Patents
Literature
1935 results about "Co-Culture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A mixture of two or more different kinds of cells that are grown together.
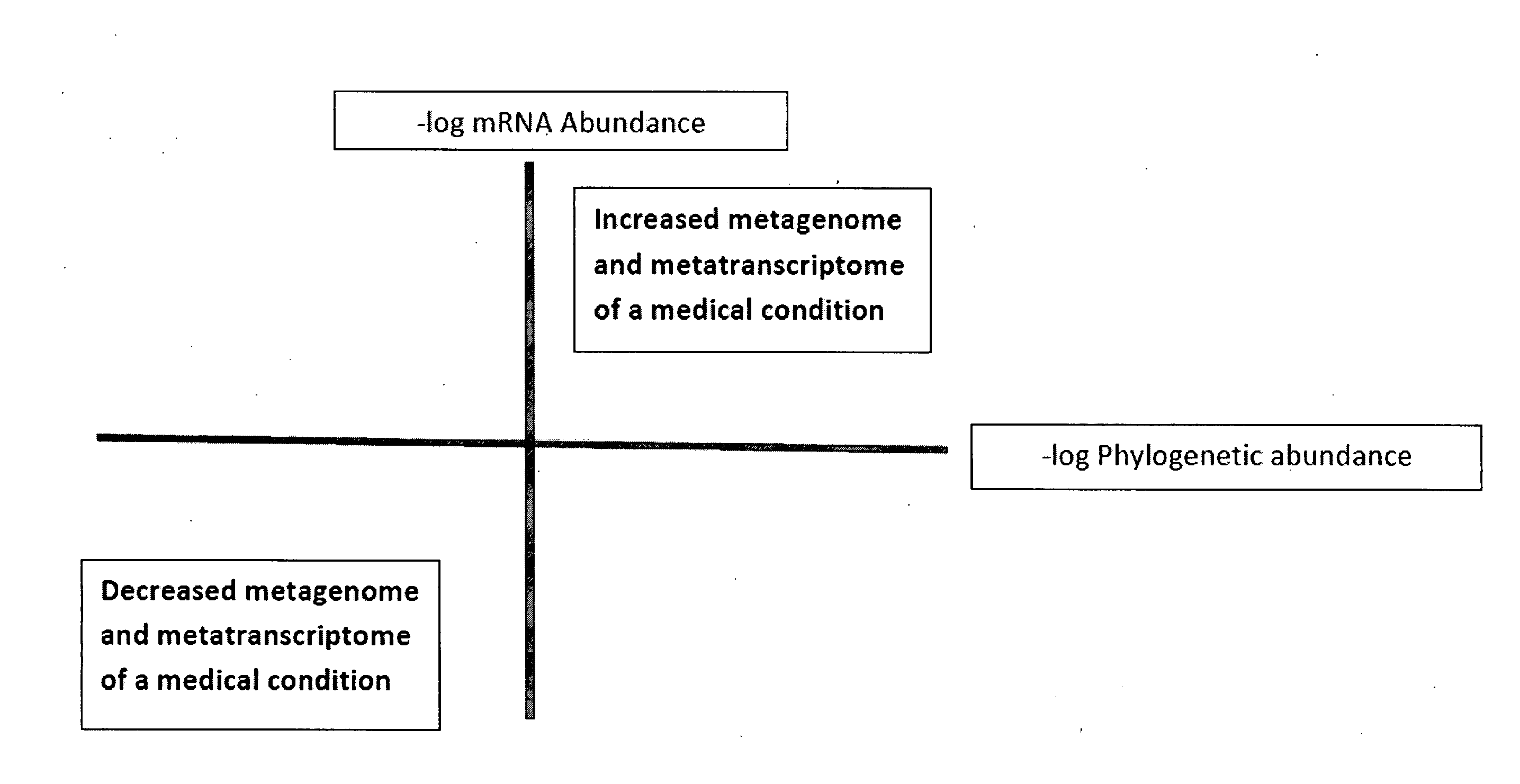
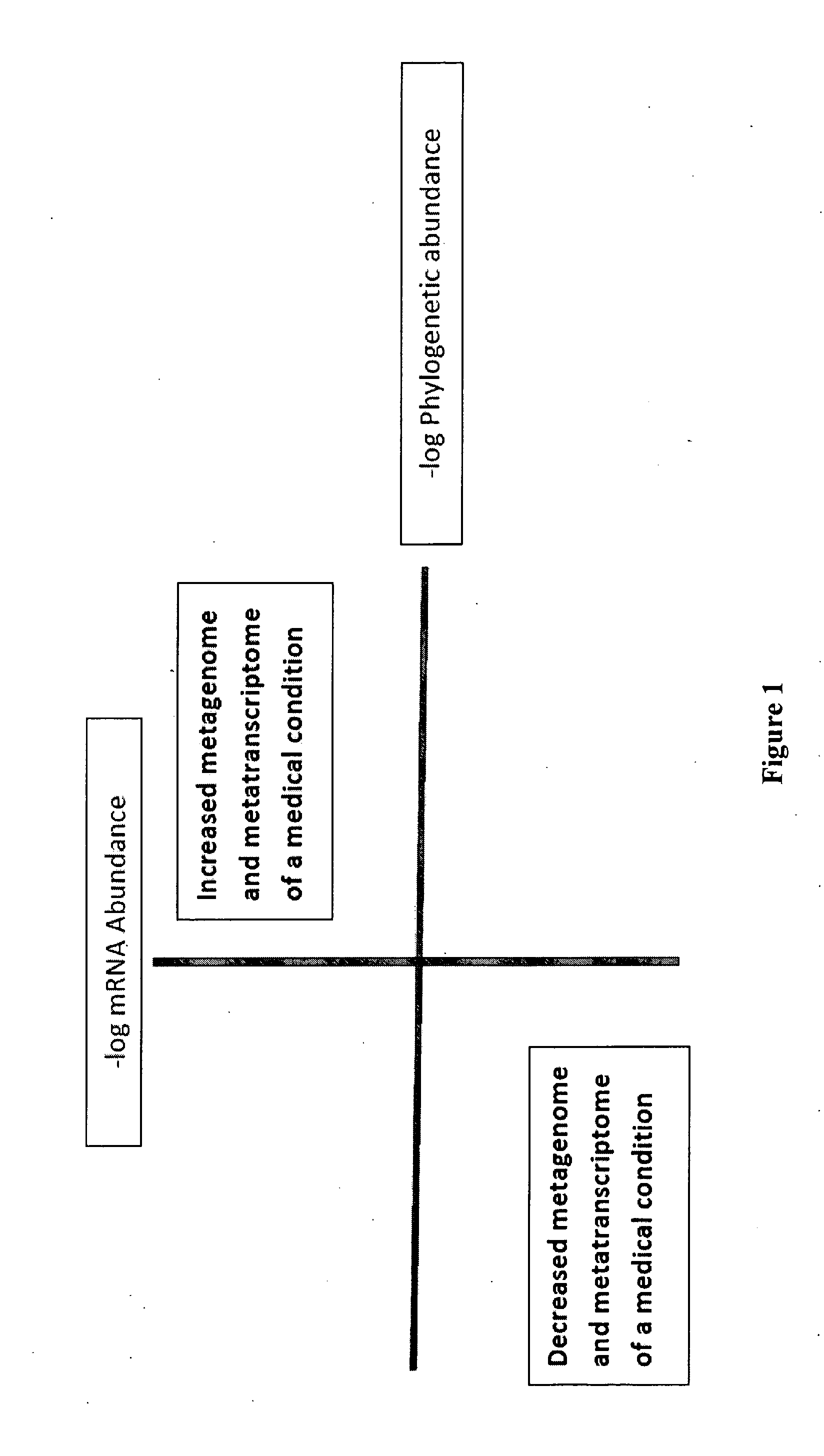
Methods of combining metagenome and the metatranscriptome in multiplex profiles
The present invention describes changes in bacterial gastrointestinal, cutaneous and nasal microbiota associated various mammalian medical conditions. Described are diagnostic tests that arise from combining phylogenetic information about the families, genus, and species of the microbiome and their relative abundance with the metabolic information contained in the metatranscriptome to determine the presence and absence of a disease or medical condition. Provided are compositions of bacteria, co-cultures of bacteria and a carrier for use in treating the disclosed medical conditions. The described compositions restore or correct disease- or medical condition-related imbalances in the microbiome profile with culture-conditioned formulations in which the transcriptome activity of the administered organisms is optimized. Alternatively, formulations of metabolites that drive changes in the metatranscriptome native to the mammal that treat disease or a medical condition or restore health are taught.
Owner:ENSISHEIM PARTNERS
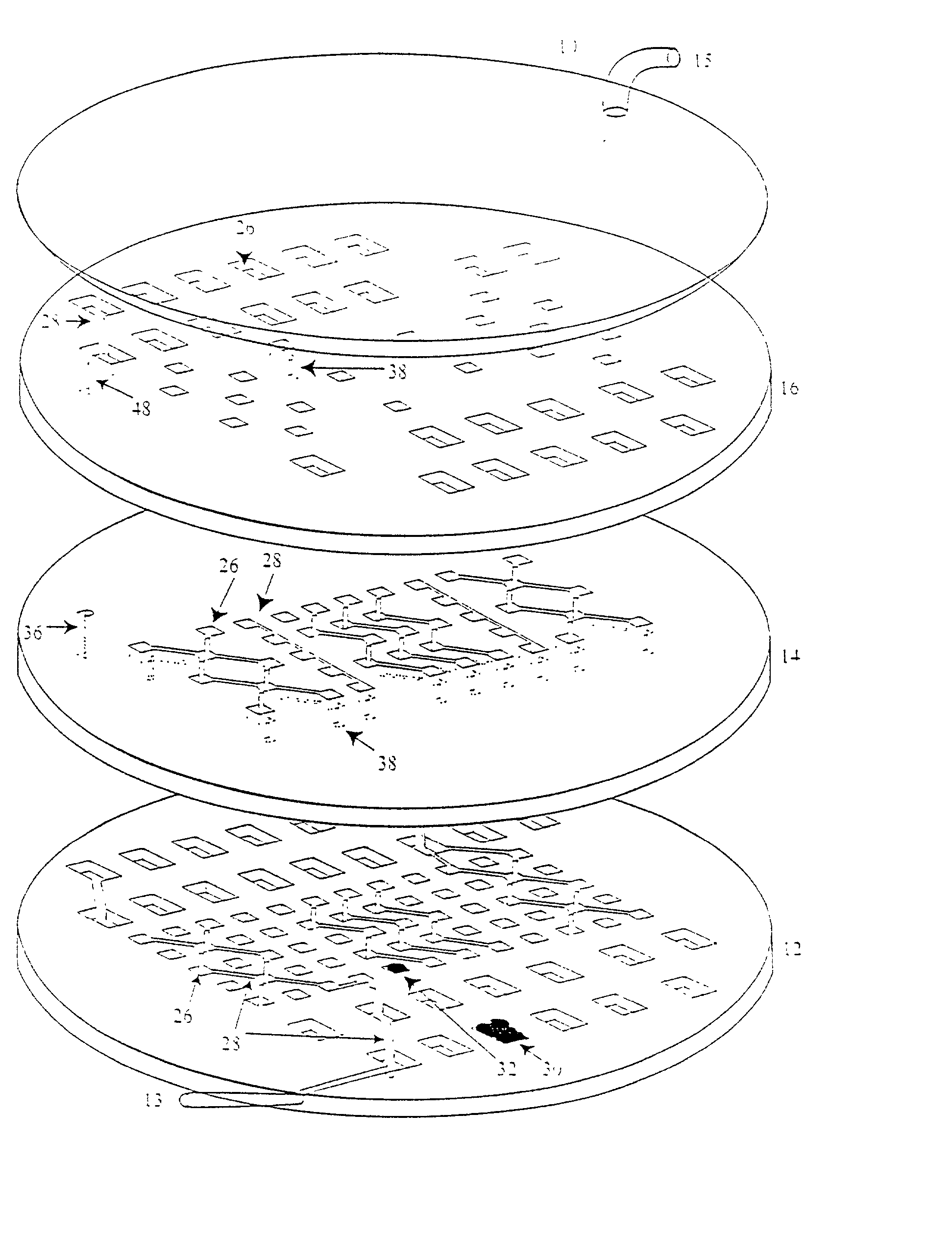
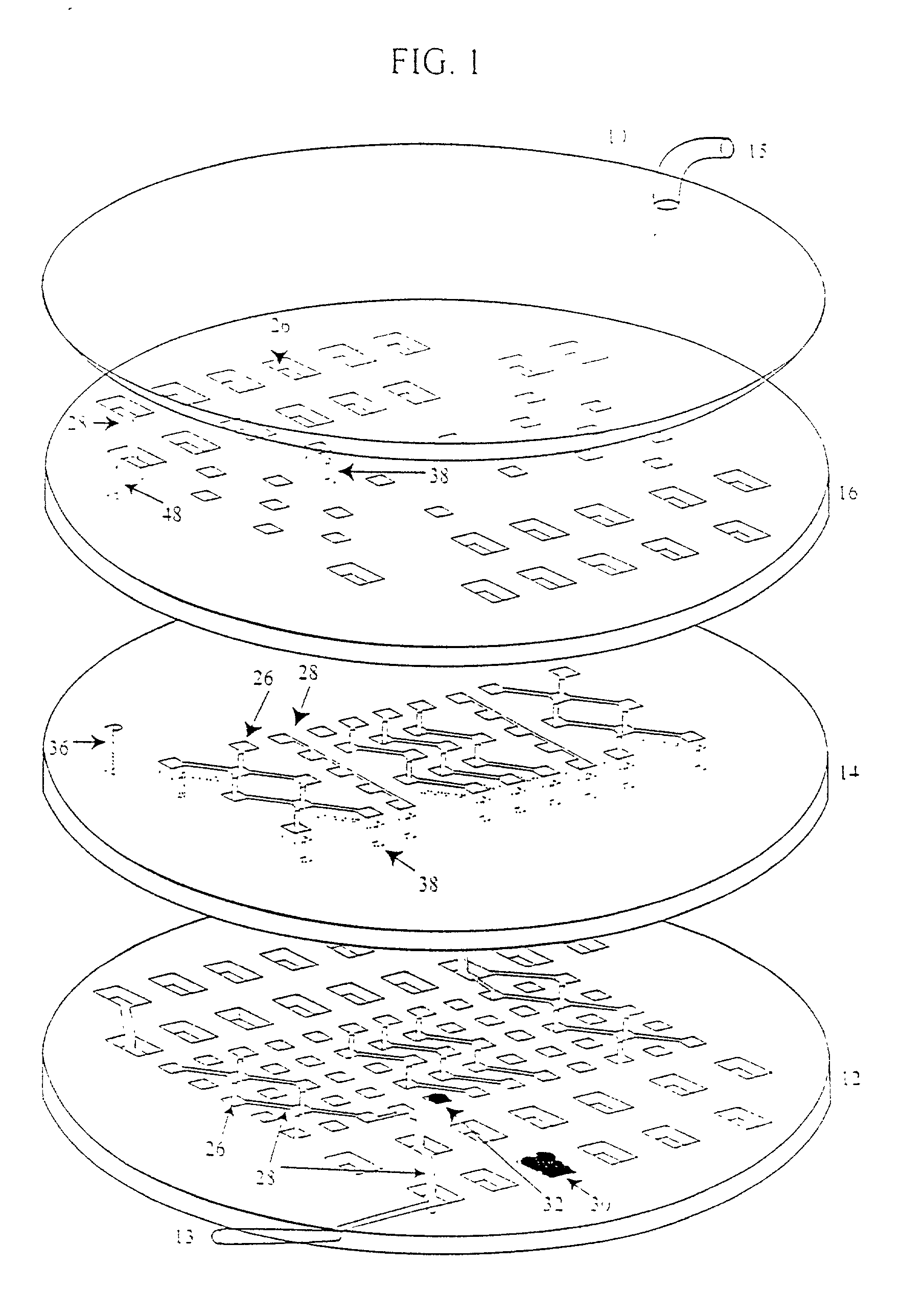
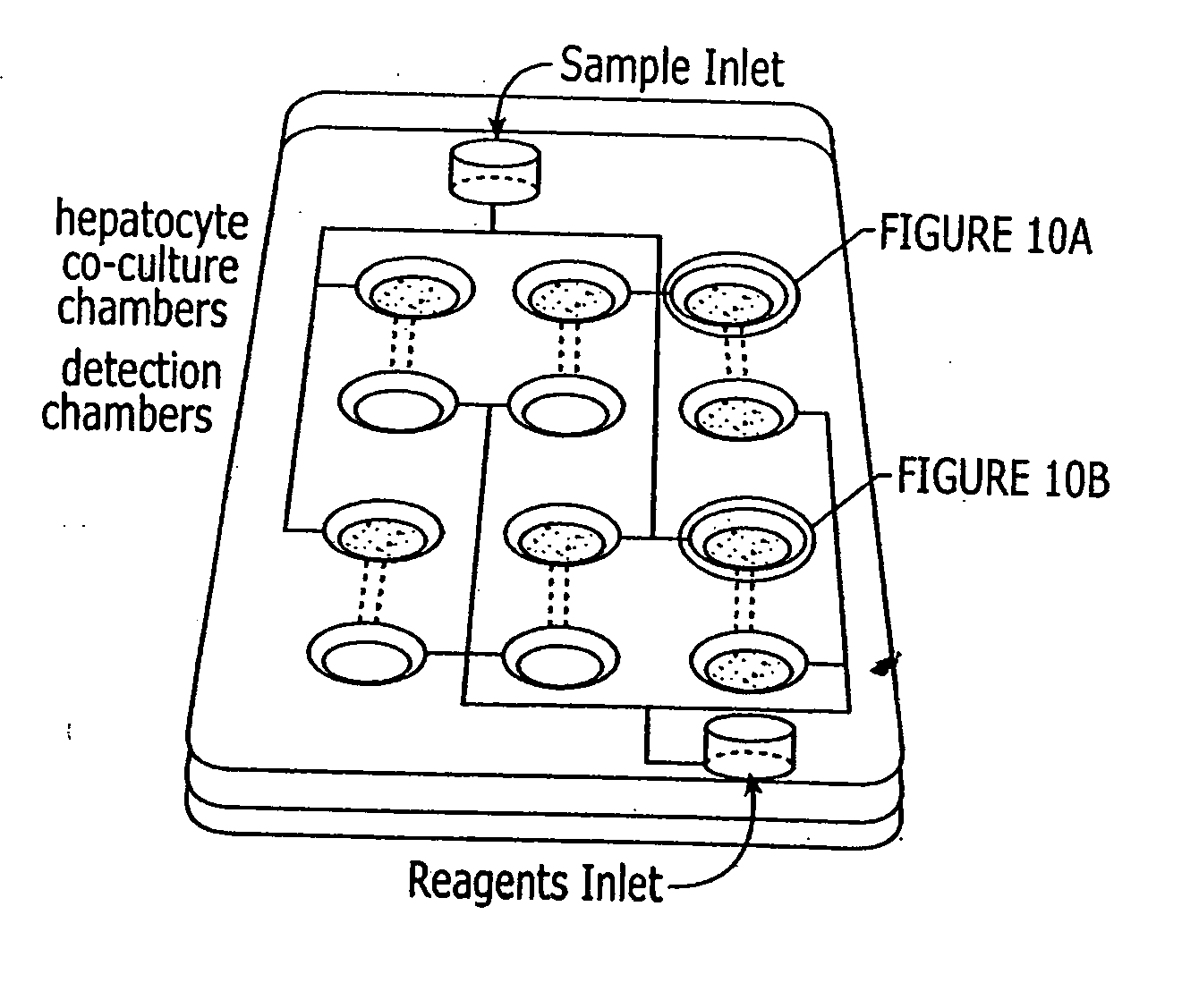
Device and method or three-dimensional spatial localization and functional interconnection of different types of cells
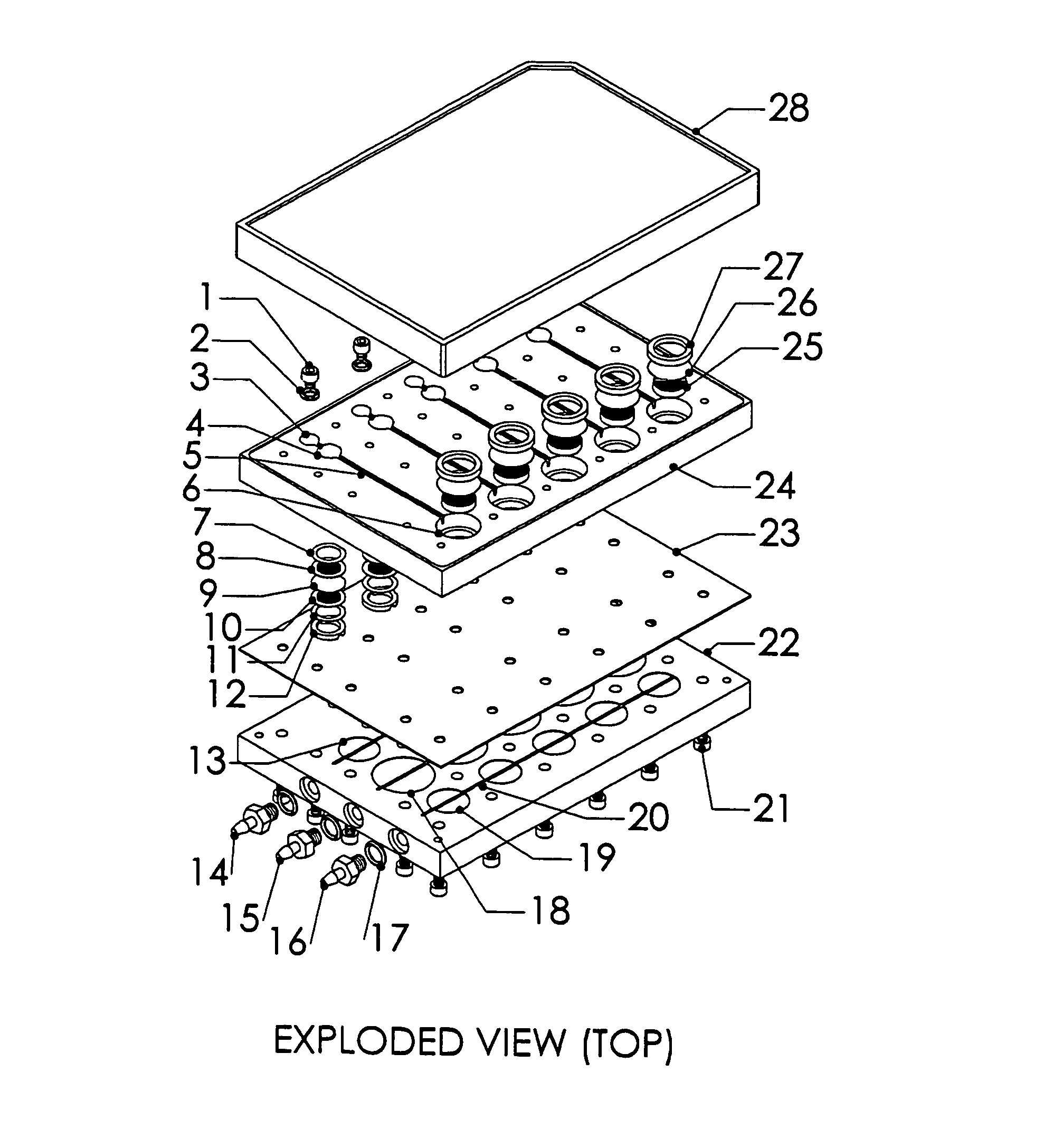
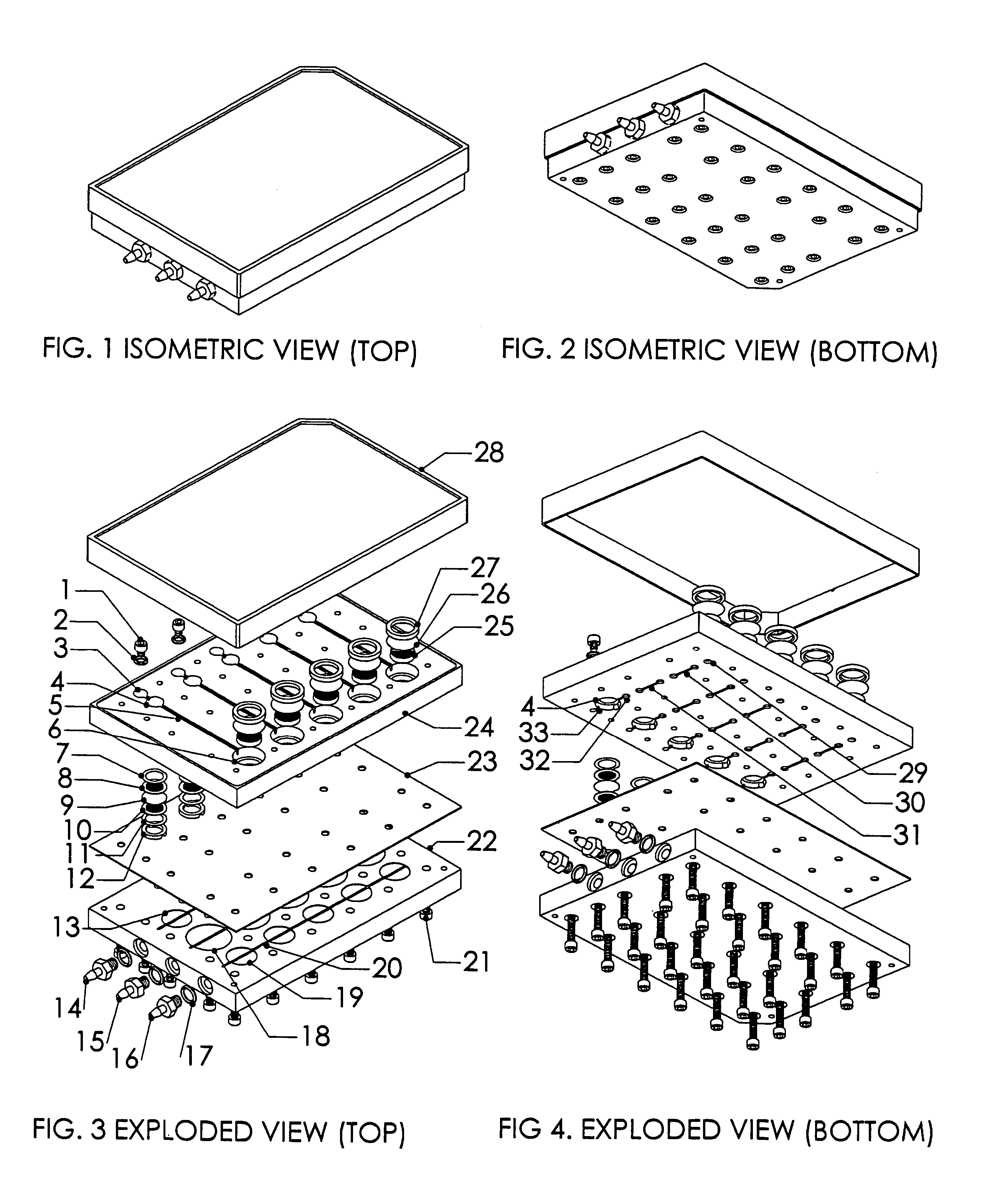
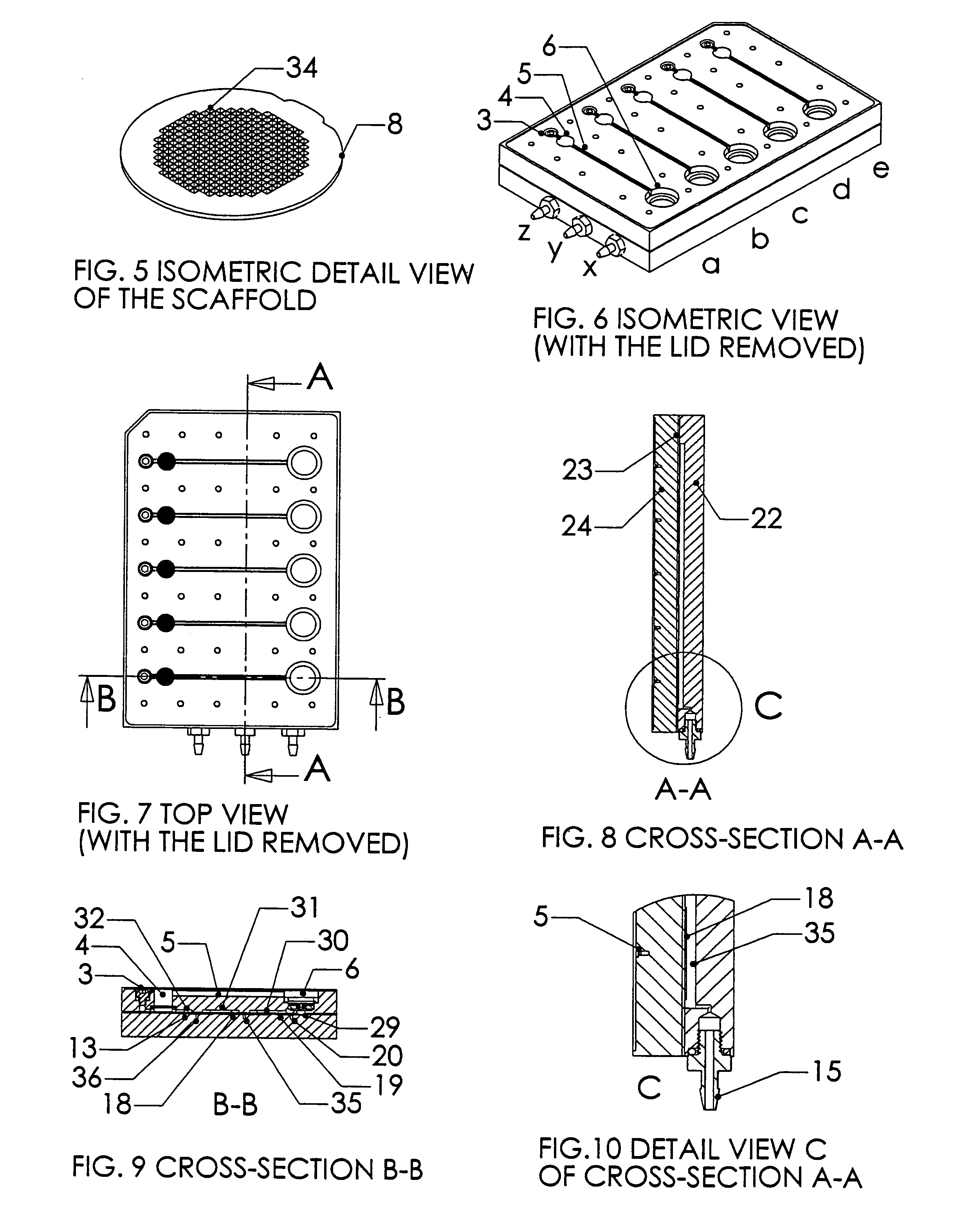
InactiveUS20020173033A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSpatial OrientationsMetabolite
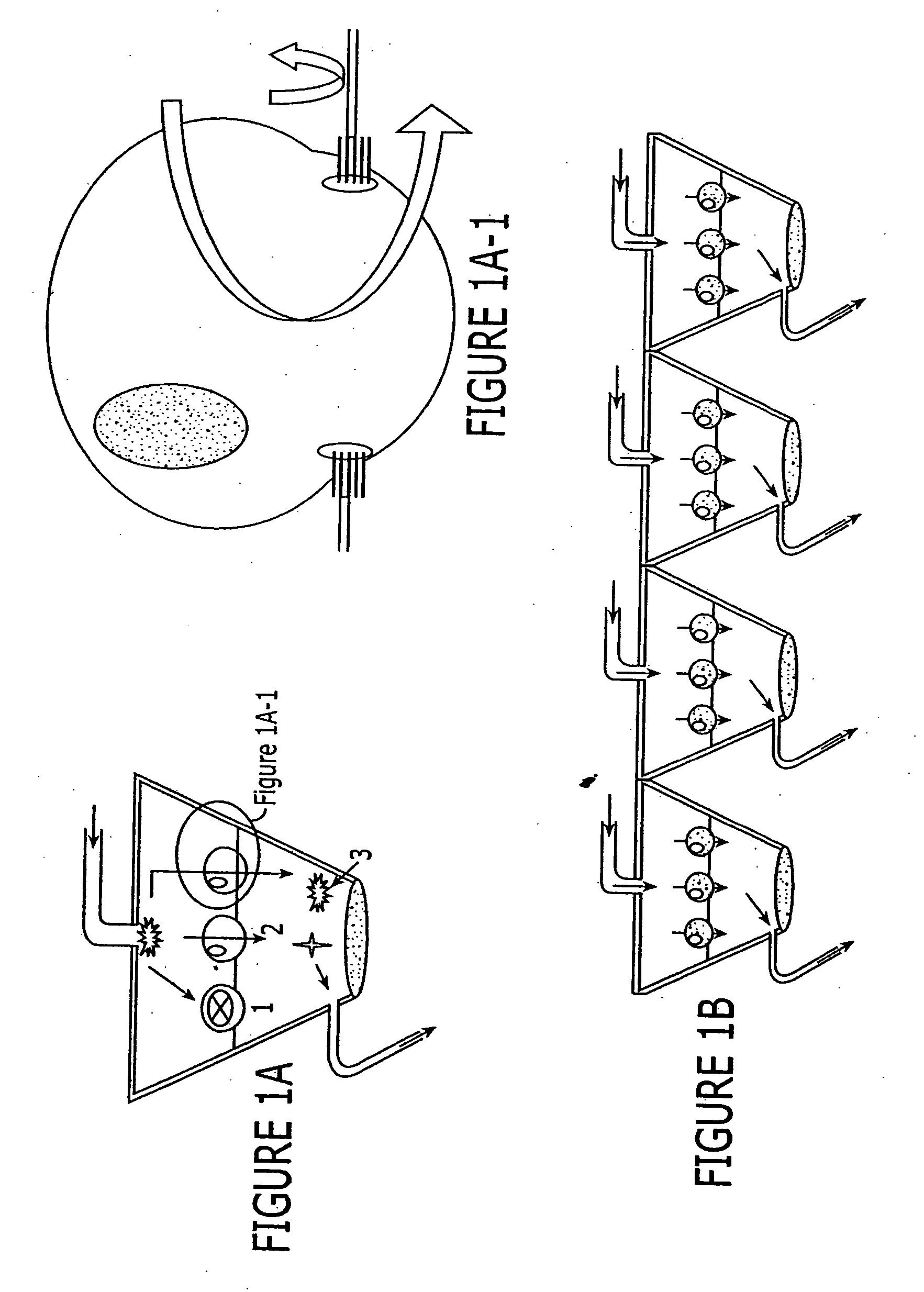
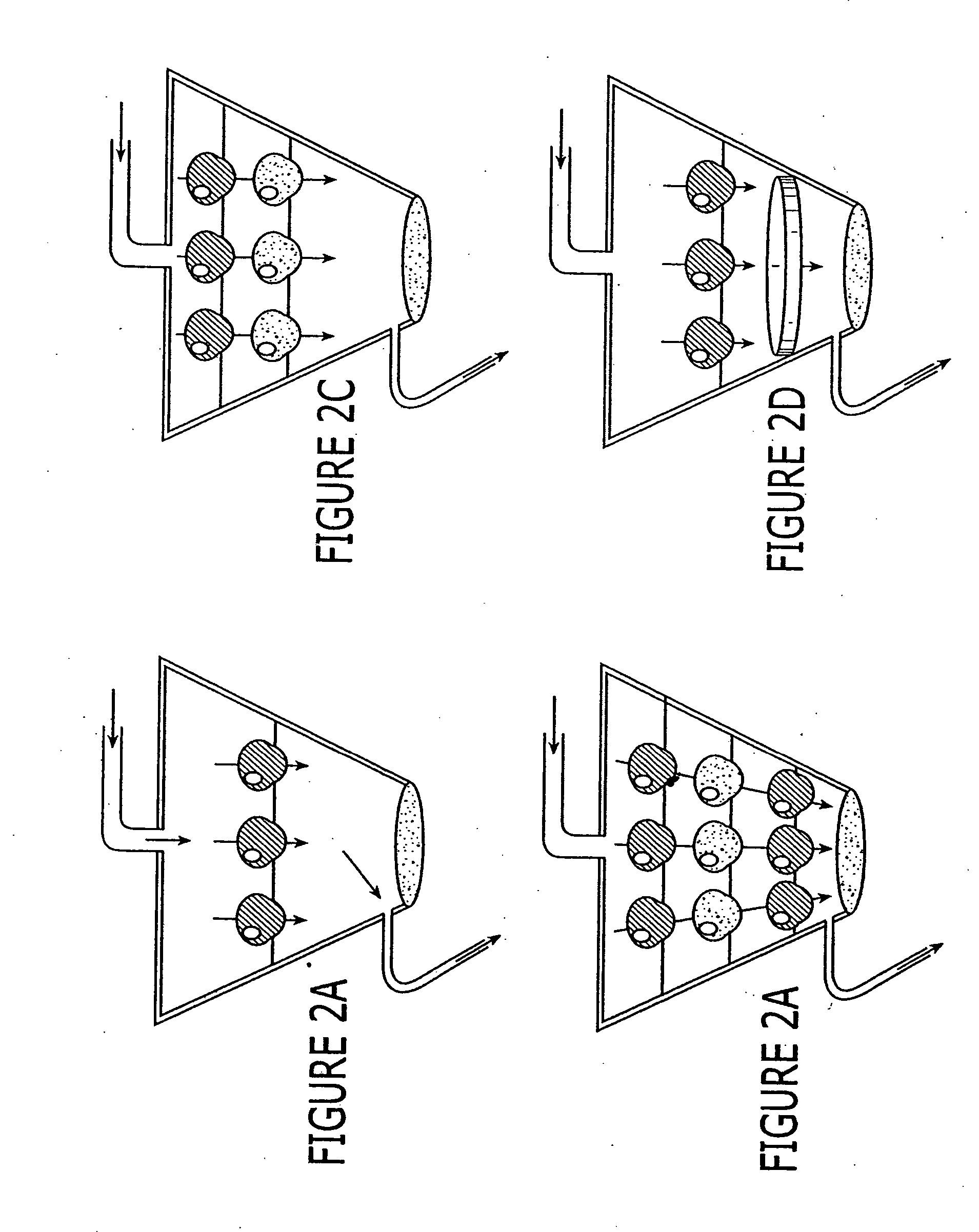
A device, method and process for three-dimensional spatial localization and functional interconnection of the same or different types of cells. The two or three-dimensional device comprising multiple layers containing wells for cell deposition where both the wells and layers are interconnected through microfluidic channels. A process for fabricating the three-dimensional device and a method for depositing different types of cells within the device in a functional interdependent spatial orientation thereby mimicking physiological functions. The device is useful for diagnostic assays, determination of dysfunction of certain cells in the system, quantification of production of cellular proteins, metabolites, hormones or other cellular products, for organ or tissue replacement, for co-culturing different cells, for testing pharmaceutical agents and as a bioreactor for production of biologicals.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Perfused three-dimensional cell/tissue disease models
ActiveUS20050260745A1Easy to primeBioreactor/fermenter combinationsBiological substance pretreatmentsLymphatic SpreadPathology diagnosis
A system has been constructed that recapitulate the features of a capillary bed through normal human tissue. The system facilitates perfusion of three-dimensional (3D) cell monocultures and heterotypic cell co-cultures at the length scale of the capillary bed. A major feature is that the system can be utilized within a “multiwell plate” format amenable to high-throughput assays compatible with the type of robotics commonly used in pharmaceutical development. The system provides a means to conduct assays for toxicology and metabolism and as a model for human diseases such as hepatic diseases, including hepatitis, exposure-related pathologies, and cancer. Cancer applications include primary liver cancer as well as metastases. The system can also be used as a means of testing gene therapy approaches for treating disease and inborn genetic defects.
Owner:MASSACHUSETTS INST OF TECH +1
Perfused three-dimensional cell/tissue disease models
ActiveUS8318479B2Easy to primeBioreactor/fermenter combinationsBiological substance pretreatmentsLymphatic SpreadPathology diagnosis
A system has been constructed that recapitulate the features of a capillary bed through normal human tissue. The system facilitates perfusion of three-dimensional (3D) cell monocultures and heterotypic cell co-cultures at the length scale of the capillary bed. A major feature is that the system can be utilized within a “multiwell plate” format amenable to high-throughput assays compatible with the type of robotics commonly used in pharmaceutical development. The system provides a means to conduct assays for toxicology and metabolism and as a model for human diseases such as hepatic diseases, including hepatitis, exposure-related pathologies, and cancer. Cancer applications include primary liver cancer as well as metastases. The system can also be used as a means of testing gene therapy approaches for treating disease and inborn genetic defects.
Owner:MASSACHUSETTS INST OF TECH +1
Methods of ex vivo progenitor and stem cell expansion by co-culture with mesenchymal cells
Methods of ex-vivo expansion and at the same time inhibiting differentiation of stem cells by co-culture with mesenchymal cells, transplantable populations of renewable progenitor and stem cells expanded thereby, and their uses in therapeutic applications.
Owner:GAMIDA CELL
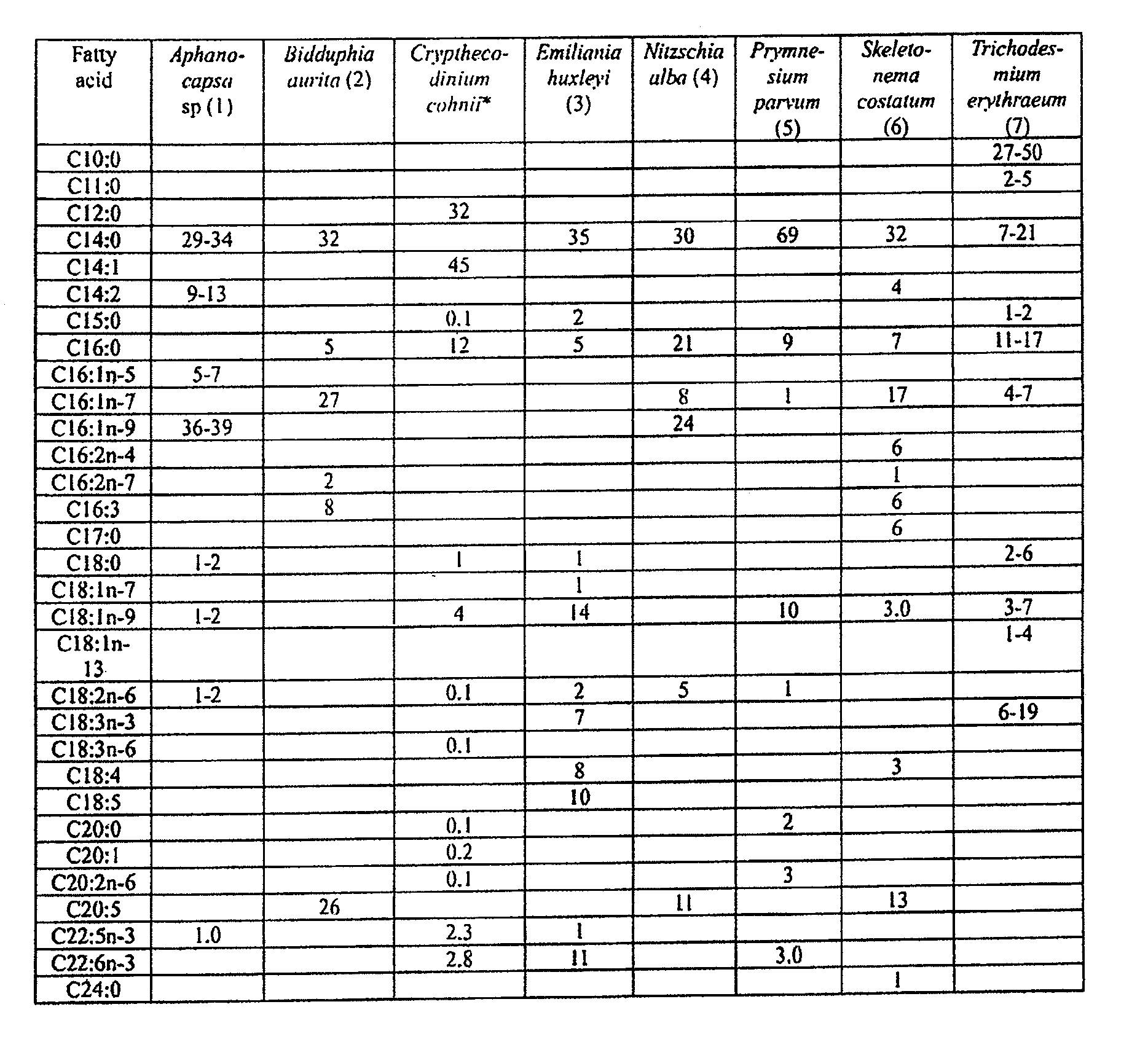
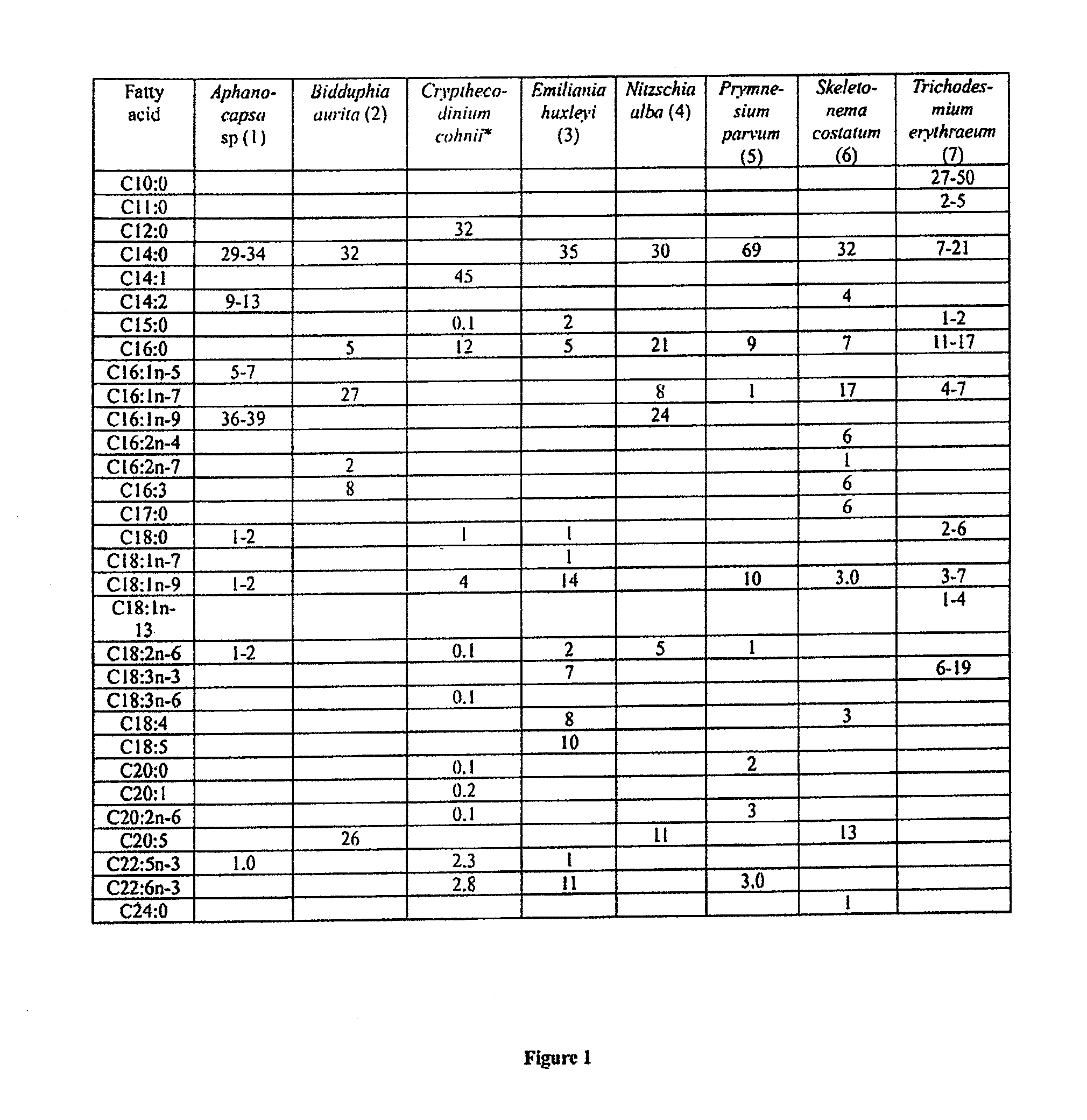
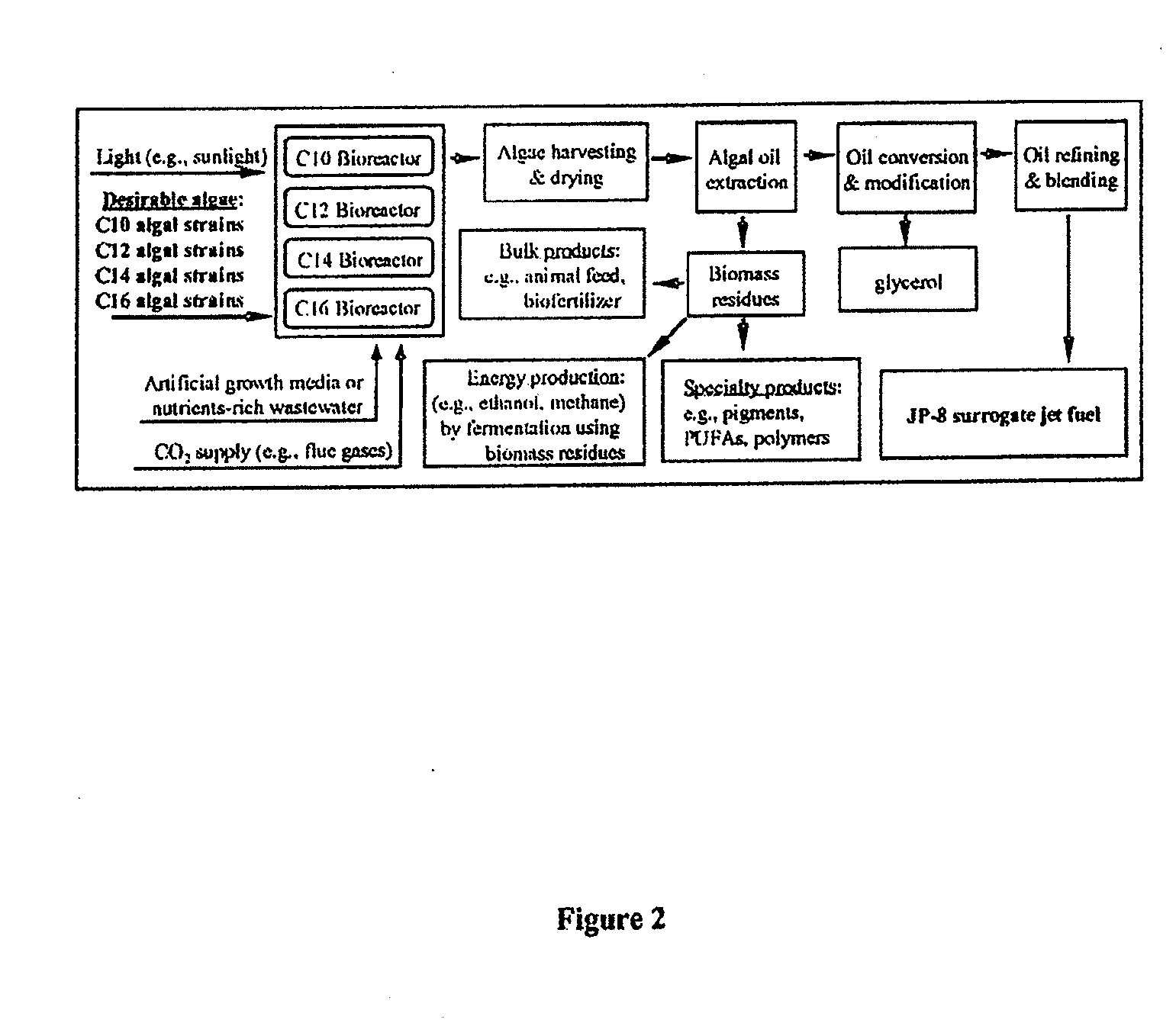
Co-culturing algal strains to produce fatty acids or hydrocarbons
Owner:ARIZONA STATE UNIVERSITY
Targeted methods of drug screening using co-culture methods
The present invention provides methods of screening for a molecule that inhibits the expression or activity of a protein encoded by a target gene which affects the fitness of a cell. The methods are based on a co-culture assay, and entail culturing together two cell populations, each of which is a population of identical cells, of the same species that differs substantially only in the expression or activity of the gene to be targeted or its encoded protein and the presence or absence of a reporter gene. The screen can be applied to cultured cells, unicellular and multicellular organisms. Manipulating the expression or activity of the target gene sensitizes the host to a molecule which inhibits the target gene or its encoded protein such that the cell or organism comprising the manipulated target gene grows at a different rate from the cell or organism comprising the unmanipulated gene in response to exposure to the molecule. The methods of the invention can be used for identifying drugs, proteins or any other molecules that inhibit the function of proteins encoded by target genes.
Owner:ROSETTA INPHARMATICS LLC
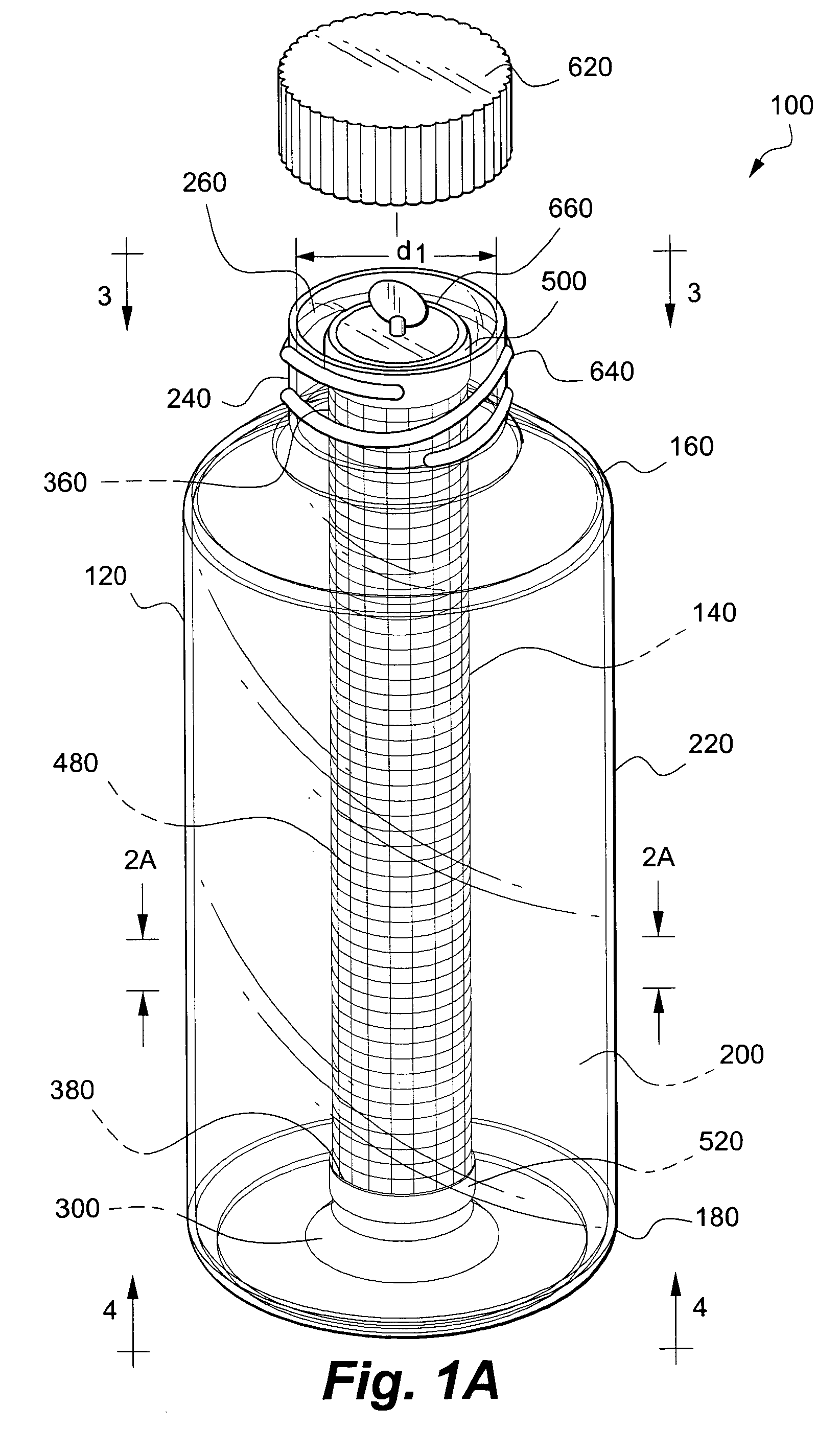
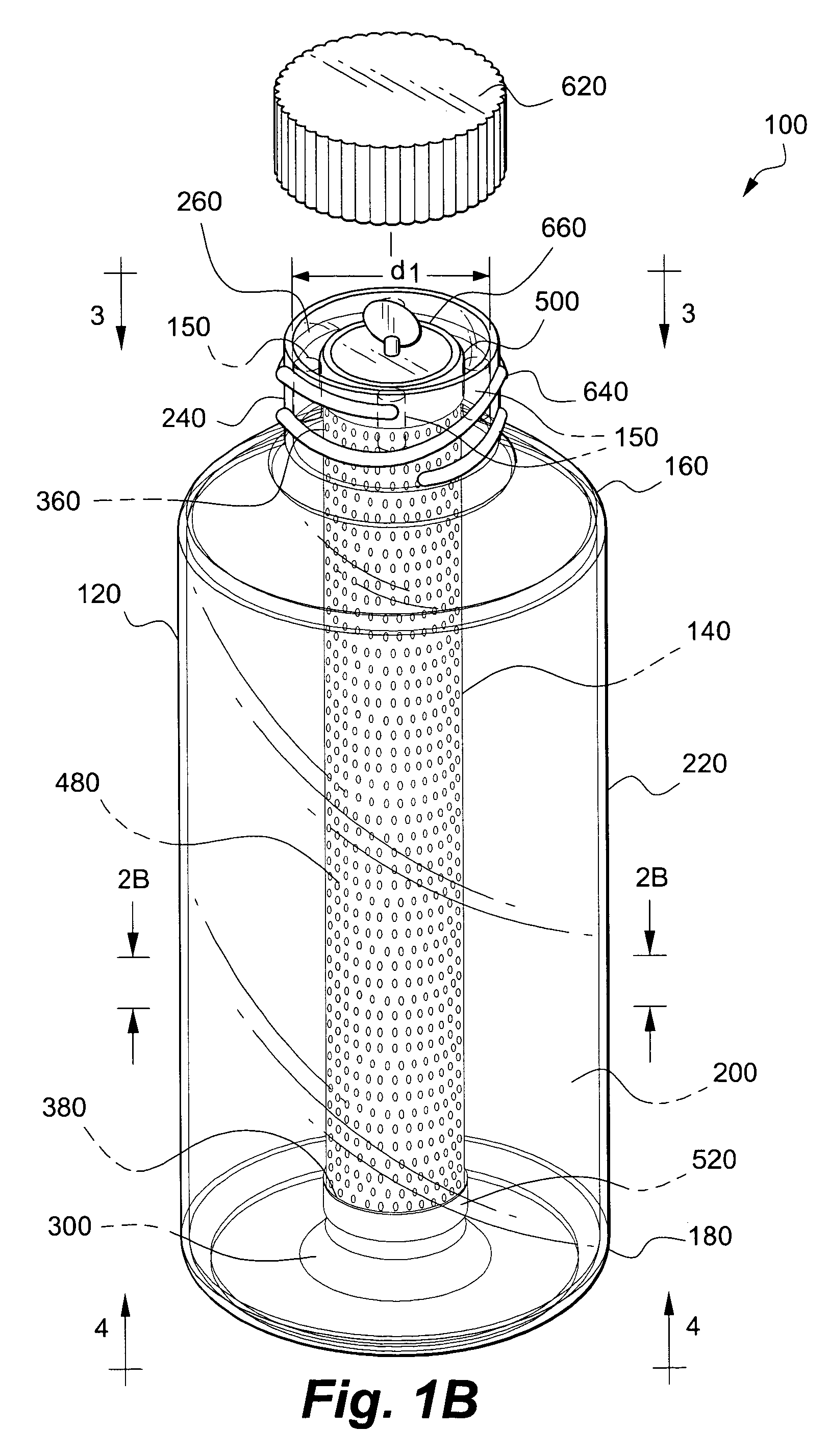
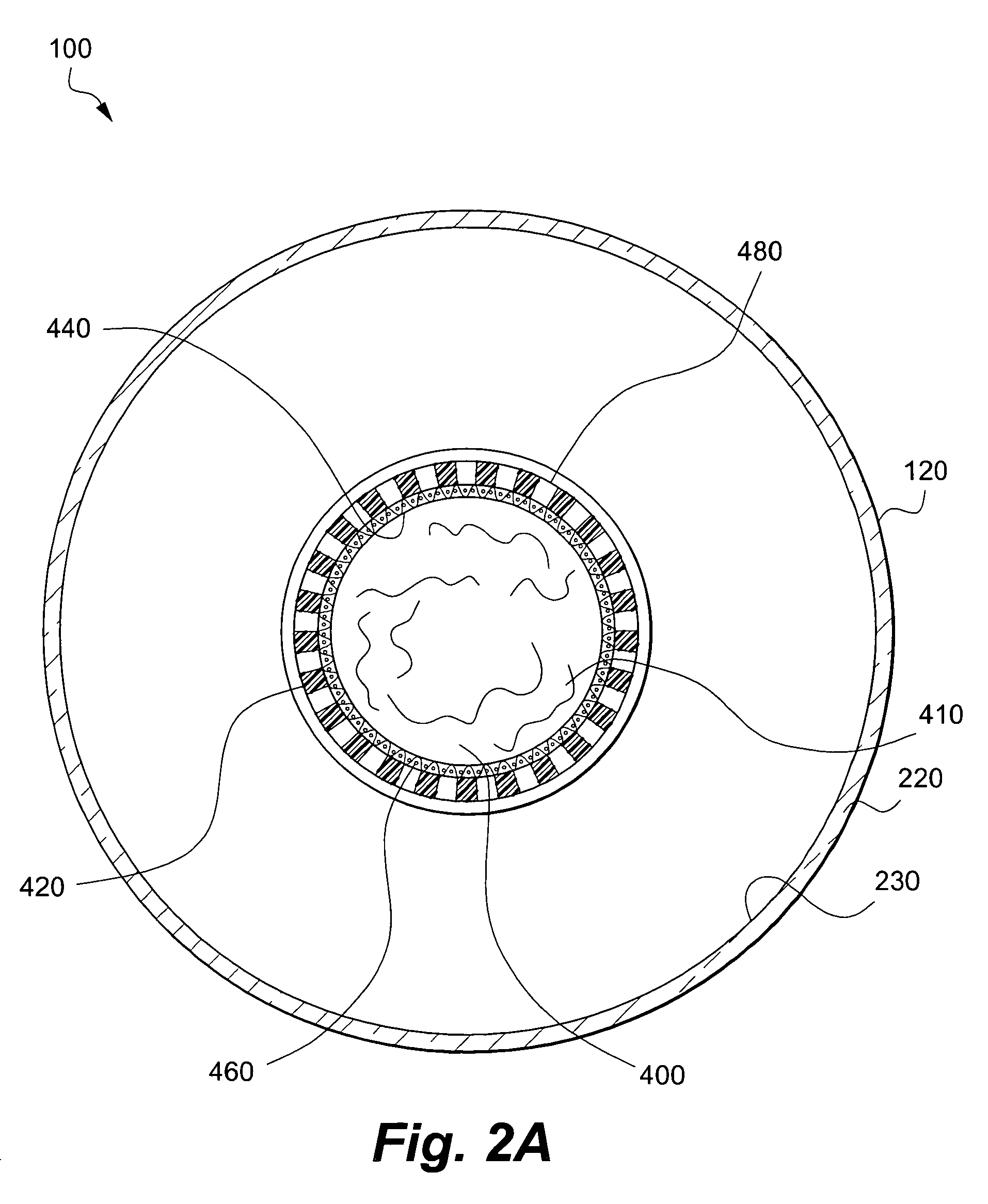
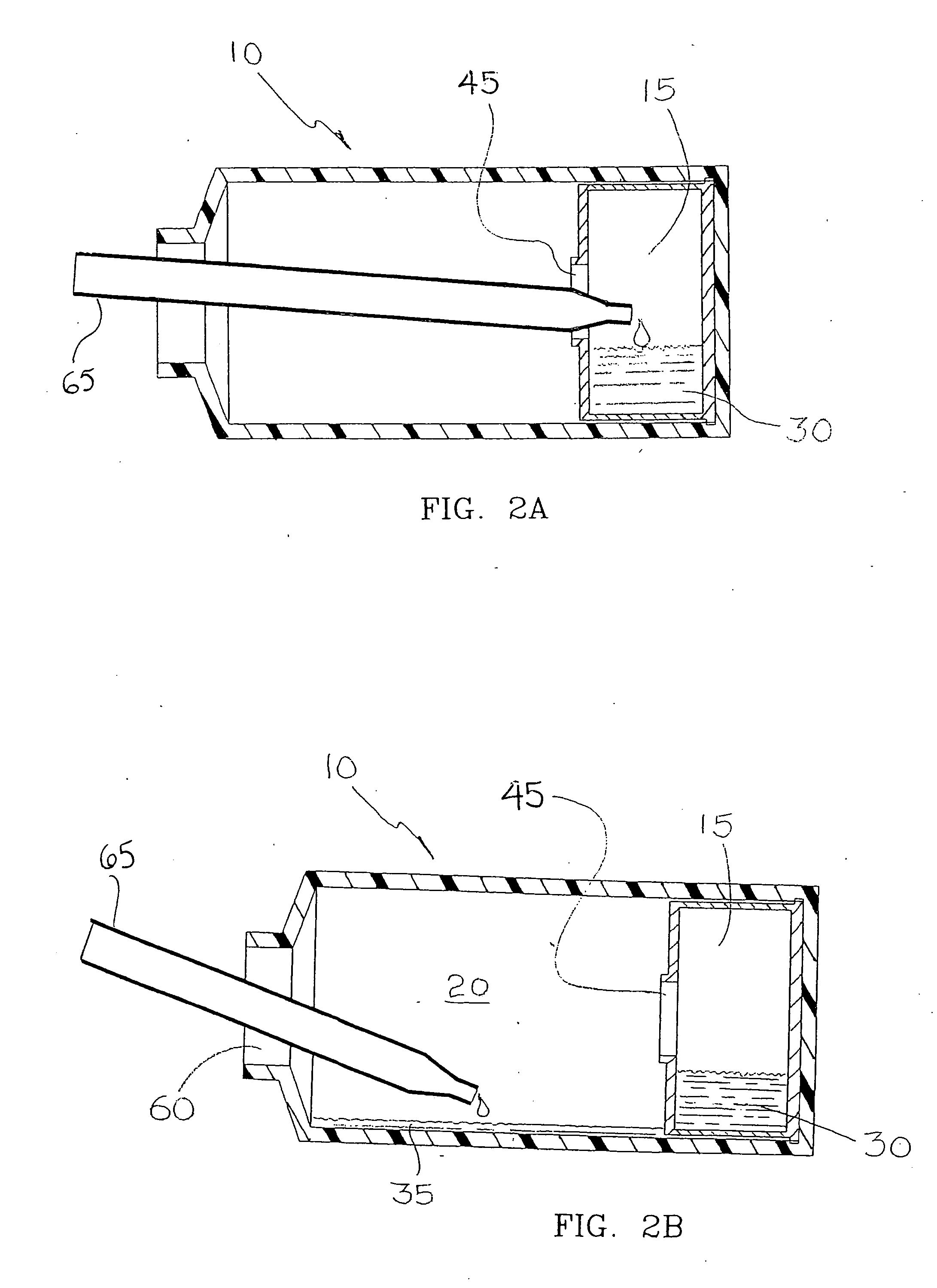
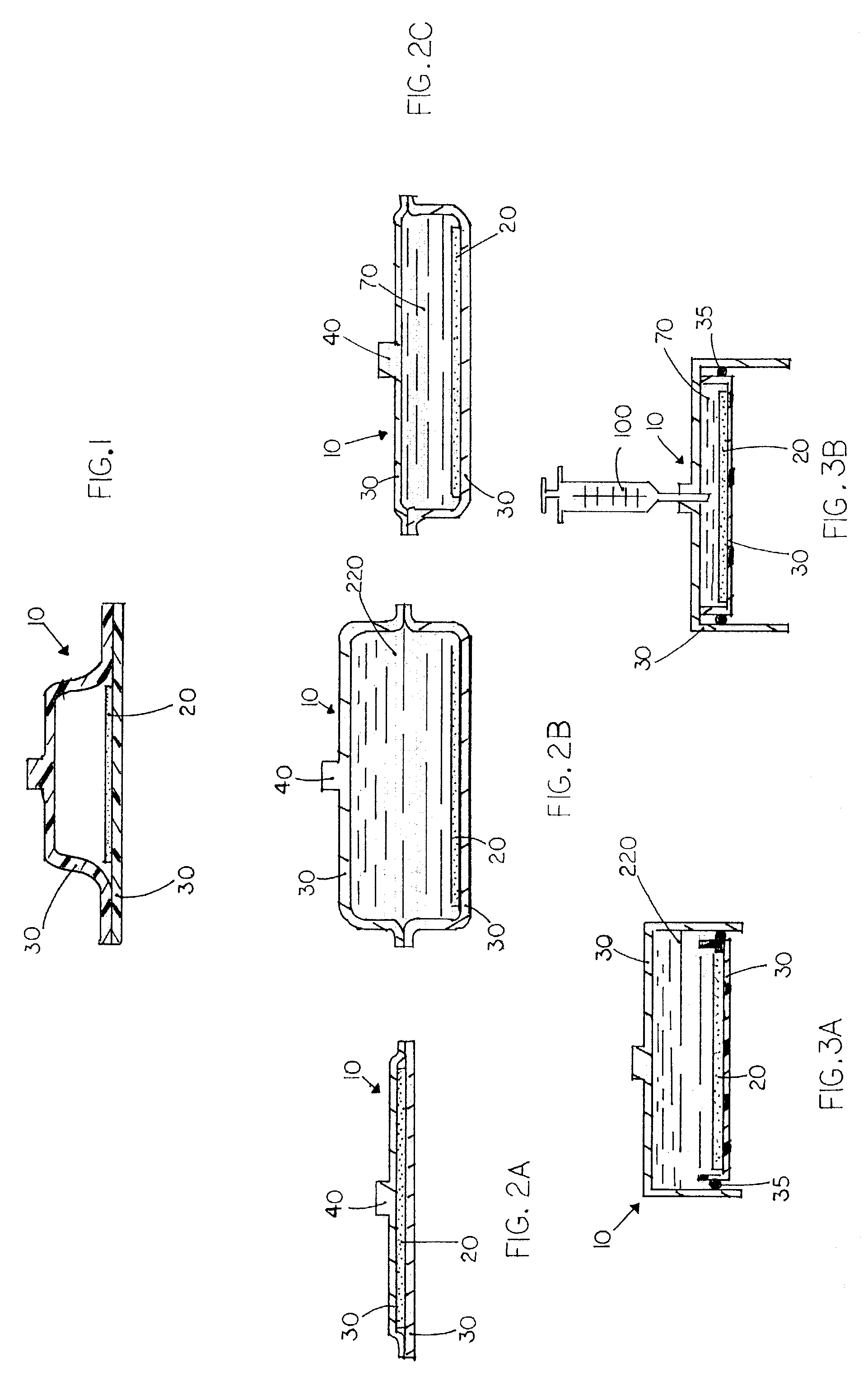
Apparatus and method for co-culturing of cells
InactiveUS7022518B1Bioreactor/fermenter combinationsBiological substance pretreatmentsProgenitorCulture cell
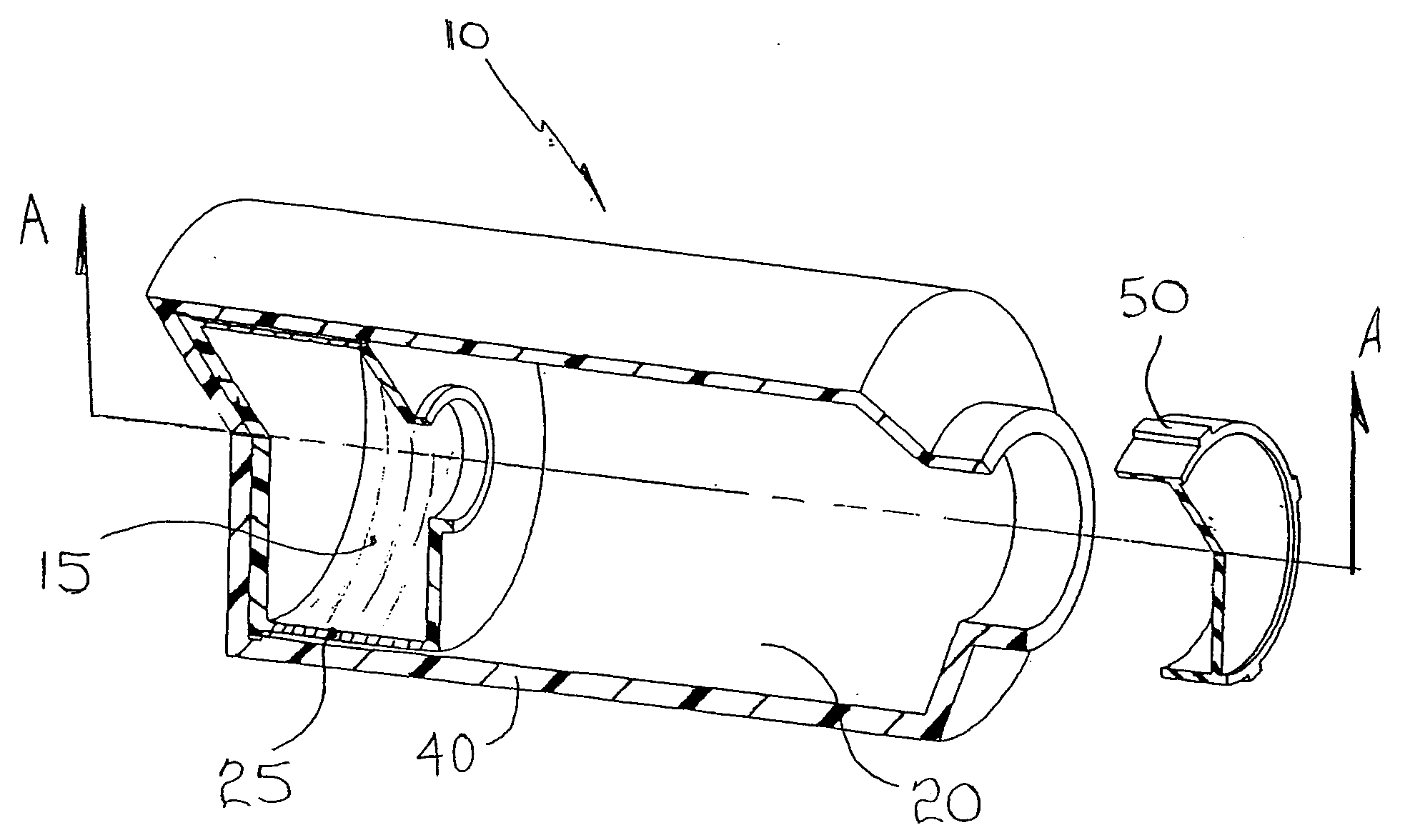
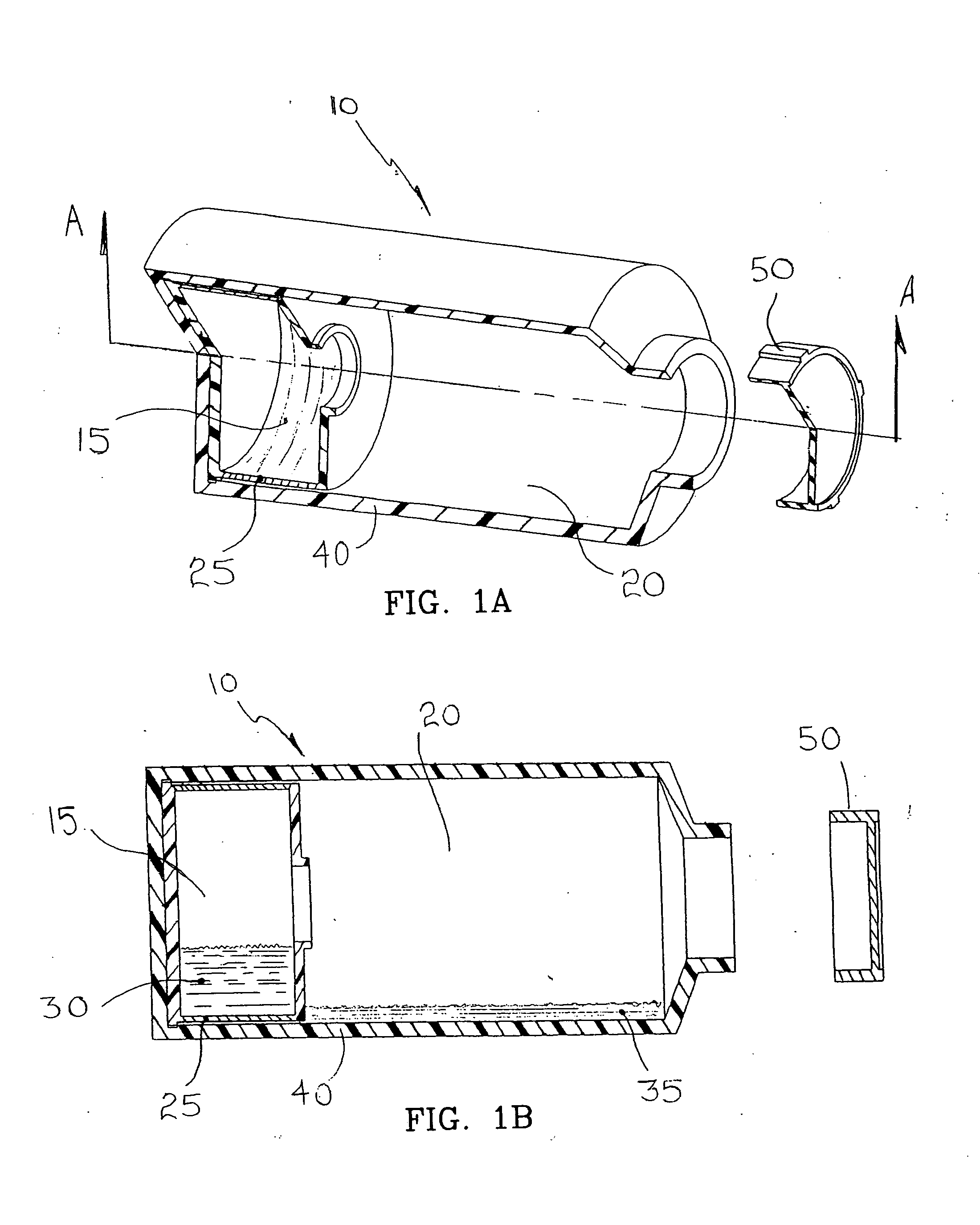
A co-culture apparatus and method for, among other things, converting a progenitor cell line into a target cell line. The apparatus comprises an exterior receptacle, a removable cartridge that fits into the exterior receptacle, and a closure member to seal the apparatus. The invention is also directed to a method for transforming a progenitor cell line into a target cell line. In one embodiment of the invention, the method comprises the steps of: establishing a progenitor cell line; establishing a support cell line; co-culturing said progenitor and support cell lines; establishing a transformation cell line; and co-culturing said progenitor and transformation cell lines, wherein the transformation cell line supports the transformation of the progenitor cell line into a target cell line.
Owner:FEYE GLEN
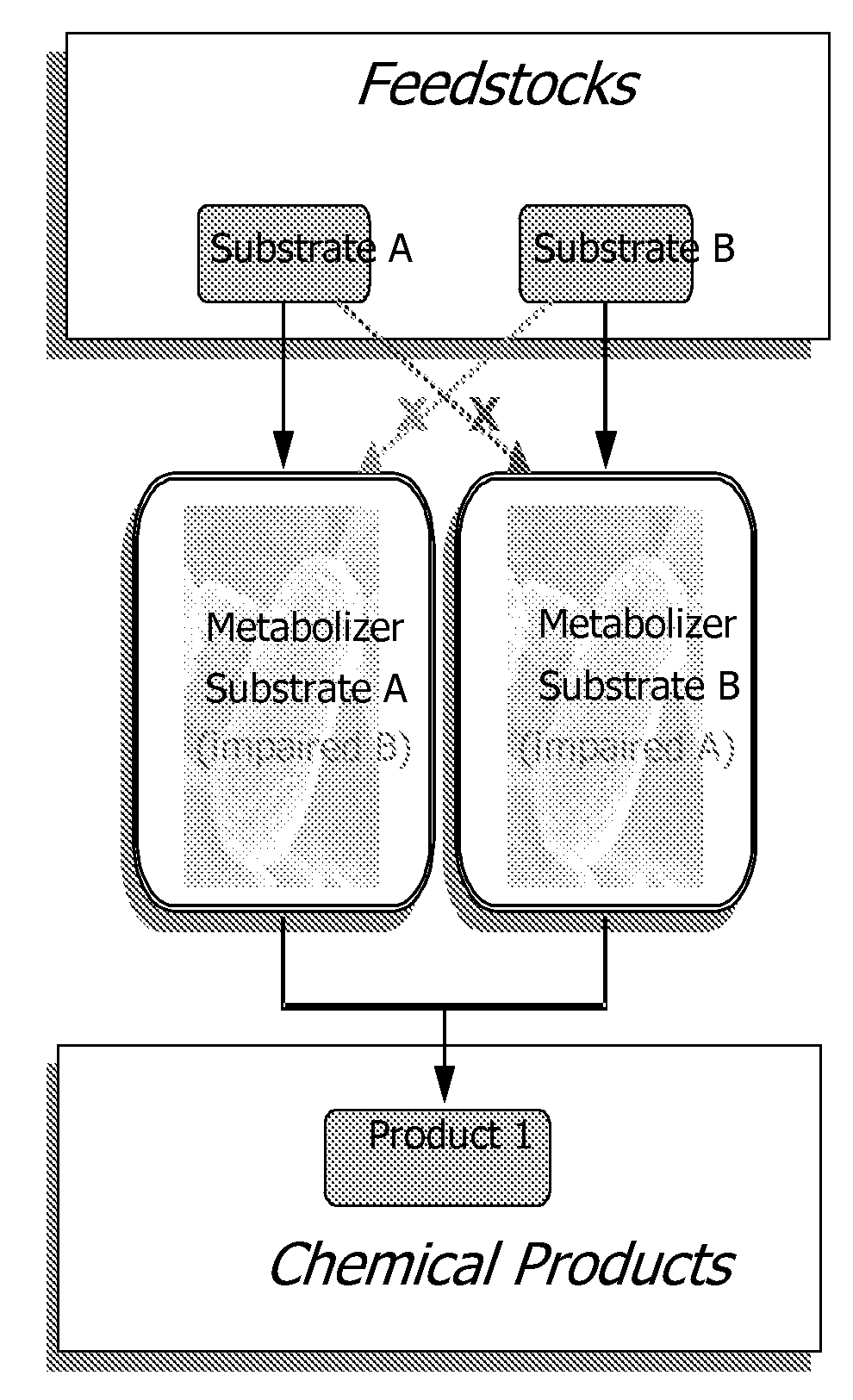
Complementary metabolizing organisms and methods of making same
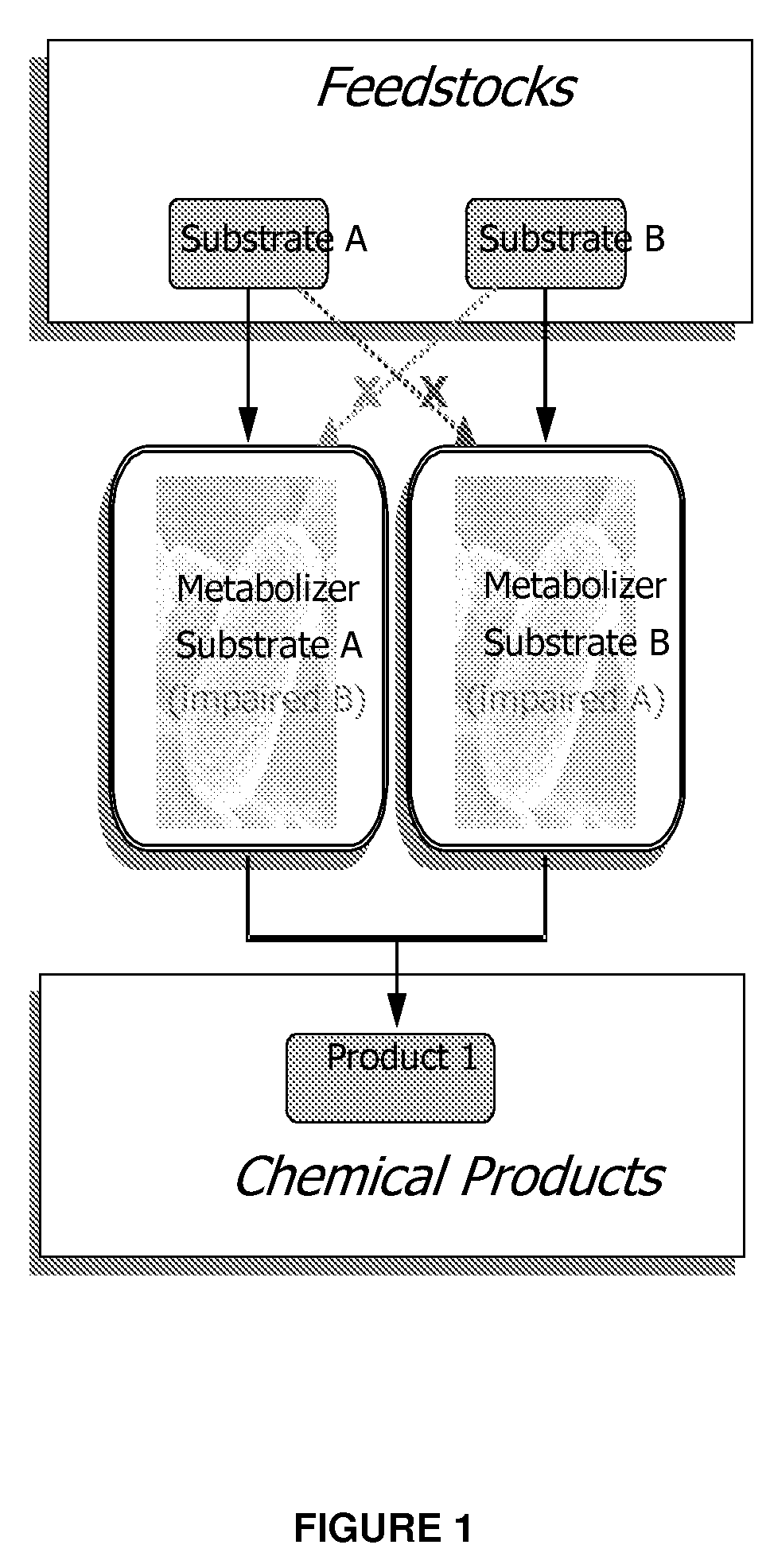
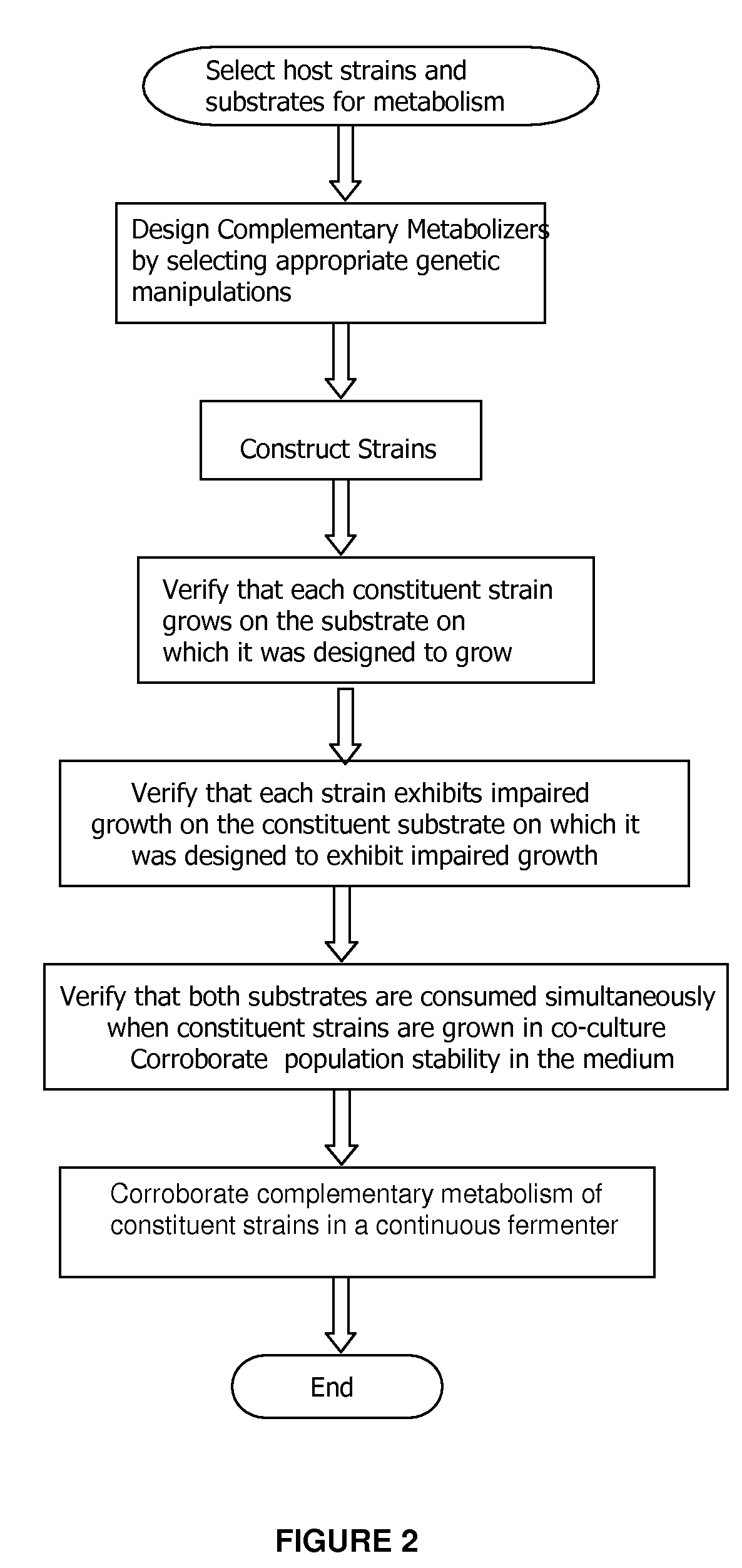
InactiveUS20090023182A1Impaired metabolic capacityIncrease metabolic rateFermentationMicrobiology processesOrganismBiology
The invention provides a non-naturally occurring set of microbial organisms. The set of organisms includes: at least a first constituent complementary metabolizing organism (CMO) exhibiting the ability to metabolize a first carbon substrate and having substantially impaired metabolic capacity for a second carbon substrate, and at least a second constituent complementary metabolizing organism (CMO) exhibiting the ability to metabolize the second carbon substrate and having substantially impaired metabolic capacity for the first carbon substrate, wherein a co-culture of the at least first and second CMOs exhibit simultaneous metabolism of a mixture having the first and second carbon substrates compared to either CMO alone. Simultaneous metabolism of a mixture having first and second carbon substrates can include an enhanced rate of metabolism of the first and second substrates compared to either CMO alone. Also provided is a bioprocess for producing a chemical compound. The bioprocess includes co-culturing a non-naturally occurring set of microbial organisms in a mixture having at least a first and a second carbon substrate under conditions sufficient for biosynthesis of a target chemical compound, the set of non-naturally occurring microbial organisms including: at least a first constituent complementary metabolizing organism (CMO) exhibiting the ability to metabolize the first carbon substrate and having substantially impaired metabolic capacity for the second carbon substrate, and at least a second constituent complementary metabolizing organism (CMO) exhibiting the ability to metabolize the second carbon substrate and having substantially impaired metabolic capacity for the first carbon substrate, wherein a co-culture of the at least first and second CMOs exhibit simultaneous metabolism of a mixture having the first and second carbon substrates compared to either CMO alone. Simultaneous metabolism of a mixture having first and second carbon substrates can include an enhanced rate of metabolism of the first and second substrates compared to either CMO alone.
Owner:GENOMATICA INC
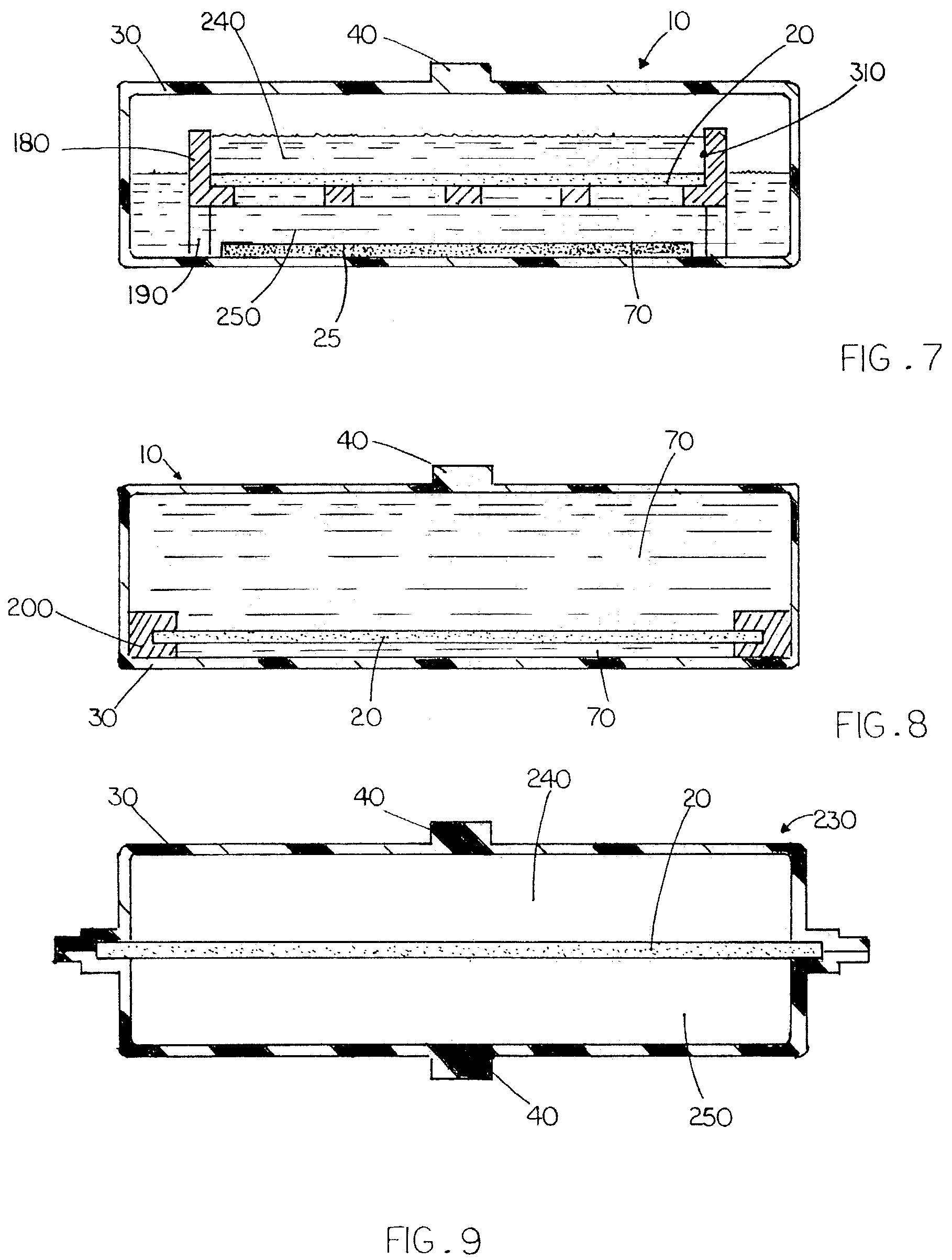
Compartmentalized device for cell culture, cell processing, and sample dialysis
ActiveUS20050101009A1More efficient dialysis of laboratory samplesImprove concentrationBioreactor/fermenter combinationsBiological substance pretreatmentsHigh cellVolumetric Mass Density
A versatile compartmentalized cell culture device, with a selectively permeable membrane separating the compartments, provides many attributes relative to traditional devices. It can be configured for high-density cell culture, co-culture, and sample dialysis while rolling or standing still. It can also be configured for continuous movement of liquid between compartments. The wide combination of attributes not found in other membrane based cell culture and bioprocessing devices includes more cell capacity, more cell secreted product capacity, higher cell and product density, increased medium capacity, minimized use of exogenous growth factors, compatibility with standard cell culture equipment and protocols, increased scale up efficiency, capacity to function when rolling or standing still, capacity for perfusion without the need for pumps, and more efficient sample dialysis.
Owner:WHEATON INDS
Assay device that analyzes the absorption, metabolism, permeability and/or toxicity of a candidate compound
InactiveUS20070166816A1Precise absorptionMaterial nanotechnologyBioreactor/fermenter combinationsAssayCell type
This invention provides device for co-culturing at least two different cell types in a two-dimensional configuration, methods of patterning at least two different cell types in a two-dimensional co-culture configuration, and uses of these devices and methods for analyzing an effect of candidate compound on such cellular cocultures. Also provided is a transmigration and extravasation device. Assay devices for analyzing the absorption, permeability, metabolism and / or toxicity of a candidate compound by a cell are provided. A microfluidic network, which is adaptable for integration with a device for coculturing is provided.
Owner:SURFACE LOGIX INC
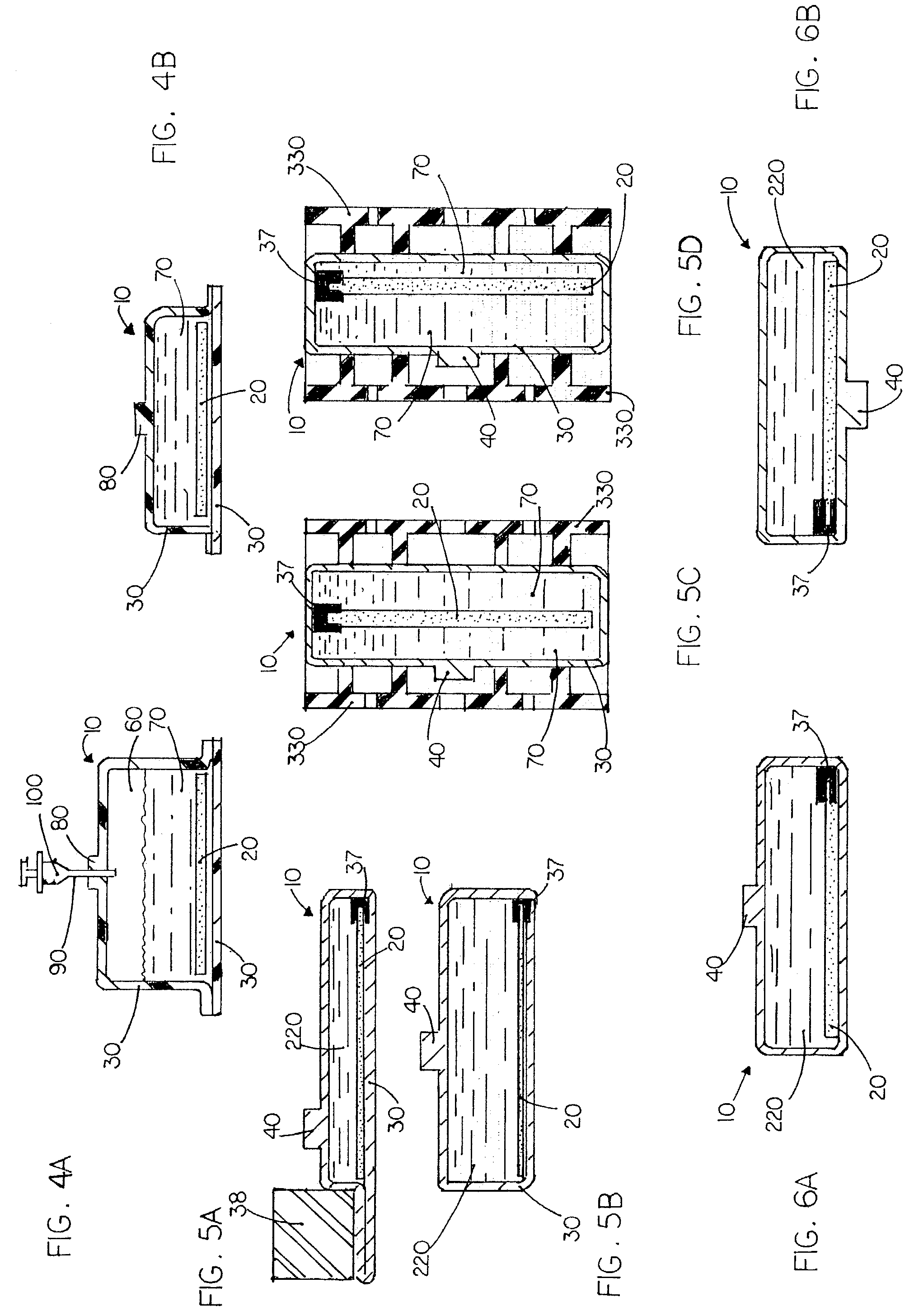
Apparatus and method for culturing and preserving tissue constructs
ActiveUS7229820B2Avoid enteringAvoid damageBioreactor/fermenter combinationsBiological substance pretreatmentsPipetteOptimal growth
A disclosure is made of various apparatus and methods for culturing and preserving cells and tissue in ways that minimize contamination potential, direct cells to reside in desired areas, allow uniform cell distribution during seeding, provide optimal growth conditions by controlling the amount of medium residing in proximity of cells, allow desired compounds and molecules to reside in proximity of the cells, allow co-culture, provide for efficient scale up, allow a desired shape of tissue to be created while retaining a closed system, and allow cryopreservation and reconstitution of cell and tissue while retaining a closed system. The apparatus and methods can be combined to prevent the need to remove the tissue from the enclosure at any point during the sterilization, seeding, culturing, cryopreservation, shipping, or restoration process. Also disclosed is an apparatus and method of pipette interface with a container in a manner that blocks contaminants from entering the container.
Owner:WILSON WOLF MFG
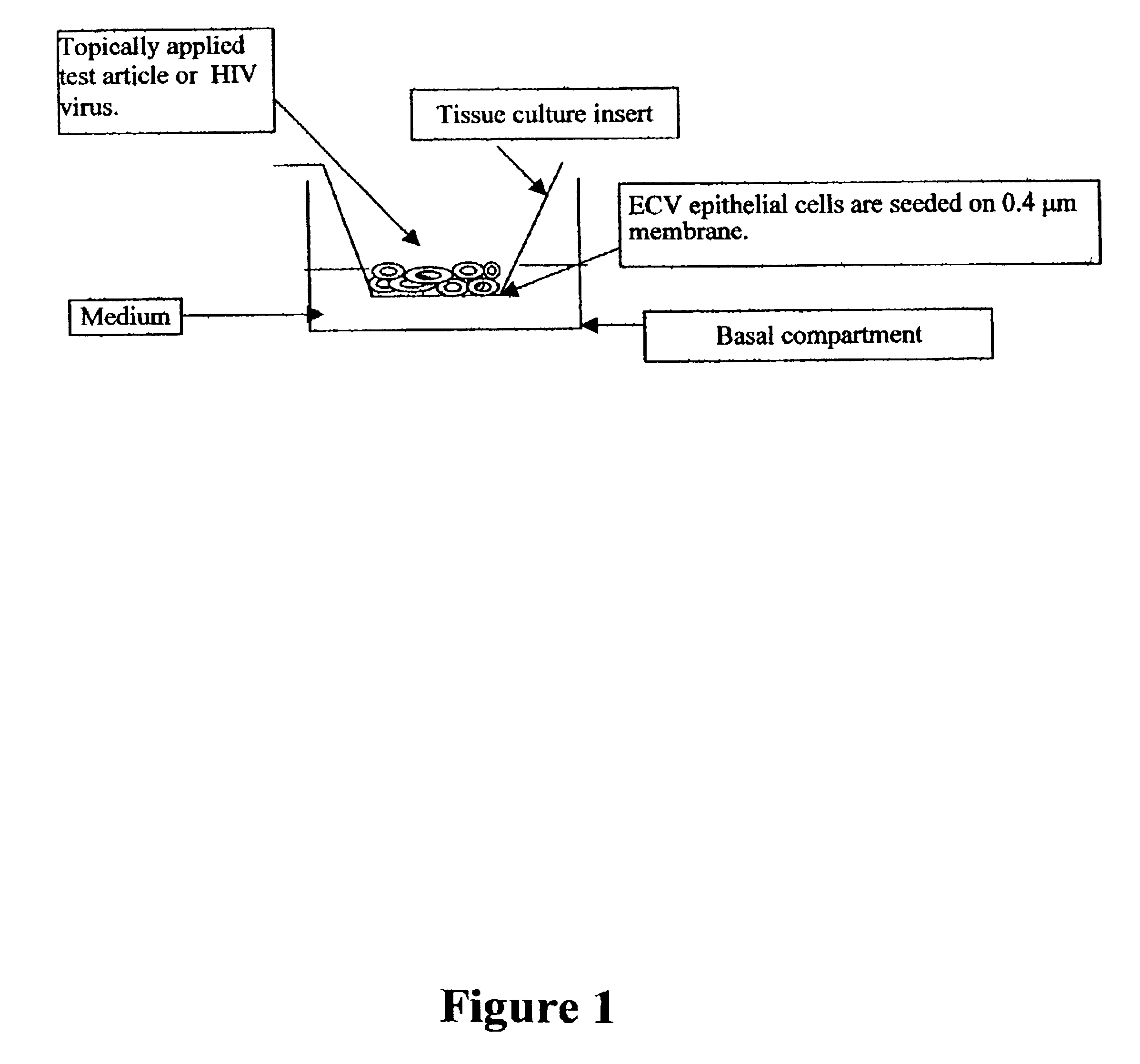
Three dimensional vaginal tissue model containing immune cells
InactiveUS6943021B2Improve survivabilityInduced proliferationBiocideEpidermal cells/skin cellsSerum free mediaAir liquid interface
Disclosed is a cervico-vaginal tissue equivalent comprised of vaginal epithelial cells and immune cells, cultured at the air-liquid interface. The tissue equivalent is capable of being infected with a sexually transmitted pathogen such as a virus (e.g., HIV), a bacteria, a helminthic parasite, or a fungus. The tissue equivalent is also capable of undergoing an allergic-type reaction or an irritant-type reaction. The tissue equivalent is characterized as having nucleated basal layer cells and nucleated suprabasal layer cells, and further as having cell layers external to the suprabasal layer progressively increasing in glycogen content and progressively decreasing in nuclei content. Immune cells of the tissue equivalent are primarily located in the basal and suprabasal layers. Also disclosed are methods for producing the tissue equivalent. The methods involve providing vaginal epithelial cells and immune cells, seeding the cells onto a porous support, and co culturing the seeded cells at the air-liquid interface under conditions appropriate for differentiation. One such method disclosed is for generation of the tissue equivalent in serum free medium. Specific cells from which the tissue equivalent is generated, and also specific preferred components of the medium in which the tissue equivalent is generated are provided. Also disclosed is a cervico-vaginal tissue equivalent produced by the methods disclosed herein.
Owner:MATTEK CORP
Cell culture apparatus and culture methods using same
InactiveUS20150072413A1Improve surface adhesionEffective medium supplyBioreactor/fermenter combinationsBiological substance pretreatmentsMicrobial fuel cellSemipermeable membrane
Cell culture apparatus comprising at least two adjacent cell cultivation channels separated by a permeable or semipermeable membrane, wherein at least one channel, for the majority of its length, has a cross sectional area of no more than 1 mm2, said channel being provided with entrance and exit means to permit the passage of media therethrough, allows co-culture of separate cell types, e.g. human and microbial cells, without mingling, allowing monitoring of cell cultures and chemical exchanges between the respective cell cultures.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +2
Methods for using dendritic cells to activate gamma/delta-T cell receptor-positive T cells
InactiveUS6821778B1Control progressIncrease the number ofArtificial cell constructsBlood/immune system cellsAdoptive cellular immunotherapyDendritic cell
This invention relates to methods of using human dendritic cells to present antigens for the induction of antigen-specific T cell-mediated immune responses. In particular, it relates to the isolation of dendritic cells from human blood, exposing the cells to antigens, co-culturing the antigen-pulsed dendritic cells with gammadelta-T cell receptor-positive-T cells (gammadelta-TCR<+> T cells) obtained from unprimed or weakly primed individuals for the stimulation of antigen-specific T cell proliferative and cytotoxic activities. The dendritic cell antigen presentation system described herein has a wide range of applications, including but not limited to, activation and expansion of large numbers of antigen-specific major histocompatibility complex-unrestricted T cells for use in adoptive cellular immunotherapy against infectious diseases and cancer.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
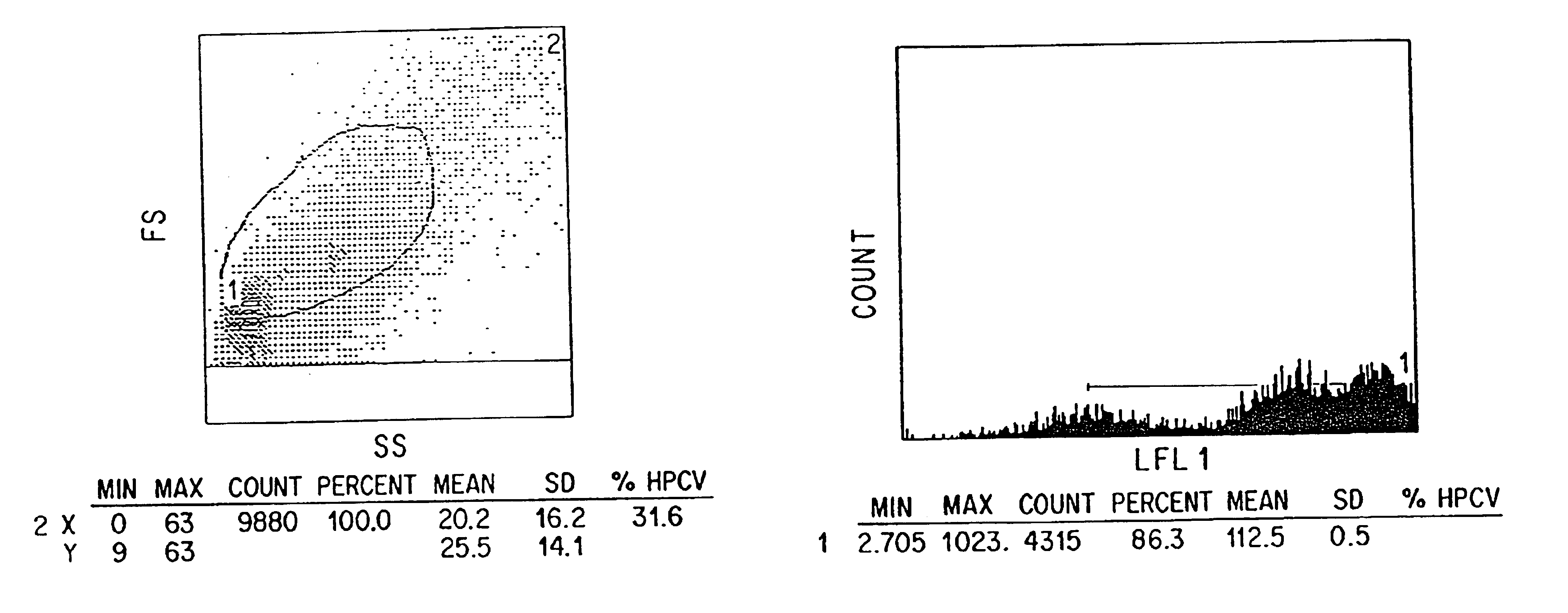
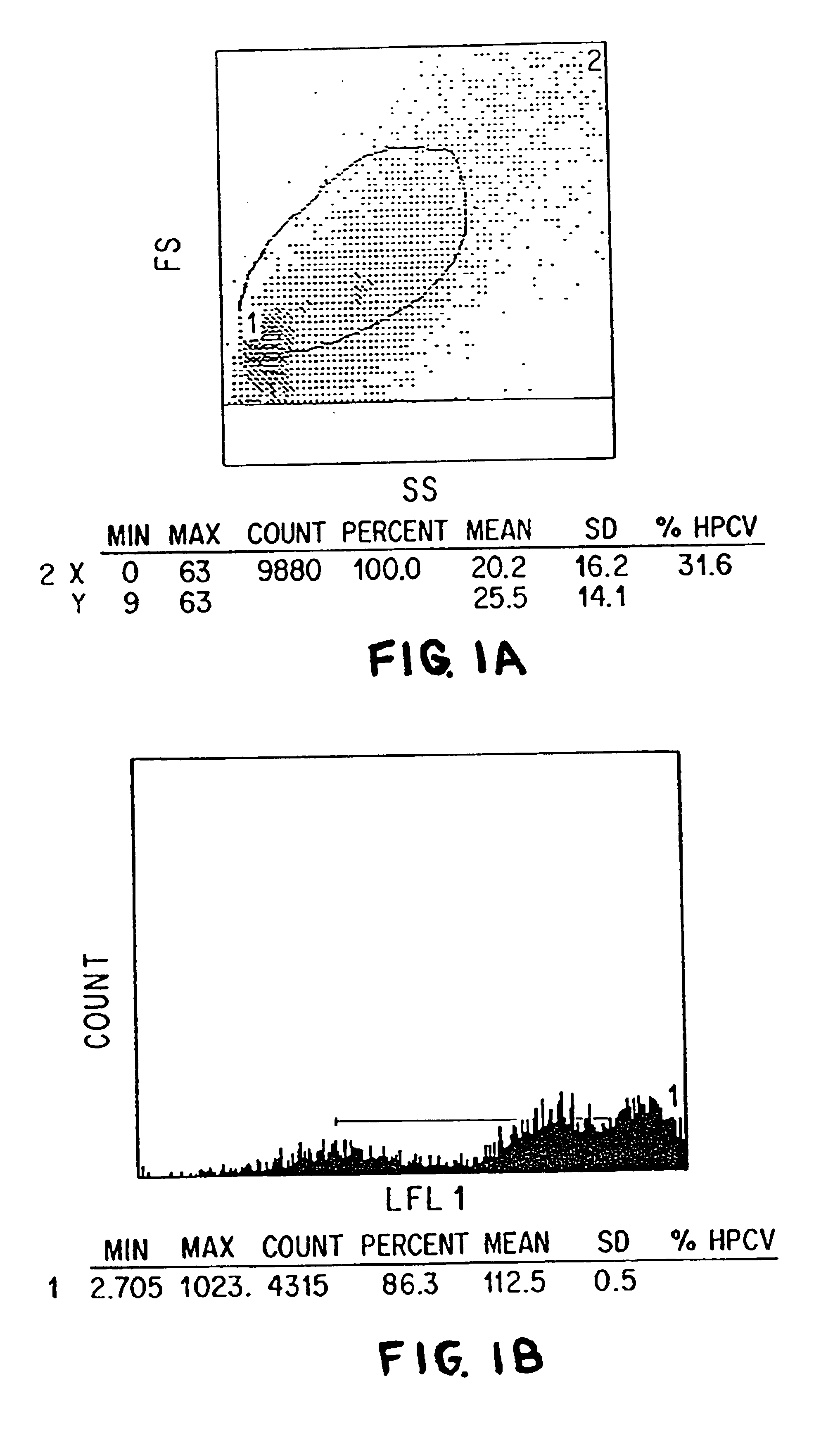
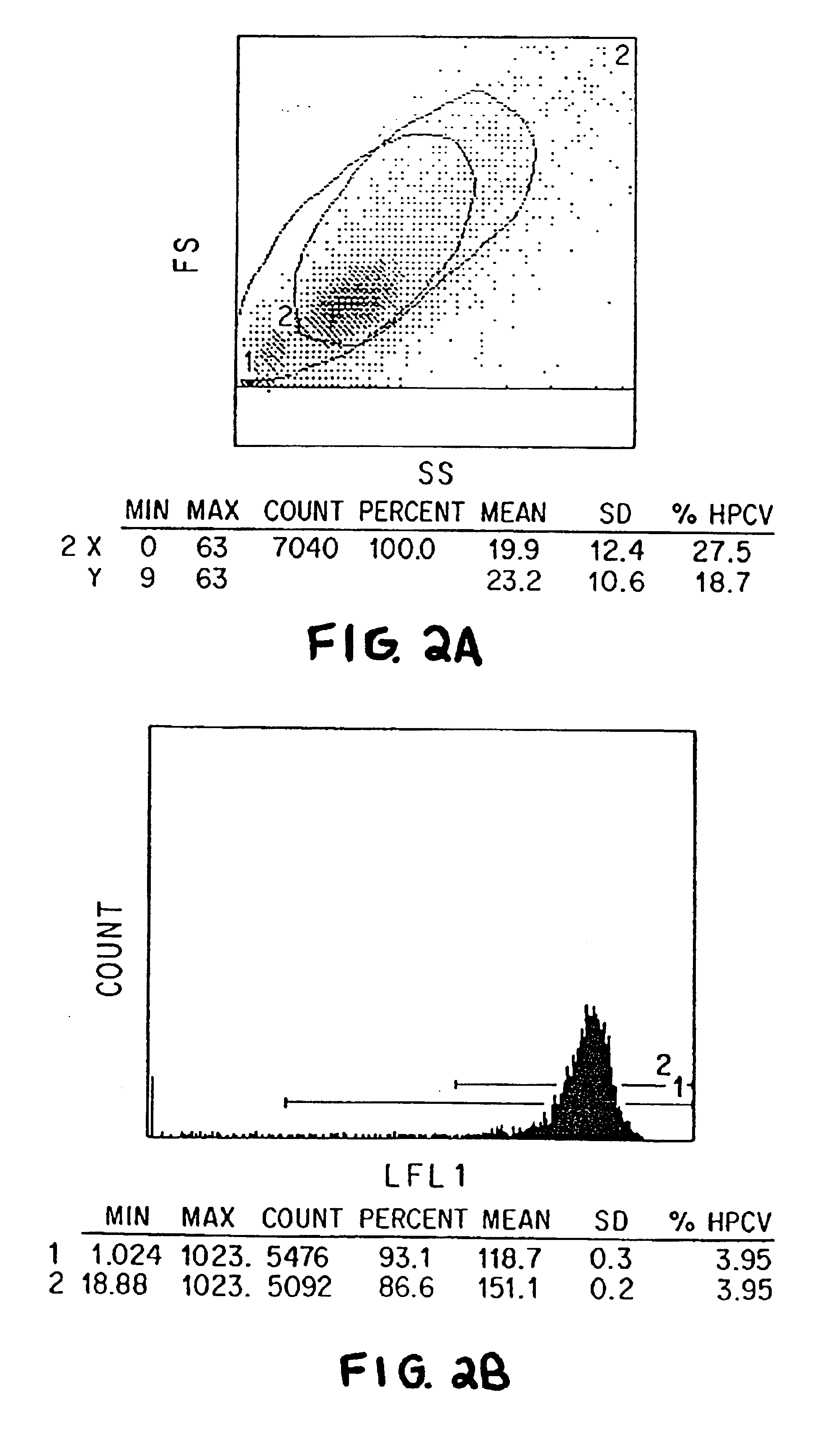
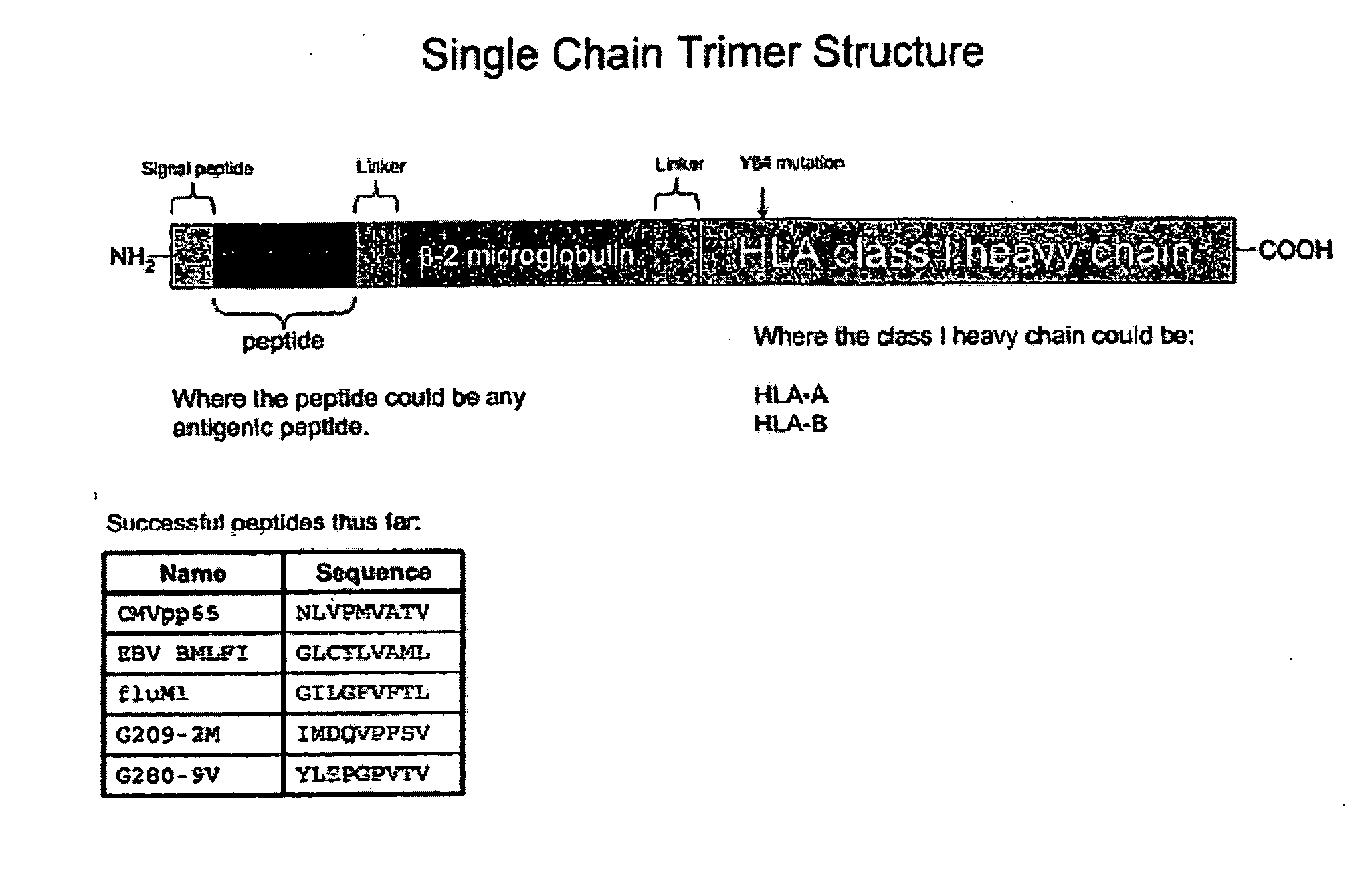
Single chain trimers and uses therefor
Owner:WASHINGTON UNIV IN SAINT LOUIS
Cell cultivation-support material, method of cocultivation of cells and cocultivated cell sheet obtained therefrom
InactiveUS20030036196A1Rapid responseA large amountNervous system cellsArtificial cell constructsPolymer sciencePolymer chemistry
By using a bed material for cell culture having a surface composed of two domains of domain A coated with a temperature-responsive polymer and domain B composed of any one or a combination of a domain coated with a polymer having high affinity with cells, a domain coated with the temperature-responsive polymer in an amount different from the amount of the temperature-responsive polymer of domain A, and a domain coated with a polymer which responds to a temperature different from the temperature to which domain A responds, a method for the co-culture of a plurality of kinds of cells which has heretofore been difficult becomes possible.
Owner:CELLSEED
Methods of isolating t cell receptors having antigenic specificity for a cancer-specific mutation
Owner:UNITED STATES OF AMERICA
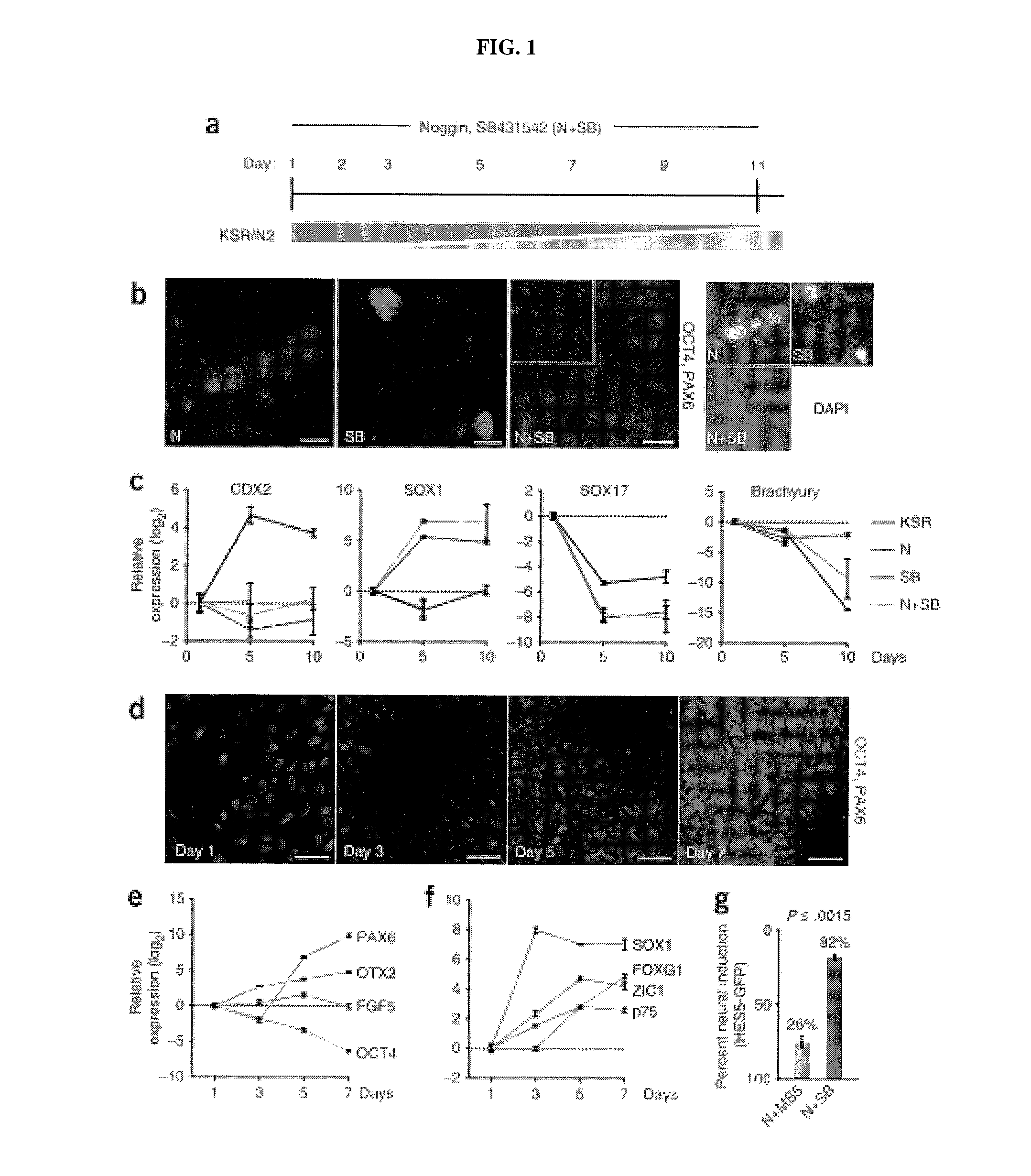
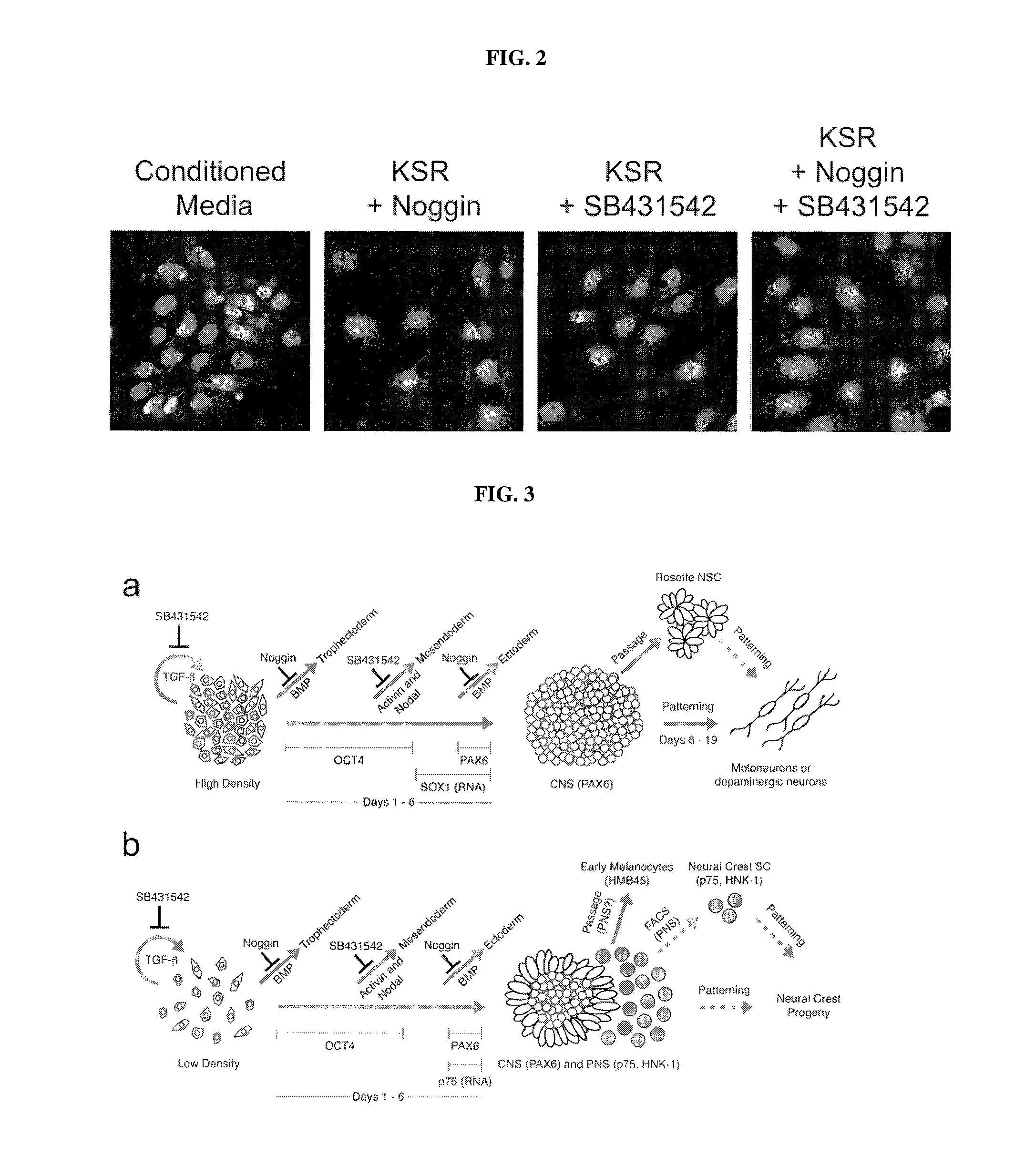
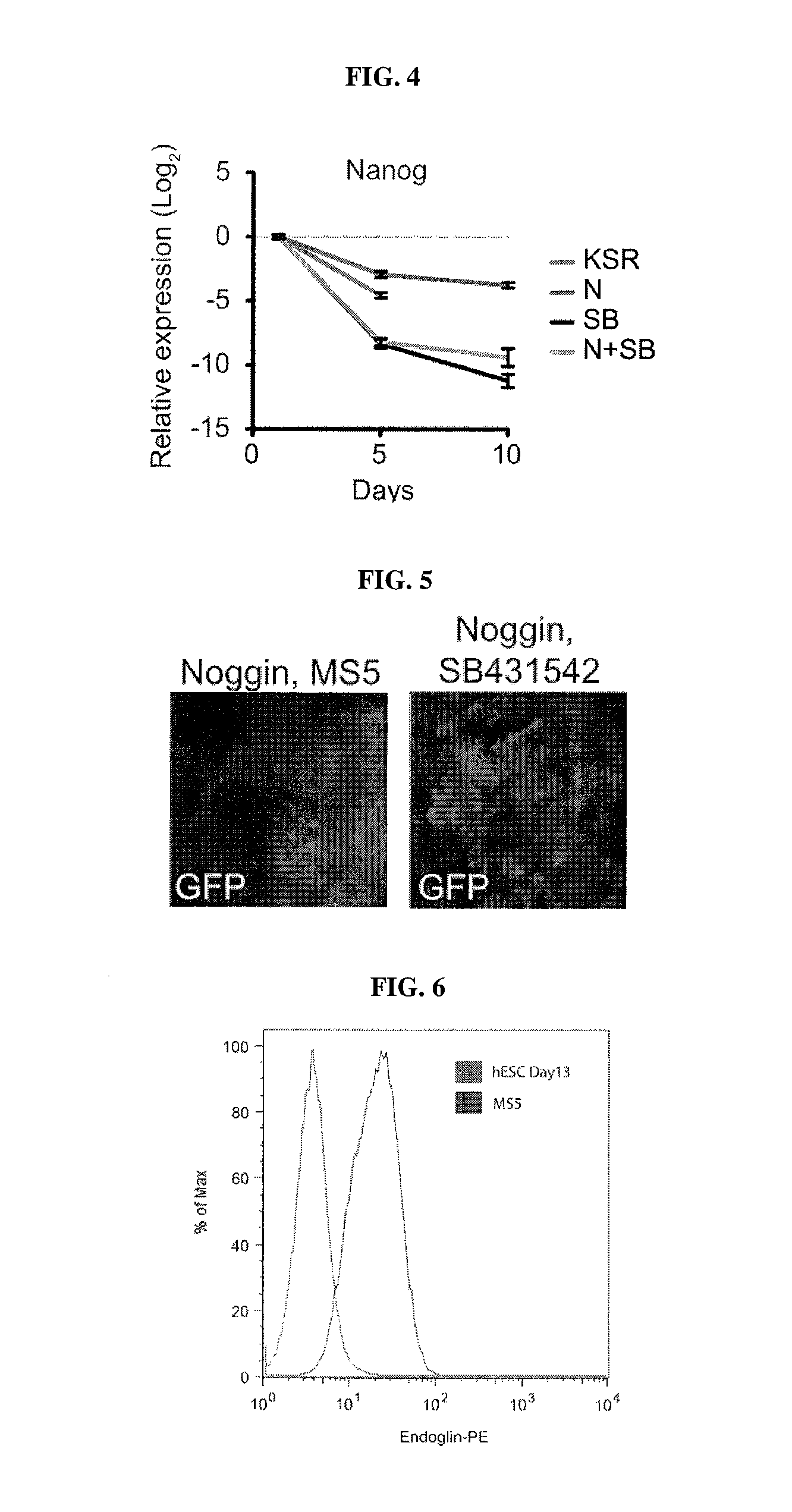
Methods of neural conversion of human embryonic stem cells
ActiveUS20120094381A1Reducing DKK- protein functionImprove featuresCulture processNervous system cellsNeural plateDirected differentiation
The present invention relates generally to the field of cell biology of stem cells, more specifically the directed differentiation of pluripotent or multipotent stem cells, including human embryonic stem cells (hESC), somatic stem cells, and induced human pluripotent stem cells (hiPSC) using novel culture conditions. Specifically, methods are provided for obtaining neural tissue, floor plate cells, and placode including induction of neural plate development in hESCs for obtaining midbrain dopamine (DA) neurons, motorneurons, and sensory neurons. Further, neural plate tissue obtained using methods of the present inventions are contemplated for use in co-cultures with other tissues as inducers for shifting differentiation pathways, i.e. patterning.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
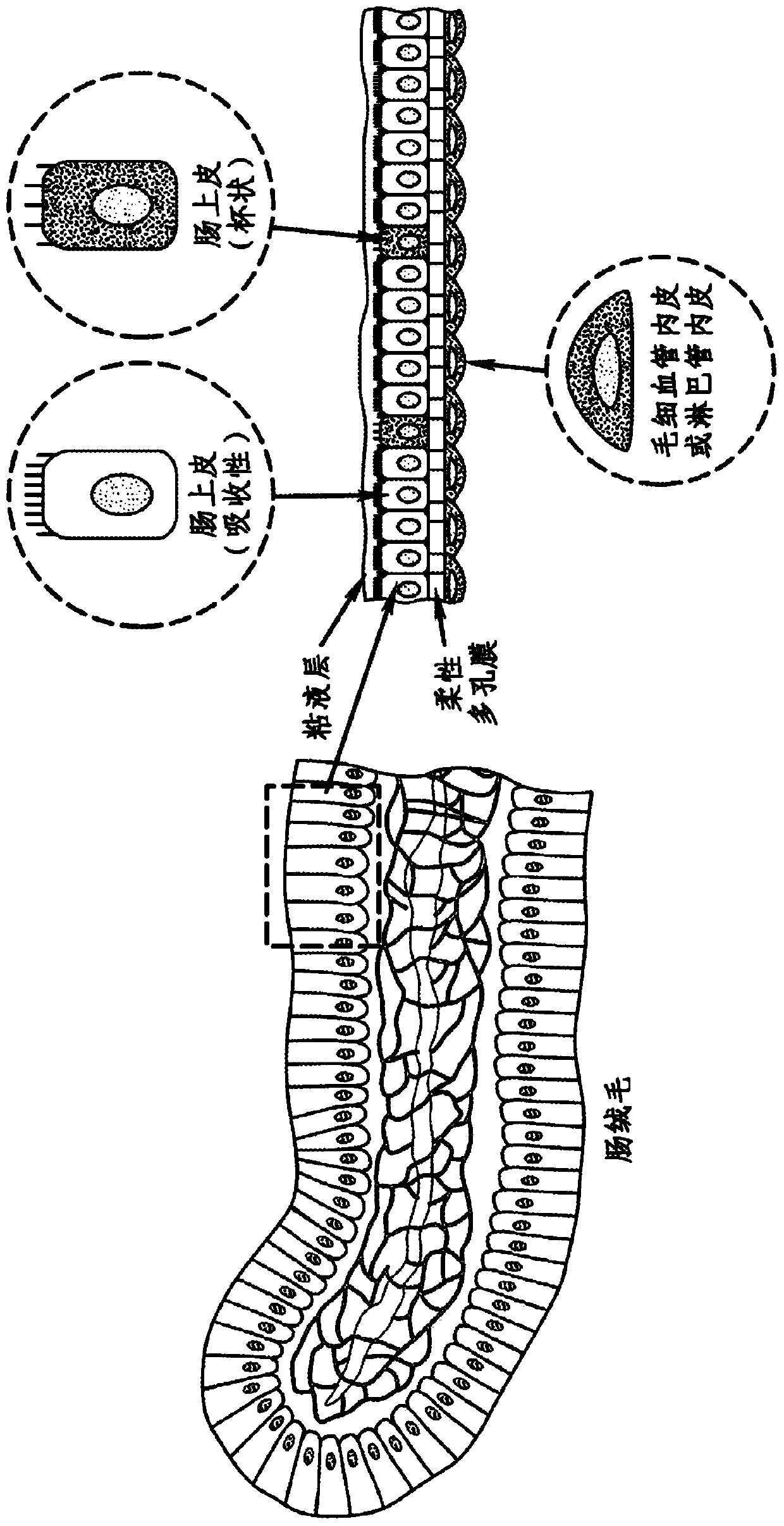
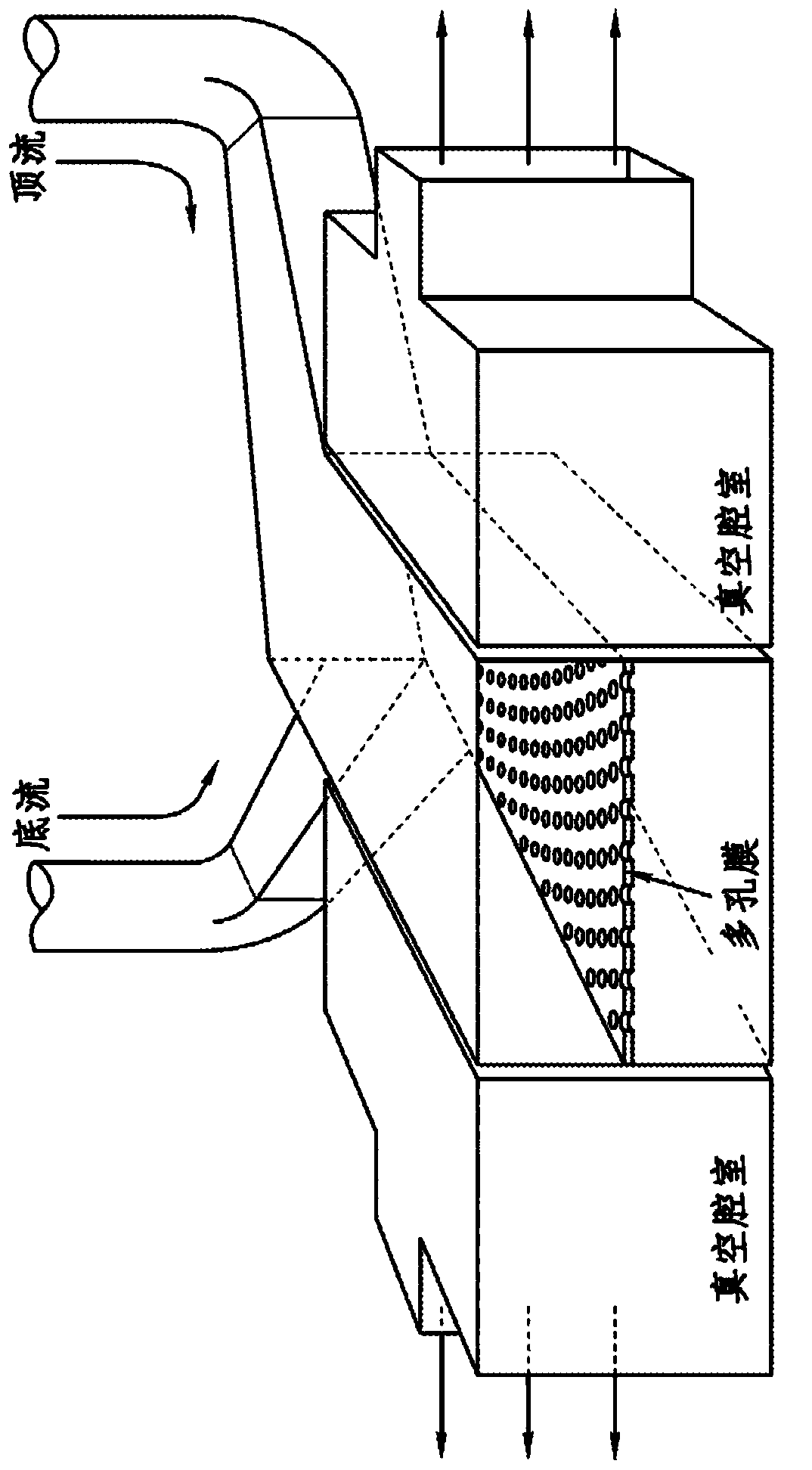
Cell culture system
The embodiments of the invention described herein relate to systems and methods for culturing and / or maintaining intestinal cells, tissues and / or organoids in vitro. The cells, tissues and / or organoids cultured according to the methods and systems described herein can mimic or reproduce natural intestinal epithelial structures and behavior as well as support co-culture of intestinal microflora.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
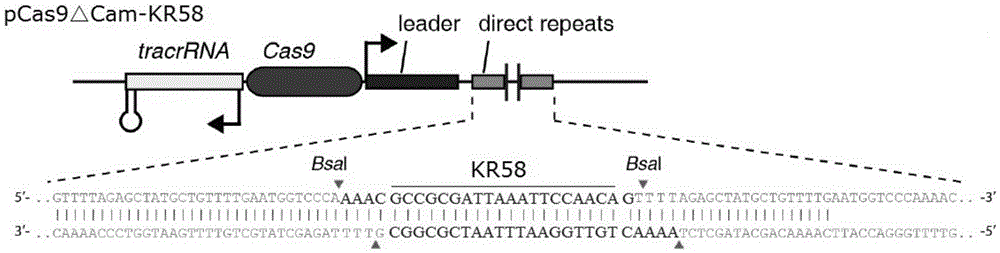
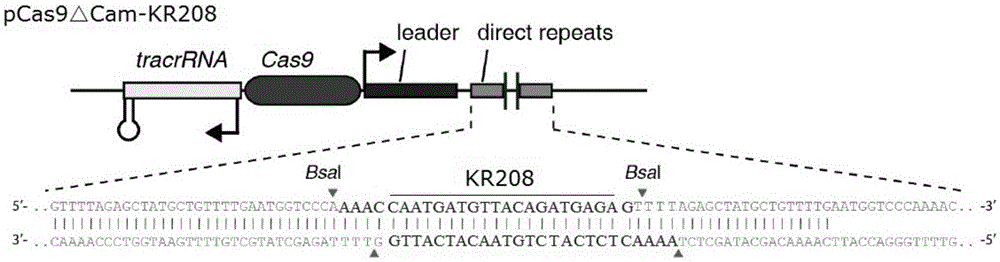
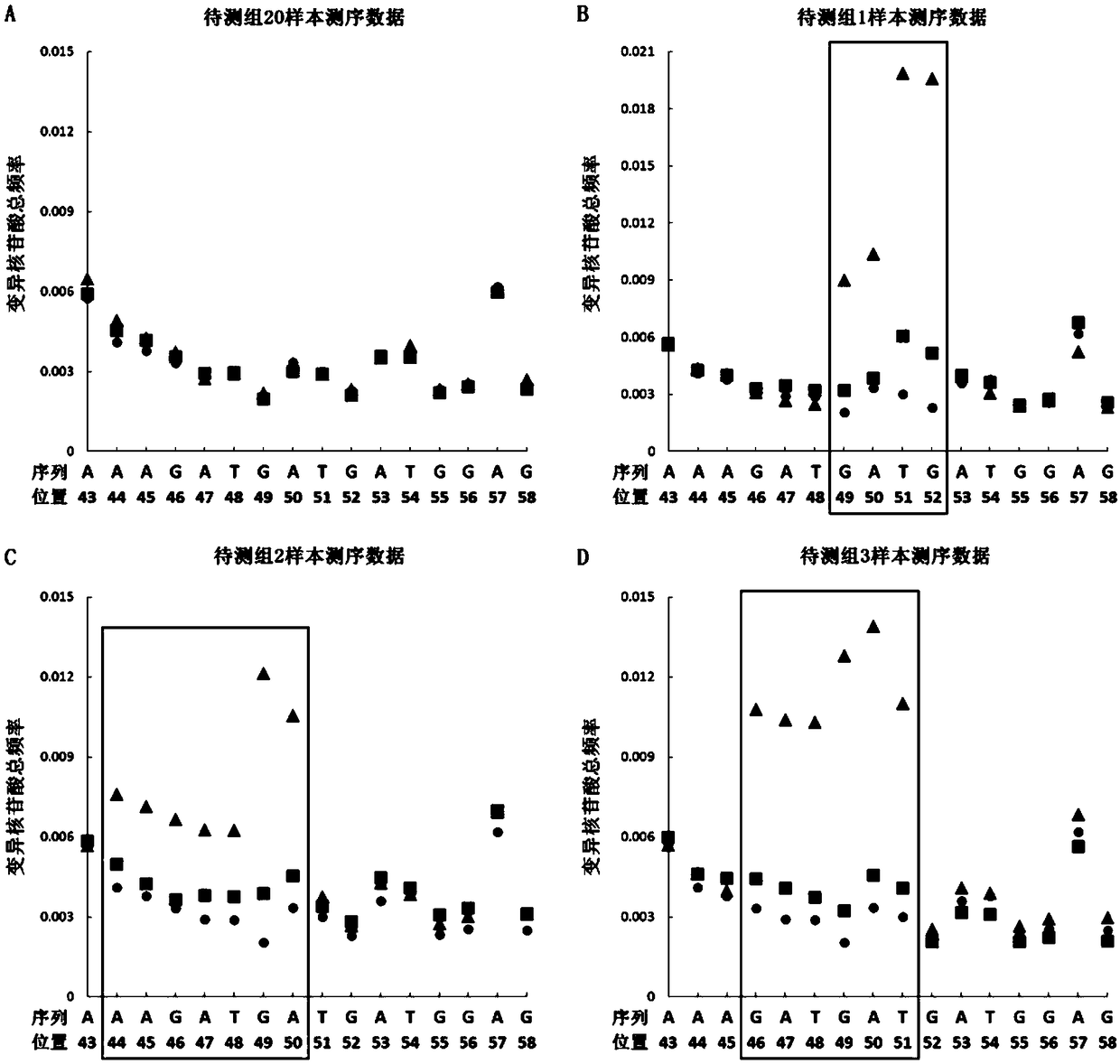
Recombinant vector for eliminating activity of kanamycin drug resistance gene and building method of recombinant vector
InactiveCN105463003AInhibitory activityHigh copy numberNucleic acid vectorVector-based foreign material introductionNovel geneOrganism
The invention provides a recombinant vector for eliminating the activity of a kanamycin drug resistance gene, and aims at eliminating drug resistance germs in organisms and solving the problem of kanamycin drug resistance of the germs. The recombinant vector for eliminating the activity of the kanamycin drug resistance gene is characterized by comprising a pCas9 vector subjected to chloramphenicol resistance elimination and a gRNA nucleotide sequence KR58 or KR208 aiming at a kanamycin resistance gene kan; the concrete nucleotide sequence of the KR58 is GCCGCGAT TAAATTCCAACA, and the concrete nucleotide sequence of the KR208 is CAATGATG TTACAGATGAGA. A building method of the recombinant vector mainly comprises the steps of carrying intergenic region nucleic acids by a novel gene editing tool CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) / Cas9 system; removing a chloramphenicol resistance gene on the recombinant vector; transforming the gene into vaccine vector bacteria such as attenuated salmonella typhimurium; performing co-culture on the recombinant bacteria and the kanamycin drug resistance gene so that the recombinant vector in the recombinant bacterium cell enters the kanamycin drug resistance bacteria in an engaging mode. The activity of the kanamycin resistance gene kan is effectively inhibited, so that the original drug resistance bacterium cannot grow on a kanamycin culture medium.
Owner:YANGZHOU UNIV
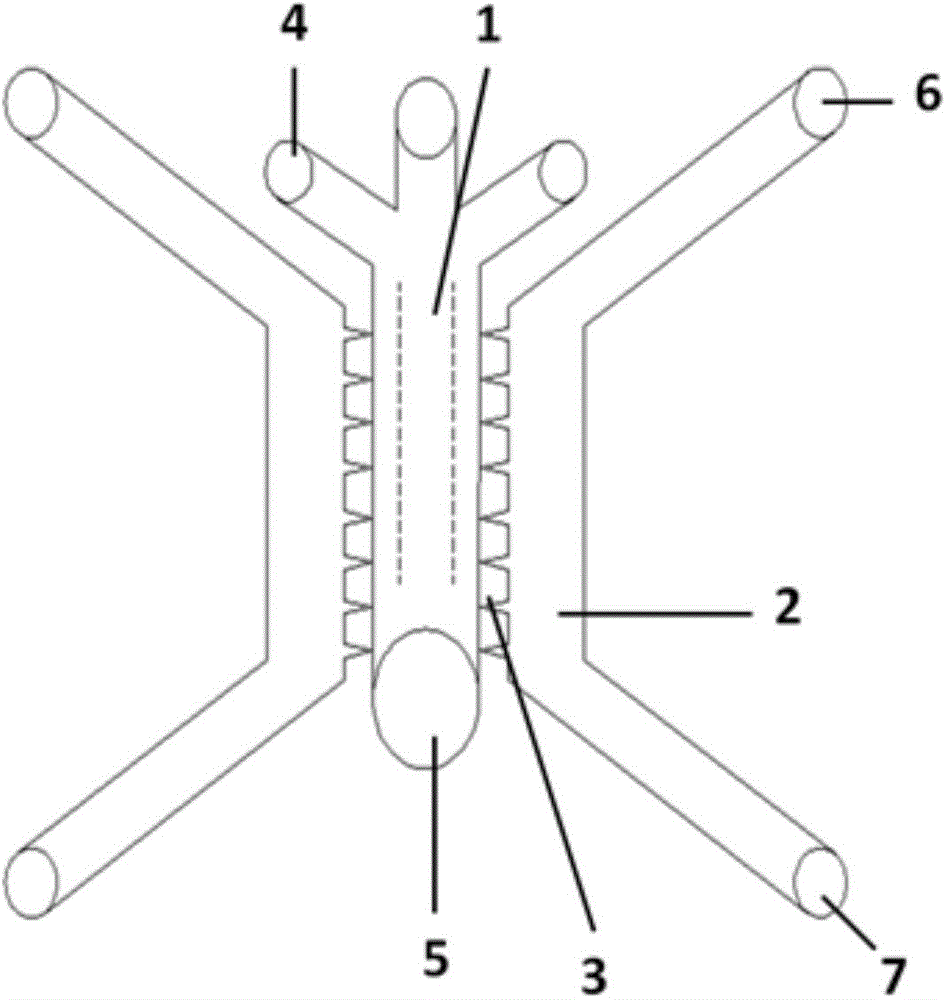
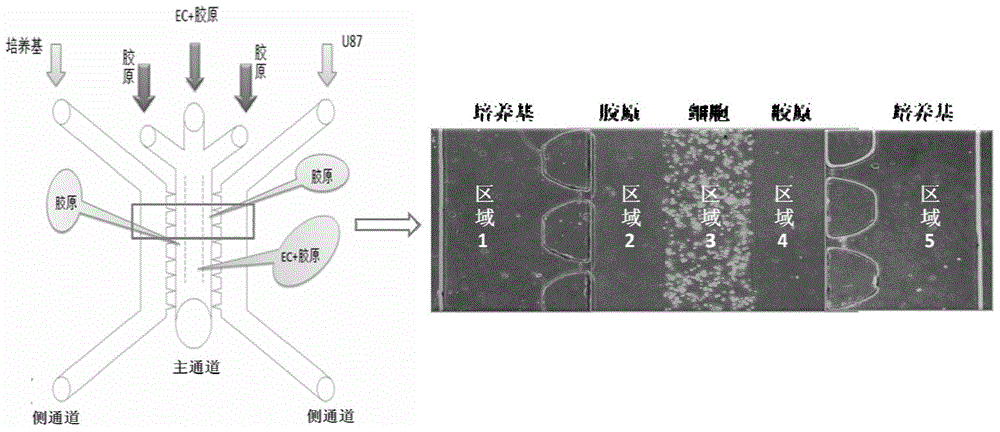
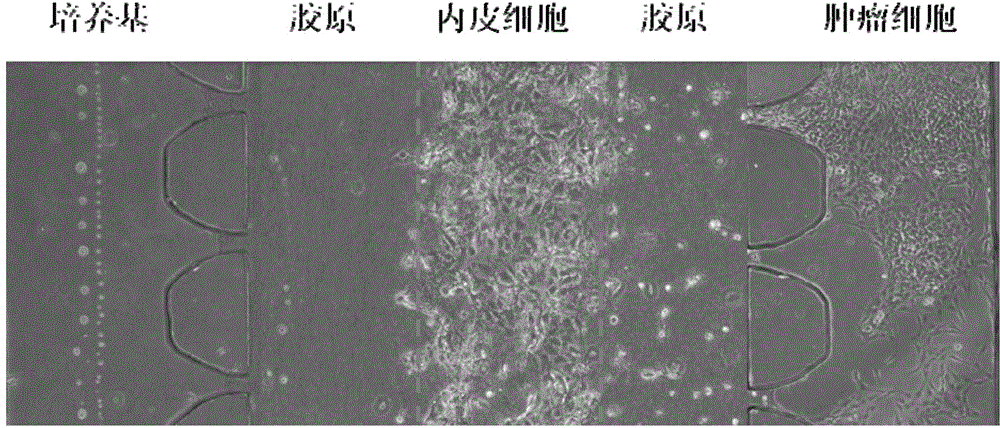
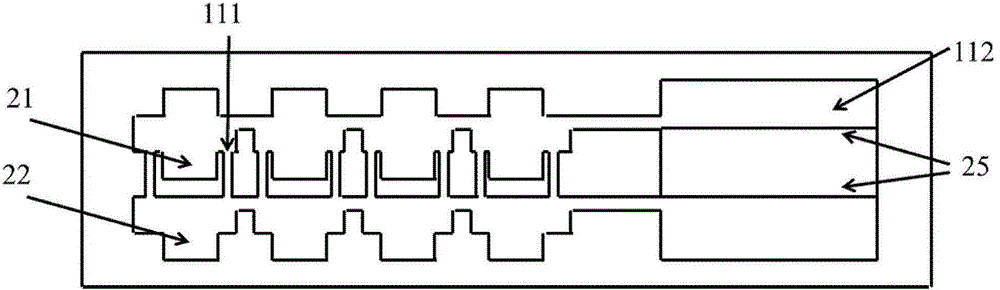
Multi-functional-region cell three-dimensional co-culture method based on micro-fluidic chip
ActiveCN105713835AAchieve inoculationAchieve perfusionArtificial cell constructsTissue/virus culture apparatusMain channelEngineering
The invention provides a multi-functional-region cell three-dimensional co-culture method based on a micro-fluidic chip. The chip is composed of a central main channel which is relatively low and side channels which are relatively high and are arranged on two sides, wherein the main channel and the side channels are connected by virtue of trumpet-shaped grid structures. Specifically, the method comprises the following steps: (1) adding collagen solutions different in composition to the sample inlet of the main channel of the chip and applying negative pressure to the outlet of the main channel, wherein the three collagen solutions flow into the main channel and functional regions, which keep distinct boundaries, are formed on the basis of a lamina flow principle; and (2) sterilizing the chip for a whole night through ultraviolet radiation, and directly blending cells to the collagen solutions which are injected into the main channel, so that cell inoculation can be achieved. By virtue of the micro-fluidic chip designed by the invention, a cell three-dimensional culture system, which has a plurality of independent functional regions, can be constructed in one step; and the method has an important application potential in the construction of in vitro complex cell micro-environments.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Transplantable cell growth niche and related compositions and methods
InactiveUS20070077649A1Repair damageNervous system cellsArtificial cell constructsAdipose tissuePolymer
Provided is a method of culturing a successor cell from a precursor cell comprising co-culturing a precursor cell such as, without limitation, a human embryonic stem cell or a neuronal stem cell with an adult mesenchymal stem cell. In one embodiment, the adult mesenchymal stem cell is derived from adipose tissue. Also provided is a composition comprising mesenchymal stem cells in a growth matrix biologically-compatible with the cells. In another embodiment, a method of regenerating tissue is provided comprising introducing into a patient a cell growth niche comprising either or both of mesenchymal stem cells and neuronal stem cell or successor cell in a growth matrix biologically-compatible with the cells. In another embodiment, a method of regenerating tissue is provided comprising introducing into a patient a cell growth niche comprising matrix, cells and slow release polymer beads containing growth factors.
Owner:UNIVERSITY OF PITTSBURGH
Establishment and characterization method of in-vitro blood brain barrier model based on microfluidic chip
InactiveCN102978109AReduce consumptionResolve the secondary inoculationArtificial cell constructsVertebrate cellsVaccinationEngineering
The present invention provides an establishment and characterization method of in-vitro blood brain barrier model based on microfluidic chip. The microfluidic chip mainly comprises a cell inlet pool (1), collagen inlet pools (2), a cell culture chamber (3) and a waste liquid pool (4). The upper of the cell culture chamber (3) is connected with the cell inlet pool (1). The lower of the cell culture chamber (3) is connected with the waste liquid pool (4). Each collagen inlet pool respectively includes four observation chambers. The collagen inlet pools are communicated with the cell culture chamber (3). According to the invention, construction and characterization of the in-vitro blood brain barrier model and evaluation of the barrier function are integrated into a chip of a few square centimeters, and the chip can be used for in-vitro simulation and subsequent applications of the blood-brain barrier model. Compared the present invention with a Transwell small chamber co-culture model, the cell co-culture model secondary vaccination and time-consuming problems are solved, the flow conditions are added, the chip is closer to the true in-vivo microenvironment, the cell and reagent consumption are significantly reduced, and a plurality of experiment parameters can be obtained once and simultaneously.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Two-layer cell culture system organ chip and preparation method thereof
InactiveCN103981096AEasy to observeReduce dosageBioreactor/fermenter combinationsBiological substance pretreatmentsHuman bodyMicro structure
The invention relates to a two-layer cell culture system organ chip and a preparation method thereof. The organ chip comprises a two-layer cell culture system, wherein each layer comprises a culture solution micro-fluid channel, a medicament micro-fluid channel, cell culture chambers and a medicament testing tank; a micro structure and micro-fluid channels are designed on the organ chip, two cells are respectively fixed on each specific cell culture chamber, and intercellular signal transmission and interaction between the cells are performed by virtue of the micro-fluid channels. The organ chip realizes the parallel implantation and co-culture of two or more cells, is simple in operation, reduces the dose of practical samples, simplifies the cell implantation process, has the characteristics of portability, economic performance, high efficiency and accuracy, and can be used for independently performing cell seeding and culture and detection of medicament toxicity or pharmacological activity. The two-layer cell culture system organ chip is a novel organ chip with miniaturization, automation and visualization, prepared by simulating the structures and functions of a human organ, and can be used for providing the effective theoretical basis for tissue and regeneration engineering, organ transplantation and medicament evaluation.
Owner:SOUTHEAST UNIV
Stat3 antagonists and their use as vaccines against cancer
The present invention relates to methods for treating and / or preventing cancer. In particular the present invention relates to ex vivo immunotherapeutic methods. The methods comprise decreasing Stat3 (signal transducer and activator of transcription3) expression and / or function in tumor cells and the administration of such cells to a subject in need of treatment and / or prevention. Other methods of the invention comprise activating T-cells by co-culturing the T-cells with the tumor cells with decreased Stat3 expression or function. The invention further encompasses methods comprising decreasing Stat3 expression or function in antigen-presenting cells and co-administering tumor cells and the antigen-presenting cells with decreased Stat3 function to a patient. The invention further relates to methods for stimulating dendritic cell differentiation.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
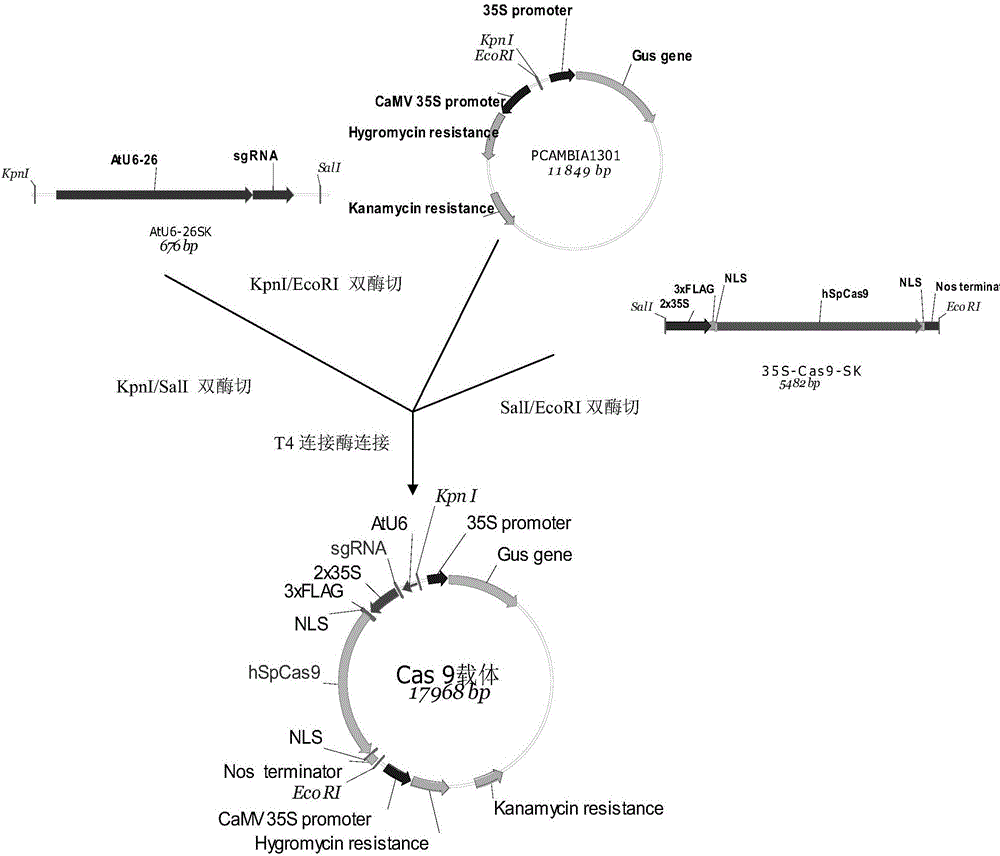
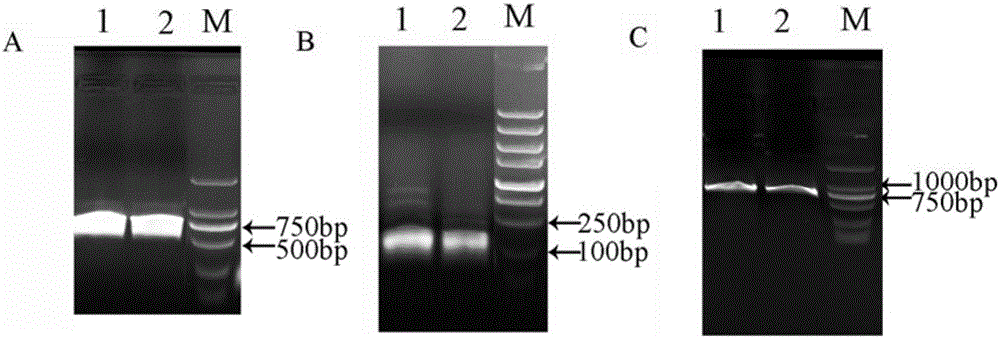
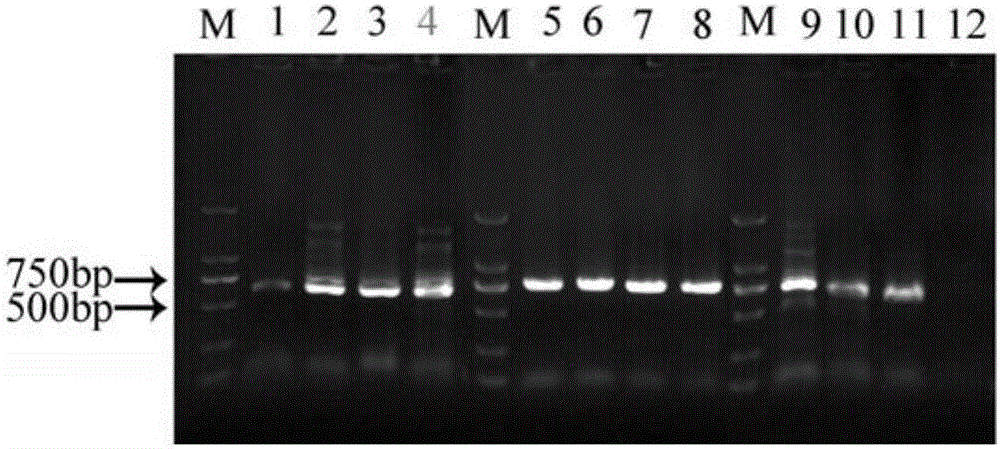
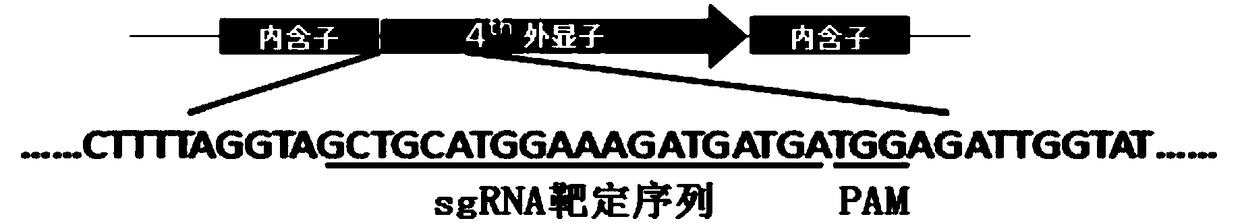
Cas9 mediated carnation gene editing carrier and application
The invention relates to a Cas9 mediated carnation gene editing carrier and application. The application comprises the following steps: firstly, establishing a CRISPR-Cas9 system of carnation-containing target gene sites, introducing the Cas9 expression carrier into an agrobacterium tumefaciens C58 strain, putting roots of carnation leaves into a pre-culture medium, culturing with light for 3-4 days at 22+ / -2 DEG C, activating agrobacterium containing the Cas 9 expression carrier, dipping the pre-cultured explant into the activated agrobacterium solution for 20-30 minutes, completely absorbing the agrobacterium solution, transferring into a co-culture medium, performing dark culture for 3-4 days at 22+ / -2 DEG C, further transferring into a screening culture medium to culture, performing light culture at 22+ / -2 DEG C so as to differentiate regeneration buds, further transferring the regeneration buds into a multiplication medium for multiplication screening culture, detecting positive transgenosis regeneration plants, sequencing target sites, and detecting mutation strain systems of carnation target gene sites.
Owner:FLOWER RES INST OF YUNNAN ACAD OF AGRI SCI
Preparation method of non-transgenic CRISPR mutant
InactiveCN108611364AReduce frequencyImprove accuracyNucleic acid vectorVector-based foreign material introductionScreening methodSexual reproduction
The invention provides a preparation method of a non-transgenic mutant based on a CRISPR-Cas9 technology. Specifically, the preparation method comprises a construction method and a screening method. The construction method is characterized in that CRISPR-Cas9 and sgRNA are preassembled and are located at T-DNA of agrobacterium tumefaciens; site-directed change of a target gene of a target plant can be realized by infection of the target plant by the agrobacterium tumefaciens and coculture without integrating the sequences of the CRISPR-Cas9 and the sgRNA into a genome of the target plant, so that an obtained site-directed mutant material of the target gene does not need the processes of sexual reproduction, segregation posterity, population screening and the like or does not contain any exogenous gene sequence. The obtained regeneration seedlings are screened by a high-throughput sequencing and high-resolution melting curve technology provided by the invention; mutant plants of which target genes are mutated can be obtained by identifying the regeneration seedlings efficiently and quickly at the current generation of transgene, even if low-proportion mutants of which the proportionis 1 / 100 of a mixed population can be screened. The screening method provided by the invention still can ensure excellent accuracy and sensitivity.
Owner:NANJING AGRICULTURAL UNIVERSITY
Cell culture tool and method
ActiveUS20050101010A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBiologyCell material
A cell co-culture tool includes a body, an outer wall surrounding the body, and more than one vessel within the perimeter of the outer wall. Each vessel has a top edge below a rim of the outer wall. A method of interacting a substance with more than one type of cell material in a culture dish having a plurality of wells includes depositing a different type of the cell material in separate wells of the culture dish, interconnecting the wells with a fluid medium, and adding the substance to the fluid medium.
Owner:DISCOVERY LIFE SCI LLC
Isolation and cultivation of microorganisms from natural environments and drug discovery based thereon
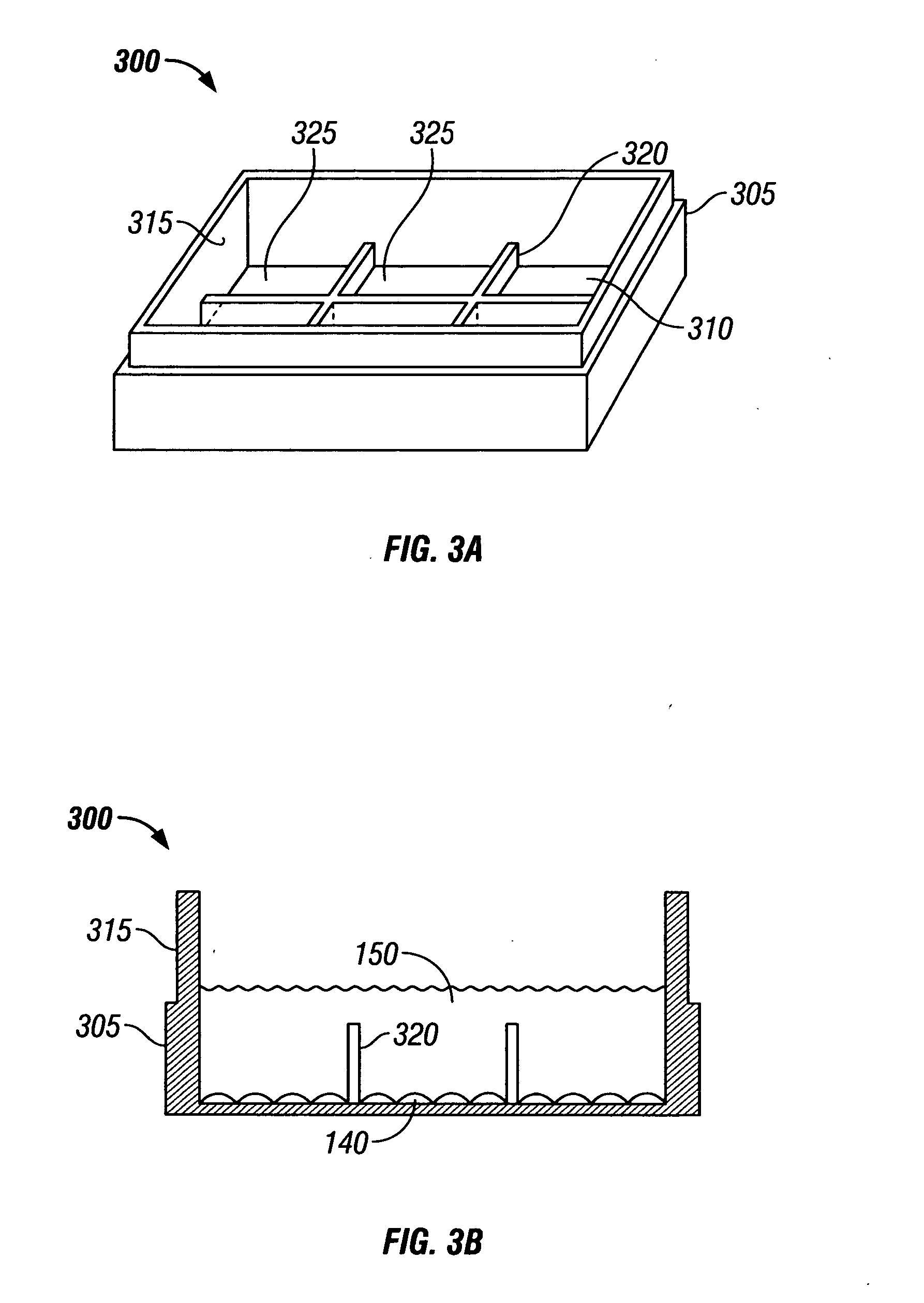
InactiveUS7011957B2Simple wayCompound screeningBioreactor/fermenter combinationsMicroorganismMicrobiology
The method of the invention is directed to the novel use of a diffusion chamber within which previously “uncultivatible” microorganisms can be isolated. Rather than attempting to replicate the natural environment of an unknown microorganism, the method of the invention provides for exposing dividing microorganisms to all the components of the original environment while simultaneously containing the resulting colonies so that they can be isolated. The method of the invention can take advantage of the recognition that the preponderance of difficult-to-grow microorganisms do not form colonies visible to the naked eye. Therefore, these organisms must be isolated under a compound microscope as “microcolonies.” In addition, methods according to the invention permit the isolation of novel microorganisms capable of growing in artificial media only in co-culture in the presence of a companion microorganism.
Owner:NORTHEASTERN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com