A kind of chimeric antigen receptor for the treatment of liver cancer and its application
A chimeric antigen receptor and carrier technology, which is applied in liver cancer vaccines, antibody mimics/scaffolds, and introduction of foreign genetic material using carriers, which can solve the problems of increasing CAR-T cells, toxic side effects, and insufficient sustainable viability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Example 1. Preparation of CAR-T cells
[0155] 1. Construction of retroviral vector
[0156] 1. Optimization of the full-length cDNA sequence of wild-type human IFNα2b gene
[0157] The full-length sequence of wild-type human IFNα2b gene cDNA is called nIFNα2b. In order to make nIFNα2b more suitable for expression in human cells, under the condition that the amino acid sequence encoded by nIFNα2b remains unchanged, the codon optimization of nIFNα2b sequence was carried out on the website http: / / sg.idtdna.com / site to obtain oIFNα2b, The nucleotide sequence of oIFNα2b is shown in 2701-3264 of SEQ ID NO.1.
[0158] 2. Design and synthesis of GPC3-CAR-IFN gene sequence
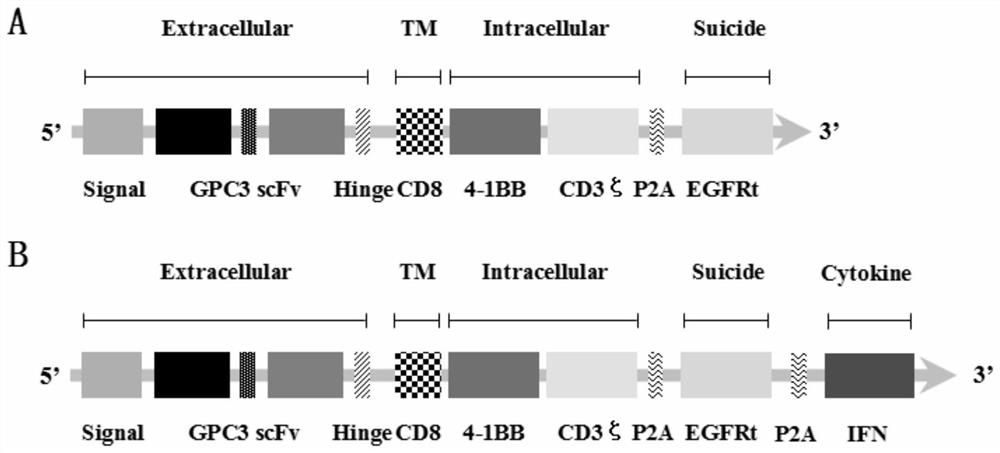
[0159] The GPC3-CAR-IFN gene sequence sequentially includes the coding gene sequence of human CD8 leader peptide, the coding gene sequence of GPC3 scFv, the coding gene sequence of human CD8 hinge transmembrane region, the coding gene sequence of human 4-1BB intracellular region, the coding gene sequence...
Embodiment 2
[0192] Example 2, CFSE labeling method to detect the specific killing effect of CAR-T cells on tumor cells
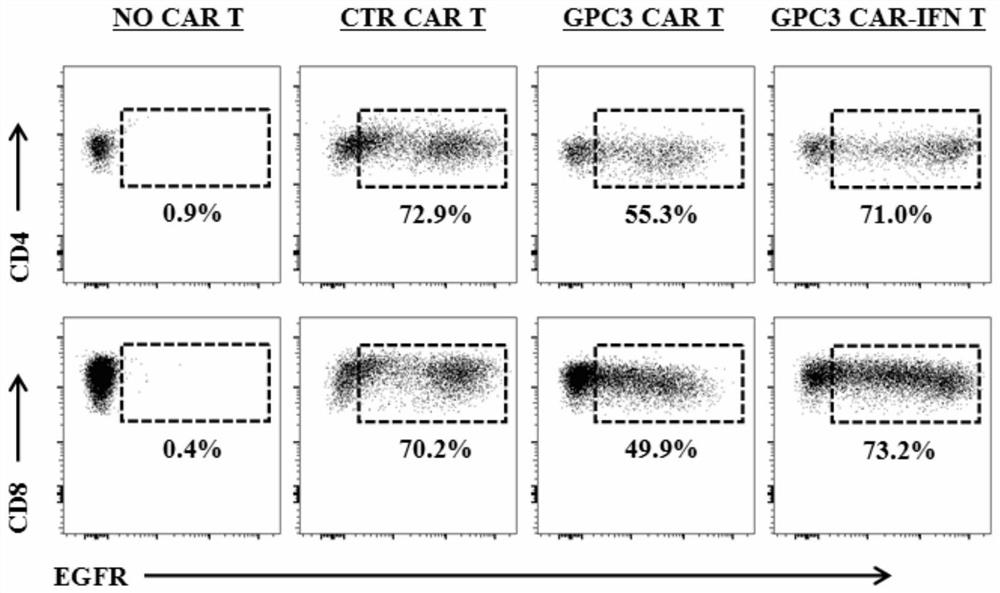
[0193] CFSE (CFDA-SE) is a cell staining reagent that can fluorescently label living cells. It can easily penetrate the cell membrane, covalently bind to intracellular proteins in living cells, and release green fluorescence after hydrolysis. The principle of CFSE labeling living cells can be used to label and quantify tumor cells, so as to detect the killing efficiency of CAR-T cells on tumor target cells. The specific method is: the target cells are equally divided into two groups and adjusted to the same cell density. Stained with low concentration and high concentration of CFSE respectively, wherein high concentration stained target cells and non-stained immune cells were co-cultured according to a certain ratio. After a period of incubation, the high-concentration stained tube of target cells (along with immune cells) was mixed in equal volume with the low-concent...
Embodiment 3
[0207] Example 3. Tumor transplantation model to detect the tumor killing effect of CAR-T cells in animals
[0208] Experimental materials: B-NDG severe combined immunodeficiency mice aged 5-6 weeks and weighing 18-22 g (Biocytogen Biotechnology Co., Ltd.).
[0209] Experimental grouping: The experimental materials were randomly divided into 3 experimental groups, with 5 mice in each group. The specific treatment methods for each group are as follows:
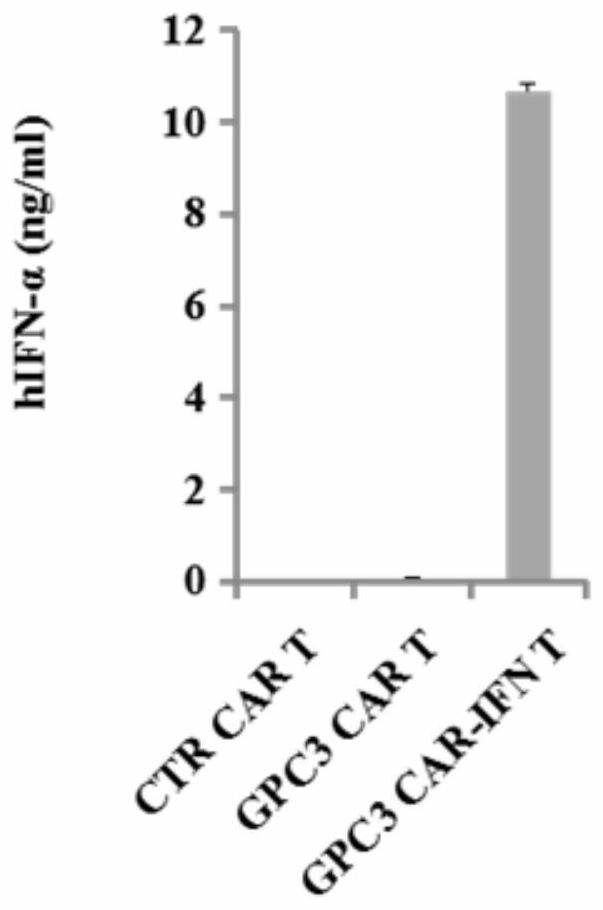
[0210] GPC3 CAR-IFN T group: B-NDG severe combined immunodeficiency mice were subcutaneously inoculated with HepG2 tumor cell solution (solvent is PBS), the inoculation volume was 0.3ml (containing 1×10 7 HepG2 cells). Five days after tumor cell inoculation, the GPC3 CAR-IFN T cell solution (solvent is PBS) prepared in Example 1 was injected into the mouse tail vein, and the injection volume was 0.2ml (containing 5×10 6 GPC3 CAR-IFN T cells).
[0211] GPC3 CAR T group: B-NDG severe combined immunodeficiency mice were subcut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com