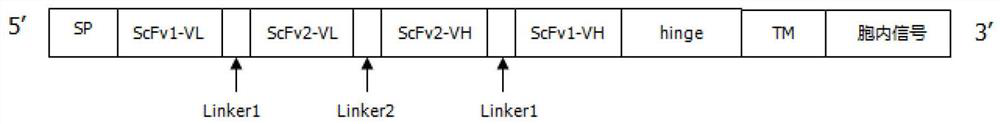
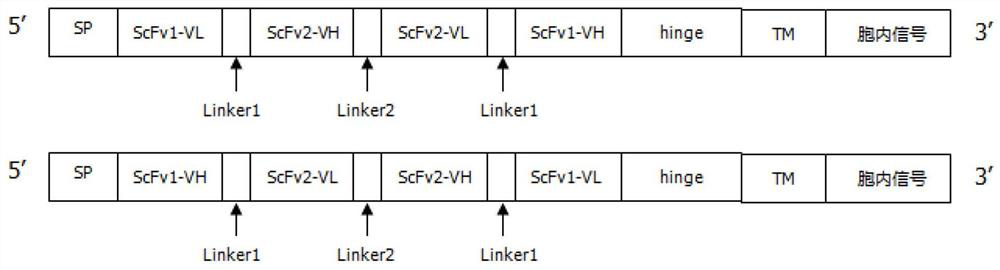
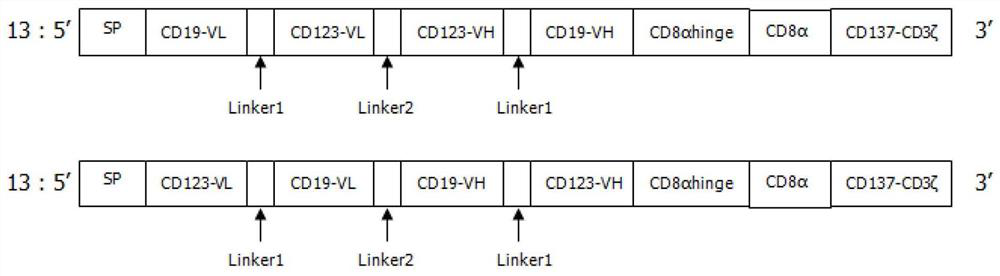
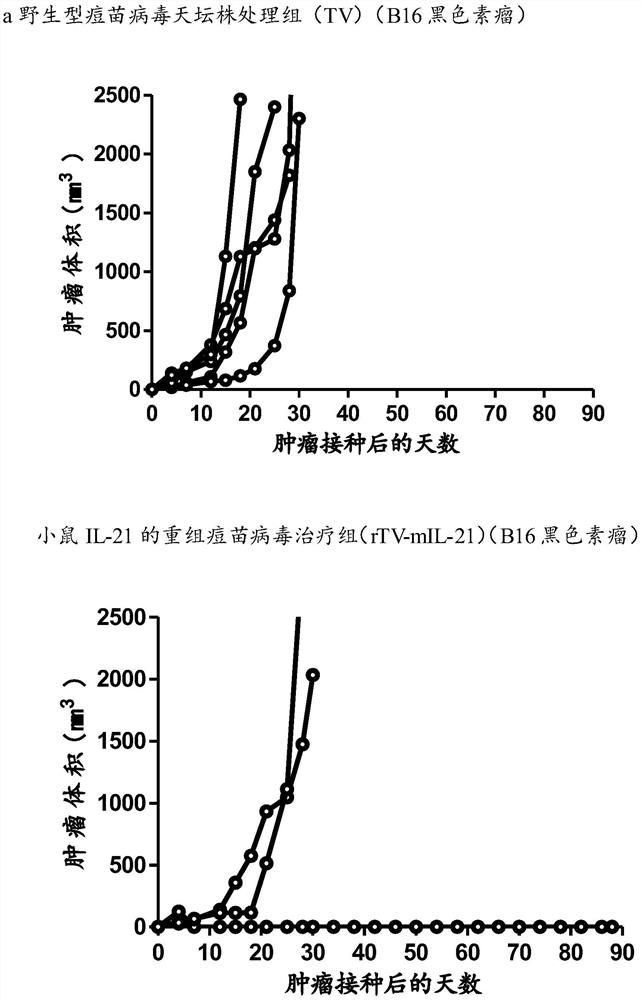
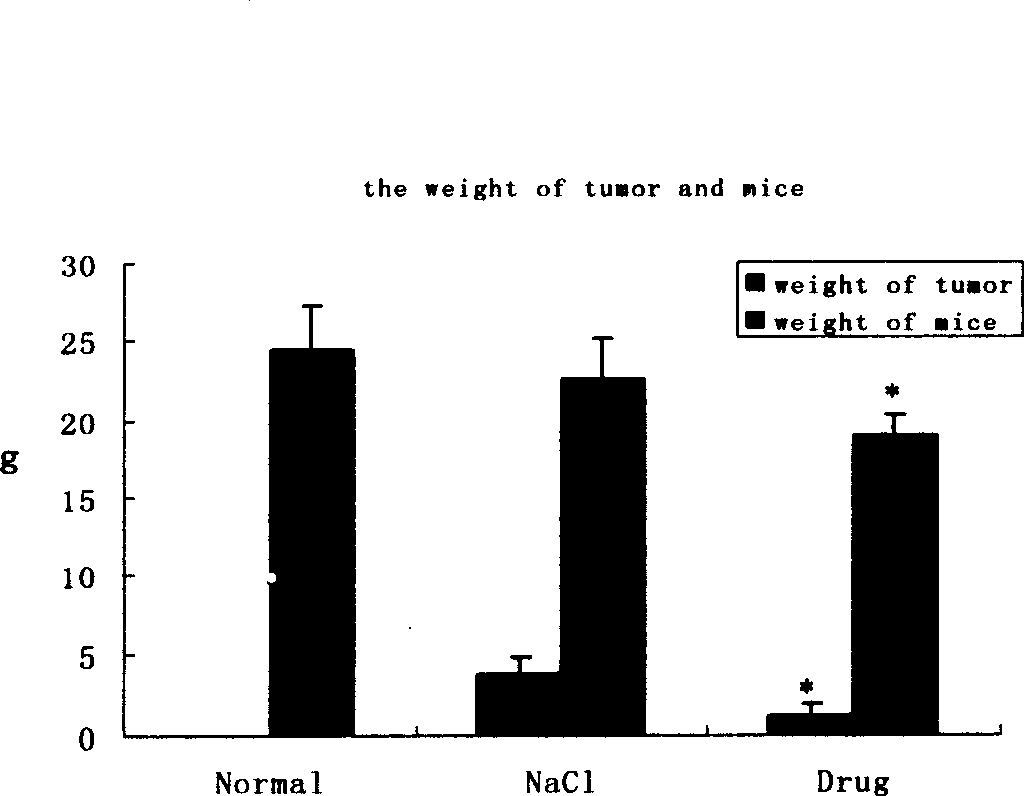
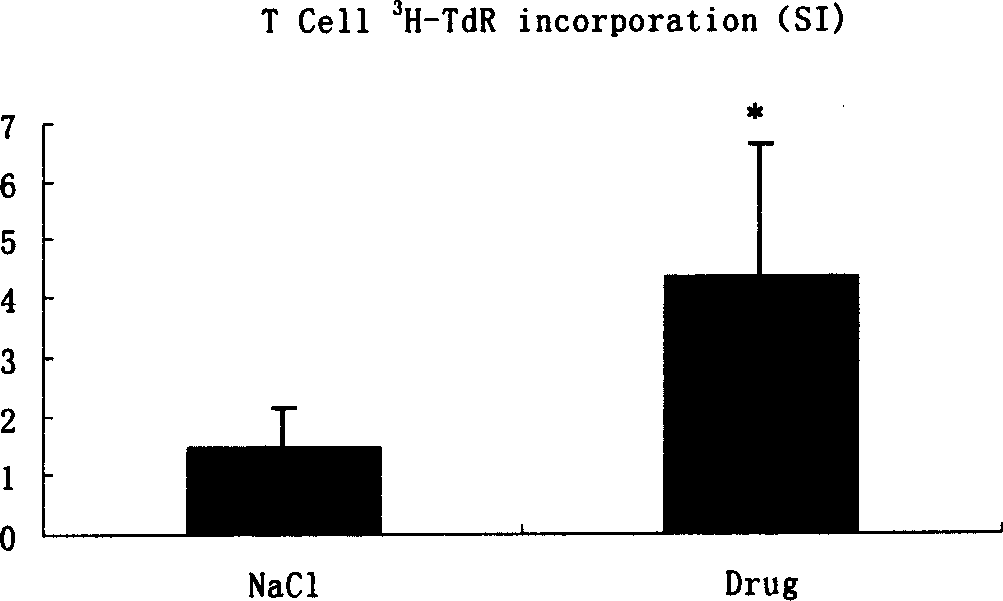
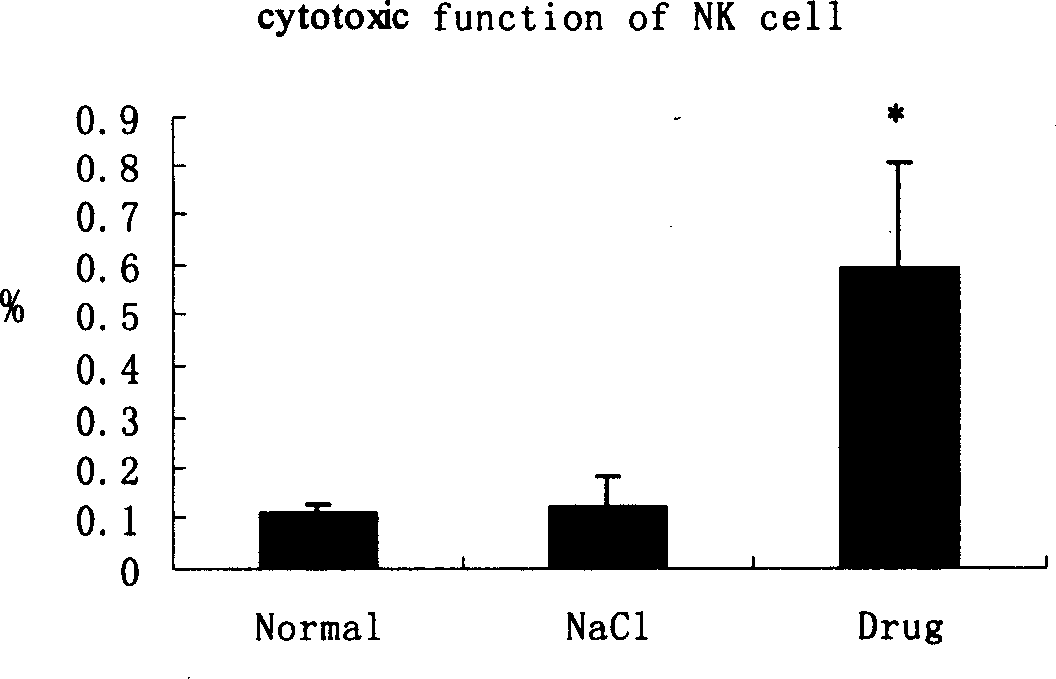
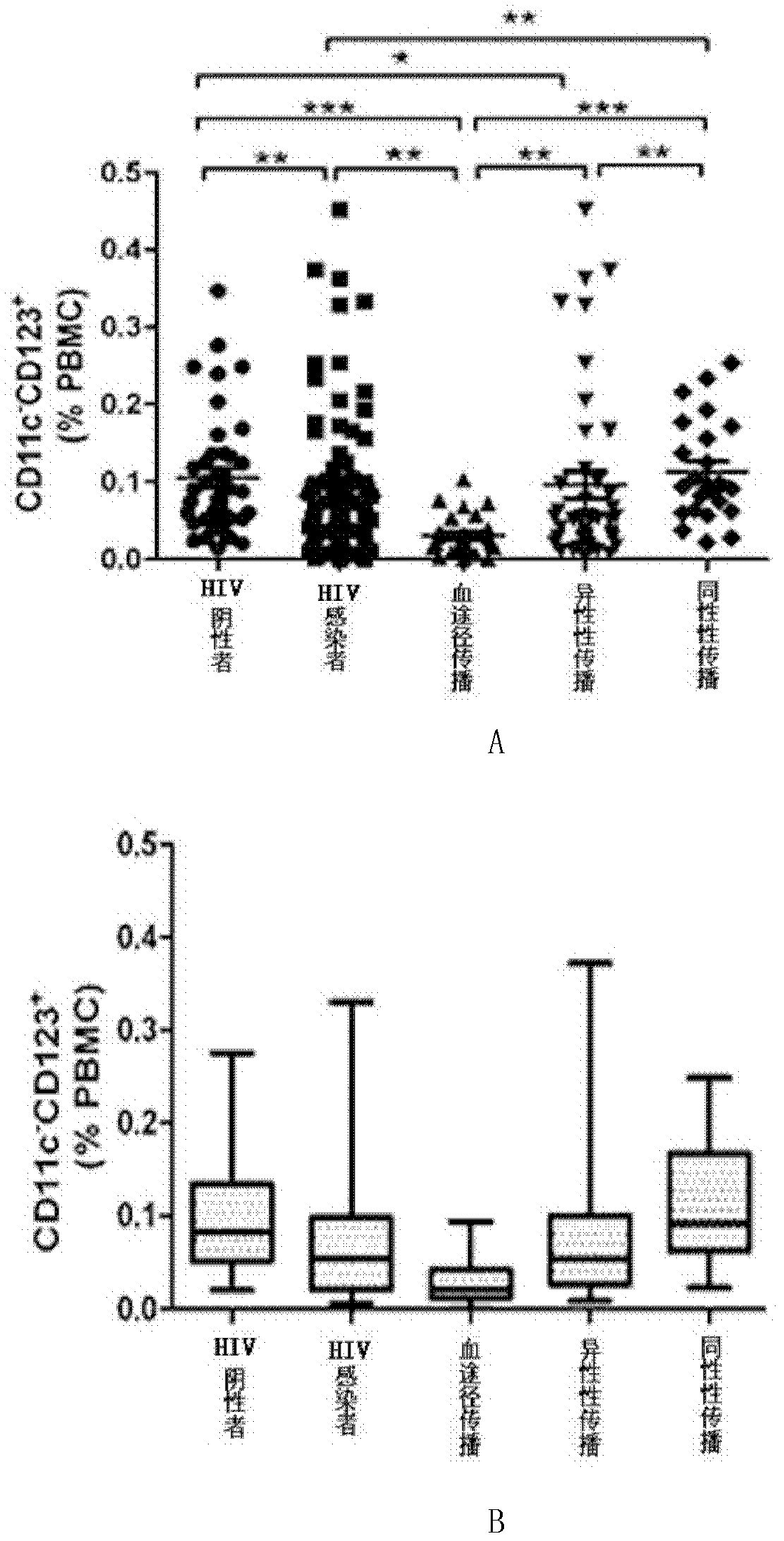Patents
Literature
35results about How to "Enhanced lethality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
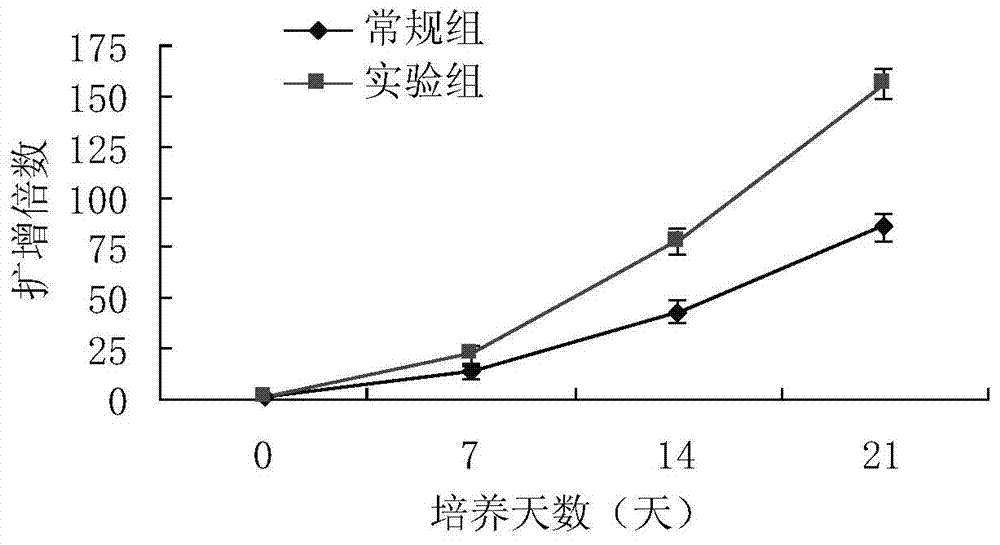
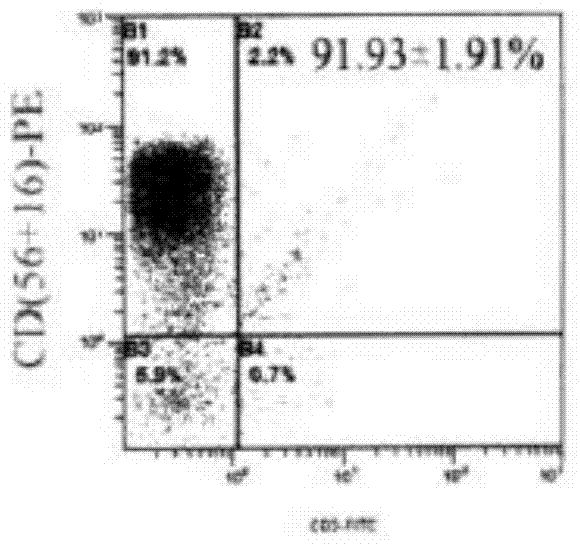
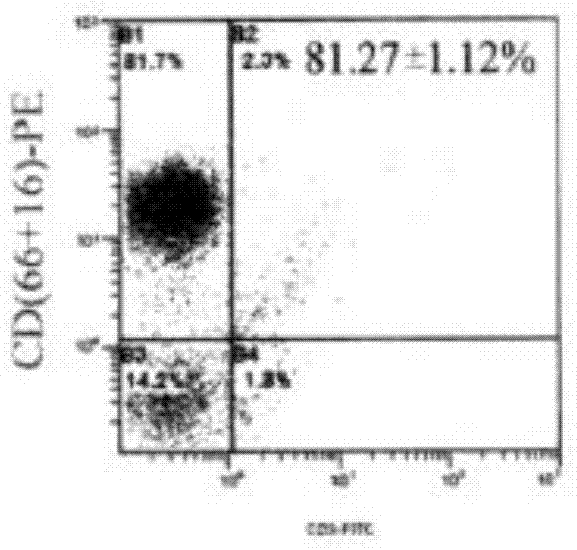
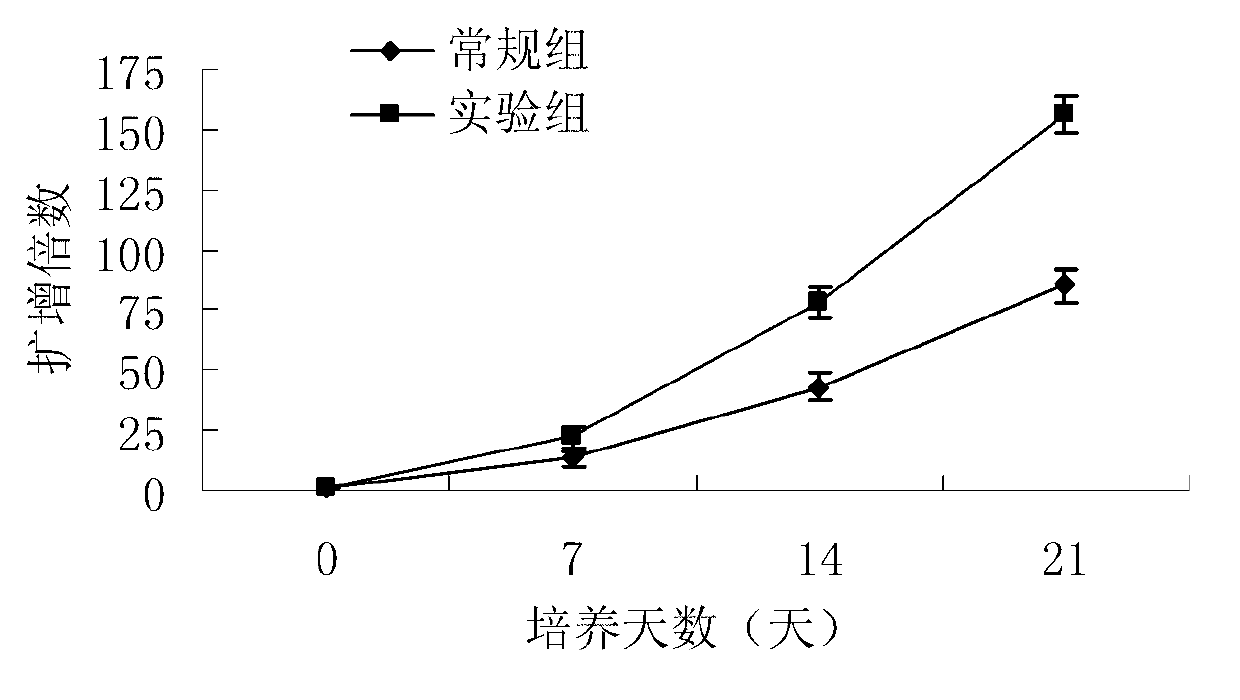
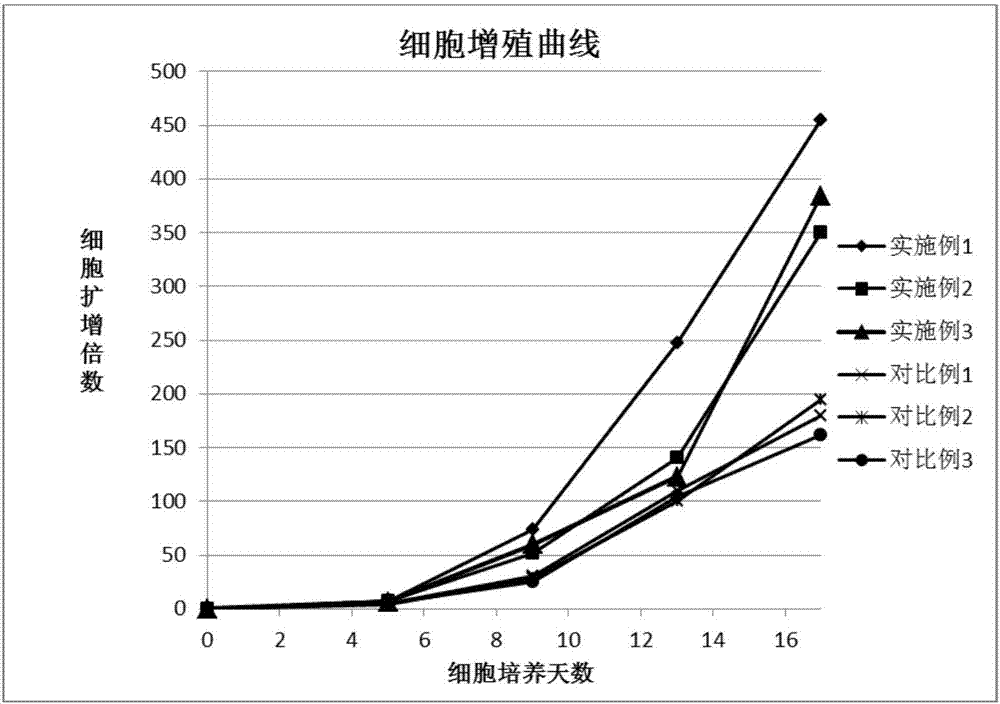
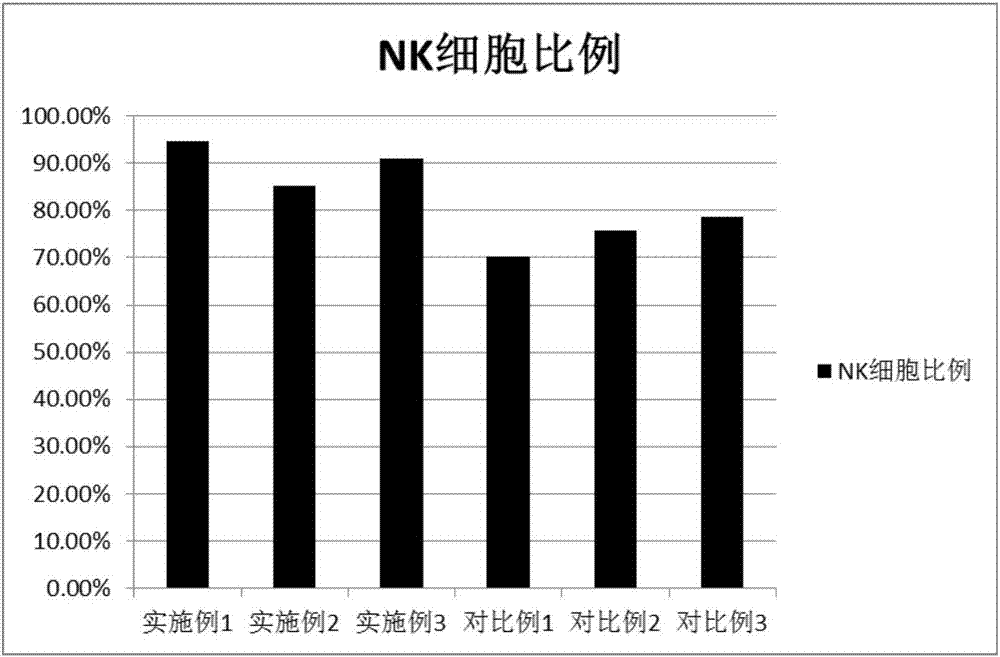
Method used for in vitro proliferation of NK cells
ActiveCN103756963AIncrease lethalityEasy to synthesizeBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
Owner:SHANGHAI CLAISON BIOTECH
Method for in-vitro amplification of NK cells
InactiveCN102994449AEasy to synthesizeIncrease lethalityBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
The invention relates to a method for in-vitro amplification of NK cells, and in particular relates to a method for massive in-vitro amplification of NK cells, wherein the method comprises the following steps of: a, inoculating a peripheral blood mononuclear cell in a CD3McAb and CD226McAb pre-coated culture bottle for coculture; b, adding 1L-2 and 1L-18, coculturing for 72hours to stimulate amplification of NK cells; c, transferring the NK cells, K562 cells after lethal treatment and a serum-free medium containing 1L-2 and 1L-18 in a cell culture bag for coculture; and d, collecting the NK cells. According to the method for in-vitro amplification of the NK cells, two antibodies CD3McAb and CD226McAb are simultaneously coated, so the cell factor synthesis and ADCC effect are promoted, and killing toxicity of the NK cells is remarkably improved; the activation and amplification on the NK cells are achieved just by the 1L-2 and 1L-18 cell factors, so the amplification multiple and cell toxicity of the NK cells are guaranteed, and the cost of cell culture is reduced.
Owner:SHANGHAI CLAISON BIOTECH
Method for efficiently amplifying NK cells
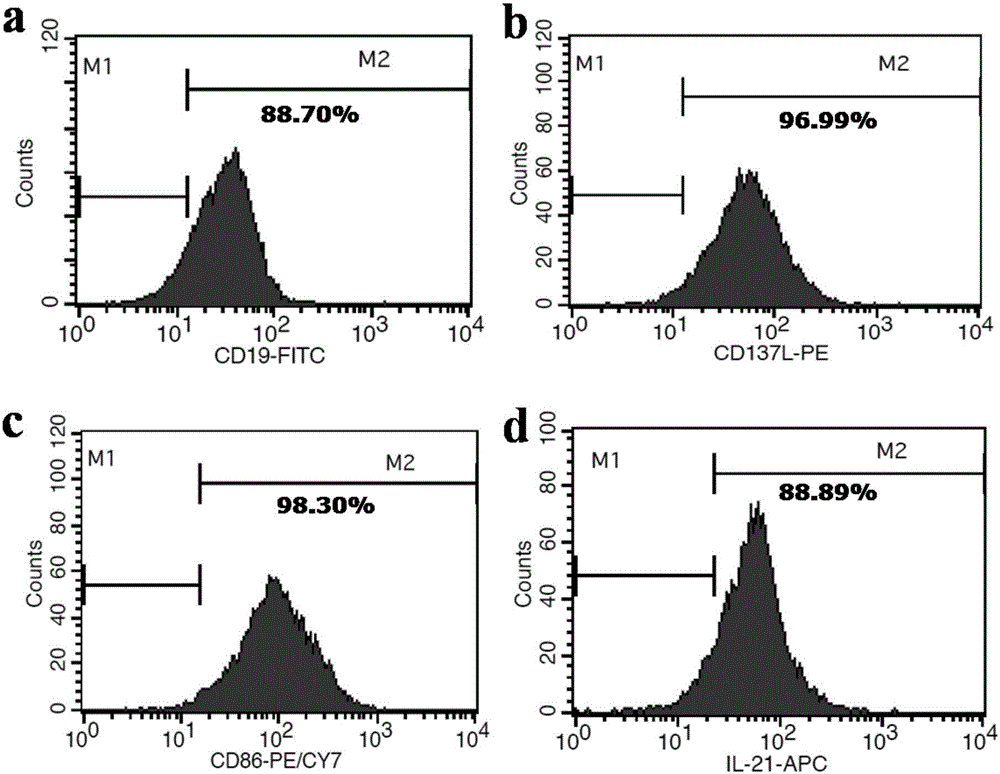
InactiveCN106754730AEnhanced lethalityEnhanced ADCC effectBlood/immune system cellsCell culture active agentsNatural Killer Cell Inhibitory ReceptorsCD86
The invention discloses a method for efficiently amplifying NK cells, in particular to a method for efficiently amplifying NK cells using K562 engineered cells of high expression membrane proteins CD19, CD137L, CD86, CD64 and transmembrane protein IL- 21 and combining with human IL-2 mutants. The method has the advantages of simple operation, low cost, large number of obtained NK cells, high purity and good killing effect; the method is suitable for large-scale preparation of the NK cells and lays a good foundation for the application of NK cell adoptive immunotherapy in clinical practice.
Owner:SHANGHAI BIOMED UNION BIOTECHNOLOGY CO LTD
Macrophages membrane coated breast cancer targeted nanoparticles and preparation method thereof
InactiveCN109953972AEnhanced lethalityImprove the effect of treatmentOrganic active ingredientsPharmaceutical non-active ingredientsSolventBiocompatibility Testing
The invention belongs to the field of pharmaceutical preparations, and relates to macrophages membrane coated breast cancer targeted nanoparticles and a preparation method thereof. The macrophage membrane coated paclitaxel-loaded nanoparticles are prepared into a paclitaxel-loaded nanometer medicine delivery system from paclitaxel, macrophage membranes, amphiphilic high molecular materials, an injection solvent and the like. The macrophage membrane coated medicine carrying nanoparticles are good in biocompatibility, simple in preparation method and uniform in nanoparticle size distribution. Tumor cells are actively recognized through biological molecules on the surface of the macrophage membranes, so that active collection of medicine-containing carriers to a tumor region is effectively increased, retention of the medicine-containing carriers in tumor tissue is increased, controllability of medicine release is realized, and the anti-tumor treatment effects of medicines are increased.
Owner:FUDAN UNIV
Peripheral blood NK cell in-vitro efficient expansion method
ActiveCN107083363AEnhanced lethalityMeet clinical needsBlood/immune system cellsCell culture active agentsCell expansionCD16
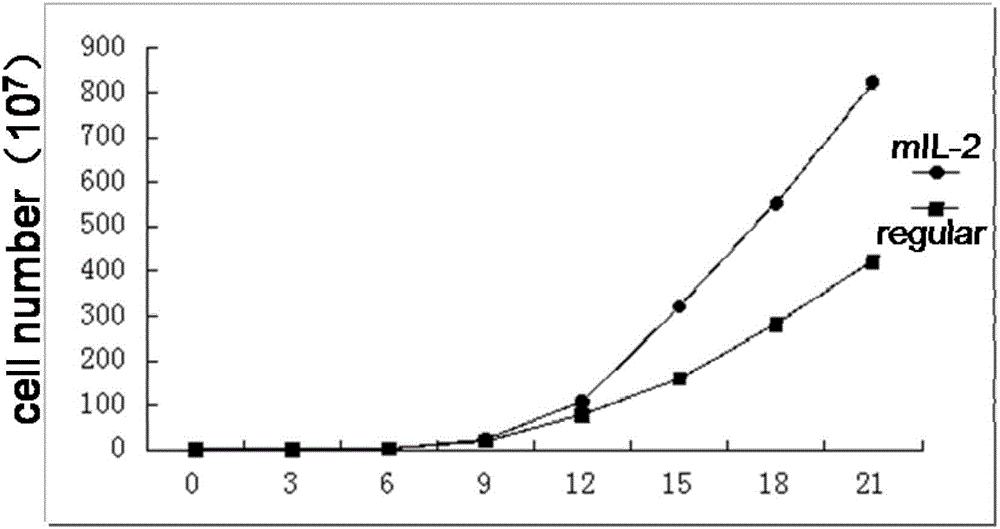
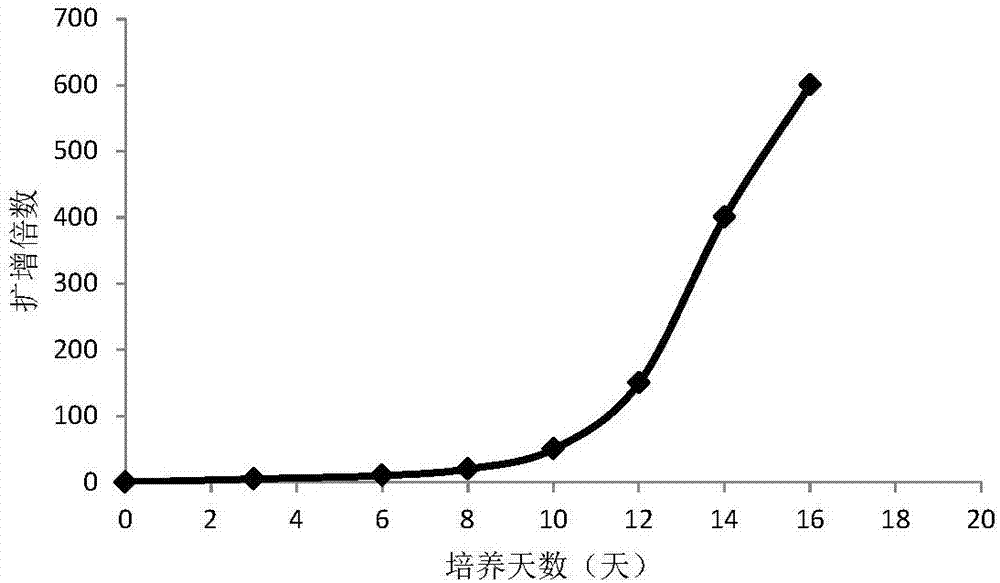
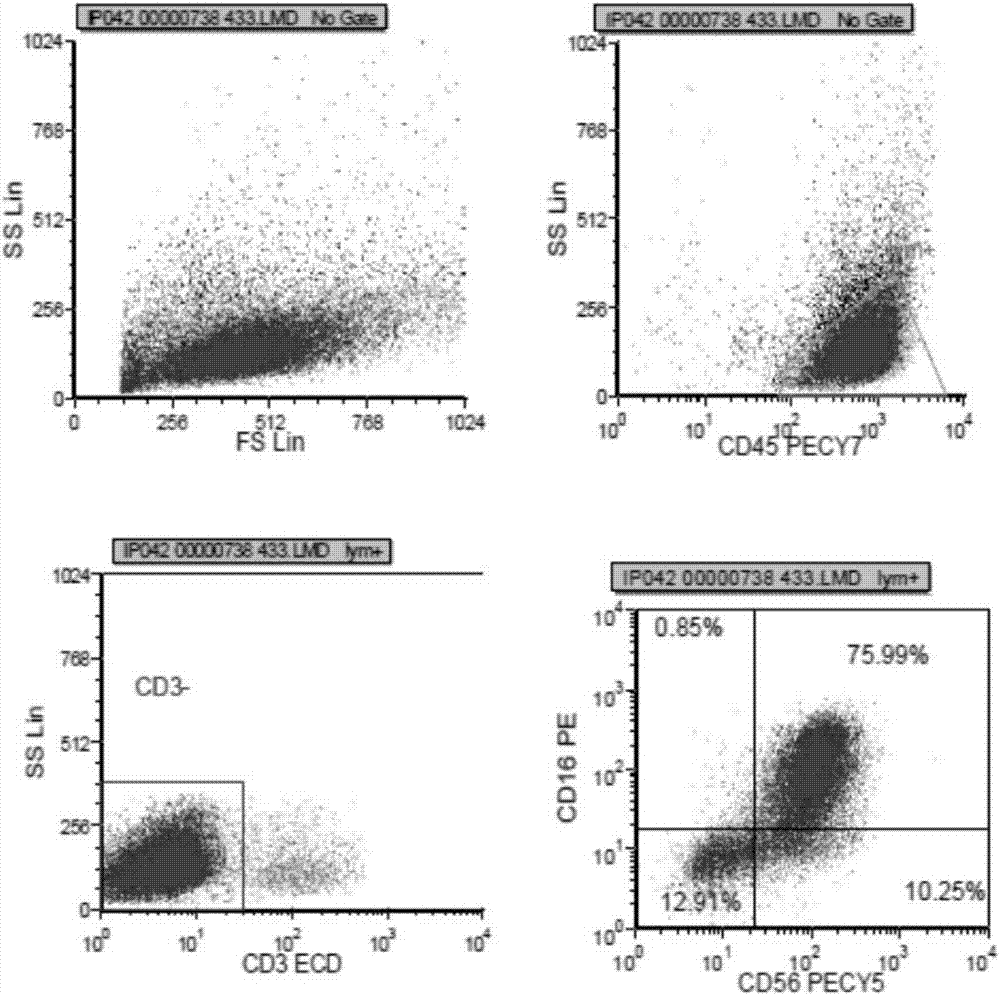
The invention provides a peripheral blood NK cell in-vitro efficient expansion method. According to the peripheral blood NK in-vitro efficient expansion method, mononuclear cells (PBMC) are separated from peripheral blood through a lymph separation solution, the separated mononuclear cells are added to a CD16 monoclonal antibody, and a CD56 monoclonal antibody and a low-concentration CD3 monoclonal antibody are subjected to stimulation and co-culture in a culture bottle which is coated in advance, so that NK cells are preferentially expanded; then, IL-15 is used for activating the cultured cells on the fifth day, and culture continues to obtain a large number of high-purity NK cells. According to the method, the PBMCs do not need to be purified, a large number of high-purity NK cells can be obtained without using feeder cells, NK cells with the purity of 90% or above can be obtained after culture is conducted for 17 days, the cell expansion multiple of the cells is 400 times or above, requirements of clinical application can be met, the cost is reduced, steps are simple, and operability is high.
Owner:青岛麦迪赛斯医疗技术有限公司
Combination of medication for reducing poison and synergic action in radiotherapy or chemotherapy as well as its preparing method
InactiveCN1480208AEnhanced lethalityGood immune regulationUnknown materialsImmunological disordersMedicineLycium barbarum fruit
A detoxicating synergistic Chinese medicine for radiotherapy or chemicotherapy is prepared from astragalus root, wolfberry fruit and ganoderma. Its advantage is high curative effect.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Cell culture method
ActiveCN107083361AEnhanced lethalityIncrease proliferation rateCulture processBlood/immune system cellsPeripheral blood mononuclear cellMicrobiology
The invention belongs to the field of cell culture, and particularly relates to a cell culture method. The cell culture method comprises the following steps that 1, a peripheral blood mononuclear cell is separated; 2, the peripheral blood mononuclear cell separated in the first step is added into a plasma-containing activated culture medium to activate NK cells; 3, a proliferation culture medium is added into the peripheral blood mononuclear cell which is cultured for 10 days in the second step to promote proliferation of the NK cells. According to the cell culture method, the technical defect that the amplified cell volume is low when the NK cells are subjected to in vitro expansion can be effectively overcome.
Owner:深圳市沃英达生命科学有限公司
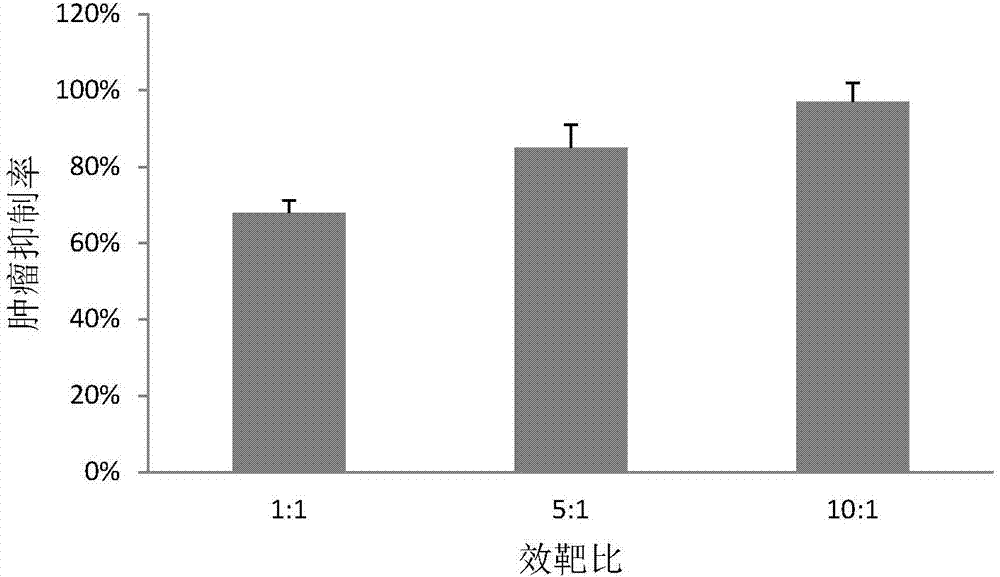
New human cell factor VSTM1-v2 and application thereof
The invention relates to a new human cell factor BSTM1-v2 and application thereof, in particular to a gene or a protein of a transcript VSTM1-v2 of VSTM1 or immune fragments of the gene and the protein, and application of the gene or protein of the VSTM1-v2 or immune fragments of the gene and the protein in promoting Th17 differentiation and the function of killing CD8+ lymphocytes, and in preparing medicinal compositions for preventing and / or treating immune related diseases. The invention also relates to an antagonist of the VSTM1-v2, such as a monoclonal antibody or a polyclonal antibody, which comprises a vector, a host cell or a composition of the VSTM1-v2, a reagent for detecting the VSTM1-v2 or immune fragments thereof, and application of the antagonist and the reagent.
Owner:PEKING UNIV
Human IL1RAP (IL-1 receptor accessory protein) specific CAR (chimeric antigen receptor) and application thereof
ActiveCN106831998AIncrease T cell activation rateEnhanced lethalityImmunoglobulin superfamilyCancer antigen ingredientsMemory T cellVariable region gene
The invention discloses a human IL1RAP (IL-1 receptor accessory protein) specific CAR (chimeric antigen receptor) and application thereof. The CAR consists of an IL1RAP specific single-chain antibody scFV, a costimulatory molecule and a T cell activated CD3zeta chain, wherein the IL1RAP specific single-chain antibody scFV comprises variable zone gene segments of a heavy chain and a light chain of an IL1RAP specific single-chain antibody McAb; the costimulatory molecule is CD137. Compared with the prior art, the human IL1RAP specific CAR has the advantages that (1) by introducing the CD137 cooperative costimulatory molecule, the activating rate of T cell transfected by an IL1RAP specific CAR virus is improved, and the ability of killing tumor cells is improved; (2) the function of killing leukemia cells is realized, the long-life antigen specific memory T cell can be formed in a human body, and the new leukemia cell is timely cleared.
Owner:苏州艾凯利元生物科技有限公司
Chimeric antigen receptor and application thereof in preparation of product for treatment of tumors
ActiveCN111892661APromote growthReduce the risk of inhibitionVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
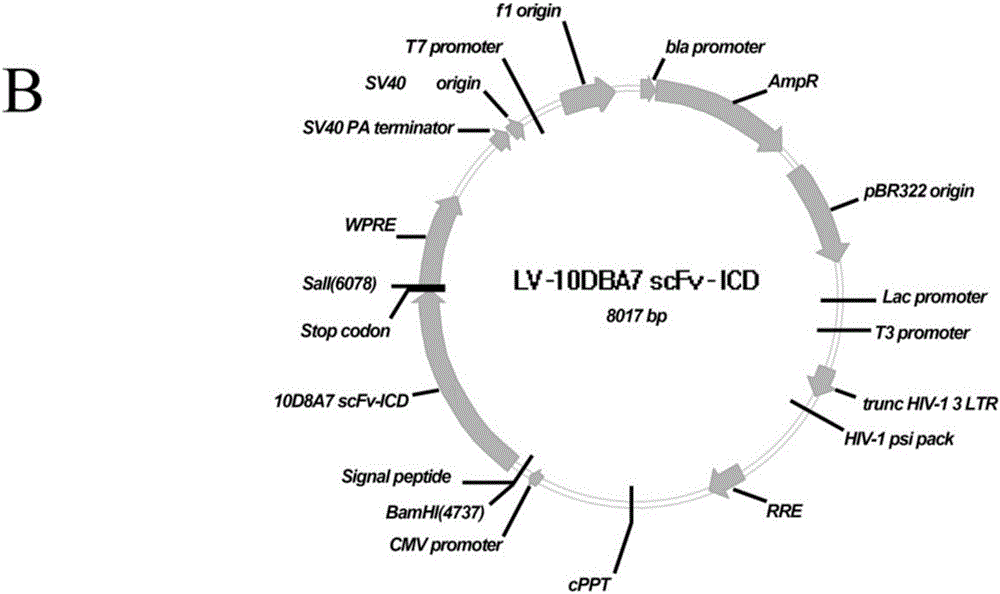
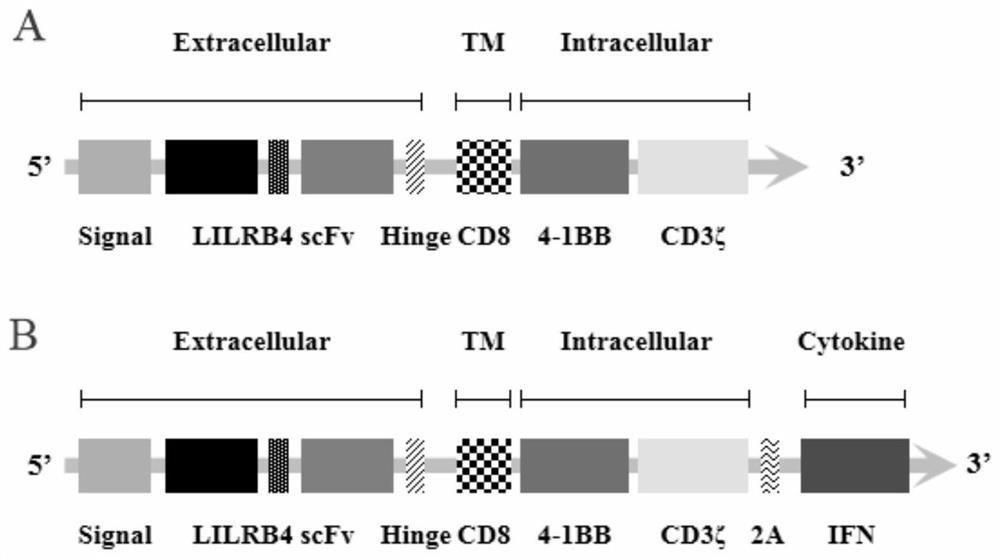
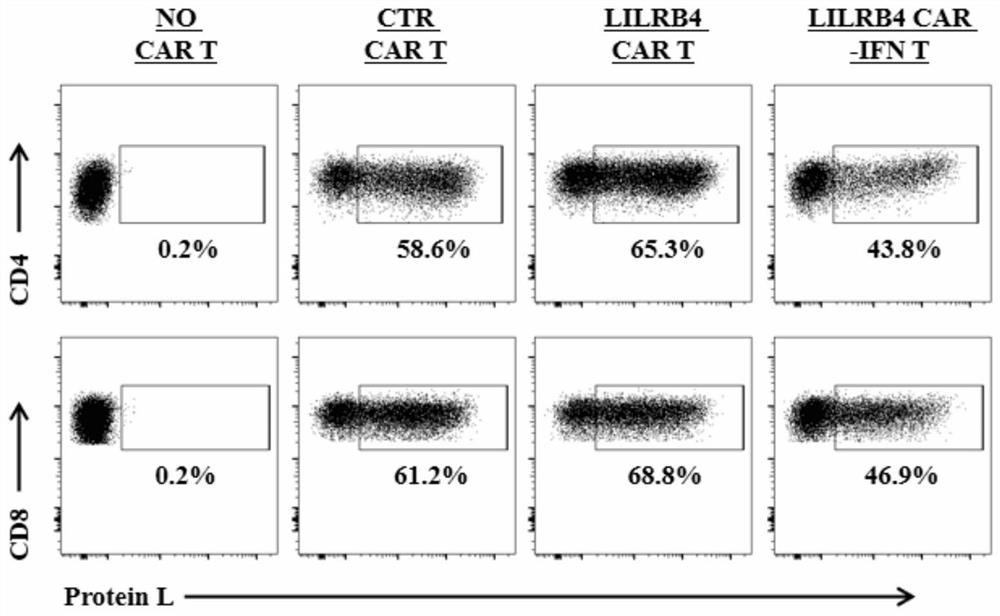
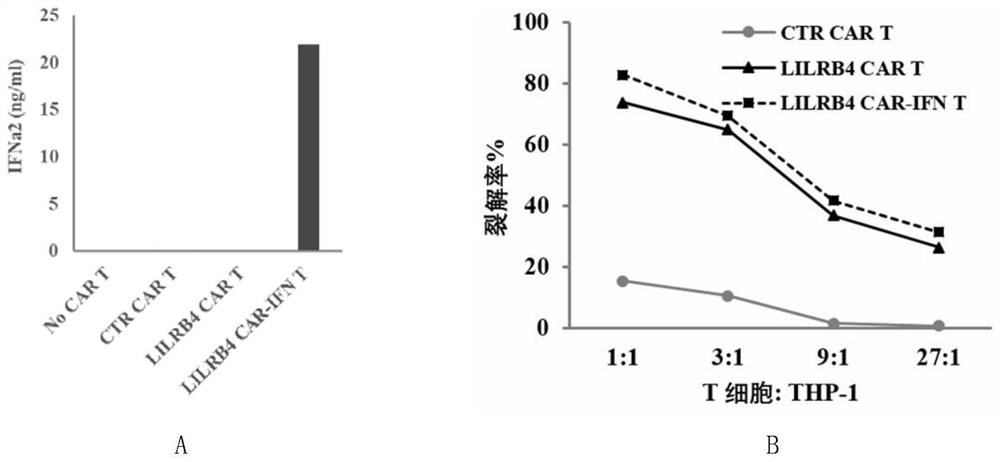
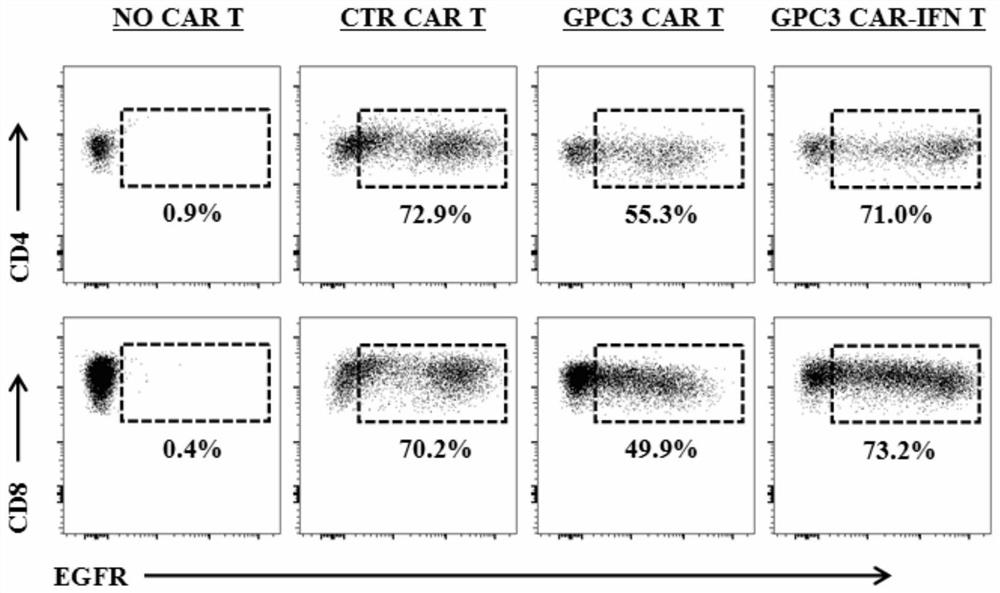
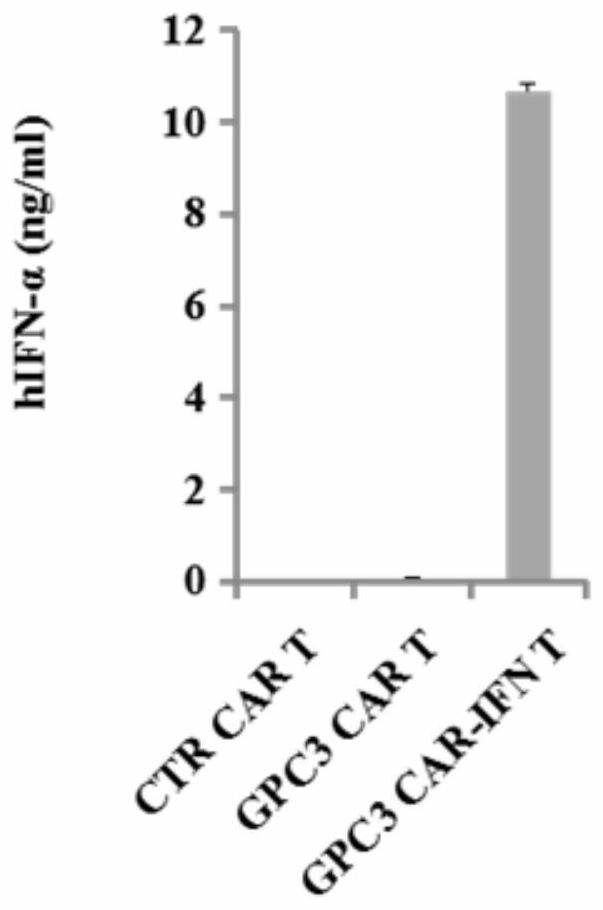
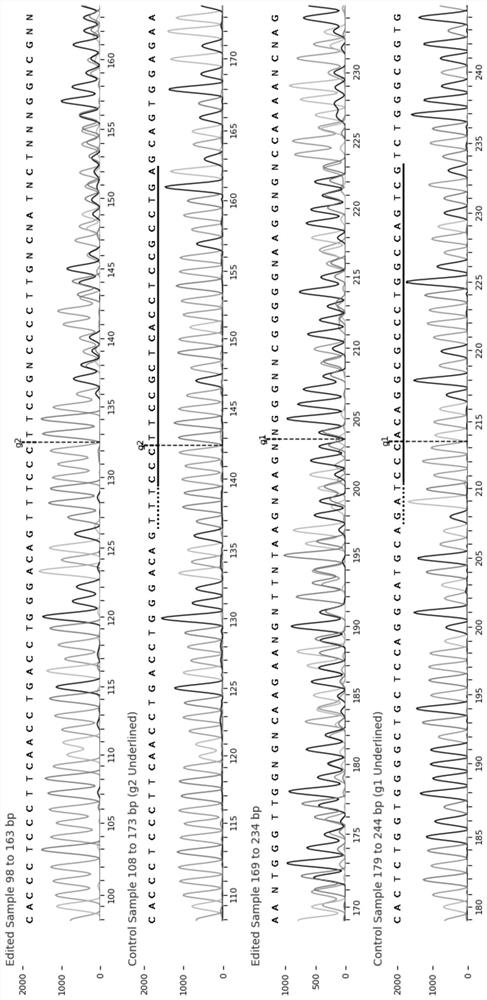
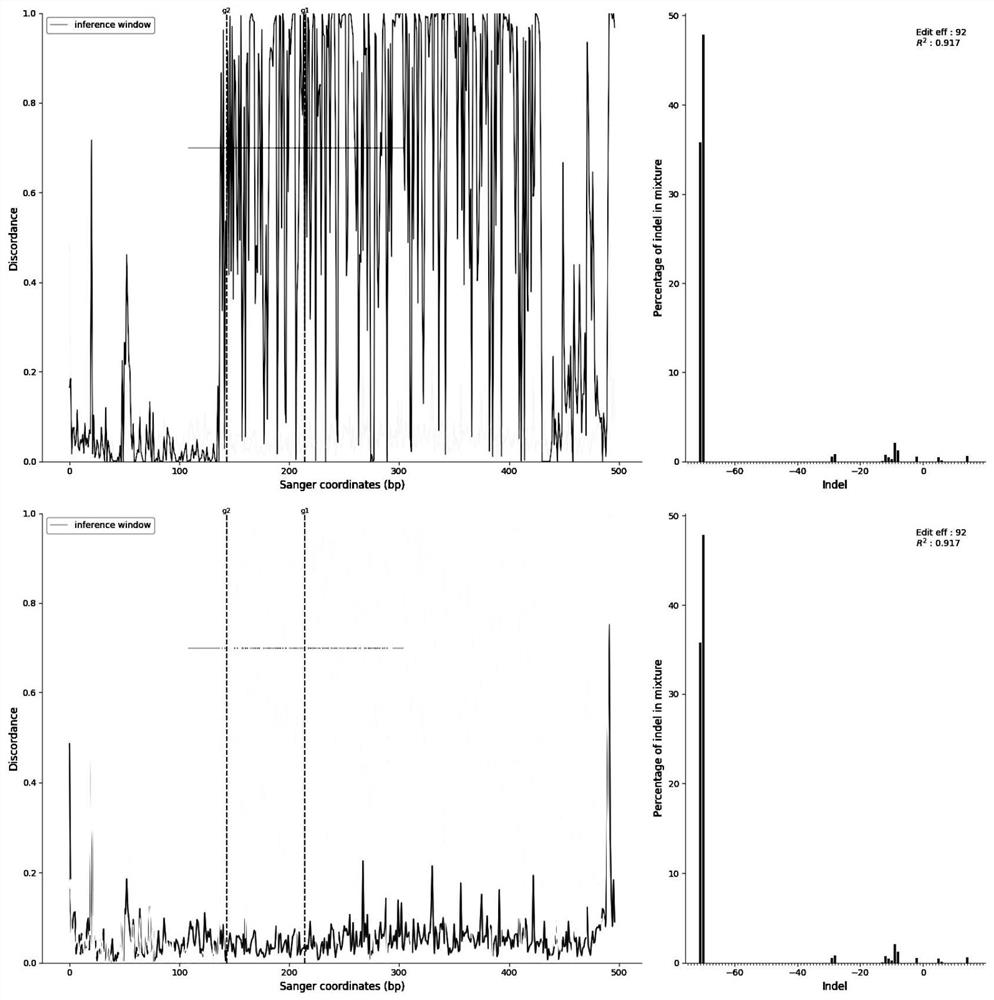
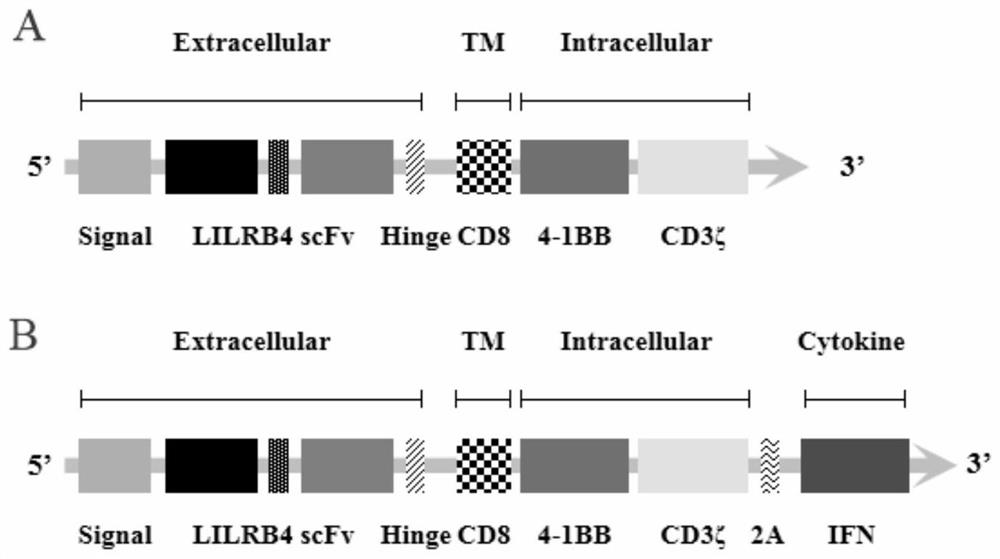
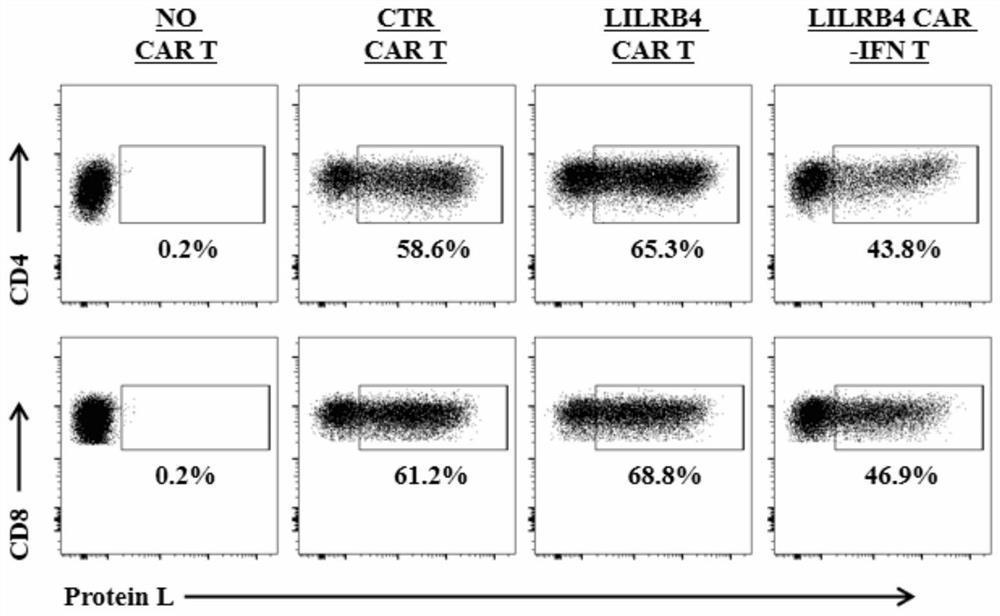
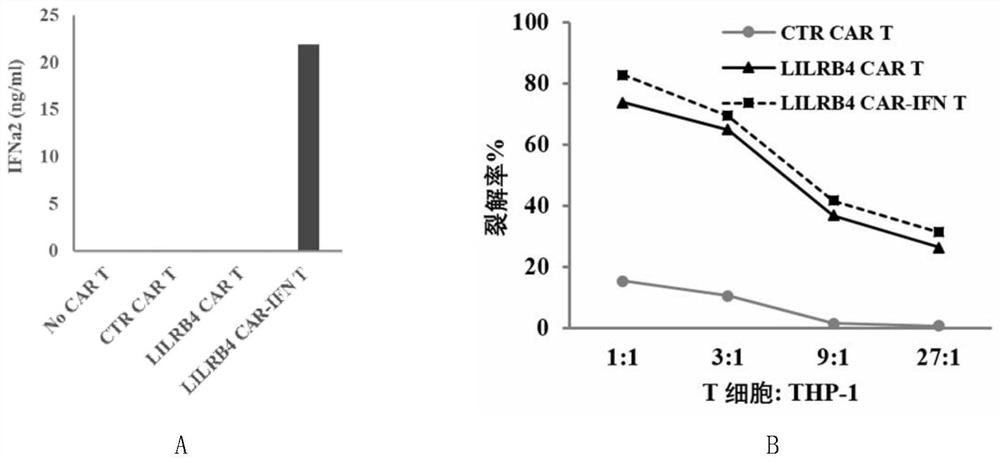
The invention discloses a chimeric antigen receptor and an application thereof in preparation of a product for treatment of tumors. The chimeric antigen receptor sequentially comprises a lead peptide,an anti-LILRB4 single-chain antibody, a human CD8 hinge transmembrane region, a human 4-1BB intracellular region, a human CD3 zeta intracellular region, a self-cleaving peptide and human IFN full length. A new generation of CAR-T cell treatment product is designed and developed by using LILRB4 as an antigen target for the first time, and a gene-optimized human full-length interferon (IFN) fragment is added to the C terminal of the LILRB4-CAR. Compared with a CAR-T cell which only expresses the LILRB4-CAR, the CAR-T cell which expresses the LILRB4-CAR-IFN has higher tumor killing capacity, andthe safety and effectiveness of the CAR-T cell in tumor treatment are further improved.
Owner:CARBIOGENE THERAPEUTICS CO LTD
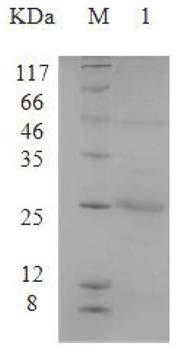
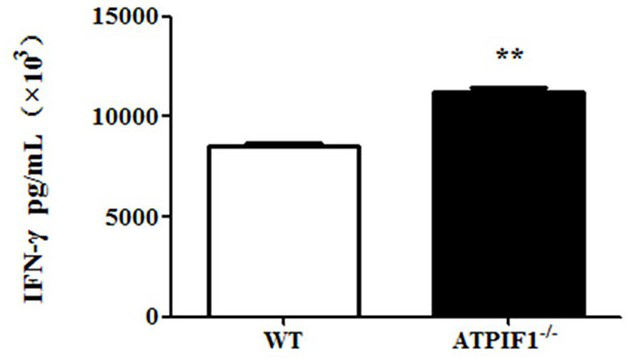
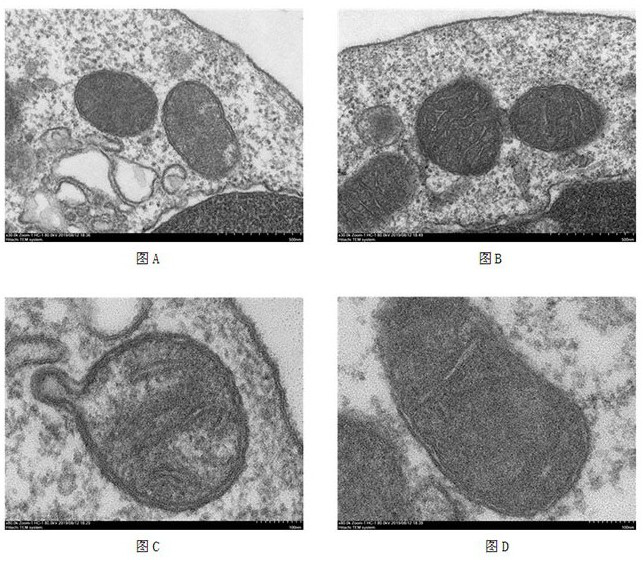
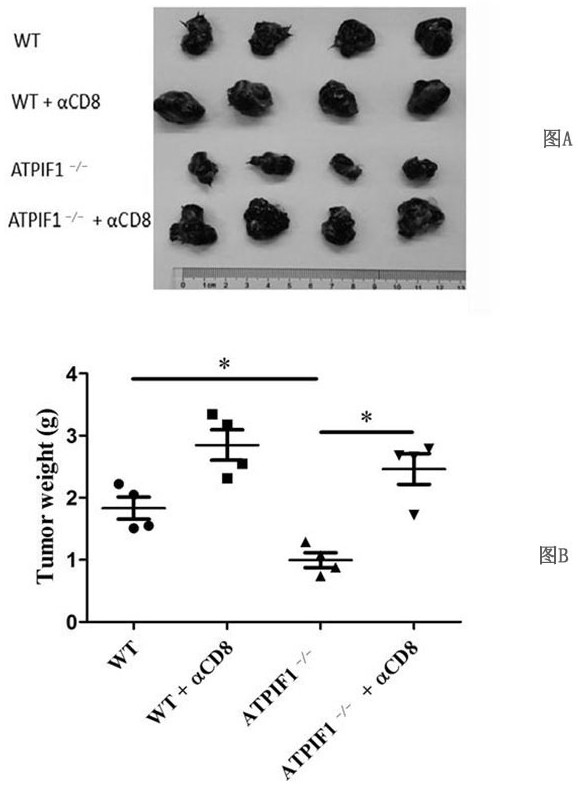
Application of ATPIF1 gene silenced T cells in preparation of antitumor drugs
ActiveCN110604744AEnhanced lethalityGenetically modified cellsStable introduction of DNAGene silencingT cell
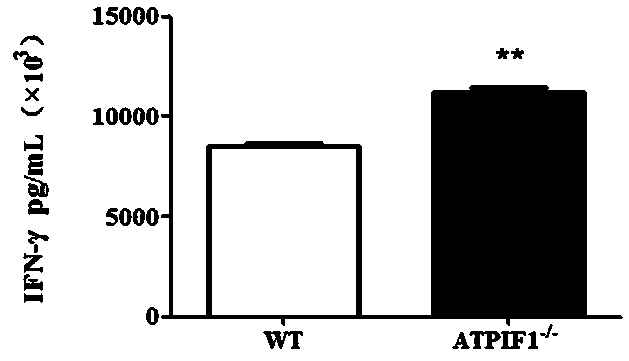
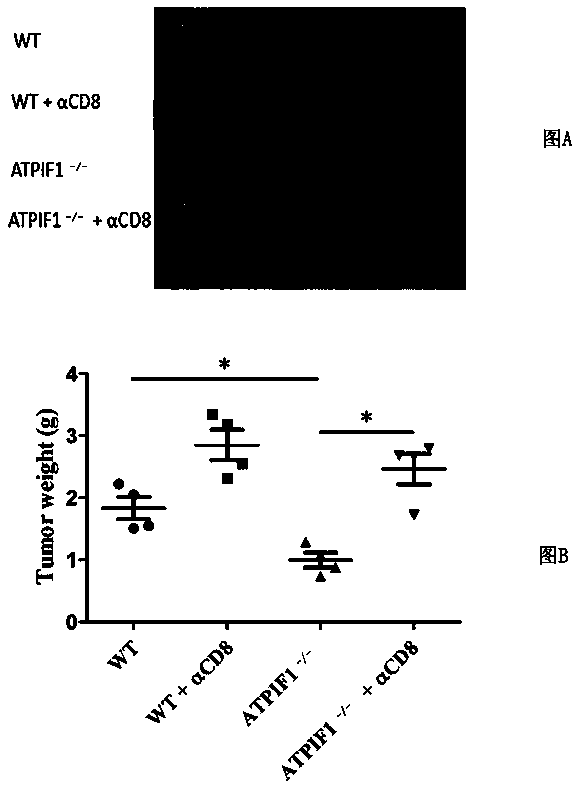
The invention discloses a method for enhancing the activity of T cells. By silencing an ATPIF1 gene in the T cells, the T cells can rapidly proliferate, activate, improve the effector function of theT cells and enhance the killing effect of the T cells on tumor cells in the immune process. The invention further discloses application of the ATPIF1 gene silenced T cells in the preparation of antitumor drugs. A new direction is provided for the research and development of tumor adoptive immunotherapy.
Owner:XINXIANG MEDICAL UNIV
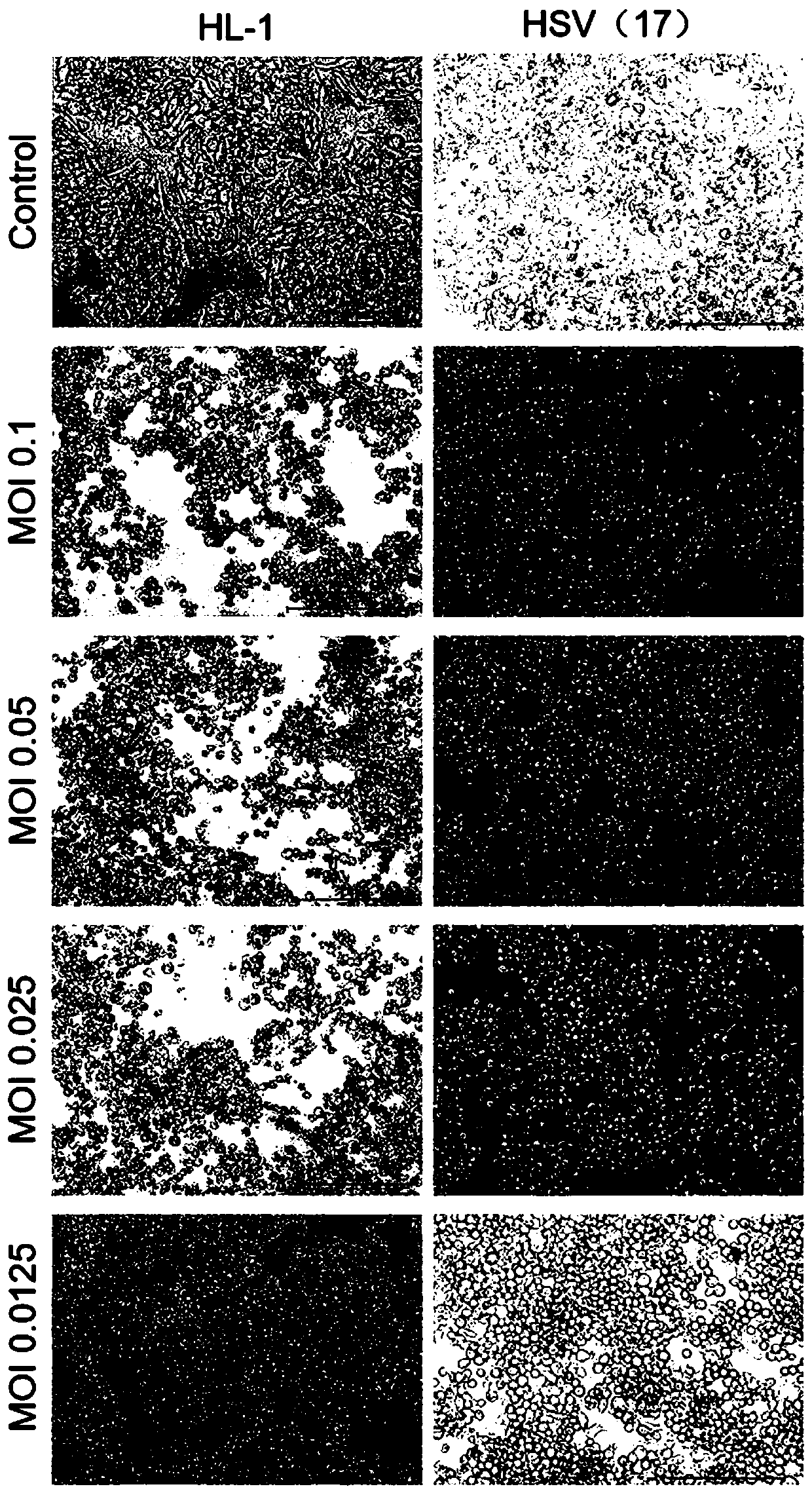
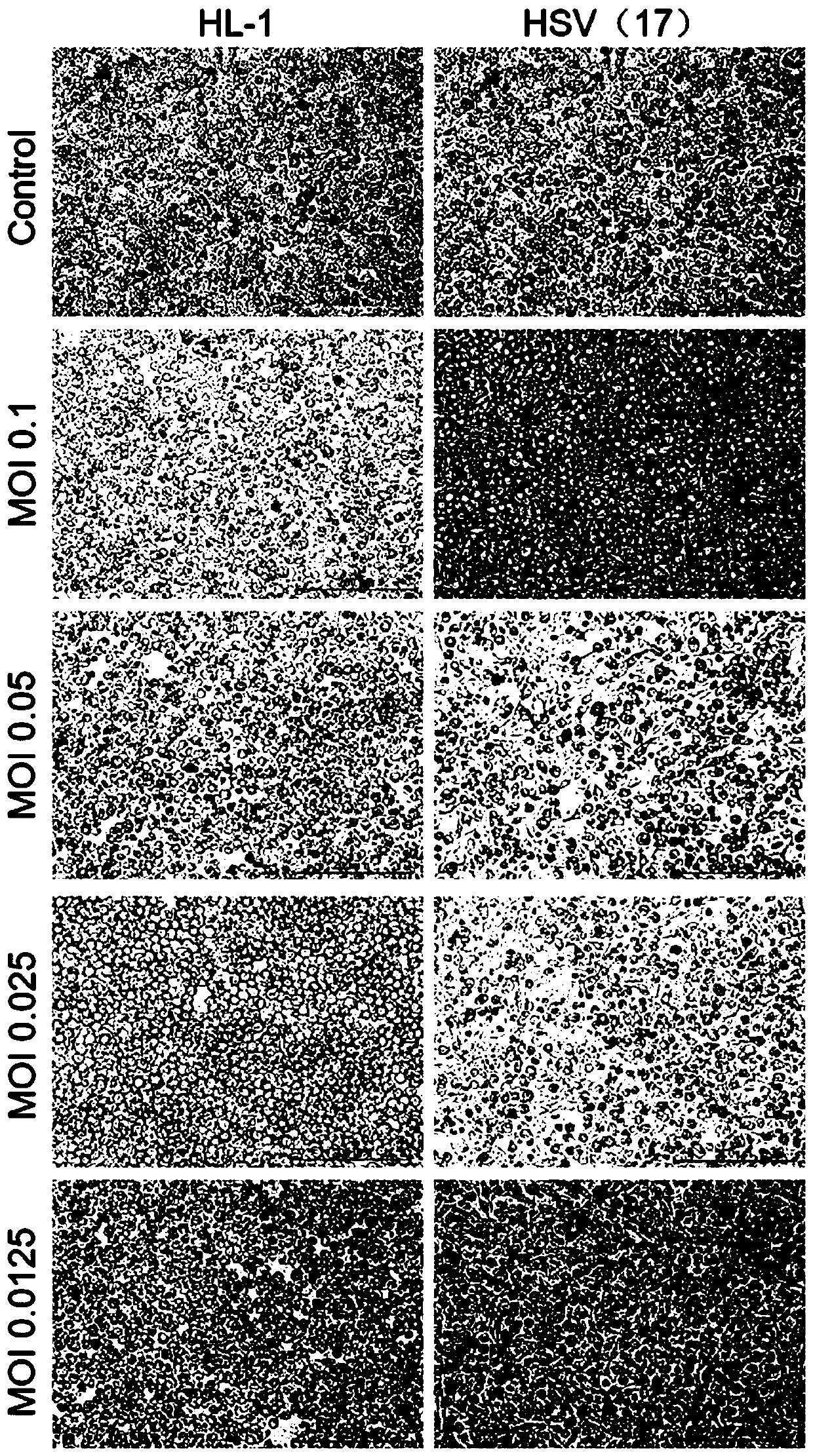
Herpes simplex virus and application thereof
ActiveCN110982795APromote replicationEnhanced lethalityPeptide/protein ingredientsMicroorganism based processesHerpes simplex virusAttenuated strain
Owner:BEIJING WELLGENE CO LTD
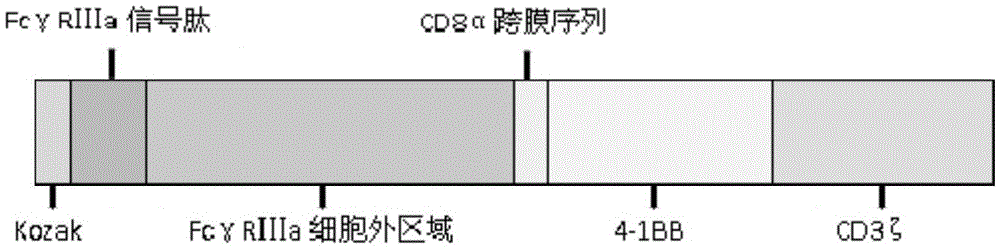
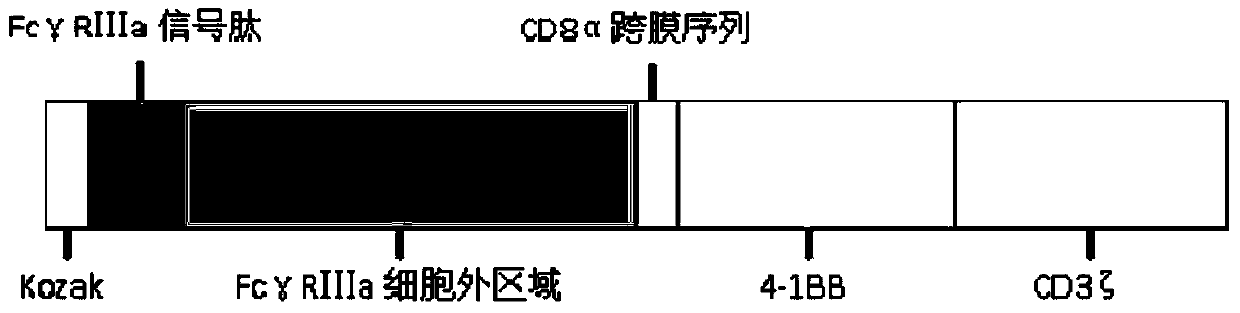
Fc gamma RIII a-based chimeric gene and application thereof
ActiveCN105647946AEfficient killing functionImprove clinical efficacyPolypeptide with localisation/targeting motifPeptide/protein ingredientsSequence signalAntigen receptors
The invention relates to an Fc gamma RIII a-based chimeric gene and application thereof. The chimeric gene comprises an Fc gamma RIII a signal peptide, an Fc gamma RIII a extracellular area, a CD8alpha cross-film area and an intracellular signal transmission structural domain, wherein the Fc gamma RIII a extracellular area is directly connected with the CD8alpha cross-film area. According to the mosaic gene, the Fc gamma RIII a extracellular area is directly connected with the CD8alpha cross-film area, and a CD8alpha hinge area in a conventional chimeric antigen receptor (CAR) molecule design is deleted, so that an Fc gamma RIII a-CAR molecule is better for activating effector cells, and the killing capability of the Fc gamma RIII a-CAR molecule to tumor cells is remarkably improved; the designed Fc gamma RIII a-CAR molecule combined with a monoclonal antibody drug can be generally used in cell therapy of various tumors.
Owner:JIANGSU PURECELL BIOMEDICAL TECH CO LTD
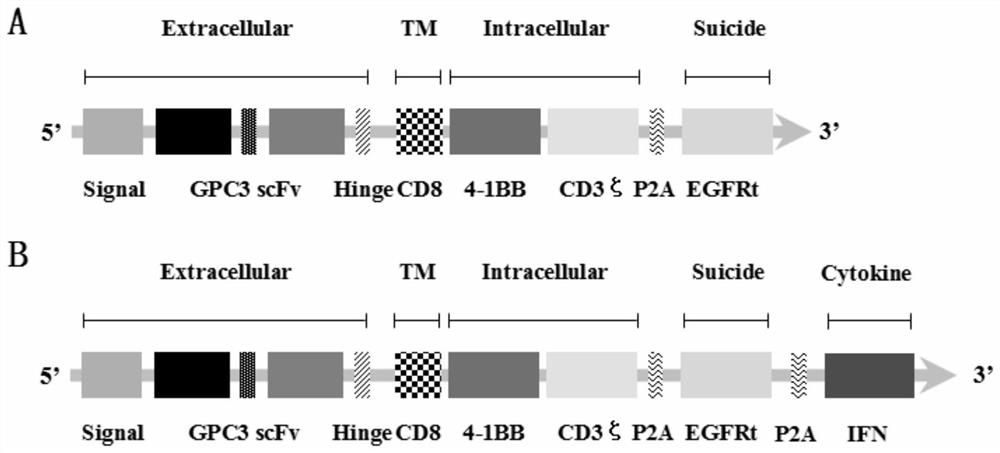
Chimeric antigen receptor for hepatocellular carcinoma treatment and application of chimeric antigen receptor for hepatocellular carcinoma treatment
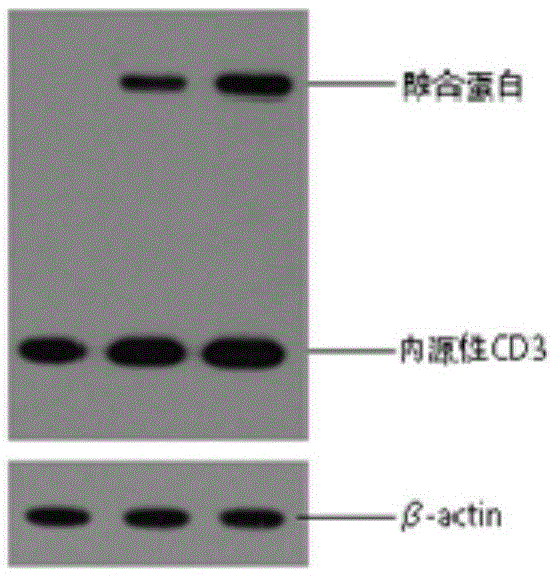
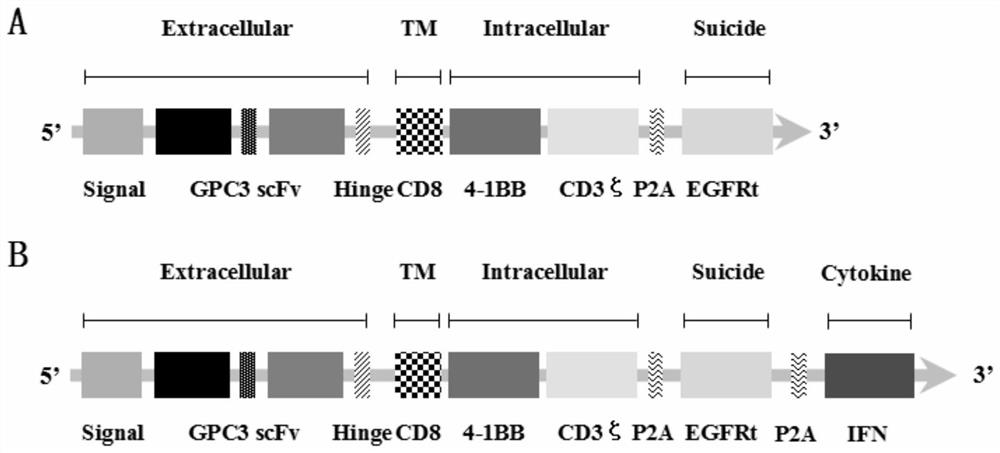
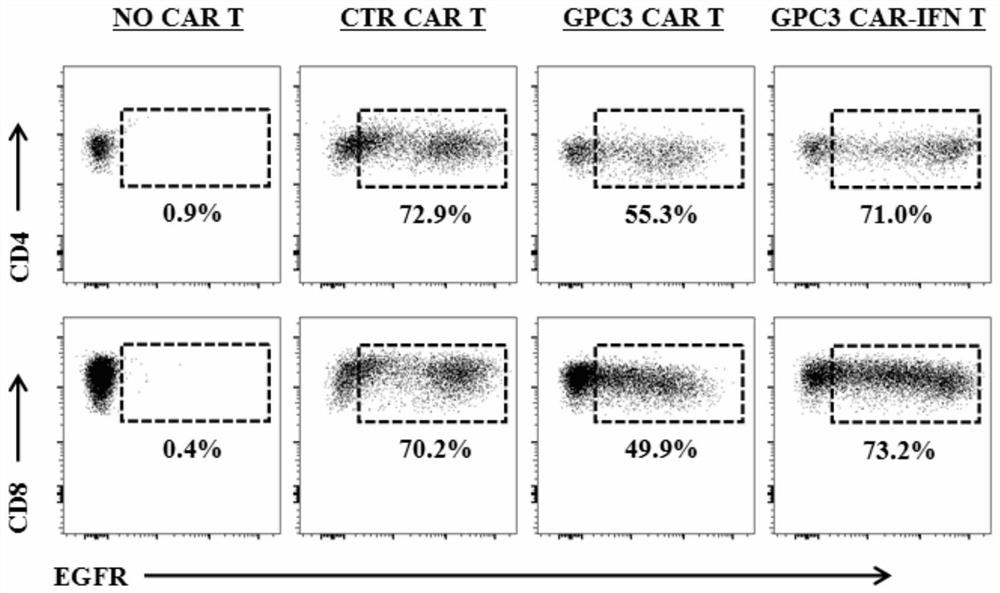
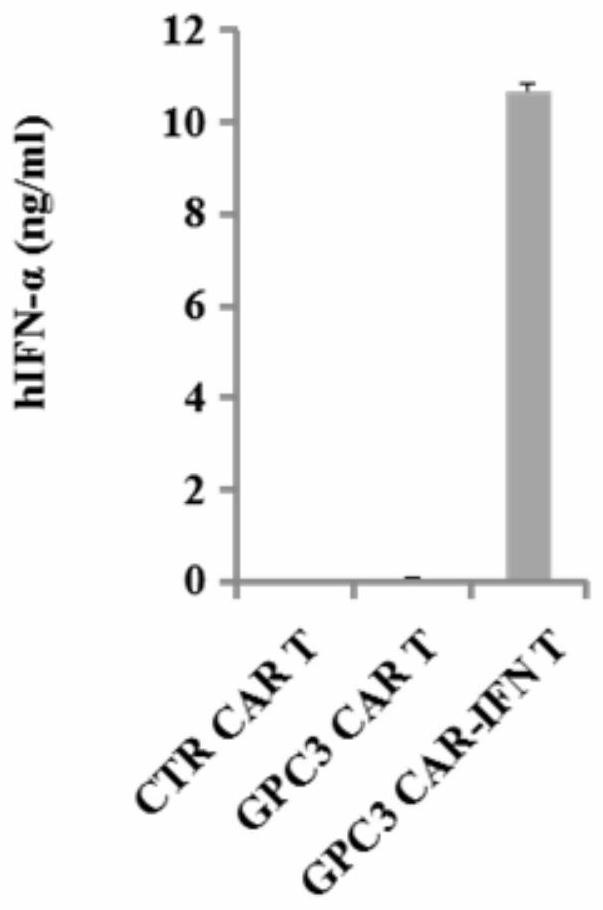
ActiveCN112079932AStrong killing functionEnhanced lethalityVirusesAntibody mimetics/scaffoldsAntigenCD3
The invention discloses a chimeric antigen receptor for hepatocellular carcinoma treatment and application of the chimeric antigen receptor for hepatocellular carcinoma treatment. The chimeric antigenreceptor sequentially comprises a leader peptide, an anti-GPC3 single-chain antibody, a human CD8 hinge transmembrane region, a human 4-1BB intracellular region, a human CD3[zeta] intracellular region, a self-cleavage peptide, a signal peptide, an EGFRt protein, a self-cleavage peptide and a human IFN full length. GPC3 is used as an antigen target to design and develop a new generation of CAR-T cell treatment products, and in addition, a gene-optimized human full-length interferon (IFN) fragment is added to the C terminal of GPC3-CAR. Compared with a CAR-T cell only expressing the GPC3-CAR, the CAR-T cell expressing GPC3-CAR-IFN has stronger tumor killing ability, so that the safety and effectiveness of the CAR-T cell in tumor treatment are further improved.
Owner:CARBIOGENE THERAPEUTICS CO LTD
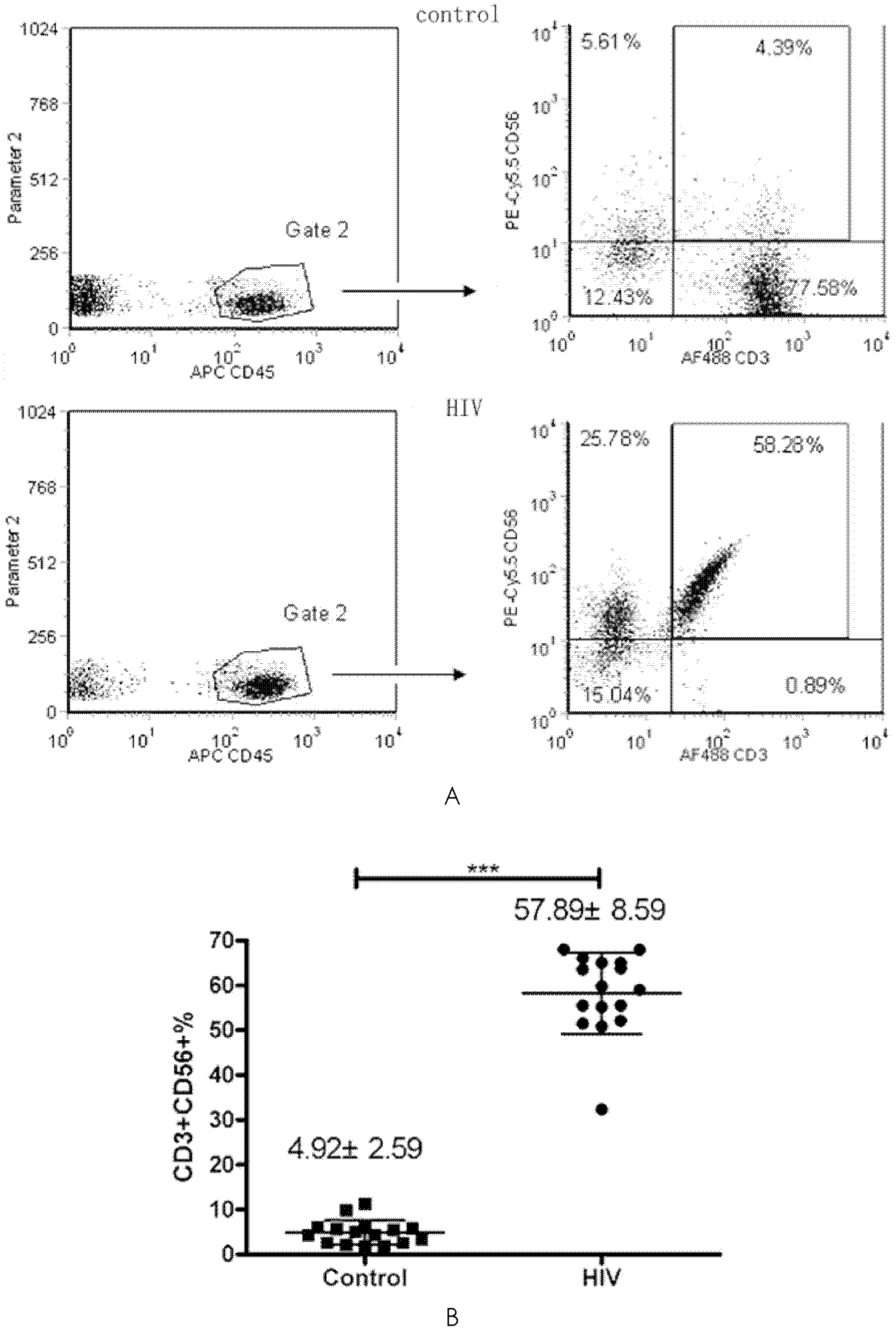
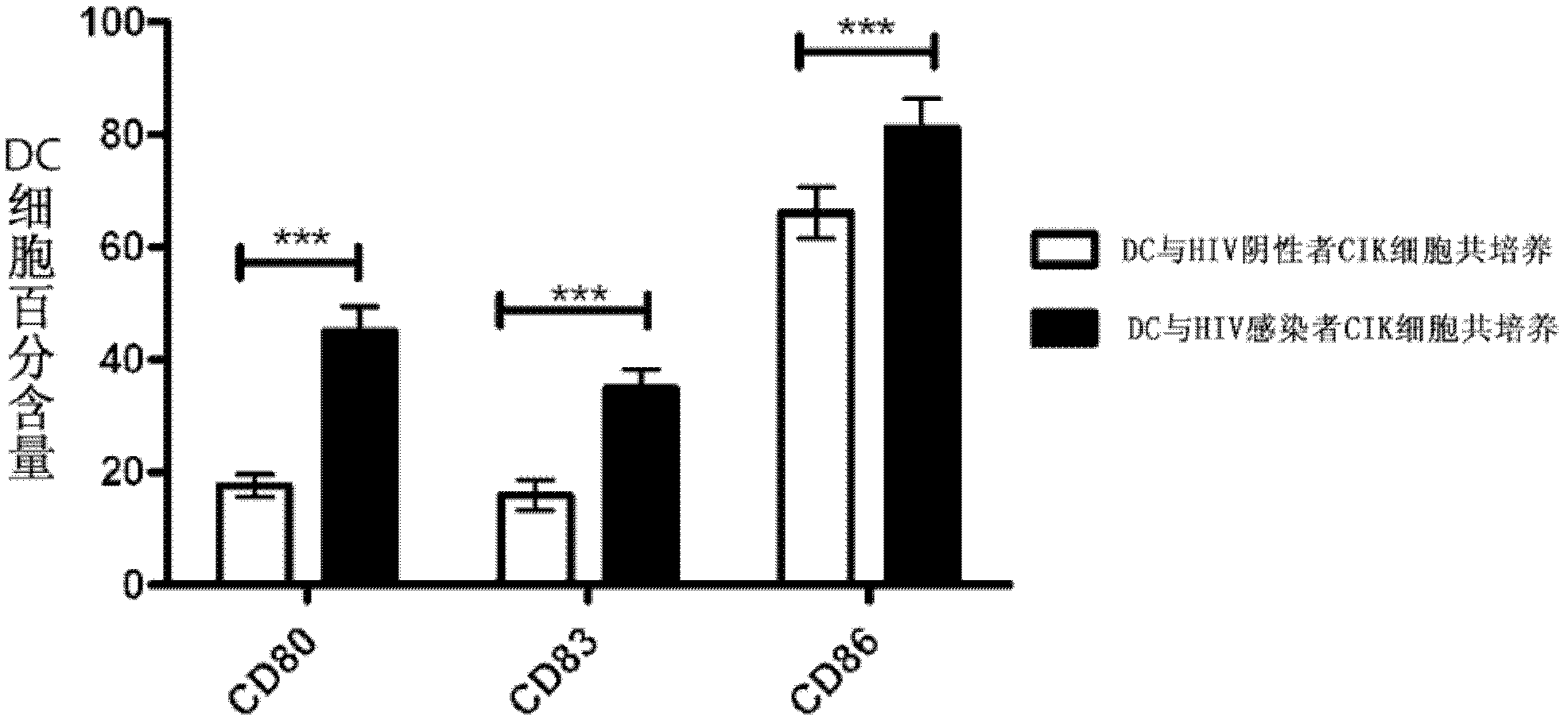
Application of DC-CIK (Dendritic Cell-Cytokine-Induced Killer) cells in preparation of medicine used for resisting HIV (Human Immunodeficiency Virus) infection
InactiveCN102512448AHas a lethal effectPromote maturityAntiviralsMammal material medical ingredientsDendritic cellHuman immunodeficiency
The invention belongs to the field of biomedicine and discloses application of DC-CIK cells in preparation of a medicine used for resisting HIV infection. Through a lot of experiments, the inventor discovers that when CIK cells impacted by HIV and DC cells are co-cultured, the CIK cells can obviously promote maturation of the DC cells. When the DC and CIK cells are combined for use, the DC and CIK cells have mutual promotion presentation and a function for killing cells infected by HIV. After 30 days of the DC-CIK cell infusion, the number of CD4T cells of a HIV infector is obviously increased, the killing function of NK (Natural Killer) and CIK cells is enhanced, and HIV viral load is obviously restrained. Thus, kill cells induced by co-cultured dendritic cells and cytokines can be applied to the preparation of the medicine used for resisting HIV infection.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
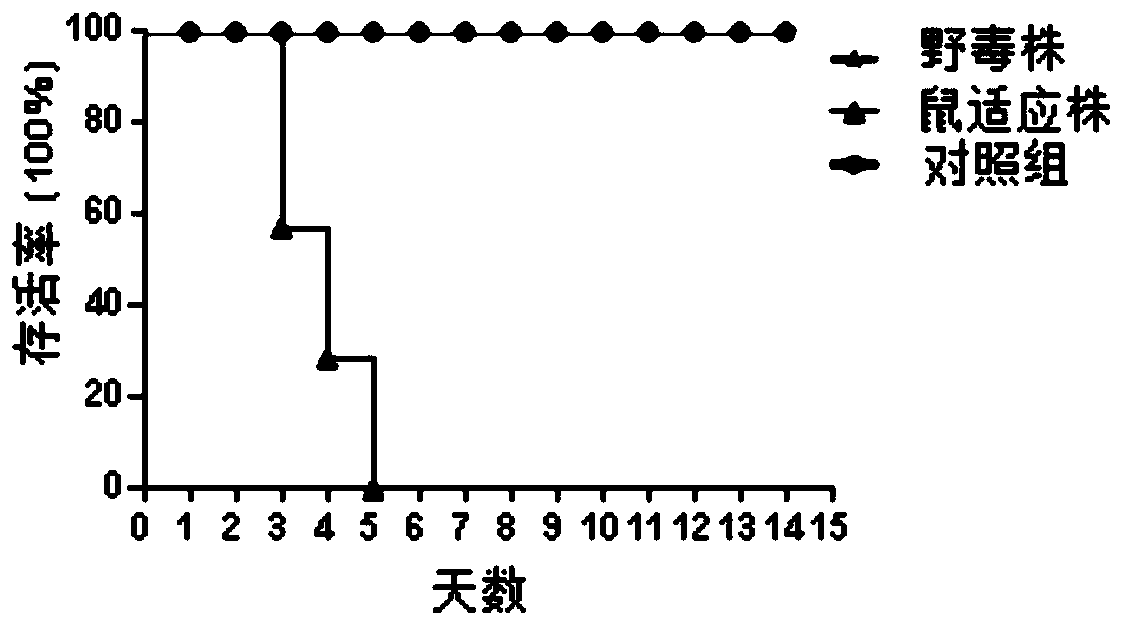
H3N2 subtype canine influenza virus mouse adaptive virulent strain and application thereof
ActiveCN110468109AIncrease virulenceEnhanced lethalitySsRNA viruses negative-senseBiological material analysisVirus influenzaAntibody level
The invention discloses a H3N2 subtype canine influenza virus mouse adaptive virulent strain and application thereof. The techniques of virus isolation, culture, infection of animals and the like areadopted, enhanced virulence and pathogenic lethal amino acid mutations and deletions are obtained through adaptation of mice, and the H3N2 subtype canine influenza virus mouse adaptive virulent strainis obtained; the adaptive strain can cause lethal infection in mice, and a powerful animal model is provided for evaluation and development of a vaccine, effectiveness evaluation of prevention and treatment drugs and the like, influenza virus infection and pathogenic and lethal mechanism research. In addition, due to the fact that the mouse adapts to the virulent strain, an HA gene is not mutated, the antigenicity is unchanged, and the strain can serve as an antigen for basic experimental research such as preparation and / or detection based on antibody levels. In conclusion, the adaptive virulent strain provides the powerful technical support for research and development of the H3N2 subtype canine influenza virus vaccine and research of screening, infection, virulence enhancing and other mechanisms of the high-efficiency prevention and / or treatment drugs.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
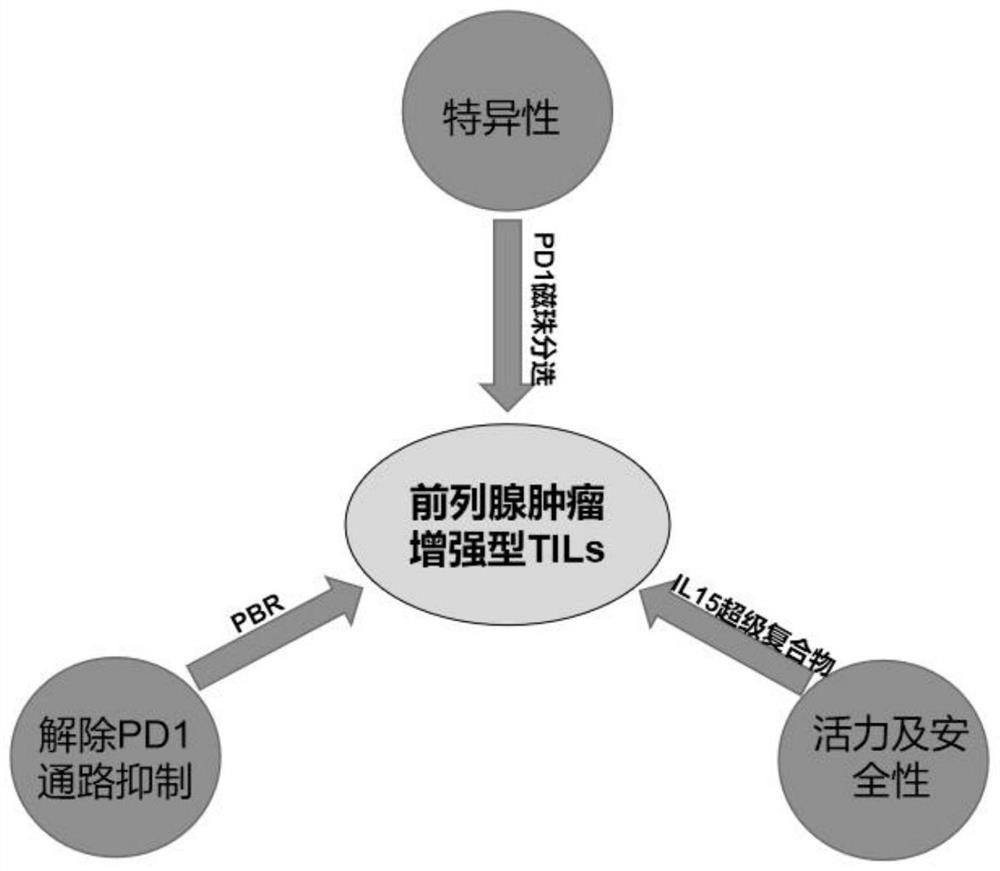
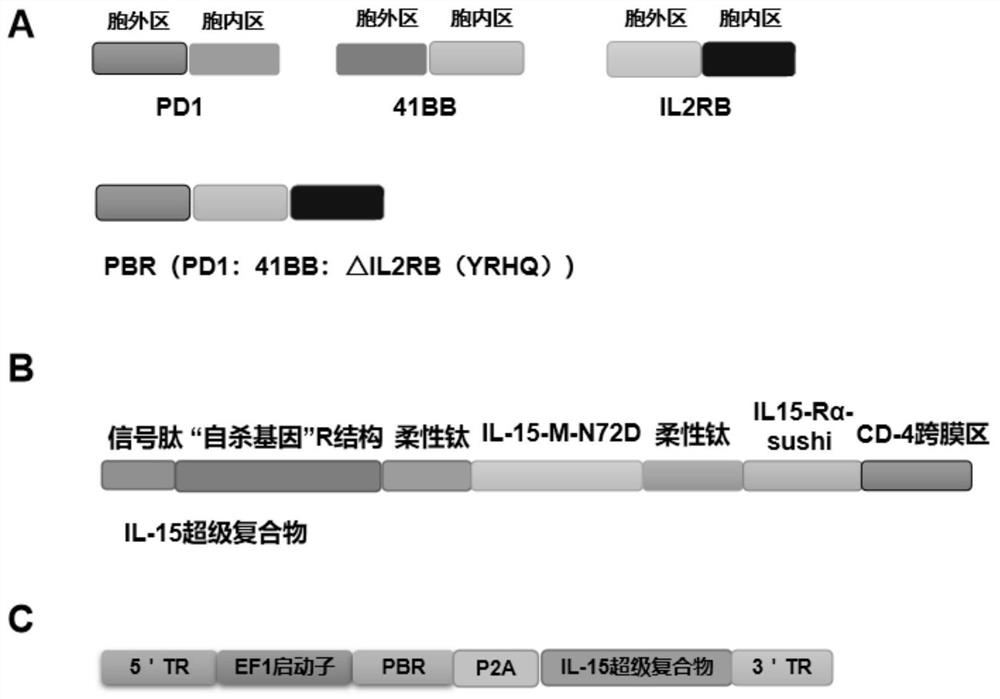
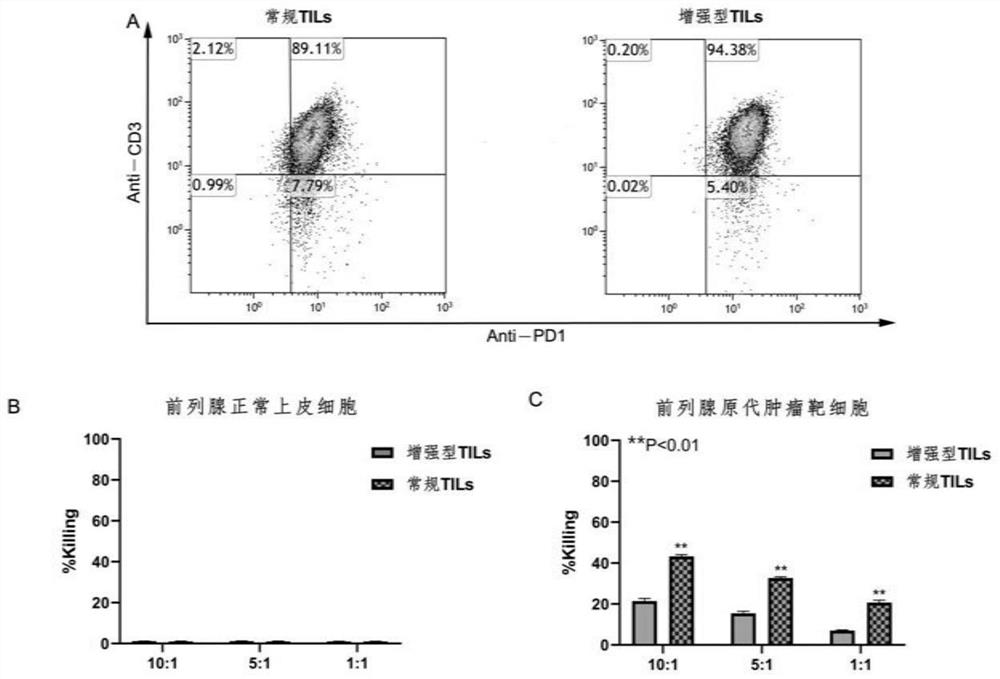
Preparation method of tumor-enhanced tumor-infiltrating lymphocytes
ActiveCN113755529AEnhanced lethalityImprove tumor killing activityPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsTumor specificGene Modification
The invention discloses a preparation method of tumor-enhanced tumor-infiltrating lymphocytes. The preparation method comprises the following steps: (1) extracting tumor-infiltrating lymphocytes TILs from tumor tissues; (2) sorting and enriching PD1 positive T cells in the TILs; (3) integrating a PBR transformed fusion protein and an IL-15 super compound into the PD1 positive T cell at one time; and (4) carrying out multiplication culture to obtain the tumor enhanced TILs. The preparation method has the advantages that (1) by sorting the PD1 positive T cells, the proportion of tumor-specific T cells is increased; (2) the designed PBR fusion protein can relieve the inhibition from a PD1 pathway in a tumor microenvironment and improve the killing ability of the TILs to tumor cells; (3) the IL-15 super compound not only can improve the tumor killing activity of T cells, but also can stimulate other immune cells in the body, such as endogenous NK, T cells and the like, and accelerates the removal of the tumor cells in the body; and (4) the added 'suicide gene' R structure solves the problem of subsequent clinical safety after T cell gene modification.
Owner:THE FIRST AFFILIATED HOSPITAL OF WANNAN MEDICAL COLLEGE YIJISHAN HOSPITAL OF WANNAN MEDICAL COLLEGE
Optimized connecting peptide combination and application thereof
PendingCN113493518AEnhanced lethalityReduce chance of immune escapeHybrid immunoglobulinsGenetically modified cellsMolecular biologyImmune escape
The invention belongs to the technical field of gene engineering, and particularly relates to an optimized connecting peptide, a bispecific single-chain antibody connected with the optimized connecting peptide, a CAR (Chimeric Antigen Receptor) structure containing the bispecific single-chain antibody, an expression vector, an immune cell and application. In the bispecific single-chain antibody, two linkers are used for connecting heavy chains or light chains of two single-chain antibodies; the construction of the CAR structure overcomes the technical effect defect of a double-target CAR in the prior art; two different tumour antigens can be targeted at the same time; killing of tumour cells is improved; and the probability of immune escape of the tumour cells is reduced.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Composition capable of enabling skin to defend pigments, and application
PendingCN112294720AInhibition formationReduce pigmentationCosmetic preparationsToilet preparationsIndometacinVitamin C
The invention discloses a composition capable of enabling the skin to defend pigments, and application. The composition comprises 1-4% of glutathione, 1-3% of arbutin, 0.2-0.6% of vitamin C, 0.1-2% ofvitamin E, 0.05-0.1% of indomethacin, 0.01-0.05% of 9-carotene, 0.1-0.6% of green tea, 0.2-0.7% of fructus mume extract, 0.2-0.9% of cortex cinnamomi extract, 2-12% of glycyrrhizae radix oil, 1-6% ofsoluble extract and the balance of water. Since the glutathione and the arbutin are added, formation of melanin is inhibited and disturbed so as to reduce skin pigment deposition and remove colored patches and freckles, the vitamin C, the vitamin E, the fructus mume extract and the soluble extract are combined to increase oxidation resistance capacity, the composition is prevented from being oxidized, the composition is guaranteed to still own an effect of inhibiting the formation of the melanin in the sun, and the skin fully defends the pigments.
Owner:珂蓝(重庆)化妆品有限公司
A novel oncolytic virus and its preparation method and application
ActiveCN110218707BUpregulation of immune responseEnhanced lethalityPeptide/protein ingredientsAntibody ingredientsRegulatory T cellNatural Killer Cell Inhibitory Receptors
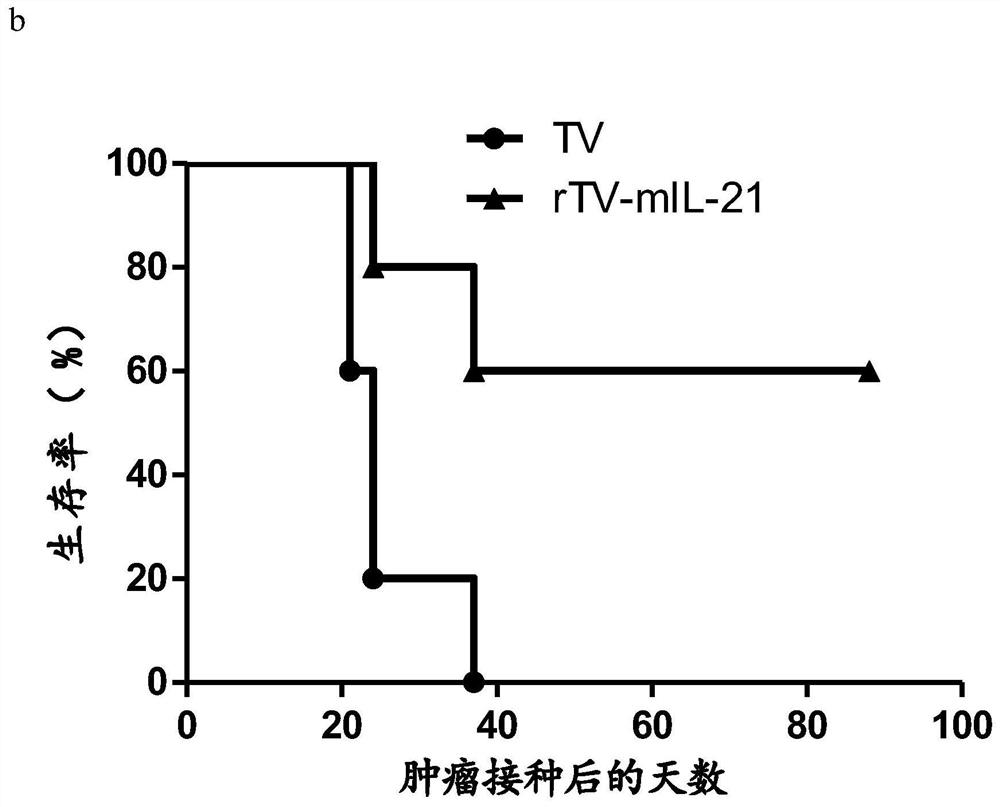
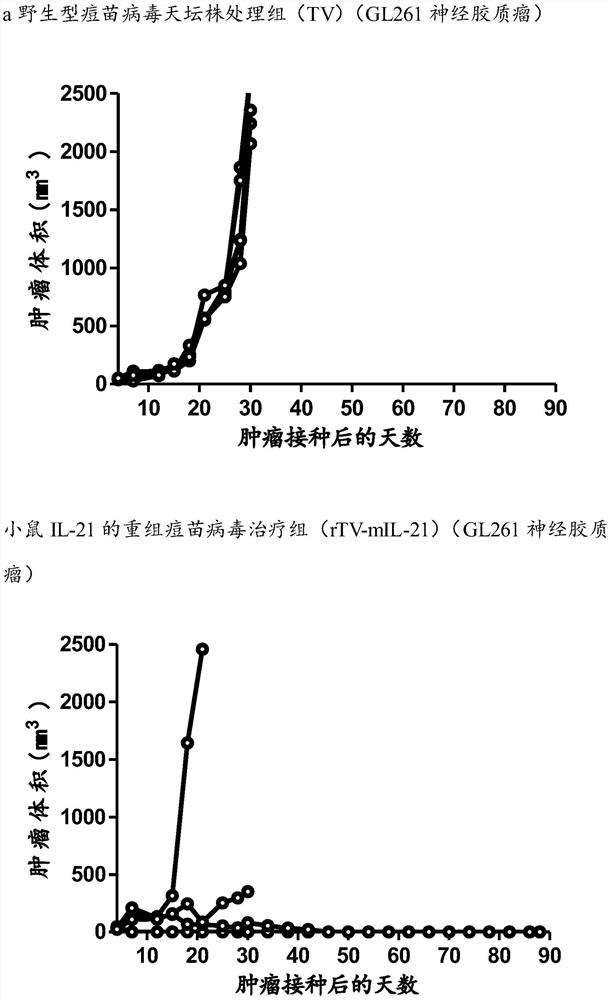
The invention discloses a novel oncolytic virus and its preparation method and application. The oncolytic virus genome contains gene coding sequences of anti-human CTLA4 antibody and human IL-21, and can express anti-human CTLA4 antibody and human IL-21 21. Combining the tumor-suppressing effect of immunotherapy with the oncolytic effect of viral therapy, an oncolytic virus capable of highly expressing the αCTLA4-Fc(ALIE)-IL21 gene was prepared, and the oncolytic virus exerted an oncolytic effect to lyse the tumor At the same time, it can kill regulatory T cells (Regulatory T Cells, Treg) in the tumor microenvironment by expressing a large amount of αCTLA4‑Fc (ALIE), or block its inhibitory effect, thereby up-regulating the immune response of the tumor microenvironment and improving the anti-tumor effect At the same time, the oncolytic virus expresses a large amount of IL21, further activates the killing function of T cells and NK cells in the tumor microenvironment, and exerts multiple anti-tumor effects. Compared with simple gene therapy or virus therapy, it has enhanced its ability to kill malignant tumors.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
A bispecific antibody for multiple myeloma and its application
ActiveCN112159476BEnhanced lethalityHybrid immunoglobulinsAntibody ingredientsProtein targetCell recruitment
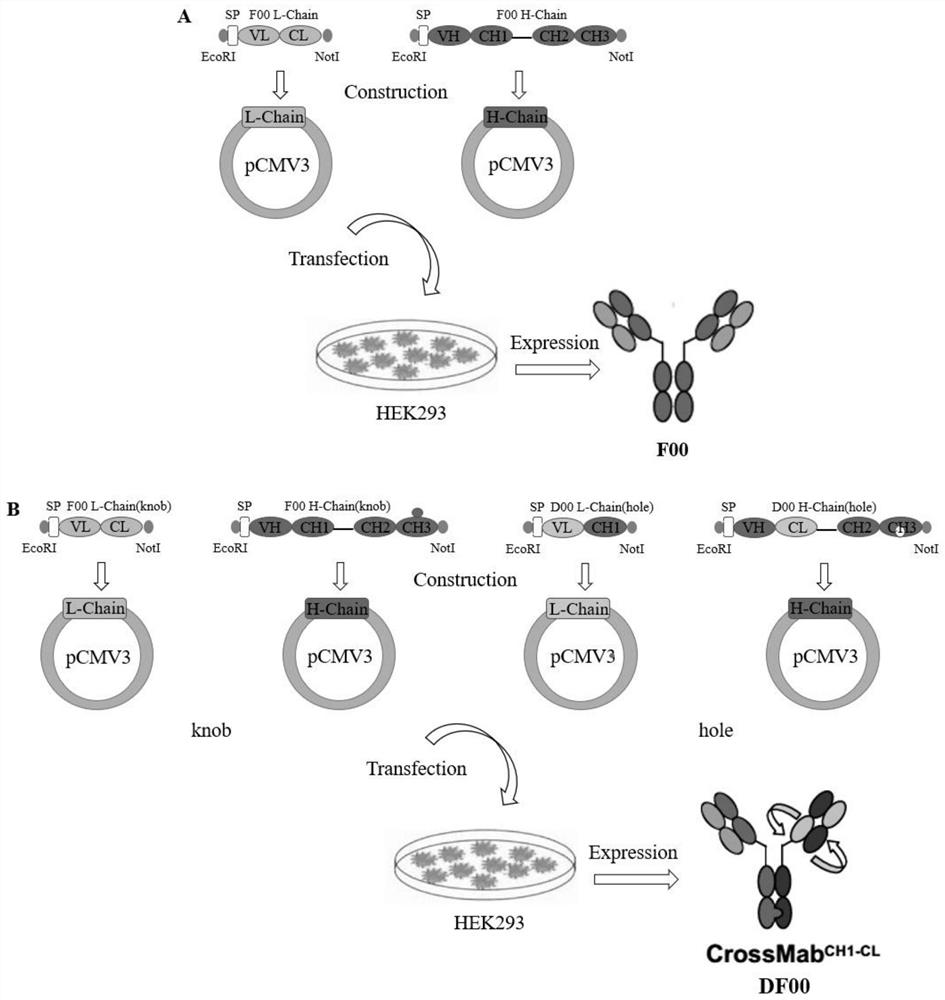
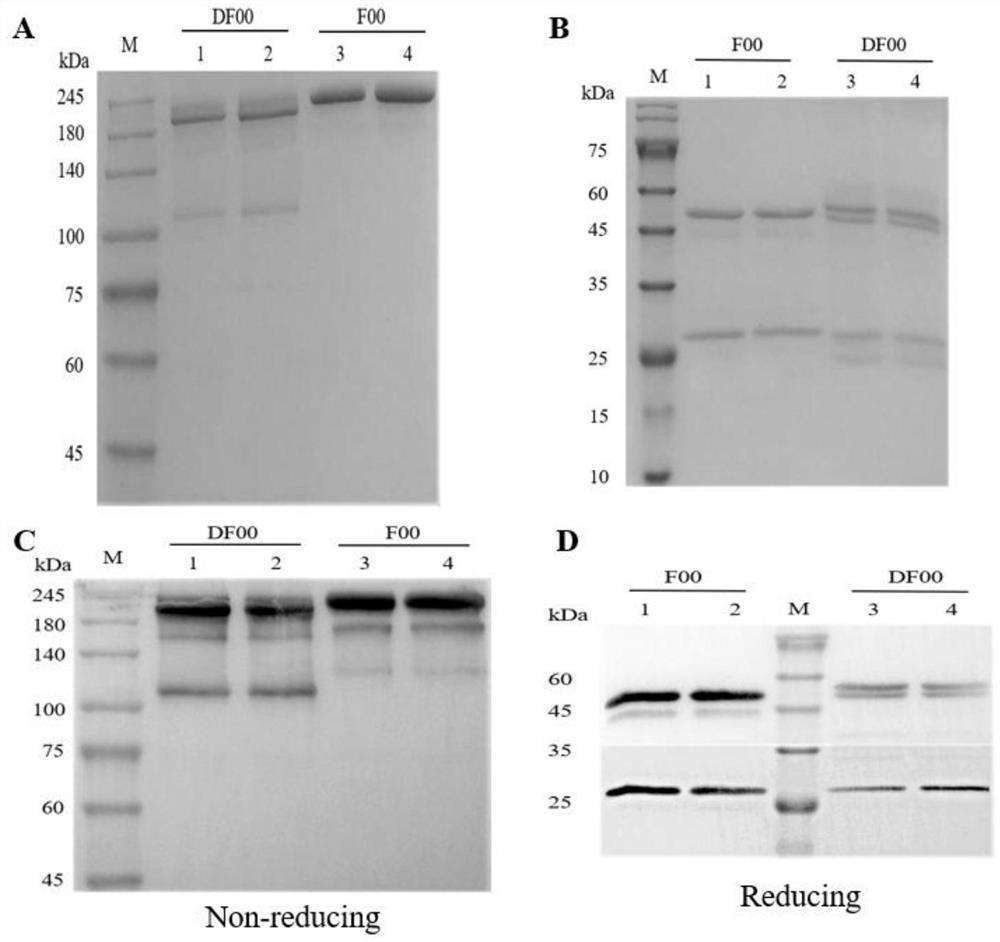
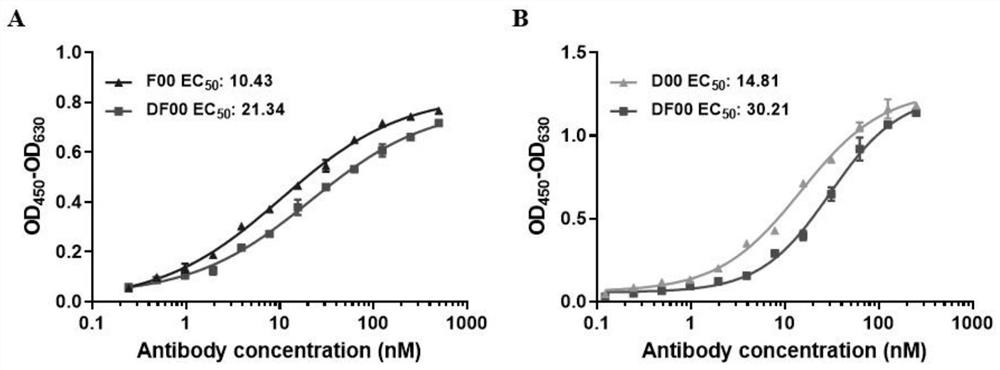
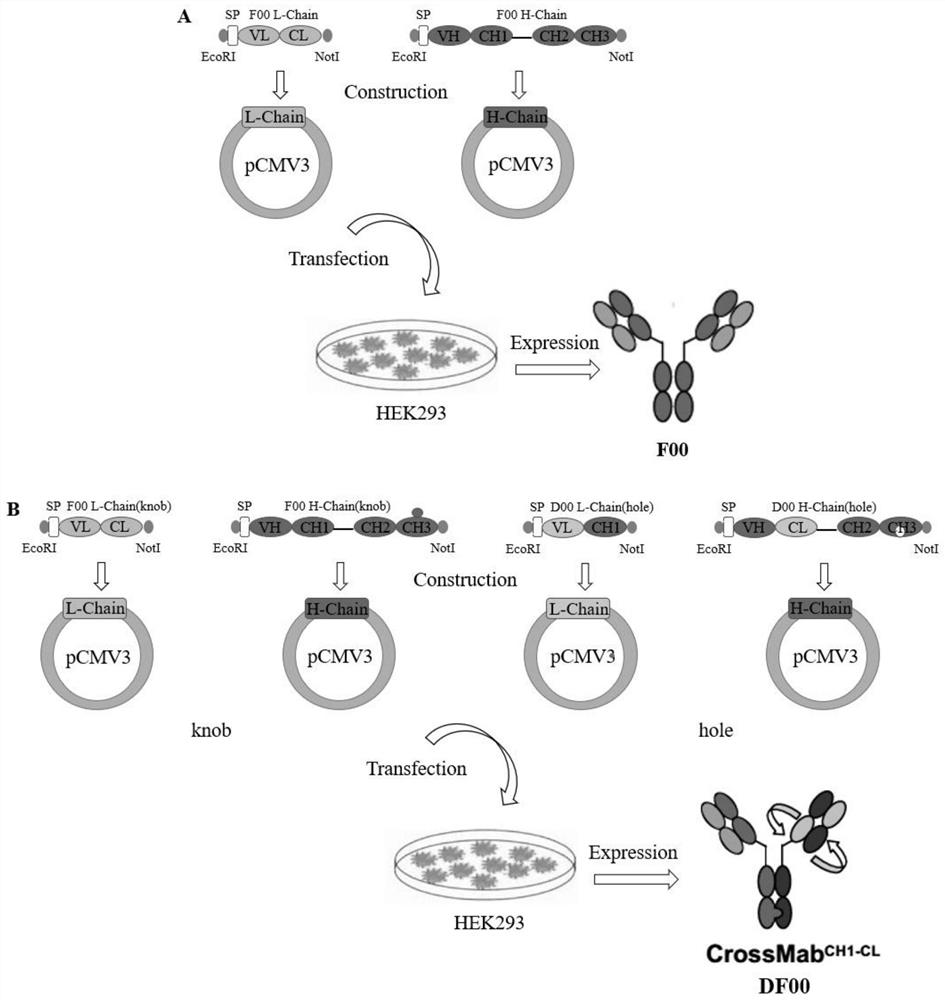
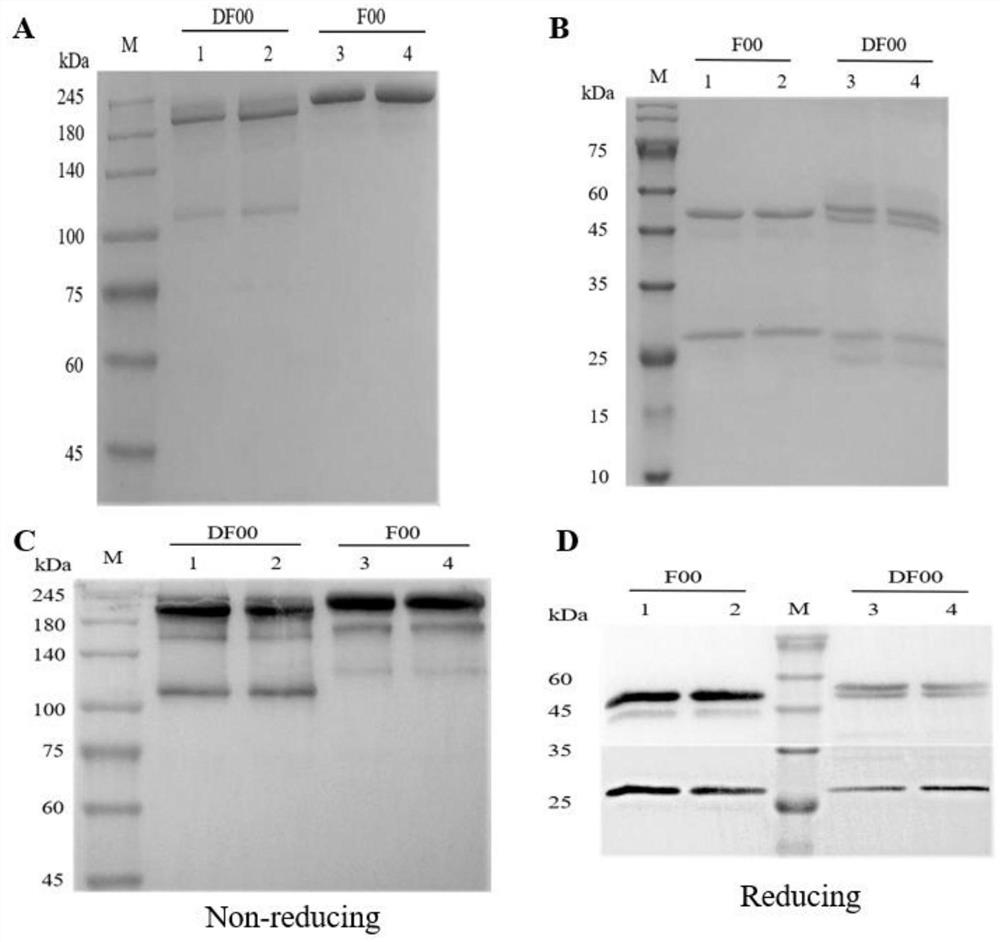
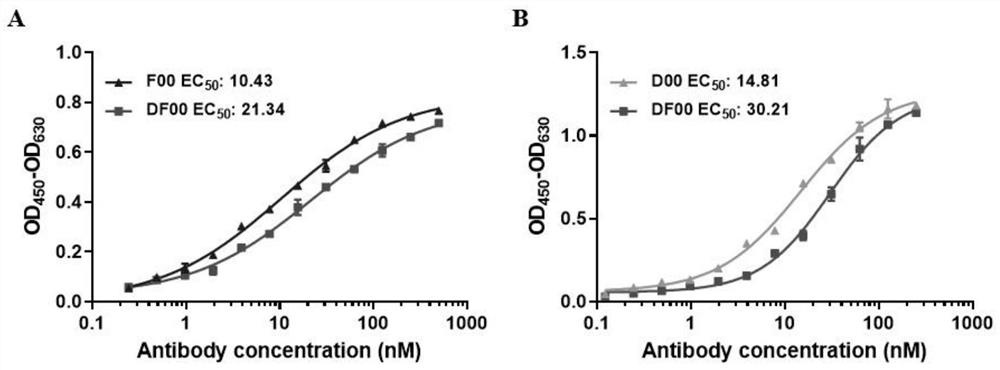
The invention discloses a bispecific antibody for multiple myeloma and its application, and belongs to the technical field of genetic engineering antibodies. In the present invention, the antibody F00 targeting human BCMA and the antibody D00 targeting human PD-1 are used as parent antibodies, and 'knob' and 'hole' ends are respectively introduced into the two heavy chain Fc according to the CrossMab platform using genetic engineering technology. Animal eukaryotic expression system expresses DF00. The present invention also provides a method for expressing and purifying the bispecific antibody DF00. The target protein is obtained by secreting expression of HEK293 cells and purifying by affinity chromatography. The bispecific antibody DF00 can specifically bind to both the BCMA molecule on the surface of multiple myeloma (MM) cells and the immune checkpoint molecule PD-1 on the surface of activated T cells, inhibiting the immunosuppressive microenvironment in tumors and promoting T cells Recruited to surrounding multiple myeloma cells, remodeling the function of T cells to kill MM cells.
Owner:CHINA PHARM UNIV
Bispecific antibody for multiple myeloma (MM) and application of bispecific antibody
ActiveCN112159476AEnhanced lethalityHybrid immunoglobulinsAntibody ingredientsProtein targetCell recruitment
The invention discloses a bispecific antibody for multiple myeloma (MM) and application of the bispecific antibody, and belongs to the technical field of genetic engineering antibodies. An antibody F00 targeting human BCMA and an antibody D00 targeting human PD-1 are used as parent antibodies, a 'knob' end and a 'hole' end are respectively introduced to two heavy chains Fc by a gene engineering technology according to a CrossMab platform, and a mammal eukaryotic expression system expresses DF00. The invention further provides a method for expressing and purifying the bispecific antibody DF00.A target protein is obtained through HEK293 cell secretory expression and affinity chromatography purification. The bispecific antibody DF00 can specifically bind to a BCMA molecule on the surface ofMM cells and an immune checkpoint molecule PD-1 on the surface of activated T cells at the same time, inhibit an immunosuppressive microenvironment in tumors and recruit T cells around the MM cells toremodel the MM cell killing function of the T cells.
Owner:CHINA PHARM UNIV
A chimeric gene based on fcγriiia and its use
ActiveCN105647946BHigh kill ratePromote activationPolypeptide with localisation/targeting motifPeptide/protein ingredientsSequence signalAntigen receptors
The invention relates to an Fc gamma RIII a-based chimeric gene and application thereof. The chimeric gene comprises an Fc gamma RIII a signal peptide, an Fc gamma RIII a extracellular area, a CD8alpha cross-film area and an intracellular signal transmission structural domain, wherein the Fc gamma RIII a extracellular area is directly connected with the CD8alpha cross-film area. According to the mosaic gene, the Fc gamma RIII a extracellular area is directly connected with the CD8alpha cross-film area, and a CD8alpha hinge area in a conventional chimeric antigen receptor (CAR) molecule design is deleted, so that an Fc gamma RIII a-CAR molecule is better for activating effector cells, and the killing capability of the Fc gamma RIII a-CAR molecule to tumor cells is remarkably improved; the designed Fc gamma RIII a-CAR molecule combined with a monoclonal antibody drug can be generally used in cell therapy of various tumors.
Owner:JIANGSU PURECELL BIOMEDICAL TECH CO LTD
A kind of chimeric antigen receptor for the treatment of liver cancer and its application
ActiveCN112079932BStrong killing functionEnhanced lethalityVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
Owner:CARBIOGENE THERAPEUTICS CO LTD
Protein nanoparticles carrying antitumor peptide and preparation method and application of protein nanoparticles
ActiveCN111110654ASmall toxicityGood tumor treatmentPeptide/protein ingredientsAntibody mimetics/scaffoldsEscherichia coliLysosome
The invention belongs to the technical field of biomedicines and in particular relates to protein nanoparticles carrying an antitumor peptide and a preparation method and application of the protein nanoparticles. The preparation method comprises the following steps: according to gene sequences of a pET25b(+)-HSP16.5 expression vector, synthesizing a single stranded DNA (deoxyribonucleic acid) sequence corresponding to an apoptosis-promoting peptide, namely KLAK peptide, performing annealing treatment, performing HindIII and XhoI double-digestion on the product and the vector, performing gel recycling on the digestion product, connecting the KLAK sequence with the vector, transforming the connection product into a competence of DH5a escherichia coli, picking a monoclonal bacterial colony, performing overnight bacterium shaking, extracting a plasmid, and performing sequencing; and transforming a pET25b(+)-HSP-KLAK plasmid with correct sequencing into a competence of BL21 escherichia coli, amplifying culture, performing ultrasonic bacterium crushing so as to obtain a corresponding crude product, performing purification, and performing dialysis, concentration and freeze-drying, so as to obtain an HSP-KLAK protein. The heat shock protein nanoparticles carrying the antitumor peptide, which are provided by the invention, can be rapidly taken in by tumor cells, after being released inlysosome, a targeting mitochondrion plays a role of tumor killing, the protein of the medicine carrier self can be rapidly degraded by cells, and thus a good tumor treatment effect can be achieved.
Owner:BEIHUA UNIV
Application of atpif1 gene silenced T cells in preparation of antitumor drugs
ActiveCN110604744BEnhanced lethalityGenetically modified cellsStable introduction of DNACell activityMedicine
The invention discloses a method for enhancing the activity of T cells. By silencing the ATPIF1 gene in T cells, the T cells can be rapidly multiplied and activated in the immune process, the effector function of T cells can be improved, and the killing effect of T cells on tumor cells can be enhanced. , the present invention also discloses the application of ATPIF1 gene silenced T cells in the preparation of anti-tumor drugs, which provides a new direction for the research and development of tumor adoptive immunotherapy.
Owner:XINXIANG MEDICAL UNIV
Application of cantharis in medicament preparation
InactiveCN1879658AEnhance pharmacological effectsGrowth inhibitionAnthropod material medical ingredientsImmunological disordersHuman bodyCantharis
The invention relates to the application of cantharis and cantharis mixture, wherein said application comprises: (1) the invention develops new application of cantharis and cantharis mixture; (2) the inventive cantharis and cantharis mixture have low toxicity and strong pharmacological function, and wider application; (3) the invention can restrain cancer growing and adjust the immunity of human body, to support clinical cancer treatment.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
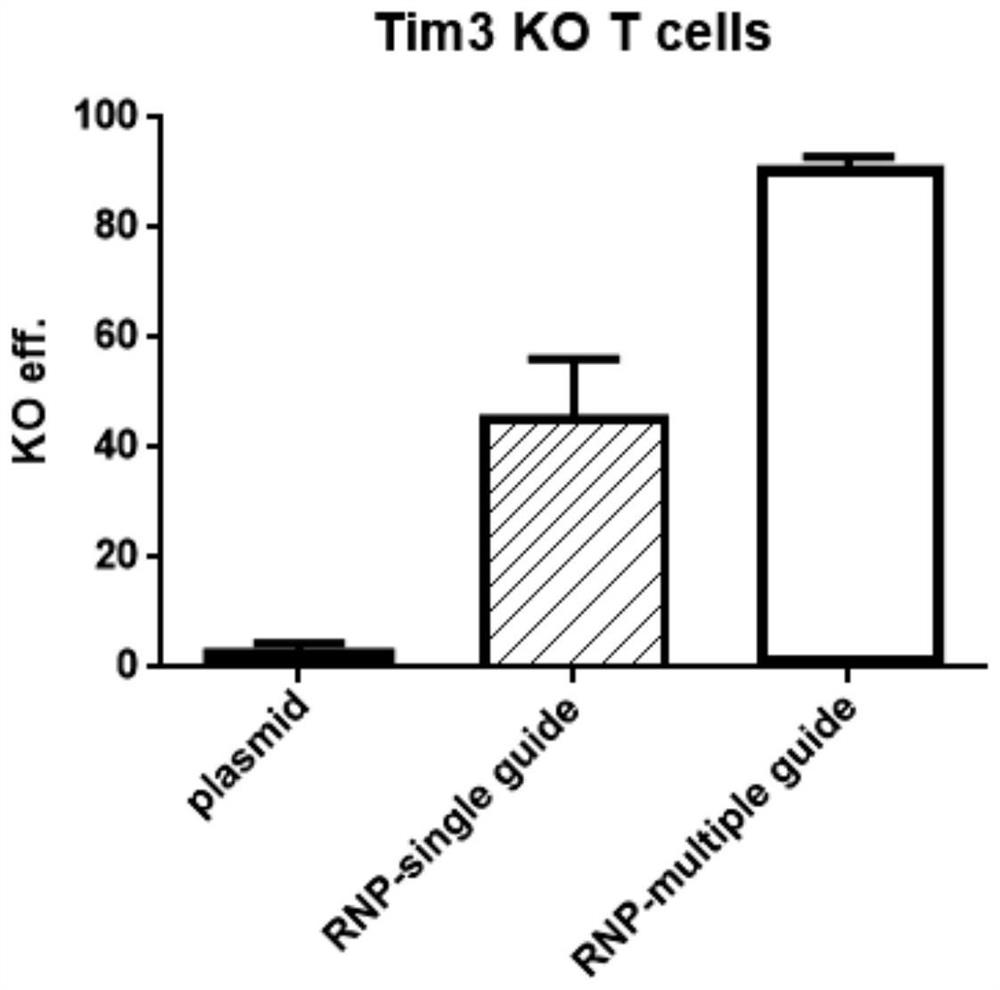
Method for knocking out Tim-3 gene to prolong survival of T cell in vivo
ActiveCN113061624AProlong survival timeKnockout High EfficiencyGenetically modified cellsBlood/immune system cellsHuman cellBiochemistry
The invention relates to a method for knocking out a Tim-3 gene to prolong in-vivo survival of a T cell, in particular to a method for knocking out a TIM-3 gene of the T cell. The method comprises the following steps: preparing CRISPR-Cas9 protein and gRNA into an RNP complex, and then conveying the RNP complex into a T cell in an electroporation manner. According to the method disclosed by the invention, the survival time of a T cell is prolonged by specifically knocking out a HAVCR2 gene in the human T cell by utilizing CRISPR-Cas9 and sgRNA, so that the effect of efficiently knocking out the gene can be ensured to be realized.
Owner:矫士平 +1
A chimeric antigen receptor and its application in the preparation of products for treating tumors
ActiveCN111892661BPromote growthReduce the risk of inhibitionVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
The invention discloses a chimeric antigen receptor and its application in preparing products for treating tumors. The chimeric antigen receptor sequentially comprises a leader peptide, an anti-LILRB4 single-chain antibody, a human CD8 hinge transmembrane region, a human 4-1BB intracellular region, a human CD3ζ intracellular region, a self-cleaving peptide and a full-length human IFN. This invention uses LILRB4 as an antigen target for the first time to design and develop a new generation of CAR-T cell therapy products, and adds a gene-optimized human full-length interferon (IFN) fragment to the C-terminus of LILRB4-CAR. Compared with CAR-T cells expressing only LILRB4-CAR, CAR-T cells expressing LILRB4-CAR-IFN have stronger tumor killing ability, which further improves the safety and effectiveness of CAR-T cells in treating tumors.
Owner:CARBIOGENE THERAPEUTICS CO LTD
Chimeric antigen receptor containing c5ar intracellular domain, lentiviral vector, expressing cell and drug
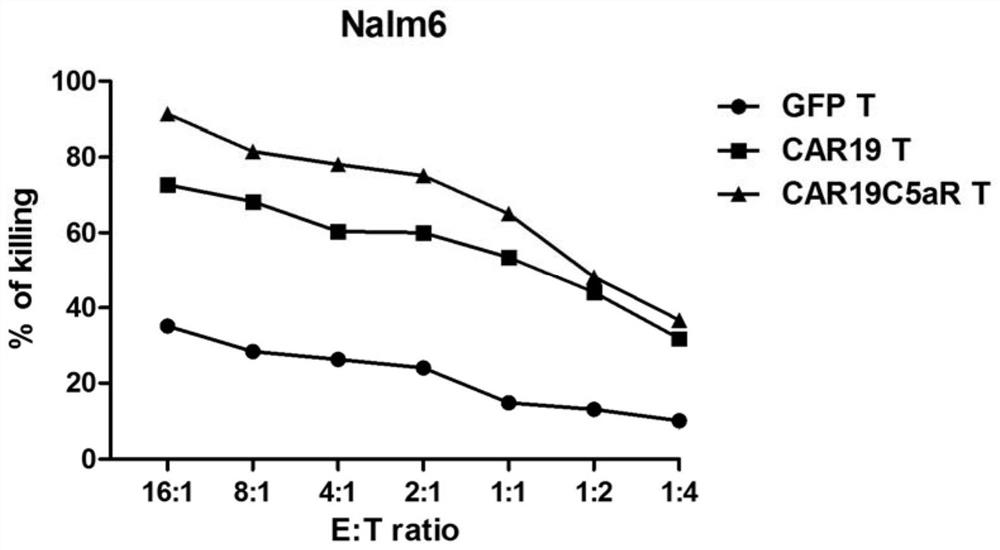
ActiveCN108017716BEnhanced lethalityEliminate immunosuppressionImmunoglobulin superfamilyGenetically modified cellsAntigenAntigen receptor
The invention provides a chimeric antigen receptor including a C5aR intracellular domain, a lentiviral vector, an expression cell and a medicine. The chimeric antigen receptor including a C5aR intracellular domain includes an extracellular protein capable of binding to an antigen. structural domain, a transmembrane domain and at least one intracellular domain, and the at least one intracellular domain refers to the C5aR intracellular domain, or the intracellular domain of the signaling region in series with the C5aR intracellular domain. The chimeric antigen receptor of the present invention can increase the T17 cell subpopulation, eliminate the immunosuppressive effect of regulatory T cells, can significantly improve the in vitro killing efficiency of tumor target cells, and significantly improve the killing effect of second-generation CAR T cells on tumors. Provide a new idea and choice for the field of CAR‑T cell therapy.
Owner:赖沛龙
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com