One-way amnion decellularization method
A decellularization and amniotic membrane technology, applied in the field of tissue engineering, can solve problems such as no solution is given, achieve the effect of simple operation, ensure stability, and facilitate large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
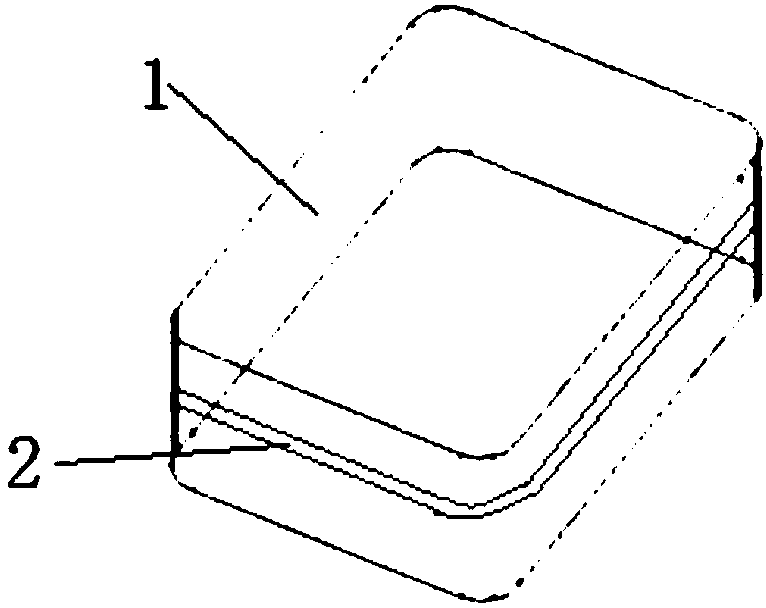
[0084] Take the placenta of healthy puerpera, strip the amniotic membrane from the placenta, wash it with 0.9% saline for several times in a biological safety cabinet, and use sterile medical tweezers to peel off the residual chorionic material and blood components. Spread the amniotic membrane on a sterile square one-way decellularization device (20x20x2cm, figure 1 as shown, figure 11 refers to the plane, 2 refers to the groove on the side) (the stroma layer is down), and use a sterile elastic medical latex tube to wrap the edge of the amniotic membrane on the groove of the device and fix it, use sterile medical scissors along the groove Unfixed amniotic membrane was cut off. Put the fixed amnion into TrypLE TM In the Select Enzyme solution, shake at 37°C for 2 hours. After shaking and washing with sterile normal saline for 5 times, the fixed amniotic membrane was put into Super Nuclease solution and treated with shaking at 37°C for 3 hours. Finally, the human amniotic ...
Embodiment 2
[0092] Take the placenta of healthy puerpera, strip the amniotic membrane from the placenta, wash it with 0.9% saline for several times in a biological safety cabinet, and use sterile medical tweezers to peel off the residual chorionic material and blood components. Spread the amniotic membrane on a sterile cylindrical one-way decellularization device (15 cm in diameter and 2 cm in height) (with the stroma layer facing down), and wrap the edge of the amnion on the groove of the device with a sterile elastic medical rubber band to fix it. Cut off the unfixed amniotic membrane along the groove with sterile medical scissors. Put the fixed amniotic membrane into the neutral protease solution, and shake at 37°C for 30 minutes. After shaking and washing with sterile normal saline for 5 times, the fixed amnion was put into Super Nuclease solution and treated with shaking at 37°C for 1 hour. Finally, the human amniotic membrane extracellular matrix scaffold material was obtained by s...
Embodiment 3
[0097] Take the placenta of healthy puerpera, strip the amniotic membrane from the placenta, wash it with 0.9% saline for several times in a biological safety cabinet, and use sterile medical tweezers to peel off the residual chorionic material and blood components. Spread the amniotic membrane on the sterile rectangular one-way decellularization device (20x15x3cm) (stromal layer down), wrap the edge of the amniotic membrane on the groove of the device with a sterile elastic medical rubber band and fix it with sterile medical scissors Cut off the unfixed amniotic membrane at the groove. Put the fixed amniotic membrane into CHAPS solution, shake at 37°C for 12h. After shaking and washing with sterile normal saline for 5 times, the fixed amniotic membrane was put into Benzonase Nuclease solution and treated with shaking at 37°C for 8 hours. Finally, the human amniotic membrane extracellular matrix scaffold material was obtained by shaking and washing with sterile normal saline ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com