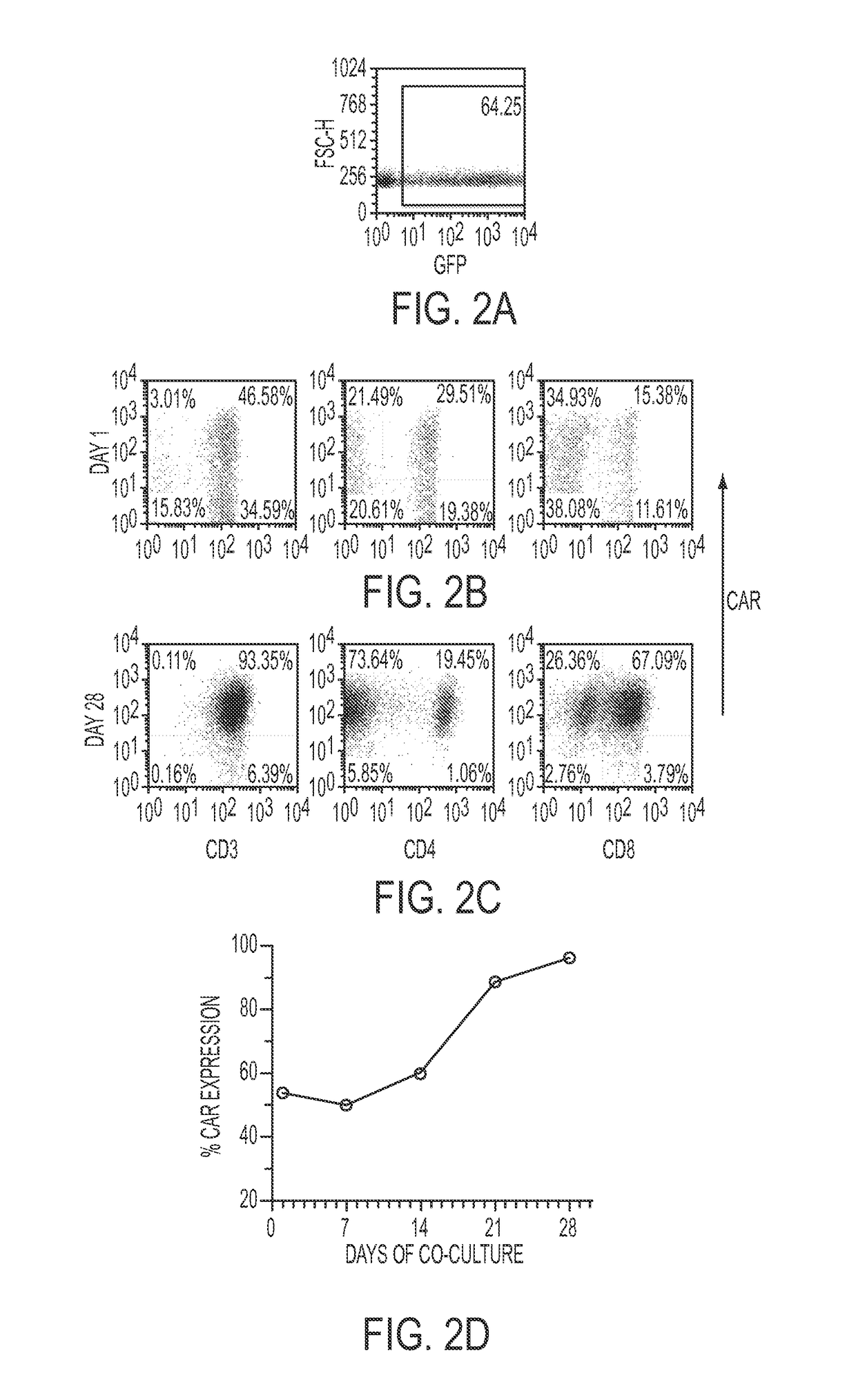
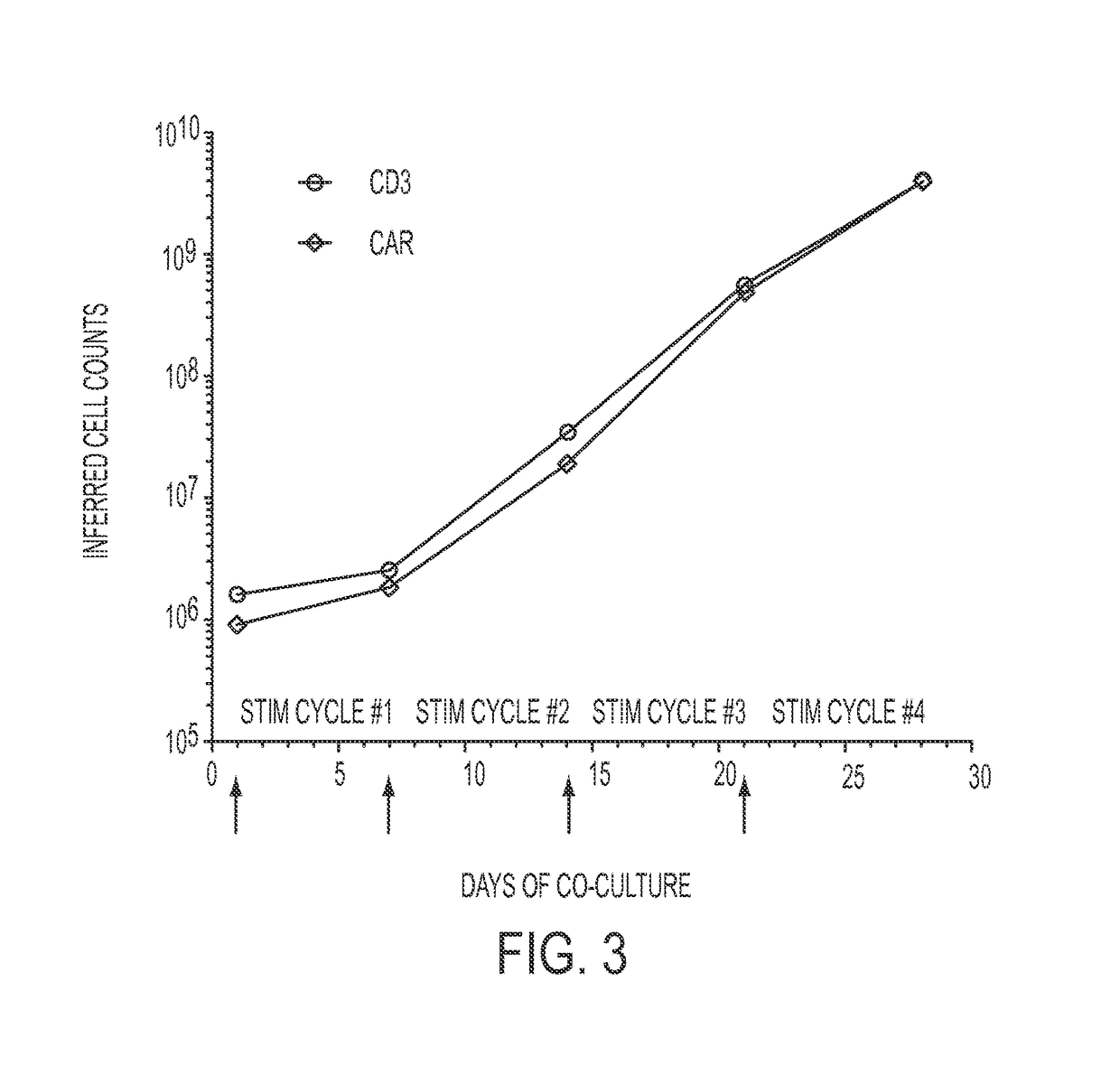
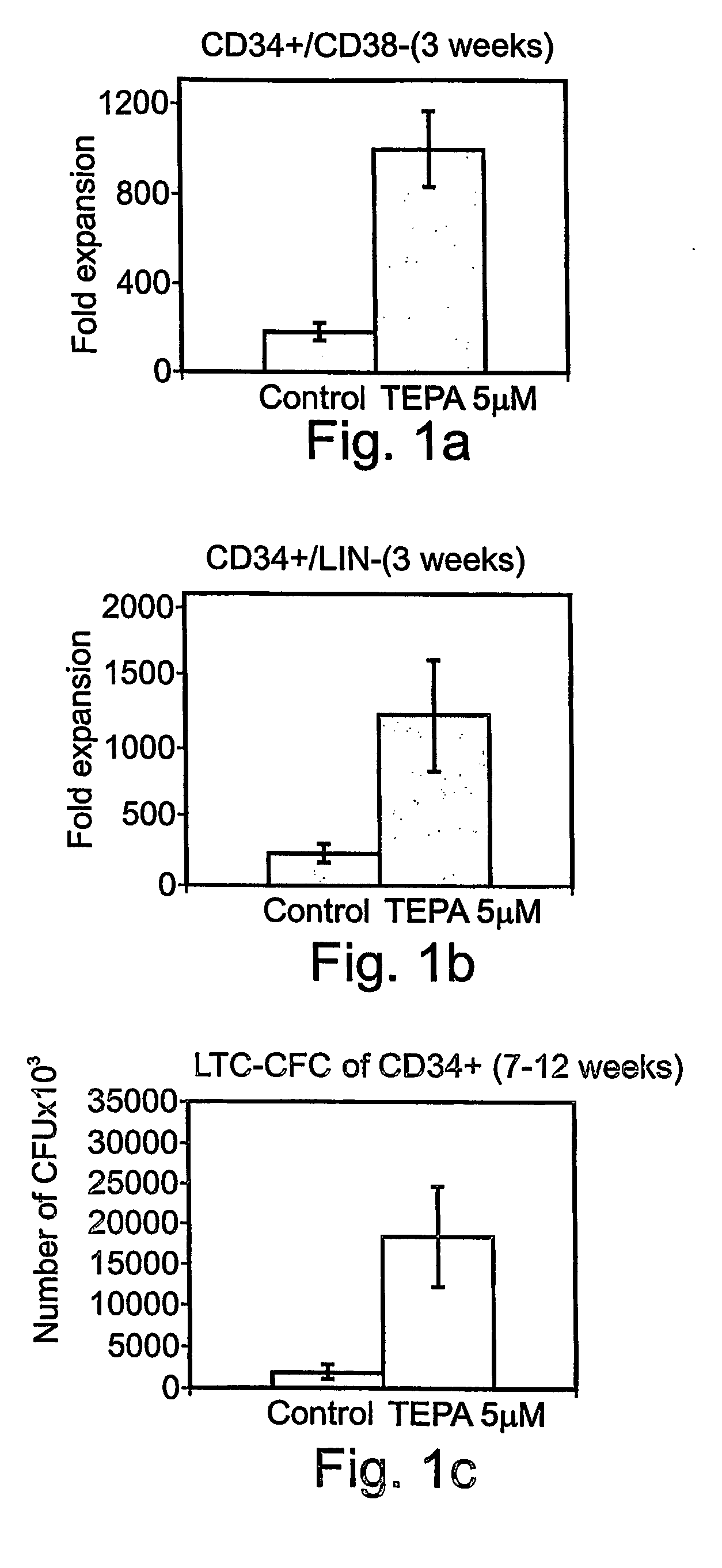
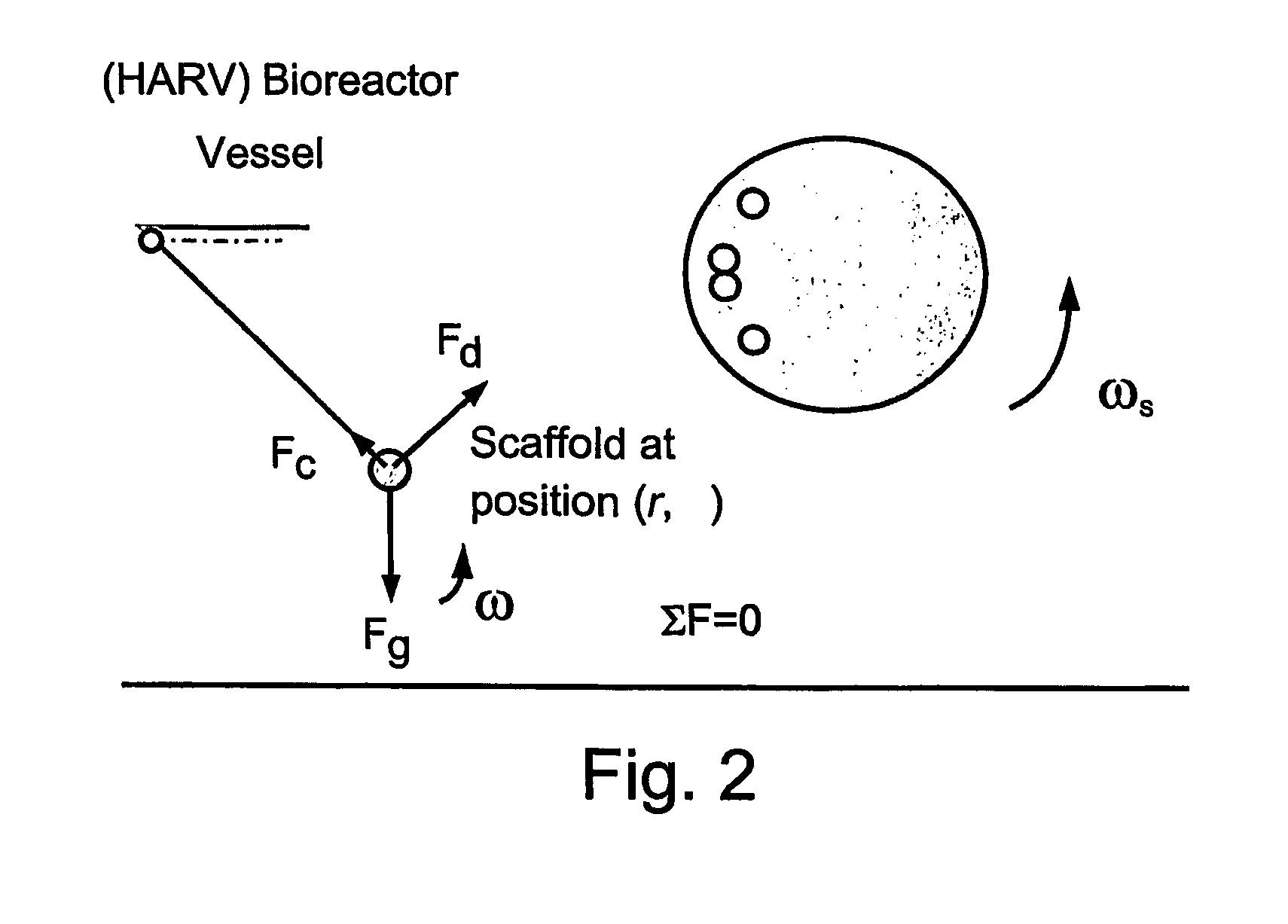
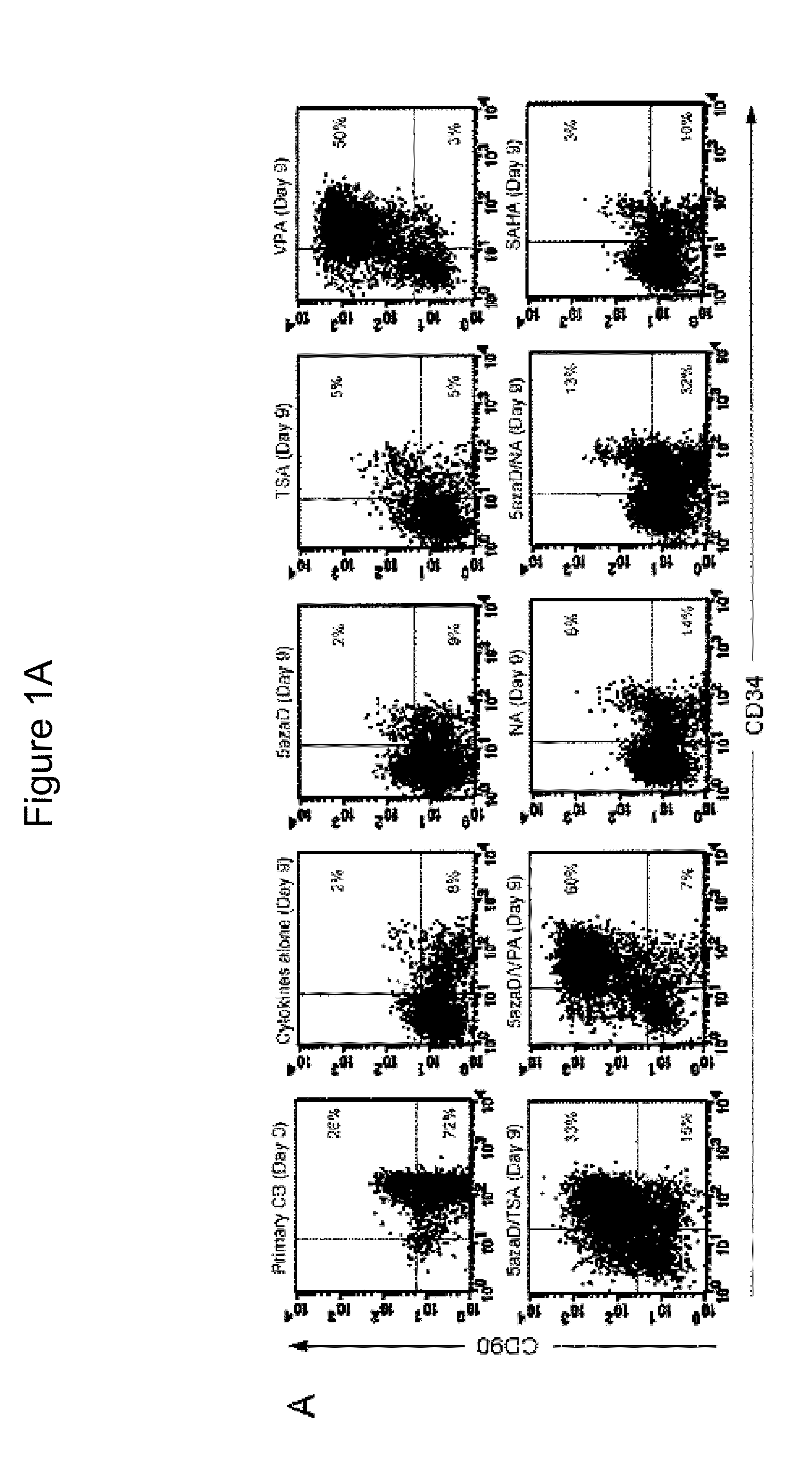
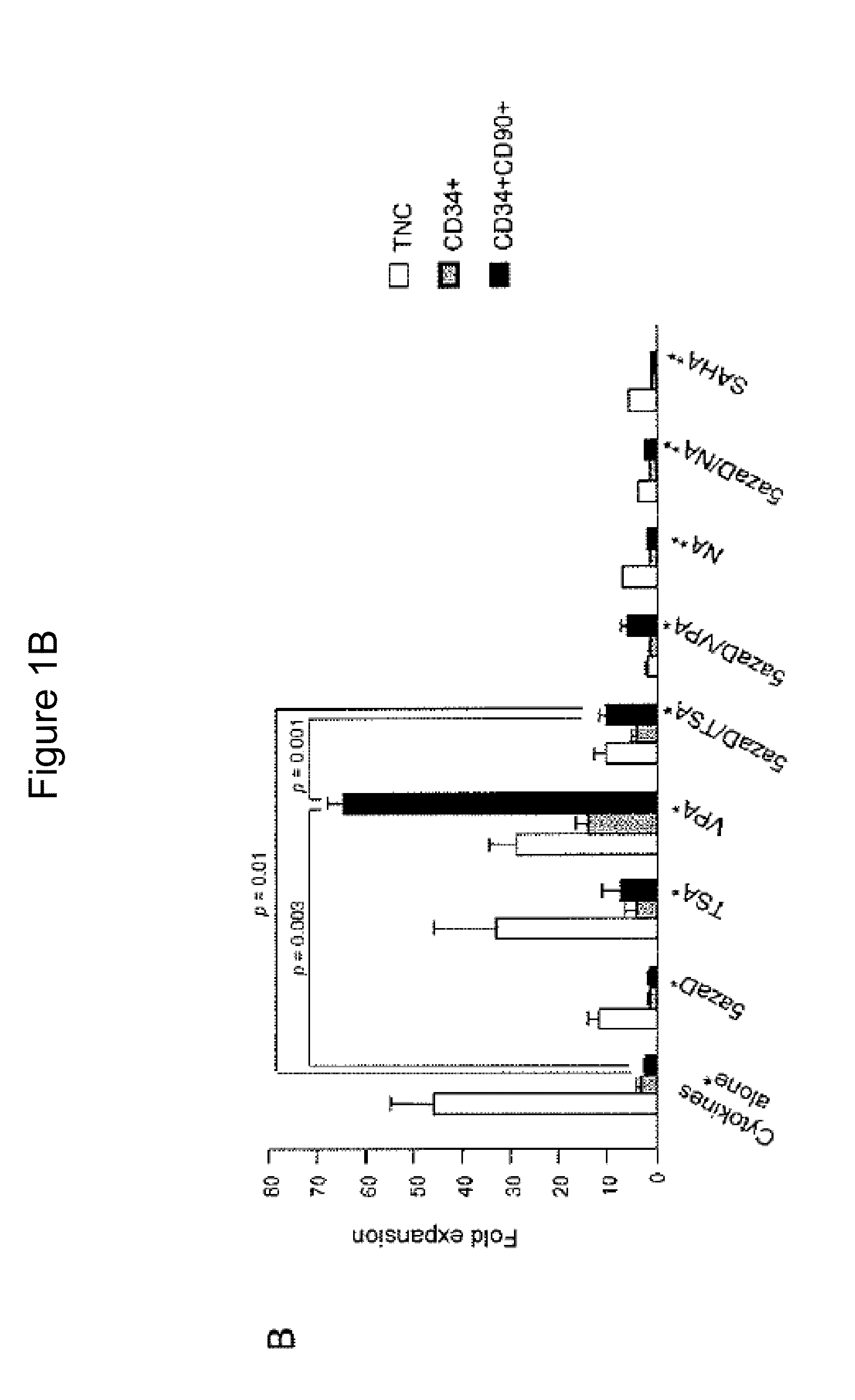
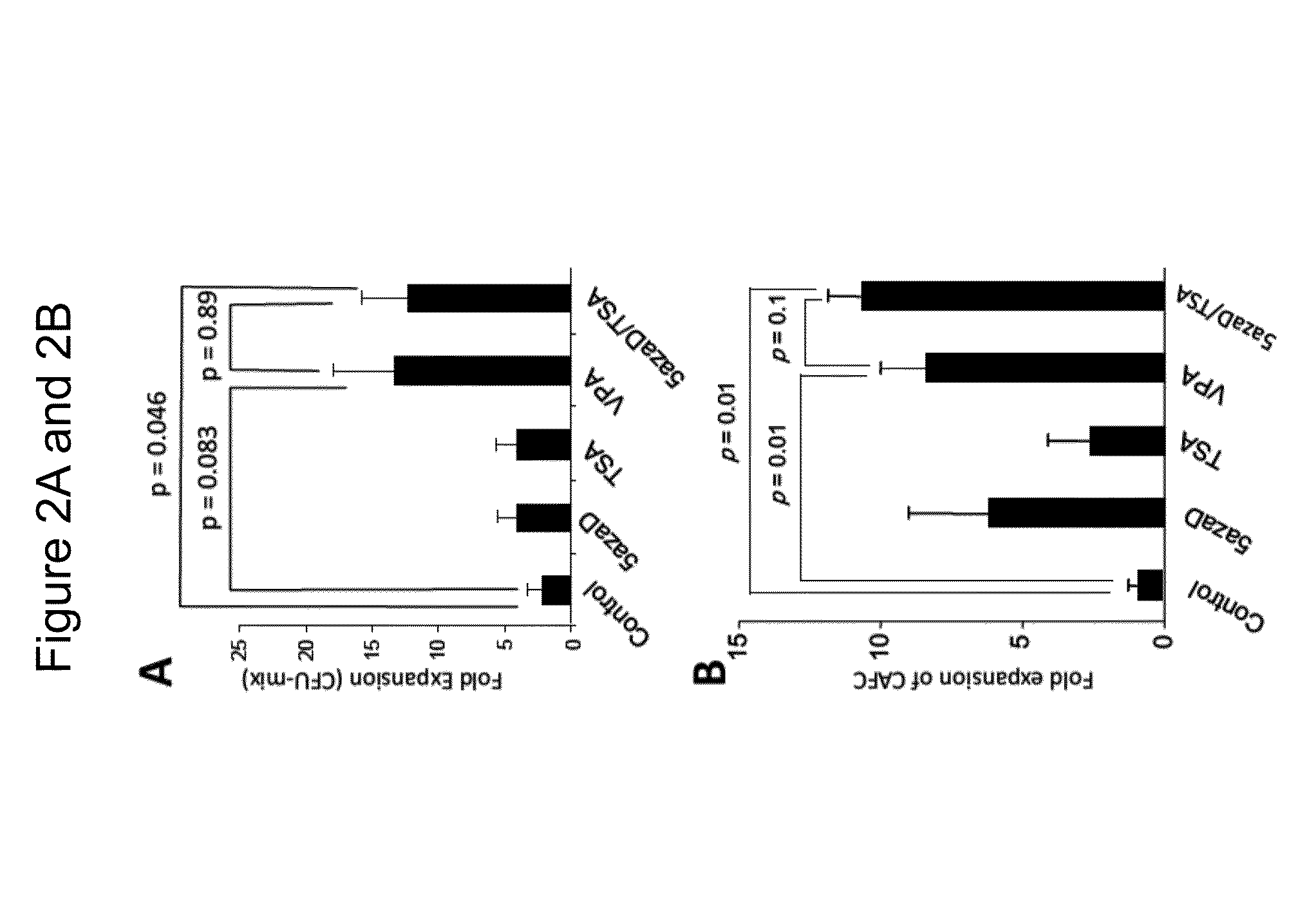
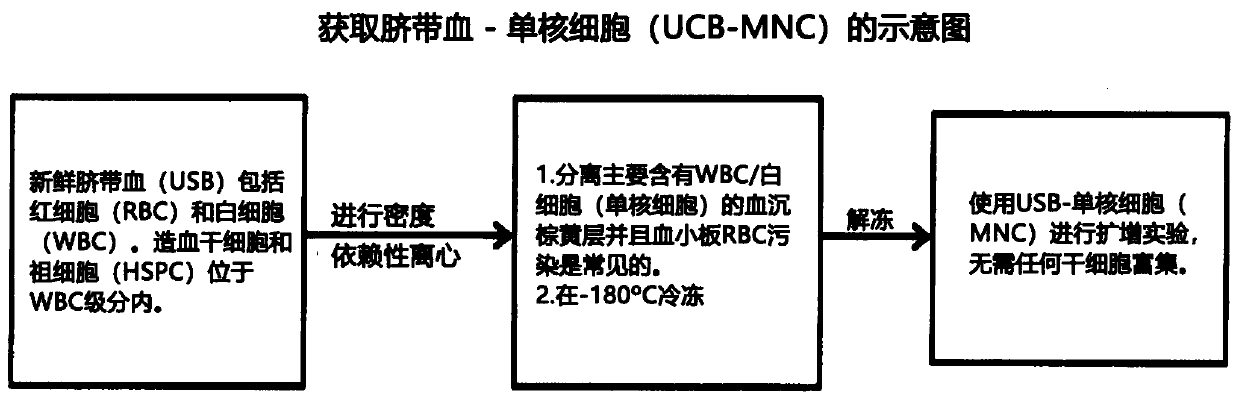
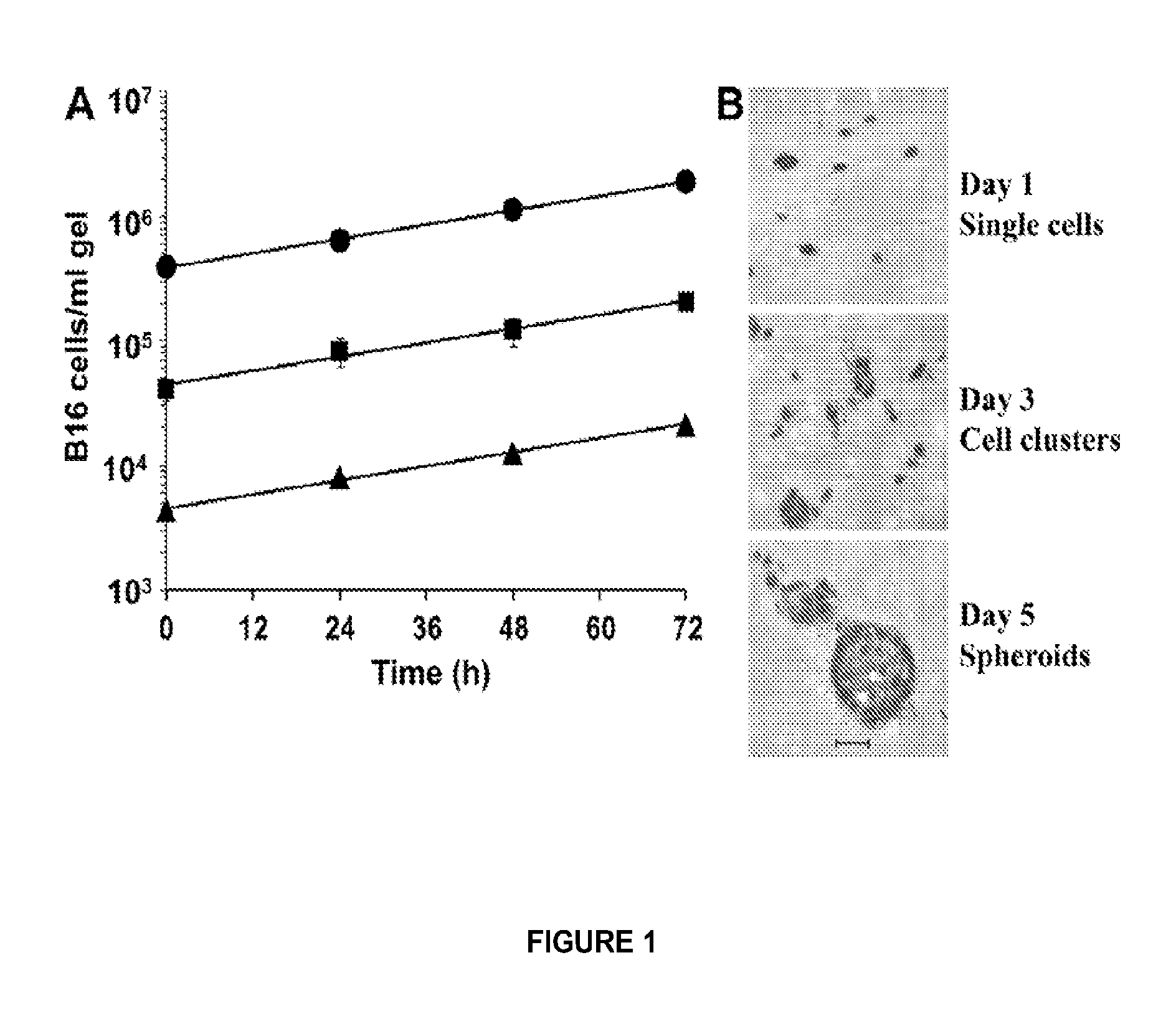
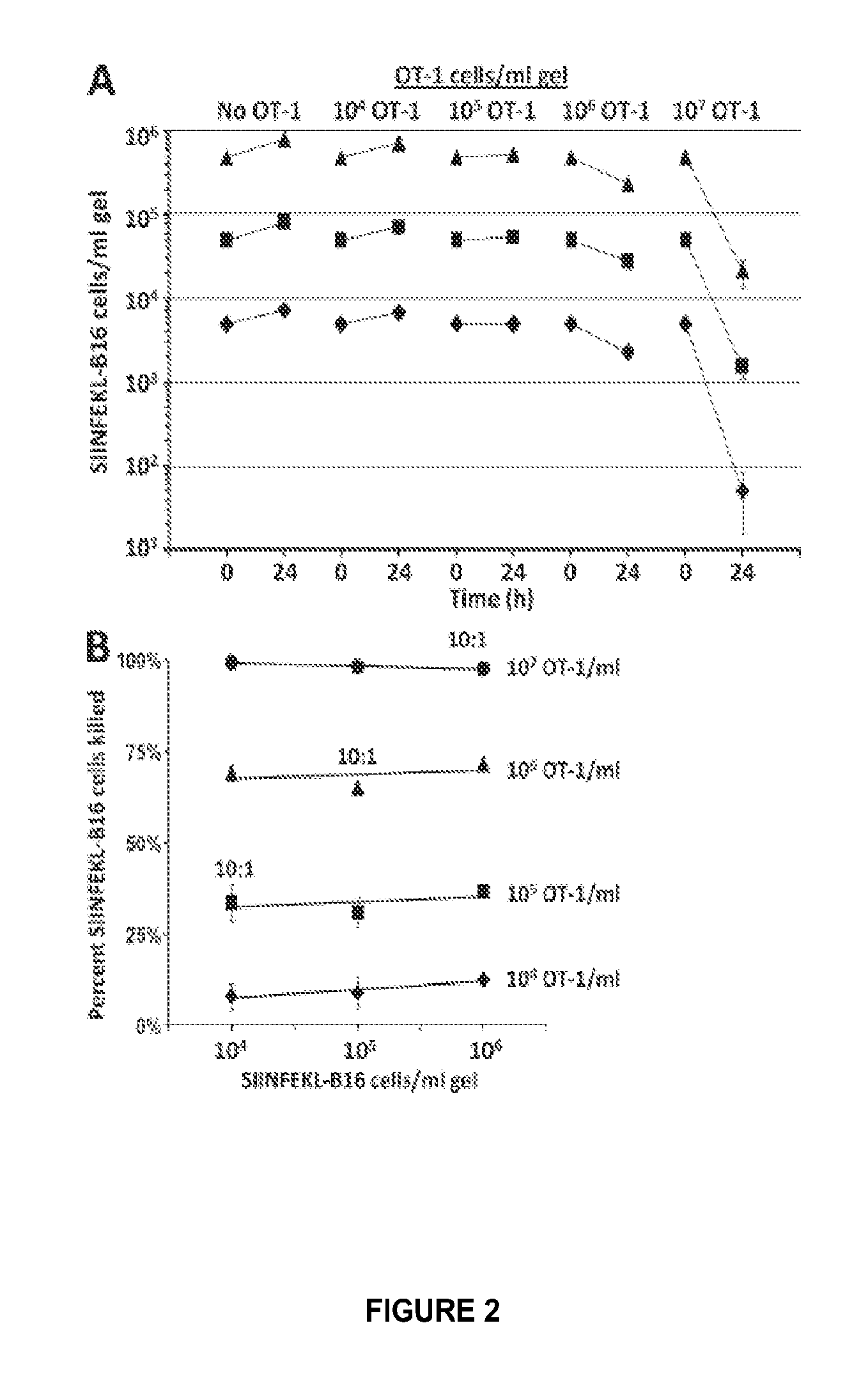
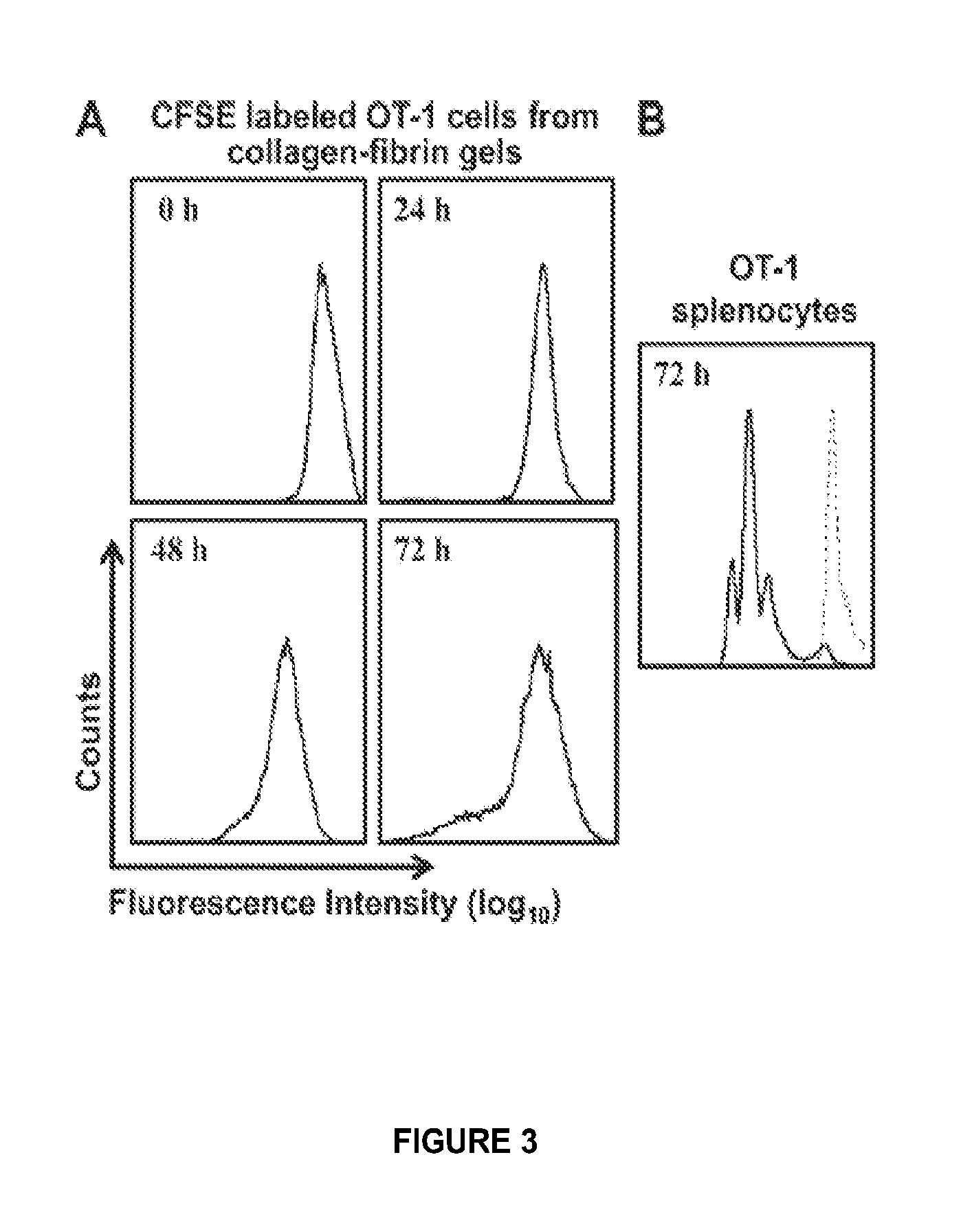
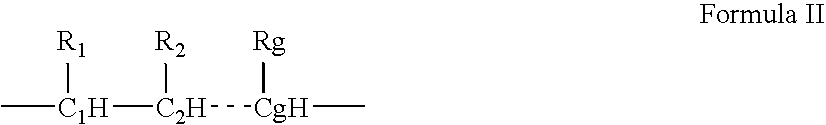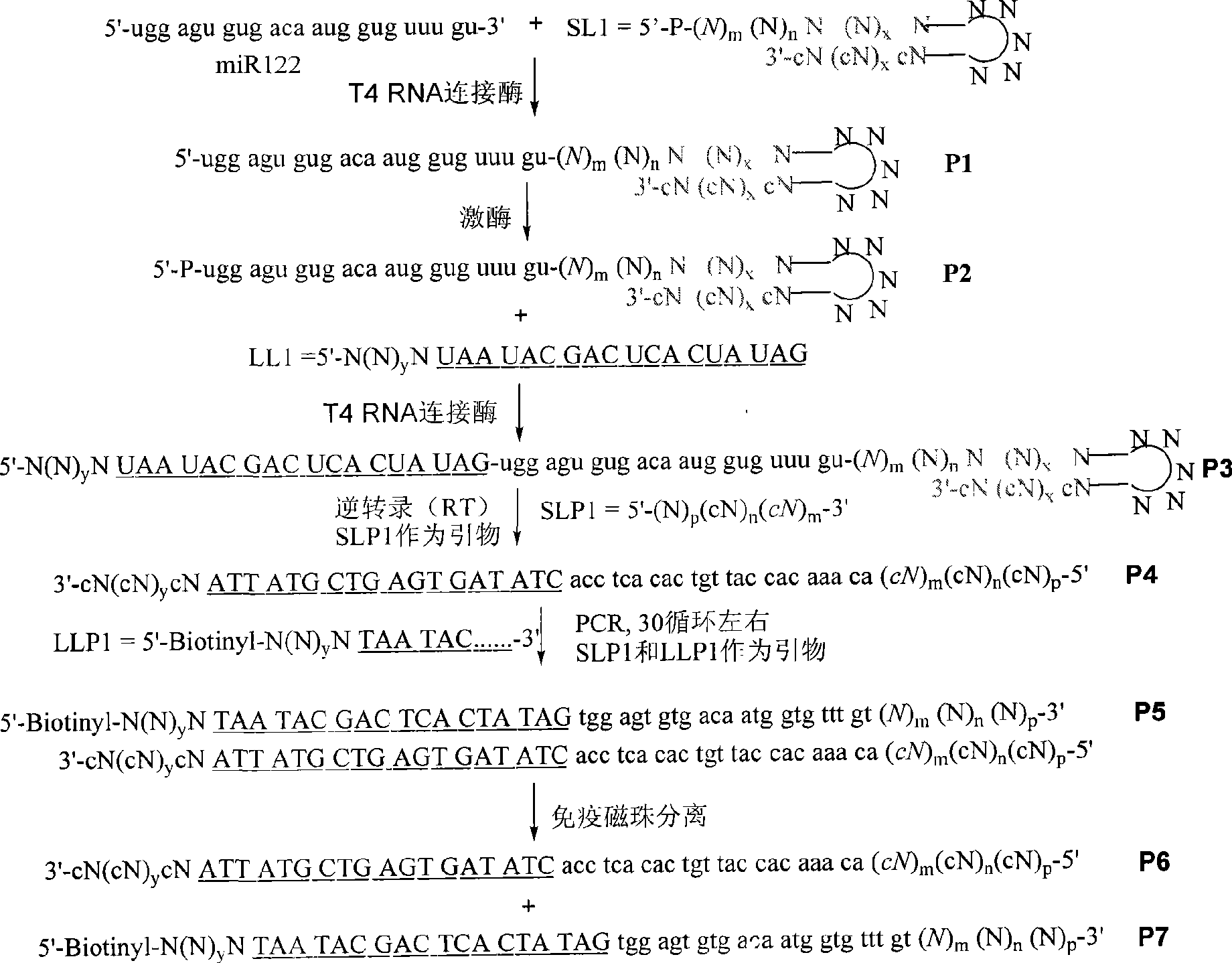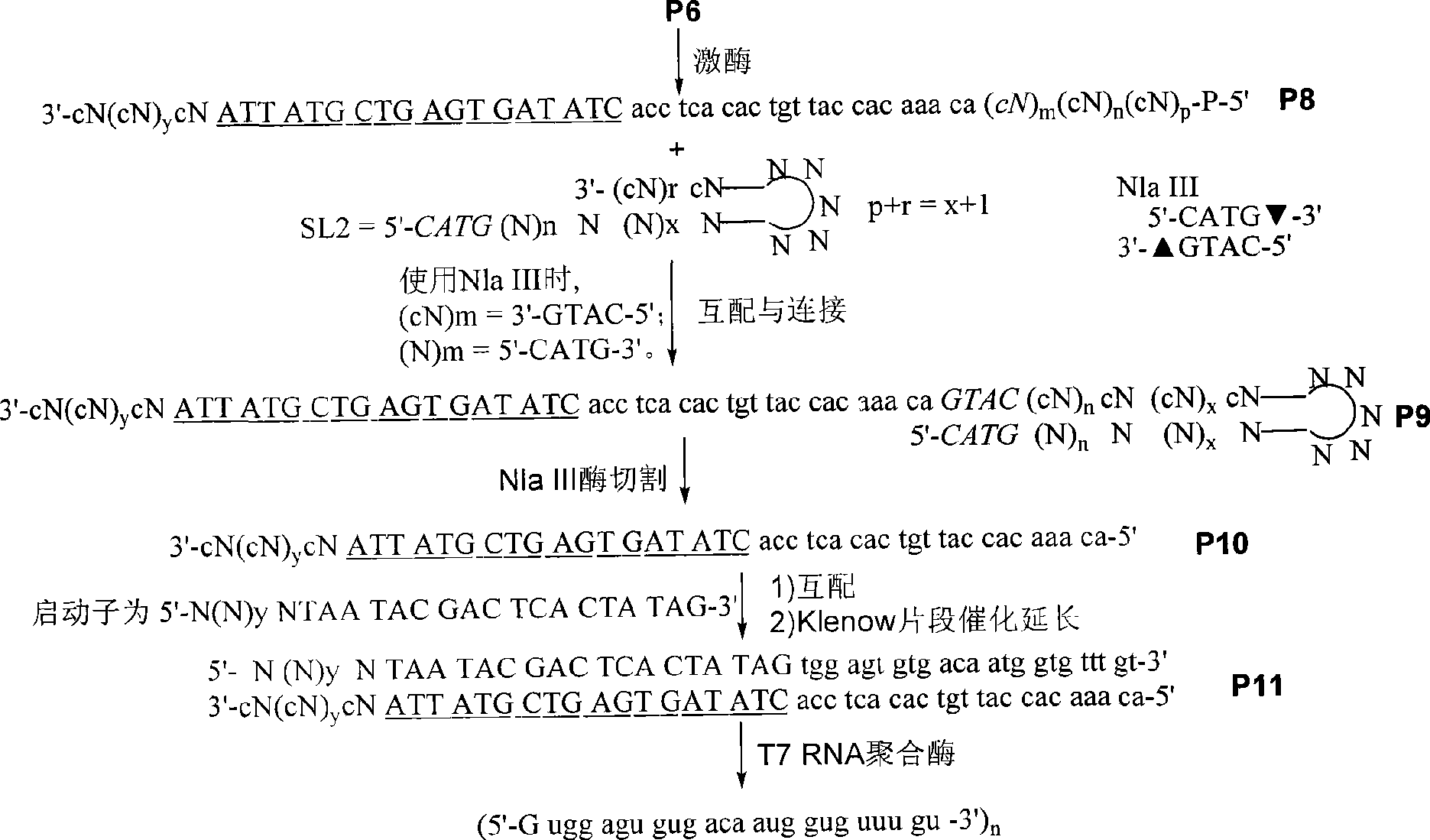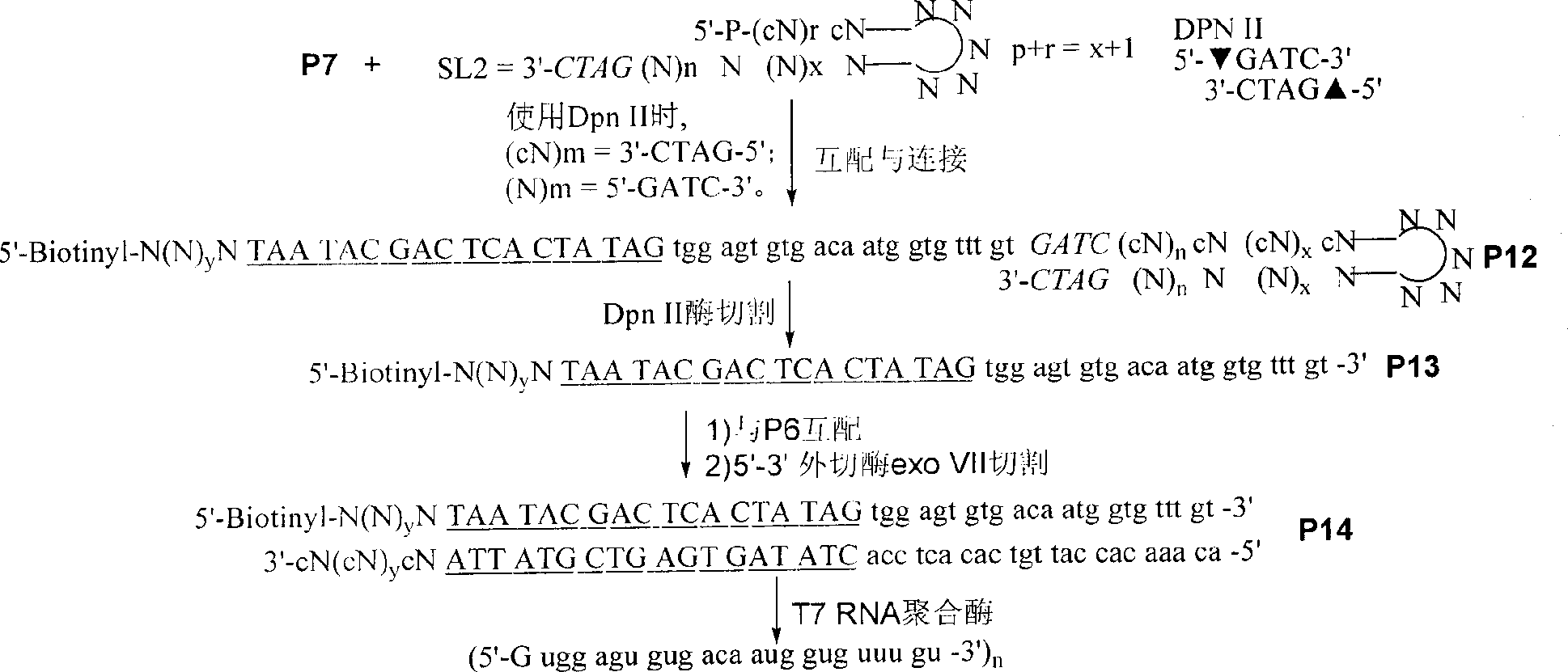Patents
Literature
55 results about "Ex vivo expansion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of ex vivo progenitor and stem cell expansion by co-culture with mesenchymal cells
Methods of ex-vivo expansion and at the same time inhibiting differentiation of stem cells by co-culture with mesenchymal cells, transplantable populations of renewable progenitor and stem cells expanded thereby, and their uses in therapeutic applications.
Owner:GAMIDA CELL
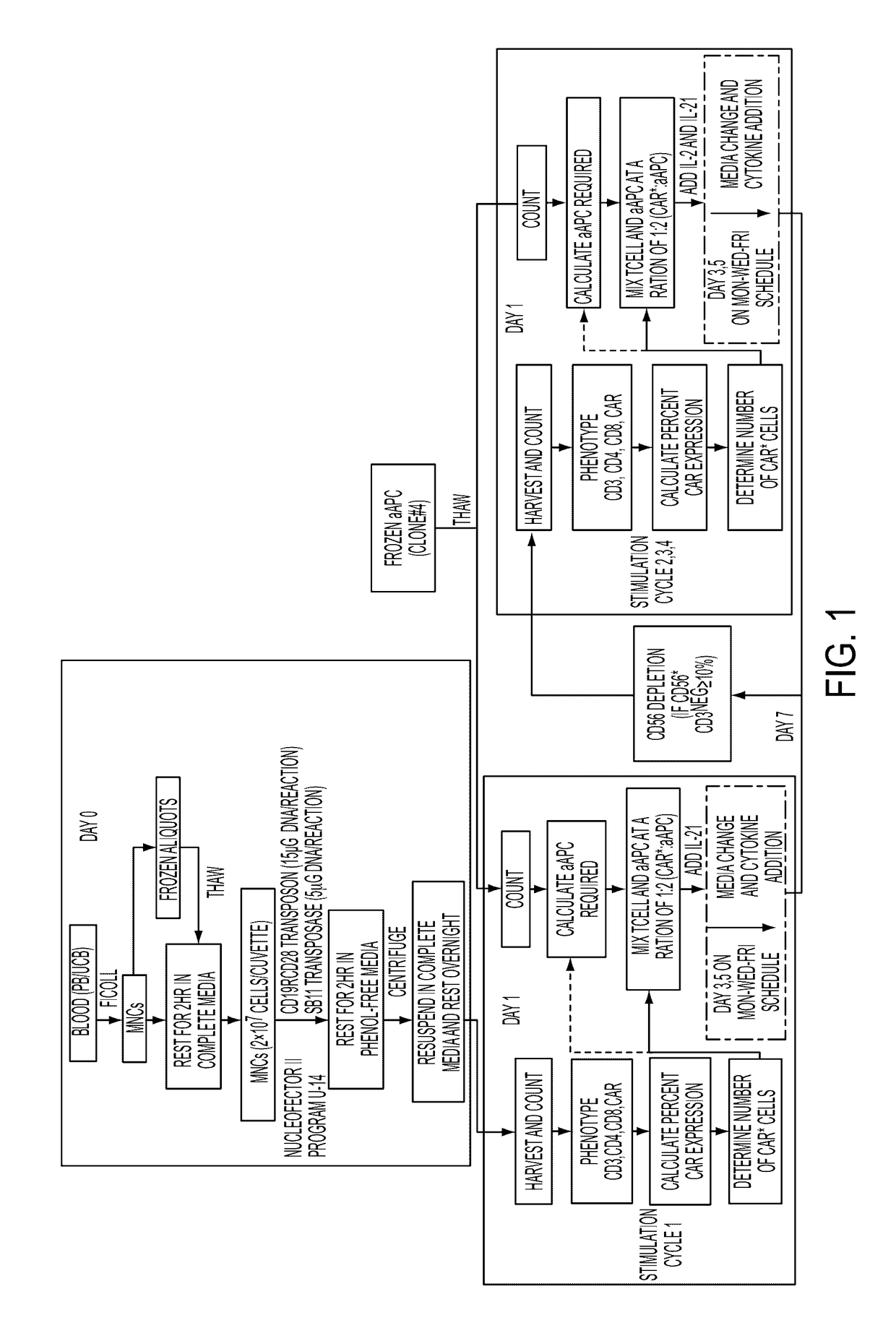
Human application of engineered chimeric antigen receptor (CAR) T-cells
ActiveUS9629877B2Promote proliferation and survivalInhibit expressionImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsHuman bodyElectroporation
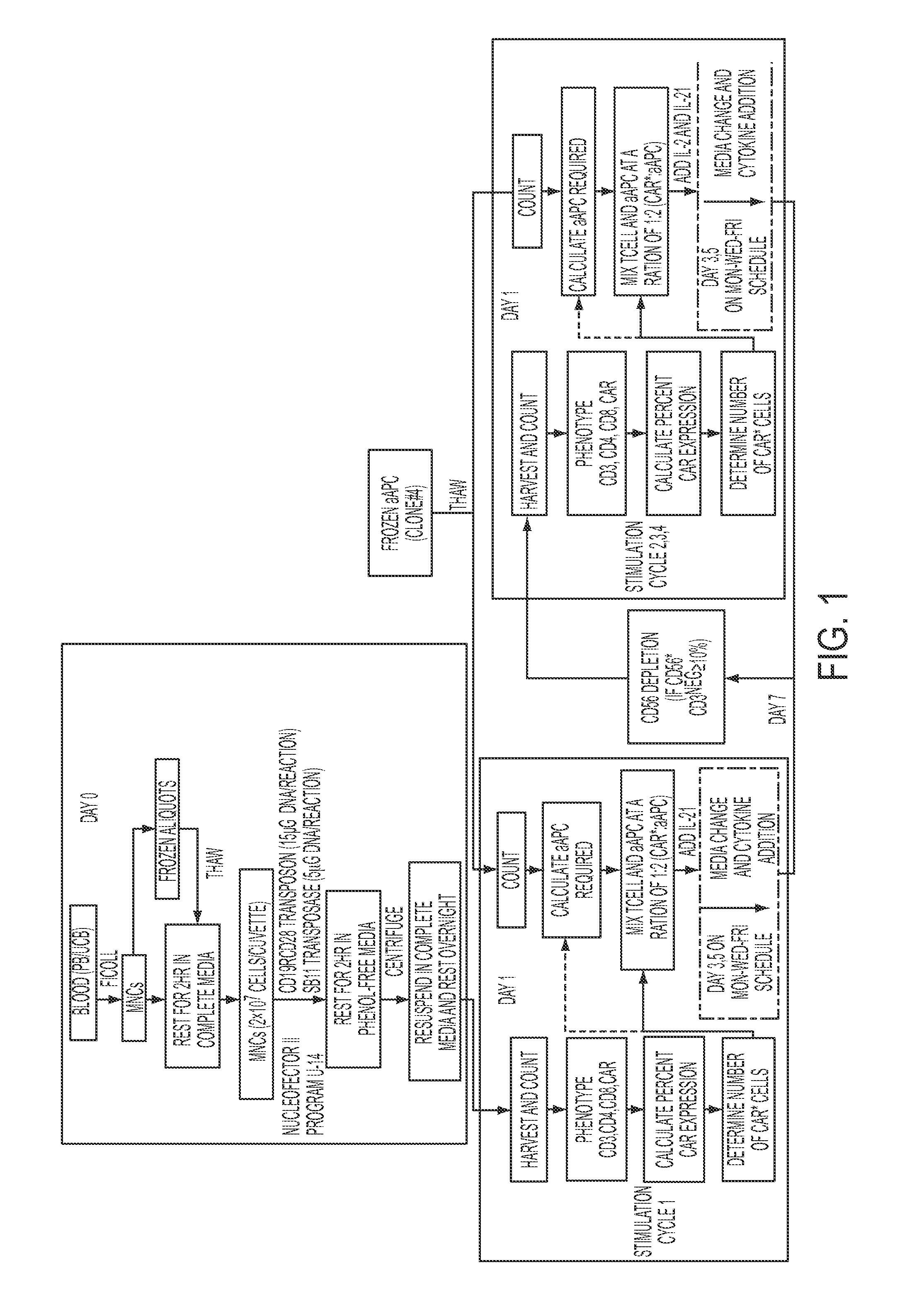
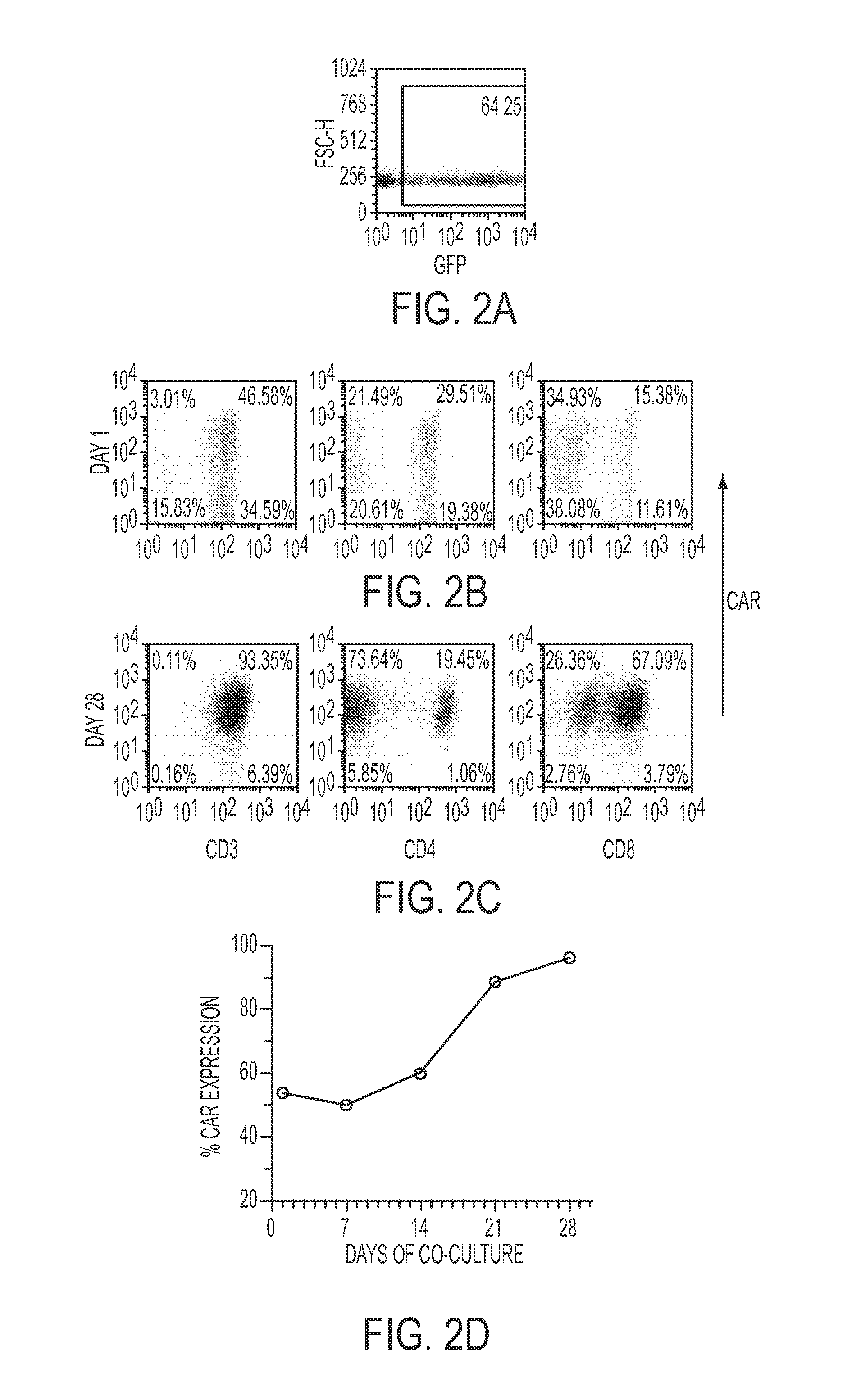
The present invention concerns methods and compositions for immunotherapy employing a modified T cell comprising a chimeric antigen receptor (CAR). In particular aspects, CAR-expressing T-cells are producing using electroporation in conjunction with a transposon-based integration system to produce a population of CAR-expressing cells that require minimal ex vivo expansion or that can be directly administered to patients for disease (e.g., cancer) treatment.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods for ex-vivo expanding stem/progenitor cells
InactiveUS20060205071A1Increase productionCulture processArtificial cell constructsProgenitorBone marrow transplantations
Methods of ex-vivo expansion of fetal and / or adult progenitor, and umbilical cord blood, bone marrow or peripheral blood derived stem cells in bioreactors for bone marrow transplantation, transfusion medicine, regenerative medicine and gene therapy.
Owner:GAMIDA CELL
Isolation and expansion of animal cells in cell cultures
Described are methods for isolating / purifying and expanding animal stem cells and stem-cell-like cells. Isolation methods include conditions comprising preferentially digesting non-stem cells and non-stem-cell-like cells in a population and preferentially adhering stem cells and stem-cell-like cells in a population. Expansion methods include culturing such cells under conditions comprising modulation of TGF-β signaling, inhibition of cell signaling mediated by p38 MAP kinase using small molecular weight inhibitors, expansion of the cells on human amniotic epithelial cells as feeder layers, control of cell seeding density, control of levels of Ca2+ in the culture media, rapid adhesion on a substrate or by a combination of such conditions. More particularly, what is disclosed relates to methods and systems for expanding animal cells in ex vivo cell cultures, while preventing cellular differentiation, and selectively enriching stem cells. The embodiments also disclose a culture system for ex vivo expansion of limbal epithelial cells or mesenchymal cells, as well as surgical grafts made there from.
Owner:TISSUETECH INC
Cultured hematopoietic stem cells and method for expansion and analysis thereof
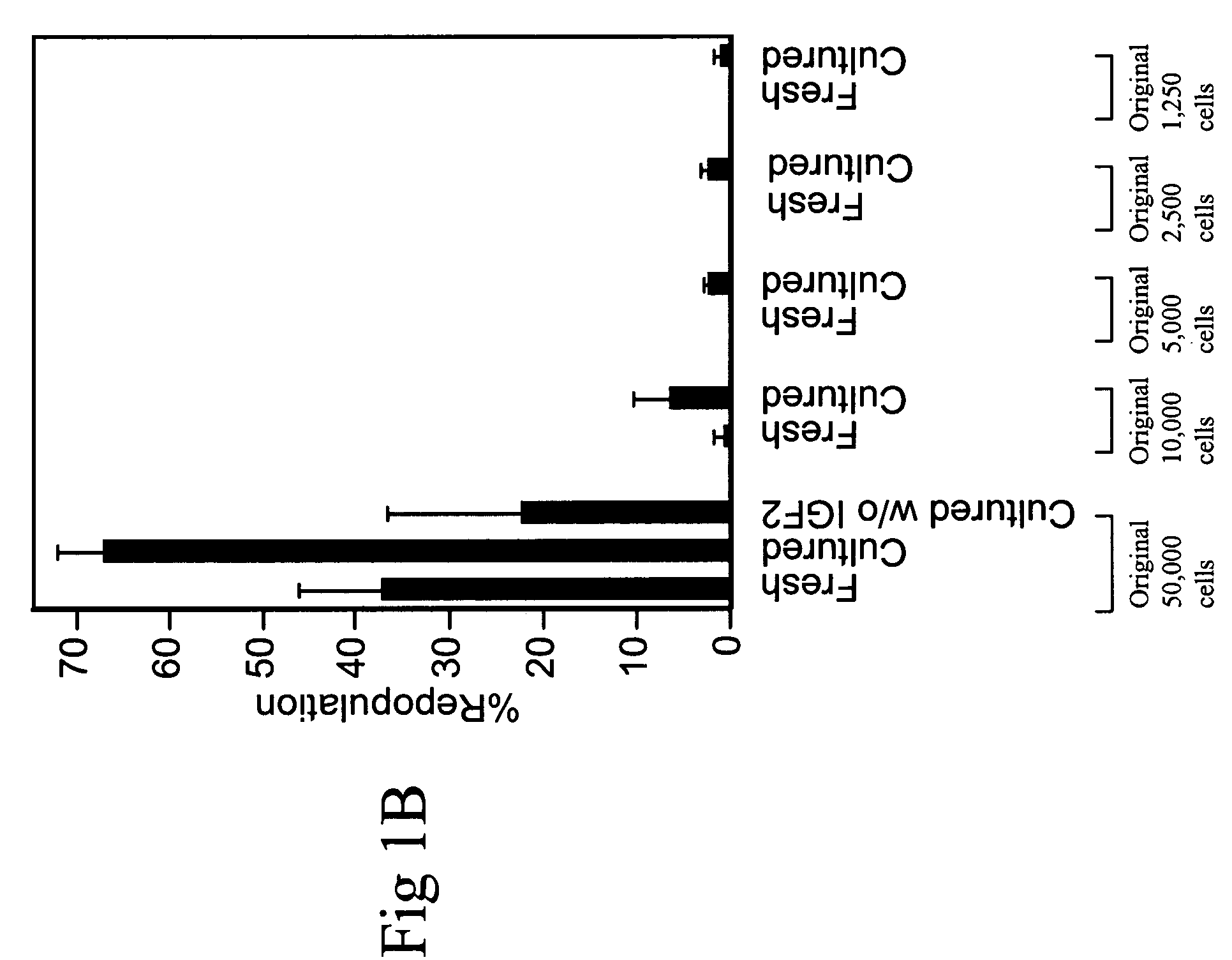
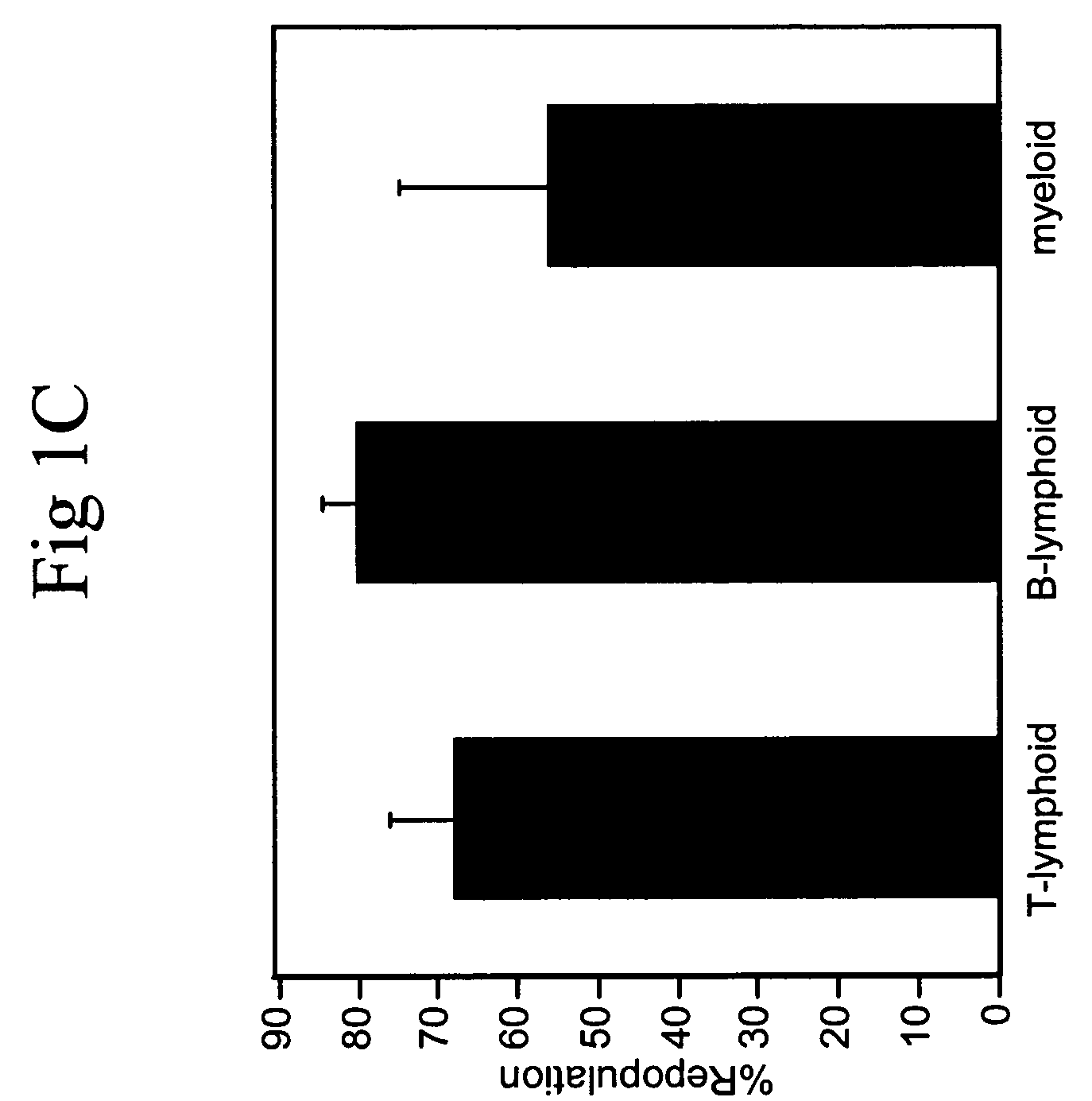
Hematopoietic stem cells and methods for ex vivo expansion of hematopoietic stem cells are provided. The methods comprise culturing the cells in a media containing an effective amount insulin-like growth factor (IGF), fibroblast growth factor (FGF), thrombopoietin (TPO), and stem cell factor (SCF), under conditions sufficient for expansion of said cells. Methods for identifying expanded hematopoeitc stem cells and kits for ex vivo expansion of hematopoietic stem cells are also provided.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Human application of engineered chimeric antigen receptor (CAR) t-cells
ActiveUS20160158285A1Easy SurvivalInhibit expressionMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseElectroporation
The present invention concerns methods and compositions for immunotherapy employing a modified T cell comprising a chimeric antigen receptor (CAR). In particular aspects, CAR-expressing T-cells are producing using electroporation in conjunction with a transposon-based integration system to produce a population of CAR-expressing cells that require minimal ex vivo expansion or that can be directly administered to patients for disease (e.g., cancer) treatment.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Preparation method of human cytokine-induced killer cells
InactiveCN102732481APromote proliferationRaise the ratioBlood/immune system cellsHybrid peptidesPeripheral blood mononuclear cellCytotoxicity
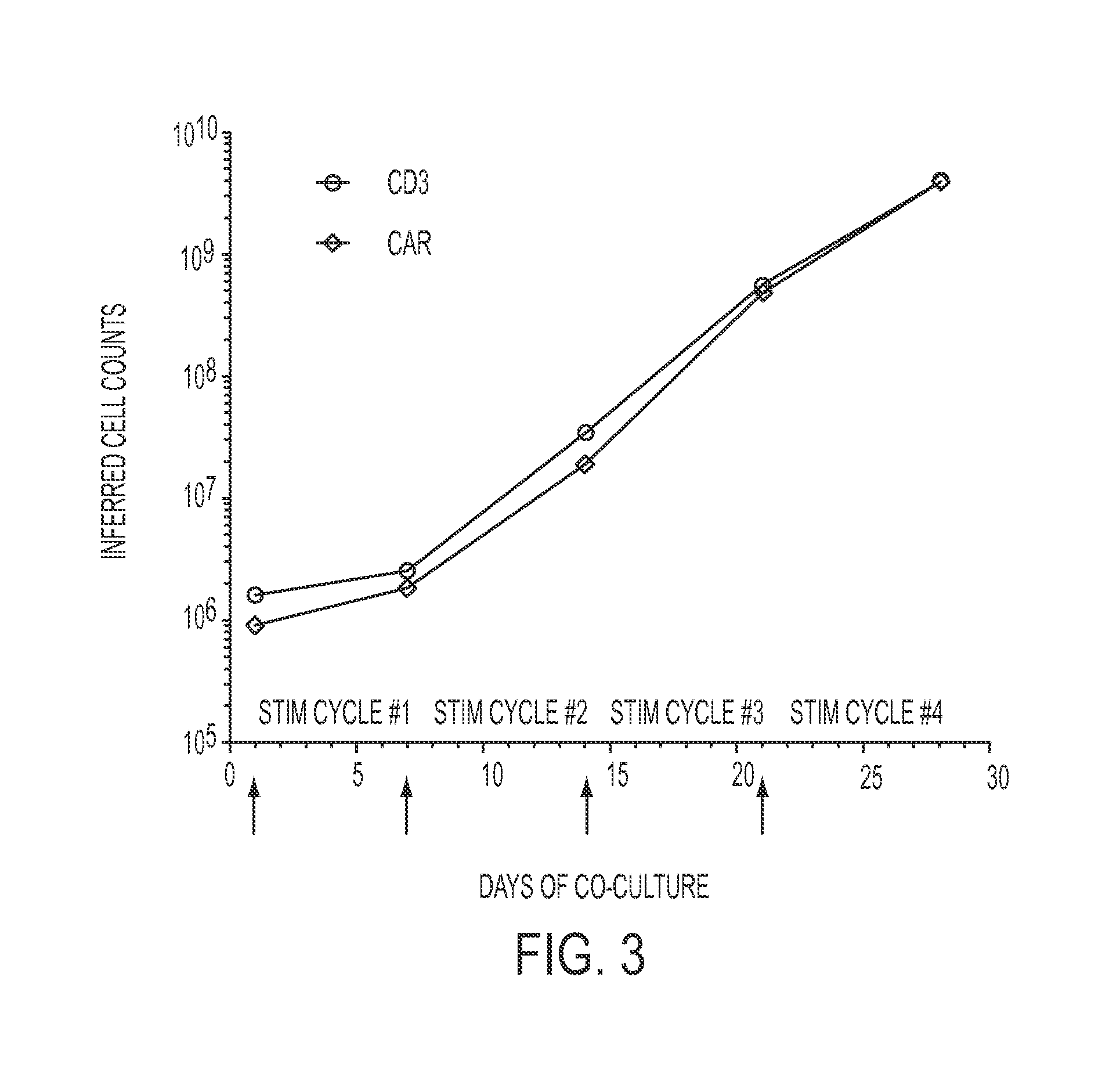
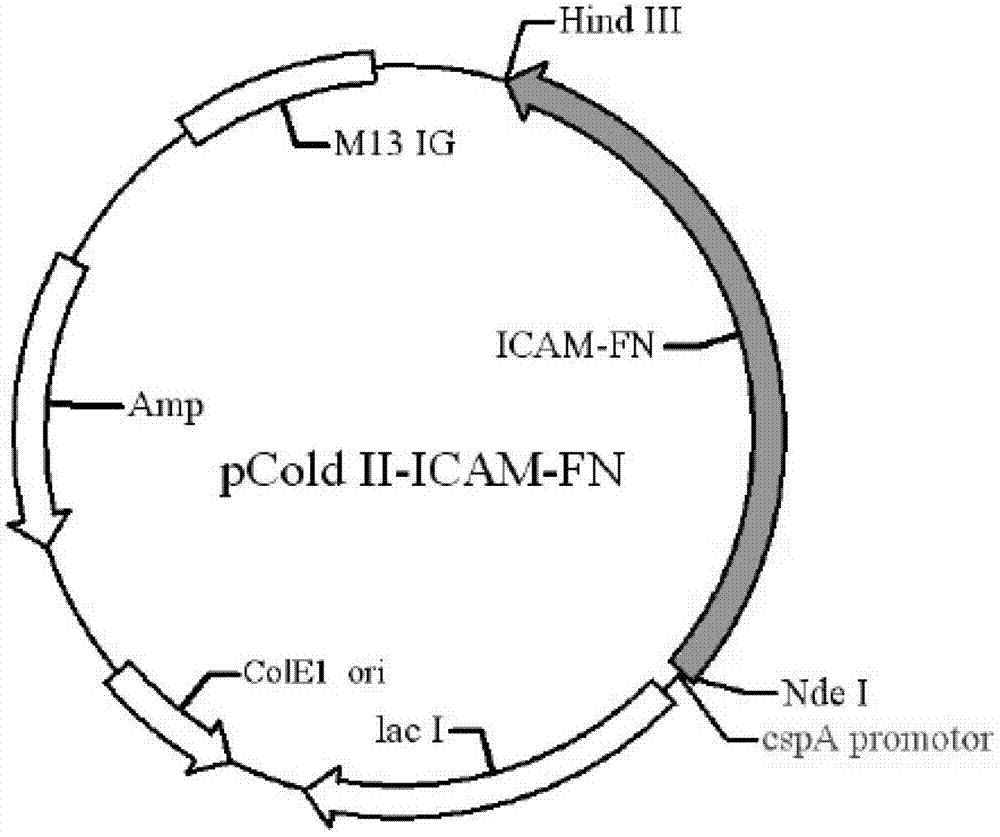
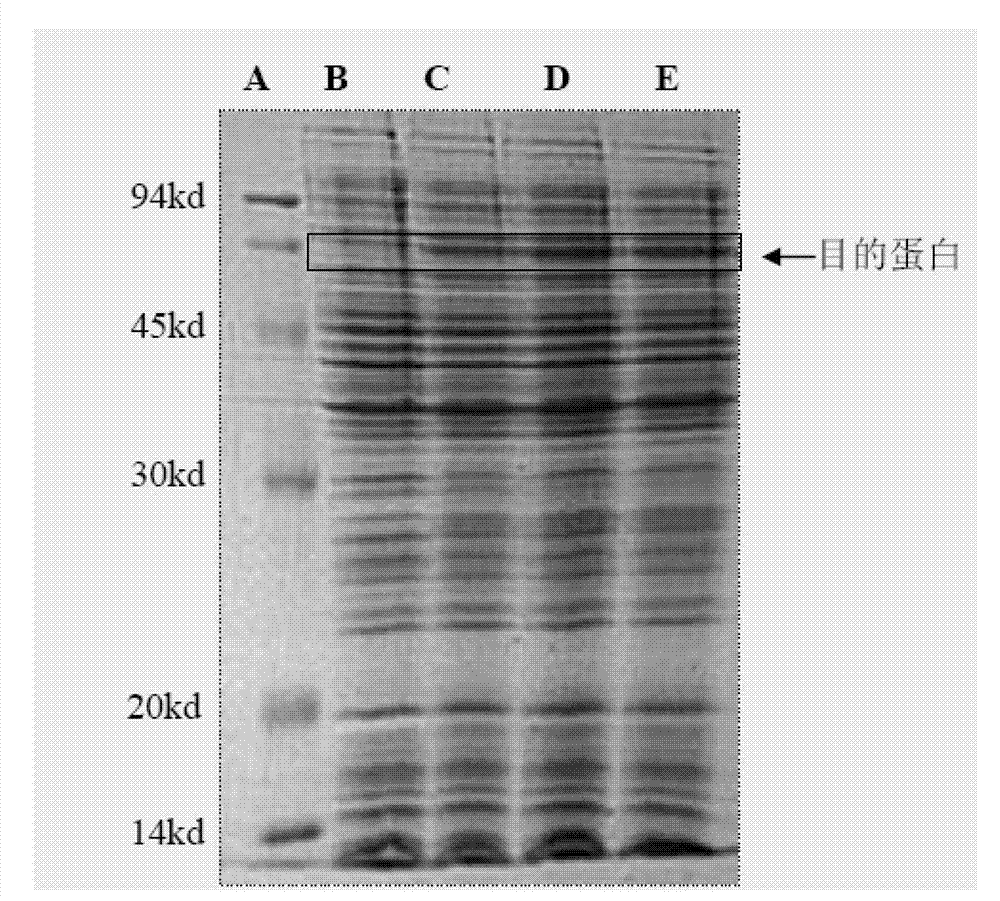
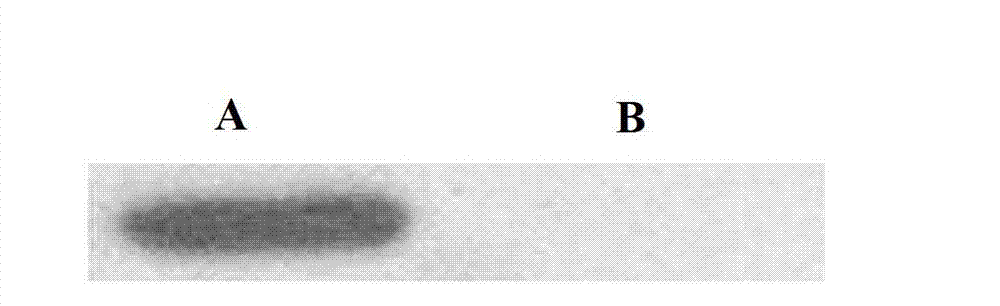
The invention discloses a preparation method of human cytokine-induced killer cells, comprising the following steps: coating a cell culture flask with a coating buffer containing effective amount of fusion protein and human CD3 monoclonal antibody before culturing precursor cells of human CIK cells, and adding the human CD3 monoclonal antibody in the whole process of inducing and culturing the human CIK cells, wherein the fusion protein is human intercellular adhesion molecule-1 functional domain and human fibronectin functional domain fusion protein, and the concentration of the human CD3 monoclonal antibody in the cell culture solution is lower than the concentration of the human CD3 monoclonal antibody in the coating buffer. According to the invention, ex-vivo expansion efficiency of peripheral blood mononuclear cells and the proportion of CD3 / CD56 double positive cells in the CIK cells are significantly raised, the cytotoxicity activity of the CIK cells is enhanced, thus the effect of cellular immunity treatment is raised.
Owner:SHENZHEN YOUNGCELL BIO TECH
Methods of Ex Vivo Expansion of Blood Progenitor Cells, and Generation of Composite Grafts
InactiveUS20130136722A1Sustained blood cell productionImprove survivalBiocideMicrobiological testing/measurementProgenitorCell type
This invention provides methods and compositions of hematopoietic progenitor cells and hematopoietic stem cells, particularly methods for expanding populations of these cells types from biological sources.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Methods of ex vivo hematopoietic stem cell expansion by co-culture with mesenchymal cells
InactiveUS8080417B2Increase the number ofBiocideCulture processProgenitorHematopoietic Stem Cell Mobilization
Owner:GAMIDA CELL
Methods and Systems for Isolating, Ex Vivo Expanding and Harvesting Hematopoietic Stem Cells
InactiveUS20100291534A1Easy to useBioreactor/fermenter combinationsBiological substance pretreatmentsTime efficientEx vivo expansion
Owner:NAT CENT UNIV
Methods of Ex Vivo Expansion of Blood Progenitor Cells, and Generation of Composite Grafts
InactiveUS20150164952A1Sustained blood cell productionImprove survivalBiocideMammal material medical ingredientsProgenitorEx vivo expansion
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Method for carrying out the ex vivo expansion and ex vivo differentiation of multipotent stem cells
The invention relates to a method for carrying out the expansion of multipotent stem cells ex vivo. Moreover, the invention relates to a two-stage method for carrying out the expansion and differentiation of multipotent stem cells ex vivo, in which it is possible for the stem cells to be gene transfected during the first stage, i.e. during the expansion phase. In this phase, the differentiation of the multipotent stem cells takes place optionally in cells of the hematopoietic, endothelial or mesenchymal cell lineage. Stem and progenitor cells as well as mature cells of the hematopoietic, endothelial and mesenchymal cell lineage, which are obtained in this way, can be used, among other things, for the prophylaxis, diagnosis and therapy of human deseases and for tissue engineering.
Owner:UNIVERSITAETSKLINIKUM HAMBURG EPPENDORF
Discovery of regulatory t cells programmed to suppress an immune response
InactiveUS20130302276A1Maintenance of toleranceEffective to ameliorateBiocideNervous disorderAntigenDisease
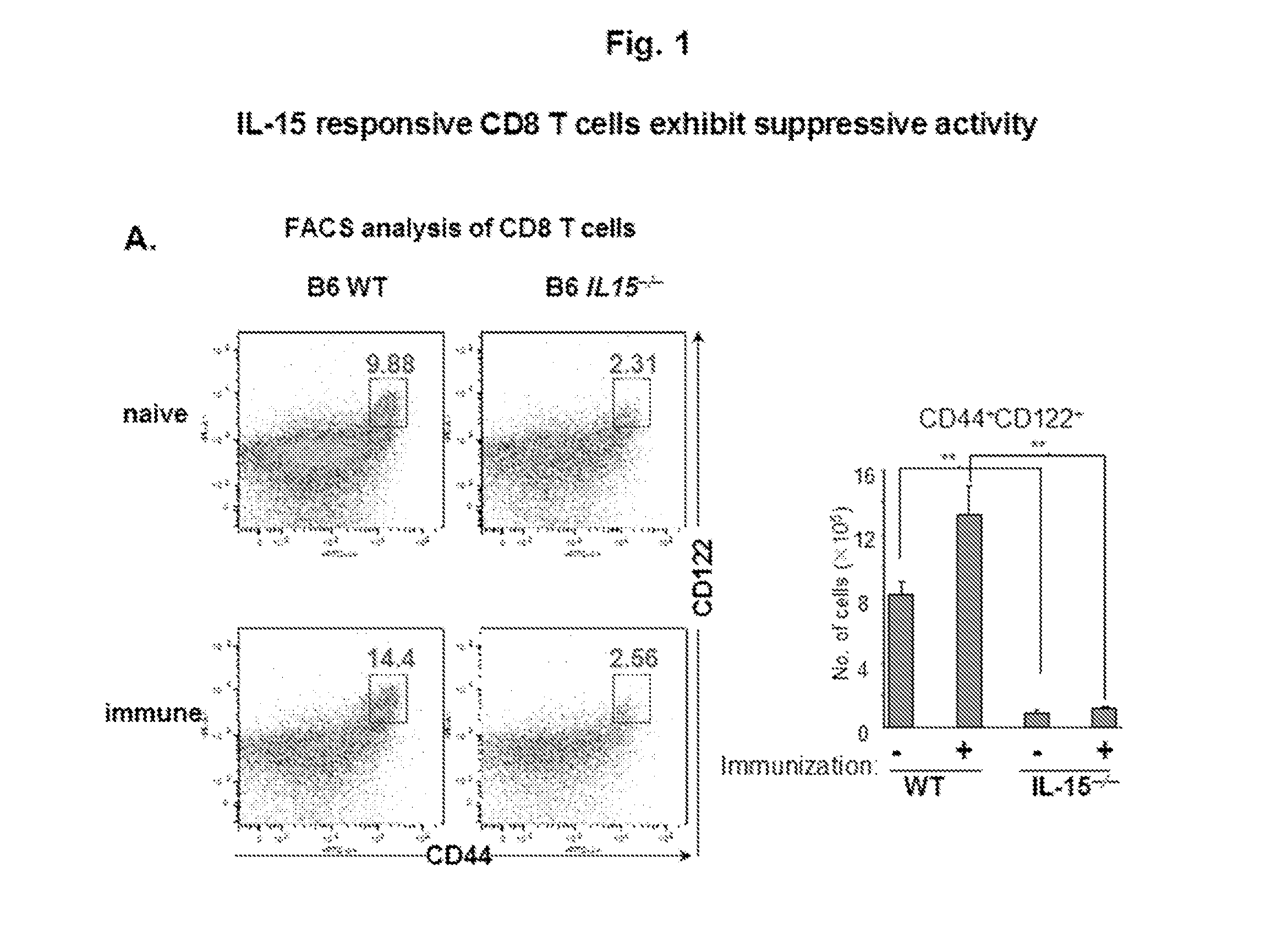
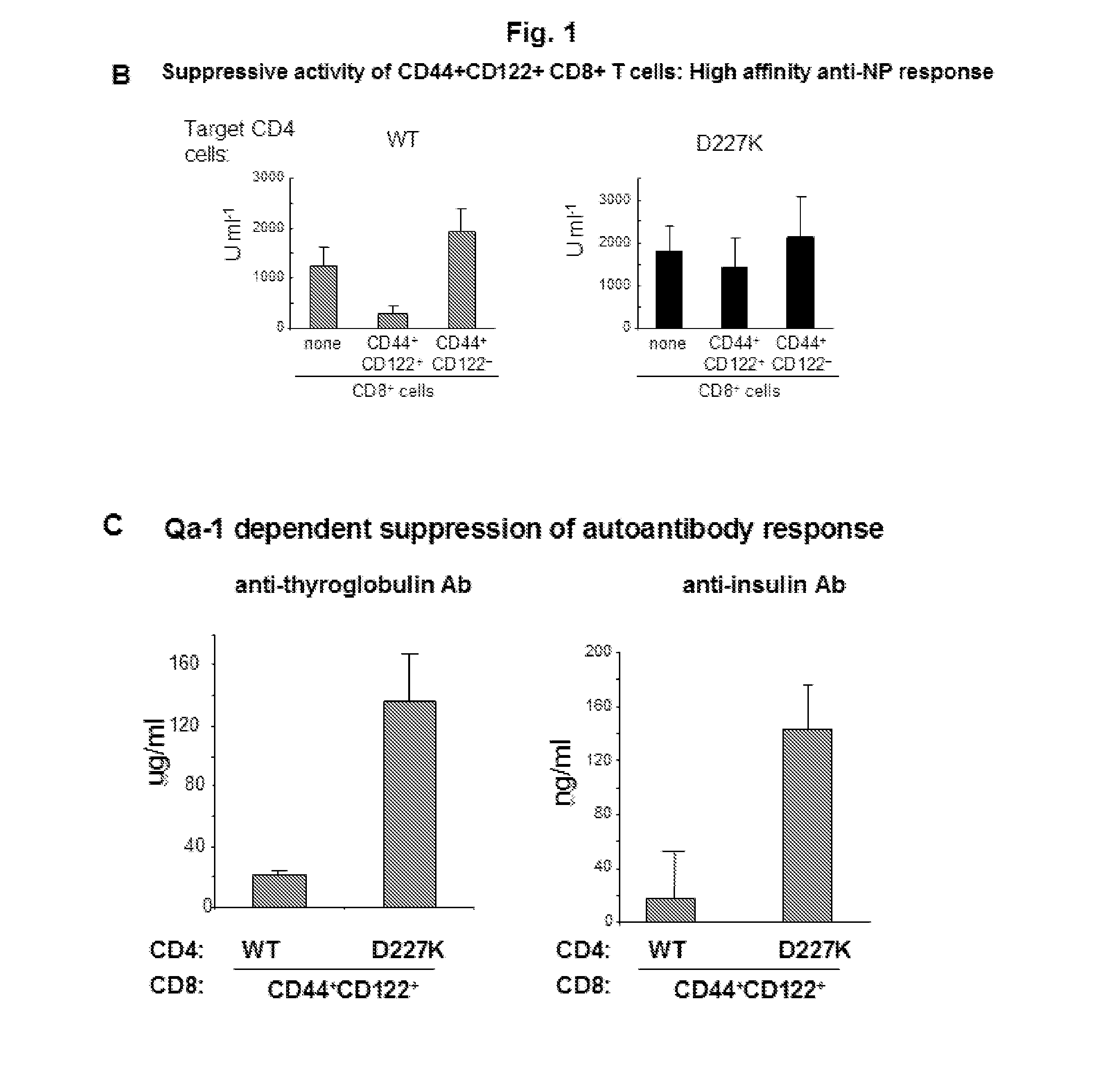
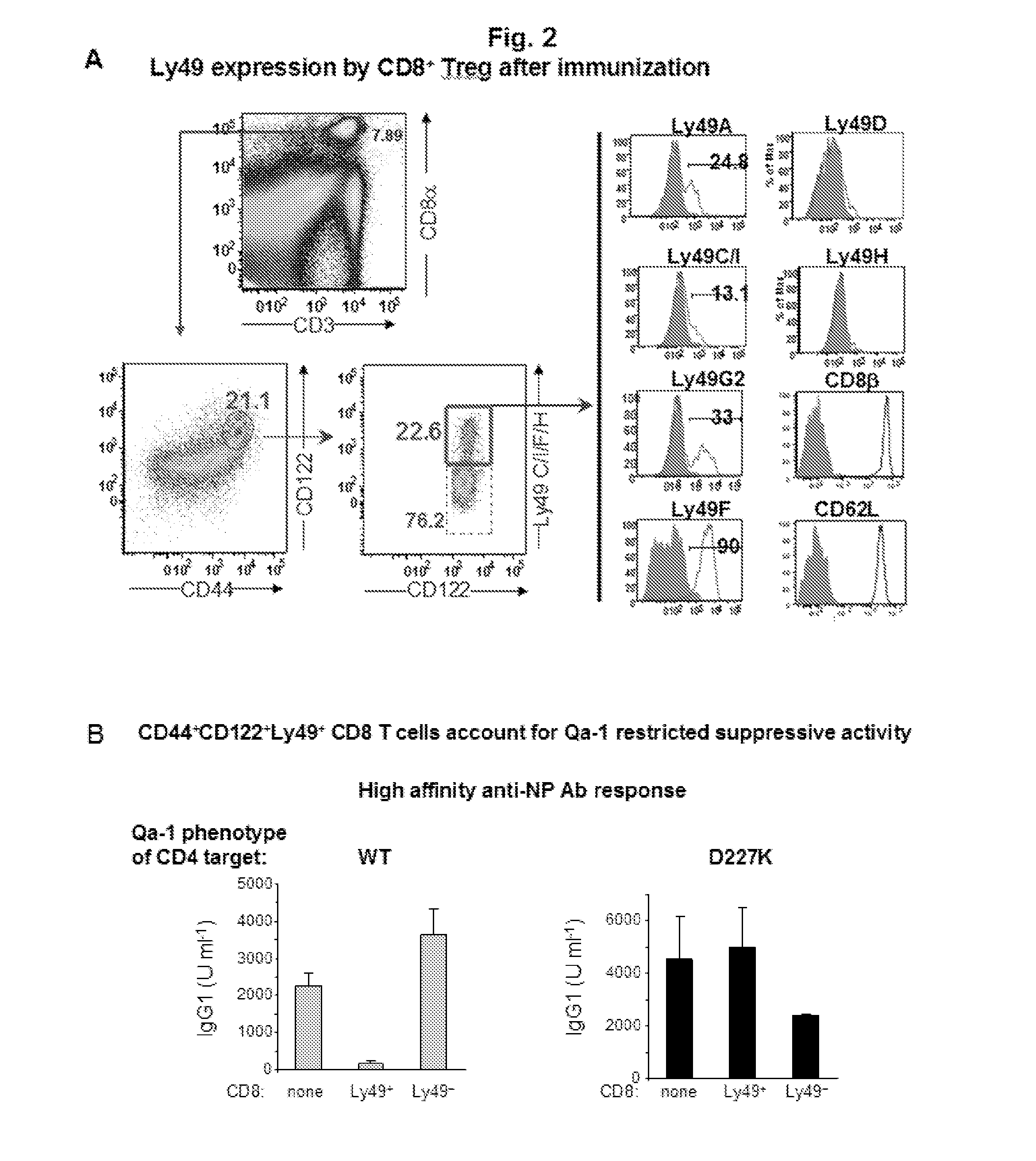
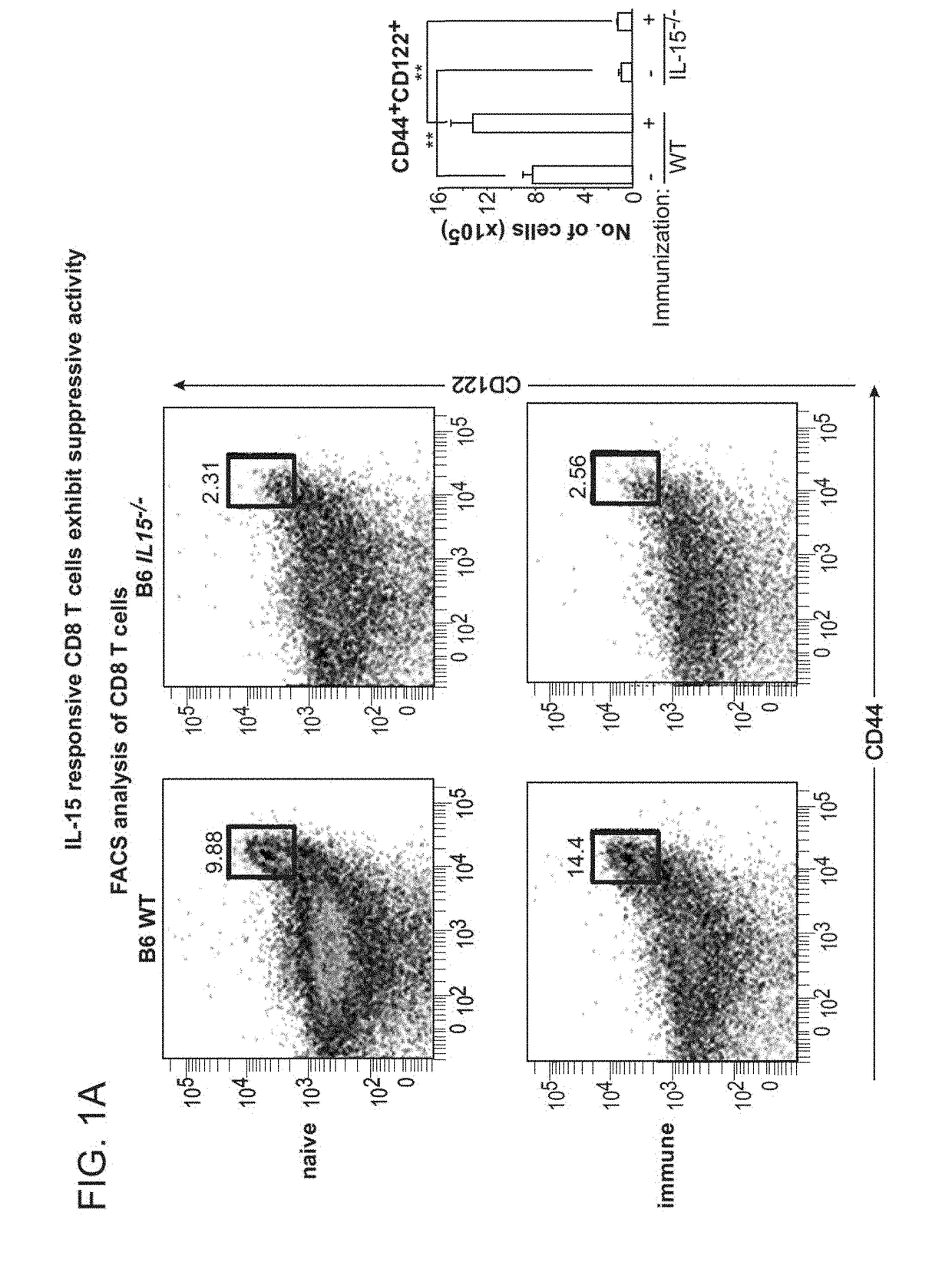
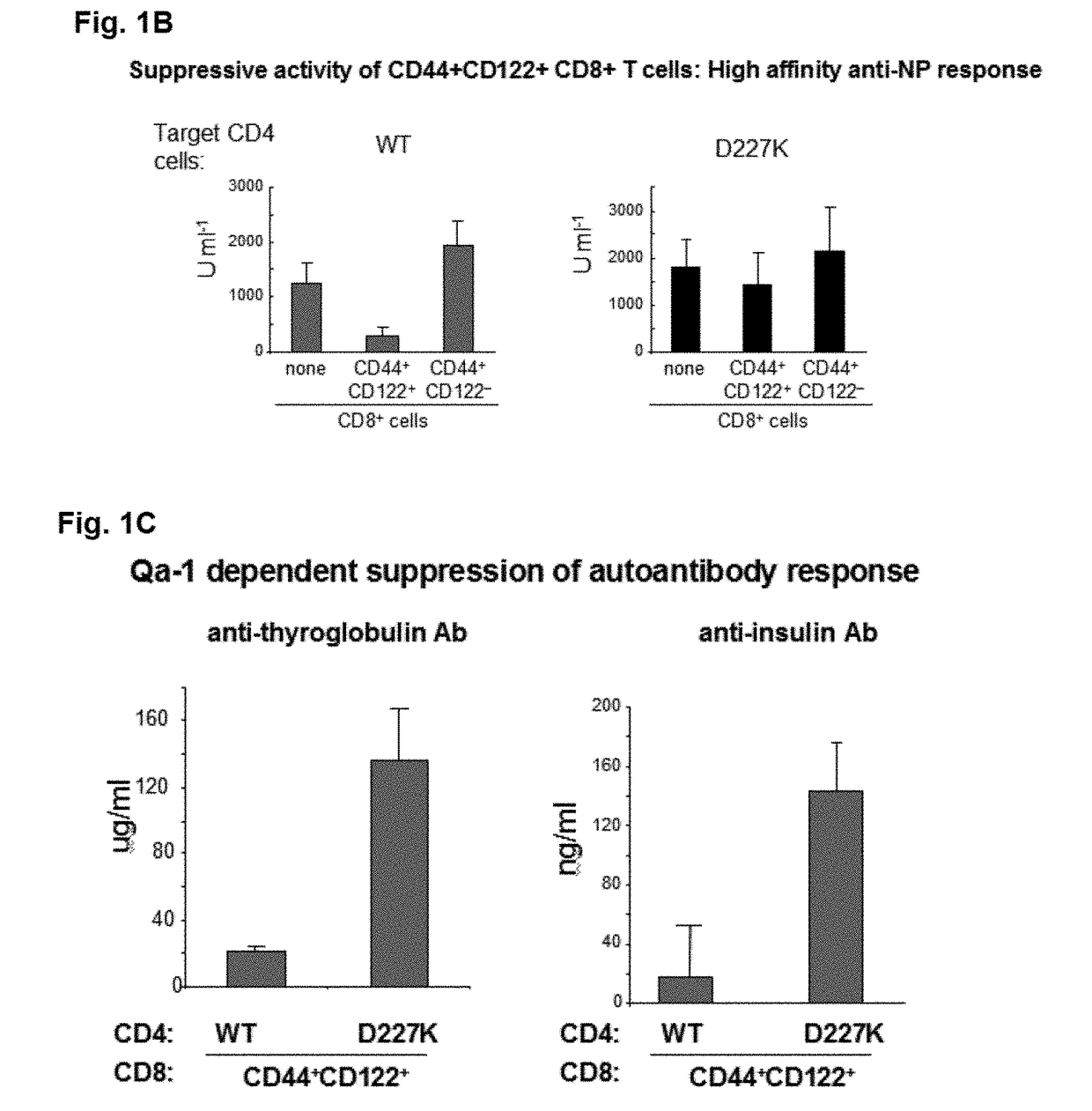
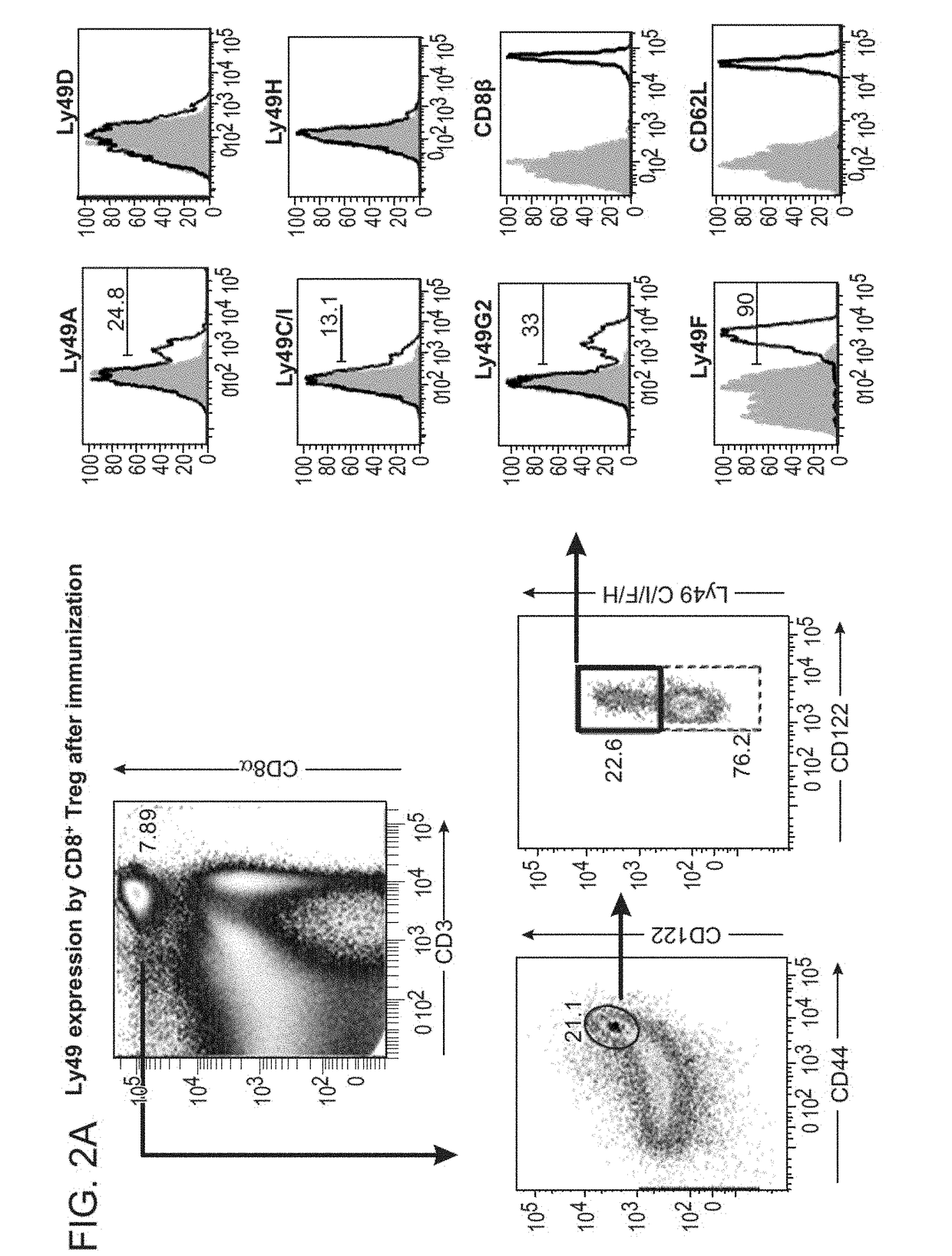
A method to treat an autoimmune disease is provided. The method involves administration of interleukin-15 receptor (IL-15R) agonists in an amount effective to ameliorate a symptom of the autoimmune disease. The invention also involves a method to treat an autoimmune disease by ex-vivo expansion of CD44+CD122+Kir+ CD8+ Treg cells and administration of the CD44+CD122+Kir+ CD8+ Treg cells. Compositions comprising CD44+CD122+Kir+ CD8+ Treg cells are also provided. Methods for stimulating an immune response to an antigen are also provided.
Owner:DANA FARBER CANCER INST INC
Relationship of ABC transport proteins with hematopoietic stem cells and methods of use thereof
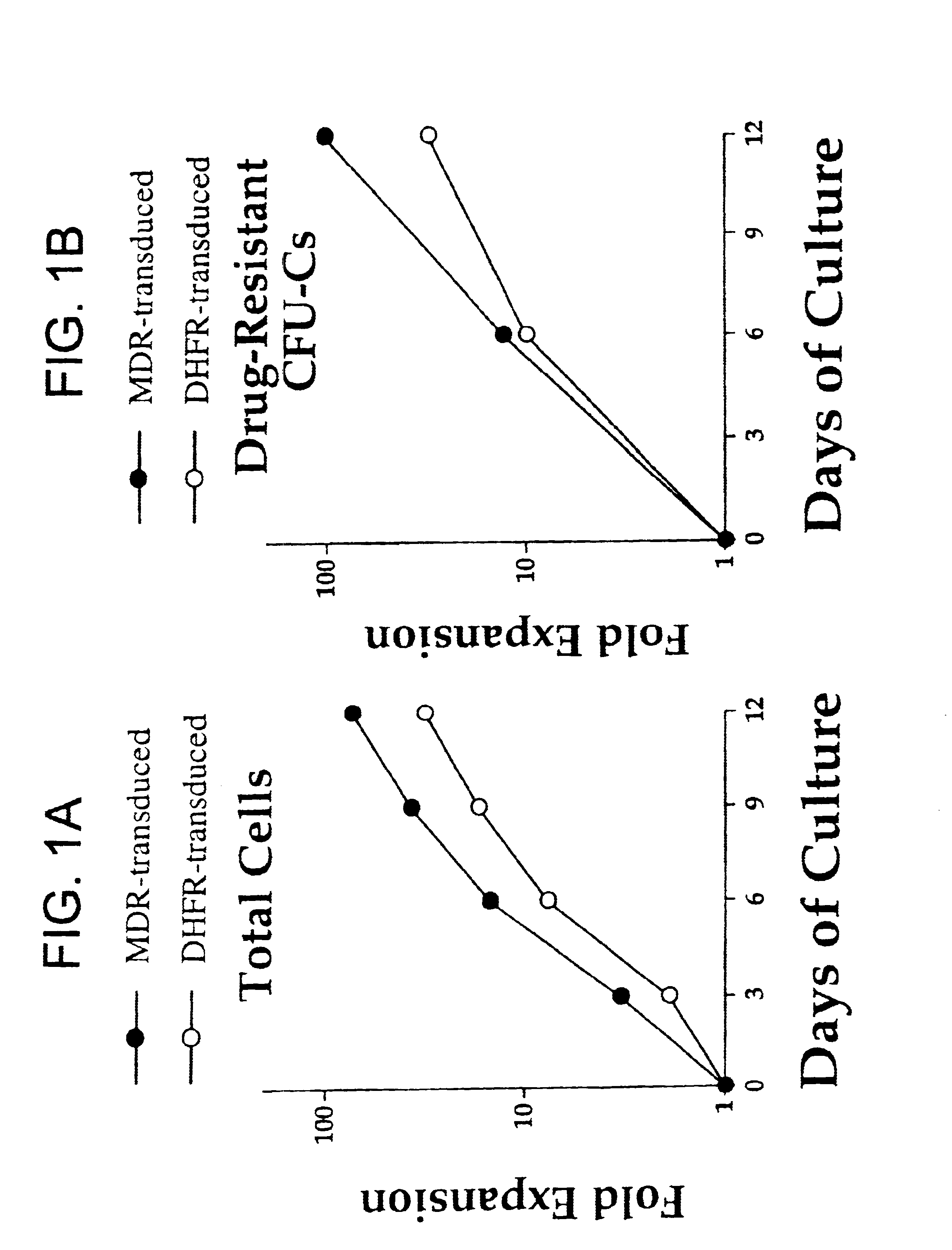
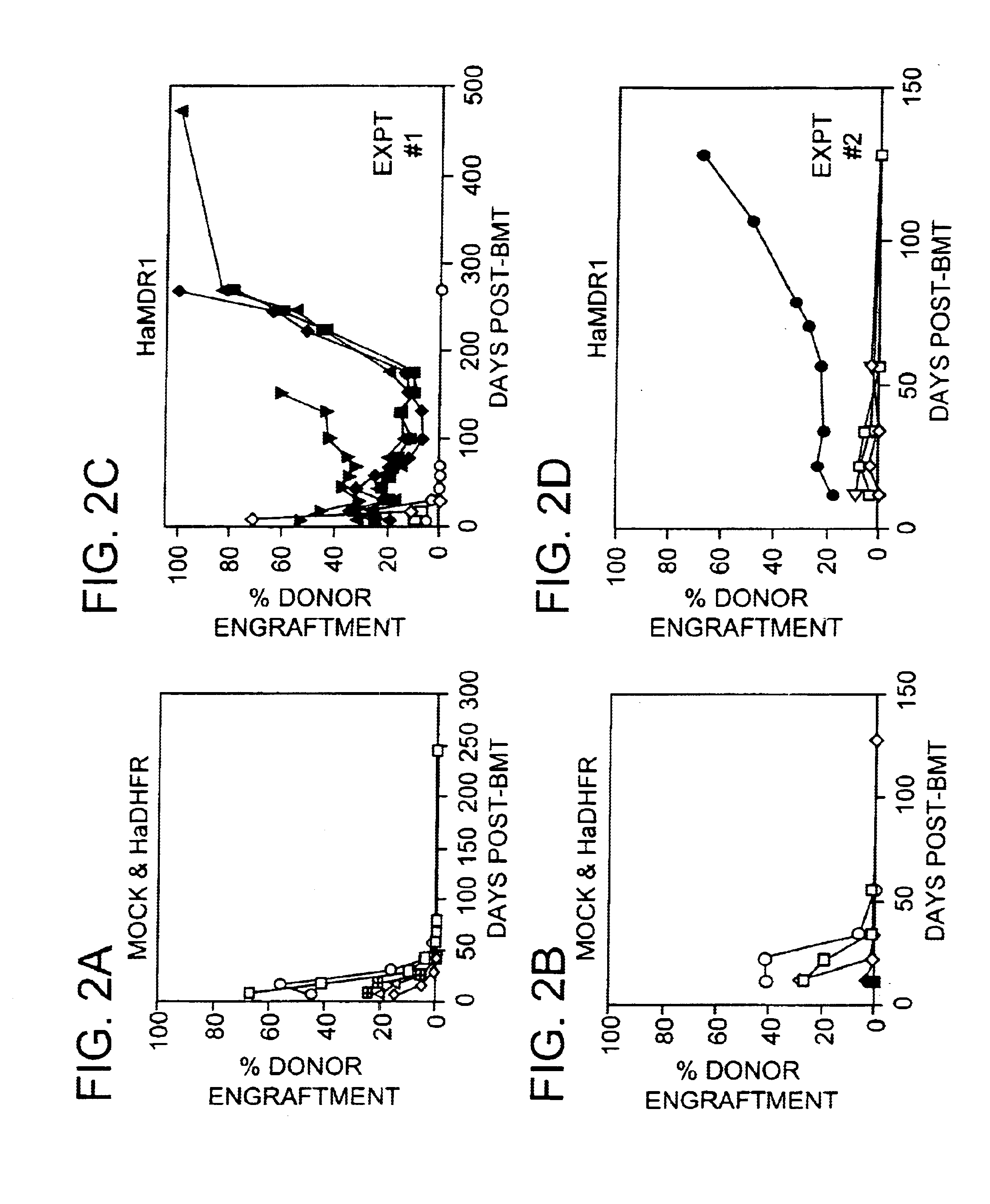
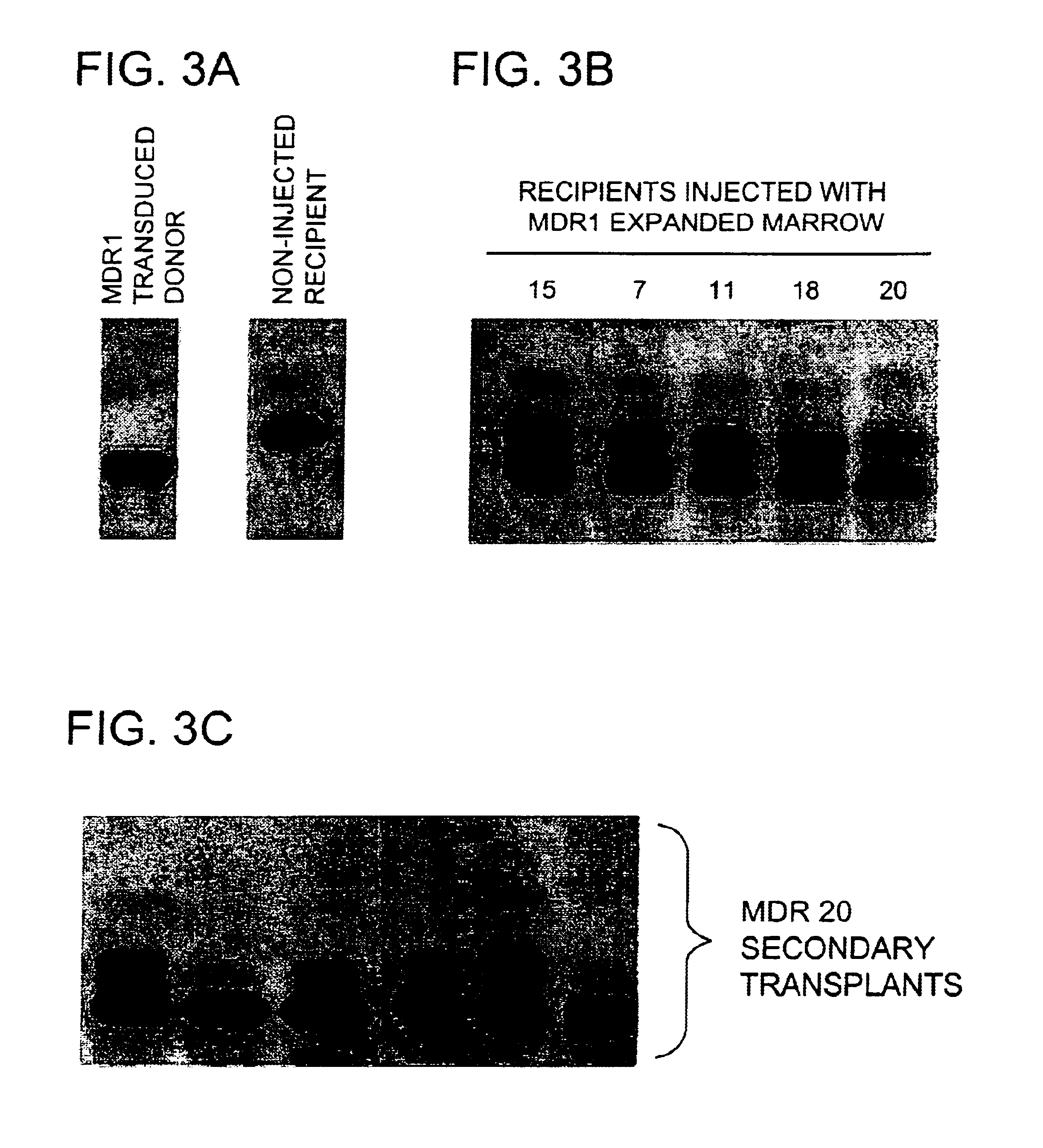
The present invention includes methods of performing ex vivo expansion of gene-modified hematopoietic stem cells which are useful for many applications involving bone marrow transplantation and ex vivo gene therapy. The present invention further includes the gene-modified hematopoietic stem cells that are used and produced by such methods. Such gene-modified hematopoietic stem cells can also contain a second heterologous gene. In addition, the present invention also includes methods of engrafting the gene-modified hematopoietic stem cells of the present invention into animals, including for ex vivo gene therapy and for reconstitution of hematopoietic cells in ablated mammals. The present invention also provides a method of isolating stem cells.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Discovery of regulatory t cells programmed to suppress an immune response
A method to treat an autoimmune disease is provided. The method involves administration of interleukin-15 receptor (IL-15R) agonists in an amount effective to ameliorate a symptom of the autoimmune disease. The invention also involves a method to treat an autoimmune disease by ex-vivo expansion of CD44+CD122+Kir+ CD8+ Treg cells and administration of the CD44+CD122+Kir+ CD8+ Treg cells. Compositions comprising CD44+CD122+Kir+ CD8+ Treg cells are also provided. Methods for stimulating an immune response to an antigen are also provided.
Owner:DANA FARBER CANCER INST INC
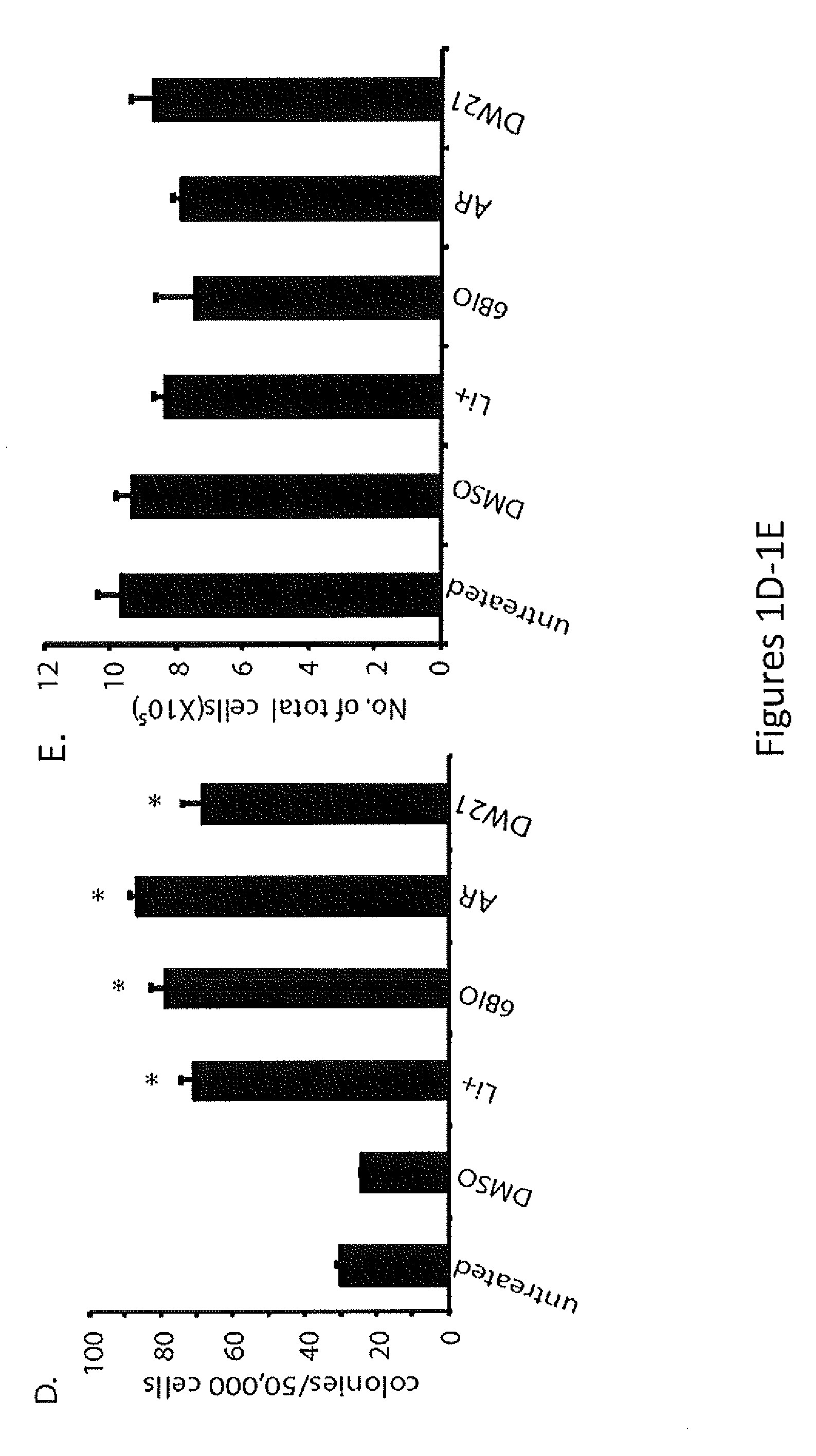
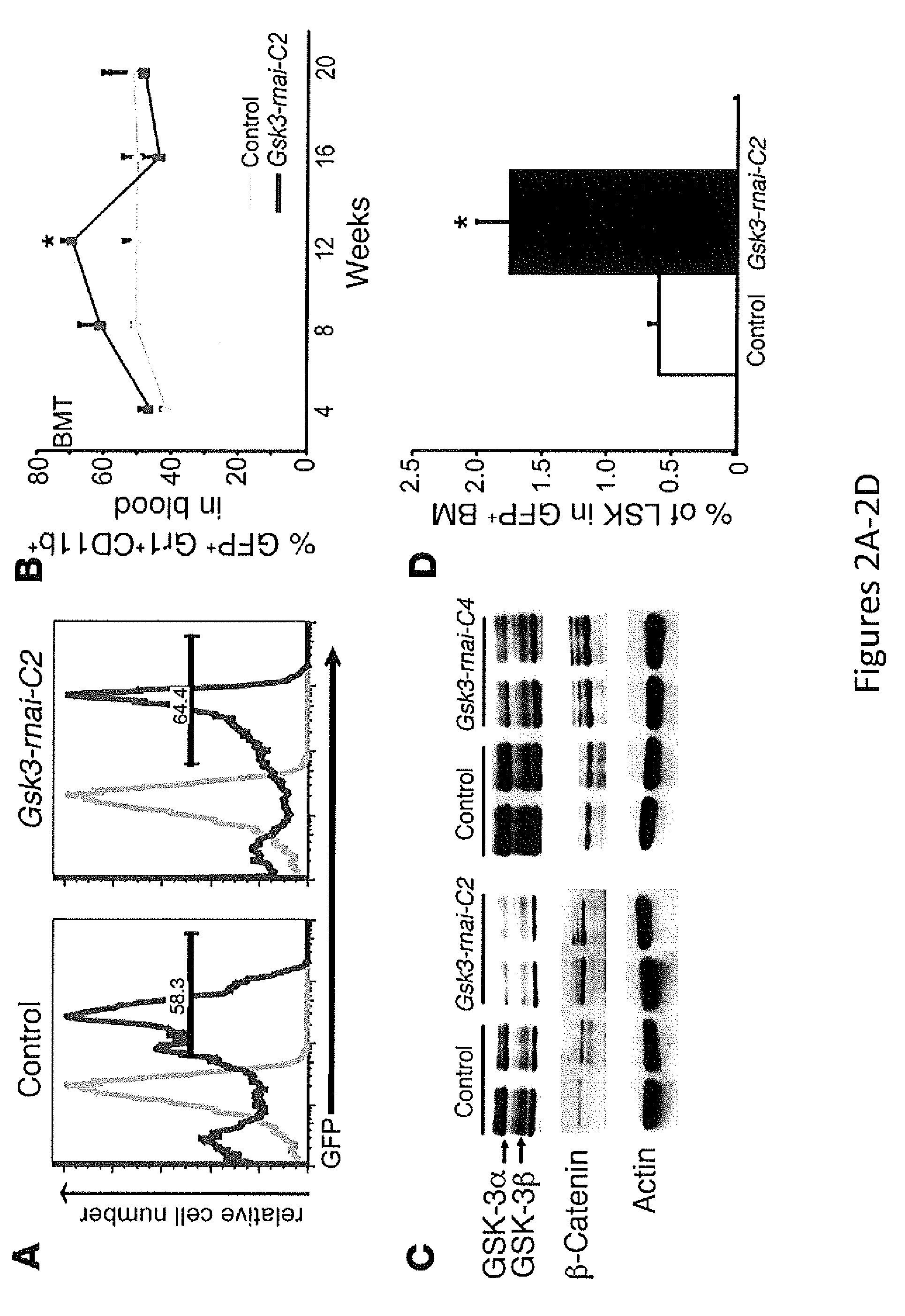
In Vivo and Ex Vivo Expansion of Hematopoietic Stem Cells With a Targeted Combination of Clinically Tested, FDA Approved Drugs
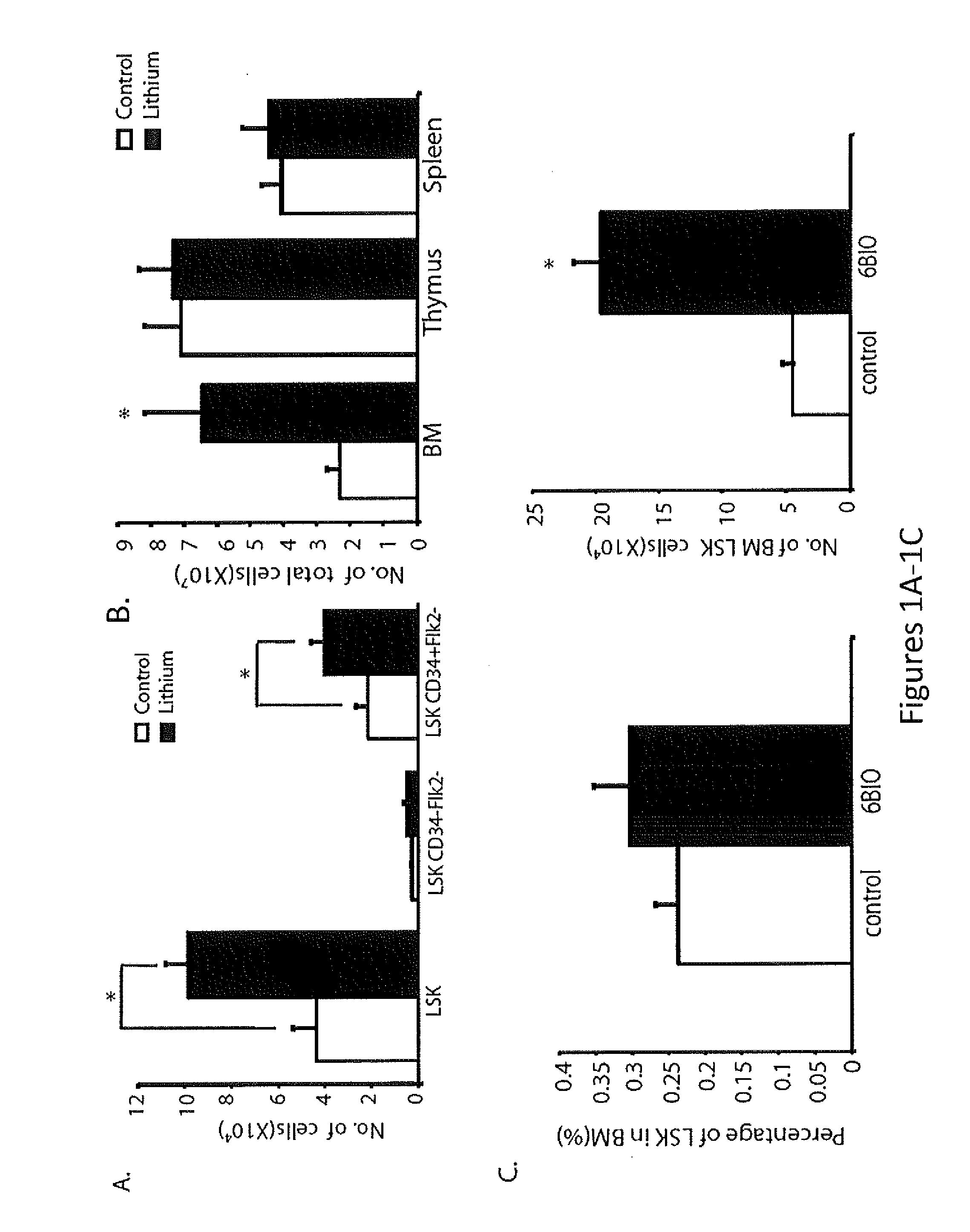
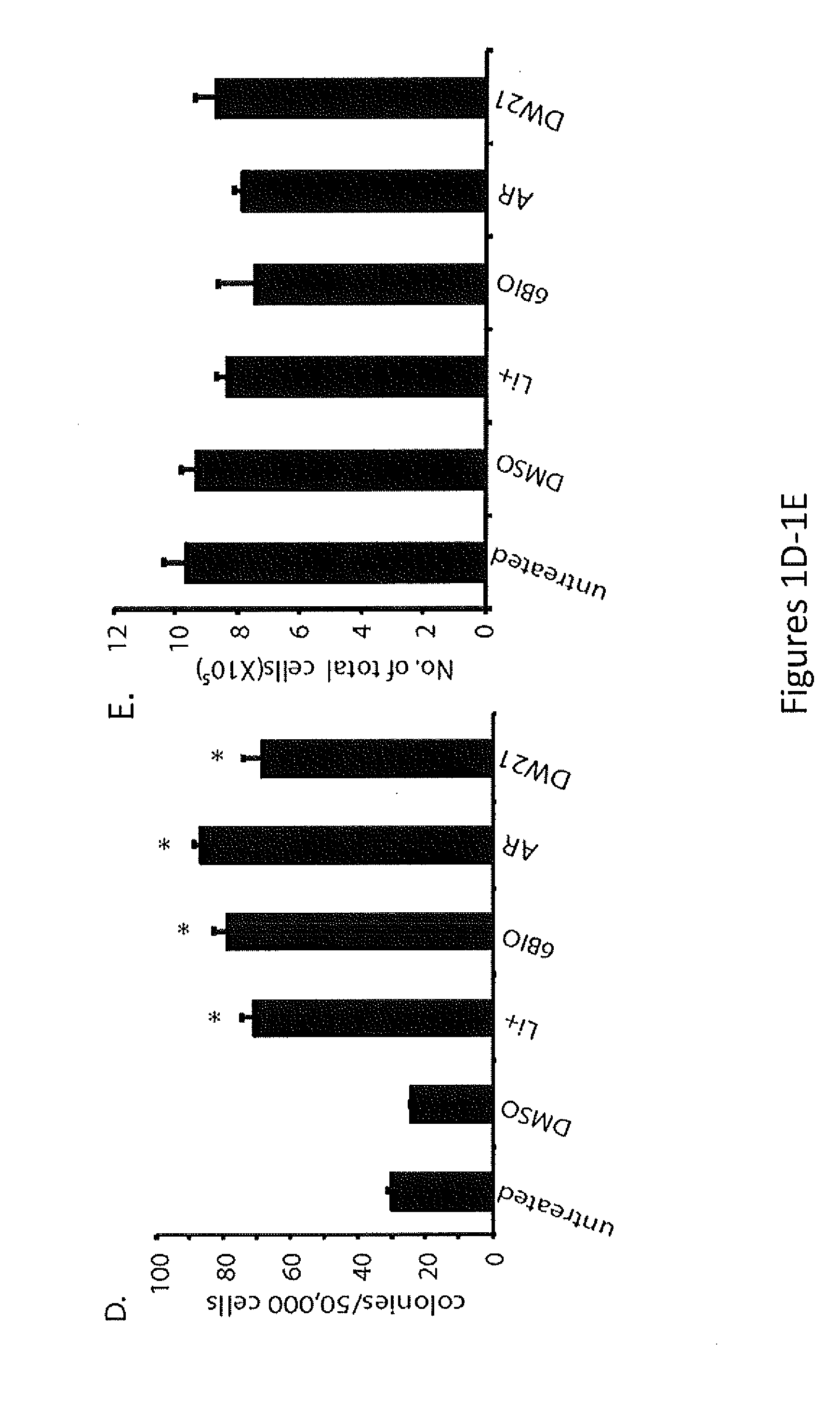
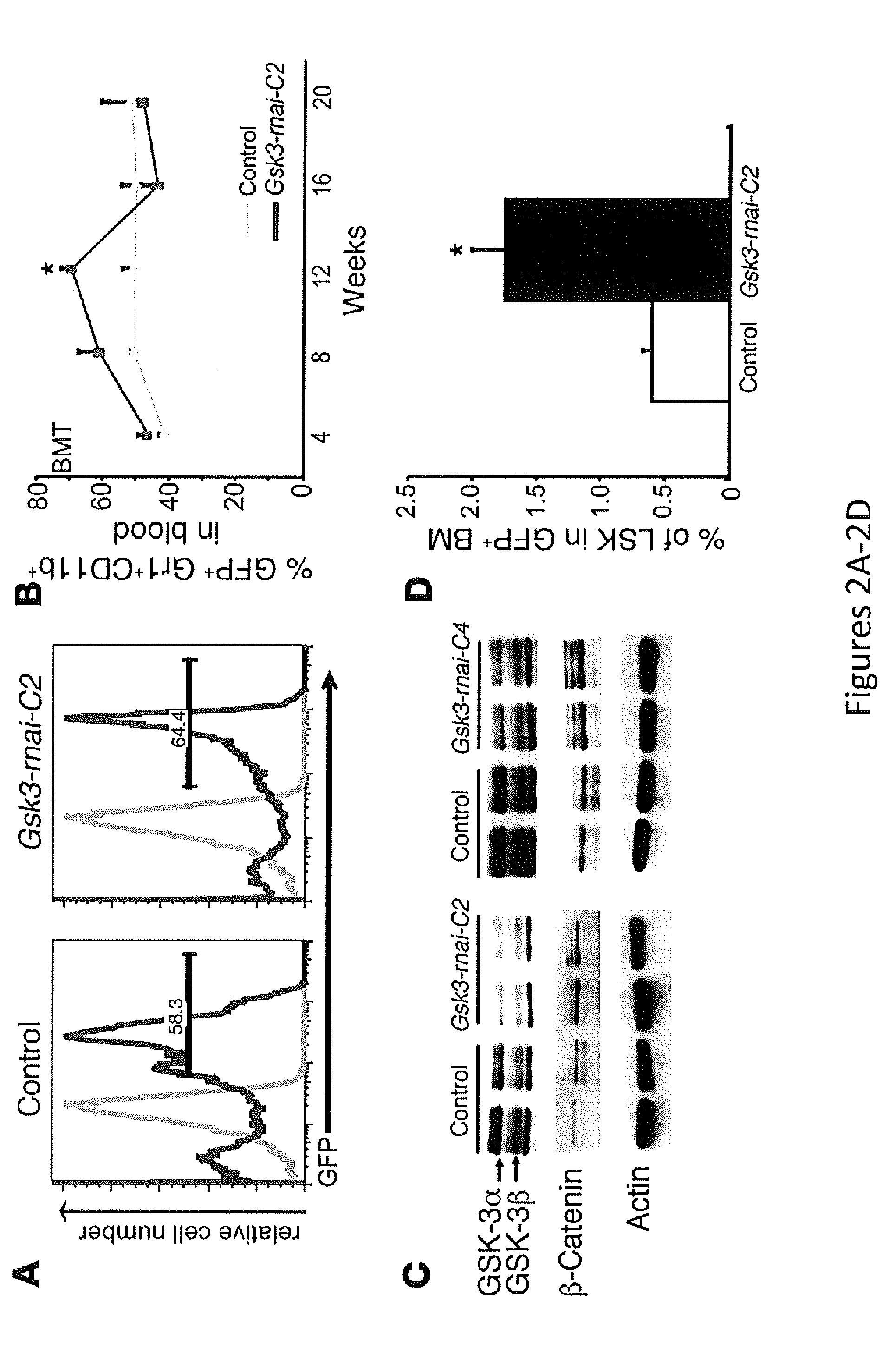
The present invention provides a therapeutic approach to maintain and expand HSCs in vivo using currently available medications that target GSK-3 and mTOR. The present invention also provides a system and method for the ex vivo culturing of HSCs, where an mTOR inhibitor is combined with a GSK-3 inhibitor within the culturing conditions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Immunocyte medicine containing B cell vaccine loaded with new antigen
PendingCN110272874AGood cancer preventionTumor rejection antigen precursorsTumor specific antigensAbnormal tissue growthTherapeutic effect
The invention provides an immunocyte medicine containing a B cell vaccine (neoB) loaded with a tumor new antigen, and a medicine combination for united use of neoB and tumor specificity T cells. The neoB vaccine can have higher ex vivo expansion properties than a DC vaccine, has higher effect of continuous activating of in vivo T cells under the condition of repeated infusion, and can become a preventive or therapeutic vaccine or an immunocyte medicine to be applied to tumor resisting treatment suitable for any cancer kind. According to the medicine combination for united use of neoB and tumor specificity T cells, disclosed by the invention, the neoB can be used for further stimulating the tumor specificity T cells in bodies, so that the number of the tumor specificity T cells in bodies can be amplified, and the treatment effect is increased.
Owner:CHINEO MEDICAL TECH CO LTD
Method for ex vivo expansion of human vascular endothelial progenitor cells in low oxygen condition
InactiveCN108753688AMaintain drynessIncrease in vitro expansionCulture processArtificial cell constructsProgenitorUbiquitin-Protein Ligases
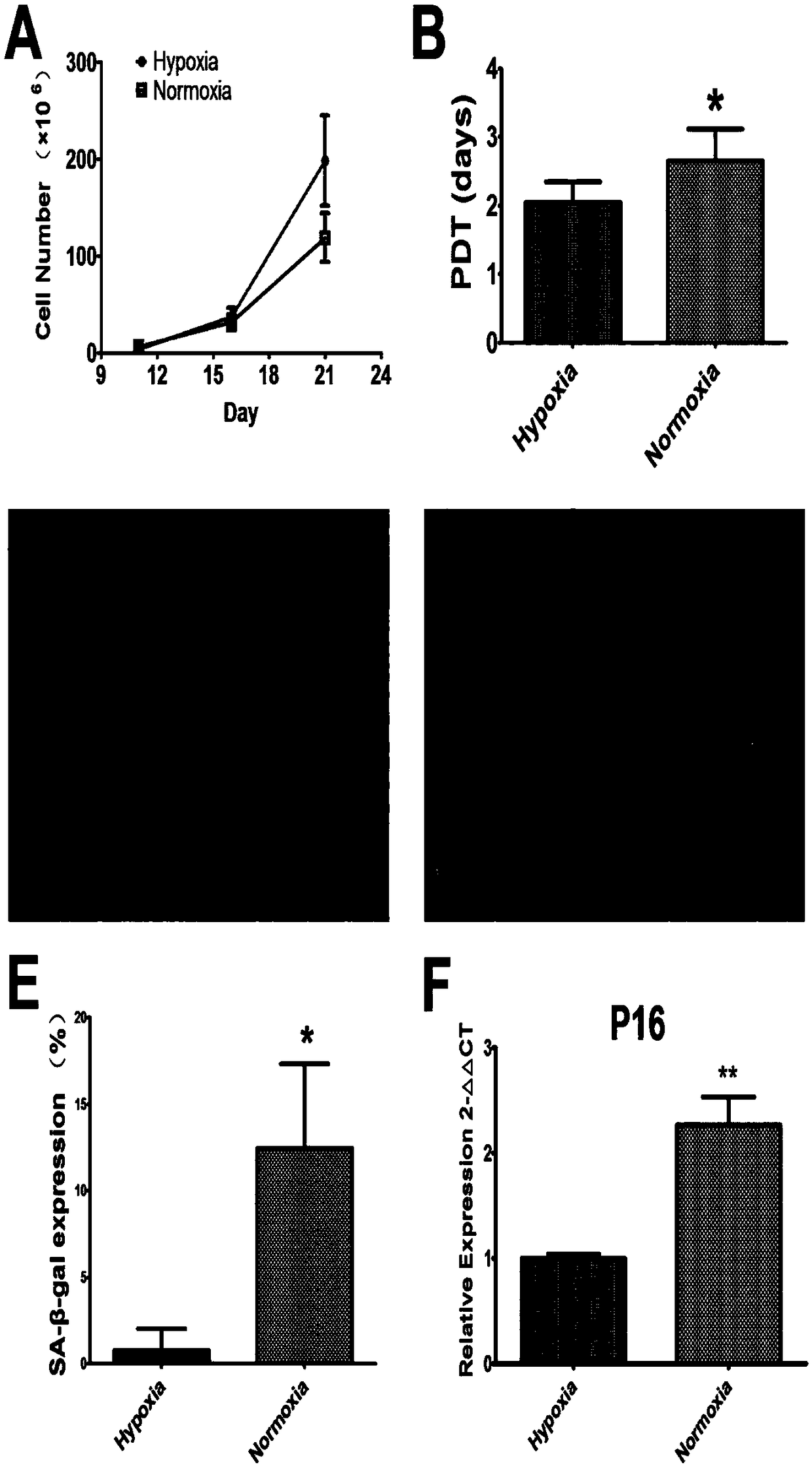
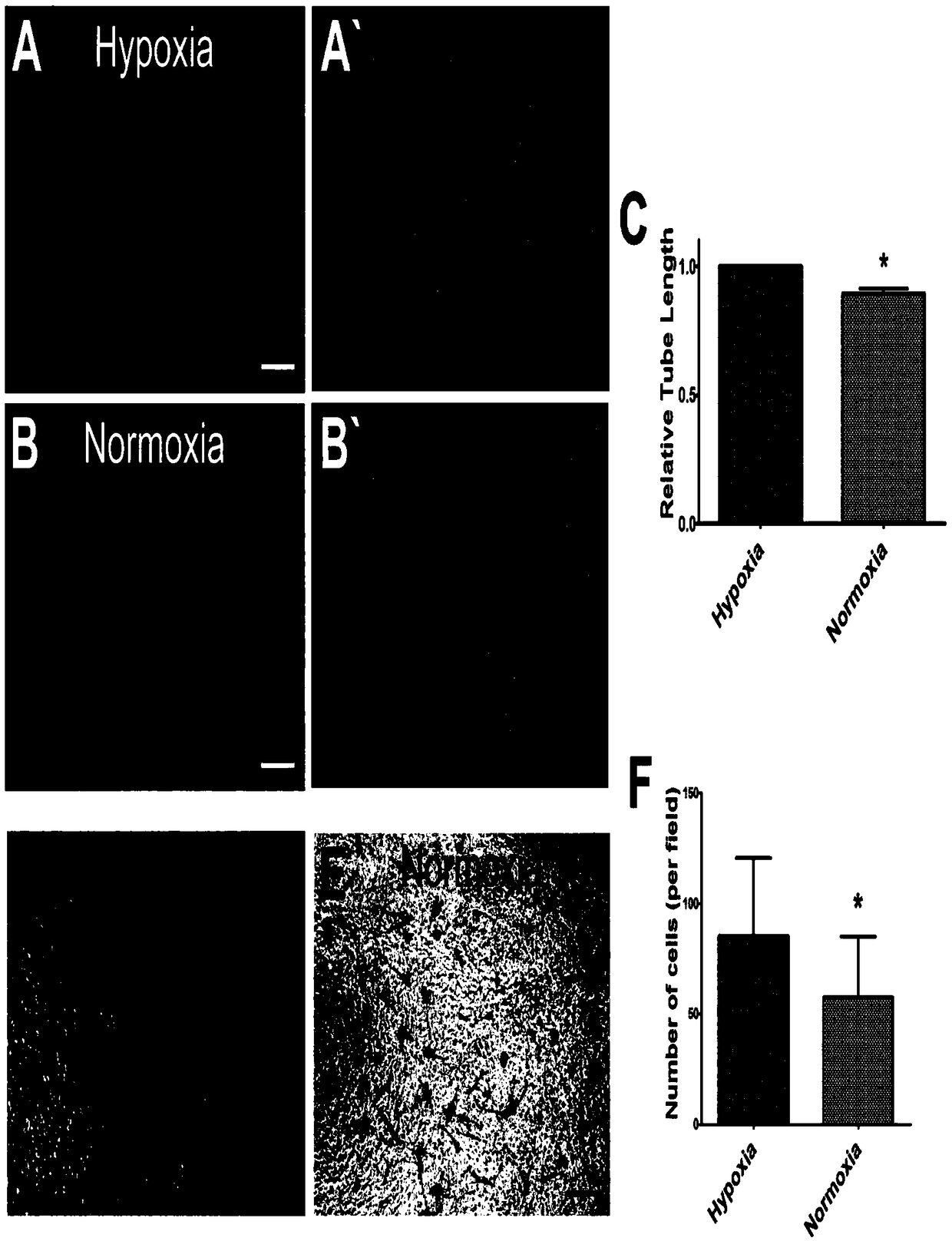
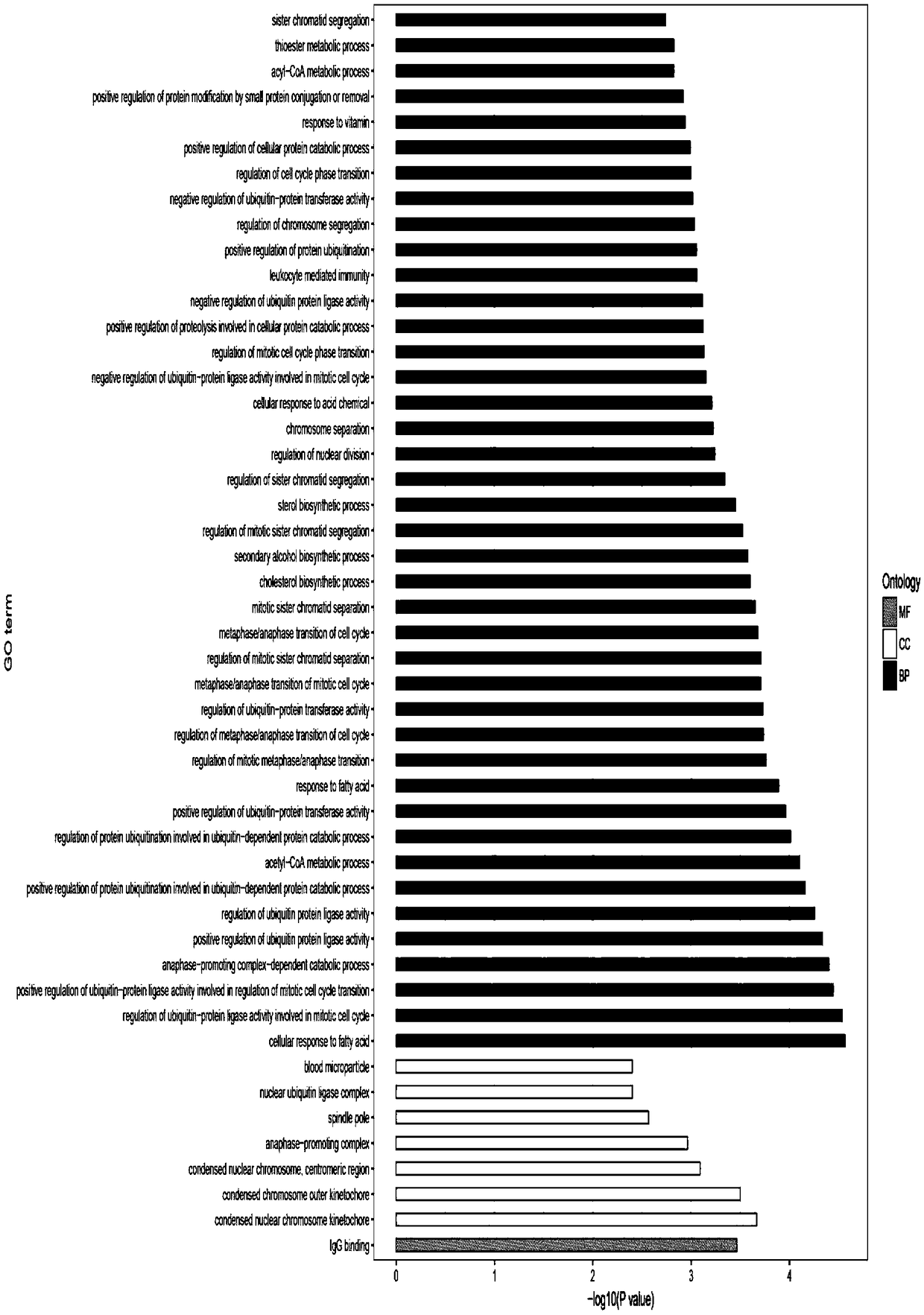
The invention provides a method for ex vivo expansion of human vascular endothelial progenitor cells in a low oxygen condition. The method comprises the following steps: separating EPCs from human bone marrow, culturing the EPCs in an incubator in the low oxygen condition, and ensuring that the low oxygen condition adopts a 1 percent O2 low oxygen condition. Gene expression to the EPCs by the lowoxygen condition comprises various gene differential expression, and at least relates to the following biological processes: reaction of cells to fatty acid, and adjustment of positive regulation of the activity of ubiquitin-protein ligase with transformed caryomitosis cell cycles. Compared with normoxia culture, the culture method has the advantages that low oxygen environment is more similar tobone marrow microenvironment, and is more beneficial to keeping the primary characteristics of the EPCs; the method performs molecular level evaluation on change of gene expression in EPCs caused by different culture methods, gives differential expression genes in which the low oxygen condition or anoxic condition affects cell dryness and molecular pathways of the differential expression genes, and is applied to improvement of the current stem cells.
Owner:广州赛琅生物技术有限公司
Reagent used for ex vivo expansion and method thereof
InactiveCN101445797AEasy to useMicrobiological testing/measurementDNA preparationAssayEx vivo expansion
The invention discloses a reagent used for ex vivo expansion and a method thereof, comprising an adaptor, a primer, a promoter, a restriction enzyme, and the like. The aim of RNA ex vivo expansion is achieved. Target RNA in the invention is connected with the adaptor and is expanded after RT-PCR under the existing of the primer. Then, a stem-loop structure is used for forming a unique site of the restriction enzyme. A product obtained through the restriction enzyme is promoted by the promoter and is transcribed to obtain a sequence which is totally the same with the target RNA sample except a G is added on 5' end and an A or C is added on few 3' ends. The RNA product can be directly used as the original sample RNA. The reagent used for the ex vivo expansion and the method thereof can be applied to various aspects related to the application of RNA, and the like, including general scientific research, medical assay and forensic identification.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Human stem cell growth factor as well as production method and application of polyethylene glycol (PEG) modified human stem cell growth factor
InactiveCN102559725AInduced expression condition optimizationHigh expressionCosmetic preparationsPeptide/protein ingredientsHalf-lifeCuticle
The invention discloses a human stem cell growth factor as well as a production method and application of a polyethylene glycol (PEG) modified human stem cell growth factor. The production method comprises the following steps of: fusing an h-SCF (Stem Cell Factor)-alpha sequence and an SUMO (Small Ubiquitin-Related Modifier) sequence and constructing to obtain an SUMO-rhSCF-alpha fused gene expression vector; transferring the SUMO-rhSCF-alpha fused gene expression vector into a host bacteria to obtain engineering bacteria; culturing the engineering bacteria and inducing to express an SUMO-rhSCF-alpha fused protein; and cutting off an SUMO part to obtain an rhSCF-alpha protein. By using the production method, the high soluble expression and large-scale purification of the rhSCF-alpha protein in cell plasmas are realized, and the activity of the obtained rhSCF-alpha protein is high. The invention also discloses the production method of the PEG modified human stem cell growth factor; the half-life period of the PEG modified human stem cell growth factor obtained by using the method is remarkably prolonged, and the activity of the PEG modified human stem cell growth factor in promoting the proliferation of red blood cells is also remarkably improved; and the PEG modified human stem cell growth factor can be applied to the preparation of medicines for hypohemia therapy, reconstruction and recovery of a hematopoietic function after chemoradiotherapy and a bone marrow transplantation operation, stem cell ex-vivo expansion and gene therapy and cosmetics for promoting the metabolism of epidermal cells, repairing aged and damaged skin cells, delaying the aging of skin and the like.
Owner:GUANGZHOU JINAN BIOMEDICINE RES & DEV CENT
Method for generating highly active human dendritic cells from peripheral blood mononuclear cells
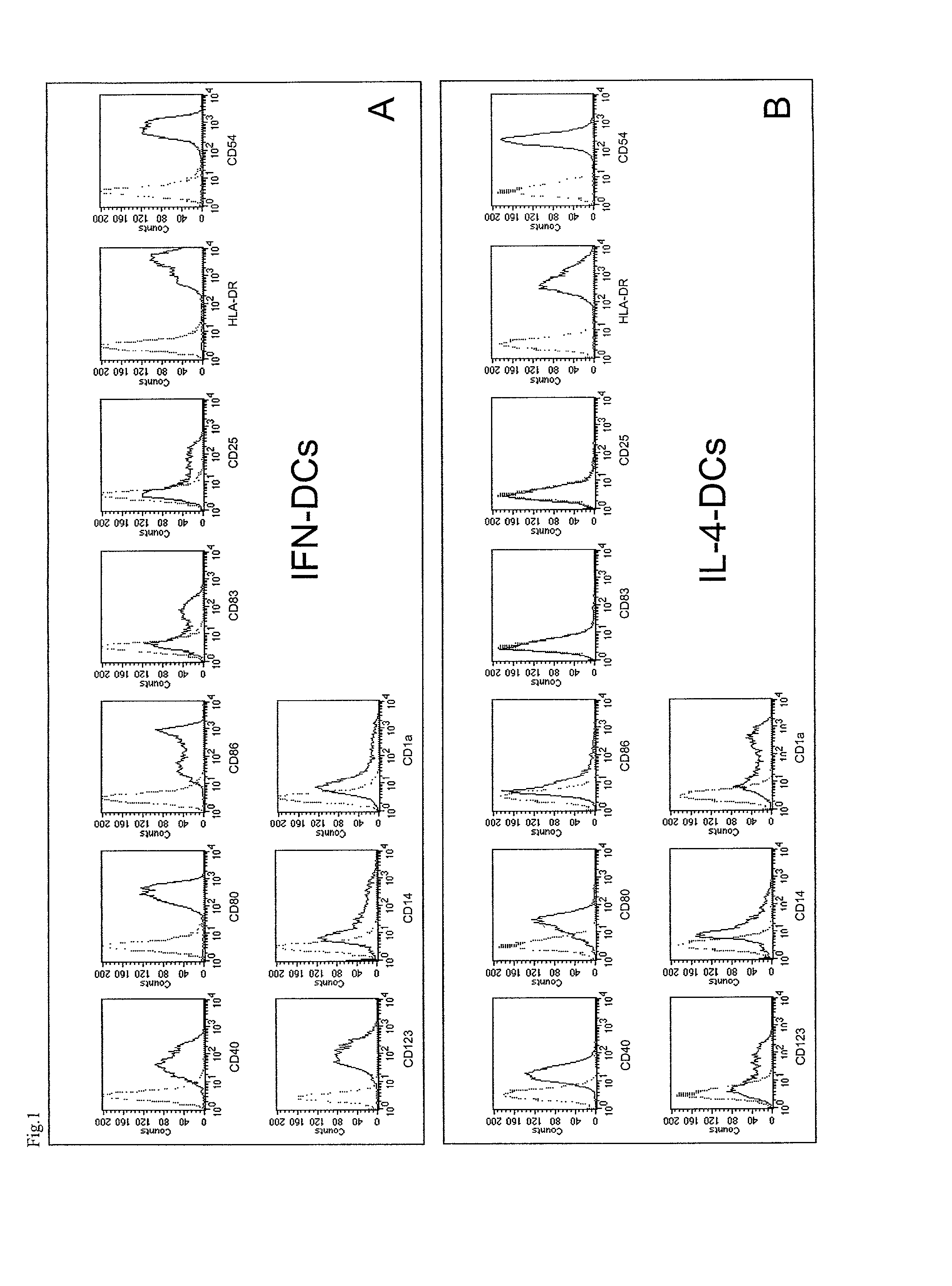
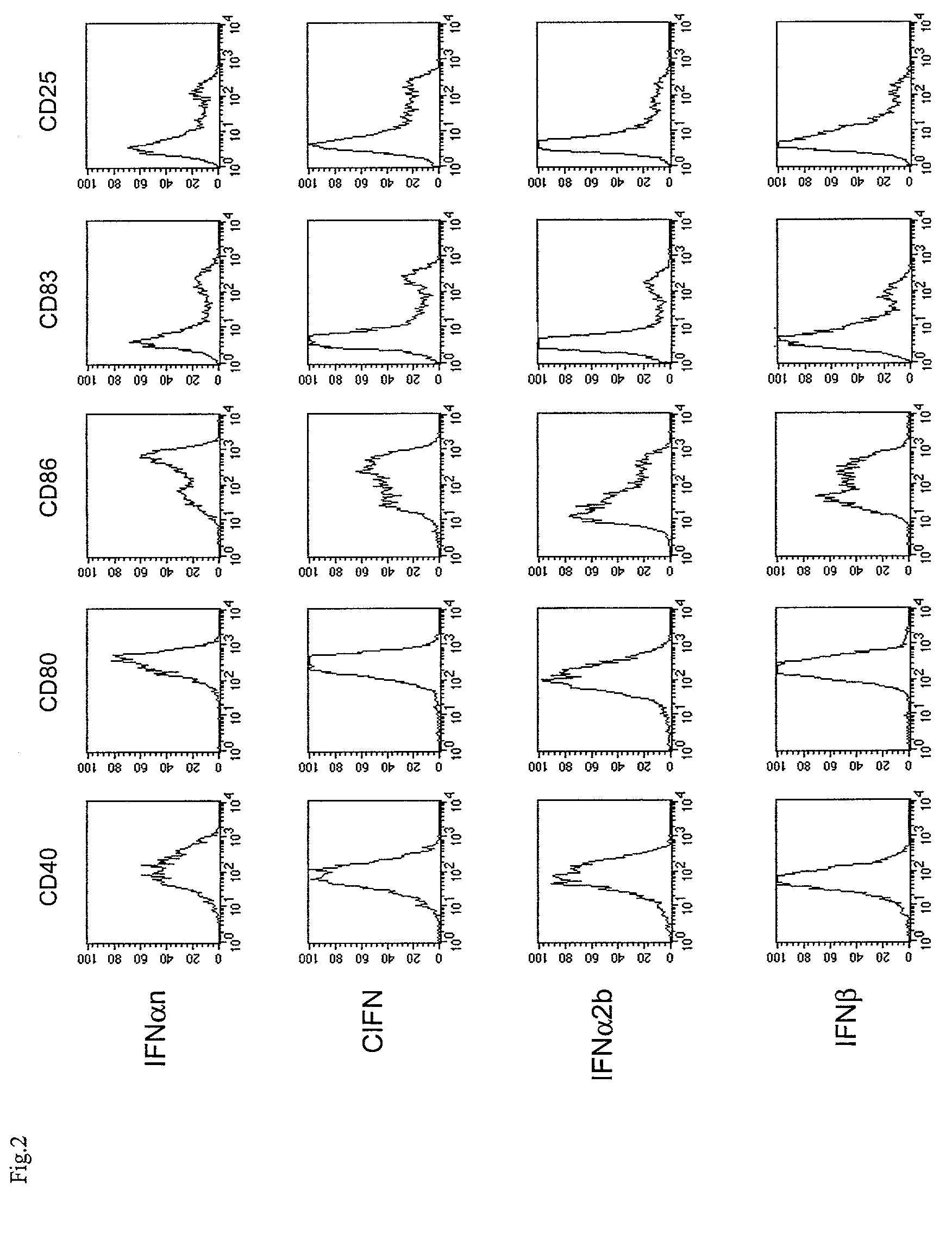
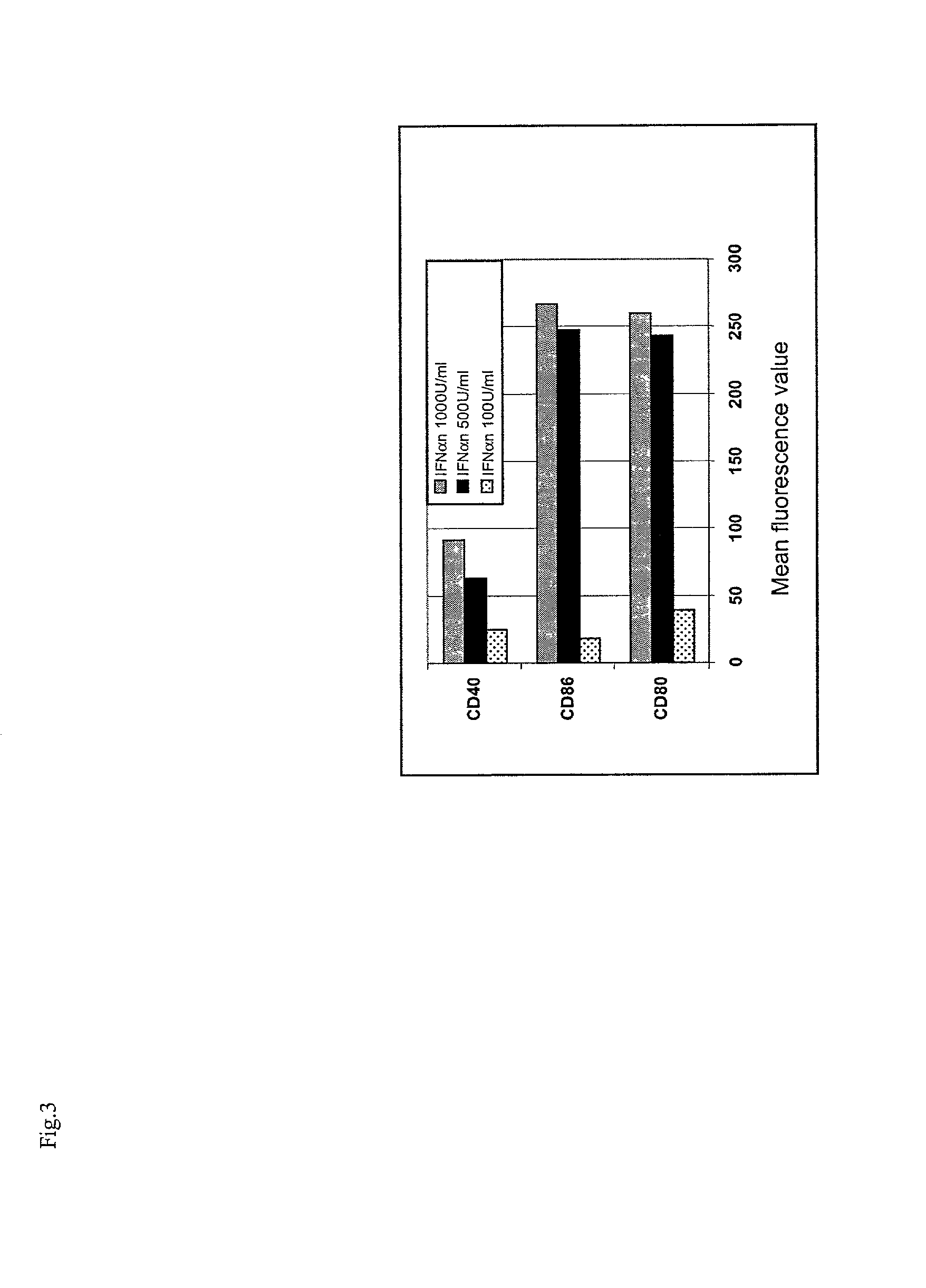
InactiveUS7566568B2Quick buildQuicker procedureViral antigen ingredientsSnake antigen ingredientsDendritic cellAdjuvant
The present invention relates to a process for deriving dendritic cells from mononuclear cells in culture comprising the step of putting in contact type I IFN with said mononuclear cells. Dendritic cells suitable as cellular adjuvants in prophylactic as well as therapeutic vaccination of animal and human beings, are obtainable thereby, after a single step treatment in a brief period of time. Dendritic cells obtainable thereby, pharmaceutical compositions including them, in particular a vaccine comprising said cells as active principle, and a method of treatment of a pathology associated with the presence of an antigen in human beings, are further objects of the invention, as well as a kit for deriving said dendritic cells and a method for the ex vivo expansion of T cells using them.
Owner:INST SUPERIORE DI SANITA
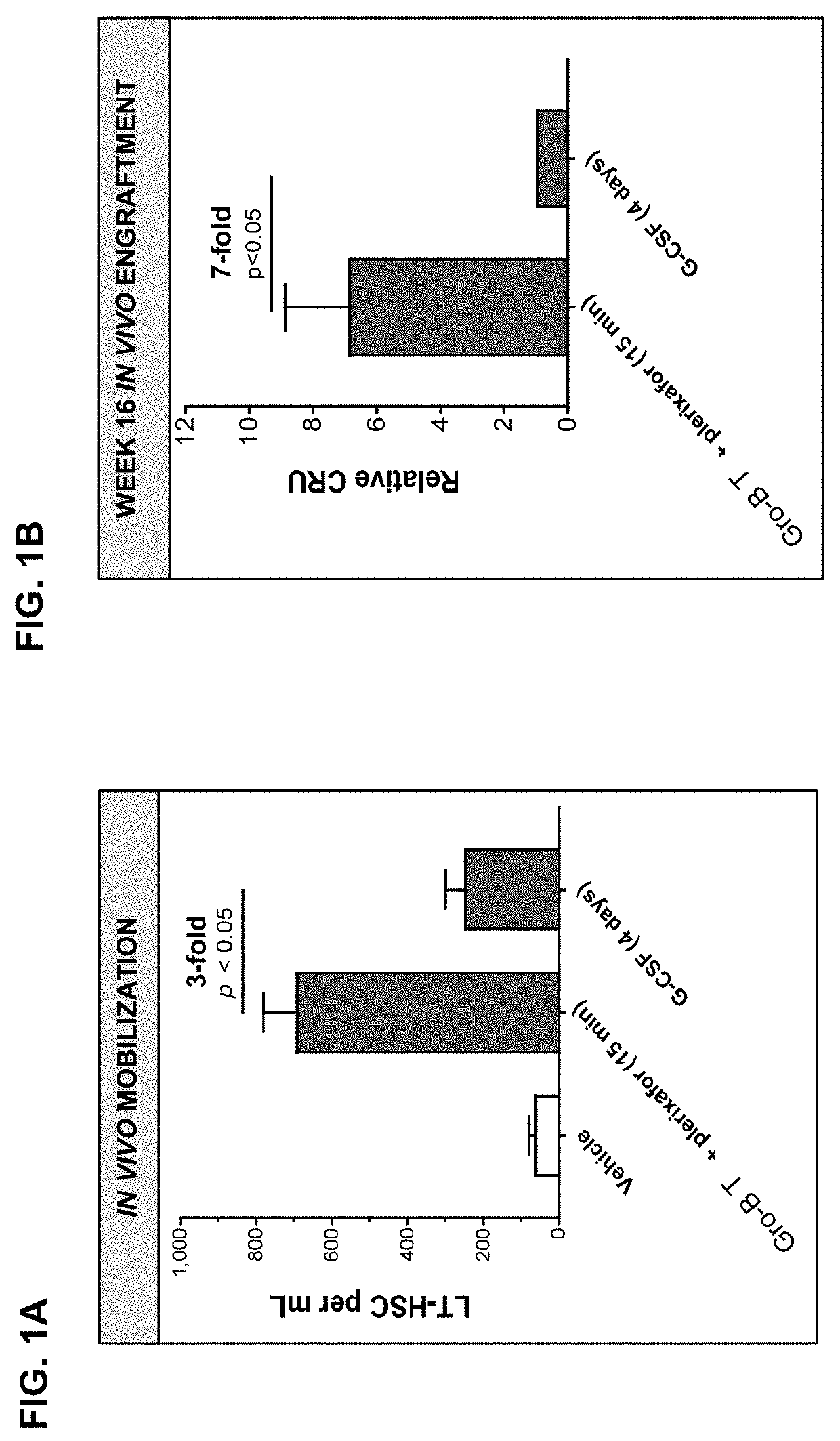
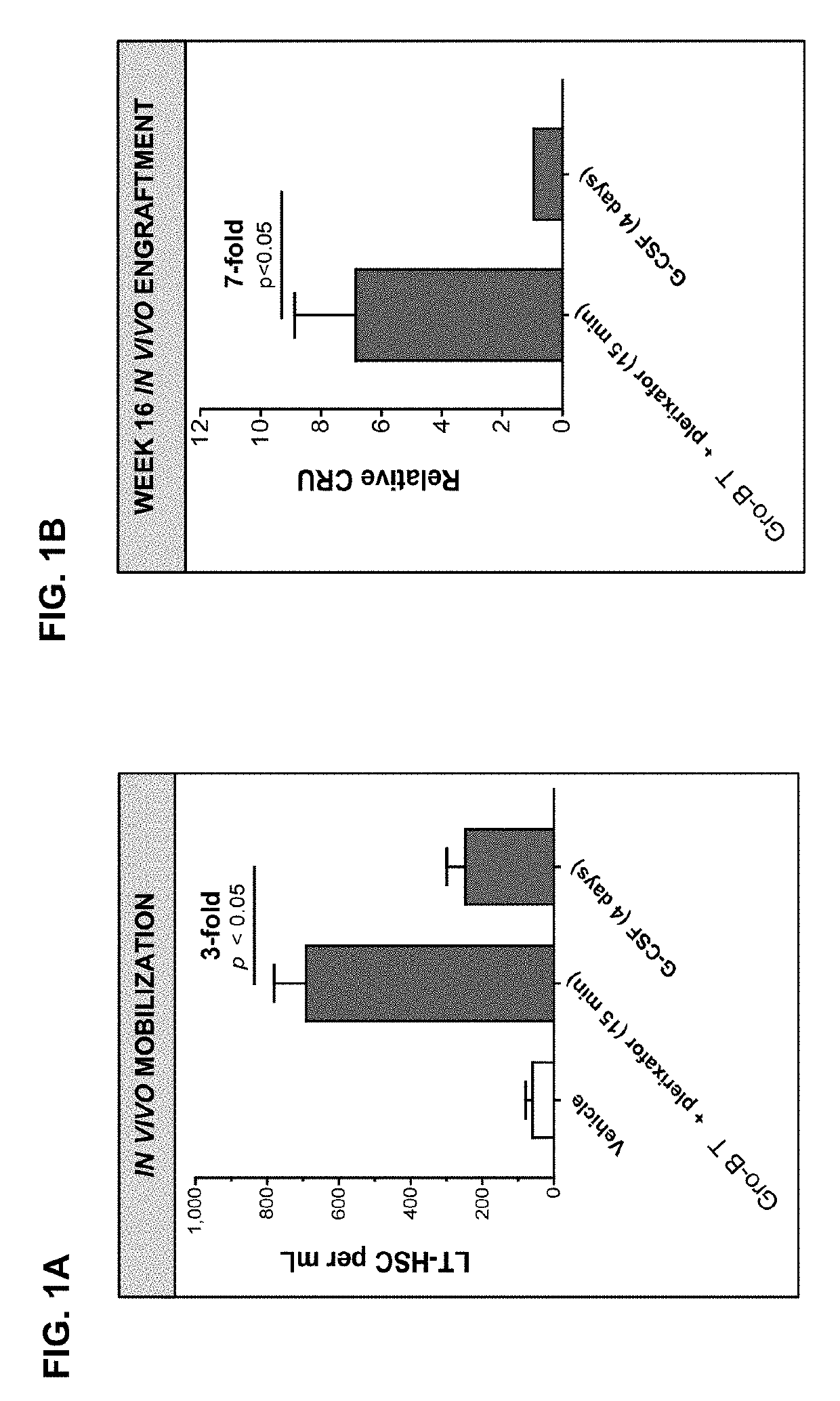
Dosing regimens for the mobilization of hematopoietic stem and progenitor cells
InactiveUS20190336536A1Reducing mobilizationIncrease the number ofOrganic active ingredientsChemokinesProgenitorRegimen
The invention provides compositions and methods useful for mobilizing populations of hematopoietic stem and progenitor cells within a donor, as well as for determining whether samples of mobilized cells are suitable for release for ex vivo expansion and / or therapeutic use. In accordance with the compositions and methods described herein, mobilized hematopoietic stem and progenitor cells can be withdrawn from a donor and administered to a patient for the treatment of various stem cell disorders, including hematopoietic diseases, metabolic disorders, cancers, and autoimmune diseases, among others. In certain embodiments, the compositions and methods described herein lead to the mobilization of a population of CD34dim cells that have immunosuppressive effects and that can reduce the incidence of graft vs. host disease.
Owner:MAGENTA THERAPEUTICS INC
STAT3 activated stem cell
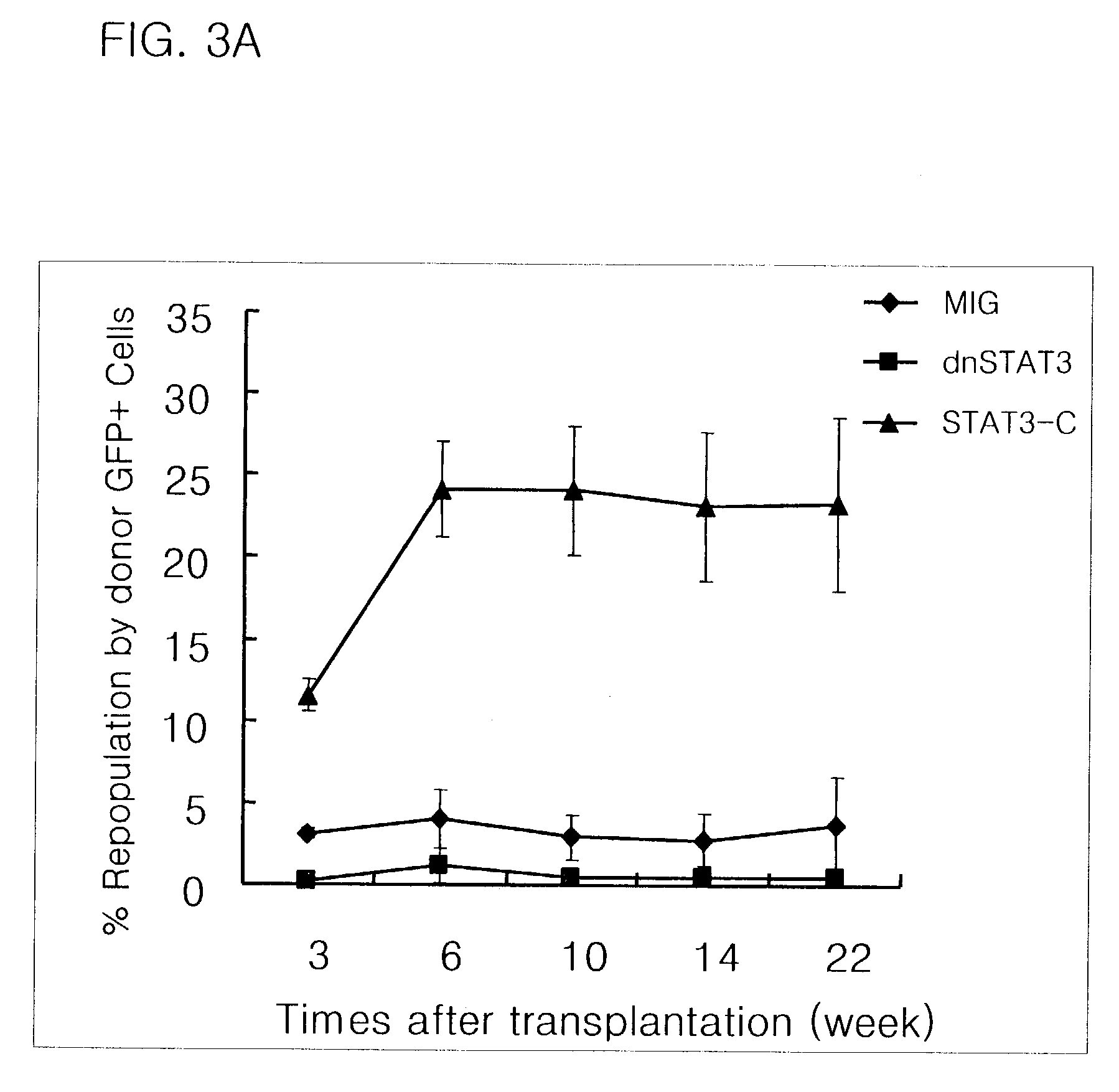
InactiveUS7510870B2Improve regenerative abilityAccelerate self-renewalPeptide/protein ingredientsGenetic material ingredientsEx vivo expansionIn vivo
Stem cells modified to express activated form of STAT3 by genetic modification or protein delivery, and stem cells co-cultured with cells expressing activated form of STAT3 exhibit increased ex-vivo expansion and enhanced in-vivo regeneration accompanied by net increase in stem cell self-renewal as compared to control group.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOPERATION FOUND
Use of endogenous viral vaccine in chimeric antigen receptor T cell therapy
ActiveUS11116834B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyViral VaccineTGE VACCINE
Provided herein are, inter alia, methods and compositions including T cells expressing (i) a recombinant CAR protein which includes a peptide binding site and is capable of specifically binding cancer-specific antigens and (ii) a T cell receptor specific for a viral antigen (e.g., a CMV pp65 protein). The engineered T cells provided herein may be used in combination with a viral vaccine (e.g. cytomegalovirus (CMV) Triplex Vaccine) to treat a variety of cancers. The methods described herein also permit in viva expansion of CMV-specific CAR T cells, instead of or in addition to ex vivo expansion, avoiding excessive T cell exhaustion that results in some cases from ex vivo manufacturing.
Owner:CITY OF HOPE
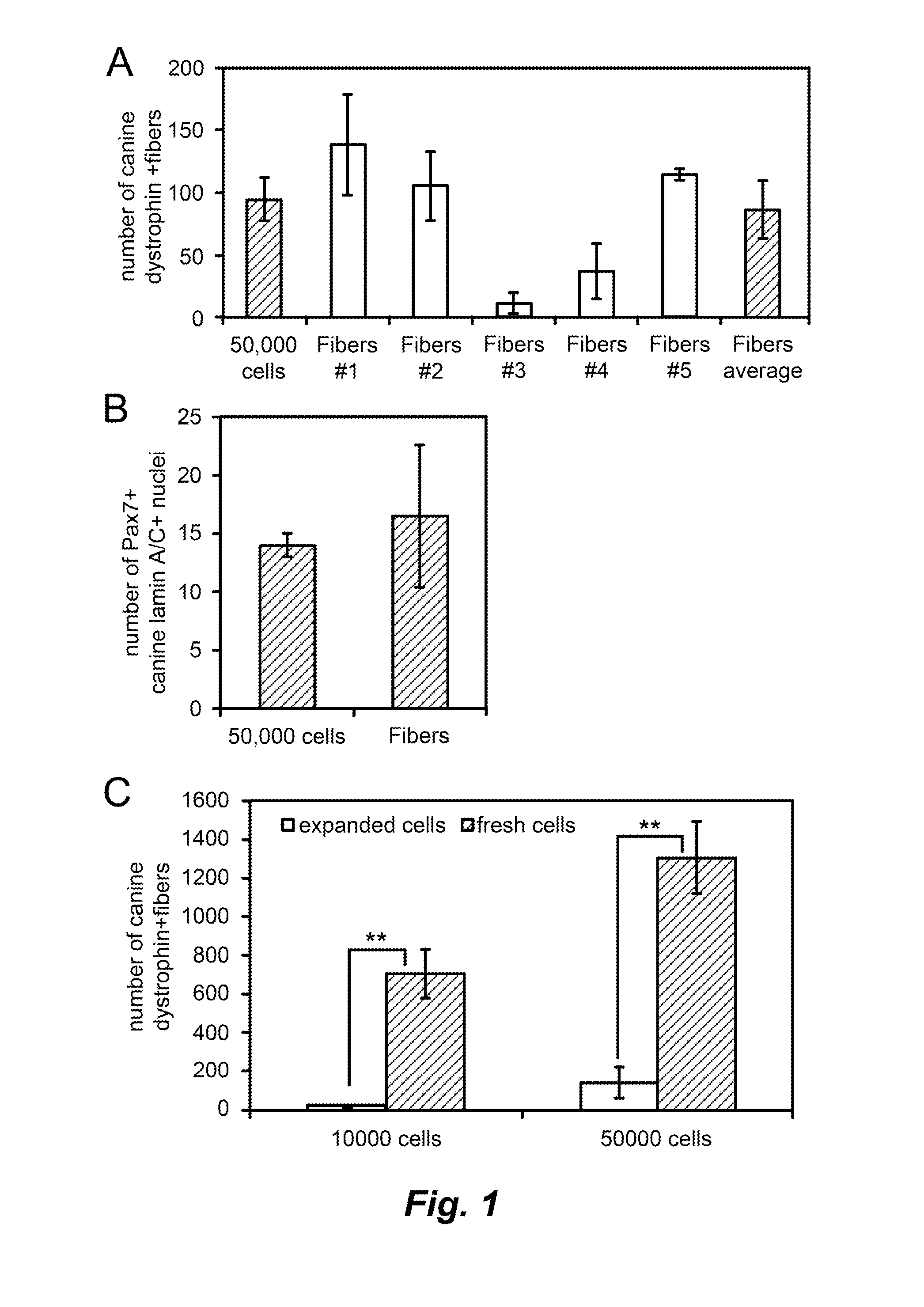
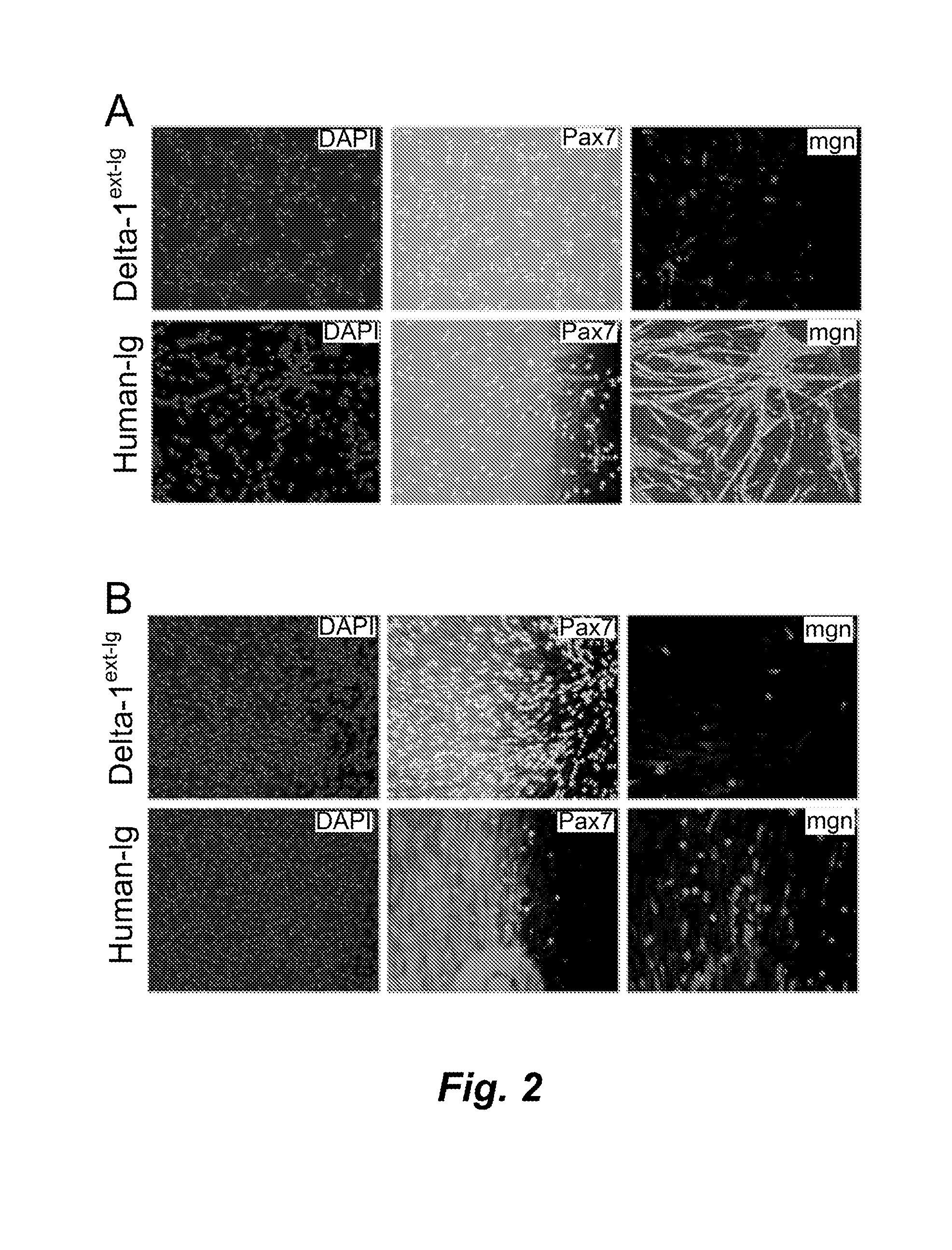
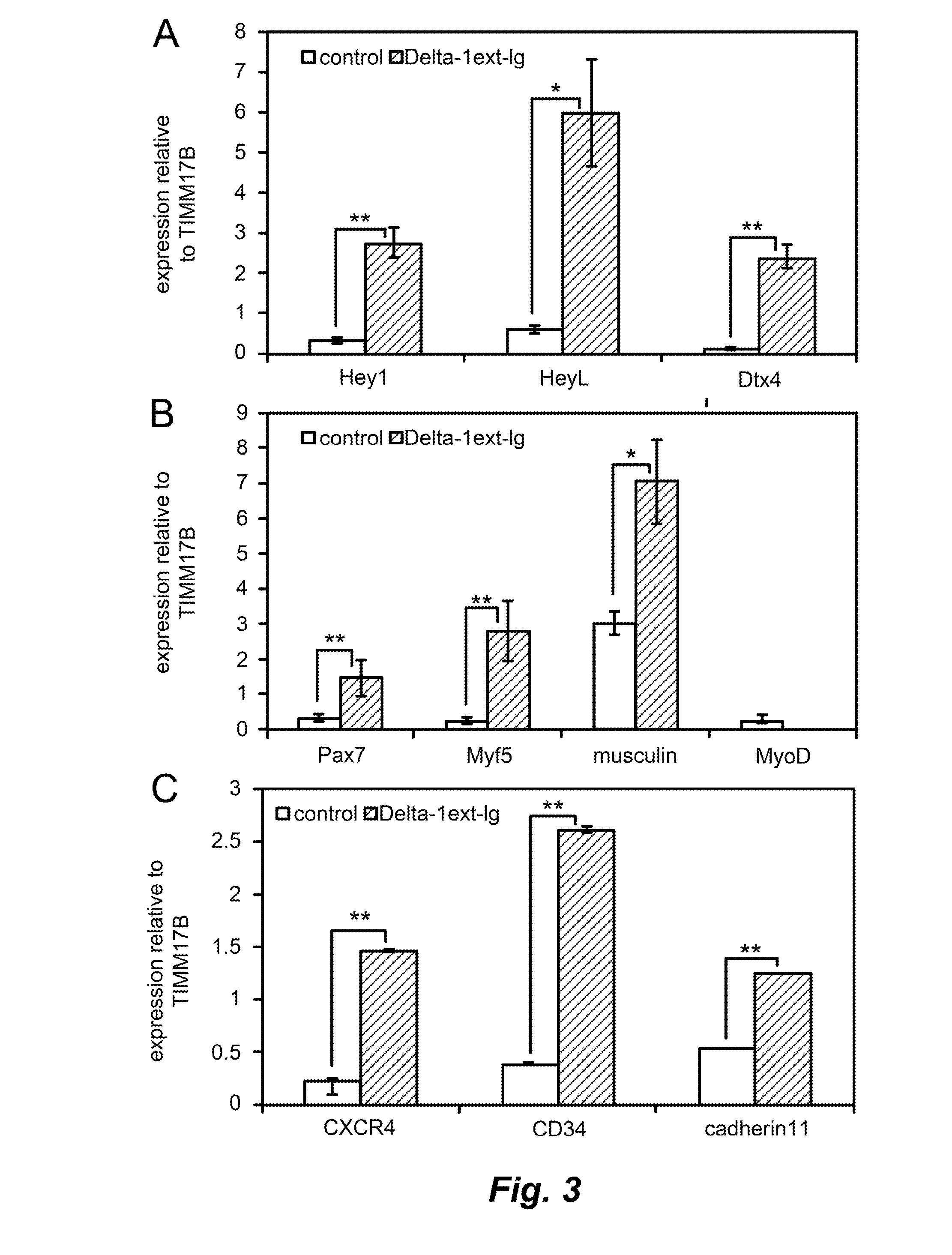
Ex vivo expansion of myogenic stem cells by notch activation
InactiveUS20150166961A1Promoting muscle tissue regenerationEasy to transplantBiocideMicrobiological testing/measurementProgenitorHuman cell
Activating Notch signaling in cultured canine muscle derived cells inhibited myogenic differentiation, and increased the number of myogenic progenitor cells that were similar to quiescent or newly activated satellite cells. Importantly, cells expanded in the presence of Notch activation maintained engraftment potential, indicating the potential for therapeutic benefit. Activation of Notch signaling to inhibit myogenic differentiation in cultured human muscle-derived cells is also contemplated, for maintaining engraftment potential using such human cells in transplantation.
Owner:FRED HUTCHINSON CANCER RES CENT
Dosing regimens for the mobilization of hematopoietic stem and progenitor cells
ActiveUS20190269735A1Reducing mobilizationIncrease the number ofOrganic active ingredientsPeptide/protein ingredientsProgenitorRegimen
The invention provides compositions and methods useful for mobilizing populations of hematopoietic stem and progenitor cells within a donor, as well as for determining whether samples of mobilized cells are suitable for release for ex vivo expansion and / or therapeutic use. In accordance with the compositions and methods described herein, mobilized hematopoietic stem and progenitor cells can be withdrawn from a donor and administered to a patient for the treatment of various stem cell disorders, including hematopoietic diseases, metabolic disorders, cancers, and autoimmune diseases, among others. In certain embodiments, the compositions and methods described herein lead to the mobilization of a population of CD34dim cells that have immunosuppressive effects and that can reduce the incidence of graft vs. host disease.
Owner:MAGENTA THERAPEUTICS INC
Substituted azole derivatives for generation, proliferation and differentiation of hematopoietic stem and progenitor cells
ActiveCN109890805AOrganic chemistryMammal material medical ingredientsEx vivo expansionXenograft Transplantation
The present invention relates to substituted azole derivatives and their use in the ex vivo expansion of CD34+ hematopoietic stem and progenitor cells (HSPC) in a biological sample, more particularlyin the expansion of these cells obtained from non-enriched, i.e., the mononuclear fraction of the biological sample. The present invention further describes the transplantation regimen of the expandedhematopoietic graft developed through xenotransplantation studies.
Owner:NAT UNIV OF SINGAPORE +1
Assays for determining the effect of an immune cell on a cell from an infectious or neoplastic disease
An in vitro assay is provided for determining the effect of an immune cell on a cell from an infectious or neoplastic disease. Also provided is an in vitro assay for determining the effect of an activated CD8+ T-cell on a sensitized melanoma cell. A method for improving the specific cytolytic activity (SCA) of an immune cell comprising contacting an immune cell with an antigen and an antigen-independent pro-inflammatory agent is provided. A method for ex vivo expansion of antigen-specific CD8+ T-cells with enhanced specific cytolytic activity (SCA) comprising culturing the antigen-specific CD8+ T-cells in a suitable culture media comprising an amino acid. An in vitro assay is provided for determining the effect of an immune cell on a cell from an infectious or neoplastic disease. A method of treating a subject suffering from an infectious or neoplastic disease with immuno therapy is described.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods, compositions, and assays for determining the effect of an immune cell on a cell from an infectious or neoplastic disease
An in vitro assay is provided for determining the effect of an immune cell on a cell from an infectious or neoplastic disease. Also provided is an in vitro assay for determining the effect of an activated CD8+ T-cell on a sensitized melanoma cell. A method for improving the specific cytolytic activity (SCA) of an immune cell comprising contacting an immune cell with an antigen and an antigen-independent pro-inflammatory agent is provided. A method for ex vivo expansion of antigen-specific CD8+ T-cells with enhanced specific cytolytic activity (SCA) comprising culturing the antigen-specific CD8+ T-cells in a suitable culture media comprising an amino acid. An in vitro assay is provided for determining the effect of an immune cell on a cell from an infectious or neoplastic disease. A method of treating a subject suffering from an infectious or neoplastic disease with immuno therapy is described.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
In vivo and ex vivo expansion of hematopoietic stem cells with a targeted combination of clinically tested, FDA approved drugs
The present invention provides a therapeutic approach to maintain and expand HSCs in vivo using currently available medications that target GSK-3 and mTOR. The present invention also provides a system and method for the ex vivo culturing of HSCs, where an mTOR inhibitor is combined with a GSK-3 inhibitor within the culturing conditions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com