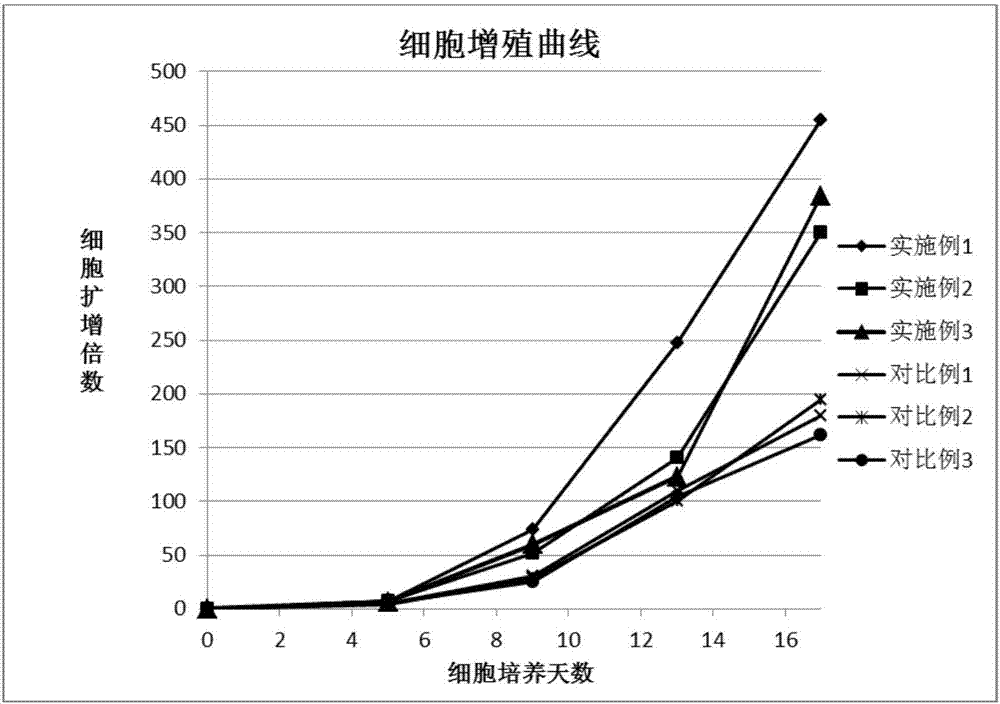
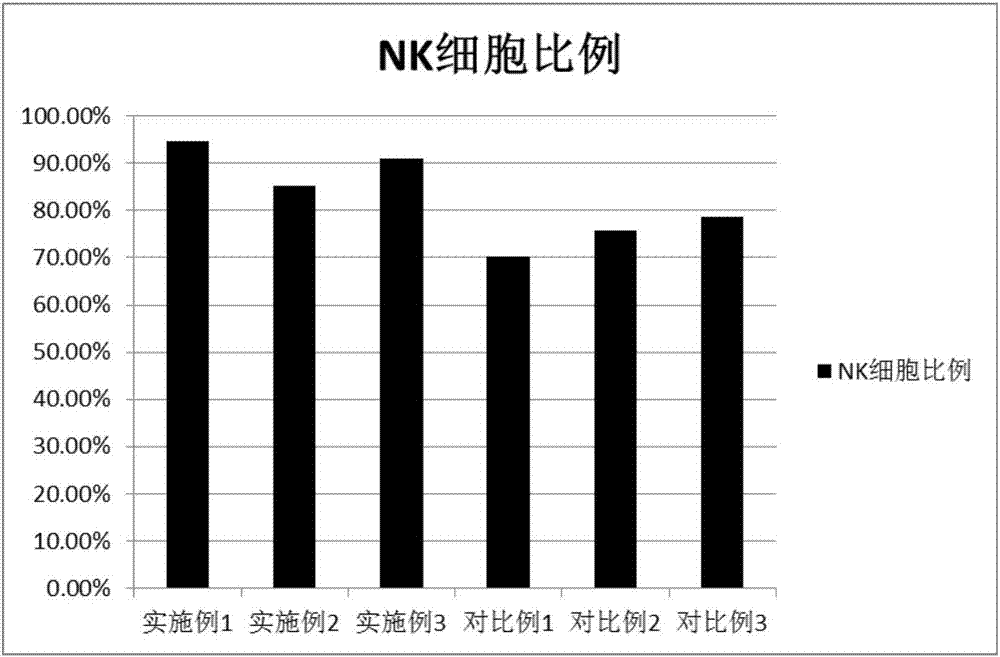
Peripheral blood NK cell in-vitro efficient expansion method
An NK cell and in vitro expansion technology, applied in the field of cell culture, can solve the problems of hidden safety hazards, complicated operation, high cost, etc., and achieve the effect of enhancing killing ability and meeting clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Coating of culture flasks: respectively, 25 micrograms of CD16 monoclonal antibody, 25 micrograms of CD56 monoclonal antibody, and 50 nanograms of CD3 monoclonal antibody were dissolved in 5 ml of PBS and fully dissolved, and added to a culture flask with a bottom area of 75 square centimeters, 4 ℃ overnight in the dark;
[0035] 2) Cell inoculation: a) Peripheral blood 40ml, whole blood centrifuged at 700g for 15min; b Autologous plasma preparation: take 21ml of upper plasma, inactivate at 56°C for 30min, let stand at 4°C for 15min, centrifuge at 900g for 15min, supernatant is autologous plasma Standby; c take about 5ml of the middle buffy coat (mononuclear cells) into 15ml of PBS, dilute and mix; d then slowly add the diluted monocytes to the surface of the lymphatic separation solution to make the gap between the buffy coat and the fence separation solution There are clear layers between them, the ratio of lymph separation liquid to mononuclear cell liquid is 3:...
Embodiment 2
[0043] In order to verify whether the combination of lower and higher concentrations of cytokines will affect the proliferation and purity of NK cells, this experiment set up two sets of examples, the first set of NK cell induction culture process in Example 2 CD3 monoclonal antibody, CD16 monoclonal antibody , the concentration of CD56 monoclonal antibody is different from Example 1, and the lower concentration in the combination is used in Example 2, wherein the final concentration of CD3 monoclonal antibody is 1ng / ml, the concentration of CD16 monoclonal antibody is the final 5μg / ml, and the final concentration of CD56 monoclonal antibody is 5μg / ml. The final concentration of antibody was 5 μg / ml. The CD3 monoclonal antibody, CD16 monoclonal antibody, and CD56 monoclonal antibody concentration in the NK cell induction culture process of the second group of Example 3 are also different from those in Example 1, and those used in Example 3 are higher concentrations in the combi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com