Ionic-liquid-base polymer electrolyte
An ionic liquid and polymer technology, applied in circuits, electrical components, secondary batteries, etc., can solve problems such as high freezing point, potential safety hazards, and easy decomposition and deterioration of electrolytes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
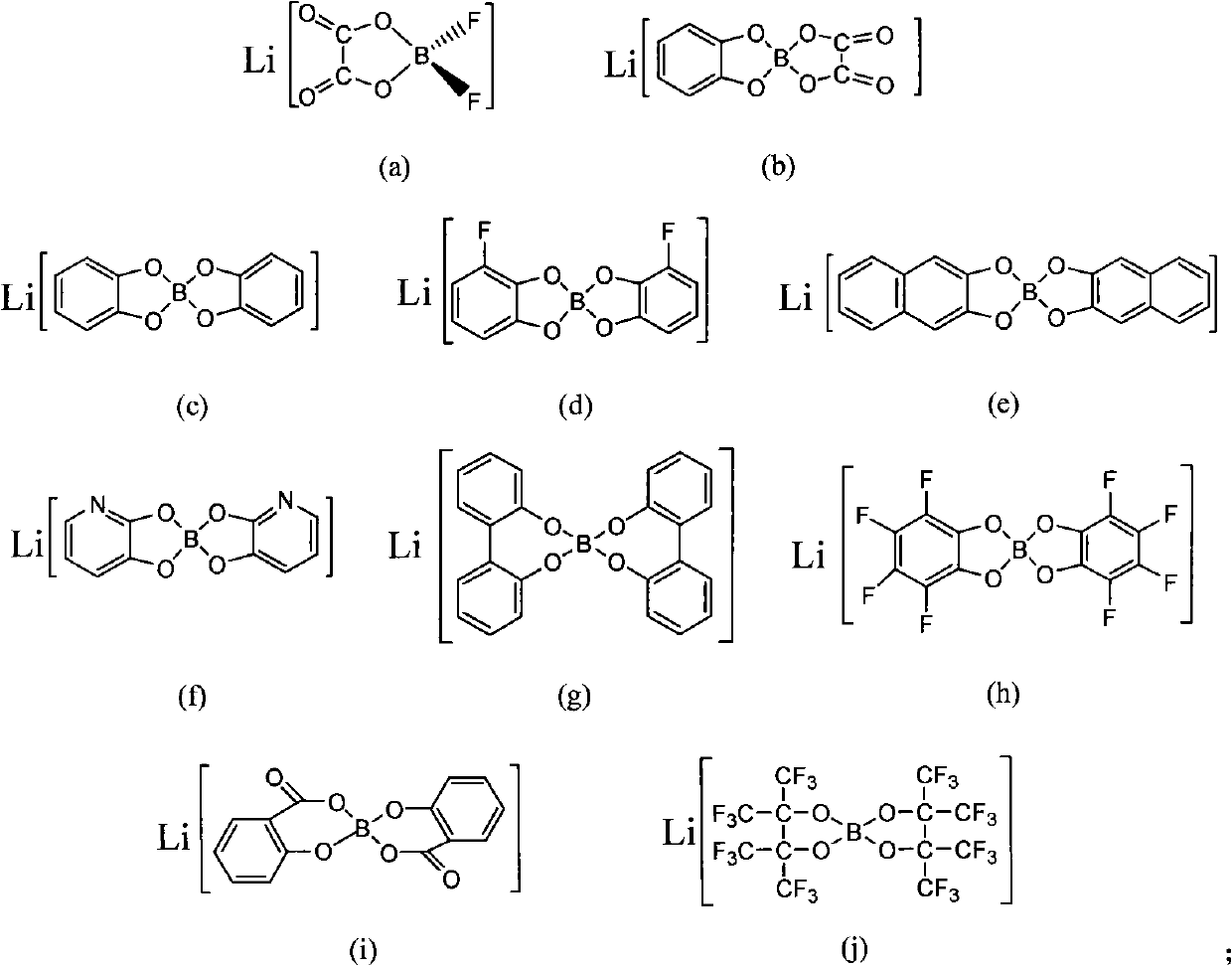
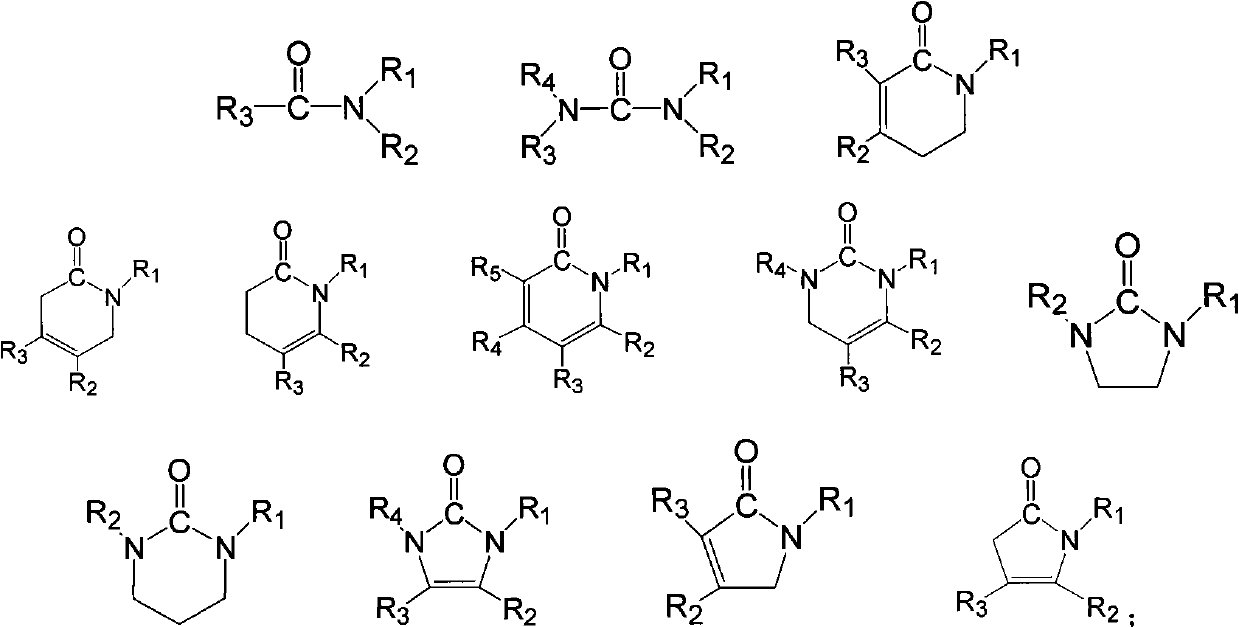
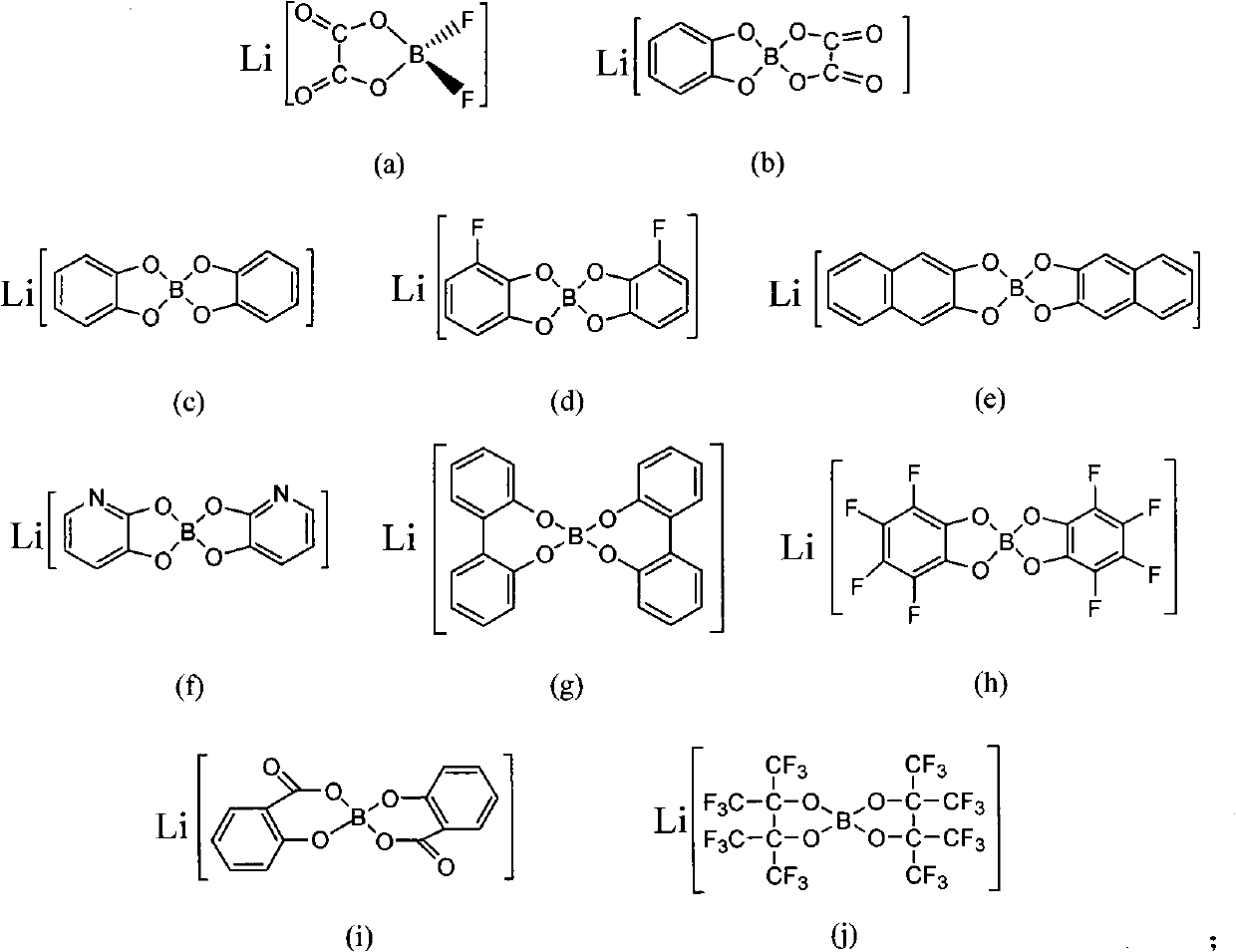
[0020] After drying organic borate lithium (a) and urea, put them into a glove box, weigh 10 g and 20 g of samples respectively, mix them in a weighing bottle, and stir fully at room temperature to form a homogeneous liquid to obtain an ionic liquid. The melting point was -25°C measured by DSC2010 differential scanning calorimeter; the conductivity (25°C) was measured as 1.6mS / cm by AC impedance method using CHI660a electrochemical workstation.
[0021]Based on the good electrochemical and thermal properties of the above-mentioned ionic liquid, it is combined with a porous polyoxyethylene membrane, and the conductivity at room temperature can reach 0.4mS / cm at 25°C; the conductivity of the sample is measured at different temperatures, and the conductivity varies with temperature. The curvilinear relationship fits the Arrhenius equation.
Embodiment 2
[0023] Dry organic borate lithium (a) and acetamide and put it in a glove box, weigh 10g and 20g samples respectively, mix them in a weighing bottle, and stir them fully at room temperature to form a homogeneous liquid to obtain an ionic liquid. The conductivity (25° C.) was measured as 2.7 mS / cm by AC impedance method using CHI660a electrochemical workstation.
[0024] Based on the good electrochemical and thermal properties of the above-mentioned ionic liquid, when it is combined with a porous polyoxyethylene membrane, the conductivity at room temperature can reach 0.9mS / cm; the conductivity of the sample is measured at different temperatures, and the conductivity varies with temperature. The curvilinear relationship fits the Arrhenius equation.
Embodiment 3
[0026] Based on the good electrochemical and thermal properties of the ionic liquid prepared in Example 2, it is compounded with a polyacrylonitrile membrane, and the conductivity at room temperature can reach 0.85mS / cm at 25°C; the conductivity of the sample is measured at different temperatures, and the conductivity varies with The relationship of the temperature change curve conforms to the Arrhenius equation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com