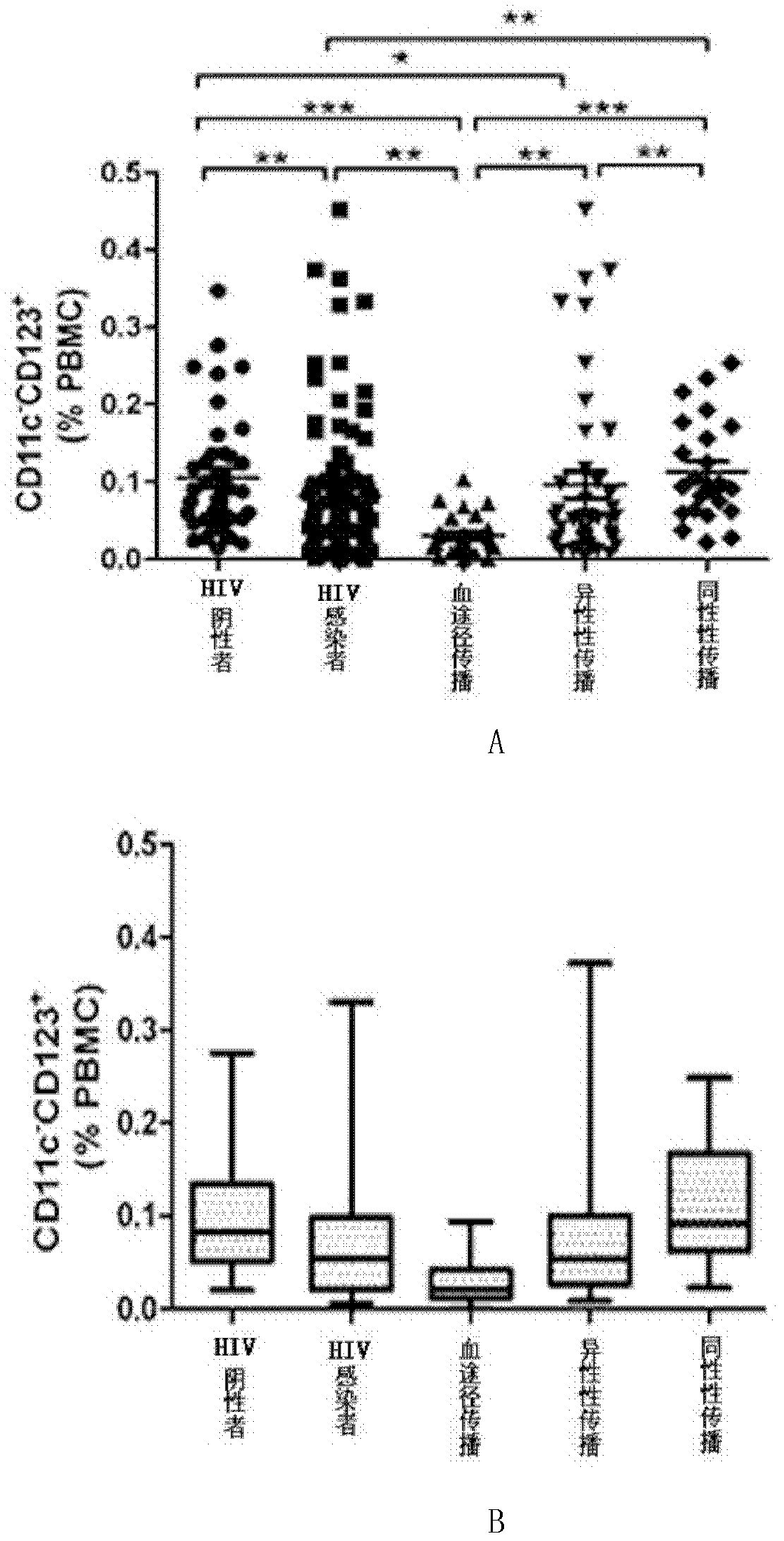Application of DC-CIK (Dendritic Cell-Cytokine-Induced Killer) cells in preparation of medicine used for resisting HIV (Human Immunodeficiency Virus) infection
A technology of drugs and cytokines, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1HI
[0026] Example 1 Detection of DC and CIK cell content and function in HIV-infected persons / AIDS patients
[0027] 1. Materials: Take 10ml of peripheral blood from HIV-negative patients, HIV-infected patients and AIDS patients transmitted by different ways, anticoagulate with EDTA-2K (BD Vacutainer USA), and use 1×PBS phosphate buffer saline (Invitrogen, USA) 1: 1 dilution, and then slowly add the diluted blood to the top of the lymphocyte separation medium (GE Healthcare, Sweden), centrifuge at 1500rpm for 30 minutes to separate peripheral blood lymphocytes (PBMC), wash 2 times with 1×PBS, and prepare for the following analysis .
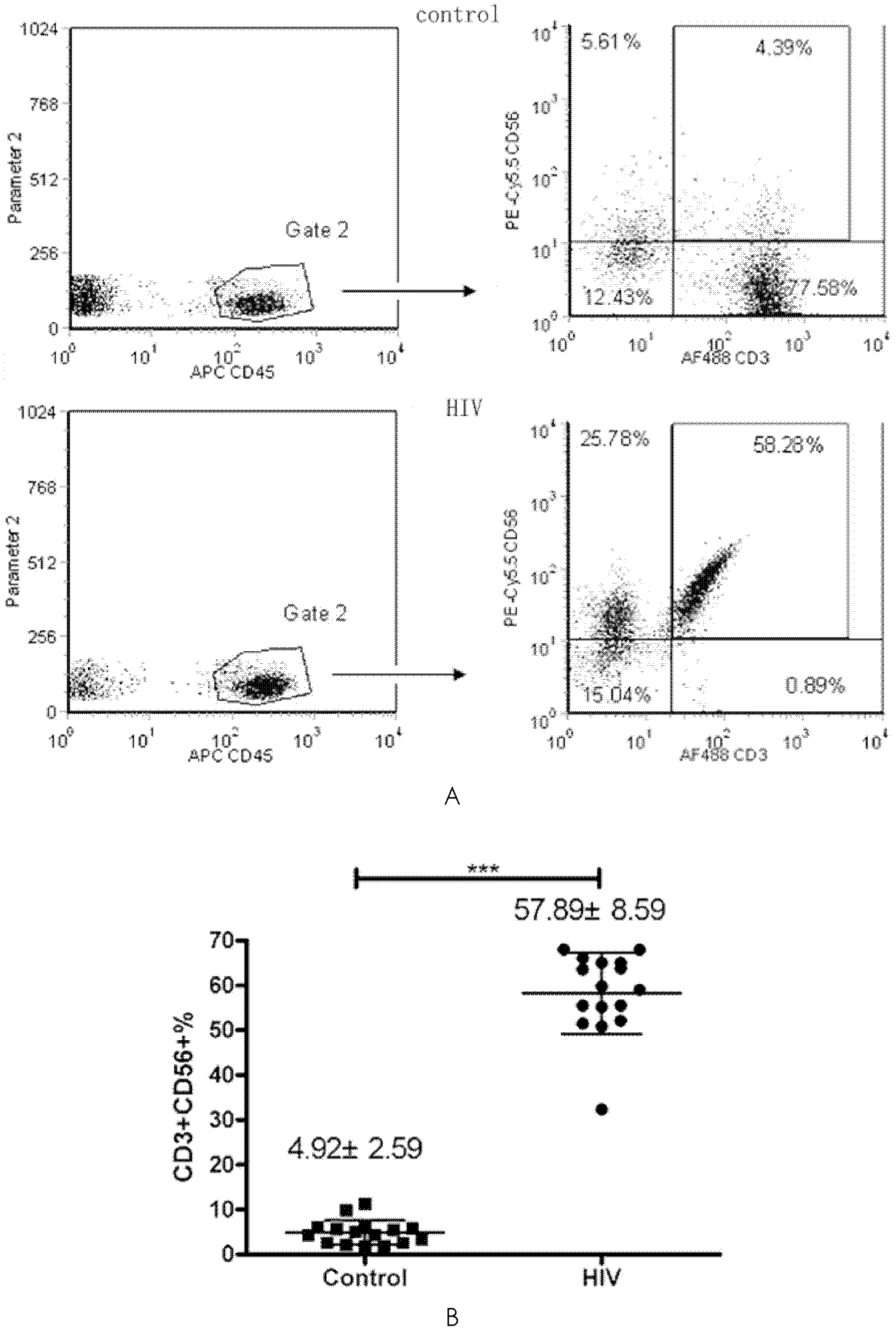
[0028] 2. Detection of the content of DC and CIK cells in peripheral blood: take the above separated and washed PBMCs, and the total number of cells is 106. Dilute to 100 μl with 1×PBS. DC cells were labeled with PE-Cy5.5-HLA-DR (Pharmingen USA), APC-CD11c (Pharmingen) and PE-CD123 (Pharmingen). CD11c+CD123+ positive cells were defined as DC cells...
Embodiment 2
[0033] Example 2 Preparation and reinfusion of autologous DC-CIK cells
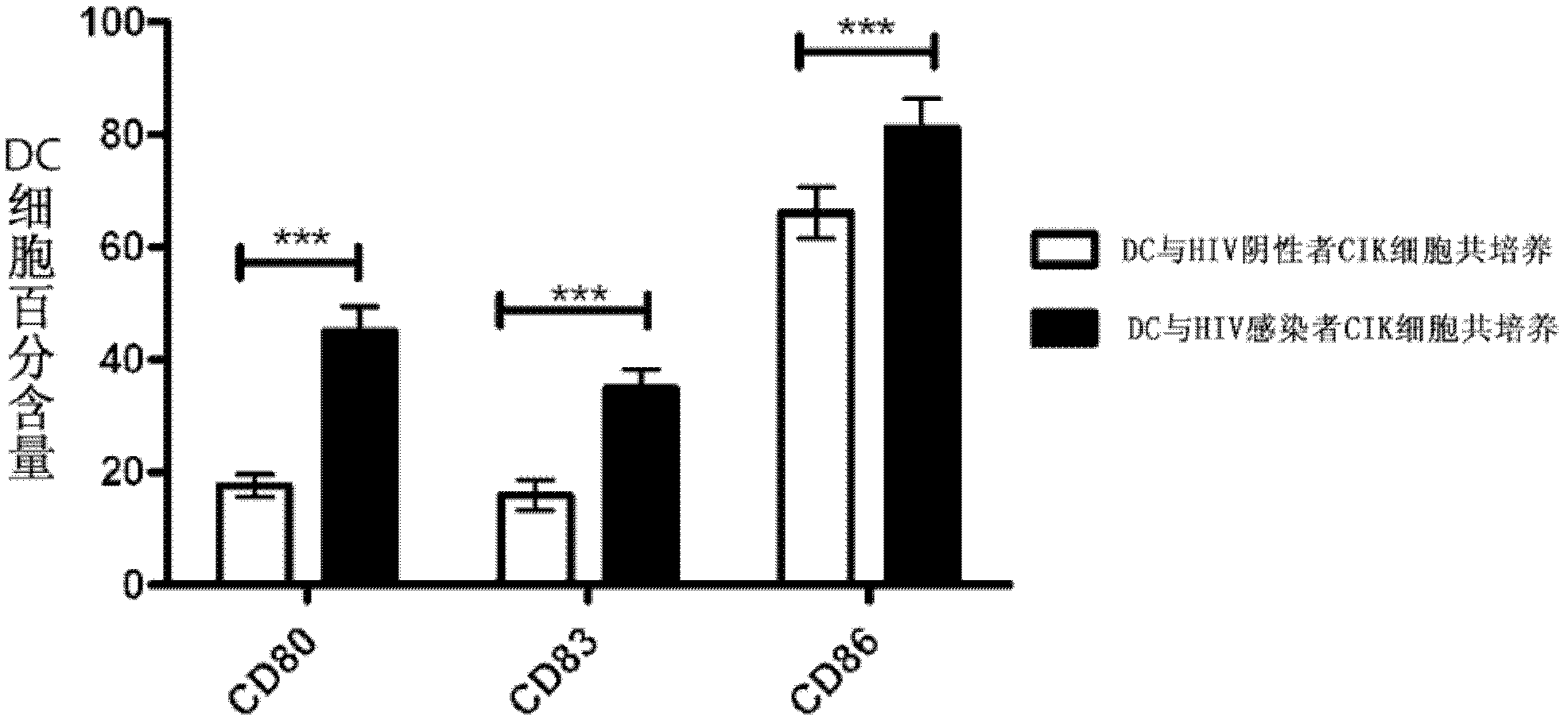
[0034] 1. Preparation of DC and CIK cells: Use a blood cell separator to collect peripheral blood PBMCs from patients. After removing plasma, separating, washing and counting, respectively culture DC and CIK cells according to the method in Example 1, and culture them for 6 days DC and CIK cells were co-cultured for 3 days. The separation and other operations after cell collection should be performed in a sterile laboratory, and each link must be operated in strict accordance with the aseptic rules, and a sterility test should be performed on each batch of cells to ensure that the cells are not contaminated.
[0035] 2. Reinfusion of DC and CIK cells: collect the co-cultured DC and CIK cells, and digest the DC cells with 0.25% trypsin for 5 minutes before collecting them. The collected DC and CIK cells were washed 3 times with serum-free 1640 medium. Immediately after collection, the DC cells were subcu...
Embodiment 3
[0036] Example 3 Observation of curative effect of patients after autologous cell reinfusion
[0037] 1. Follow-up of patients after reinfusion of autologous cells: medical follow-up of patients was carried out on days 0, 3, 5, 10, 30, 60, 90, 120, 240 and 360 after reinfusion of cells. The main content of the follow-up visit is: the follow-up visit on days 0-10 is mainly to understand the side effects of the patient after the reinfusion of cells. During the follow-up visit, 10ml of blood is collected from the patient and anticoagulated with EDTA. The following medical follow-up is mainly to observe the curative effect after the reinfusion of cells, and to understand the relevant information of the patient after the reinfusion of cell therapy, such as whether there are related opportunistic infections, whether there is high-risk sexual behavior, etc. At the same time, 10ml of blood is collected from the patient. EDTA anticoagulation. The anticoagulated blood collected above n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com