Application of cantharis in medicament preparation
The technology of cantharidin and norcantharidin is applied in the field of cantharidin mixture and cantharidin, which can solve the problems of affecting life and work, affecting appearance and beauty, and achieve the effect of strong pharmacological action and good medicinal prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Mylabris in the preparation of treatment or prevention of H 22 Drug application in liver cancer
[0037] 1 Materials and methods
[0038] 1.1 Experimental materials
[0039] 1.1.1 Preparation of Mylabris: Take the decoction pieces of Chinese herb Mylabris, put them in a decoction container, add cold water equivalent to 5-7 times the amount of medicinal materials, soak for 1-2 hours, boil for 30 minutes, and filter. Add 3-5 times the amount of water to the dregs and continue to decoct, boil for 20 minutes, and filter. The two filtrates were combined, concentrated on a water bath to a liquid medicine equivalent to 0.01g / ml of the original medicinal material, cooled and filled into sterilized medicine bottles, and placed in a 4°C refrigerator for later use.
[0040] 1.1.2 Animals: C57BL / 6 mice, male, 10 weeks old, purchased from Animal Center of Shanghai Chinese Academy of Sciences.
[0041] 1.1.3 Tumor Strain: Liver Cancer H 22 Tumor strains were provided by Shanghai...
Embodiment 2
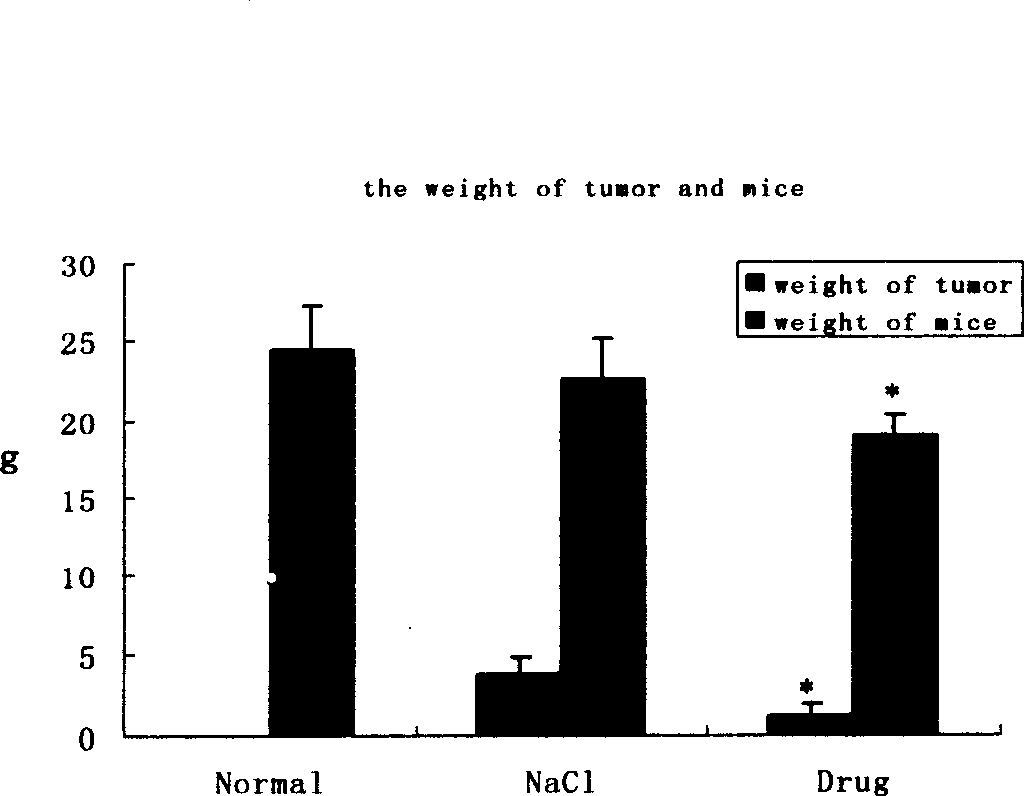
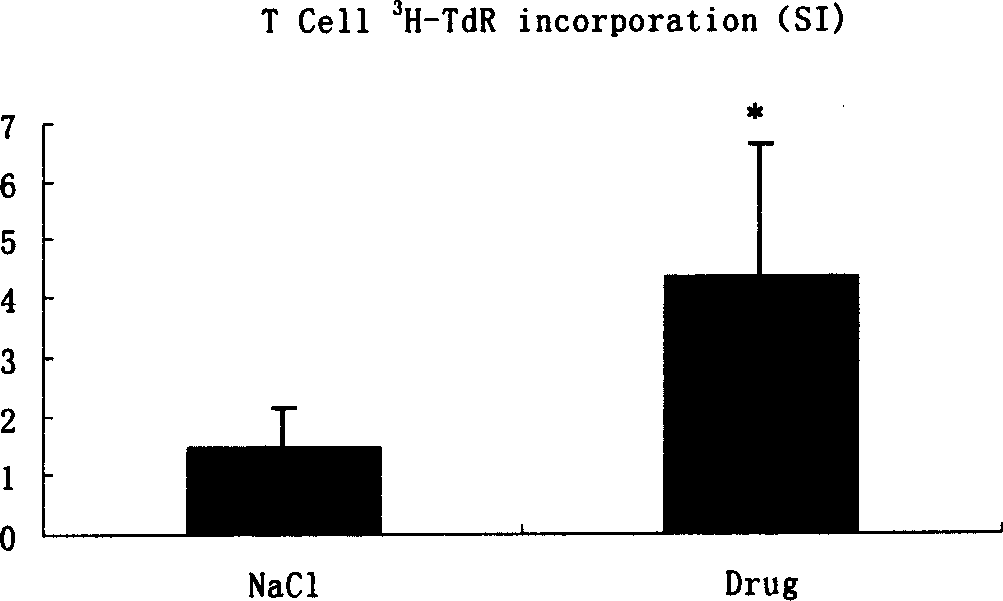
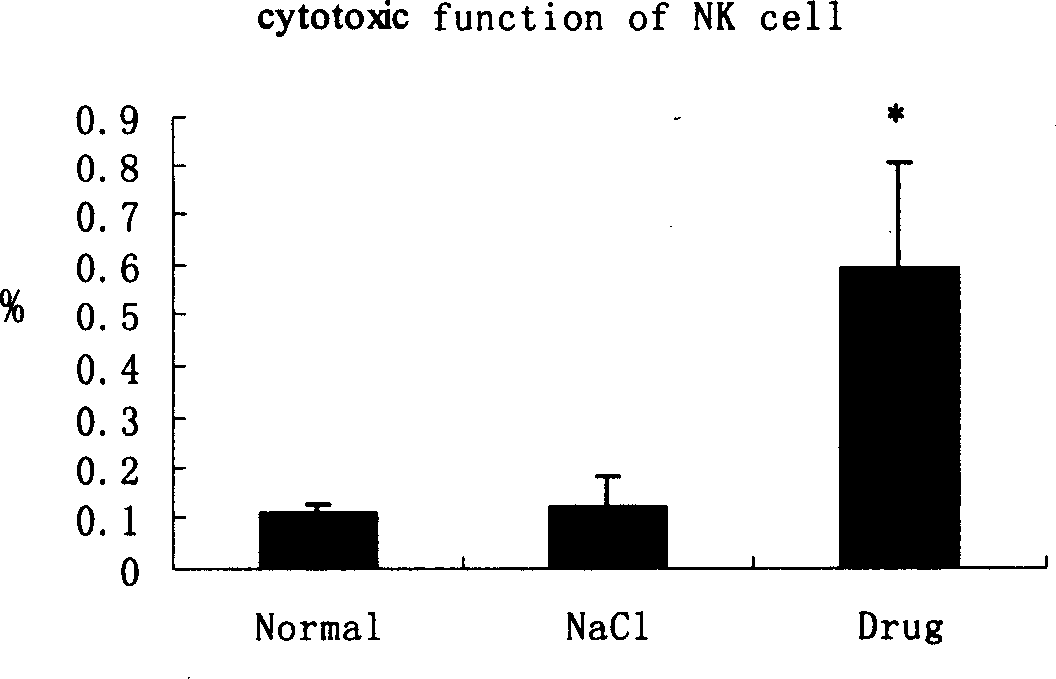
[0067] The cantharidin mixture containing hydroxycantharidin is used in the preparation of treatment or prevention of H 22 Drug application in liver cancer
[0068] 1 Materials and methods
[0069] 1.1 Experimental materials
[0070] 1.1.1 Mylabris mixture: the content of mylabris in the mylabris mixture is 0.02g / ml.
[0071] 1.1.2 Animals: C57BL / 6 mice, male, 10 weeks old, purchased from Animal Center of Shanghai Chinese Academy of Sciences.
[0072] 1.1.3 Tumor Strain: Liver Cancer H 22 Tumor strains were provided by Shanghai Institute of Immunology.
[0073] 1.1.4 Reagents: ConA (Sigma Company), CD3, CD4, CD8 monoclonal antibodies (eBioScience Company), NK cell killing reagents (made by Shanghai Institute of Immunology), 3 H-TdR (Shanghai Institute of Atomic Energy), IFN-γ and IL-4 detection kit (Jingmei Company).
[0074] 1.2 Experimental method
[0075] 1.2.1 Modeling method: Under sterile conditions, take the H 22 Cell line, cells 5×10 6 Inoculated subcutaneousl...
Embodiment 3
[0091] Application of cantharidin in preparation of medicine for treating or preventing systemic lupus erythematosus
[0092] 1. Animals and Materials
[0093] Experimental animals: 60 systemic lupus erythematosus experimental rats, body weight 200±10g, age 160 days, 4 rats per cage, room temperature 23±2°C, humidity 30-50%, refined pellet feed,
[0094] 2. Grouping
[0095] We randomly divided the above 60 experimental mice with systemic lupus erythematosus into two corticosteroid treatment groups of 30 cases: this group was injected with corticosteroids at a dose of 0.01 g per day for 15 consecutive days.
[0096] 30 cases in the mylabris treatment group: this group was given 0.01 g / ml of mylabris water decoction, and 0.2 ml / piece was given by intragastric administration, and the administration was continued for 15 days.
[0097] 3. Observation
[0098] Mylabris treatment group: the days needed to improve the main clinical symptoms of fever, joint swelling and pain, skin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com