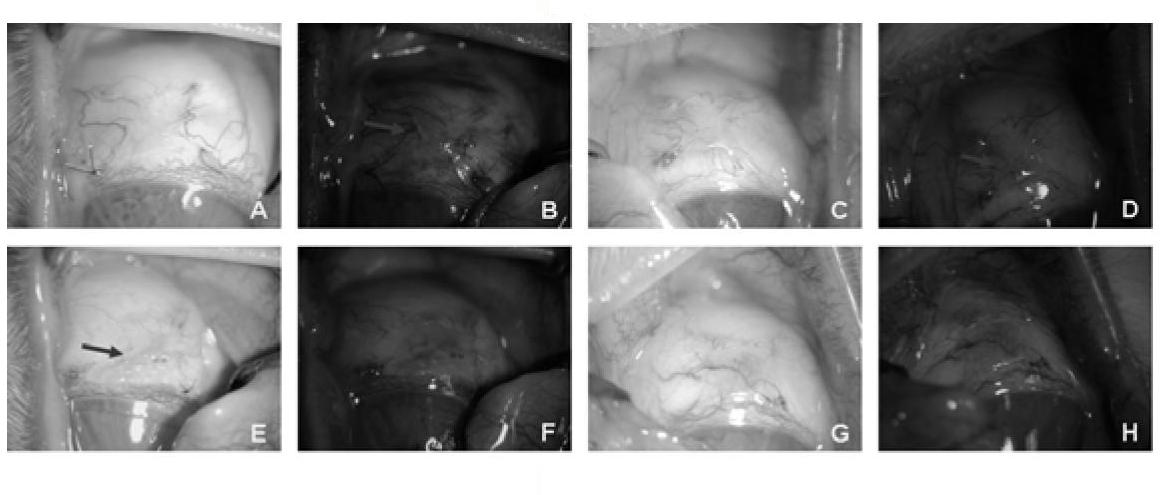
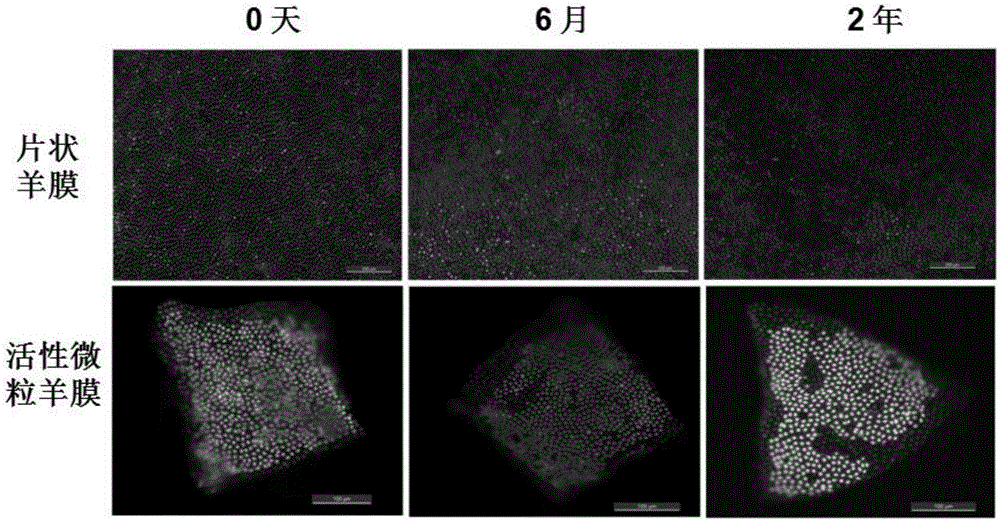
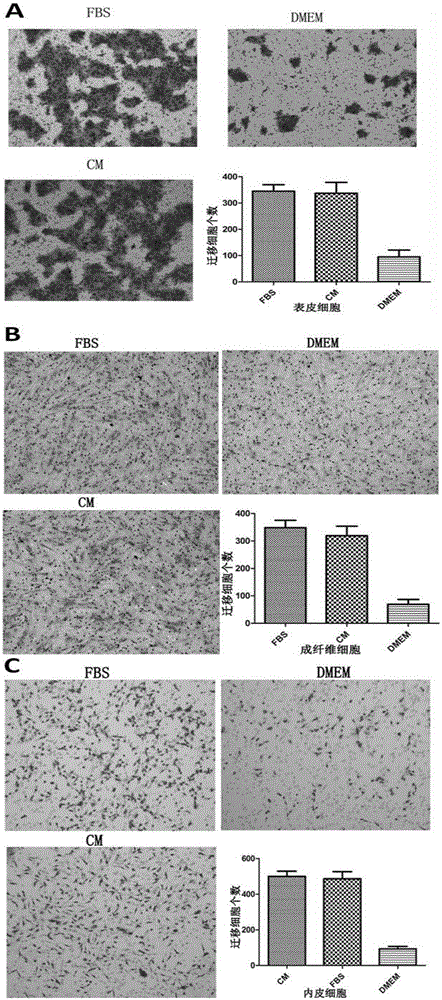
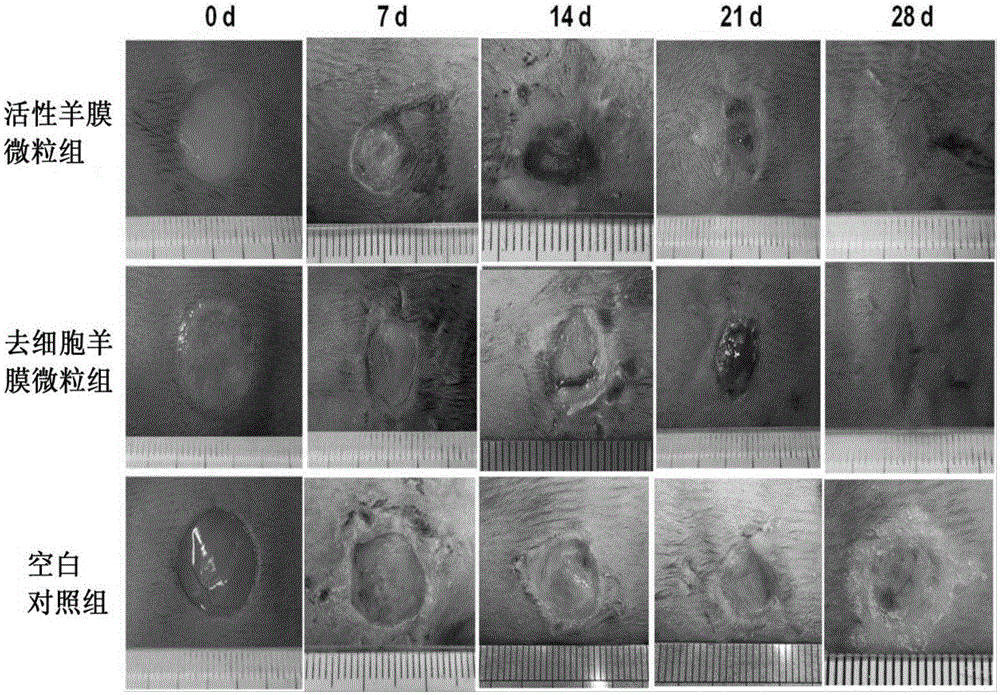
Patents
Literature
88 results about "Amniotic epithelial cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
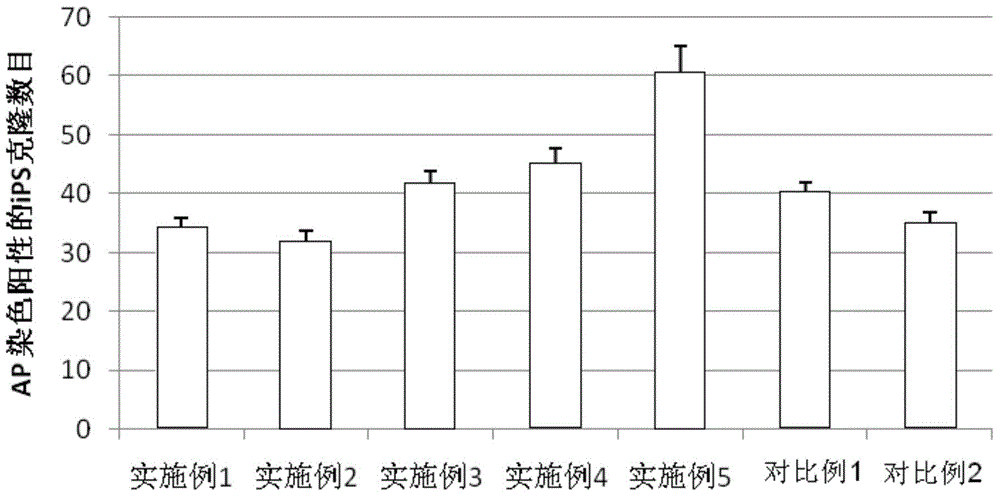
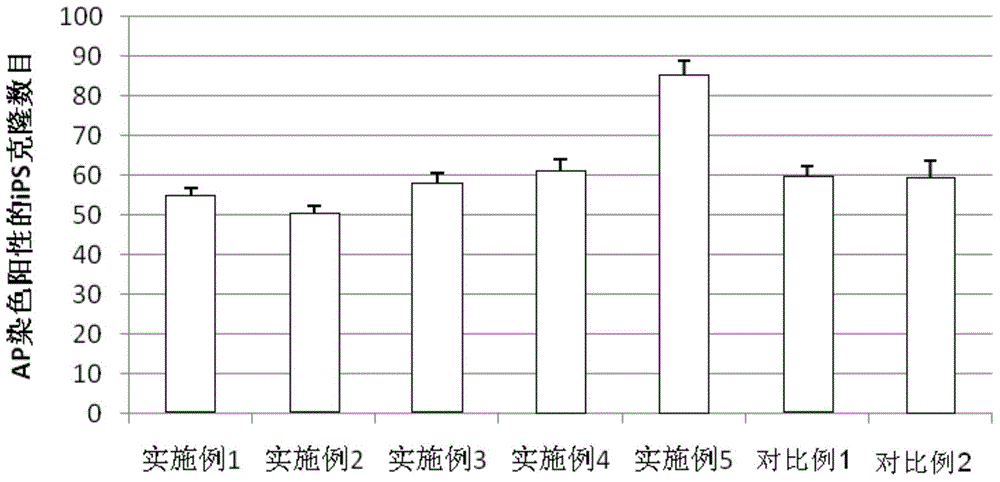
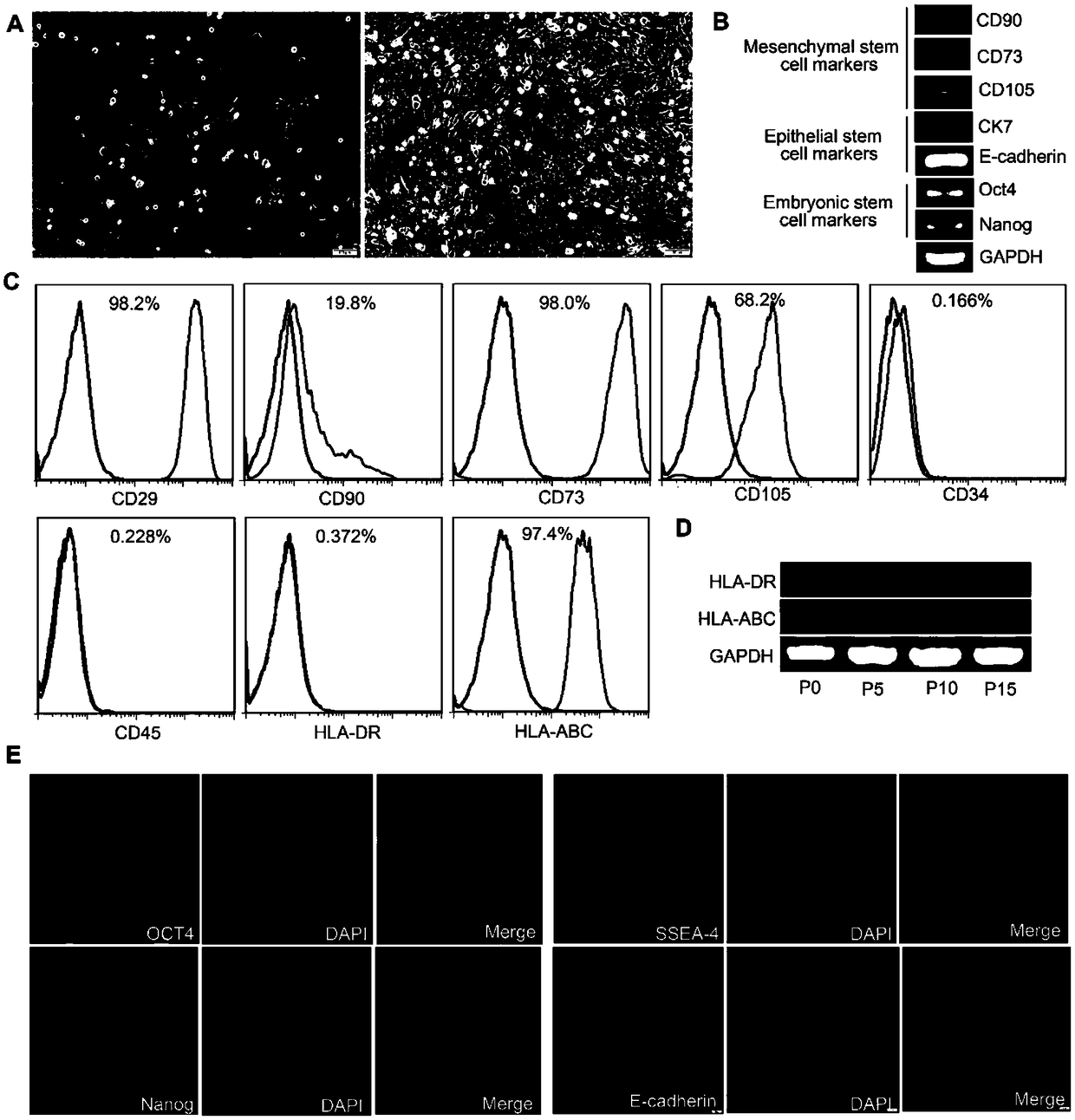
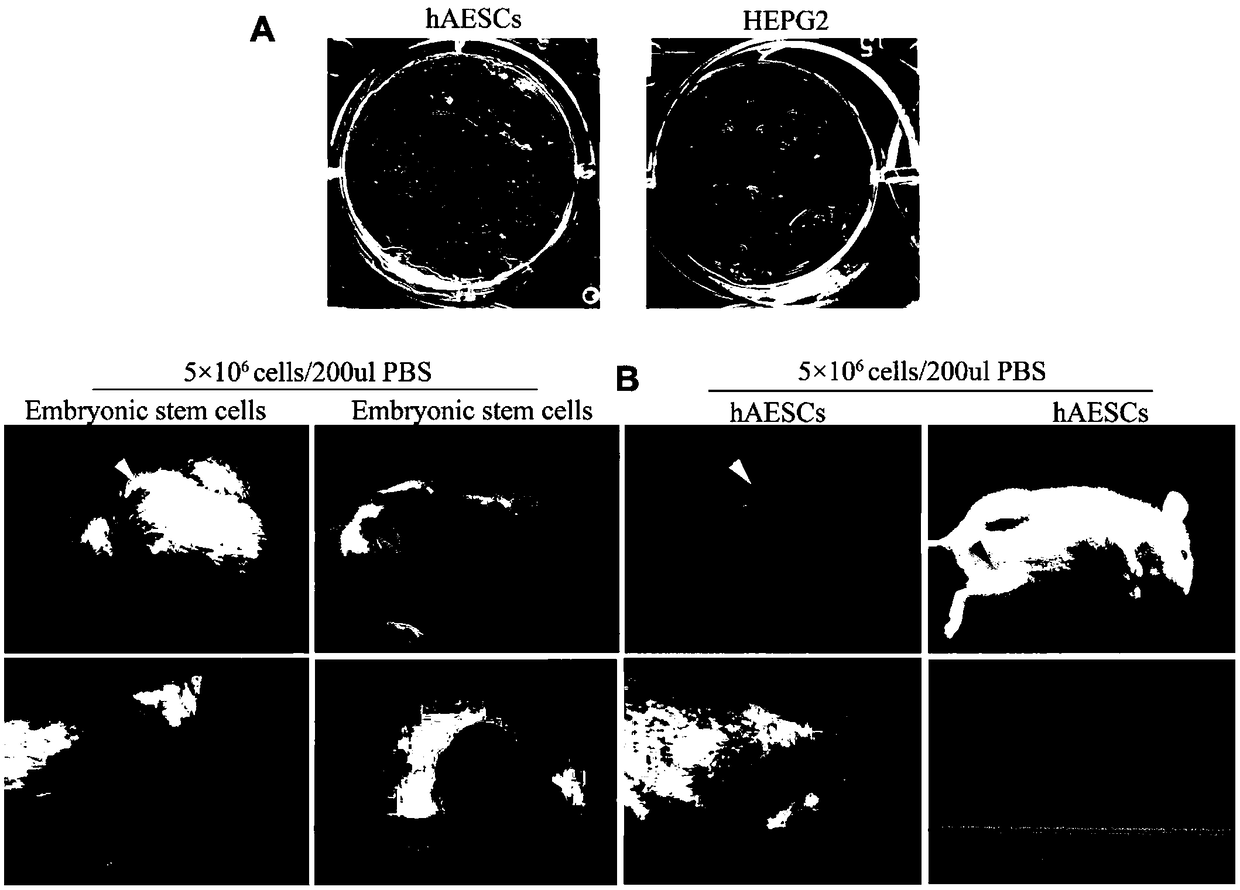
An amniotic epithelial cell is a form of stem cell extracted from the lining of the inner membrane of the placenta. Amniotic epithelial cells start to develop around 8 days post fertilization. These cells are known to have some of the same markers as embryonic stem cells, more specifically, Oct-4 and nanog. These transcription factors are the basis of the pluripotency of stem cells. Amniotic epithelial cells have the ability to develop into any of the three germ layers: endoderm, mesoderm, and ectoderm. They can develop into several organ tissues specific to these germ layers including heart, brain, and liver. The pluripotency of the human amniotic epithelial cells makes them useful in treating and fighting diseases and disorders of the nervous system as well as other tissues of the human body. Artificial heart valves and working tracheas, as well as muscle, fat, bone, heart, neural and liver cells have all been engineered using amniotic stem cells. Tissues obtained from amniotic cell lines show promise for patients suffering from congenital diseases or malformations of the heart, liver, lungs, kidneys, and cerebral tissue.
Isolation and expansion of animal cells in cell cultures
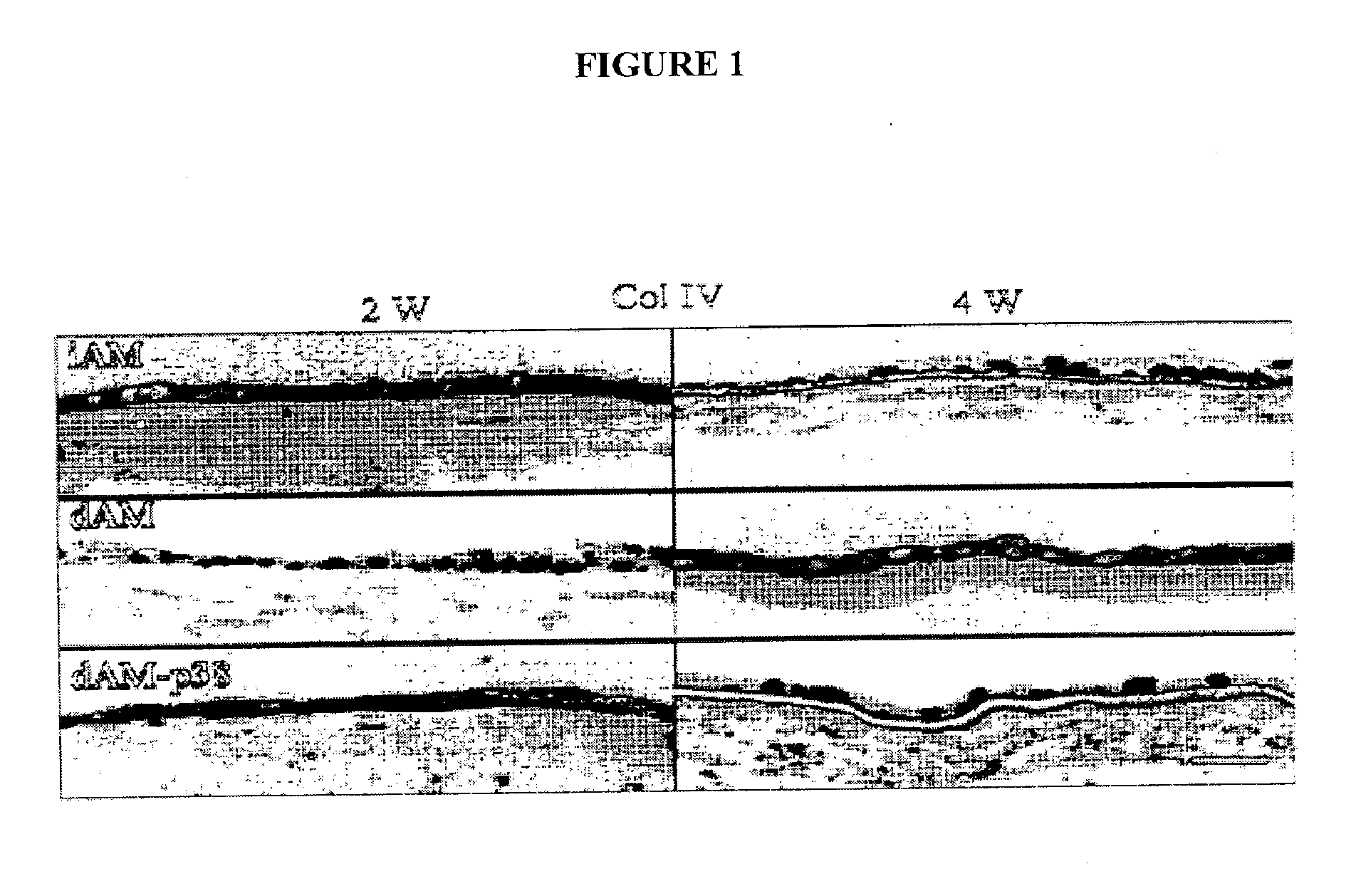
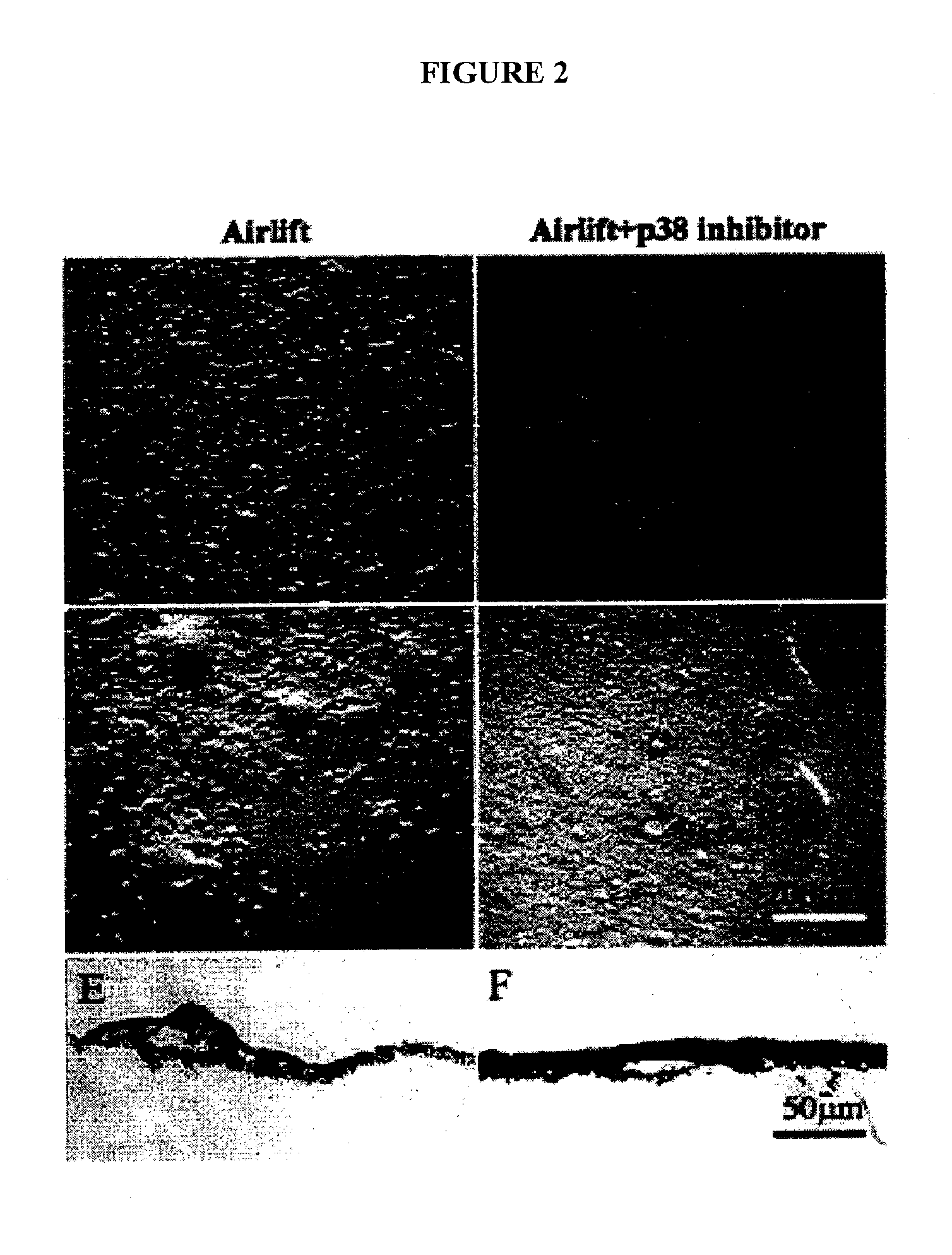
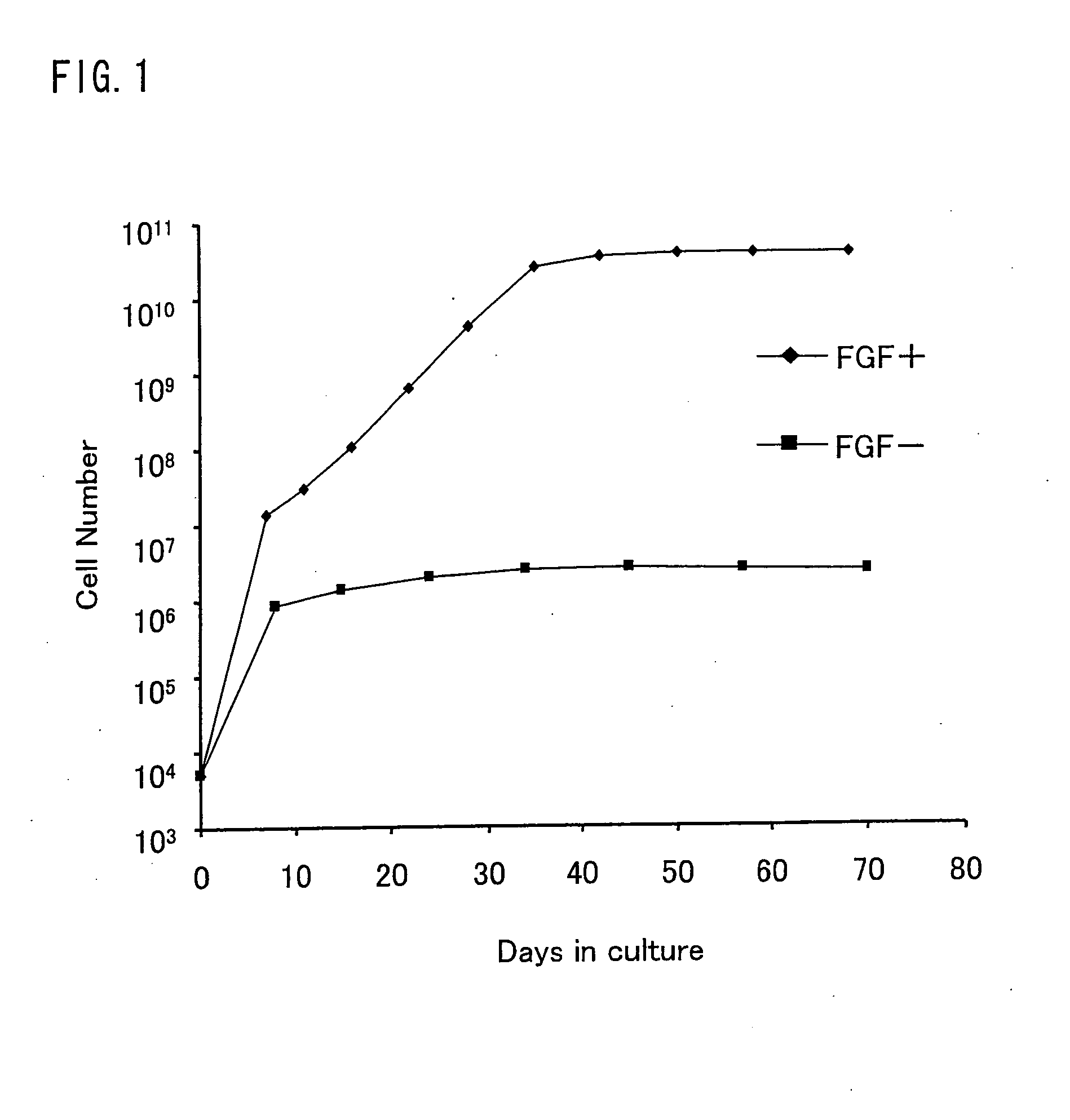
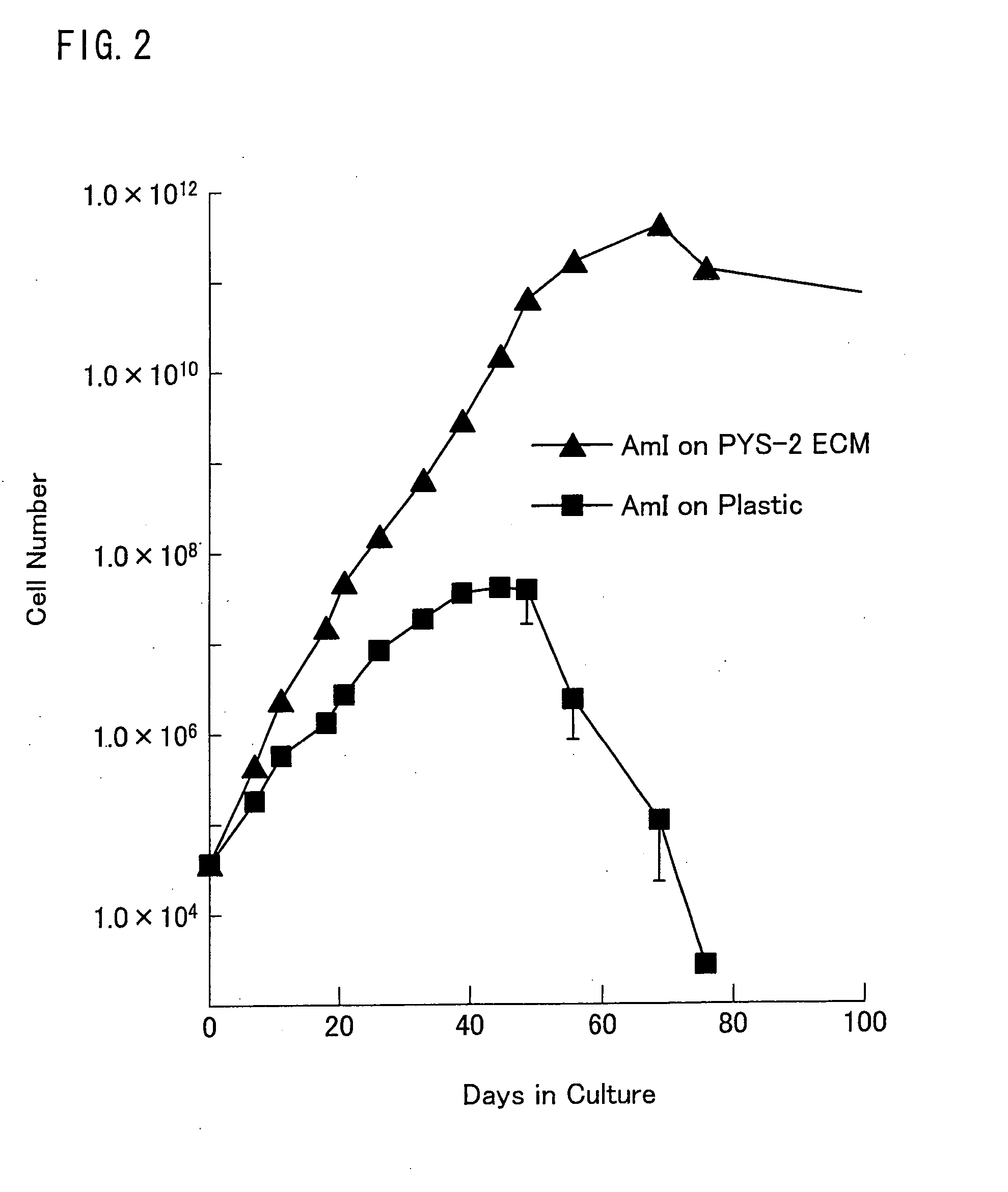
Described are methods for isolating / purifying and expanding animal stem cells and stem-cell-like cells. Isolation methods include conditions comprising preferentially digesting non-stem cells and non-stem-cell-like cells in a population and preferentially adhering stem cells and stem-cell-like cells in a population. Expansion methods include culturing such cells under conditions comprising modulation of TGF-β signaling, inhibition of cell signaling mediated by p38 MAP kinase using small molecular weight inhibitors, expansion of the cells on human amniotic epithelial cells as feeder layers, control of cell seeding density, control of levels of Ca2+ in the culture media, rapid adhesion on a substrate or by a combination of such conditions. More particularly, what is disclosed relates to methods and systems for expanding animal cells in ex vivo cell cultures, while preventing cellular differentiation, and selectively enriching stem cells. The embodiments also disclose a culture system for ex vivo expansion of limbal epithelial cells or mesenchymal cells, as well as surgical grafts made there from.
Owner:TISSUETECH INC
Method for isolating and culturing adult stem cells derived from human amniotic epithelium
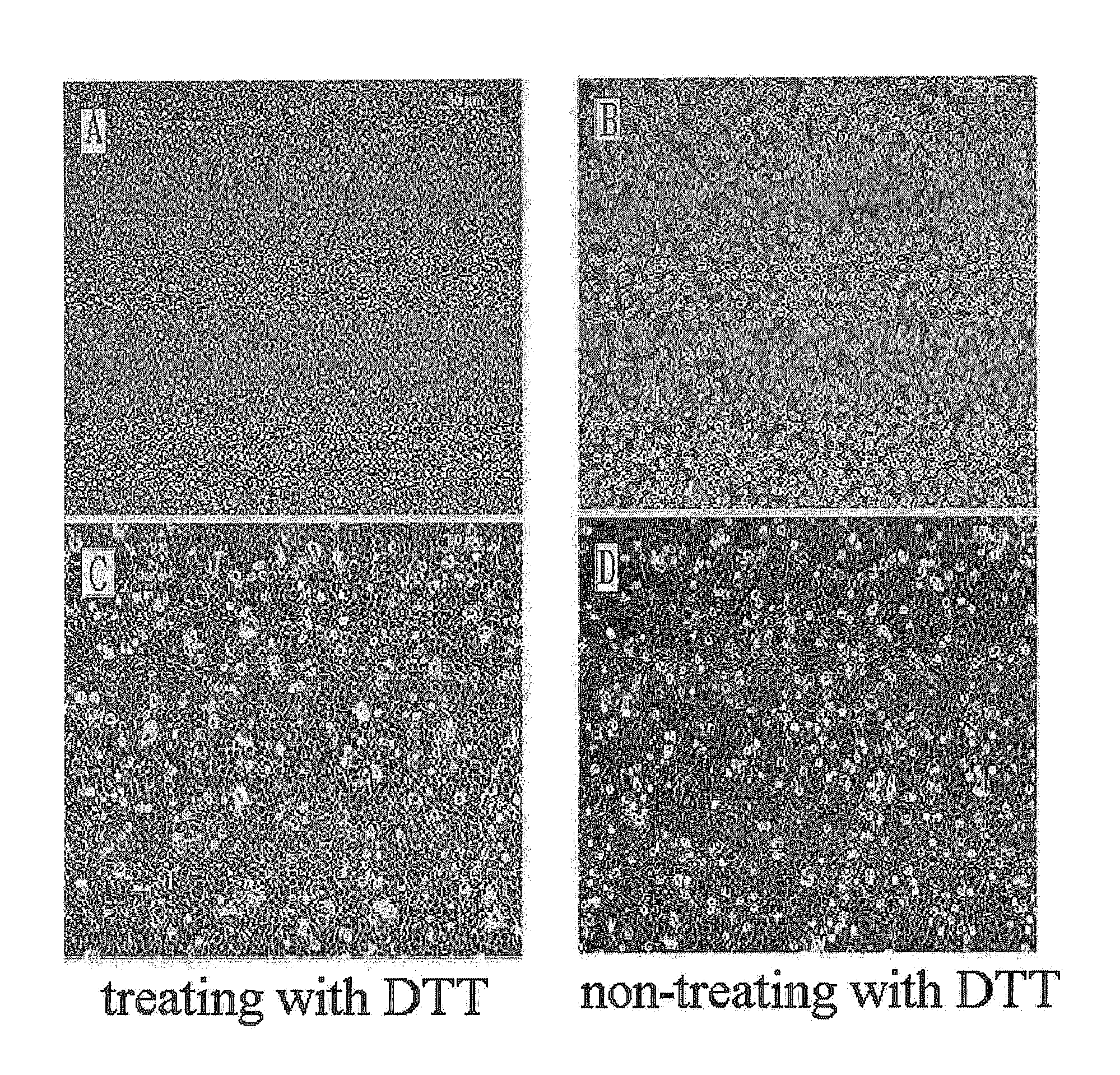
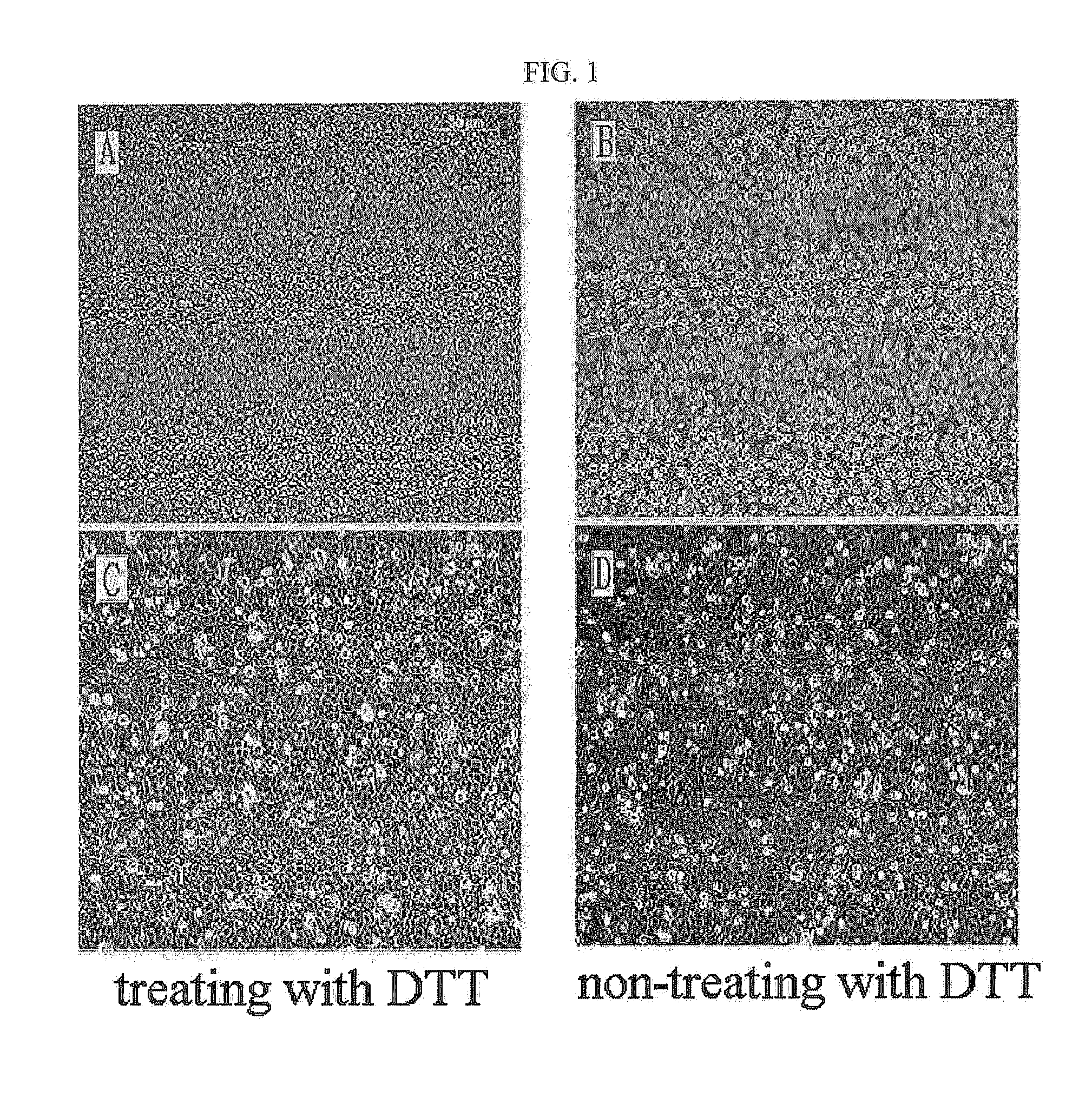
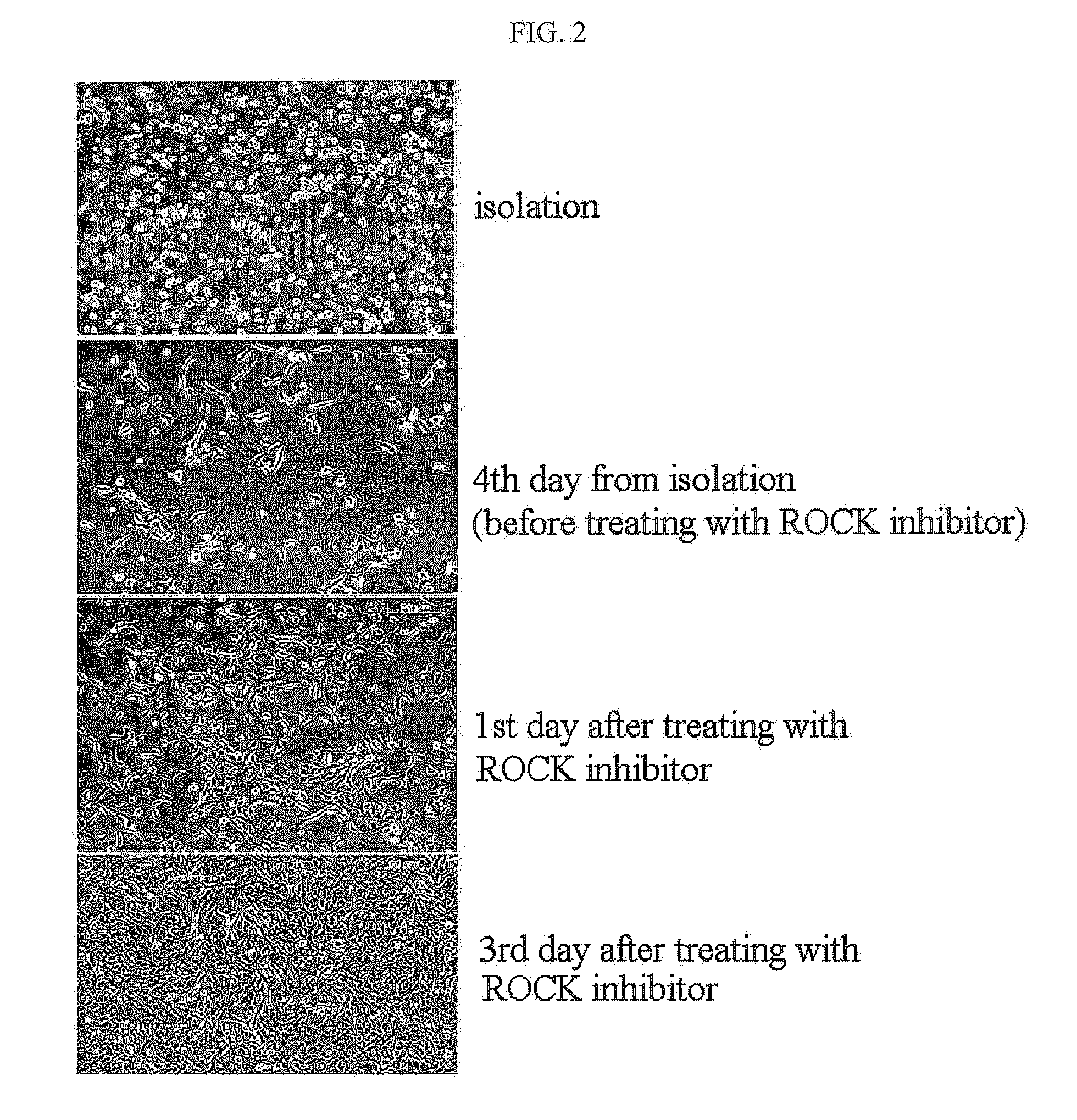
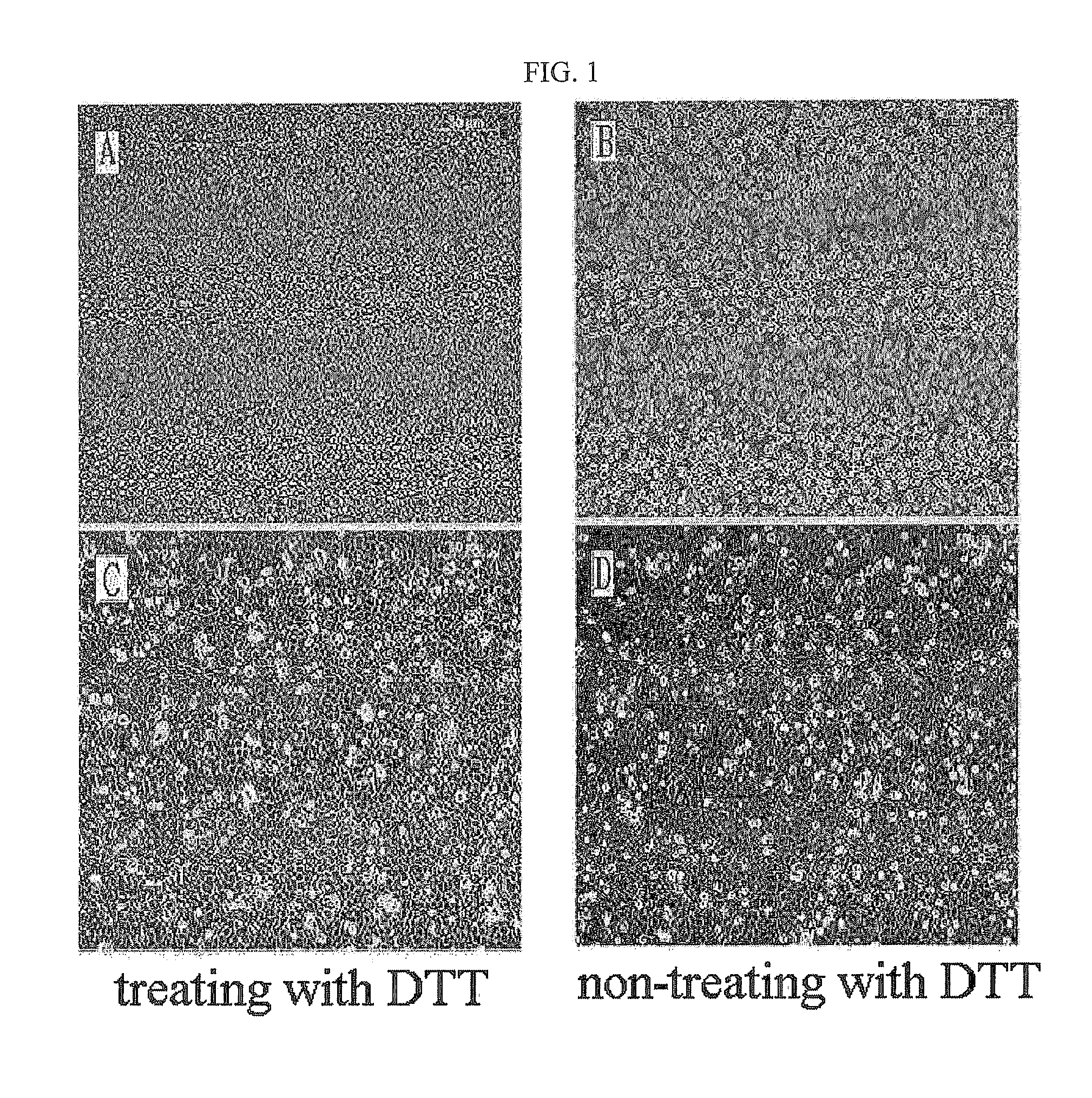
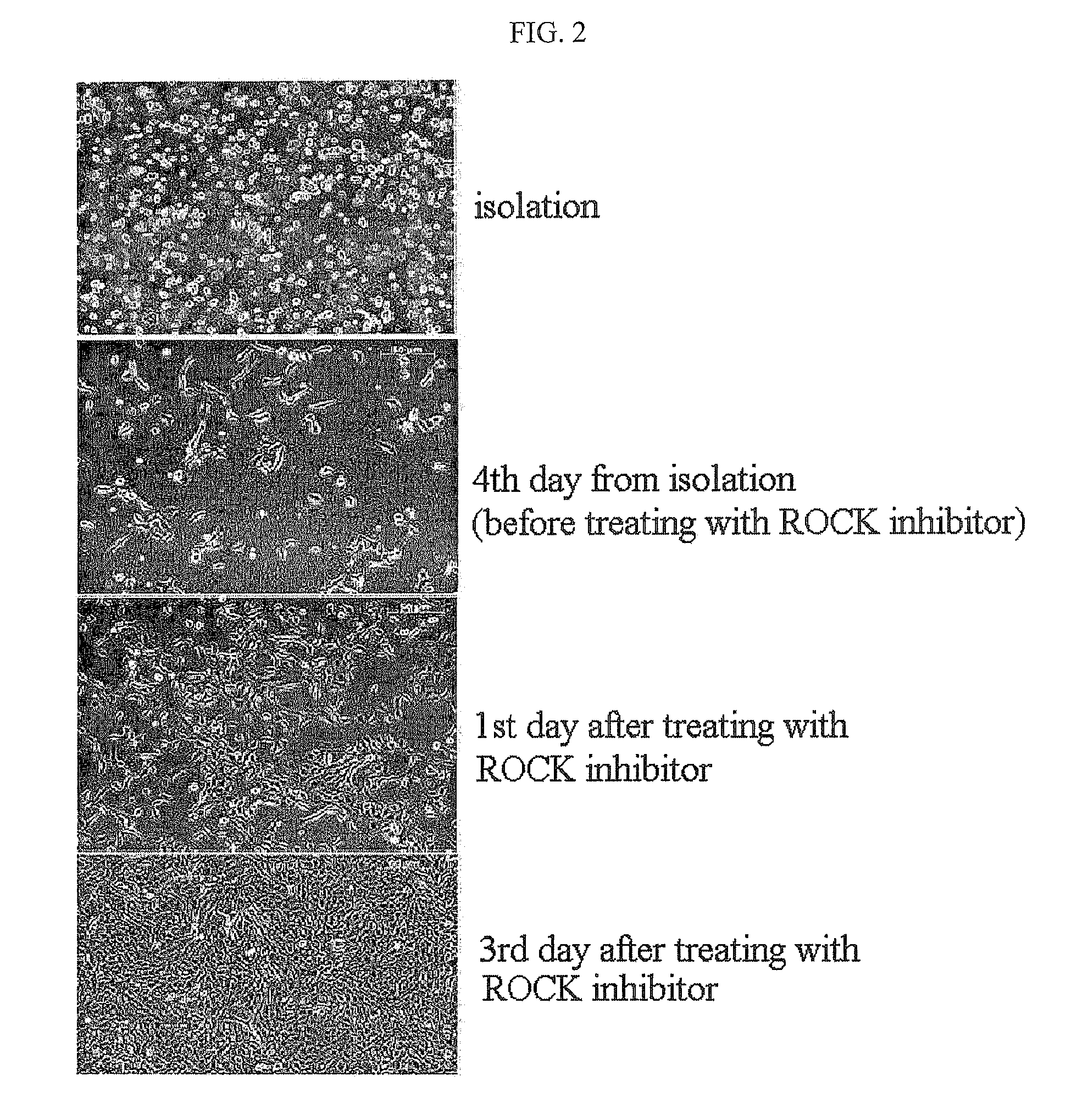
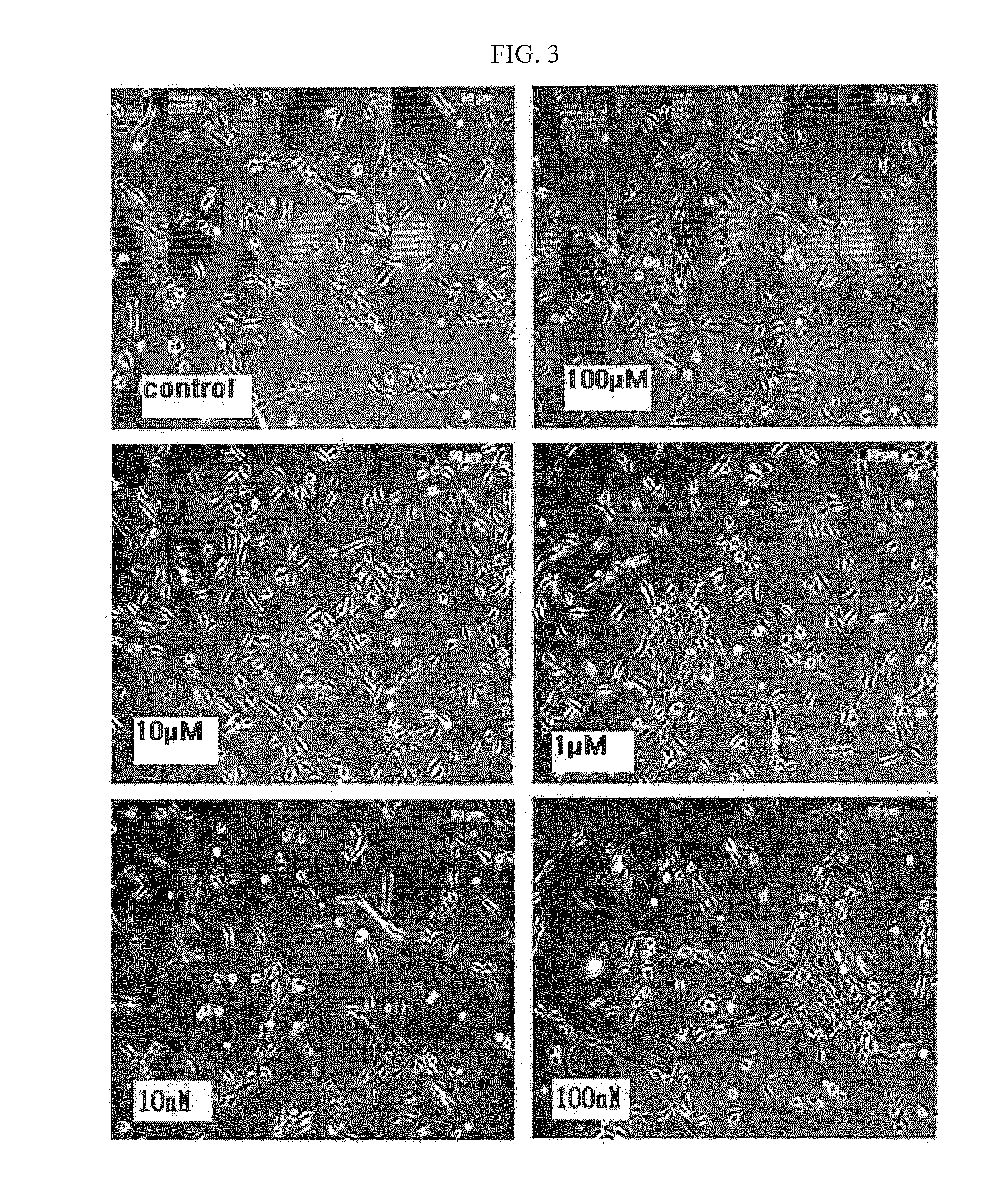
The present invention relates to a method for isolating and culturing adult stem cells derived from human amniotic membrane in high yield, and more particularly to a method for obtaining a large amount of adult stem cells, the method comprising obtaining amniotic epithelial cells from human amniotic tissue in high yield by treatment with dithiothreitol (DTT) and a low concentration of trypsin and culturing the amniotic epithelial cells in a medium containing a Rho-associated kinase inhibitor. The human amniotic epithelial cell-derived stem cells are easily extracted compared to existing therapeutic stem cells such as umbilical cord blood stem cells and bone marrow stem cells, the yield and proliferation thereof are significantly increased by DTT treatment, the addition of the ROCK inhibitor or the replacement of medium. Thus, the method can be used to efficiently prepare adult stem cells.
Owner:RNL BIO
Method for simultaneously extracting epithelial cells and mesenchymal stem cells from umbilical cord and placenta amnion tissues
InactiveCN105420179AReduce concentrationLow costCell dissociation methodsCulture processCell culture mediaUmbilical cord
The invention relates to a method for simultaneously extracting epithelial cells and mesenchymal stem cells from umbilical cord and placenta amnion tissues. The method comprises the steps of (1) sampling umbilical cord and placenta amnia and inoculating; (2) performing amplifying and passage on mesenchymal stem cells and amnion epithelial cells in a culture flask; (3) identifying the mesenchymal stem cells and the amnion epithelial cells: 1) when culture cells is transferred to the third generation, identifying the purity of culture cells after trypsinization; 2) verifying the cell purity to be 90 percent or more, namely the standard is met, and a mixture of the epithelial cells and the mesenchymal stem cells is obtained. The method is low in cost, simple and rapid, umbilical cord and placenta amnia are cut into tiny pieces by using a tissue cutting machine, which is conducive to swimming out of the tissues after the cells are directly inoculated in the culture flask; the cost is greatly lowered due to the fact that no proteinase reagent is used, and the preparation time is reduced, so that large-scale cell preparation in short time is realized; the method uses cell culture mediums being added with growth factors and other nutrients, and serum is saved.
Owner:斯坦姆(天津)生物技术研究有限公司
Separation, purification and identification methods of human amnion mesenchymal stem cells
InactiveCN102559586AMeet the treatment needsAccurate identification methodIndividual particle analysisEmbryonic cellsLow glucoseStaining
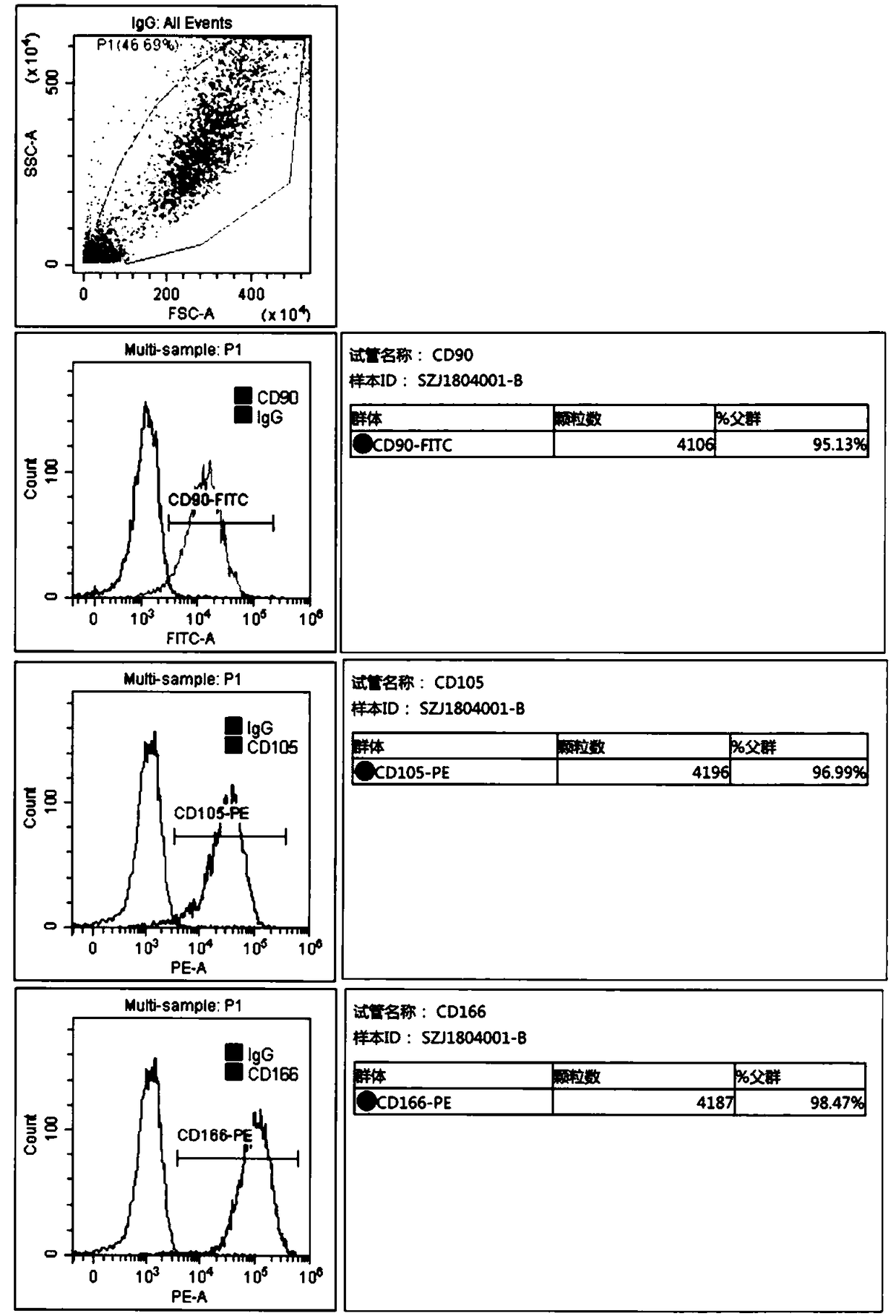
The invention discloses separation, purification and identification methods of human amnion mesenchymal stem cells. The separation method of hAMSCs (human amnion mesenchymal stem cells) comprises the following steps: fragmentating human amnion; and carrying out two-step rotating digestion with trypsin of EDTA (ethylene diamine tetra-acetic acid) and collagenase of DNaseI, filtering with a steel mesh and collecting cell filtrate namely separated original hAMSCs. The purification method of hAMSCs comprises the following steps: incubating original hAMSCs with an LG (low glucose)-DMEM (dulbecco modified eagle medium) culture medium in a CO2 incubator; removing amnion epithelial cells which do not perform complete adherence growth under an inverted microscope; replacing a new culture medium on the third day; digesting with a trypsin-EDTA solution after cell converge degree reaches 80-90%; and collecting cells so as to obtain high-purity hAMSCs. The identification method of hAMSCs comprises the following steps: identifying hAMSCs and the amnion epithelial cells by adopting immunocytochemical staining vimentin and CK19; and detecting expressions of CD29, CD44, CD166, CD34 and CD45 by adopting a flow cytometry. The separation and purification methods disclosed by the invention have the advantages of high yield, high activity and high purity of hAMSCs; and the identification method is simple, convenient and precise.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Preparation method of tissue engineering skin containing appendant organs
InactiveCN101773688AIncrease success rateMaintain structure and functionEpidermal cells/skin cellsArtificial cell constructsSweat glandTissue engineered skin
The invention relates to a preparation method of tissue engineering skin containing appendant organs. In the invention, the preparation method comprises the following steps of: inoculating amniotic mesenchyme stem cells, amniotic mesenchyme stem cells induced to the directions of hair papillae, amniotic epithelial cells and amniotic epithelial cells induced to the directions of the epithelia of the sweat gland into a gel solution, then inoculating the amniotic epithelial cells and the amniotic epithelial cells induced by keratinocyte in a warp direction on the surface of a structure of the hair follicle and the sweat gland after culturing by a dermal layer by induced culture forming the structure of the hair follicle and the sweat gland, and obtaining the tissue engineering skin with the structure of the hair follicle and the sweat gland after culturing. The skin has elasticity, toughness, vigorous cellular metabolism, strong multiplication capacity, sufficient secretion of extracellular matrices, tight linkage of the cuticular layer, the inophragma and the cells of the dermal layer and little possibility of falling off, enhances the success rate of transplantation, can promote the healing of a wound surface, enhances the effects of restoration and replacement and has the function of regulating the immunological rejection of a receptor; by the contained structure of the hair follicle and the sweat gland, the function of the skin is more complete; the adopted amniotic tissues are postpartum wastes and have extensive source, strong cell multiplication capability and multiplepassage frequency; and the invention is beneficial to the mass production of seed cells and lowers the industrialization cost.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY +1
Method Of Inducing The Differentiation Of Amnion-Derived Cells And Utilization Of The Same
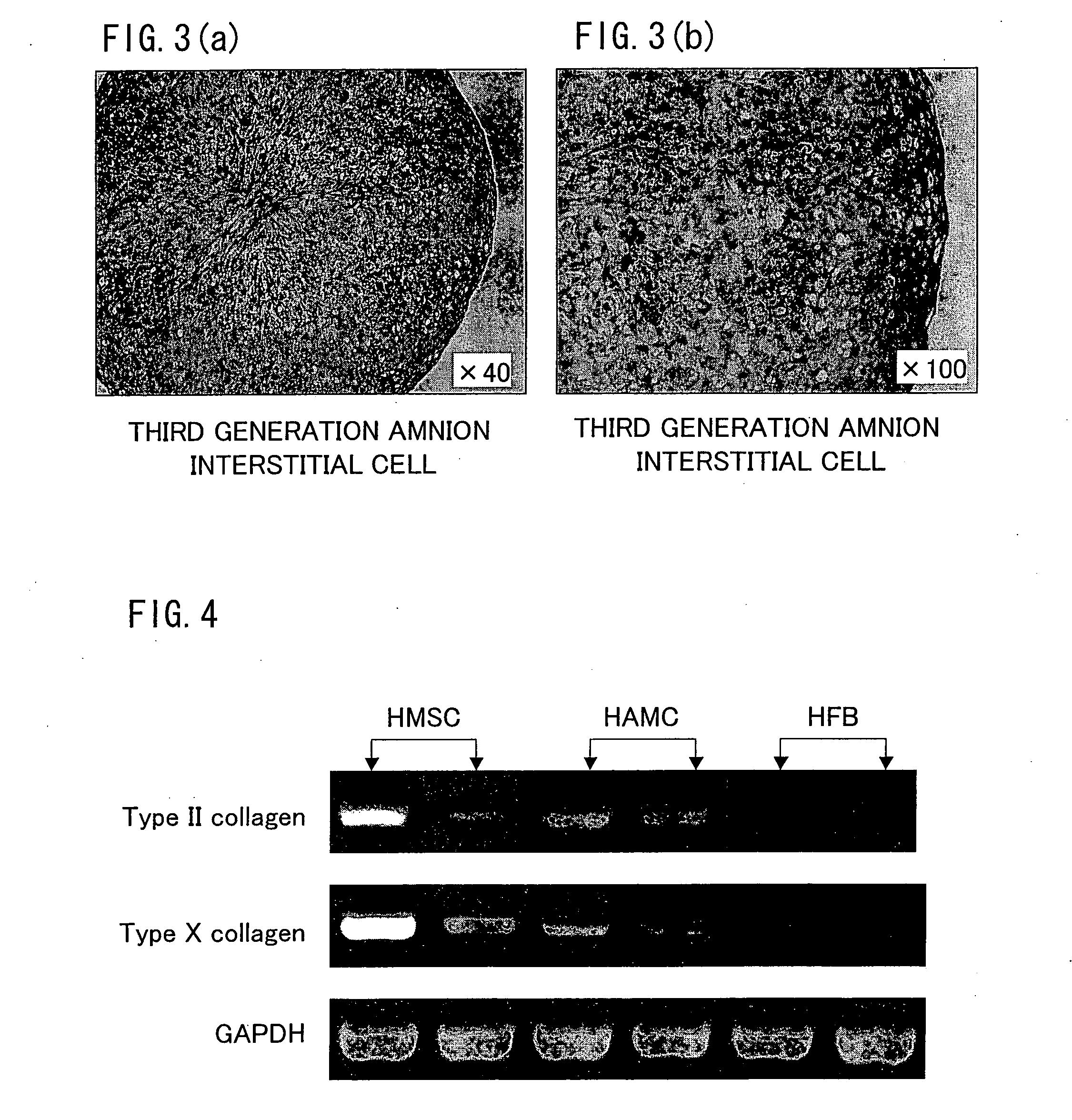
By a method of inducing differentiation of cells having: a step of co-culturing amniotic epithelial cells and amniotic interstitial cells so as to induce their differentiation into predetermined tissue cells, it is possible to efficiently induce differentiation of the amniotic epithelial cells and the amniotic interstitial cells. It is preferable that the amniotic epithelial cells or the amniotic interstitial cells are prepared by being cultured by a predetermined culture method.
Owner:HIROSHIMA UNIVERSITY
Novel bovine oocyte in vitro maturation culture solution
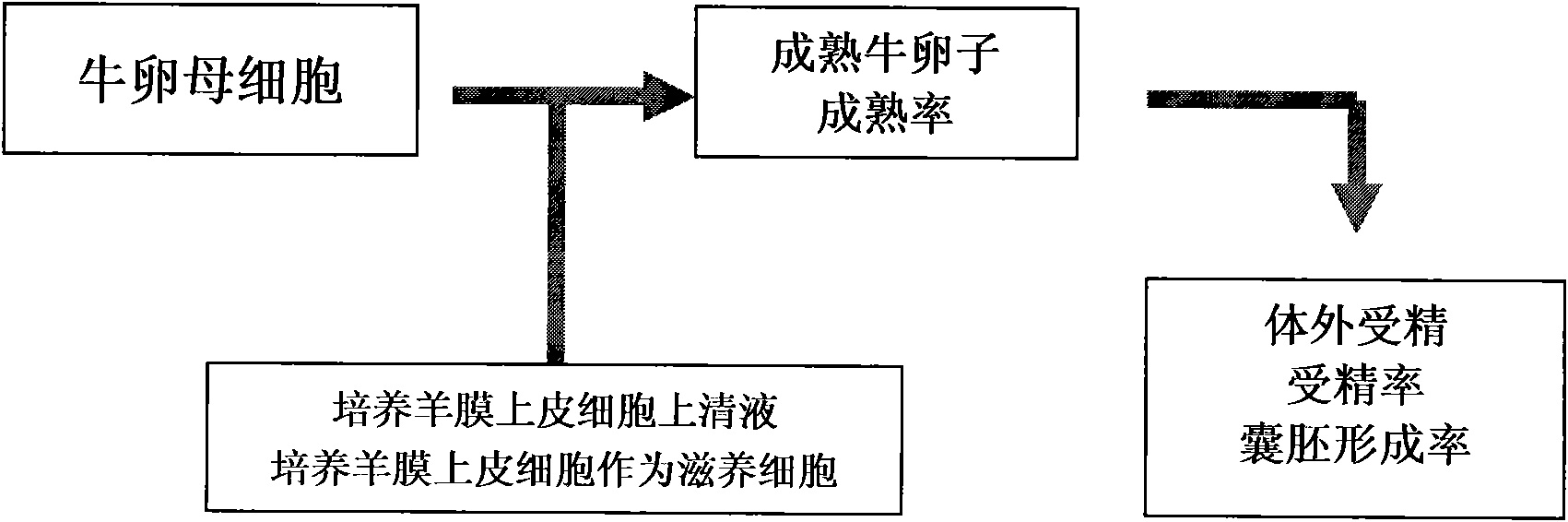
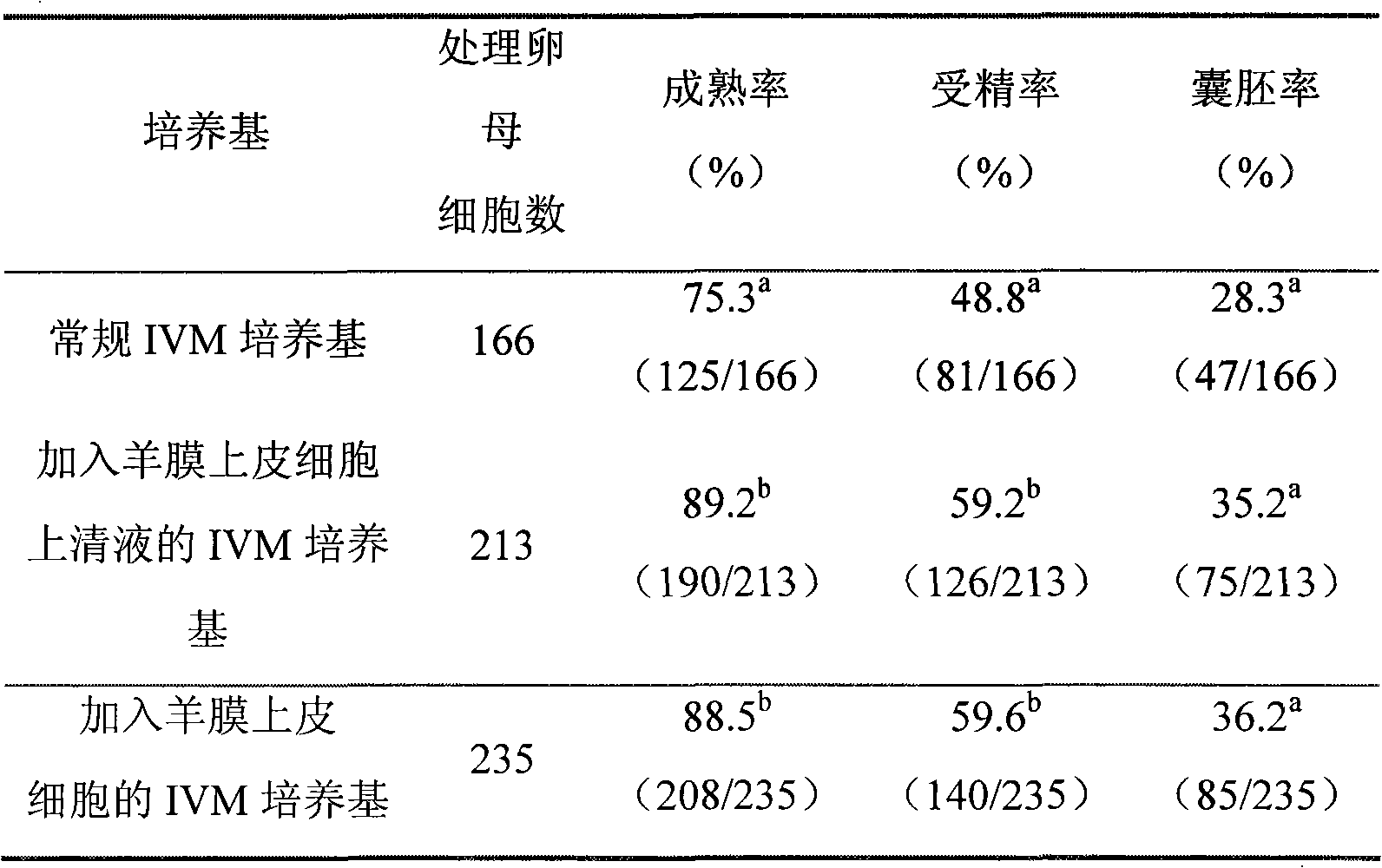
The invention relates to a novel bovine oocyte in vitro maturation (IVM) culture solution, belonging to the field of embryo biotechnolory. In the invention, the routine IVM culture solution is added with amniotic epithelia or the supernatant of the culture solution thereof to establish a novel bovine immature oocyte IVM culture system, so as to improve the quality and maturation rate of the IVM bovine oocyte, thus solving the problem of low fertilization rate and low formation rate of blastula in the existing culture method.
Owner:北京科润维德生物技术有限责任公司
Amnion-based biological material and preparation method and uses thereof
InactiveCN102631707APromote repairPromote differentiationSurgeryProsthesisBiological materialsOcular surface
The invention relates to an amnion-based biological material and a preparation method and uses thereof, and relates to a biological material. The amnion-based biological material is a de-epithelialized amniotic tissue. The preparation method of the amnion-based biological material i.e. the de-epithelialized amniotic tissue comprises the following steps that: the amniotic tissue is de-epithelialized to remove amniotic epithelial cells so as to obtain the amnion-based biological material. The amnion-based biological material can be used as a mucosal epidermis repair promoting material or epithelial cell repair promoting material so as to promote the tissue reconstruction of the ocular surface, the oral cavity, the respiratory tract, the stomach and the vaginal mucosal. The implementation process is safe, reliable and easy. The source of amnion is wide, and the de-epithelialized amnion can be conveniently prepared. The amnion transplant surgery is simple to operate and easy to implement.
Owner:XIAMEN UNIV
Amnion innate stem cell carried frozen active amnion particle and conditioned medium and application thereof
ActiveCN105018417AAvoid spreadingRetain activityDead animal preservationEmbryonic cellsDiseaseCuticle
The invention relates to the technical field of tissue engineering and medical wound repairing, in particular to an amnion innate stem cell carried frozen active amnion particle and a conditioned medium and application thereof. Discarded fresh amnions are prepared into particles and then frozen into liquid nitrogen by means of serum-free stem cell freezing liquid; thus, complete matrix components of amnion are maintained while amnion epithelial cell activity is maintained for a long time, and further, disease transmission is avoided effectively. The conditioned medium which is prepared and collected by means of the frozen active amnion particles can promote chemotaxis and migration of human epidermal cells, fibroblast and endothelial cells effectively. In terms of zoografting, wound healing can be improved by multiple ways of regulating inflammatory reaction, promoting vascularization, quickening epithelization and the like through the active amnion particles; further, the amnion matrix can be directly used as a dermis equivalent to induce dermal regeneration so as to improve wound healing quality remarkably. The amnion innate stem cell carried frozen active amnion particle and the conditioned medium thereof can provide simple but effective ways for wound repairing.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Bletilla striata polysaccharide hydrogel, culture medium and application thereof as well as method of inducing differentiation of umbilical cord mesenchymal stem cells to corneal epithelial cells
ActiveCN105085938AGood hygroscopicityImprove hydrophilicityVertebrate cellsArtificial cell constructsPorosityHuc mscs
The invention relates to the field of cell culture, in particular relates to letilla striata polysaccharide hydrogel, a culture medium and application thereof as well as a method of inducing differentiation of umbilical cord mesenchymal stem cells (hUC-MSCs) to corneal epithelial cells. The method comprises the steps of sulfating letilla striata polysaccharide and then crosslinking with Epsilon-polylysine carrying polyfunctional groups to prepare the polysaccharide hydrogel. The letilla striata polysaccharide hydrogel is of a semi-transparent membrane, has good porosities, and is suitable for the growth and the differentiation of cells. The experiment shows that by taking the letilla striata polysaccharide hydrogel provided by the invention as a scaffold, after the hUC-MSCs and amniotic epithelial cells are subjected to co-culture for 7 days, the differentiation rate of the hUC-MSCs can be up to 31.96%. The differentiation rate (p is less than 0.01) is obviously superior to that of a contrasting example which does not adopt the hydrogel scaffold. In addition, the hydrogel provided by the invention can promote the cells to grow vigorously, and shorten the induction time.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Method for obtaining amniotic epithelial cells through separation
InactiveCN104371971AGuaranteed quantityQuality assuranceEmbryonic cellsAmniotic epithelial cellsThelial cell
The invention discloses a method for obtaining amniotic epithelial cells through separation. The method includes the following steps: adding amniotic tissues into a collagenase solution, and incubating; adding the amniotic tissues into a trypsin solution, and digesting; and centrifuging the obtained supernatant to obtain the amniotic epithelial cells. The method has the advantages of simple operation, maintenance of the original activity and growth state of cells, and improvement of the yield of the amniotic epithelial cells.
Owner:EASTERN UNION STEM CELL & GENE ENG
Method for culturing stem cells
ActiveUS20120122213A1Efficient solutionReduce the cost of trainingArtificial cell constructsCell culture active agentsBiotechnologyAdditive ingredient
In the field of biological technology, a stem cell culture method is provided. The method includes preparing an amniotic epithelial cell feeder layer that is not treated to lose the division ability; and seeding the stem cells onto the amniotic epithelial cell feeder layer, and culturing in a culture medium. The stem cell culture method according to the present invention does not require the treatment of the feeder layer cells to lose the division ability, and is thus simple and safe, thereby effectively solving the problem of contamination caused by animal-derived ingredients in culture of human stem cells at present, greatly reducing the culture cost of the stem cells, and providing a safe, effective, and inexpensive stem cell culture method for the industrialization of the stem cells in the future.
Owner:SHANGHAI ICELL BIOTECH +1
Method for building tissue engineered skin by composite DED (de-epidermidalized dermis) of amnion endothelial cells
The invention discloses a method for building tissue engineered skin by composite DED (de-epidermidalized dermis) of amnion endothelial cells. The hAECs (human amnion endothelial cells) are used as seed cells; human DED is used as a stent material; the seed cells are planted onto the stent material to build the hAECs DED; the seed cells are planted onto the stent materials to build the hAECs-DED tissue engineered skin. The method comprises the following steps of (1) hAECs separation culture; (2) FB (fibroblast) separation culture; (3) DED preparation; (4) hAECs-DED tissue engineered skin building. The amnion endothelial cells are used as the seed cells; the human DED is used as a stent; the seed cells and the stent are all from the human body and have the homeology; the built tissue engineered skin has the tissue structures and functions similar to the normal skin, and also has some features of normal skin proliferation and differentiation.
Owner:GUIZHOU PROVINCIAL PEOPLES HOSPITAL
Culture medium and method for inducing differentiation of umbilical cord mesenchymal stem cells to corneal epithelial cells
ActiveCN105087466AIncrease percentageIncrease the percentage of differentiationArtificial cell constructsVertebrate cellsHuc mscsStem cell culture
The invention relates to the field of stem cell culture, in particular to a culture medium and method for inducing differentiation of umbilical cord mesenchymal stem cells to corneal epithelial cells. An epidermal growth factor and insulin are added into a basic culture solution, so that the percentage of differentiation of hUC-MSCs to the corneal epithelial cells is increased. Moreover, the culture solution does not contain heterogenous serum, so that the risk is lowered. According to the method provided by the invention, the umbilical cord mesenchymal stem cells and amniotic epithelial cells are co-cultured, so that the differentiation percentage can be increased remarkably, and the required culture period is short. The adopted umbilical cord mesenchymal stem cells are derived from umbilical cords and placentas, so that the source is wide, and legal and ethical limitations are avoided.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Amnion epithelial cell separation and culture method
ActiveCN104818244AGuaranteed smoothImprove digestion efficiencyEmbryonic cellsTrypsinizationConnective tissue
The invention relates to an amnion epithelial cell separation and culture method which aims to solve the problem of low purity of the amnion epithelial cells which are separated and cultured by the existing method. The method comprises the following steps: 1. amnion tissue separation and disinfection; 2. amnion epithelial cell separation; and 3. amnion epithelial cell purification culture to obtain the high-purity P0-generation amnion epithelial cell unicell suspension. The amnion patch prepared from the amnion tissues can ensure the flatness of the amnion epithelial surface; meanwhile, the amnion back mucus is closely attached to the connective tissue and nitrocellulose membrane to enhance the trypsinization efficiency and promote the separation between the cells and tissues, so that all the amnion epithelial cells can be collected by short-time digestion, thereby avoiding the reduction of the cell wall-attachment rate; and after the short-time culture, trypsinization is performed twice to obtain the high-purity amnion epithelial cells. The method is applicable to the fields of fundamental research and clinical transplantation.
Owner:天晴干细胞股份有限公司
Human amniotic epithelial cell as well as isolated culture method and application thereof
PendingCN106244521AStable traitsShape stableCell dissociation methodsMetabolism disorderDiseaseD-Glucose
The invention provides a human amniotic epithelial cell, as well as an isolated culture method and application thereof. In the method, the amniotic epithelial cell can be simply and conveniently obtained on a large scale by extracting and isolating the amniotic epithelial cell from a placenta, purifying the amniotic epithelial cell and then carrying out enlarged multiplication culture in vitro. Meanwhile, the human amniotic epithelial cell obtained through culture has high purity, can be used in treatment of diseases, such as diabetes mellitus, and can be used for effectively controlling the blood glucose levels of patients.
Owner:吉林省中科生物工程股份有限公司
Reprogramming culture medium, preparation method thereof and culture method of reprogramming cell
ActiveCN103602635AClear ingredientsImprove qualityForeign genetic material cellsSodium bicarbonateValproic Acid
The invention discloses a reprogramming culture medium, a preparation method thereof and a culture method of a reprogramming cell, wherein the reprogramming culture medium comprises a basal culture medium, a reprogramming additive 1 and a reprogramming additive 2, wherein the basal culture medium is a DMEM (Dulbecco Modified Eagle Medium) / F12 culture medium; the reprogramming additive 1 comprises sodium hydrogen carbonate, sodium selenate, recombinant human insulin, recombinant human basic fibroblast growth factors, recombinant human transferrin, ascorbic acid and hydrocortisone; the reprogramming additive 2 comprises sodium hydrogen carbonate, sodium selenate, recombinant human insulin, recombinant human basic fibroblast growth factors, recombinant human transferrin, ascorbic acid, valproic acid and sodium butyrate. The reprogramming culture medium disclosed by the invention is exact in component, stable in quality, high in reprogramming efficiency, wide in cell lineage and capable of reprogramming and culturing cells such as human skin fibroblasts, peripheral blood mononuclear cells, amniotic epithelial cells and the like.
Owner:BEIJING CELLAPY BIOTECH
Method and device for layering and fixing amnion and removing cell by using amnion 3D matrix tissue engineering scaffold
The invention concerns a method and a device for amnion extension and fixation to de-cell in amnion 3D matrix tissue engineering. Dipping amnion into trypsase solution, extending suspended amnion and clamping and laying on braket, rotating it through rotary body located under bracket. Then, vortex occur in trypsase solution and rinse the amnion, until the amnion epithelium and fibroblast are all washed down. The inventive method is simple, has low cost, and takes advantage of industrialized tissue engineering.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Culture medium for culturing human amniotic epithelial cells
Owner:吉林和泽生物科技有限公司
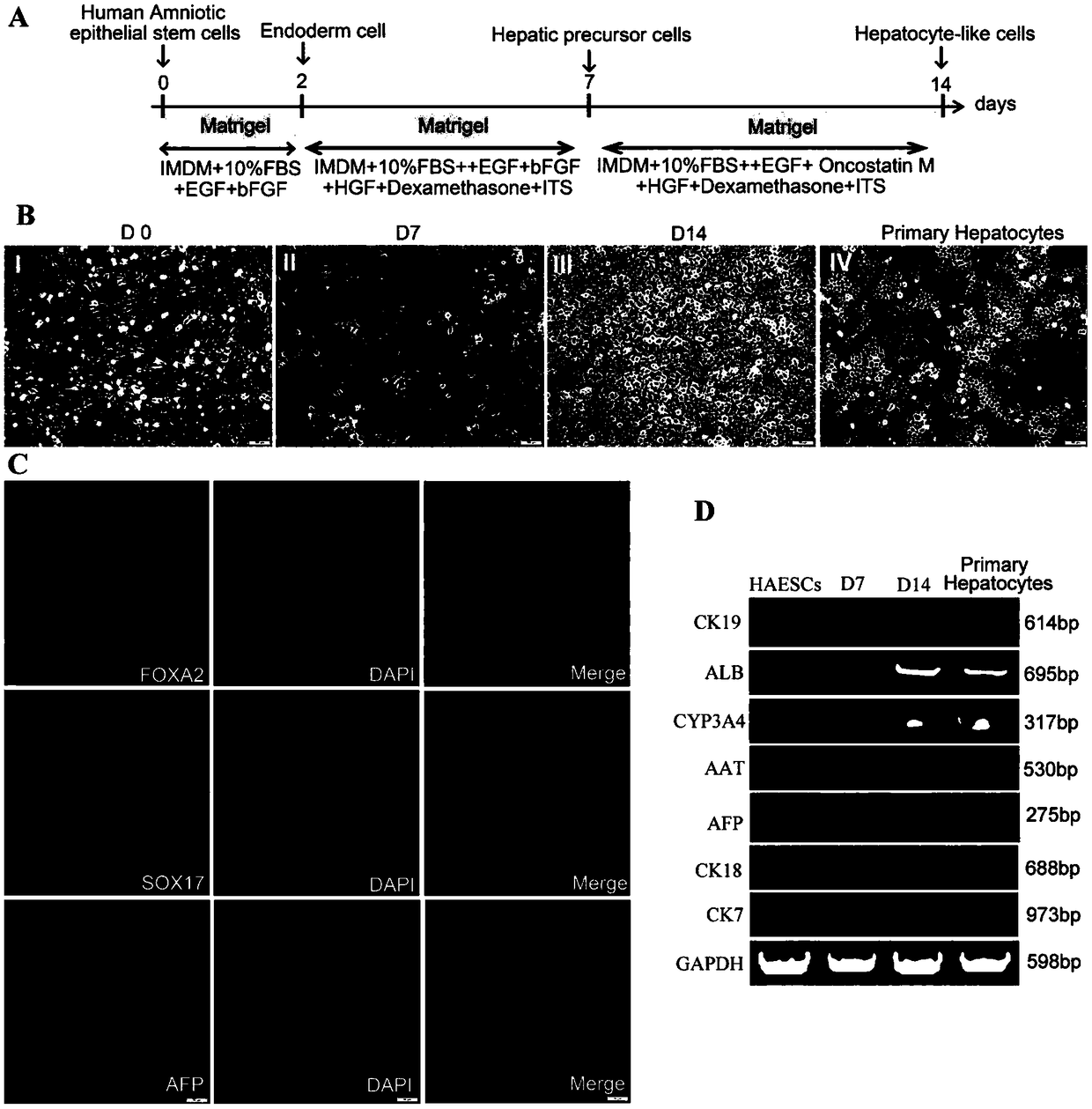
Method for inducing and differentiating amniotic epithelial stem cells into functional liver cells and application of method
The invention discloses a method for inducing and differentiating human amniotic epithelial stem cells into liver cells with biological functions. The method includes the steps: 1) separating, culturing, amplifying and identifying the human amniotic epithelial stem cells, and marking the human amniotic epithelial stem cells by GFP (green fluorescent protein); 2) in-vitro induction differentiation:using third-seventh-generation human amniotic epithelial stem cells with GFP markers for test, selecting a first system containing inducing media I, II and III or a second system containing inducingmedia IV, V and VI to perform induced differentiation, and inducing and differentiating the cells by the first system (inducing time is 14-15 days) or the second system (inducting time is 22-23 days)to obtain liver sample cells which can express specific markers of the liver cells and have normal liver cell functions; 3) in-vivo transplantation: transplanting the liver sample cells generated by inducing into a body of an acute liver injury mouse. Acute liver injury can be obviously relieved, the liver function of the mouse is restored, and the survival rate of the mouse is increased.
Owner:NANCHANG UNIV
Construction method for human amniotic epithelial cells and silk fibroin support complexus
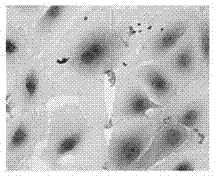
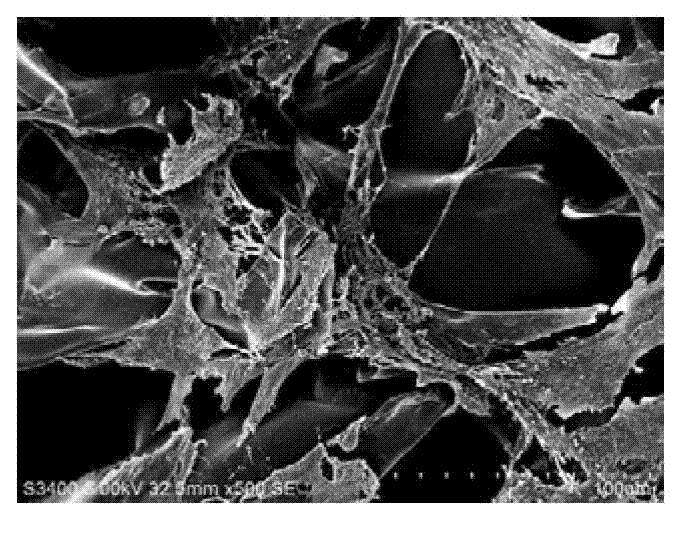
InactiveCN103045535APromote proliferationPromote secretionEmbryonic cellsDiseaseScanning electron microscope
The invention provides a method for constructing amniotic epithelial cells and silk fibroin support complexus in vitro, and the method comprises the following steps of: isolated culture of amniotic epithelial cells; purification of primary cells; identification of primary cells; amniotic cell inoculation on silk fibroin support for space cultivation; and result observation through a scanning electron microscope. According to the method, after human cesarean delivery, wasted amniotic membrane is taken as the material, the amniotic epithelial cells are obtained for cultivation in vitro, and cell purification is carried out under certain conditions, the purified cells and the silk fibroin support are used for constructing a tissue engineering material, thus building a good space growing environment for cells. The invention provides a new way for curing functional repair of all traumatic diseases of the central nervous system by using amniotic membrane cell transplantation in clinical application in the near future.
Owner:吴卫江
One-way amnion decellularization method
ActiveCN108048391APromote prolapseEasy to removeEmbryonic cellsTissue regenerationDecellularizationAmniotic epithelial cells
The invention relates to a one-way amnion decellularization method. The method comprises the following steps that a, a fresh amnion is stripped and cleaned; b, the amnion obtained in step a is laid and fixed to a supporting body with a plane; c, the amnion processed in step b is placed into a decellularization solution for treatment; d, the amnion treated in step c is washed and then placed into anuclease solution for treatment; e, the amnion treated in step d is washed, and then a human decellularized amnion is obtained. Compared with the prior art, the method is combined with structure characteristics of the amnion, and a one-way decellularization method is used for protecting a matrix layer of the amnion; the amnion is laid and expanded, an epithelial surface fully makes contact with the decellularization solution, so that epithelial cells of the amnion are thoroughly removed, and a very small number of cells of the matrix layer are removed by making contact with a small quantity of decellularization solution due to the permeability of tissue. The decellularization method is easy and convenient to operate, the stability of the process is guaranteed, and large-scale production is facilitated.
Owner:上海睿泰生物科技股份有限公司
Efficient separation and amplification method for human amniotic mesenchymal stem cells
InactiveCN109321517AHigh purityA large amountCell dissociation methodsSkeletal/connective tissue cellsEnzyme digestionTwo step
The invention provides an efficient separation and amplification method for human amniotic mesenchymal stem cells. The efficient separation and amplification method is characterized by comprising thefollowing steps: 1, preparing a reagent; 2, separating amniotic mesenchymal stem cells through a two-step enzyme digestion method; 3, carrying out subculture on the amniotic mesenchymal stem cells. Compared with an existing amniotic mesenchymal stem cell separation method, the amniotic mesenchymal stem cells are separated by adopting the two-step enzyme digestion method; amniotic epithelial cellsare removed in the first step and two times of digestion are carried out, so that the amniotic mesenchymal stem cells are more sufficiently removed and the purity of the obtained amniotic mesenchymalstem cells is higher; the method is simple to operate and tissue and cell filtering is not needed; DNase I is used and a condition that the amniotic mesenchymal stem cell enter digestion mucus and cannot be separated is avoided; more stem cells are obtained. In a whole separation process, animal-derived components do not exist, the safety is good and subsequent research and application is convenient to realize.
Owner:陕西九州细胞基因工程有限公司
Human amniotic epithelial cell separation method
ActiveCN104974980AHigh purityHigh differentiation potentialEmbryonic cellsEpithelium surfaceCell activity
Owner:CHONGQING CELL BIOENG TECH CO LTD
Method for isolating and culturing adult stem cells derived from human amniotic epithelium
The present invention relates to a method for isolating and culturing adult stem cells derived from human amniotic membrane in high yield, and more particularly to a method for obtaining a large amount of adult stem cells, the method comprising obtaining amniotic epithelial cells from human amniotic tissue in high yield by treatment with dithiothreitol (DTT) and a low concentration of trypsin and culturing the amniotic epithelial cells in a medium containing a Rho-associated kinase inhibitor. The human amniotic epithelial cell-derived stem cells are easily extracted compared to existing therapeutic stem cells such as umbilical cord blood stem cells and bone marrow stem cells, the yield and proliferation thereof are significantly increased by DTT treatment, the addition of the ROCK inhibitor or the replacement of medium. Thus, the method can be used to efficiently prepare adult stem cells.
Owner:RNL BIO
Method for manufacturing and storing corneal injury scar-free repairing device
ActiveCN103989553ABreathe freelyPromote growthMedical applicatorsEye treatmentConjunctivaTissue repair
The invention relates to a method for manufacturing and storing a corneal injury scar-free repairing device. Specially-prepared amnion elements are settled or bonded in or on the surfaces of various biological or synthetic attaching devices such as the invisible contact lens through the novel carrier controlled release technology and the biological tissue engineering technology so as to form the corneal or conjunctiva injury scar-free repairing device. At the early stage of the corneal or conjunctiva injury, the affected corneal tissue or the affected conjunctiva tissue is covered with the amnion elements of the corneal or conjunctiva injury scar-free repairing device, the amnion elements, such as the necessary cell growth factors, the tissue repair factors and protease inhibitors, of the corneal or conjunctiva injury scar-free repairing device are diffused to the affected corneal tissue or the affected conjunctiva tissue due to the special superfine structure of an amnion basilar membrane and the stem cell characteristic of amniotic epithelial cells, and therefore growth of epithelial cells of the corneal or the conjunctiva is promoted, the affected corneal tissue or the affected conjunctiva tissue is stimulated, regulated and controlled to heal orderly, and less scars are formed on the affected corneal tissue or the affected conjunctiva tissue.
Owner:广州睿辰生物科技有限公司
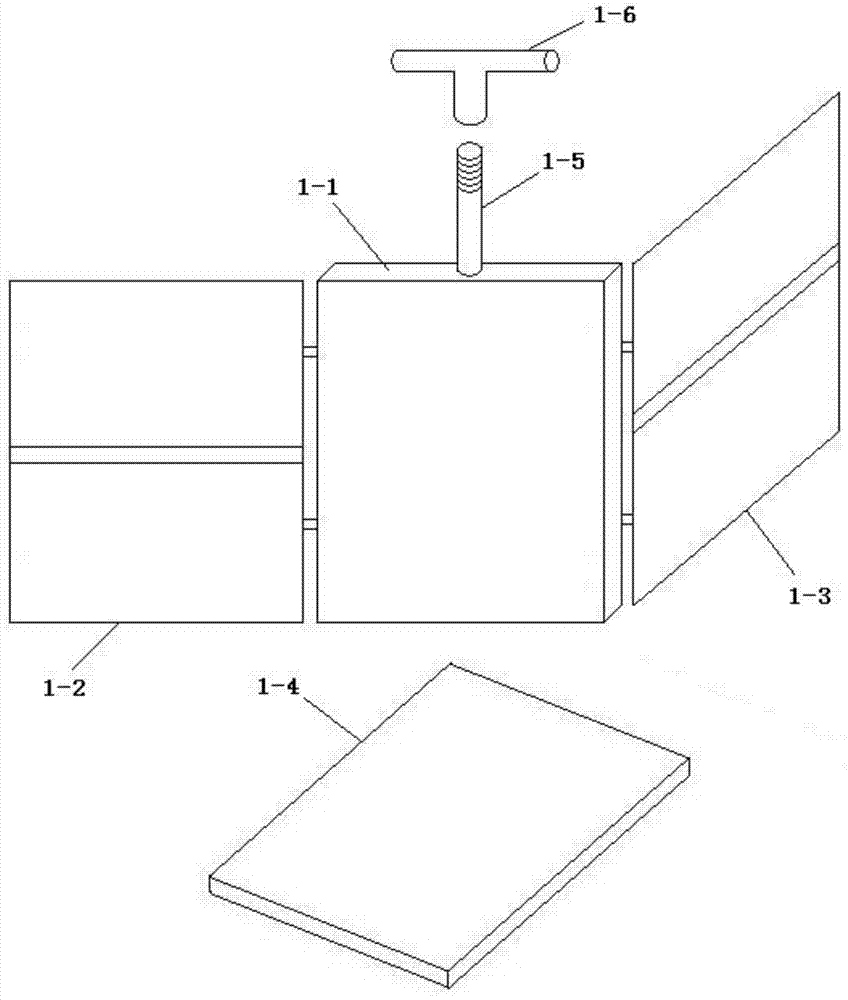
Amnion cell separation and collection purification set
ActiveCN104745473AReduce consumptionEfficient separationTissue/virus culture apparatusCylindromaDigestion
An amnion cell separation and collection purification set relates to an amnion cell separation set. The amnion cell separation and collection purification set is used for solving the problems that during amnion cell separation, an amnion is often accumulated in a digestion container, so that a lot of cells are remained among amnion tissues, the amnion cells cannot be efficiently collected, and the obtained cell line is unpurified. The set comprises an amnion cell fixing rotary frame, an amnion tissue digester, an amnion cell separator, a disposable screwed filter, a disposable screwed injector and a disposable screwed centrifuge tube; a threaded circular rod is arranged on the top of a hollow object placing groove of the amnion cell fixing rotary frame; the threaded circular rod is externally connected with a T-shaped handle; a thin plate is arranged in the object placing groove; the amnion tissue digester is of a hollow cube structure; a top cover is arranged on the top of the amnion tissue digester; a liquid inlet screwed opening is formed in one side of the upper part of the amnion tissue digester; a liquid discharge screwed opening is formed in the bottom of the amnion tissue digester; the amnion cell separator is of a hollow cylinder structure; a top cover is arranged on the top of the amnion cell separator; a liquid inlet screwed opening is formed in the side face of the upper part of the amnion cell separator; a liquid discharging screwed opening is formed in the bottom of the amnion cell separator. The amnion cell separation and collection purification set is used for separating and collecting amniotic epithelial cells.
Owner:天晴干细胞股份有限公司
Method for inducing and differentiating into dopaminergic neuron from human amniotic sepithelial cell and application for separating obtained dopaminergic neuron
The invention relates to a method for inducing and differentiating into dopaminergic neurons from human amniotic sepithelial cells, comprising the following steps of: taking a fresh human placenta; stripping a human amnion, repeatedly washing by using normal saline to remove blood clots, then soaking by using asepsis D-hank's liquor, and then rinsing by using the normal saline; placing the human amnion into 0.25 percent of a trypsin solution, and digesting at the temperature of 36.5-37.5 DEG C; stopping the digestion of the human amnion in the trypsin solution by using DMEM / F12 (Dulbecco's Modified Eagle Medium / F12) containing 10 percent of fetal calf serums, and then filtering the solution by using a cell sieve, wherein collected cells are the human amniotic sepithelial cells; separating the obtained human amniotic sepithelial cells, and carrying out in-vitro subculture on the separated human amniotic sepithelial cells by using 10 percent of culture mediums without the fetal calf serums; selecting 3 or 4 or 5 or 6 generation human amniotic sepithelial cells with good growth state, and then continuously culturing by using a neural stem cell differential medium; and harvesting dopaminergic neuron like cells.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
Application of amniotic epithelial cells in pharmacy
ActiveCN101721430AWide variety of sourcesImprove mobilityMammal material medical ingredientsCardiovascular disorderTelomeraseVascular disease
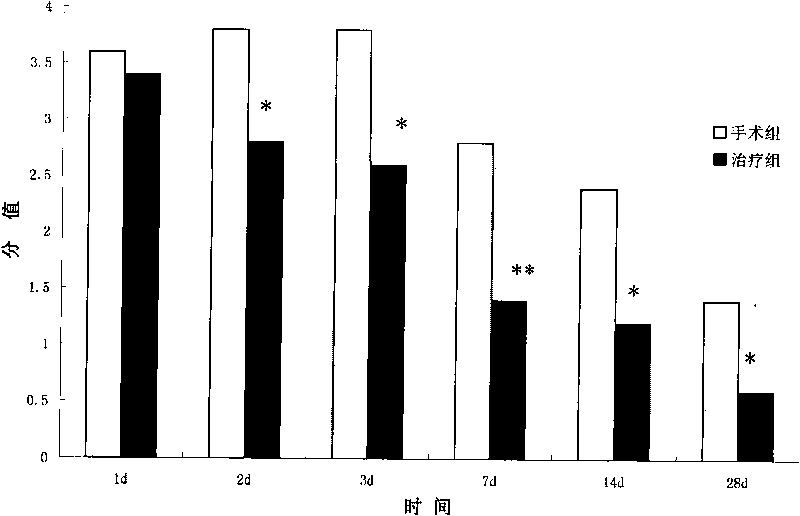
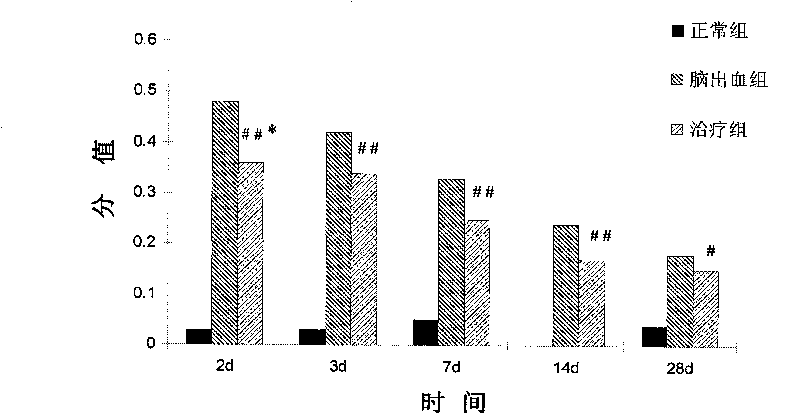
The invention relates to the technical field of biomedicine, in particular to application of amniotic epithelial cells in a medicament for treating hemorrhagic cerebral vascular diseases. The invention discloses the application of the amniotic epithelial cells in treating or improving the hemorrhagic cerebral vascular diseases. The amniotic epithelial cells can effectively treat or improve the hemorrhagic cerebral vascular diseases, obviously improve the action function of patients with the hemorrhagic cerebral vascular diseases, and obviously reduce encephaledema symptoms caused by the hemorrhagic cerebral vascular diseases; the treatment do not need match because the amniotic epithelial cells do not express or weakly express MHC; the amniotic epithelial cells also do not express telomerase, and cell proliferation is controlled, so that the transplantation treatment is safe; meanwhile, the amniotic epithelial cells have wide sources, so that the phenomenon of ethic or morality is avoided. Therefore, the amniotic epithelial cells provide a new effective choice for clinically treating or improving the hemorrhagic cerebral vascular diseases in the future.
Owner:SHANGHAI ICELL BIOTECH +5
Corneal injury scar-free repairing device and application thereof
InactiveCN103989554ABreathe freelyGood conditionMedical applicatorsEye treatmentConjunctivaTissue repair
The invention relates to a corneal injury scar-free repairing device and application thereof. Specially-prepared amnion elements are settled or bonded in or on the surfaces of various biological or synthetic attaching devices such as the invisible contact lens through the novel carrier controlled release technology and the biological tissue engineering technology so as to form the corneal or conjunctiva injury scar-free repairing device. At the early stage of the corneal or conjunctiva injury, the affected corneal tissue or the affected conjunctiva tissue is covered with the amnion elements of the corneal or conjunctiva injury scar-free repairing device, the amnion elements, such as the necessary cell growth factors, the tissue repair factors and protease inhibitors, of the corneal or conjunctiva injury scar-free repairing device are diffused to the affected corneal tissue or the affected conjunctiva tissue due to the special superfine structure of an amnion basilar membrane and the stem cell characteristic of amniotic epithelial cells, and therefore growth of epithelial cells of the corneal or the conjunctiva is promoted, the affected corneal tissue or the affected conjunctiva tissue is stimulated, regulated and controlled to heal orderly, and less scars are formed on the affected corneal tissue or the affected conjunctiva tissue.
Owner:周辉
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com