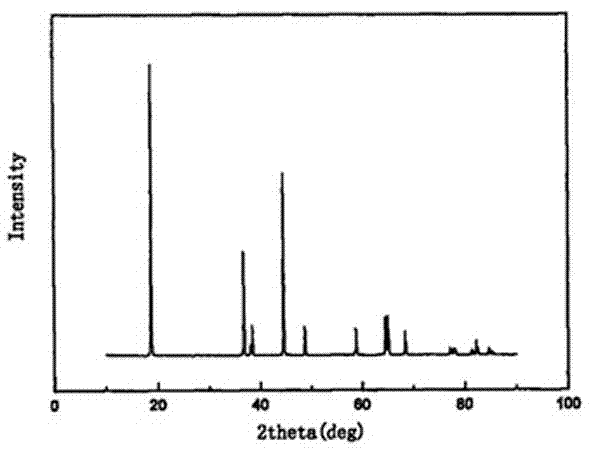
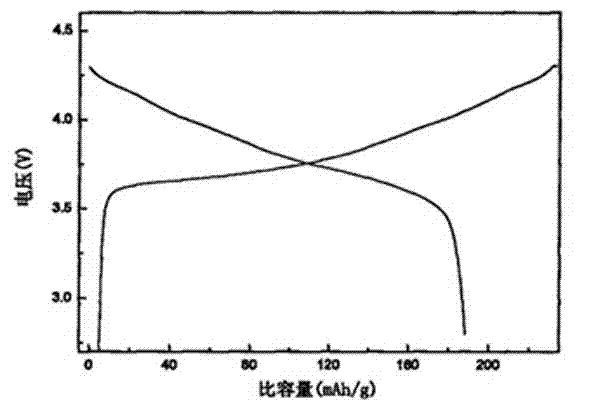
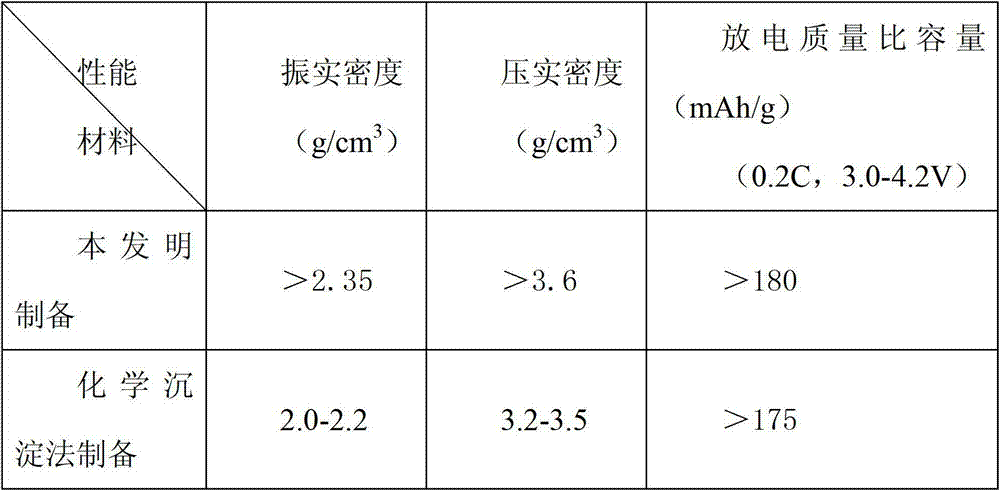
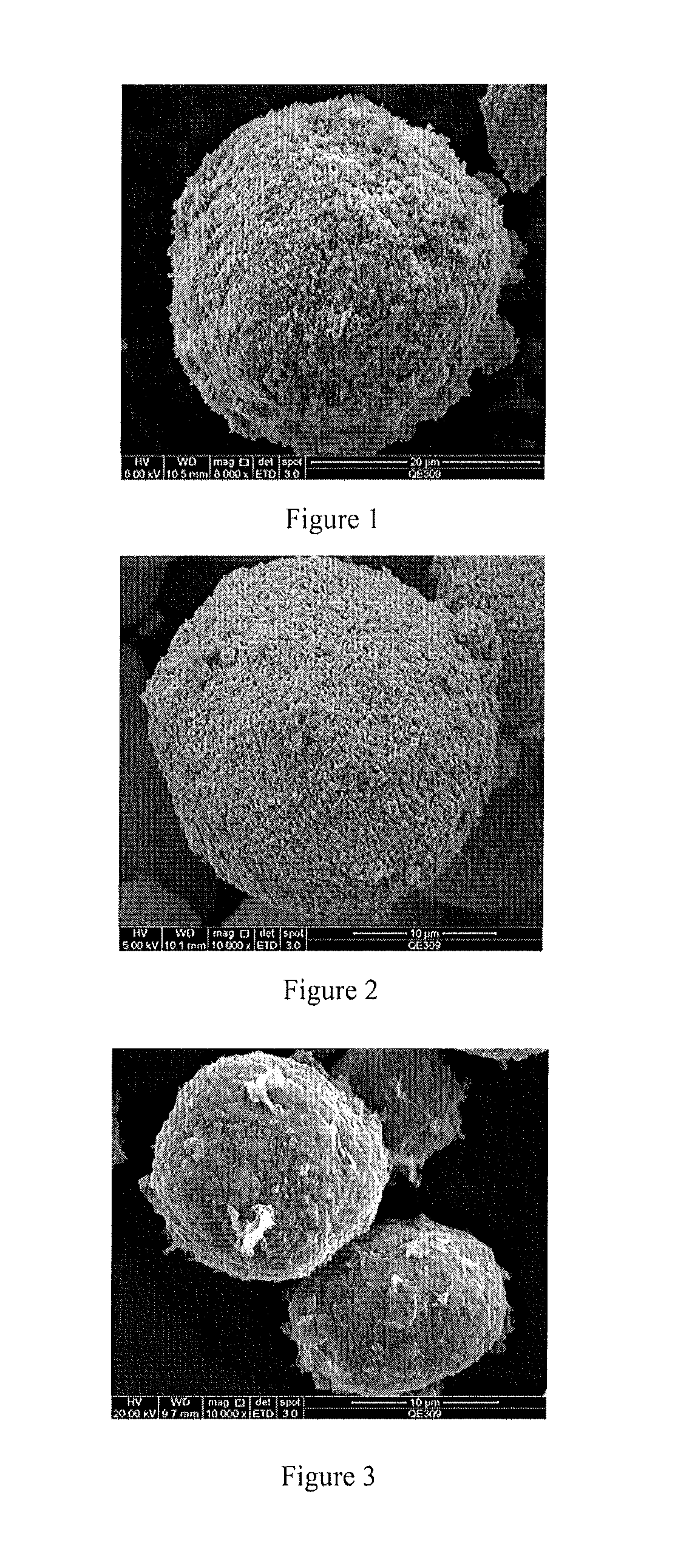
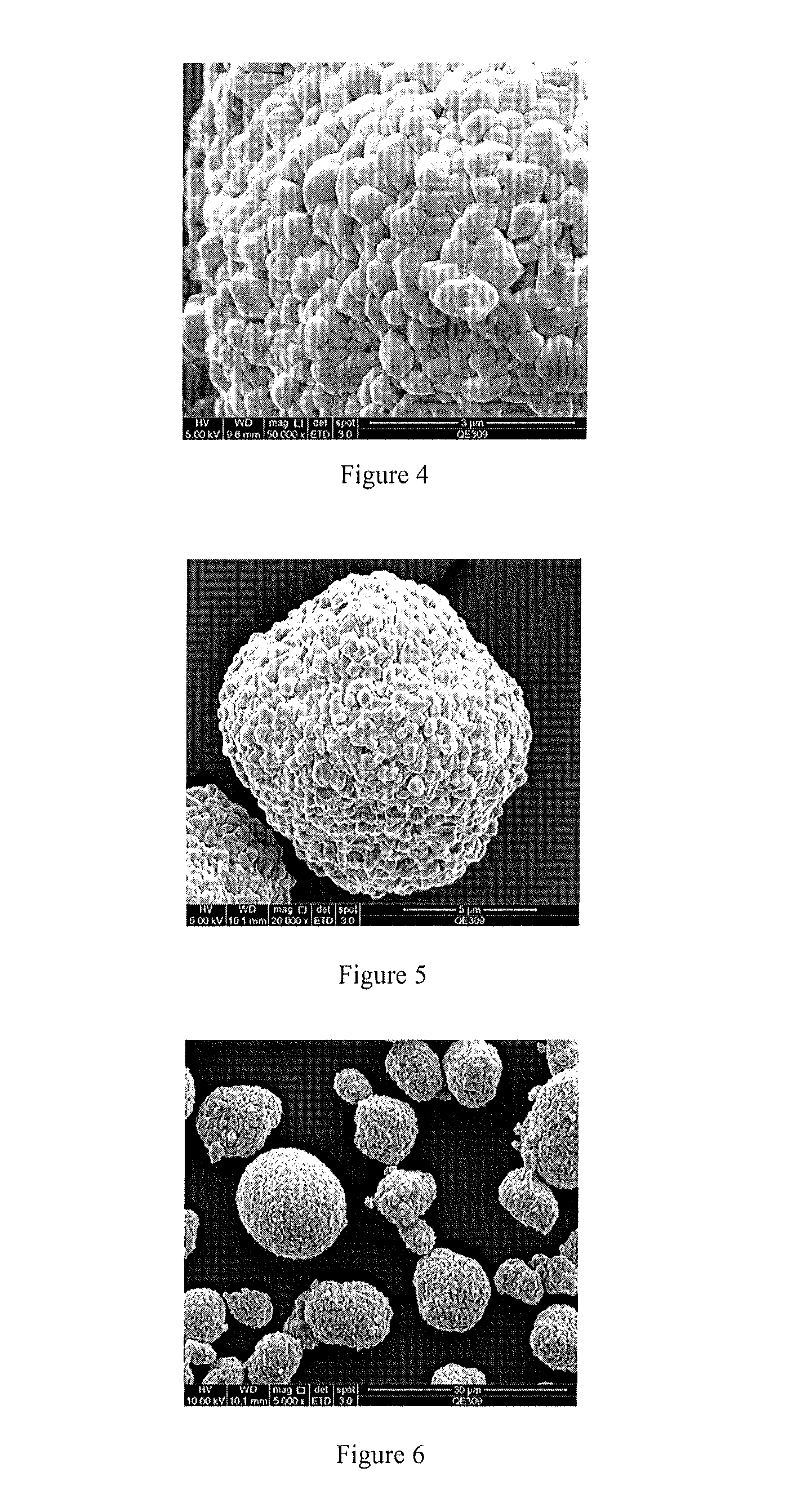
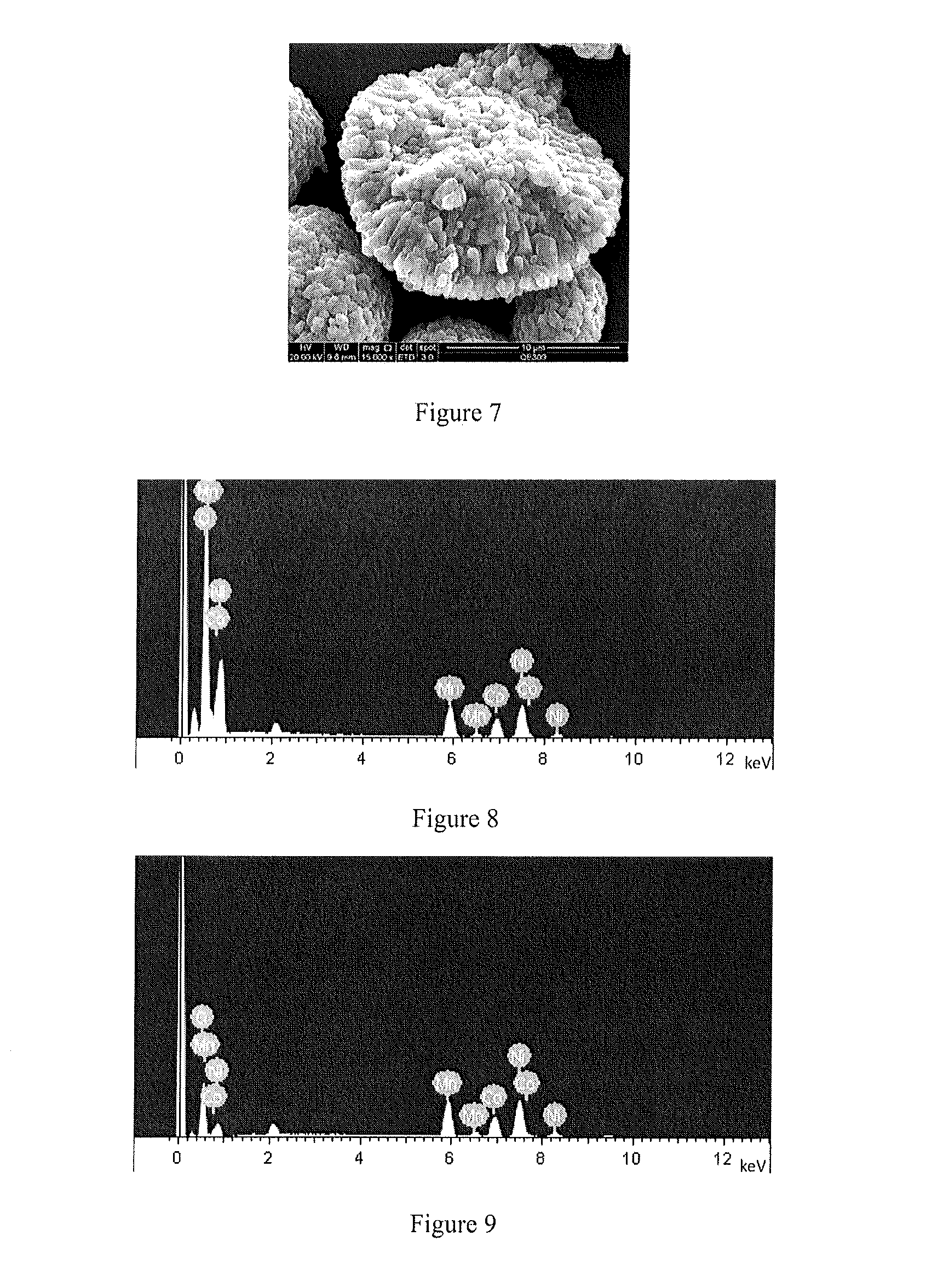
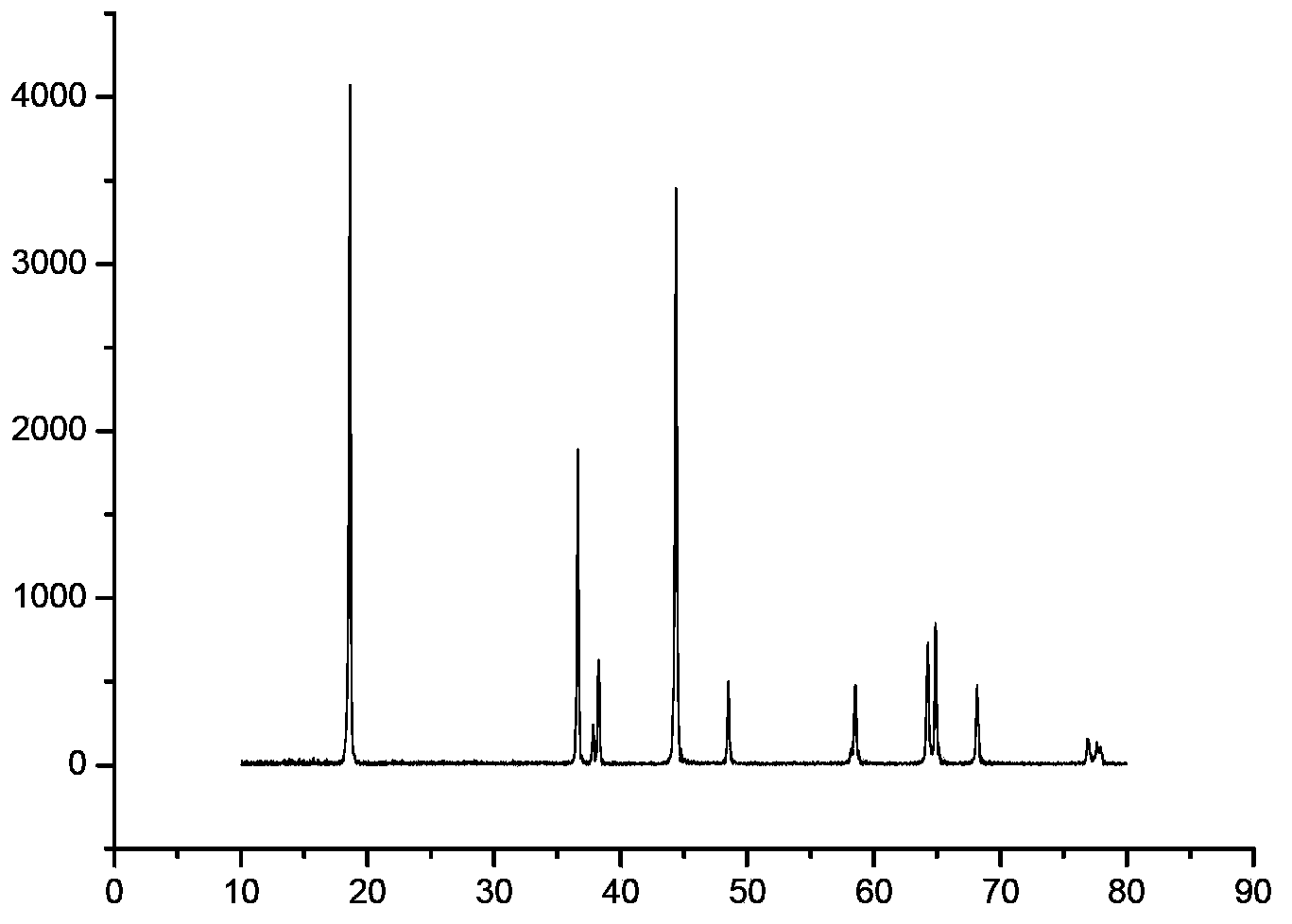
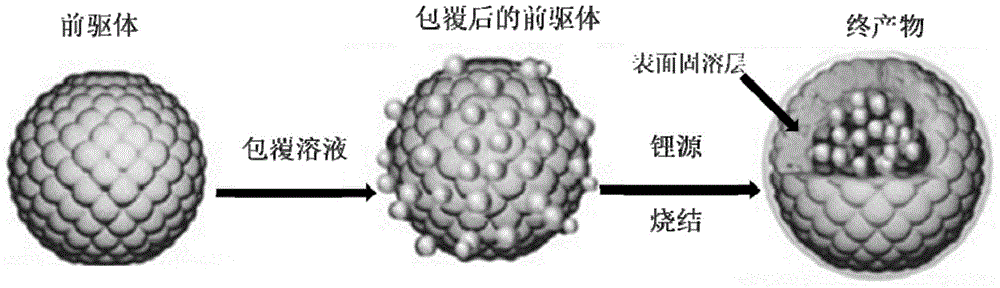
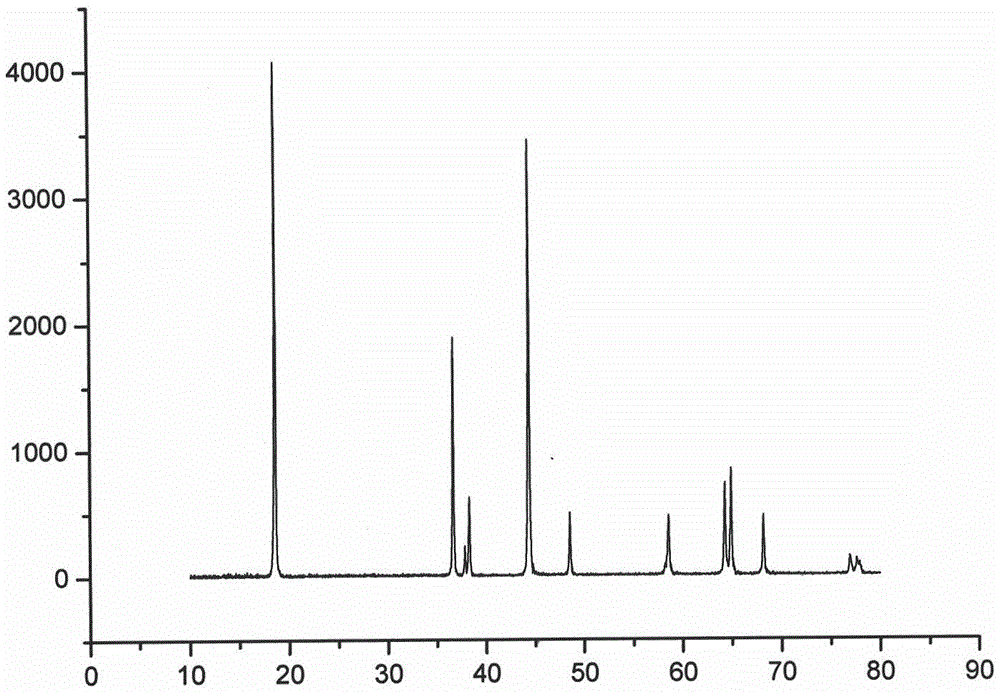
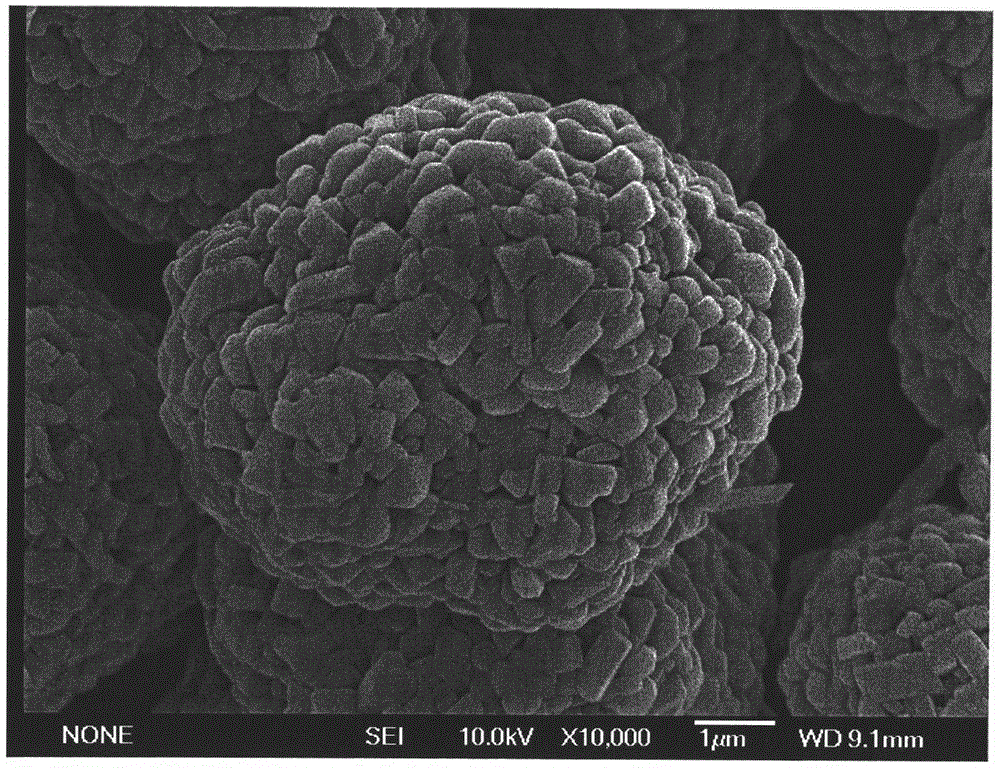
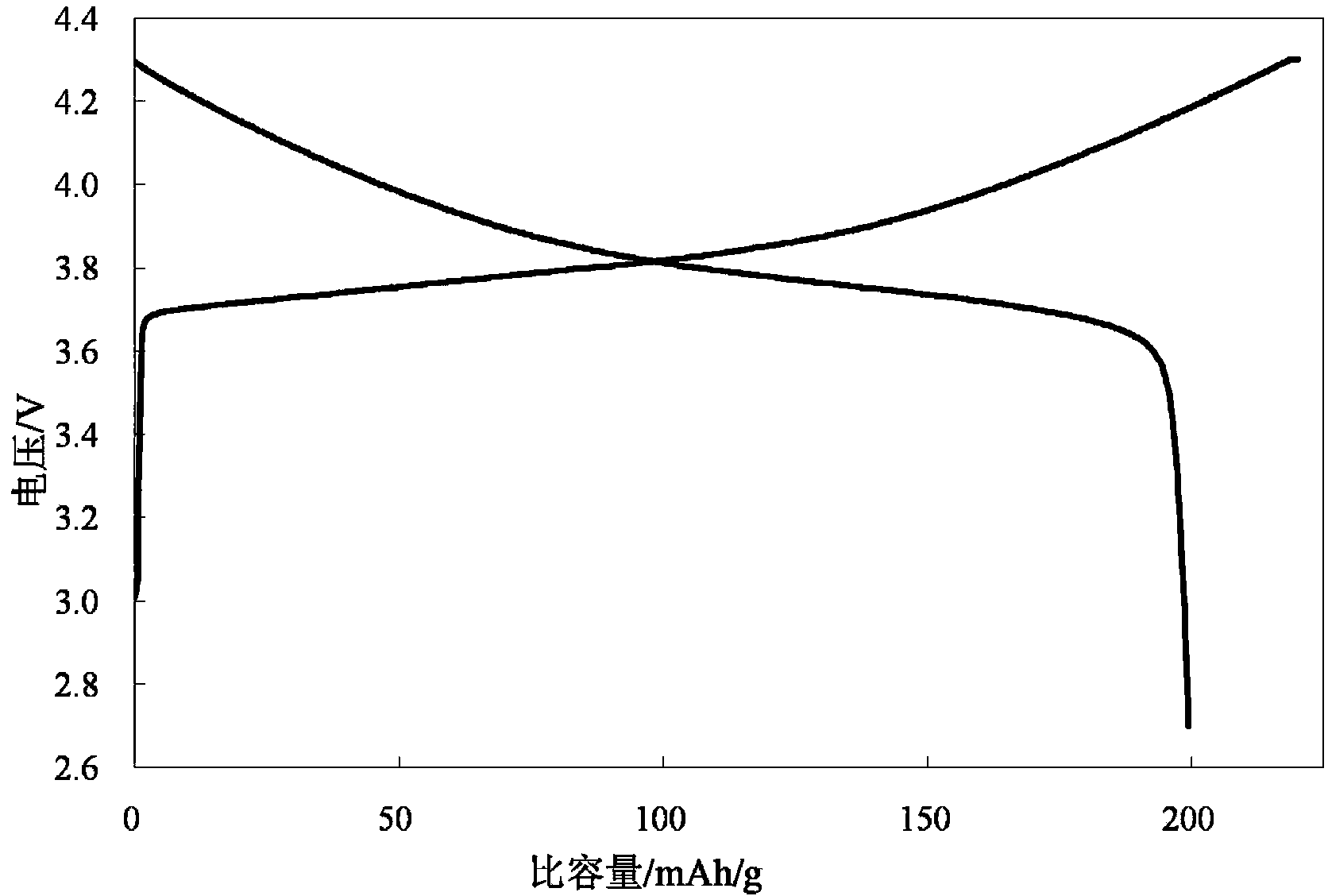
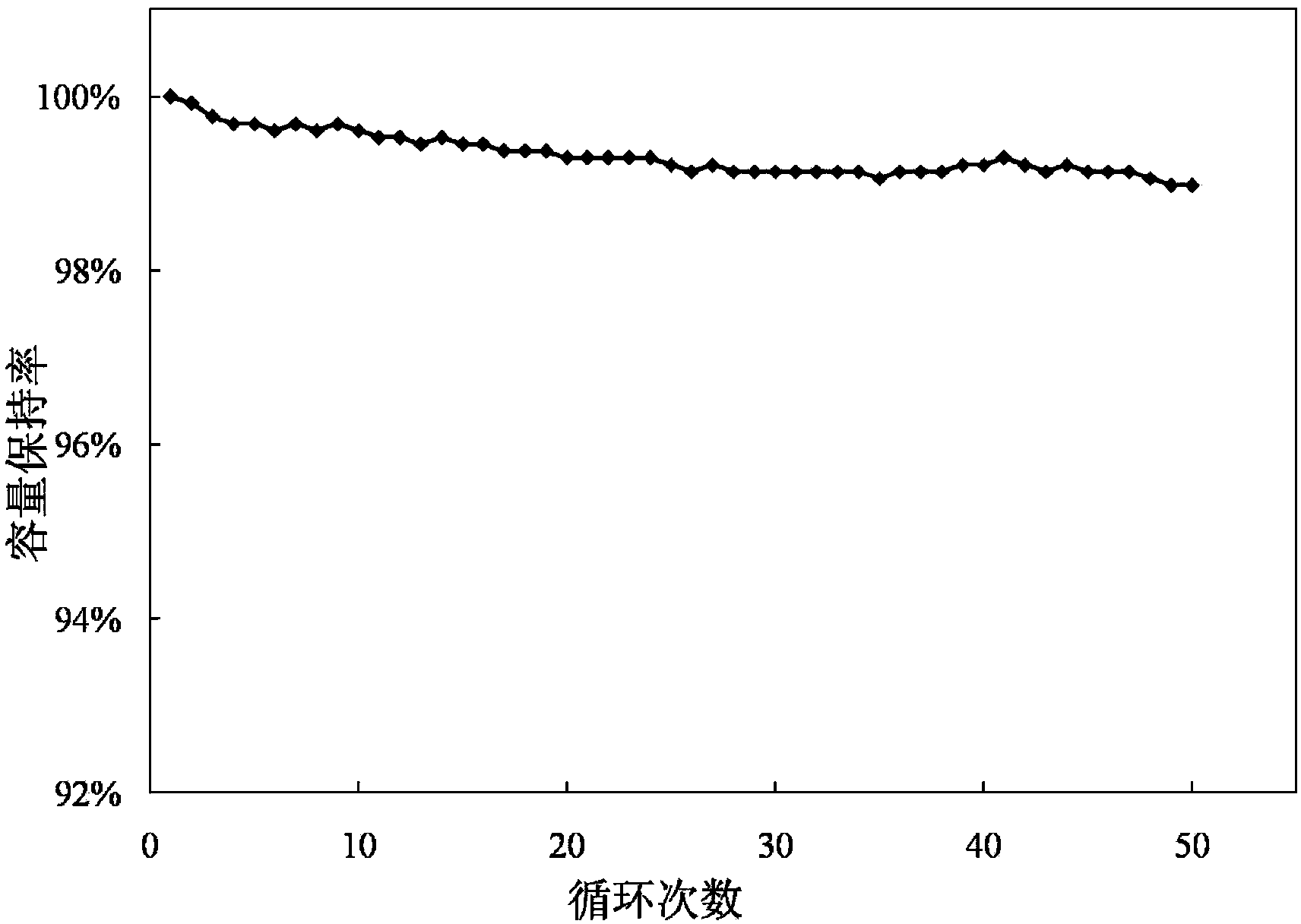
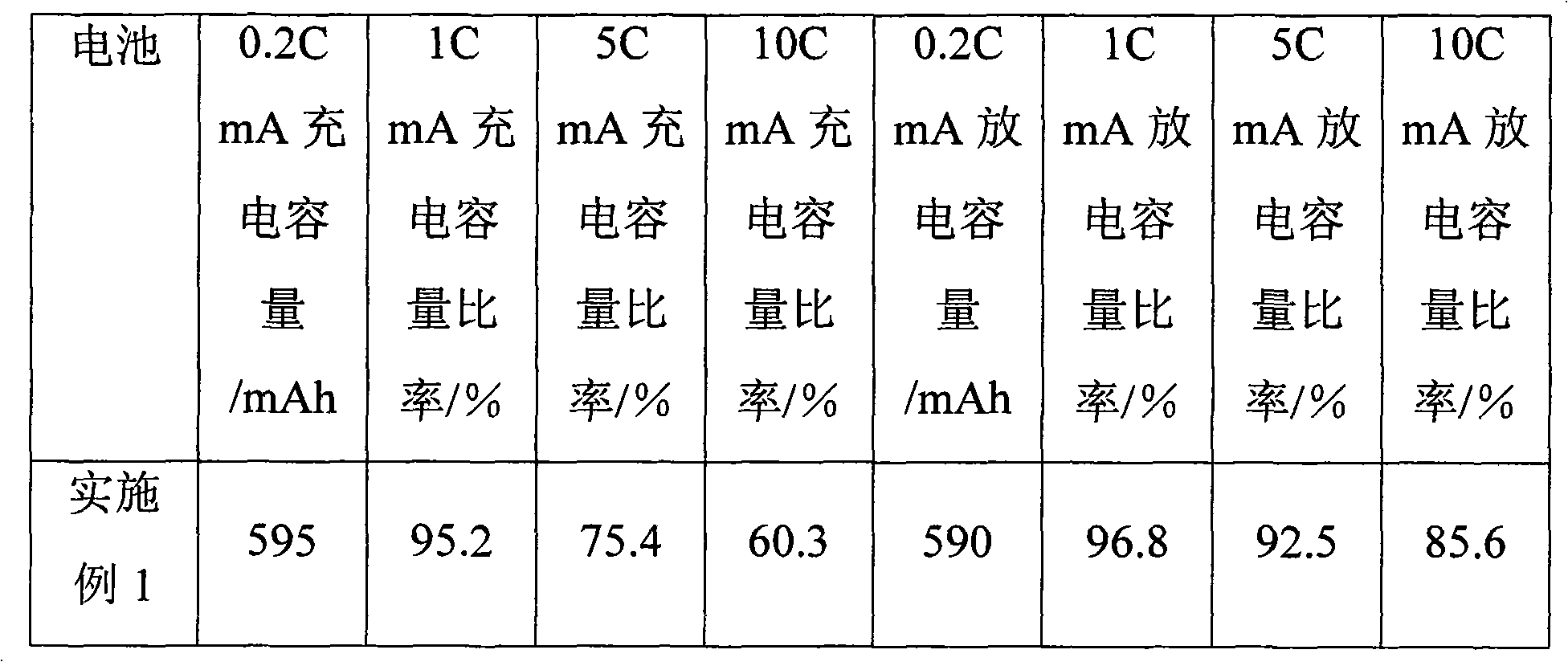
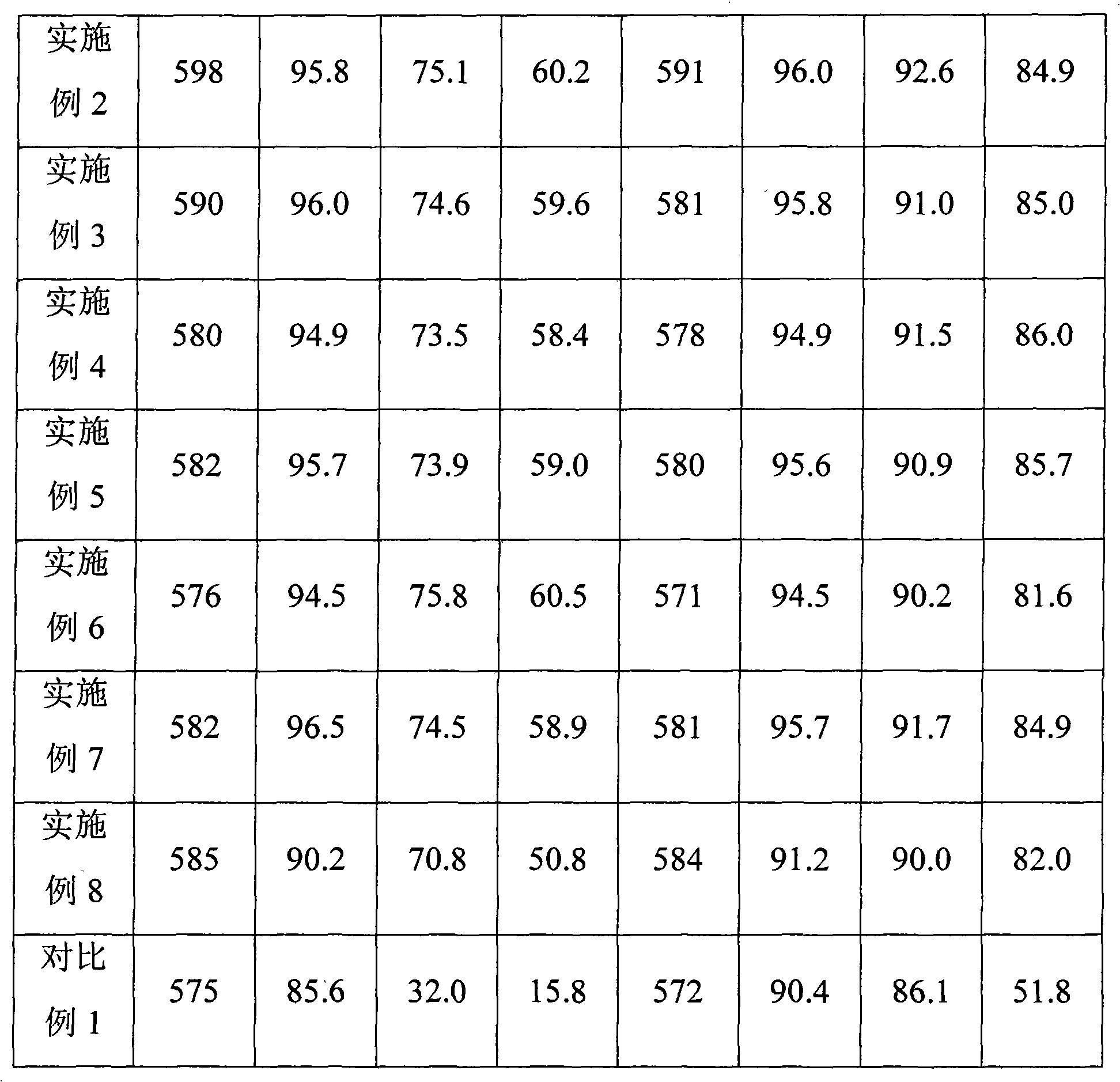
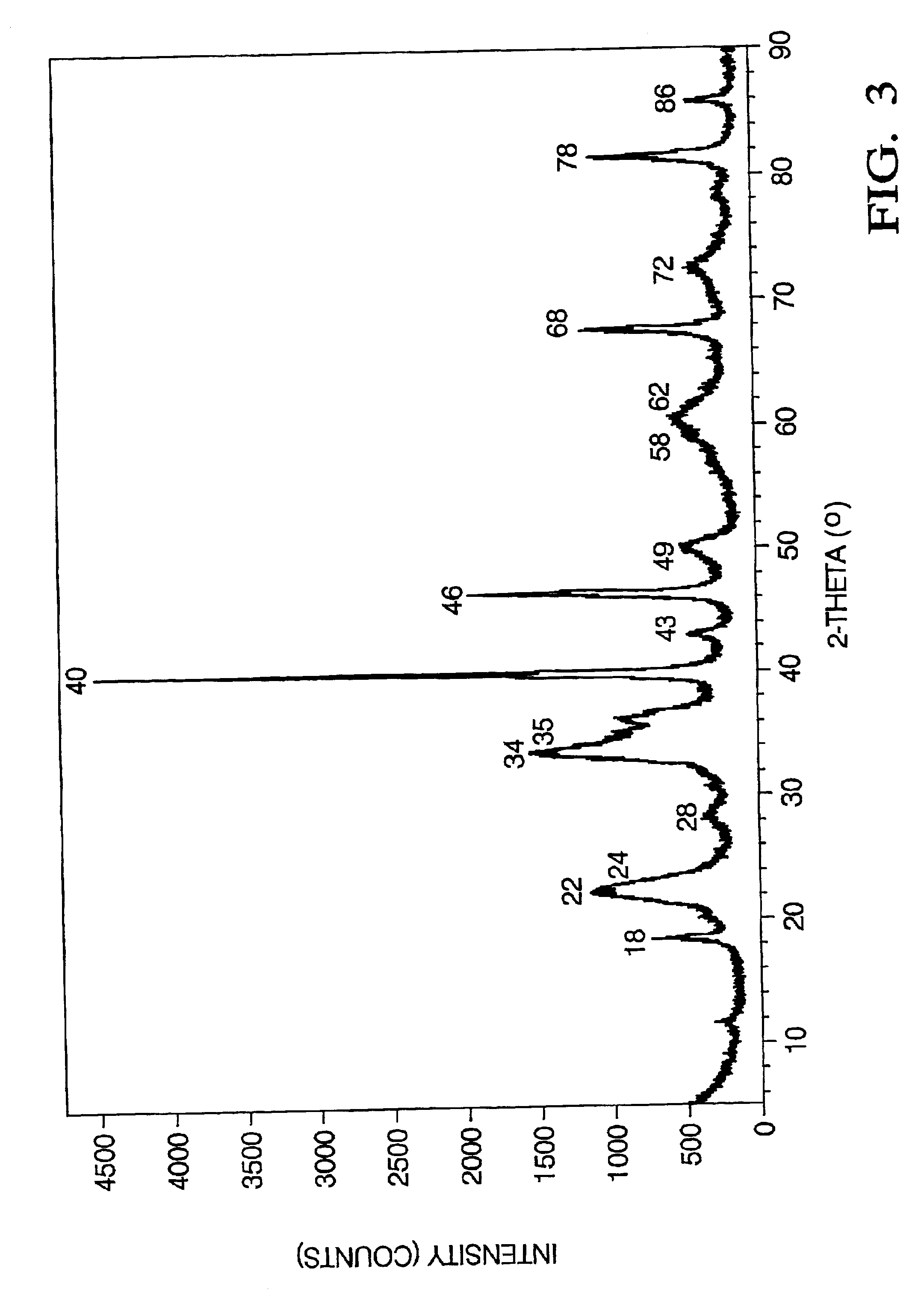
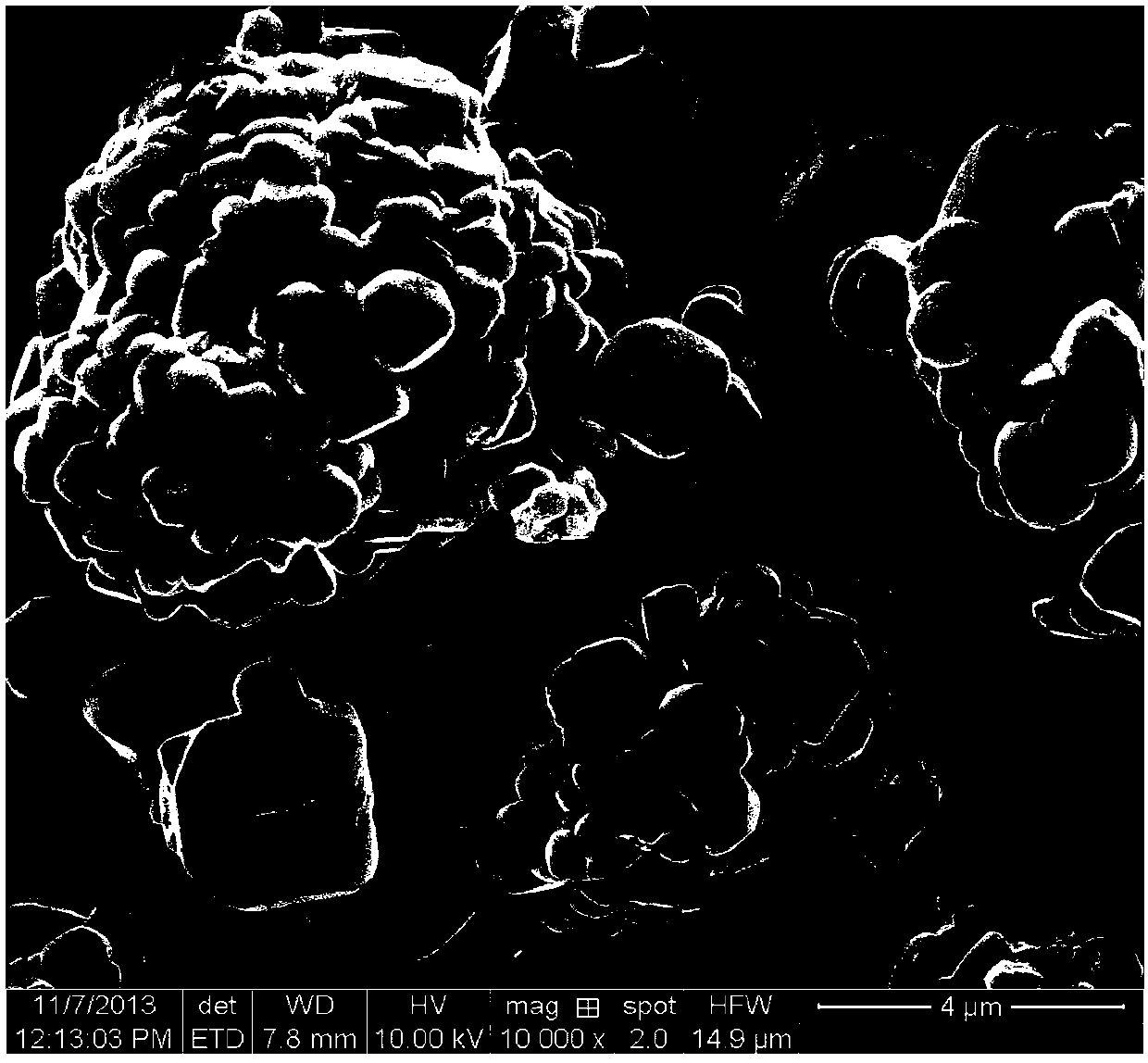
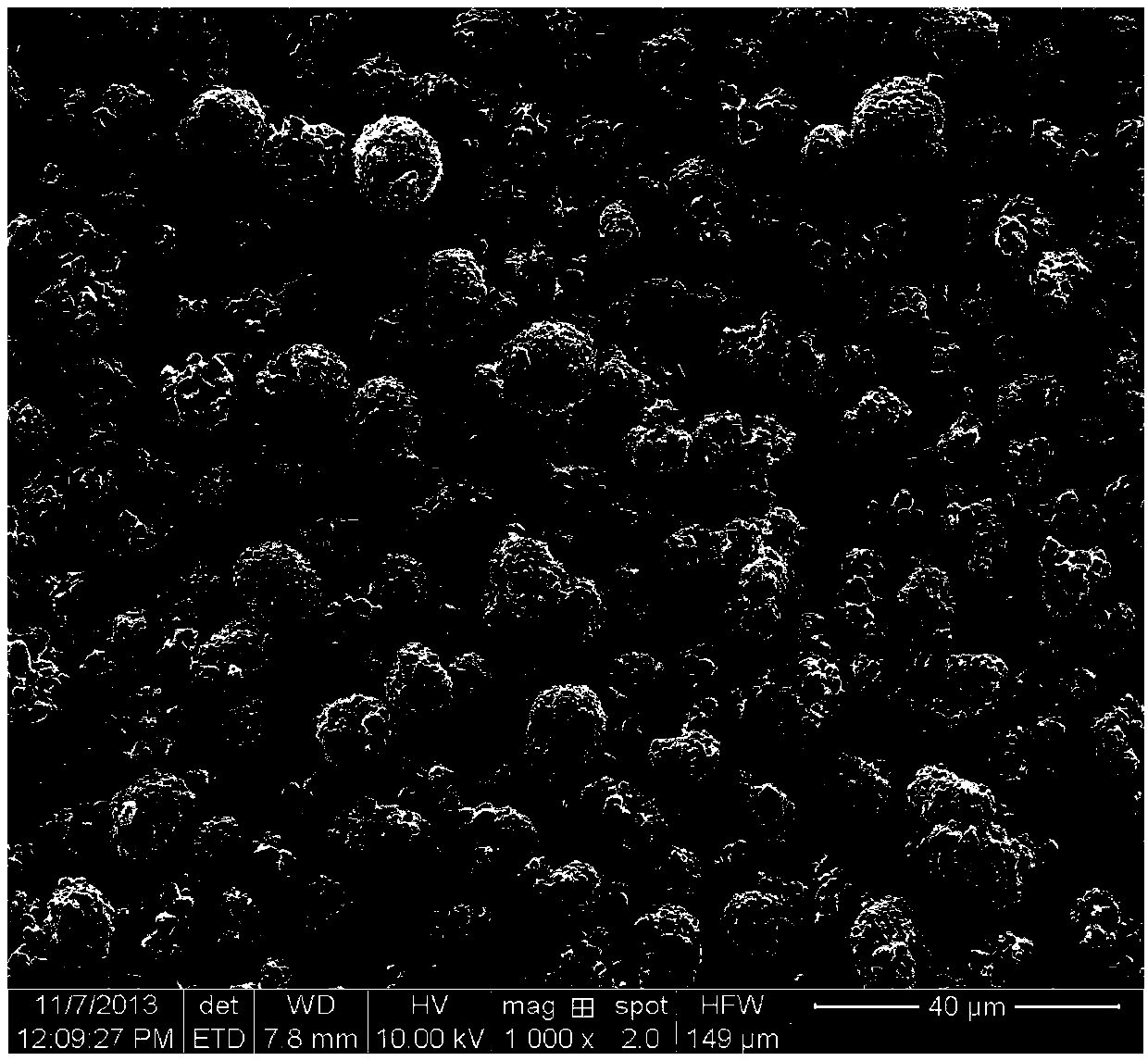
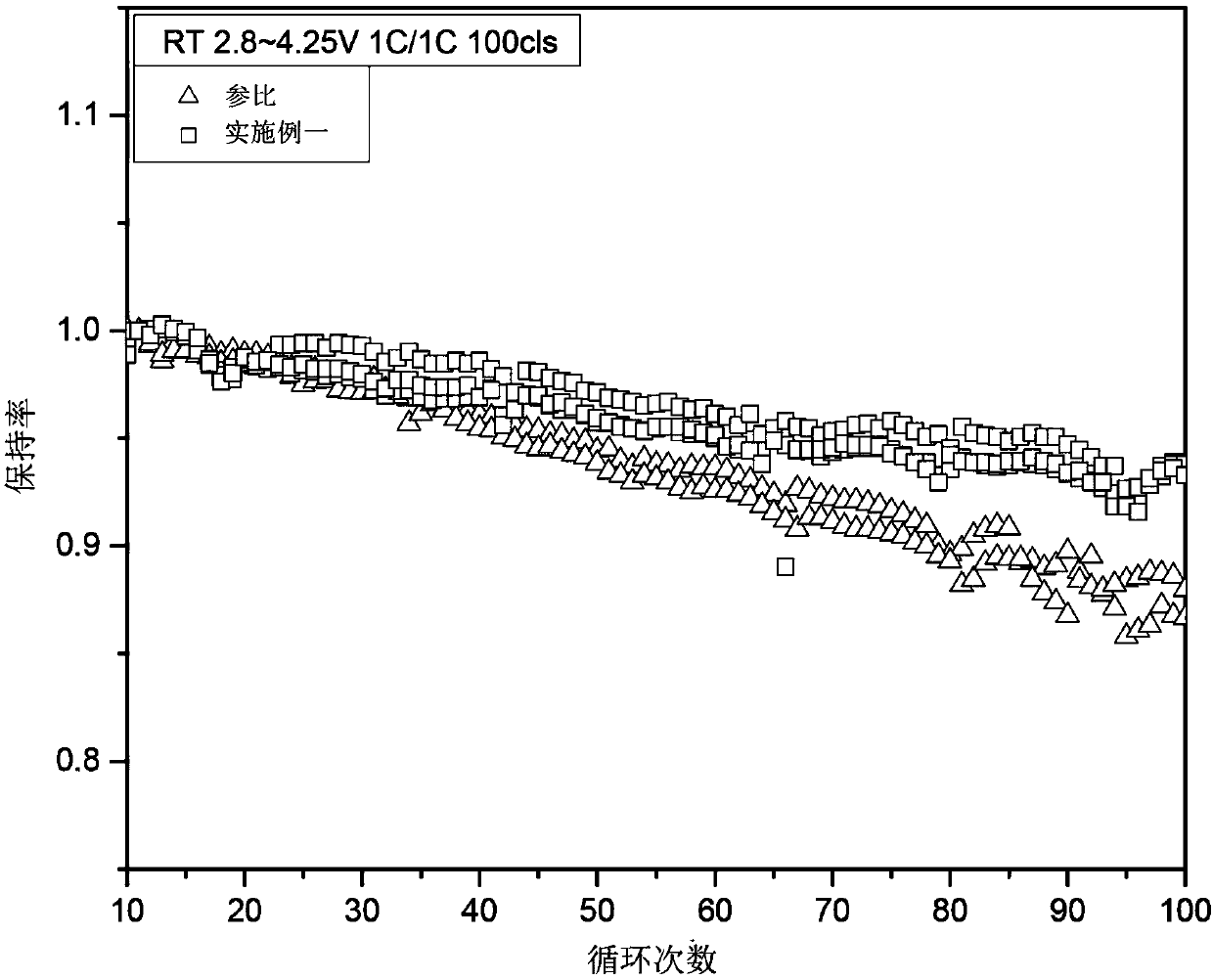
Patents
Literature
383 results about "Lithium aluminate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium aluminate (LiAlO₂), also called lithium aluminium oxide, is an inorganic chemical compound, an aluminate of lithium. In microelectronics, lithium aluminate is considered as a lattice matching substrate for gallium nitride. In nuclear technology, lithium aluminate is of interest as a solid tritium breeder material, for preparing tritium fuel for nuclear fusion.
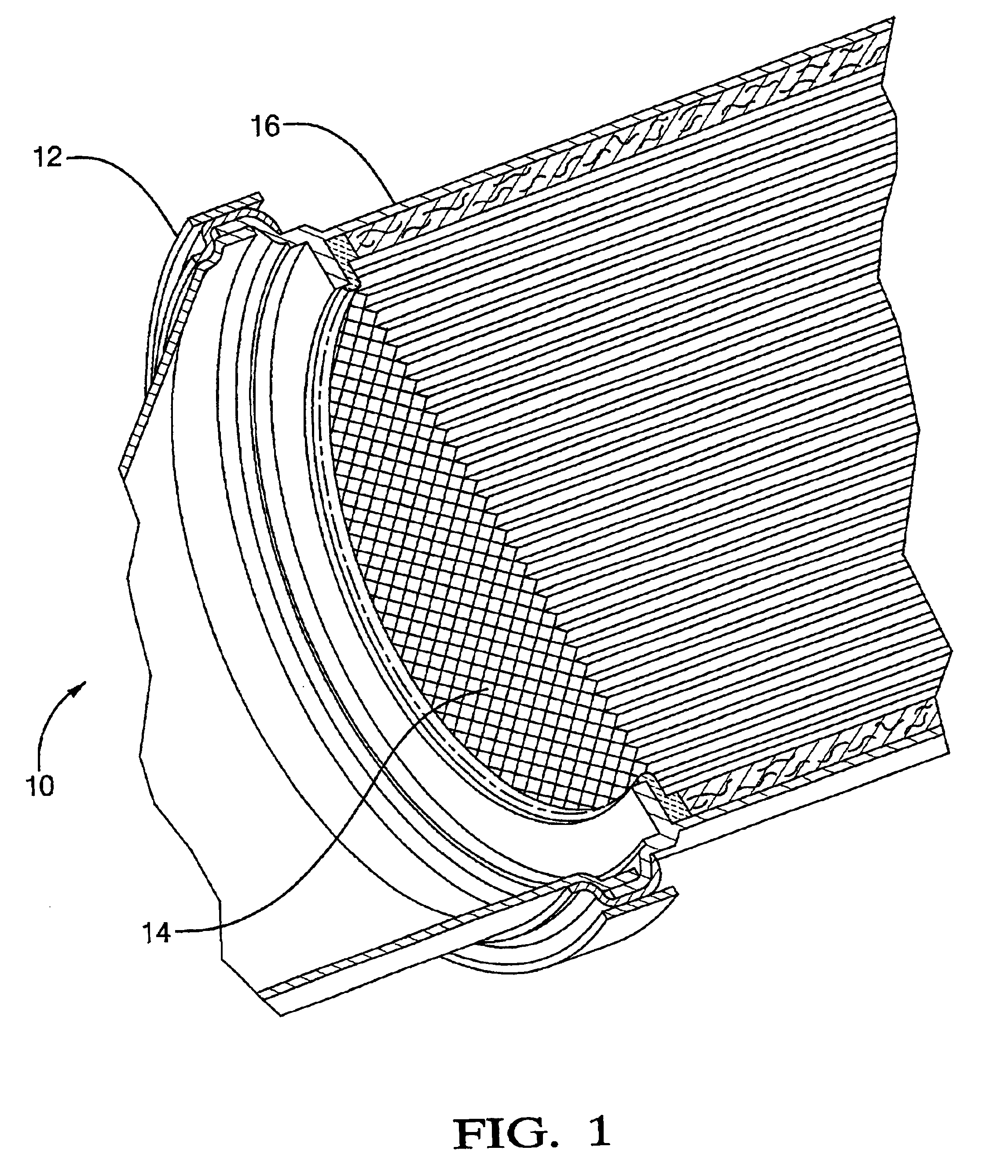
Method for making Group III nitride devices and devices produced thereby
InactiveUS7033858B2Improve cooling effectThin active areaPolycrystalline material growthSolid-state devicesLithium aluminateNitride
A method is for making at least one semiconductor device including providing a sacrificial growth substrate of Lithium Aluminate (LiAlO2); forming at least one semiconductor layer including a Group III nitride adjacent the sacrificial growth substrate; attaching a mounting substrate adjacent the at least one semiconductor layer opposite the sacrificial growth substrate; and removing the sacrificial growth substrate. The method may further include adding at least one contact onto a surface of the at least one semiconductor layer opposite the mounting substrate, and dividing the mounting substrate and at least one semiconductor layer into a plurality of individual semiconductor devices. To make the final devices, the method may further include bonding the mounting substrate of each individual semiconductor device to a heat sink. The step of removing the sacrificial substrate may include wet etching the sacrificial growth substrate.
Owner:CRYSTAL PHOTONICS
Lithium aluminate layered catalyst and a selective oxidation process using the catalyst
InactiveUS6858769B2Thermal non-catalytic crackingHydrocarbon by isomerisationHydrogenDehydrogenation
A catalyst for the selective oxidation of hydrogen has been developed. It comprises an inert core such as cordierite and an outer layer comprising a lithium aluminate support. The support has dispersed thereon a platinum group metal and a promoter metal, e.g. platinum and tin respectively. This catalyst is particularly effective in the selective oxidation of hydrogen in a dehydrogenation process.
Owner:UOP LLC
Anode active material, preparation method of anode active material, high-performance anode slurry containing anode active material, and all-solid-state lithium ion battery
ActiveCN106784798AImprove mobilityImprove electrochemical performanceCell electrodesSecondary cellsInorganic compoundSulfide
The invention relates to an anode active material, a preparation method of the anode active material, high-performance anode slurry containing the anode active material, and an all-solid-state lithium ion battery. The anode active material is a nickel-rich type core-shell structure particle or a nickel-rich type core-shell structure particle coated with an inorganic compound coating layer at the surface; an inner core of the nickel-rich type core-shell structure particle is LiNixCoyMn1-x-yO2; the shell is nickel cobalt lithium aluminate. The invention also provides the high-performance anode slurry, which comprises the anode active material, a composite conductive agent, a composite bonding agent, an additive and an organic solvent, wherein the additive is sulfide solid electrolyte; the anode slurry is used for preparing an anode plate consisting of an anode current collector, an anode slurry layer and a modification layer; the anode plate, the sulfide solid electrolyte and a cathode plate are assembled into the all-solid-state lithium ion battery. The all-solid-state lithium ion battery has the prominent advantages of high mass specific energy, high volumetric specific energy, good rate capability, good cycle performance, high safety and the like, and has wide application prospects.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Sorbent for lithium extraction
ActiveUS8753594B1Increase ionic strengthWaste water treatment from quariesAluminium compoundsSorbentLithium aluminate
This invention relates to a method for preparing a lithium aluminate intercalate (LAI) matrix solid and methods for the selective extraction and recovery of lithium from lithium containing solutions, including brines. The method for preparing the LAI matrix solid includes reacting aluminum hydroxide and a lithium salt for form the lithium aluminate intercalate, which can then be mixed with up to about 20% by weight of a polymer to form the LAI matrix.
Owner:TERRALITHIUM LLC
Method for making group ó¾ nitride devices and devices produced thereby
InactiveCN1781195AImprove cooling effectSemiconductor/solid-state device manufacturingSemiconductor devicesLithium aluminateNitride
A method of making at least one semiconductor device comprising the steps of: providing a sacrificial growth substrate of lithium aluminate (LiAlO2); forming at least one semiconductor layer comprising a III-nitride adjacent to the sacrificial growth substrate; mounting A substrate is secured adjacent the at least one semiconductor layer opposite the sacrificial growth substrate; and the sacrificial growth substrate is removed. The method may also include adding at least one contact to a surface of the at least one semiconductor layer opposite the mounting substrate, and separating the mounting substrate and the at least one semiconductor layer into a plurality of individual semiconductor devices. To fabricate the final device, the method may also include bonding the mounting substrate of each individual semiconductor device to the heat sink. The step of removing the sacrificial substrate may include wet etching the sacrificial growth substrate.
Owner:CRYSTAL PHOTONICS
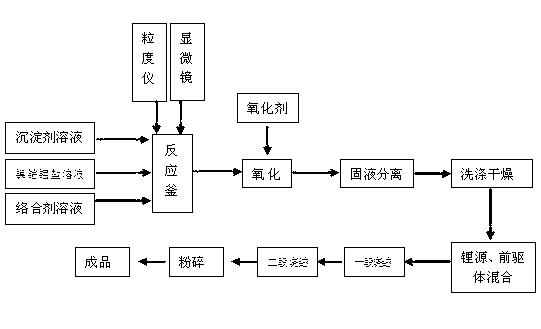
Method for preparing nickel-cobalt lithium aluminate as anode material of lithium ion battery
InactiveCN103094546AImprove electrical performanceImprove cycle performanceCell electrodesLithium aluminateOxygen
The invention discloses a method for preparing nickel-cobalt lithium aluminate as an anode material of a lithium ion battery. The method comprises the following steps: (1) mixing a nickel-cobalt metal salt water solution, a sodium metaaluminate solution, a complexing agent and a precipitant, regulating the pH value of a reaction system to be 9 to 12, and then maintaining a stirring state to carrying out a reaction at the temperature of 30 to 80 DEG C for 20 to 200 hours, thereby obtaining nickel-cobalt aluminum hydroxide precipitates; (2) washing the nickel-cobalt aluminum hydroxide precipitates by using pure water of 50 to 100 DEG C, drying, screening the part of precipitates capable of passing through a sieve being 300 meshes, adding a lithium source to the precipitates, mixing evenly, and sintering at the temperature of 600 to 1000 DEG C, wherein oxygen is filled during the sintering process; and finally sintering for 5 to 50 hours, thereby obtaining the nickel-cobalt lithium aluminate. According to the method, the sodium metaaluminate is adopted as the lithium source, so that the nickel-cobalt aluminum elements can evenly form a coprecipitation, so that the aluminum is evenly distributed in the nickel-cobalt lithium aluminate material. As a result, the electrical performance of the material is improved, and especially the cycling performance of the material is improved.
Owner:HUNAN BRUNP RECYCLING TECH +1
Method for preparing nickel cobalt lithium aluminate cathode material
The invention discloses a method for preparing a nickel cobalt lithium aluminate cathode material. The method comprises the following steps of: uniformly mixing a nickel salt solution, a cobalt salt solution and an aluminum salt solution in a certain mole ratio of metal ions; adding a complexing agent solution, a precipitator solution and a metal salt solution together into a high-speed stirring reaction kettle with a base solution, and performing precipitation reaction; after complete reaction, performing oxidizing reaction on discharged slurry and an oxidizing agent with certain concentration in an alkaline environment; after the oxidizing reaction is finished, performing solid liquid separation on the slurry, washing in pure water, and drying to obtain a nickel cobalt aluminum hydroxyl oxide precursor of a lithium ion battery cathode material; fully mixing the precursor with a lithium source, and performing multi-step sintering in an oxygen atmosphere; and performing crushing and subsequent treatment on a material obtained by sintering, and thus obtaining the nickel cobalt lithium aluminate cathode material. The method is low in equipment requirement, simple in flow, low in energy consumption and low in waste. The produced material is high in tap density and high in capacity.
Owner:HUNAN SOUNDDON NEW ENERGY
High-density lithium ion battery anode material nickel-cobalt lithium aluminate and preparation method thereof
InactiveCN103050686ANo pollution in the processImprove performanceCell electrodesHigh densityWastewater
The invention discloses a high-density lithium ion battery anode material nickel-cobalt lithium aluminate and a preparation method thereof. The preparation method for the high-density lithium ion battery anode material nickel-cobalt lithium aluminate comprises the following steps of: carrying out ball-milling and mixing, pelleting and sintering on a treated nickel source, a treated cobalt source, a treated aluminum source and a treated doping element M source in stoichiometric proportion to obtain oxides of nickel, cobalt and aluminum; and carrying out secondary calcining on the oxides of the nickel, cobalt and aluminum and a lithium source after carrying out ball-milling and uniform mixing to obtain the high-density lithium ion battery anode material nickel-cobalt lithium aluminate. According to the preparation method disclosed by the invention, the materials are sufficiently and uniformly mixed, and then sintered at a high temperature by a solid-phase process, so that the compaction density and the specific capacity of the nickel-cobalt lithium aluminate are improved by the doping element; moreover, the process is simple, free of waste water and pollution to the environment. The preparation process is easy to control and operate, low in production cost, good in industrial prospect and easy to implement large-scale industrial production.
Owner:HUNAN SOUNDDON NEW ENERGY
Oxide cathode material for lithium ion battery having high energy density and preparation process thereof
InactiveUS20150104708A1High energy densityUniform shellSecondary cellsPositive electrodesHigh energyEnergy density
Provided are a high energy density oxide anode material for lithium ion battery, preparation process and use thereof. Said anode material includes a main part of the anode material and a covering layer. Said main part includes a shell and a core inside the shell. The material of said core is Li1+x[Ni1−y−zCoyMnz]O2 wherein −0.1≦x≦0.2, 0≦y≦0.5, 0≦z≦0.5 and 0≦y+z≦0.7. The material of said shell is Li1+a[Co1−bXb]O2, wherein −0.1≦a≦0.2, 0≦b≦0.5, and X is selected from Al, Mg, Cu, Zr, Ti, Cr, V, Fe, Mn, Ni, or combination thereof. Otherwise, The material of said main part is a mixture of Li1+x[Ni1−y−zCoyMnz]O2 and LiCoO2, wherein −0.1≦x≦0.2, 0≦y≦0.5, 0<z≦0.5 and 0≦y+z≦0.7. The material of said covering layer is selected from Al2O3, ZrO2, MgO, SiO2, ZnO2, TiO2, Y2O3, LiAlO2, or combination thereof. Said anode material has the advantages of high capacity, good cycle performance, low surface activity, high voltage resistance and fine safety. The preparation process is simple, and is suitable for large-scale production.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI +1
Nickel-cobalt lithium aluminate and preparation method of precursor thereof
ActiveCN103400973AHigh crystallinityHigh charge and discharge capacityCell electrodesHigh densityCobalt salt
The invention relates to a preparation method of a nickel cobalt aluminum material precursor. The method used in the invention comprises the steps: carrying out a complexation reaction of an aluminum salt and a complexing agent to form a stable aluminum complex, then injecting the aluminum complex simultaneously with a nickel-cobalt salt solution into a reaction kettle for carrying out a co-precipitation reaction to prepare a high-density precursor of nickel-cobalt lithium aluminate, and then carrying out a lithium mixed roasting to form a high-density spherical nickel-cobalt lithium aluminate material. At the same time, the invention also discloses an application of the nickel-cobalt lithium aluminate material used in a cathode material of a lithium ion battery. The high-density spherical nickel-cobalt lithium aluminate material which has atomic level mixture of nickel cobalt aluminum elements and good crystallinity can be produced under a premise without mass increase of cost, and the charge and discharge capacity and the first time efficiency have a certain improvement compared with those of current products. The process is simple and easy to implement, and can be used in large-scale industrialized production.
Owner:郭建
Modified lithium ion battery positive electrode material and preparation method therefor
InactiveCN105470455AImprove performanceImprove cycle stabilityCell electrodesLithium aluminateLithium-ion battery
The invention provides a modified lithium ion battery positive electrode material. The modified lithium ion battery positive electrode material is characterized in that LiNi0.8Co0.15Al0.05O2 is taken as a core and a lithium-containing composite oxide is taken as a shell; and the lithium-containing composite oxide is selected from one or more of Li2TiO3, Li2SiO3, Li4SiO4, Li3PO4, Li4P2O7 and Li2ZrO3. According to the surface modified nickel cobalt lithium aluminate positive electrode material provided by the invention, a coating material with electronic conductivity or lithium ion conductivity is taken as the shell, so that the modified material has a surface layer with a lithium ion conductivity property; a surface solid solution layer with stable chemical stability endows the nickel cobalt lithium aluminate positive electrode material and an electrolyte with high interfacial compatibility and interfacial structural stability, so that the charge-discharge cycling stability of the product is improved.
Owner:ZHEJIANG FUNLITHIUM NEW ENERGY TECH CO LTD
Lithium ion battery capable of quick charging
InactiveCN104347880AIncrease gram capacityImproved magnification performanceCell electrodesSecondary cellsLithium iron phosphateAdhesive
The invention belongs to the technical field of lithium ion batteries, and particularly relates to a lithium ion battery capable of quick charging. The battery includes a positive plate, a negative plate, isolating films arranged between the positive and negative plates at intervals, and electrolyte. The positive plate includes a positive current collector and a positive electrode active material layer arranged on the surface of the positive current collector. The positive electrode active material layer includes a positive electrode active material, a positive electrode conductive agent and a positive adhesive. The positive electrode active material includes components A and B. the component A is selected from at least one of nickel-cobalt lithium aluminate, nickel-cobalt lithium manganate, lithium manganate and lithium cobaltate. The component B is selected from at least one of lithium iron phosphate and lithium titanate. The mass percent of the component B in the positive electrode active material is 5-9 percent. In comparison with the prior art, the lithium ion battery has the advantages that the time of low voltage constant current charging can be prolonged due to use of the mixed positive electrode active material, and thus the charging speed is increased.
Owner:DONGGUAN AMPEREX TECH
Preparation method of core-shell-structure nickel cobalt lithium aluminate
InactiveCN104979553AImprove cycle stabilityImprove thermal stabilityCell electrodesSolubilityHeat stability
The invention relates to a preparation method of a core-shell-structure nickel cobalt lithium aluminate and belongs to the technical field of lithium ion battery positive material application. The core-shell-structure material is in a two-layer structure, wherein the inner layer core part is LiNiaCo[1-a-b]AlbO2, a is more than 0.7, b is less than or equal to 0.05 and more than or equal to 0, and a+b is less than 1; the molecular formula of the shell part is LiNicCo[1-c-d]AldO2, c is more 0.5, d is more than 0 and less than 0.5, and c+d is less than 1. In the method, two kinds of aqueous alkali are used as precipitators, so that the materials of the core part and the shell part are consistent in crystal from and compact in structure in the crystallization process, and a solubility gradient method is adopted in the alternating process of the two kinds of aqueous alkali so that the core-shell-structure material is consistent in crystal from and high in crystallization degree, and an obvious core-shell interface does not exist. Compared with a common homogeneous phase material, the core-shell-structure material disclosed by the invention has the advantages that the high capacity is kept, the cycling stability and the heat stability are improved, the air expanding rate is obviously reduced, and the cost performance is higher, so that the core-shell-structure material is more suitable for being used in power batteries.
Owner:郭建
Lithium manganate composite positive electrode material, a preparing method thereof and a lithium-ion battery
ActiveCN104347853AHigh specific capacityCapacity decaySecondary cellsPositive electrodesManganeseManganate
The invention provides a lithium manganate composite positive electrode material, a preparing method thereof and a lithium-ion battery. The composite positive electrode material is of a core-shell structure. The inner layer of the composite positive electrode material is an in-situ composite of lithium manganate and nickel-rich concentration gradient type nickel cobalt manganese / lithium aluminate LiMn2O4-LiNi1-x-yCox(Al / Mn)yO2, wherein x is more than 0 and less than or equal to 0.25, and y is more than 0 and less than or equal to 0.15; the outer shell of the composite positive electrode material is a metal oxide coated layer. According to the lithium manganate composite positive electrode material and the preparing method thereof, the in-situ composite of lithium manganate and nickel-rich concentration gradient type nickel cobalt manganese / lithium aluminate is obtained after in-site sintering of a manganese source, a nickel-rich concentration gradient type nickel cobalt manganese / lithium aluminate precursor, and a lithium source, then shell-layer metal oxide is cladded by using spray drying, and finally the composite positive electrode material is obtained by combining a microwave sintering process. The composite positive electrode material provided by the invention has relatively high specific capacity, and excellent high temperature cycling and storage performances.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Modified nickel-cobalt lithium aluminate positive electrode material and preparation method thereof
ActiveCN103972499ASuppression of shufflingImprove electrochemical performanceCell electrodesSecondary cellsNickel saltLithium aluminate
The invention relates to a modified nickel-cobalt lithium aluminate positive electrode material and a preparation method of the material. The chemical general formula of the material is LiNi(1-a-b)CoaAlbO2 / TiO2, wherein a is greater than 0.1 and less than 0.3; b is greater than 0.01 and less than 0.2; 1-a-b is greater than 0 and less than 1; the TiO2 layer is a coating layer; the preparation method of the material comprises the following steps: preparing soluble metal nickel salt, cobalt salt and aluminium salt into a mixed salt solution, preparing the mixed salt solution, NaOH and ammonia water into a mixed alkali solution for reacting, filtering, washing, drying and then roasting the mixed alkali solution for 5-10 hours at the temperature of 400-600 DEG C in the oxygen atmosphere, then carrying out ball milling and uniformly mixing with lithium salt, roasting the mixed alkali solution for 6-16 hours at the high temperature of 800-1000 DEG C in the oxygen atmosphere, coating the mixed alkali solution by titanium dioxide to prepare the modified nickel-cobalt lithium aluminate positive electrode material. The prepared modified ternary positive electrode material of the lithium ion battery is good in electrochemical performance; the dry coating process is free of waste liquid and high-temperature sintering, so that the energy consumption and the cost are reduced.
Owner:ZHEJIANG MEIDARUI NEW MATERIAL TECH CO LTD
Preparation method of lithium-ion battery positive electrode material spherical nickel-cobalt-lithium aluminate
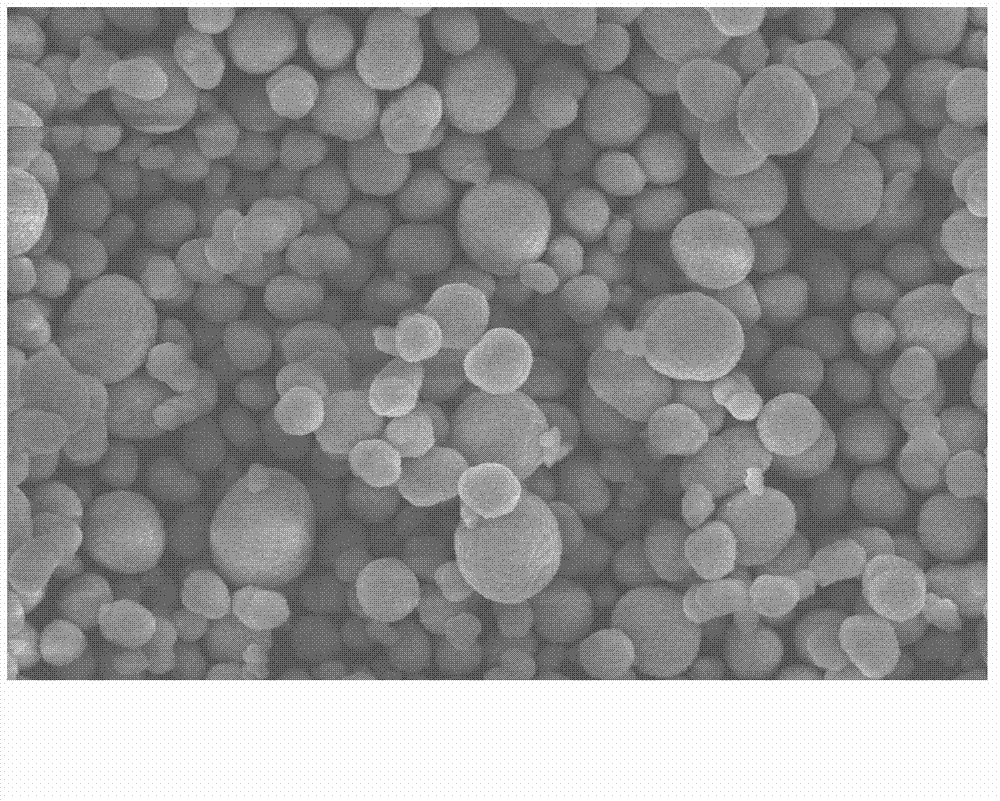
ActiveCN103296263AGuaranteed spherificationHigh tap densityCell electrodesNickel saltReaction temperature
The invention discloses a preparation method of lithium-ion battery positive electrode material spherical nickel-cobalt-lithium aluminate. The preparation method comprises the following steps of: firstly dissolving aluminum salt in deionized water, and preparing AlOOH aluminum sol by adding HNO3 or ammonium hydroxide and nitric acid; preparing nickel salt and cobalt salt into uniform aqueous solution according to a certain ratio; enabling the mixed salt solution to be collectively reacted with the aluminum sol and a mixed alkali solution, adjusting the pH value to be 9 to 12, controlling the reaction temperature, carrying out the solid-liquid separation after 20 to 30h of the reaction, and washing, filtering and drying the reaction product to obtain spherical nickel-cobalt-aluminum hydroxide precursor powder; then mixing the spherical nickel-cobalt-aluminum hydroxide precursor powder with lithium, sintering the mixture, and pulverizing and grading sintered material to obtain the lithium-ion battery positive electrode material spherical nickel-cobalt-lithium aluminate. The prepared spherical nickel-cobalt-lithium aluminate particles are controllable in shape and granularity, high in compacting density, high in specific discharge capacity, good in cycling stability and low in cost.
Owner:山东天骄新能源有限公司
A kind of separation and recovery method of NMP and catalyst lithium chloride in polyphenylene sulfide production
The invention relates to a separation and recovery method for n-methylpyrrolidone (NMP) and a lithium chloride catalyst during polyphenylene sulfide producing. The method is characterized by: directly carrying out vacuum distillation for a polyphenylene sulfide polycondensation mother liquid until the polyphenylene sulfide polycondensation mother liquid is dried to recover a solvent of the NMP, wherein the NMP can be used in the next recycling production; adding water to the distilled residues, then heating to a temperature of 40 DEG C, carrying out stirring and completely dissolving the distilled residues, carrying out filtering and washing the filter residues, mixing the resulting filtrate and the washing solution, and analyzing lithium content in the mixed solution; adding the mixed solution to a phosphoric acid solution or a sodium aluminate solution, wherein the phosphoric acid solution or the sodium aluminate solution has a lithium reaction equivalent of 105-115%, then completely stirring to enable lithium phosphate or lithium aluminate to be precipitated completely, then carrying out steps of filtering, washing, drying for the lithium phosphate or the lithium aluminate to recover the lithium salt having a purity more than 97%. According to the present invention, the operation of the method is simple; the cost is low; the recovered NMP and the recovered lithium salt havehigh purities.
Owner:NANJING UNIV
Core-shell lithium manganate composite anode material as well as preparation method and application thereof
The invention discloses a preparation method and an application of a core-shell lithium manganate composite anode material, belonging to the technical field of the preparation of the electrochemical power supply materials. The lithium manganate composite anode material is formed into a core-shell structure composed of 80-99.0wt% of core active material and 0.1-20wt% of shell layer material, wherein the core active material refers to spherical lithium manganate expressed by nominal composition formula LiaMn2-xAyO4-zB delta, while the shell layer material refers to lithium aluminate LiAlO2. The invention also discloses the method for preparing the anode material and a secondary lithium ion battery comprising the anode material. The anode material obtained by the method obviously improves the cyclic service life of the lithium manganate anode material and is capable of meeting the requirements of the common secondary lithium ion battery and the common power lithium ion battery.
Owner:甘肃大象能源科技有限公司
Ternary cathode material for lithium ion battery and preparation method thereof
InactiveCN106532035AImprove cycle performanceEasy to useCell electrodesElectrical batteryPhysical chemistry
The invention belongs to the field of electrochemical technique, and particularly relates to a ternary cathode material for a lithium ion battery and a preparation method of the ternary cathode material. The cathode material uses high-nickel nickel-cobalt lithium manganate or nickel-cobalt lithium aluminate as a basal body, and low-nickel lithium manganate is coated outside the basal body. A low-nickel ternary material is coated on the surface of a high-nickel ternary material, so that the pH and the content of residual alkali on the surface of the cathode material are reduced, the cathode material has processing performance of the low-nickel ternary material and specific capacity of the high-nickel ternary material, and the cycle performance of the cathode material can also be improved through the reduction of the surface basicity of the cathode material; and the high-nickel ternary material can be easily used in domestic enterprises, so that the invested cost of battery enterprises for using the high-nickel ternary material is greatly reduced.
Owner:WUXI JEWEL POWER & MATERIALS
Ceramic material suitable for repair of a space vehicle component in a microgravity and vacuum environment, method of making same, and method of repairing a space vehicle component
A precursor of a ceramic adhesive suitable for use in a vacuum, thermal, and microgravity environment. The precursor of the ceramic adhesive includes a silicon-based, preceramic polymer and at least one ceramic powder selected from the group consisting of aluminum oxide, aluminum nitride, boron carbide, boron oxide, boron nitride, hafnium boride, hafnium carbide, hafnium oxide, lithium aluminate, molybdenum silicide, niobium carbide, niobium nitride, silicon boride, silicon carbide, silicon oxide, silicon nitride, tin oxide, tantalum boride, tantalum carbide, tantalum oxide, tantalum nitride, titanium boride, titanium carbide, titanium oxide, titanium nitride, yttrium oxide, zirconium, diboride, zirconium carbide, zirconium oxide, and zirconium silicate. Methods of forming the ceramic adhesive and of repairing a substrate in a vacuum and microgravity environment are also disclosed, as is a substrate repaired with the ceramic adhesive.
Owner:COI CERAMICS
Preparation method of fast ion conductor and conducting polymer dual-modified ternary cathode material for lithium-ion battery
ActiveCN107706390AImprove cycle performanceExcellent rate performanceCell electrodesSecondary cellsElectrical conductorConductive polymer
The invention discloses a preparation method of a fast ion conductor and conducting polymer dual-modified ternary cathode material for a lithium-ion battery. According to the material, a ternary cathode material for a lithium-ion battery is taken as a core, a fast ion conductor is taken as a first coating layer, a conducting polymer is taken as a second coating layer and the fast ion conductor isany one of lithium vanadate, lithium metaaluminate and lithium zirconate. The fast ion conductor and the ternary cathode material are firstly mixed evenly and ground; the ternary cathode material is coated with the fast ion conductor by using a high-temperature solid state method; the conducting polymer and the ternary cathode material coated with the fast ion conductor are mixed evenly and milled; and the ternary cathode material coated with the fast ion conductor is coated with the conducting polymer to finally obtain the fast ion conductor and conducting polymer dual-modified ternary cathode material for the lithium-ion battery. The fast ion conductor is combined with the conducting polymer to modify the ternary cathode material, so that the ternary cathode material has excellent cycleperformance and good rate capability.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Titanium composite, preparation method thereof and application thereof
ActiveCN101901905AImprove performanceEasy to preparePigmenting treatmentAlkali titanatesLithium chlorideHigh rate
Owner:BYD CO LTD
Lithium silicate-coated Ni-Co lithium aluminate positive electrode material and preparation method thereof
InactiveCN107910539AImprove cycle stabilityImprove high rate discharge performanceCell electrodesSecondary cellsLithium aluminateSilicon dioxide
Owner:CENT SOUTH UNIV
Recycling method of positive electrode piece of lithium ion battery
InactiveCN105576314AEfficient removalImprove structural propertiesSolid waste disposalTransportation and packagingManganeseSodium-ion battery
The invention discloses a recycling method of a positive electrode piece of a lithium ion battery, aiming at solving the problem of recycling of a nickel cobalt lithium manganite (nickel cobalt lithium aluminate) positive electrode piece and a lithium cobaltate positive electrode piece generated in a production process of the lithium ion battery. According to the technical scheme disclosed by the invention, the recycling method comprises the following steps: 1, crushing the electrode pieces by classes; 2, immersing with an organic solvent; 3, carrying out stirring treatment; 4, filtering with a sieve net; 5, carrying out centrifugal separation; 6, immersing with an alkaline solution; 7, carrying out the centrifugal separation again; 8, drying and removing iron; 9, carrying out ICP (Inductively Coupled Plasma) analysis; and 10, calcining the materials. The recycling method disclosed by the invention can be used for effectively recycling waste materials of the positive electrode pieces of the waste lithium ion batteries, so that the cost is saved; and by immersing with the alkaline solution and carrying out a plurality of times of separation and washing, impurities, such as metal aluminum, in powder grains can be effectively removed. With the adoption of the recycling method, a positive electrode material and an aluminum foil can be completely separated, and the positive electrode material keeps a relatively good structure and electrochemical properties; and the synthesis of a precursor is not needed and the adding amount of a lithium salt is relatively less.
Owner:SHANDONG GOLDENCELL ELECTRONICS TECH
Nickel-cobalt lithium aluminate composite positive electrode material and preparation method thereof
InactiveCN104600290AImprove cycle performanceImproved magnification performanceCell electrodesSecondary cellsPower batteryLithium aluminate
The invention relates to a nickel-cobalt lithium aluminate composite positive electrode material and a preparation method thereof. The nickel-cobalt lithium aluminate composite positive electrode material is prepared from the following components: (a) a nickel-cobalt lithium aluminate positive electrode material, (b) a metal oxide, and (c) a carbon source; the metal oxide and the carbon are orderly compounded on the surface of the nickel-cobalt lithium aluminate positive electrode material to obtain the nickel-cobalt lithium aluminate composite positive electrode material. According to the composite material, the cyclic performance and the rate performance of the positive electrode material are improved obviously, and the composite material can be applied to power batteries.
Owner:BTR NEW MATERIAL GRP CO LTD
Lithium ion battery negative pole material capable of improving safety performance and preparation method thereof
The invention relates to a preparation method of a lithium ion battery negative pole material capable of improving the safety performance. The method comprises the following steps of: (1) slurry configuration, (2) coating, and (3) roasting and drying. According to the invention, as a negative pole tab is coated with a lithium metaaluminate film, which not only has a good electronic insulation effect, but also can effectively obstruct battery short circuit after a battery diaphragm PP (Polypropylene) or PE (polyethylene) is punctured, and at the same time the lithium metaaluminate has good ionic conductivity, thus increasing the transmission velocity of the lithium ion battery, and reducing the probability that dendritic crystal generated by lithium separation of the lithium ion battery pierces the diaphragm. The composite negative pole tab prepared by the method plays a very important role in improving high-capacity lithium ion batteries.
Owner:JIANGSU LENENG BATTERY INC
Catalyst for the combustion of diesel soot, methods for making the catalyst and methods of using the catalyst
A catalyst composition is disclosed for the reduction of soot and undesirable gaseous emissions from engine exhaust, particularly exhaust from diesel engines. The catalyst contains an alkali metal catalytic metal oxide, preferably lithium platinum oxide, in which the catalytic metal is atomically isolated. For improved performance in a diesel particulate filter, the alkali catalytic metal oxide is uniformly dispersed on an alkali metal aluminate such as lithium aluminate. Also disclosed is the catalytic device comprising this catalyst.
Owner:UMICORE AG & CO KG
Lithium ion battery positive electrode material and preparation method thereof
ActiveCN107681128AReduce residual alkali contentPromote circulationCell electrodesSecondary cellsSpherical shapedSingle crystal
The present invention provides a lithium ion battery positive electrode material. The chemical formula of the material is Li<1+n>Ni<0.8+x>Co<0.2-x-y>Al<y>M<z>O<2>, wherein x+y+z<0.2, 0<=x<0.2, 0.01<=y<=0.05, 0.01<=z<=0.05, and -0.1<=n<=0.1; and the M is one kind or several kinds of cobalt, aluminum, magnesium, titanium, zirconium and boron. The lithium ion battery positive electrode material is mixed by single-crystal-shaped nickel cobalt lithium aluminate particles and spherical-shaped nickel cobalt lithium aluminate particles, the median diameter of the spherical-shaped nickel cobalt lithiumaluminate particles is 8-15 [mu]m, and the median diameter of the single-crystal-shaped nickel cobalt lithium aluminate particles is 0.5-6 [mu]m. The present invention also provides a preparation method of the lithium ion battery positive electrode material.
Owner:PULEAD TECH IND
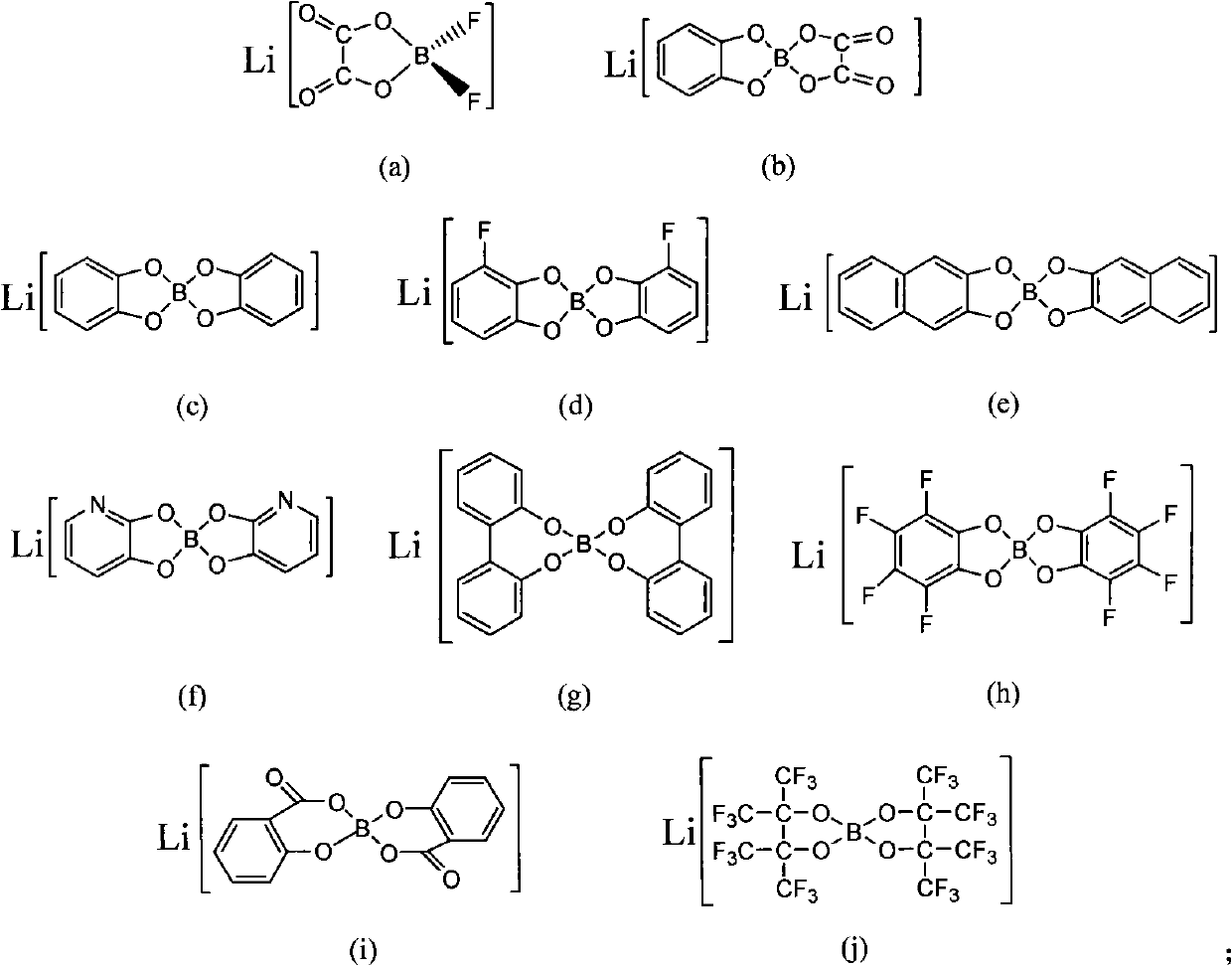
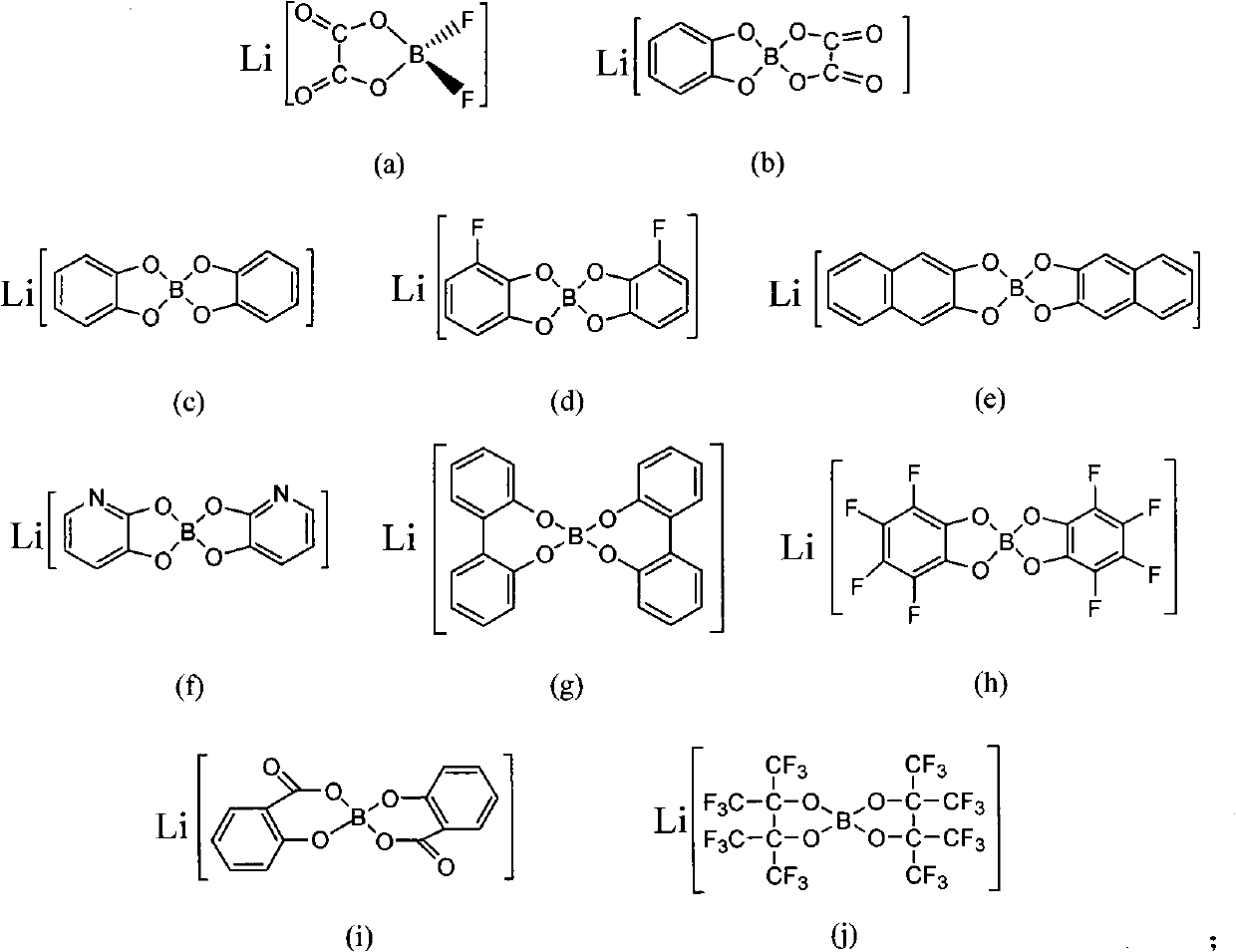
Ionic-liquid-base polymer electrolyte
An ionic liquid material, which is formed by the reaction of organic lithium borates or lithium aluminates and organic compounds containing amido functional groups, is used as a base and compounded with polymer materials to obtain the all-solid-state or gel-state polymer electrolyte material. The polymer electrolyte material has the advantages of favorable thermostability and favorable electrochemical performance. The addition reaction is carried out by regulating the composition of the ionic liquid and the proportion of ionic liquid to polymers and adding a right amount of plasticizer; or the conducting salts and the polymer materials containing amido functional groups are compounded to directly prepare the gel-state or all-solid-state polymer electrolyte with favorable performance. The polymer electrolyte has wide application prospects in the fields of chemical energy sources and especially new electrochemical energy storage systems aiming at high power, high energy density and high security requirement.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
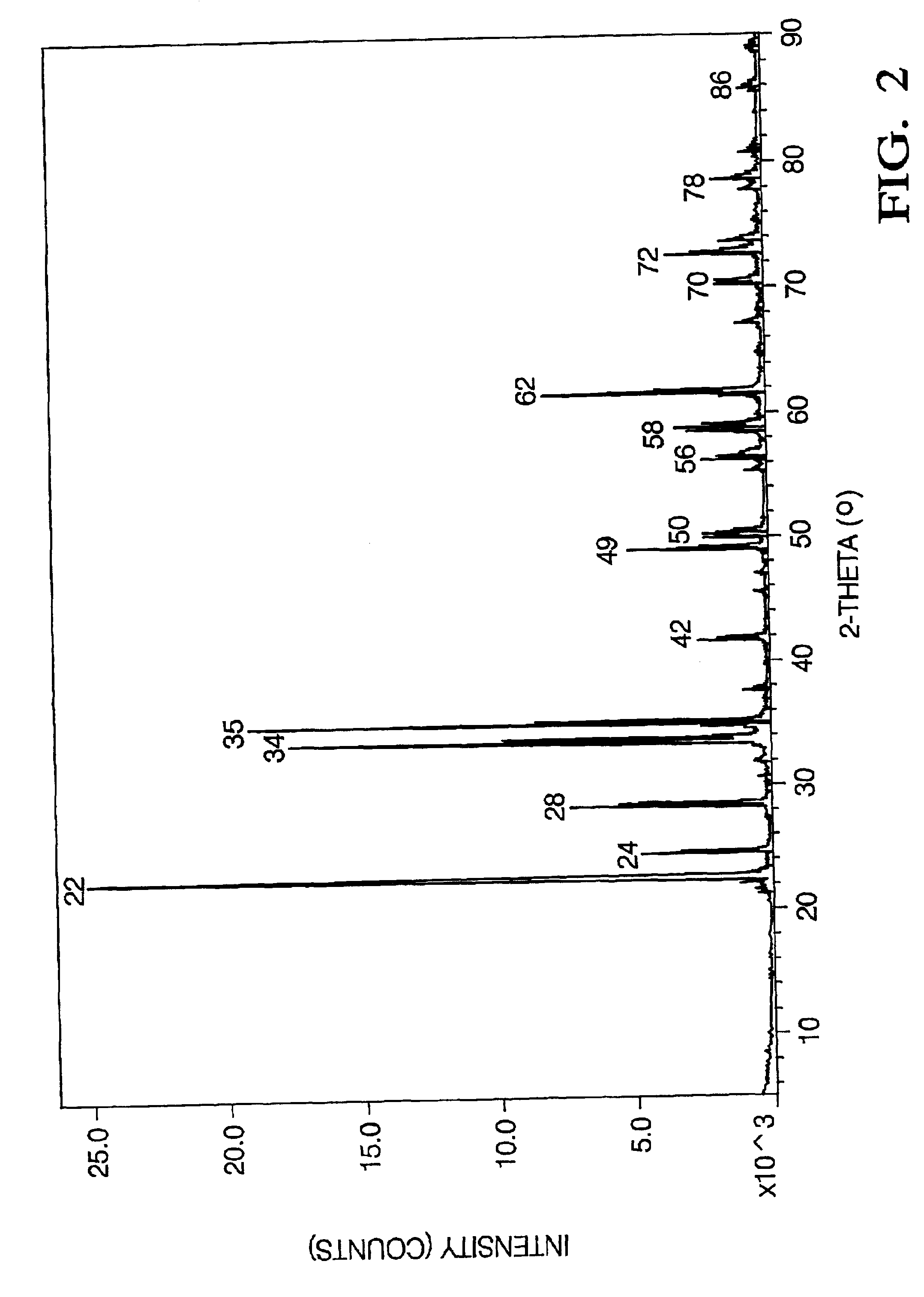
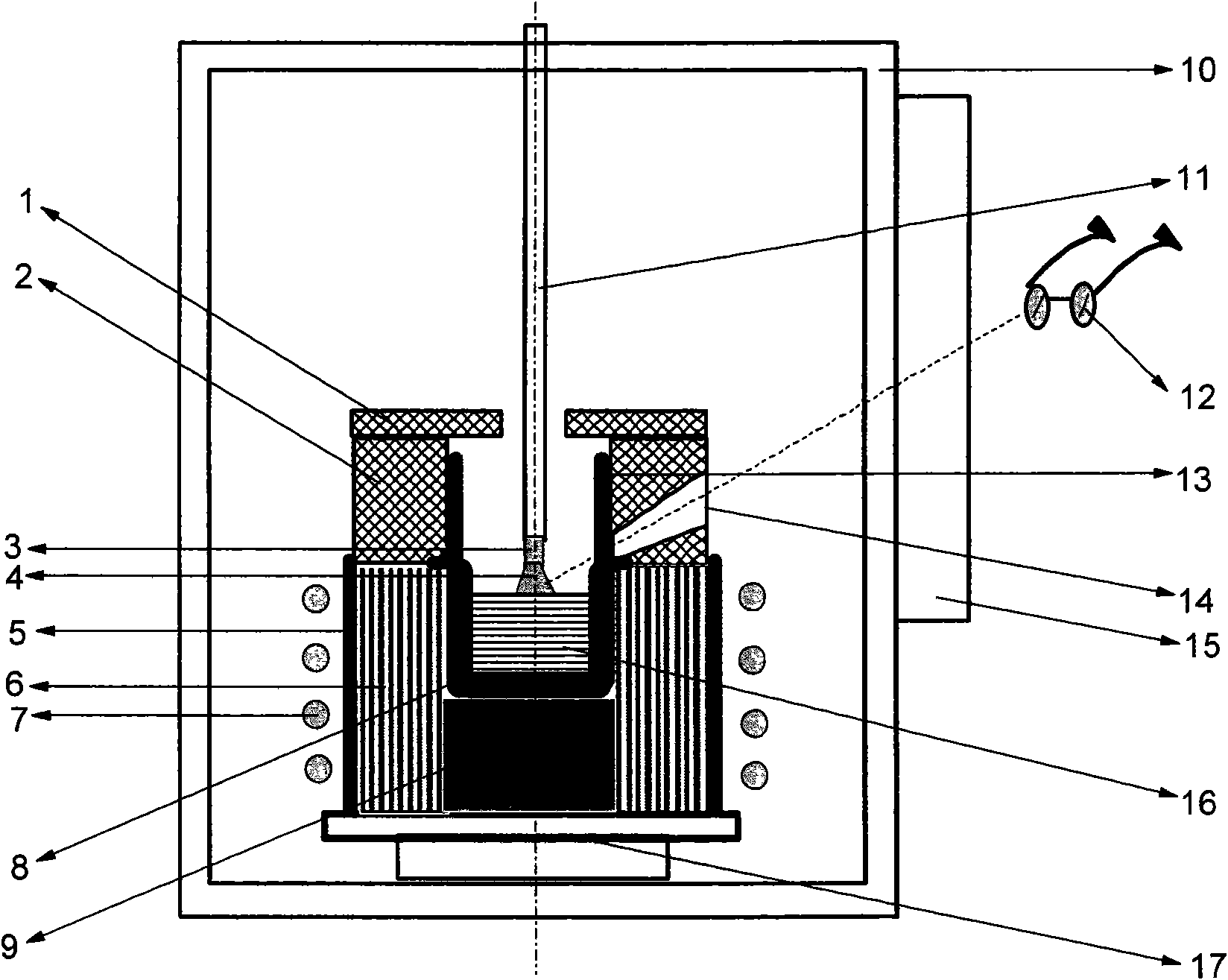
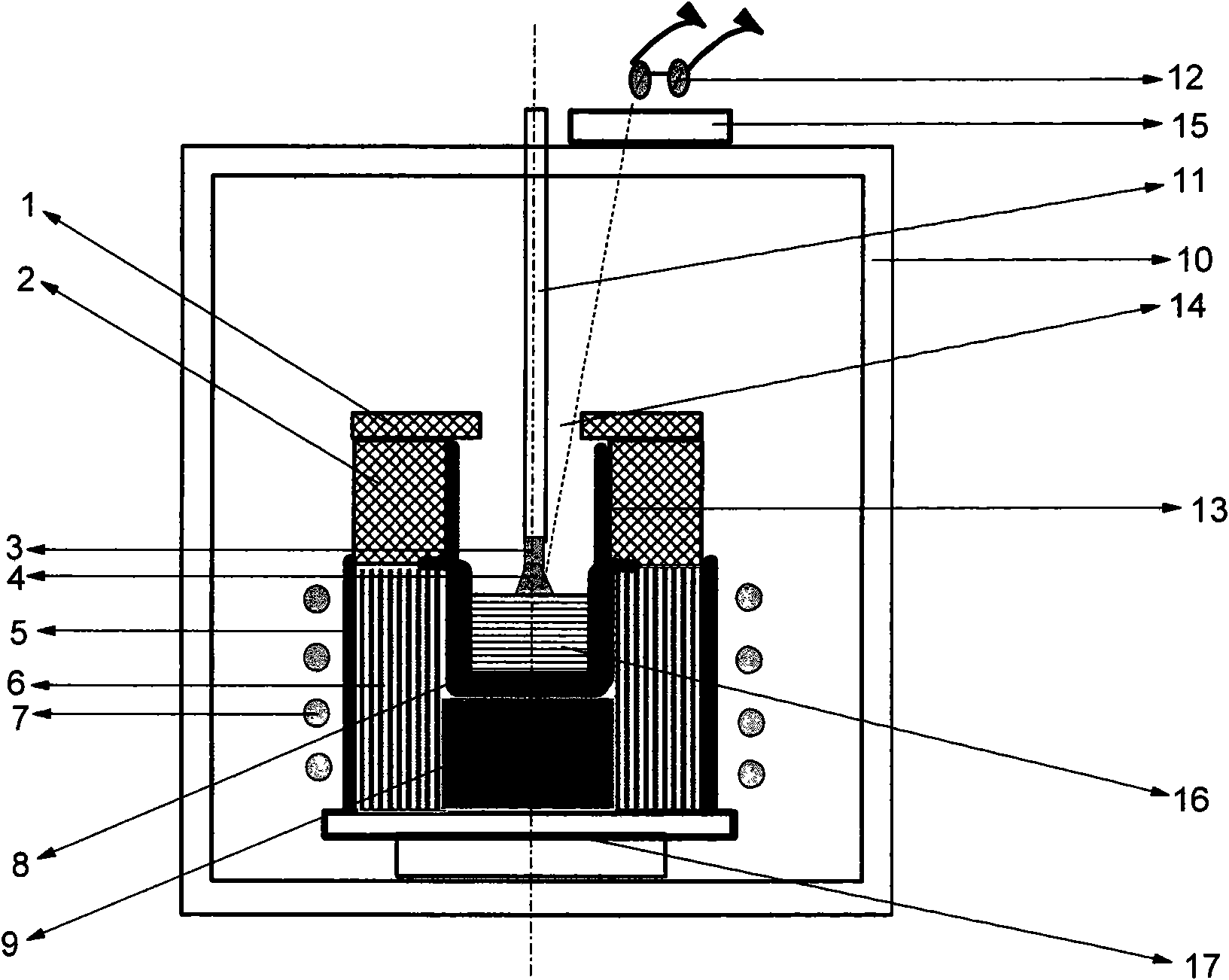
Method for lifting crystal growth by induction heating in reducing atmosphere
A method for lifting crystal growth by induction heating in reducing atmosphere is characterized by placing a tungsten crucible or a molybdenum crucible on a hard graphite felt; wrapping a plurality of layers of soft graphite felt around the hard graphite felt and the crucible; sleeving the wrapped hard graphite felt and the crucible in a quartz cylinder; wholly putting the quartz cylinder in an induction coil inside a crystal-lifting furnace, especially on a tray at the bottom of hearth; charging materials to the crucible; arranging and covering a hard graphite felt insulation cover or a zirconia insulation cover above the crucible; closing the door of the furnace and carrying out vacuumization; injecting shielding gas till positive pressure is reached; carrying out crystal growth by using a conventional method; and carrying out anneal in the atmosphere of air, oxygen or other atmosphere after the crystal growth is finished. The method has the advantages of utilizing the graphite felt to provide the reducing atmosphere so as to be capable of adopting the low-cost tungsten crucible or molybdenum crucible and realize the growth of sapphire and lithium aluminate and other crystals with low cost, thus having better universality, requiring simple equipments and especially being suitable for production of large scale.
Owner:SHANGHAI INST OF OPTICS & FINE MECHANICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com