Application of amniotic epithelial cells in pharmacy
A technology of amniotic membrane epithelial cells and uses, applied in the field of biomedicine, can solve problems such as limited clinical application and source problems, and achieve the effects of wide sources, improvement of mobility function, and reduction of cerebral edema symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1. hAEC isolation and culture
[0043] Take the placenta of healthy caesarean section pregnant women, and strip the amniotic membrane from the chorion. Rinse with PBS to remove blood cells, digest with 0.25% trypsin at 37°C for 30 min, pipette, add RPMI1640 containing 10% FBS to stop the digestion, filter through a 100-mesh sieve, centrifuge at 1,500 rpm, discard the supernatant, and dilute the mixture with 1×10 4 / cm 2 Inoculate in 10cm dish. hAECs were placed in RPMI-1640 (10% FBS, 100 μg / ml streptomycin, 100 U / ml penicillin, 10 ng / ml EGF and 0.3 mg / ml glutamine) at 37°C, 5% CO 2 cultured in a cell culture incubator.
Embodiment 2
[0044] Example 2. Preparation of hAEC cell preparation
[0045] Example 1 hAEC cultured for 24-72 hours, remove the medium, digest the adherent hAEC with 0.25% trypsin and centrifuge, then rinse with 2ml of PBS solution and centrifuge again, resuspend in PBS and adjust the cell concentration to 105 / μl for later use, namely hAECs cell preparations were obtained.
Embodiment 3
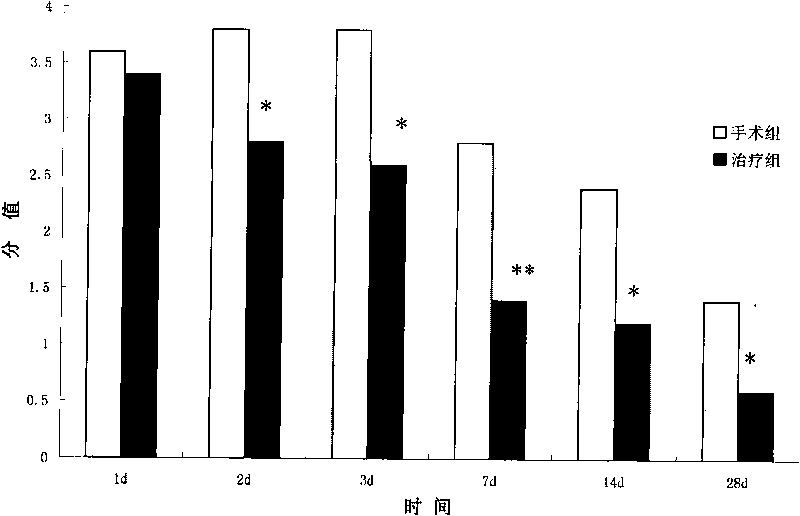
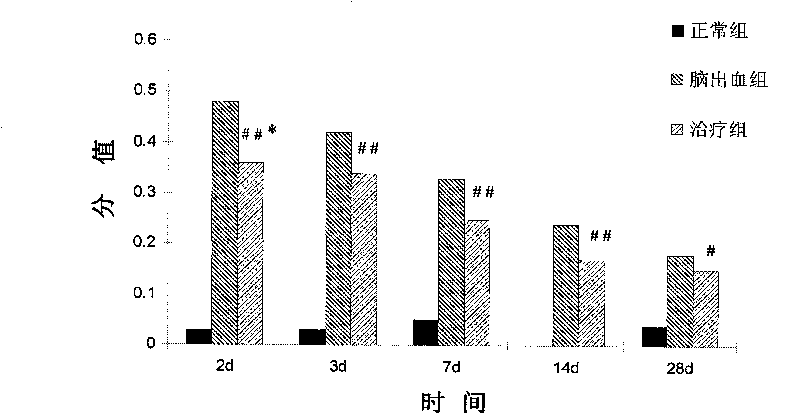
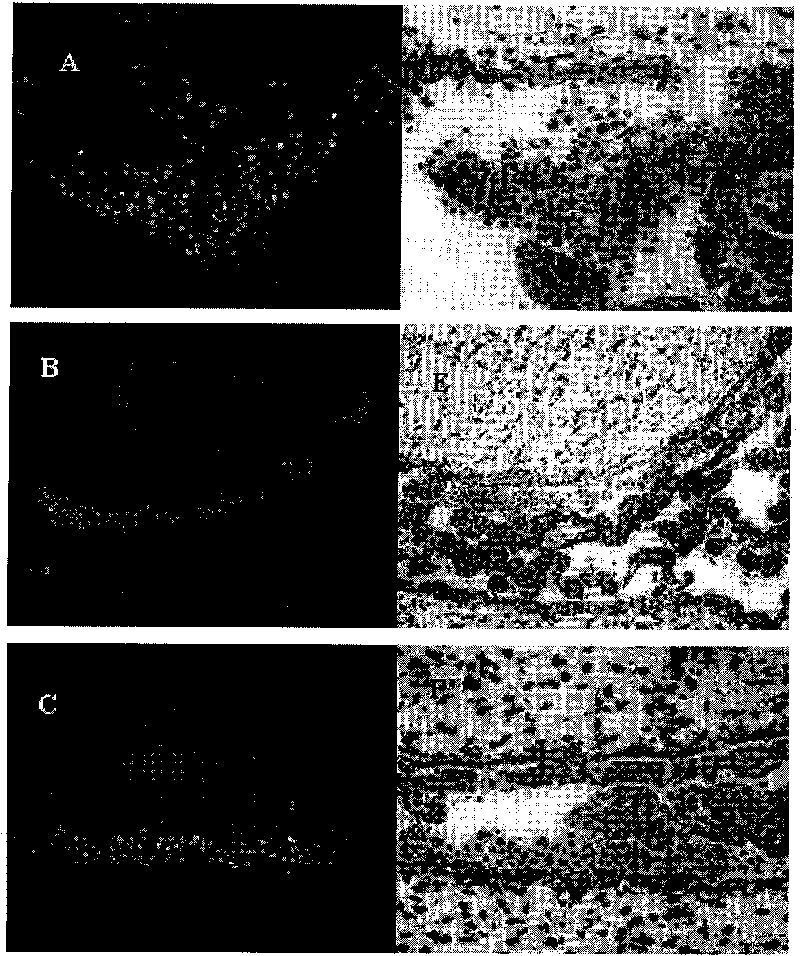
[0046] Example 3. hAECs treat cerebral hemorrhage model in rats
[0047] 1 animal
[0048] SPF grade female adult Sprague-Dawley rats were used in the experiment, purchased from Shanghai Slack Experimental Animal Center, weighing 250±10g, reared in a single cage, free to ingest water and food, and lighted to form a day / night cycle of 12h-12h. The experimental animals were divided into control group, operation (cerebral hemorrhage) group and treatment (cell transplantation) group, wherein the operation group and treatment group were divided into post operation and treatment 2d, 3d, 7d, 14d and 28d.
[0049] 2 Research Methods
[0050] (1) Preparation of cerebral hemorrhage model in rats
[0051] Refer to the methods of CrystalL [Crystal L MacLellan, Gergely Silasi, Candice C Poon, et al. Journal of Cerebral Blood Flow & Metabolism, 2007: 1-10] and make improvements. Rats were anesthetized with chloral hydrate (10% chloral hydrate, injected intraperitoneally by 0.3ml / 100g bod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com