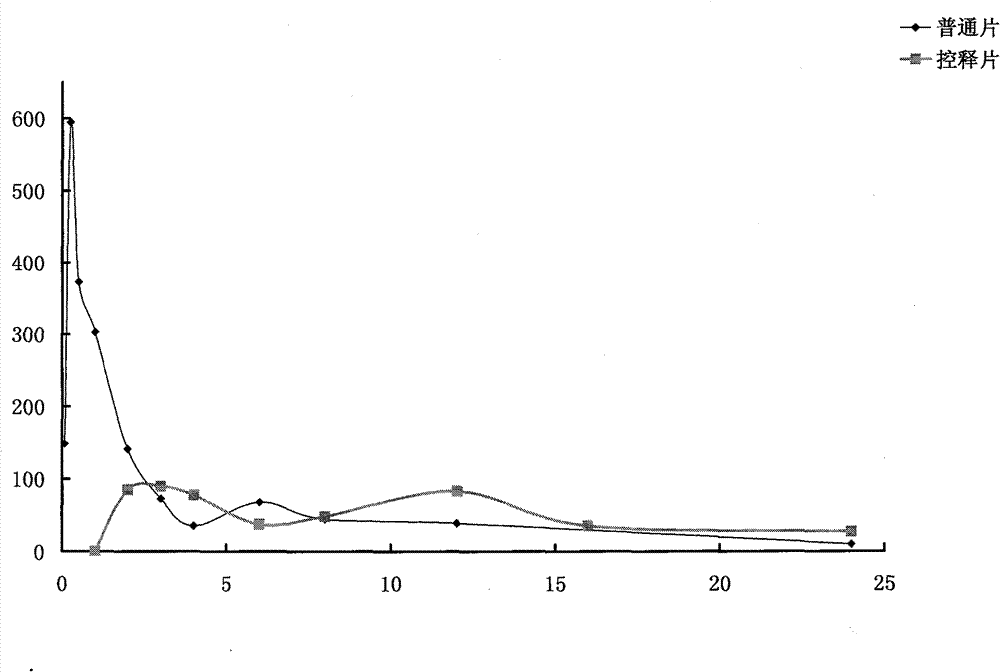
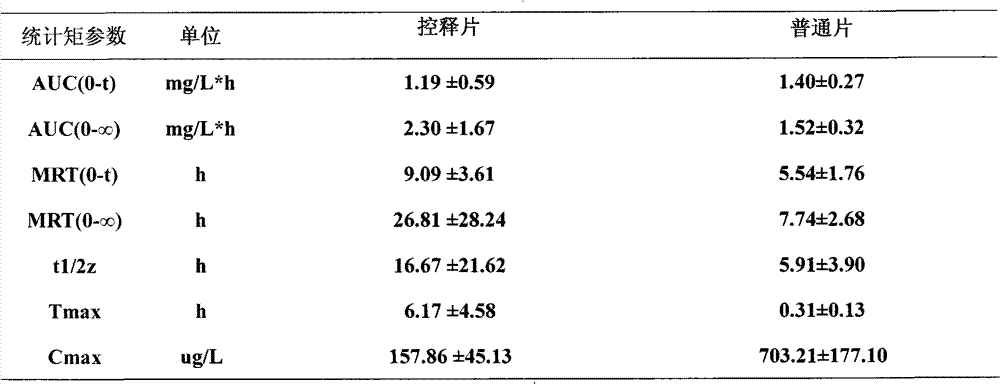
Image
Examples
Embodiment 1
[0032] Drug-containing layer
[0033] Bicyclol 75mg
[0034] Polyoxyethylene 134mg
[0035] PVP K30 24mg
[0036] Sodium chloride 5mg
[0037] Magnesium Stearate 2mg
[0038] The total weight of the drug-containing layer is 240mg
[0039] booster layer
[0040] Polyoxyethylene 64mg
[0041] PVP K30 10mg
[0042] Sodium chloride 35mg
[0043] Iron Oxide Red 0.6mg
[0044] Magnesium stearate 0.4mg
[0045] Booster layer total weight 110mg
[0046] semipermeable membrane coating
[0047] Cellulose acetate 3.9g
[0048] PEG 4000 0.2g
[0049] 95% acetone / water 1000ml
[0050] Preparation:
[0051]1) Preparation of the drug-containing layer: uniformly mix the prescribed amount of bicyclol and other auxiliary materials except magnesium stearate, and add an appropriate amount of ethanol to granulate. Dry at room temperature or in drying equipment.
[0052] 2) The dried main drug layer is sieved, then fully mixed with the magnesium stearate of the prescribed amount. ...
Embodiment 2
[0062] Drug-containing layer
[0063] Bicyclol 75mg
[0064] Polyoxyethylene 139mg
[0065] Hypromellose 24mg
[0066] Magnesium Stearate 2mg
[0067] The total weight of the drug-containing layer is 240mg
[0068] booster layer
[0069] Polyoxyethylene 64mg
[0070] Hypromellose 10mg
[0071] Sodium chloride 35mg
[0072] Iron Oxide Red 0.6mg
[0073] Magnesium stearate 0.4mg
[0074] Booster layer total weight 110mg
[0075] semipermeable membrane coating
[0076] Cellulose acetate 95g
[0077] PEG 3350 5g
[0078] Dichloromethane / methanol (2:10) 2000ml
[0079] Preparation:
[0080] 1) Preparation of the drug-containing layer: uniformly mix the prescribed amount of bicyclol and other auxiliary materials except magnesium stearate, and add an appropriate amount of ethanol to granulate. Dry at room temperature or in drying equipment.
[0081] 2) The dried main drug layer is sieved, then fully mixed with the magnesium stearate of the prescribed amount.
[0082]...
Embodiment 3
[0091] Drug-containing layer
[0092] Bicyclol 165mg
[0093] Polyoxyethylene 393mg
[0094] HPMC 70mg
[0095] Magnesium Stearate 7mg
[0096] The total weight of drug-containing layer is 635mg
[0097] booster layer
[0098] Polyoxyethylene 140mg
[0099] Hypromellose 20mg
[0100] Sodium chloride 71mg
[0101] Iron Oxide Red 1.5mg
[0102] Magnesium Stearate 2.5mg
[0103] Booster layer total weight 235mg
[0104] semipermeable membrane coating
[0105] Cellulose acetate 95g
[0106] PEG 5g
[0107] Dichloromethane / methanol (2:10) 2000ml
[0108] Preparation:
[0109] Same as Example 2, first prepare the drug-containing layer and the booster layer, then compress the double-layer tablet, and finally coat the semi-permeable membrane, laser perforate and moisture-proof isolation layer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com