Chimeric antigen receptor for targeting CD19 and interferon synergy and application of chimeric antigen receptor
A single-chain antibody and fusion protein technology, which is applied in interferon, targeted specific cell fusion, medical preparations containing active ingredients, etc., can solve toxic and side effects, increase the dosage of CAR-T cells, and prevent tumor recurrence And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
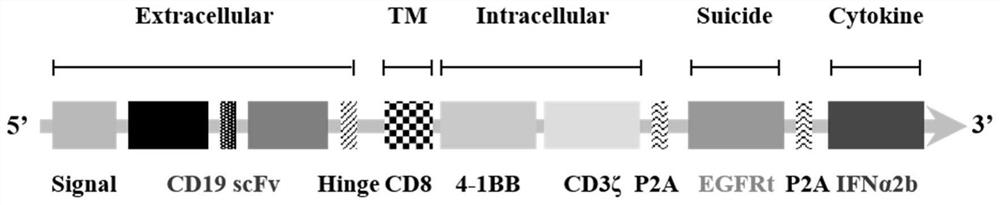
[0064] Example 1: Determination of CD19-CAR-EGFRt-IFNα2b gene sequence and construction of retroviral vector
[0065] The full-length cDNA sequence information of human CD8 hinge transmembrane region, human 4-1BB intracellular region, human CD3ζ intracellular region, EGFRt and human IFNα2b was searched from the NCBI website database. The full-length sequence of wild-type human IFNα2b gene cDNA is called nIFNα2b. The nIFNα2b sequence was codon-optimized on the website http: / / sg.idtdna.com / site to obtain oIFNα2b, ensuring that it is more suitable for expression in human cells when the encoded amino acid sequence remains unchanged.
[0066] According to the sequence of CD19 scFv, human CD8 hinge transmembrane region, human 4-1BB intracellular region, human CD3ζ intracellular region, human P2A peptide, EGFRt, human P2A peptide, oIFNα2b, the full-length polynucleoside of CD19-CAR-EGFRt-IFNα2b was obtained acid sequence. At the same time, the full-length polynucleotide sequence of...
Embodiment 2
[0071] Embodiment 2: Retrovirus packaging and establishment of toxin-producing strains
[0072] Using the retroviral vector comprising CD19-CAR-EGFRt-IFNα2b and CD19-CAR-EGFRt prepared in Example 1, the two retroviruses were packaged according to the following method:
[0073] 1. Day 1: Phoenix Ecotropic (ECO) cells should be less than 20 passages and not overgrown. Take 0.6×10 6 / ml cell density plating, add 10ml DMEM medium to a 10cm dish, mix the cells well, and culture overnight at 37°C;
[0074] 2. Day 2: ECO cell confluence reaches about 90% for transfection (usually about 14-18 hours after plating); prepare plasmid MP71-target gene 12.5μg, 1.25M CaCl 2 250 μl, H 2 O 1ml, the total volume is 1.25ml; add 2× HBS equal to the volume of the plasmid complex in another tube, and vortex for 20 seconds while adding the plasmid complex. Gently add the mixture to the ECO dish along the side, incubate at 37°C for 4 hours, remove the medium, wash with PBS, and add pre-warmed f...
Embodiment 3
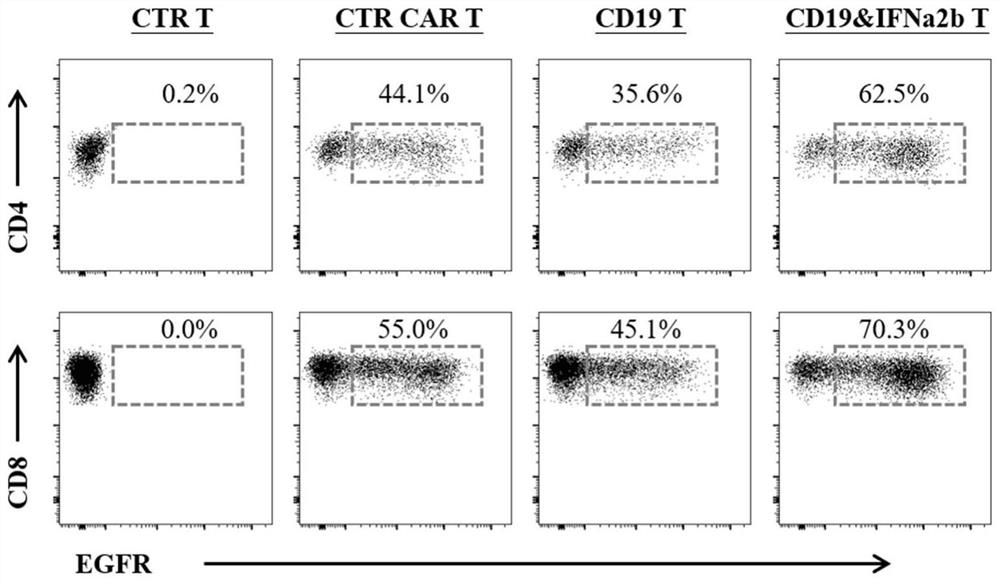
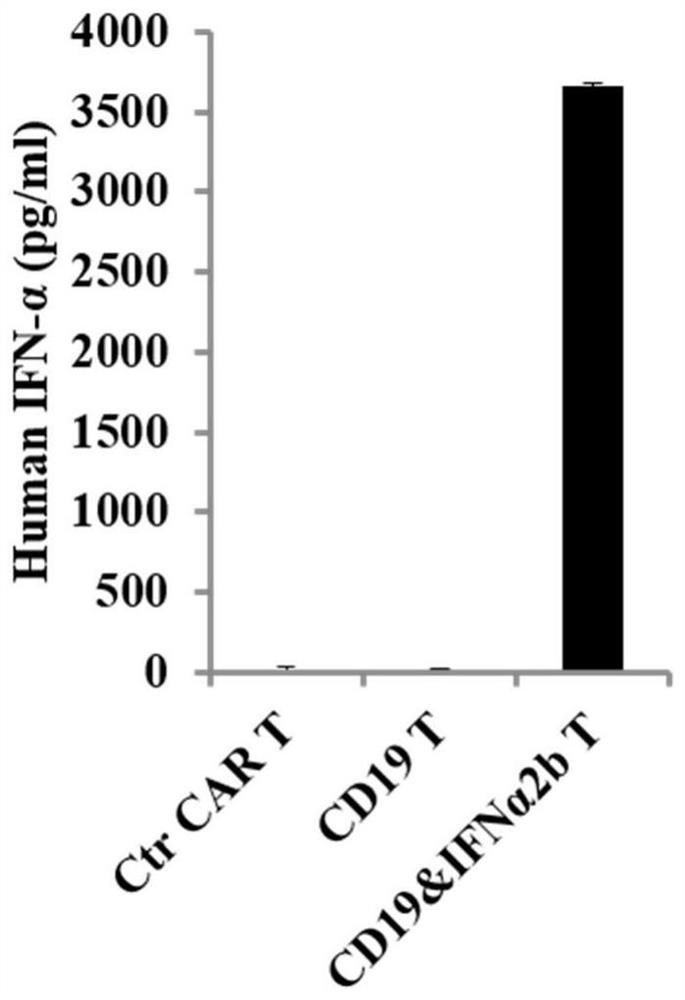
[0077] Example 3: Retrovirus infection of human T cells
[0078] 1. Throw frozen PBMCs from healthy human peripheral blood, and adjust the cell density to 1-2×10 with RPMI-1640 complete medium containing 10% FBS 6 / ml.
[0079] 2. Ficoll separation solution (Tianjin Haoyang) was used to collect PBMC, and the magnetic bead method was used to obtain relatively pure CD3 + T cells, by magnetic beads: CD3 + T cells were activated by adding clinical grade Dynabeads Human T Expander CD3 / CD28 magnetic beads (Invitrogen) at a cell ratio of 3:1.
[0080] 3. On the second day after T cell activation, coat non-tissue-treated culture plates with Retronectin (Takara) diluted in PBS to a final concentration of 15 μg / ml, 1.2 ml per well of a 6-well plate. Protected from light, overnight at 4°C for later use.
[0081] 4. After T cell activation and culture for two days, take out the coated 6-well plate, discard the coating solution, and add PBS to wash the plate once.
[0082] 5. Add the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com