Method for preparing high-rate nickel cobalt lithium aluminate anode material
A technology of nickel-cobalt-lithium-aluminate and positive electrode materials, applied in battery electrodes, electrical components, electrochemical generators, etc., can solve the problems of inability to promote the migration rate of lithium ions, unsatisfactory rate performance of materials, and obstacles to electron and lithium ion conduction To achieve the effect of inhibiting cation mixing, improving cycle performance and rate performance, and promoting structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
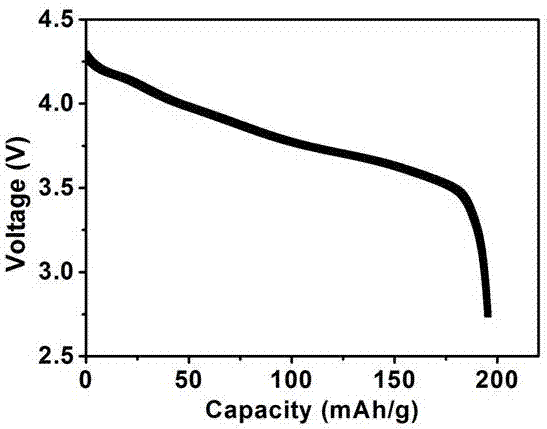
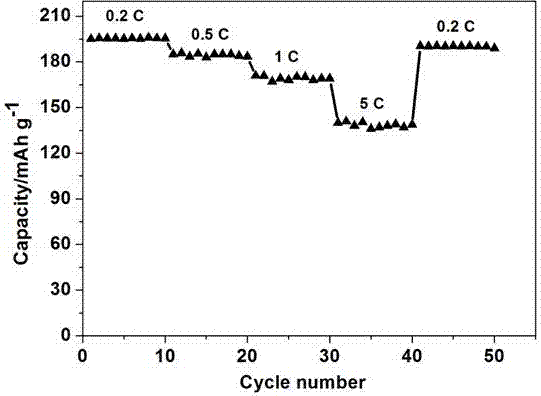
Image
Examples
Embodiment 1
[0025] The preparation method of the high-rate nickel-cobalt lithium aluminate positive electrode material in this embodiment has the following steps:
[0026] (1) Preparation of nickel-cobalt-lithium-aluminate precursor: Weigh nickel nitrate and cobalt nitrate crystals at a molar ratio of 85:15, dissolve them in water and mix them uniformly to obtain a mixed solution A with a metal ion concentration of 1.0 mol / L. Weigh 5 mol of aluminum nitrate dissolved in 2 mol / L ammonia water and 4 mol / L sodium hydroxide to prepare mixed solution B, inject mixed solution A and mixed solution B into the reaction kettle containing the bottom liquid at the same time, and control the whole reaction The pH value of the system is 11.0±0.02, the stirring speed is 500 r / min, the reaction is 12 h, the temperature is 55 °C, the reaction product is aged, washed, filtered, and dried at 120 °C to obtain the nickel cobalt lithium aluminate precursor body material;
Embodiment 2
[0031] The preparation method of the high-rate nickel-cobalt lithium aluminate positive electrode material in this embodiment has the following steps:
[0032] (1) Preparation of nickel-cobalt-lithium-aluminate precursor: Weigh nickel sulfate and cobalt sulfate crystals at a molar ratio of 85:15, dissolve them in water and mix them evenly. The metal ion concentration is 1.0 mol / L, and weigh 5 mol of sulfuric acid Dissolve aluminum in a mixed solution of 2 mol / L ammonia water and 4 mol / L sodium hydroxide, inject the two mixed solutions into the reaction kettle containing the bottom liquid at the same time, control the pH value of the entire reaction system at 11.5±0.02, and the stirring speed The temperature is 700 r / min, the reaction is 16 h, the temperature is 50 ° C, the reaction product is aged, washed, filtered, and dried at 100 ° C to obtain the nickel cobalt lithium aluminate precursor material;
[0033] (2) Lithium doping modification of potassium ions: the obtained nic...
Embodiment 3
[0037] The preparation method of the high-rate nickel-cobalt lithium aluminate positive electrode material in this embodiment has the following steps:
[0038] (1) Preparation of nickel-cobalt-lithium-aluminate precursor: Weigh nickel nitrate and cobalt nitrate crystals at a molar ratio of 85:15, dissolve them in water and mix them evenly. The metal ion concentration is 5.0 mol / L, and weigh 5 mol of nitric acid Dissolve aluminum in a mixed solution of 10 mol / L ammonia water and 20 mol / L sodium hydroxide, inject the two mixed solutions into the reaction kettle containing the bottom liquid at the same time, control the pH value of the entire reaction system at 12.0±0.02, and the stirring speed The temperature was 800 r / min, the reaction was 8 h, the temperature was 55 °C, the reaction product was aged, washed, filtered, and then dried at 80 °C to obtain the nickel cobalt lithium aluminate precursor material;
[0039] (2) Lithium doping modification of potassium ions: the obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com