A bispecific antibody for multiple myeloma and its application
A bispecific antibody and antibody technology, applied in the fields of antibody, application, fermentation, etc., can solve problems such as the complex structure of bispecific antibodies, and achieve the effect of enhancing the killing function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
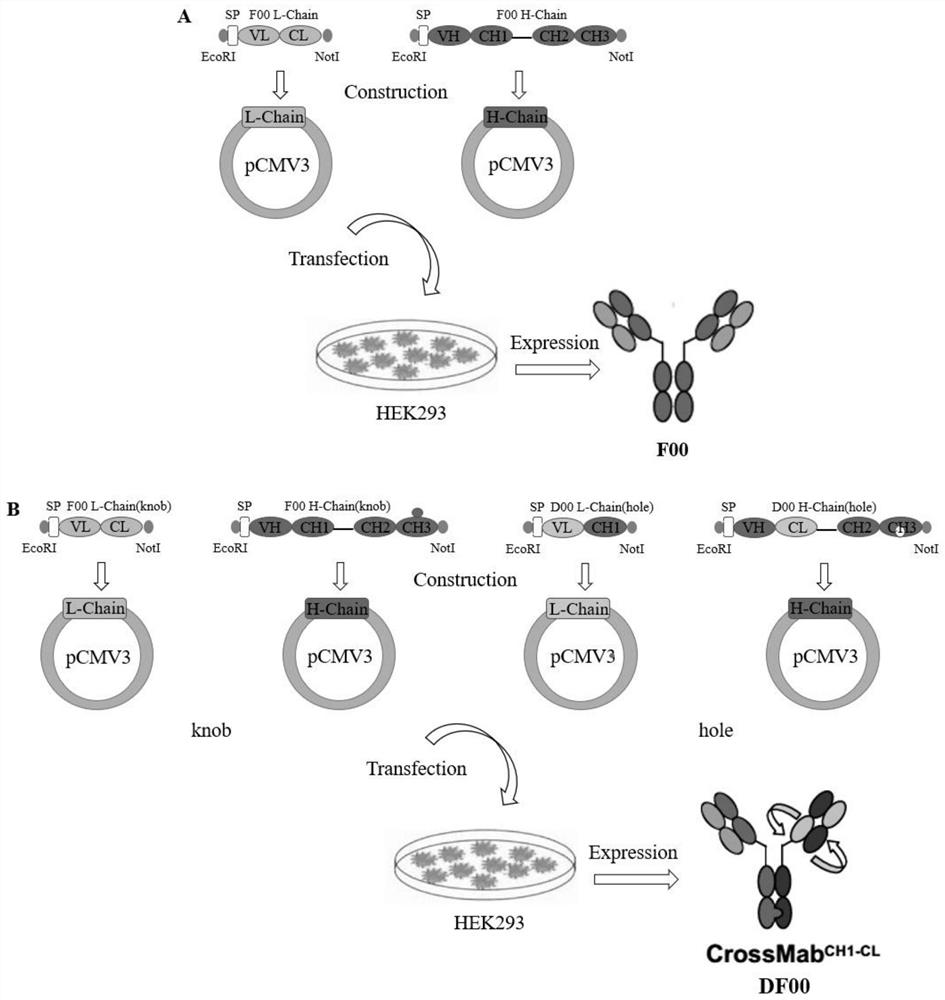
[0035] Example 1 Construction of bispecific antibody DF00.
[0036] First, the BCMA-targeting single-chain antibody 2A9 and the PD-1-targeting antibody D00, which were screened by the laboratory through phage display technology and optimized by the company, were used as templates to design primers to retrieve the heavy and light chain variable region genes. The IgG4 constant region (Fc segment is mutated by knobs-into-holes, Fab segment is modified by Crossover) gene is used as a template to design primers to retrieve the heavy and light chain constant region genes, and the variable regions and constant regions of the heavy and light chains are respectively passed through overlap PCR technology The heavy and light chain genes of bispecific antibody DF00 were constructed by ligation; the obtained PCR product and pCMV3 vector were double digested with EcoRI and NotI respectively, and after recovering the target fragment and double digested vector, T4 DNA ligase was ligated at 16 ...
Embodiment 2
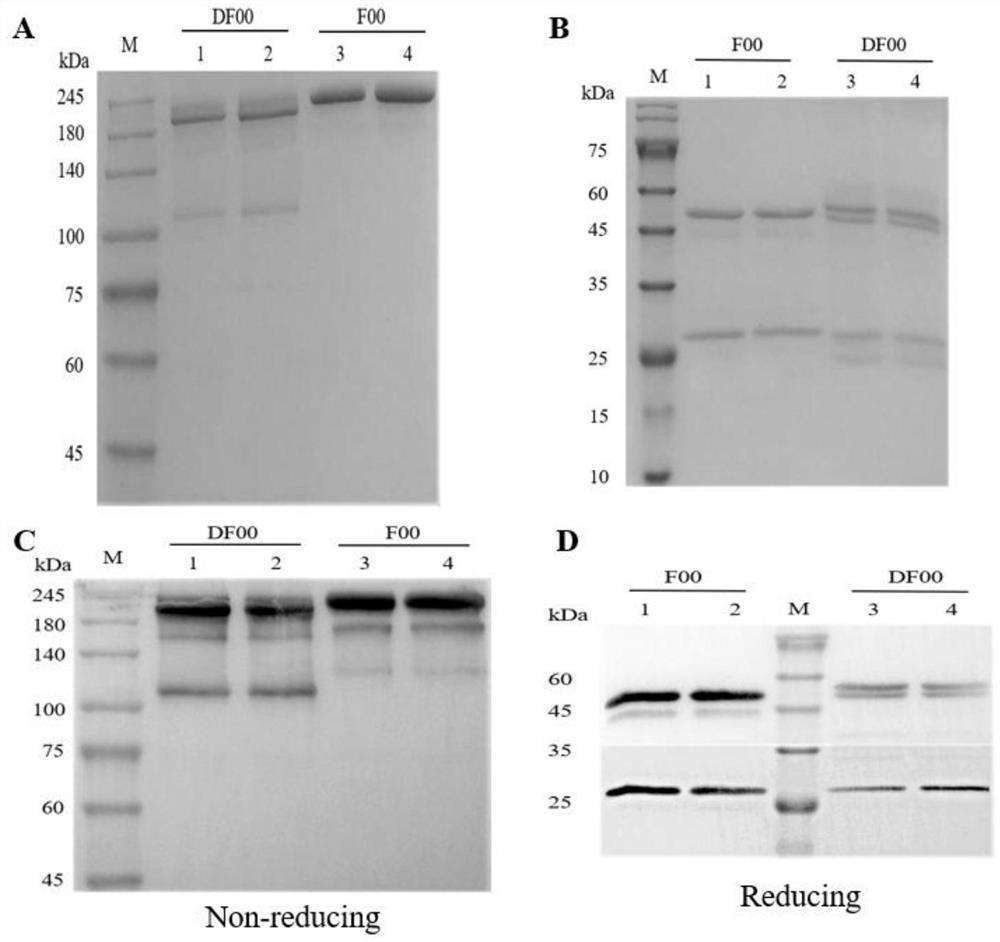
[0037] Example 2 Expression, purification and identification of bispecific antibody DF00.
[0038] First, the four recombinant plasmids pCMV3-F00-H / L-Chain (knob end) and pCMV3-D00-H / L-Chain (hole end) were transfected into HEK293 cells by PEI transfection reagent in a certain proportion. The medium was changed daily and the cells were passaged, and the scale of cell fermentation was gradually expanded. The cell culture medium was collected, and the samples were filtered with a 0.22 μm filter membrane and then purified by ProteinA column affinity chromatography to obtain a large amount of the target protein. 8% non-reducing and 12% reducing SDS-PAGE electrophoresis were performed respectively, and the molecular weight and assembly were identified by Western blotting, and the target protein was preliminarily verified. see the results figure 2 , F00 and DF00 were successfully expressed and assembled correctly.
Embodiment 3
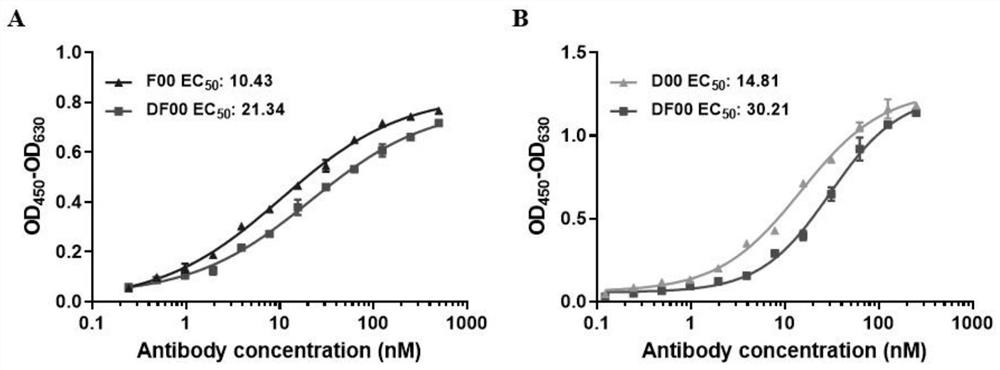
[0039] Example 3 The binding affinity of DF00 to BCMA and PD-1 was verified by ELISA analysis.
[0040]The antigens BCMA and PD-1 were first mixed with 50mM NAHCO 3 The buffer was diluted to 1 μg / ml and coated on the activated ELISA plate overnight at 4°C; after washing with PBS to remove the antigen not bound to the ELISA plate, 5% skim milk was blocked at 37°C for 2h; the plate was washed again with PBST Then start to incubate a series of diluted antibodies (500nM-0.244nM / 250nM-0.122nM) at 37°C for 2h; then wash the plate with PBST again, wash away the antibodies that are not bound to the antigen, and use HRP-labeled goat antibody Human IgG (H+L) was incubated at 37°C for 2h; after washing the plate with PBST, the color was developed with TMB chromogenic solution. When there was an obvious color gradient, the reaction was terminated with 2M dilute sulfuric acid, and the OD was detected by a microplate reader. 450 -OD 630 value as the final result. see the results image ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com