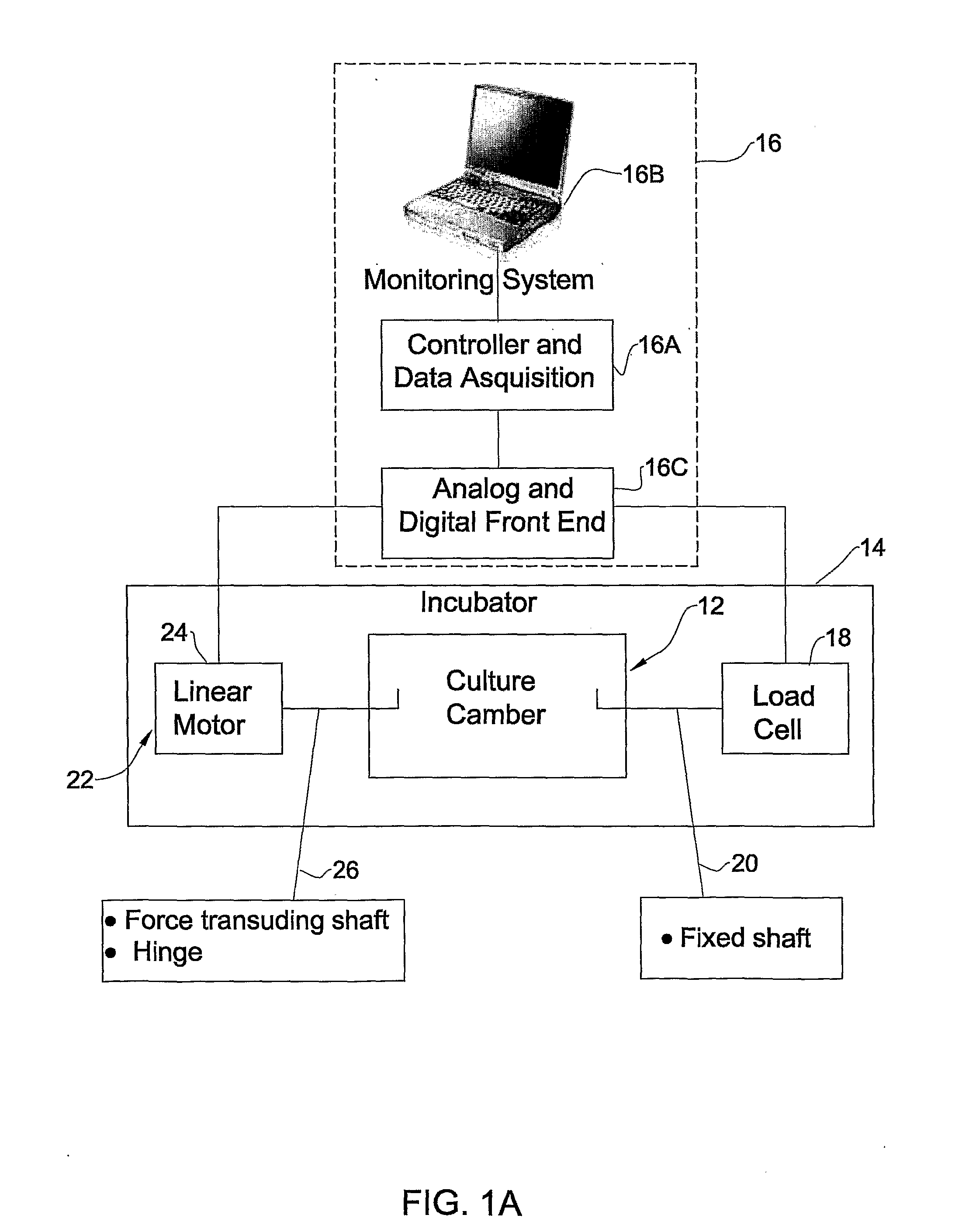
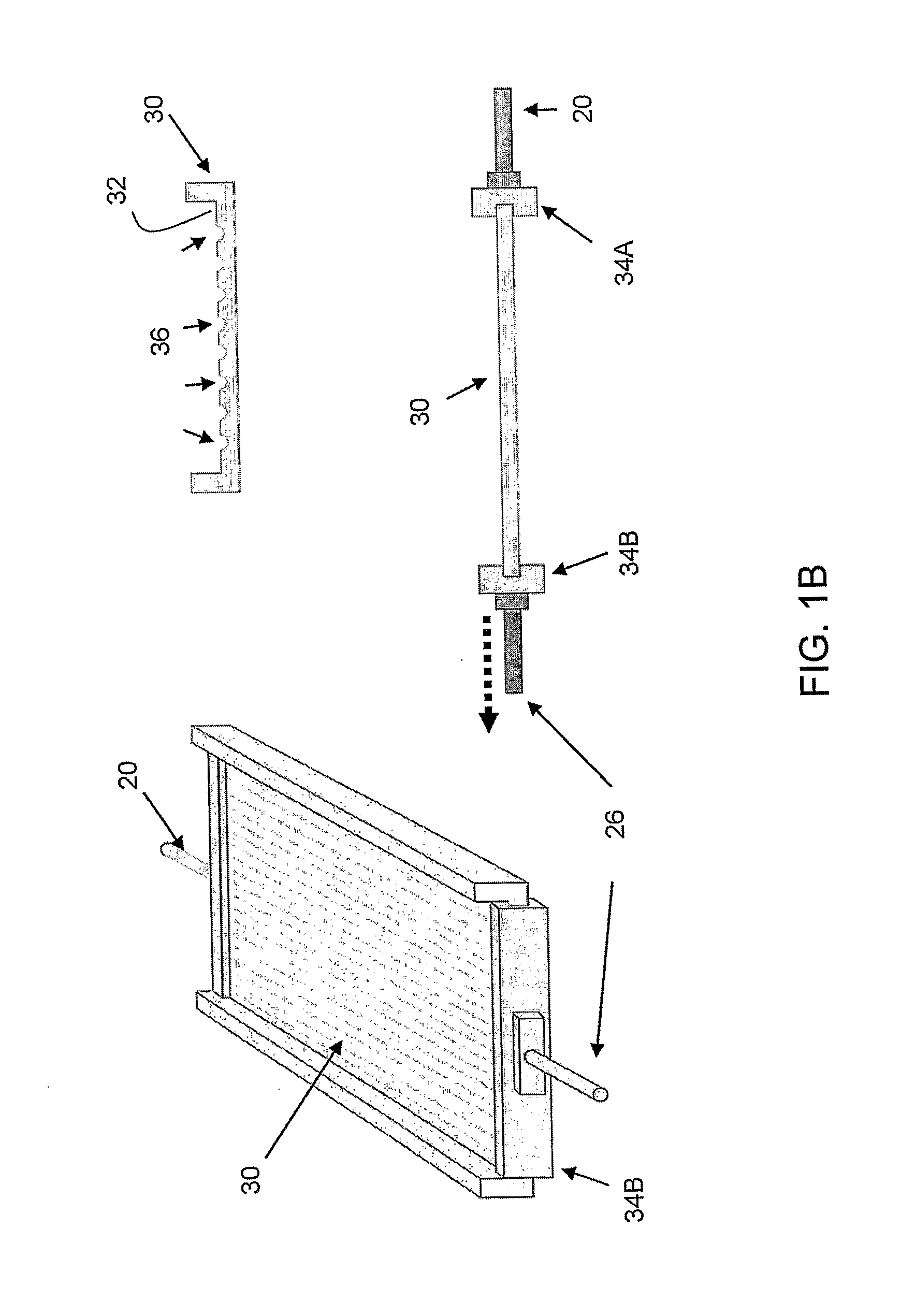
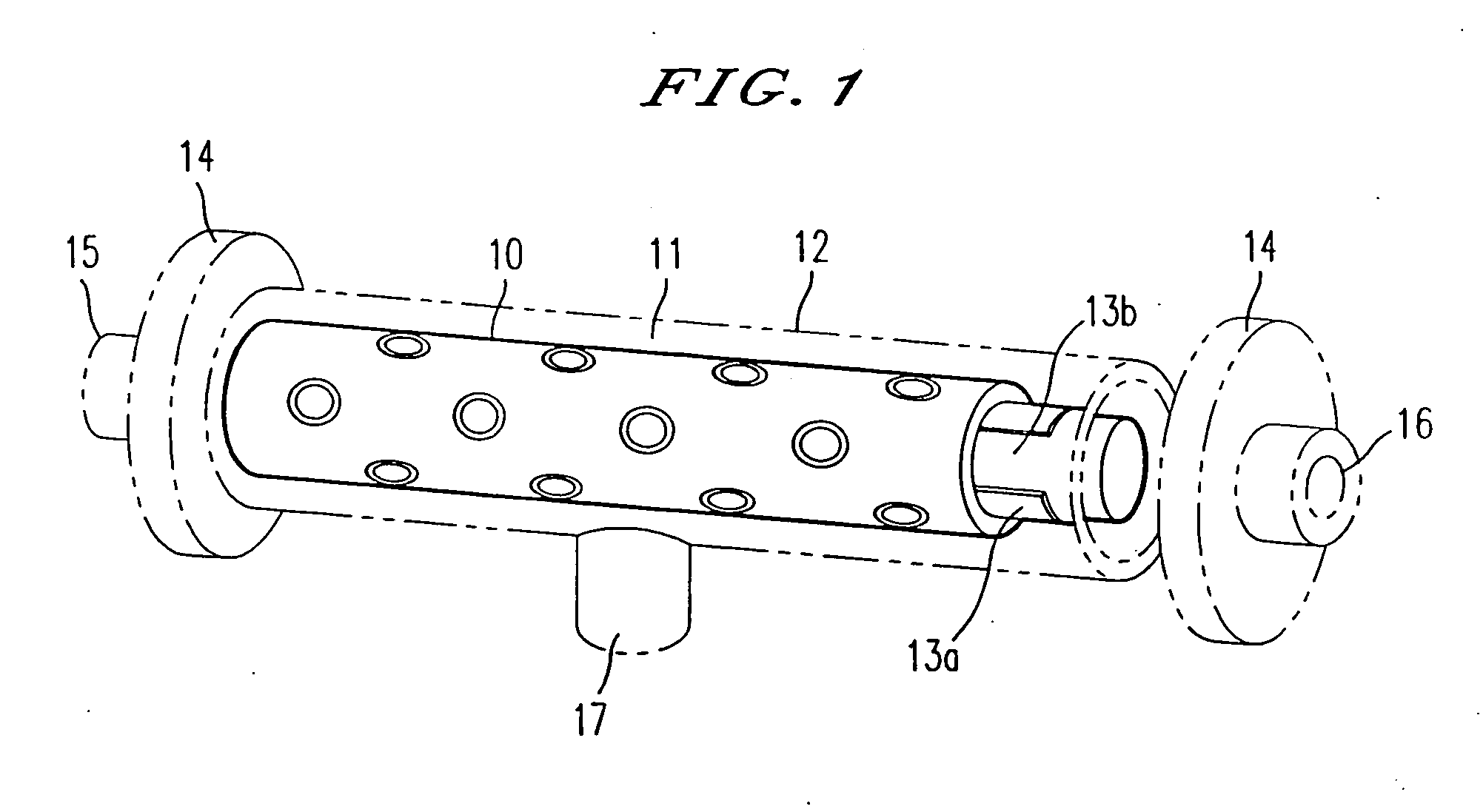
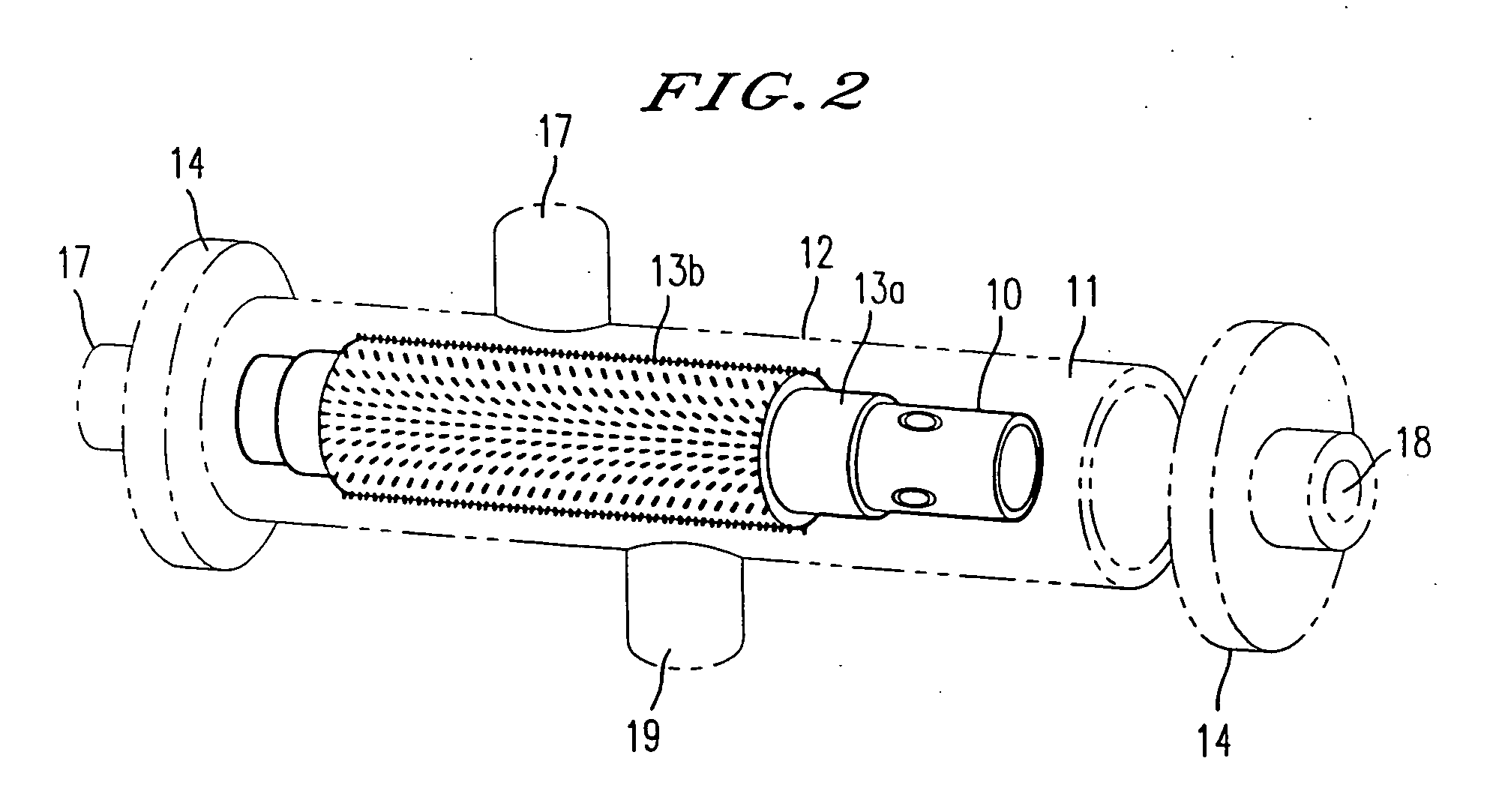
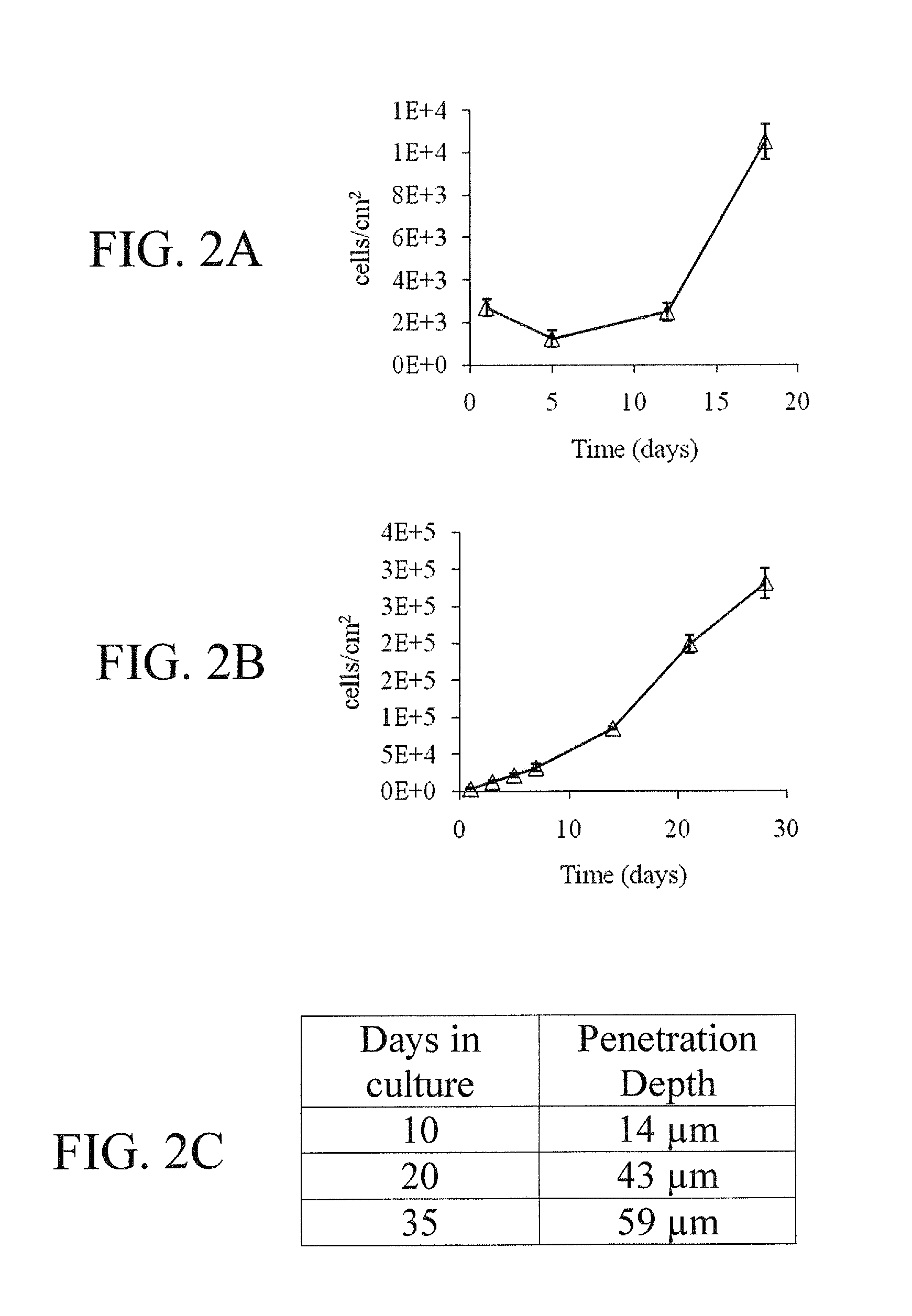
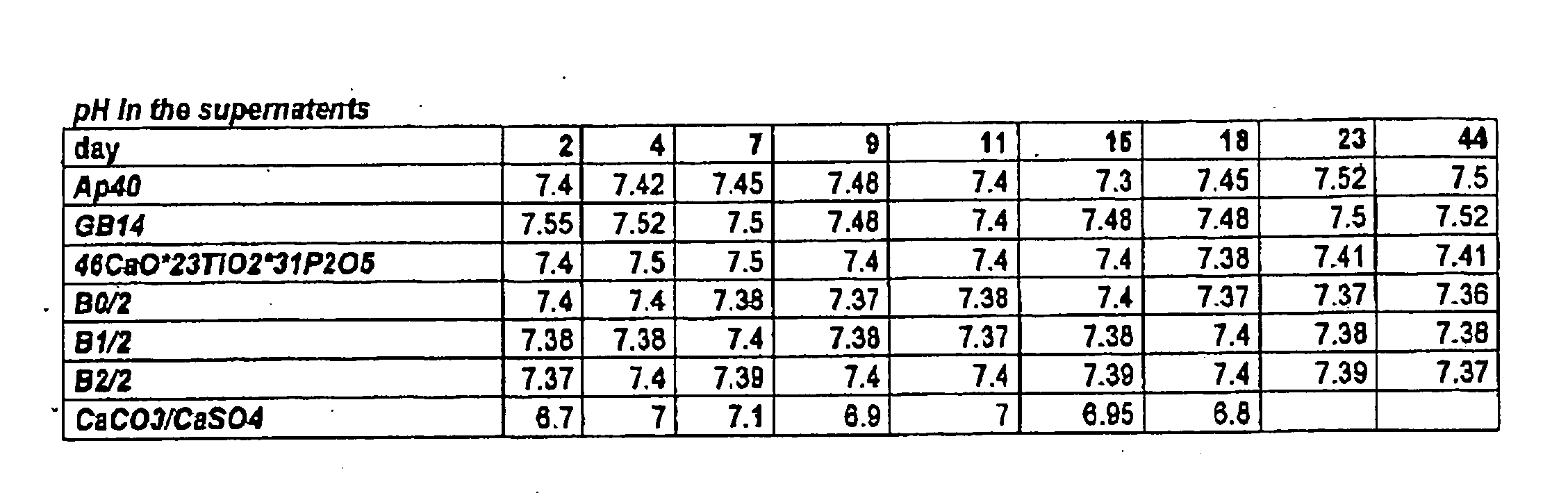
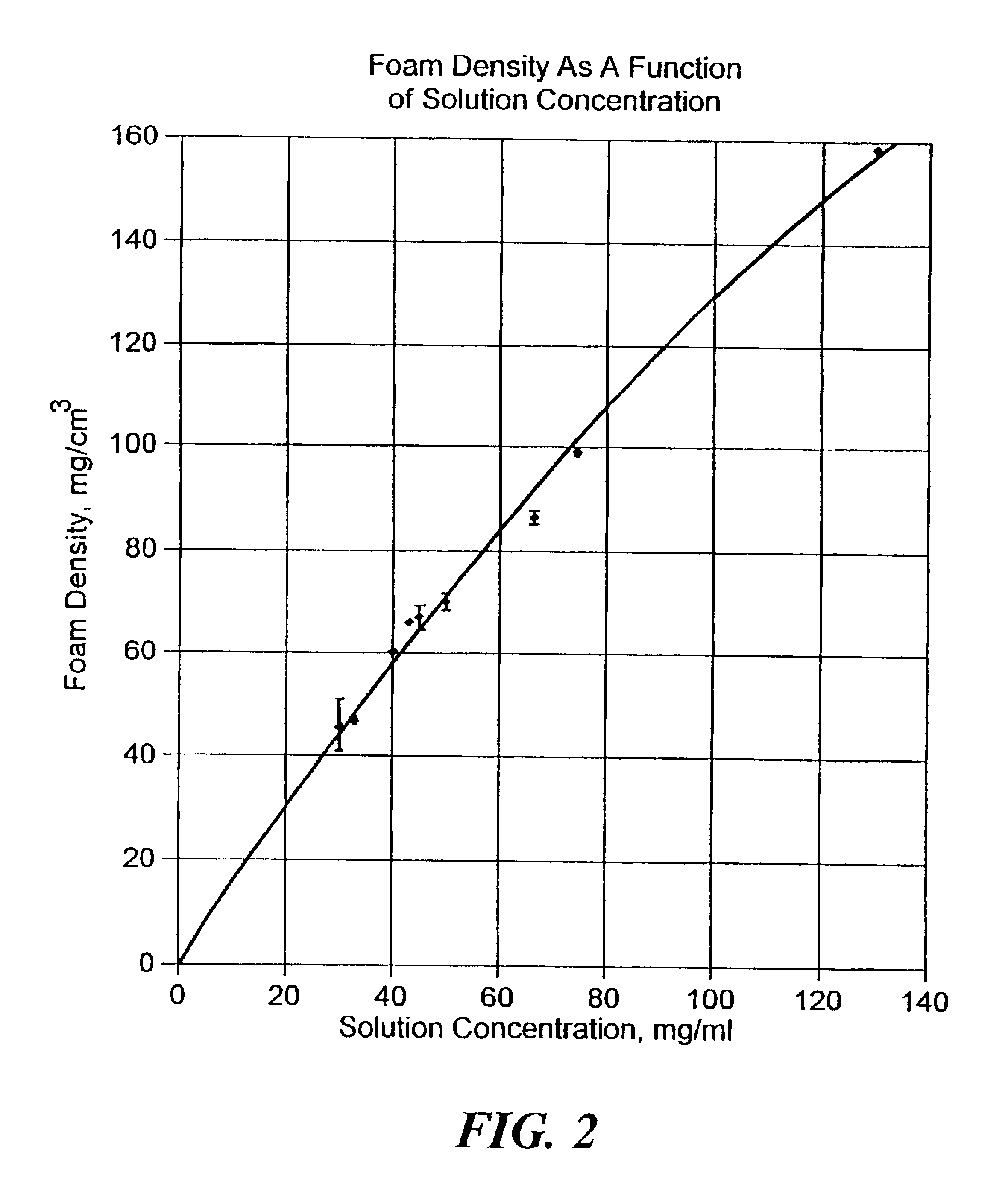
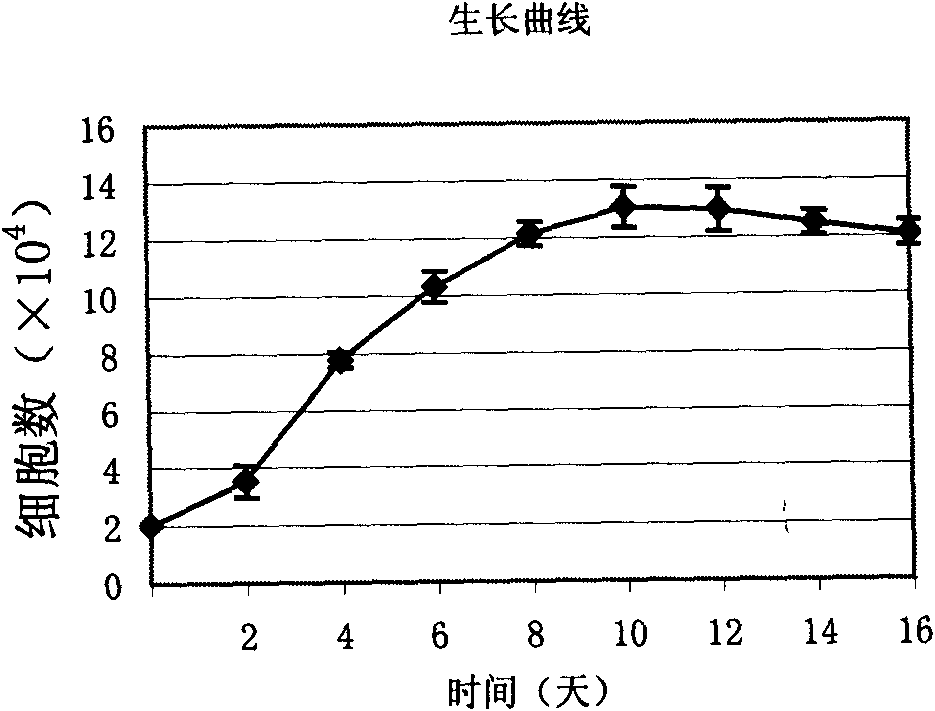
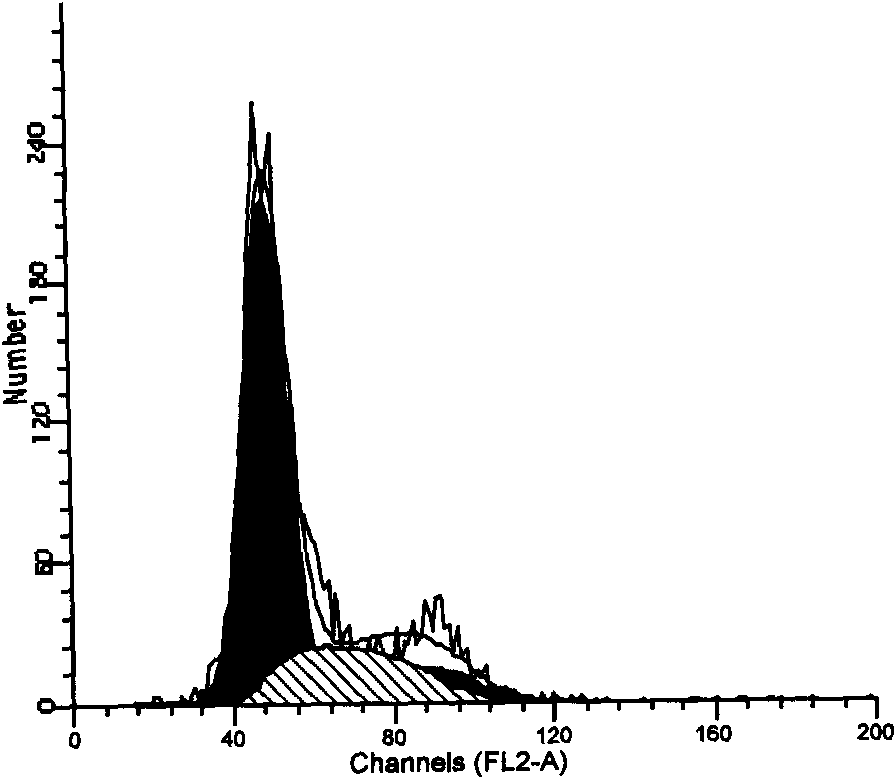
Patents
Literature
337 results about "Cell seeding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Seeding simply means to spread a defined amount (volume or cell number) of a cell suspension into a new flask or onto a plate etc. When you work with adherent cell cultures you have to trypsinize them first to get a cell suspension.
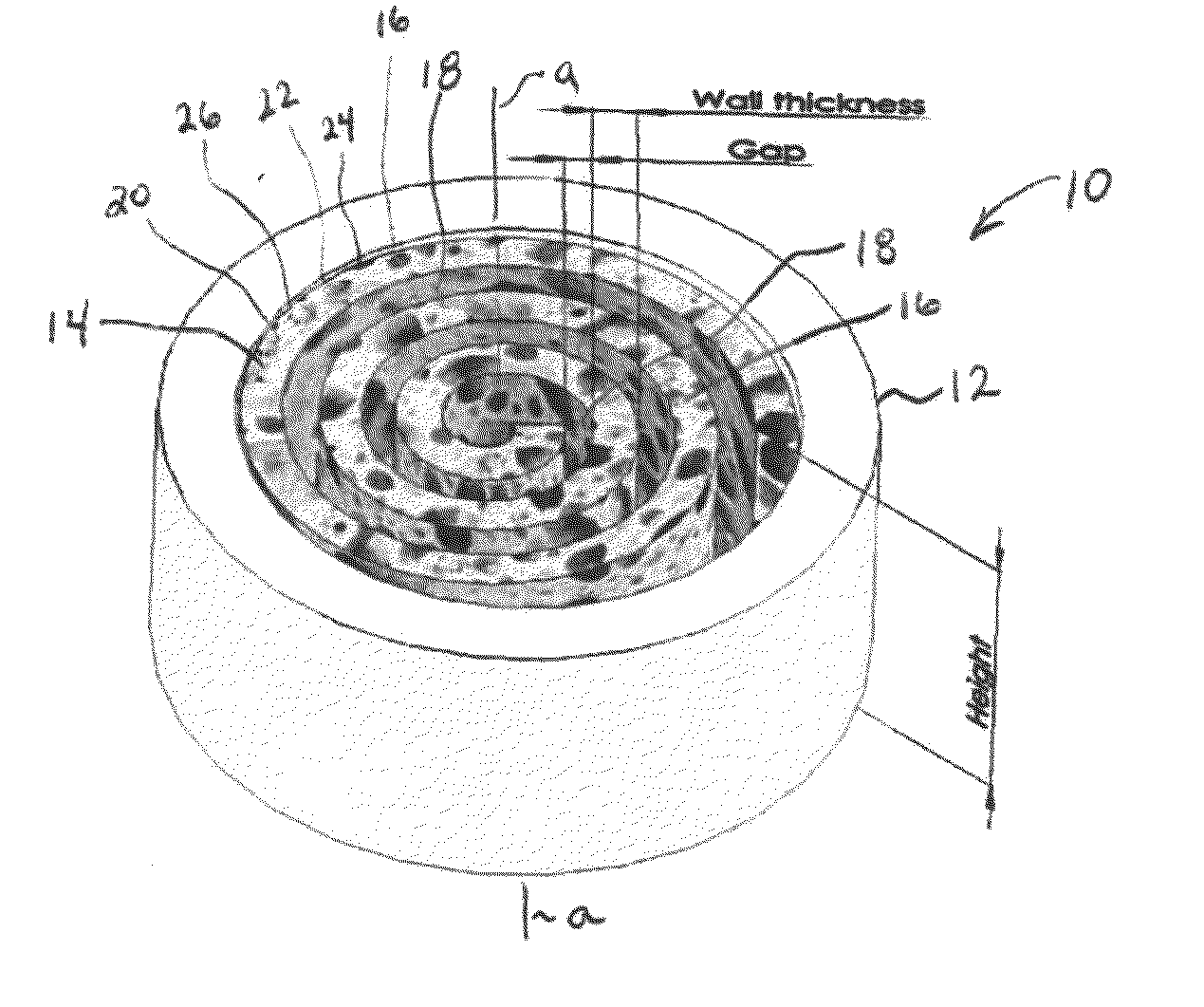
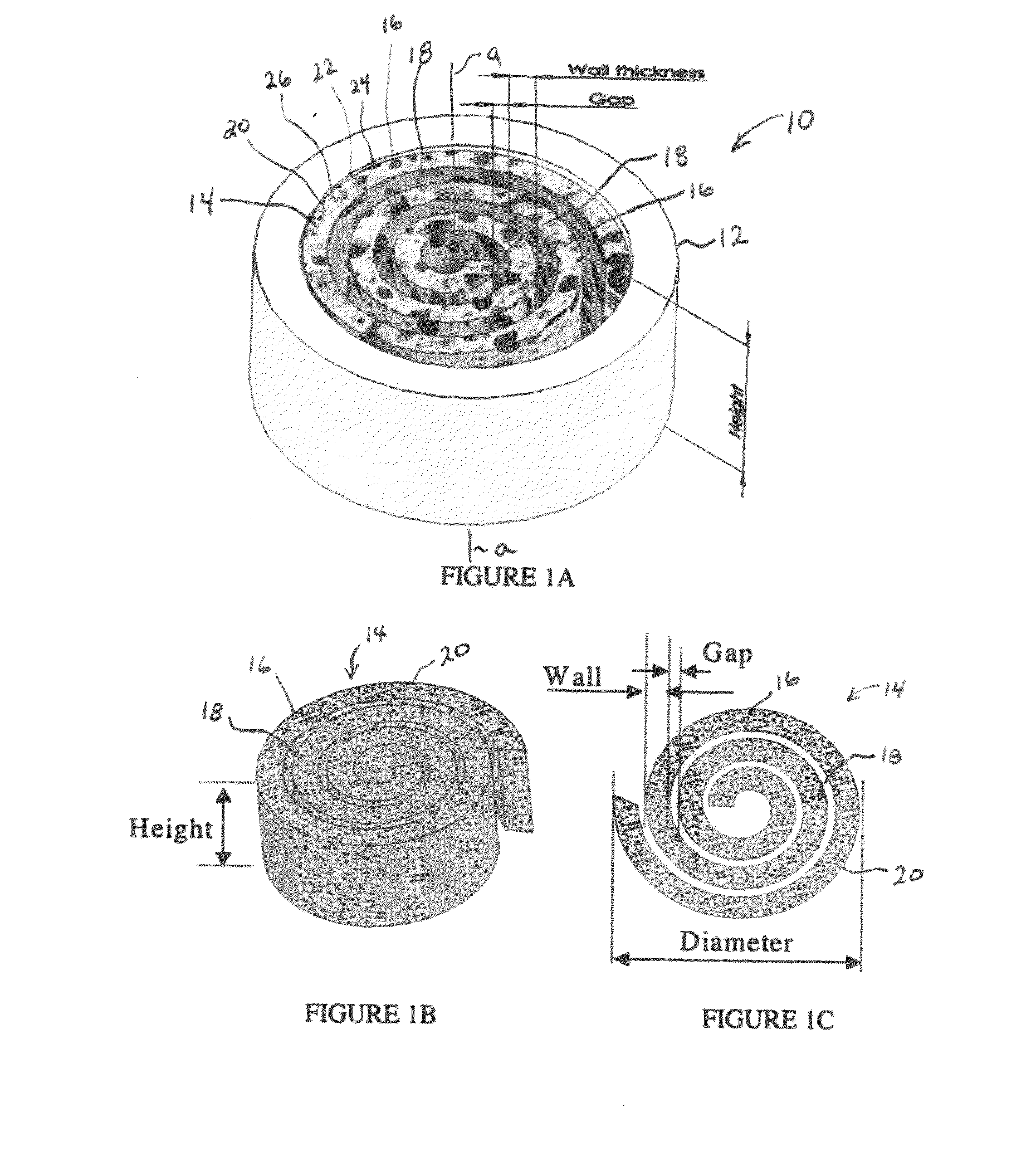
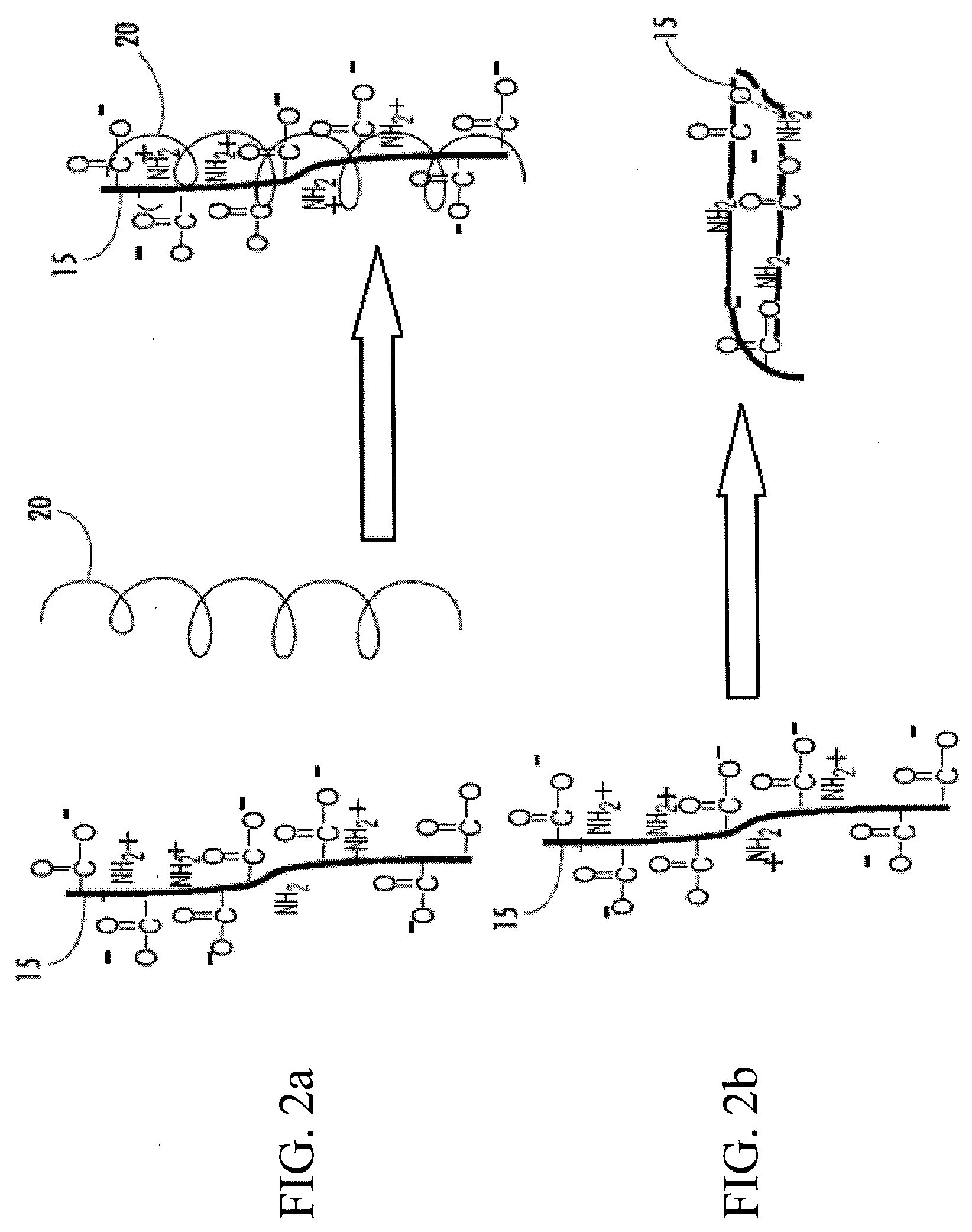
Synergetic functionalized spiral-in-tubular bone scaffolds
InactiveUS20100310623A1Increase the number ofIncrease alkaline phosphatase activityPeptide/protein ingredientsBone implantPorous sheetCell seeding
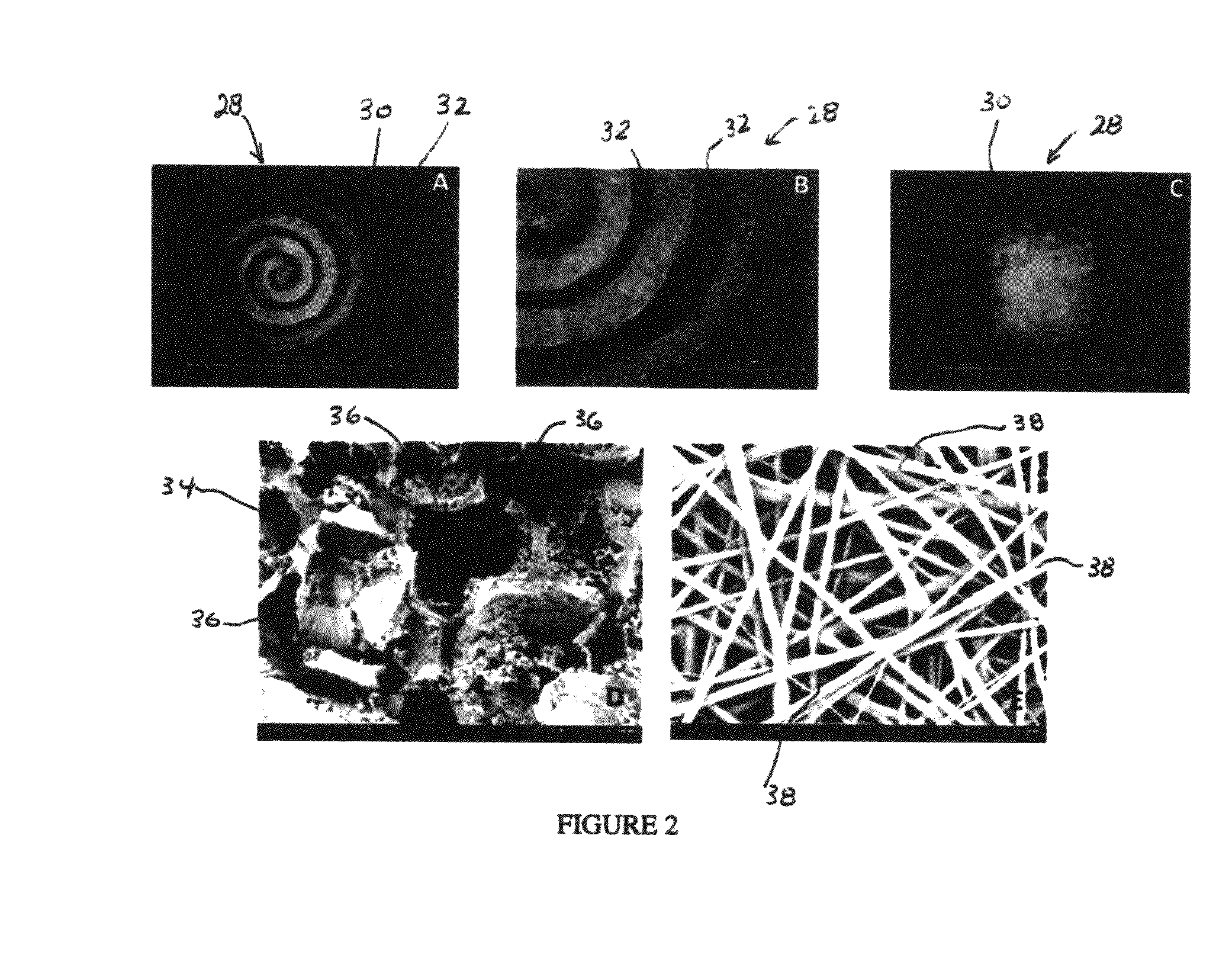
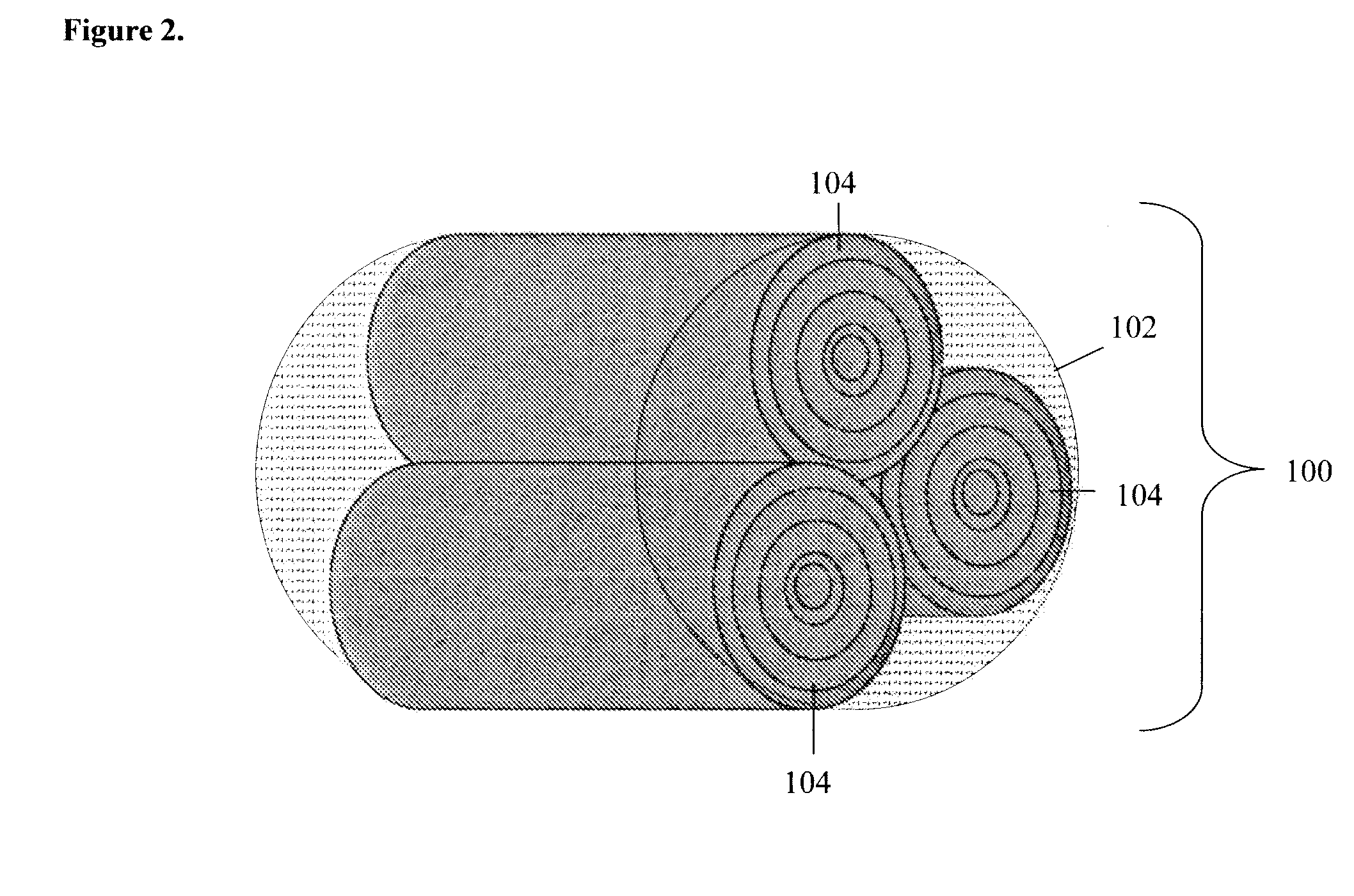
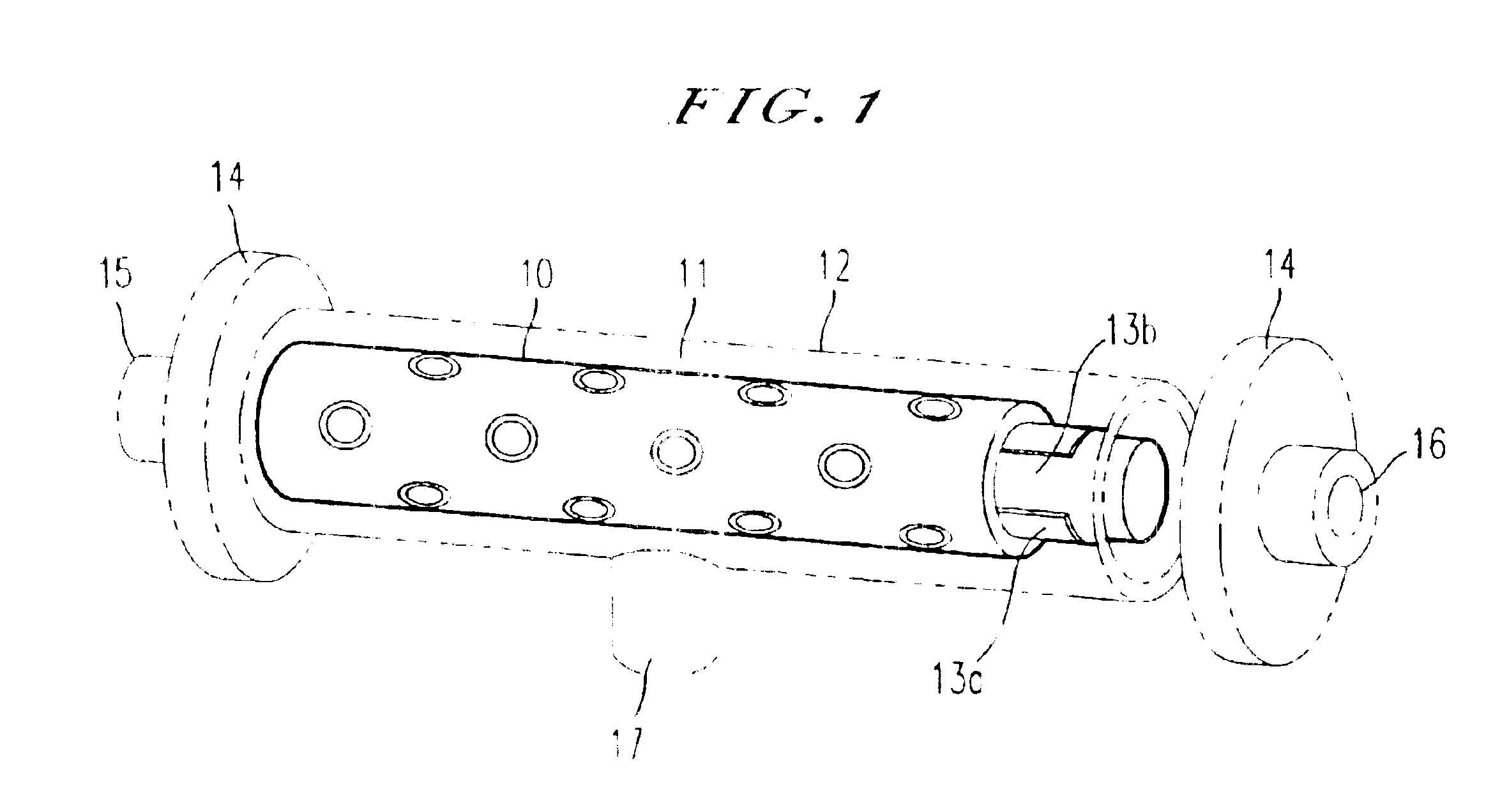
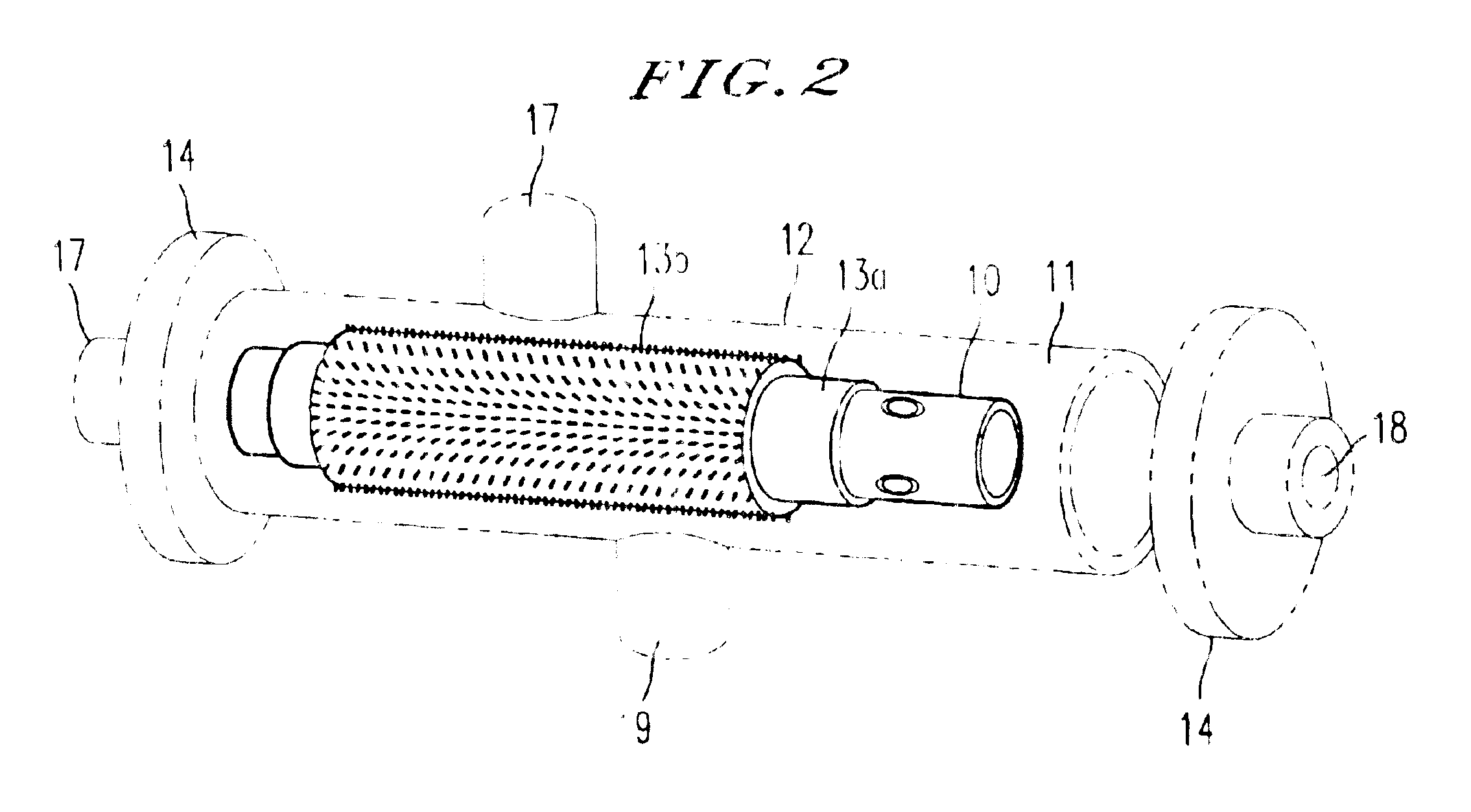
An integrated scaffold for bone tissue engineering has a tubular outer shell and a spiral scaffold made of a porous sheet. The spiral scaffold is formed such that the porous sheet defines a series of spiral coils with gaps of controlled width between the coils to provide an open geometry for enhanced cell growth. The spiral scaffold resides within the bore of the shell and is integrated with the shell to fix the geometry of the spiral scaffold. Nanofibers may be deposited on the porous sheet to enhance cell penetration into the spiral scaffold. The spiral scaffold may have alternating layers of polymer and ceramic on the porous sheet that have been built up using a layer-by-layer method. The spiral scaffold may be seeded with cells by growing a cell sheet and placing the cell sheet on the porous sheet before it is rolled.
Owner:UNIV OF CONNECTICUT
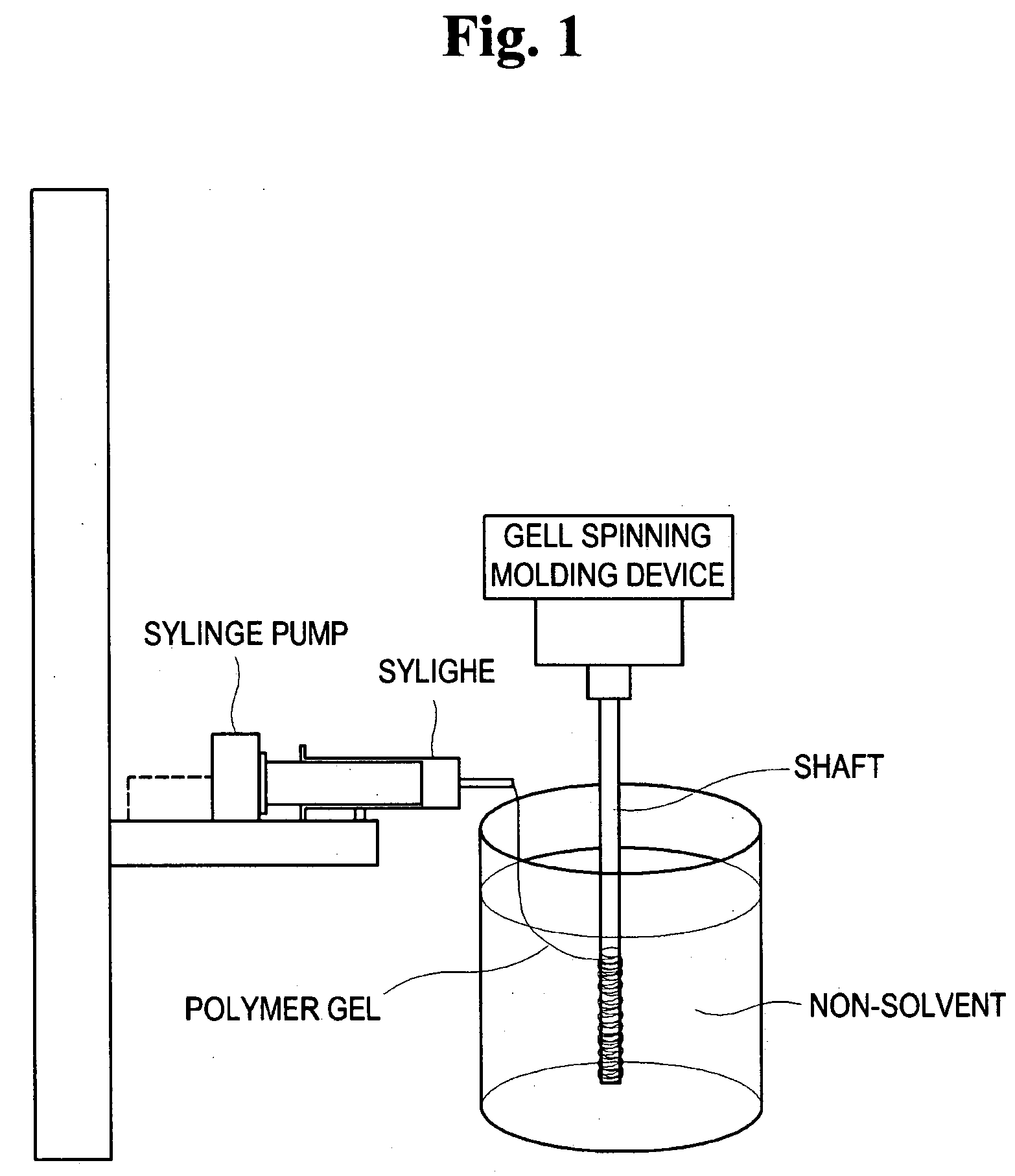
Method for preparing porous polymer scaffold for tissue engineering using gel spinning molding technique
InactiveUS20070009570A1Uniform pore sizeImprove interconnectivitySuture equipmentsCeramic shaping apparatusPolymer scienceSpinning
The present invention relates to a method of preparing a porous polymer scaffold for tissue engineering using a gel spinning molding technique. The method of the present invention can prepare a porous polymer scaffold having a uniform pore size, high interconnectivity between pores and mechanical strength, as well as high cell seeding and proliferation efficiencies, which can be effectively used in tissue engineering applications. Further, the method of the present invention can easily mold a porous polymer scaffold in various types such as a tube type favorable for regeneration of blood vessels, esophagus, nerves and the like, as well as a sheet type favorable for regeneration of skins, muscles and the like, by regulating the shape and size of a template shaft.
Owner:KOREA INST OF SCI & TECH
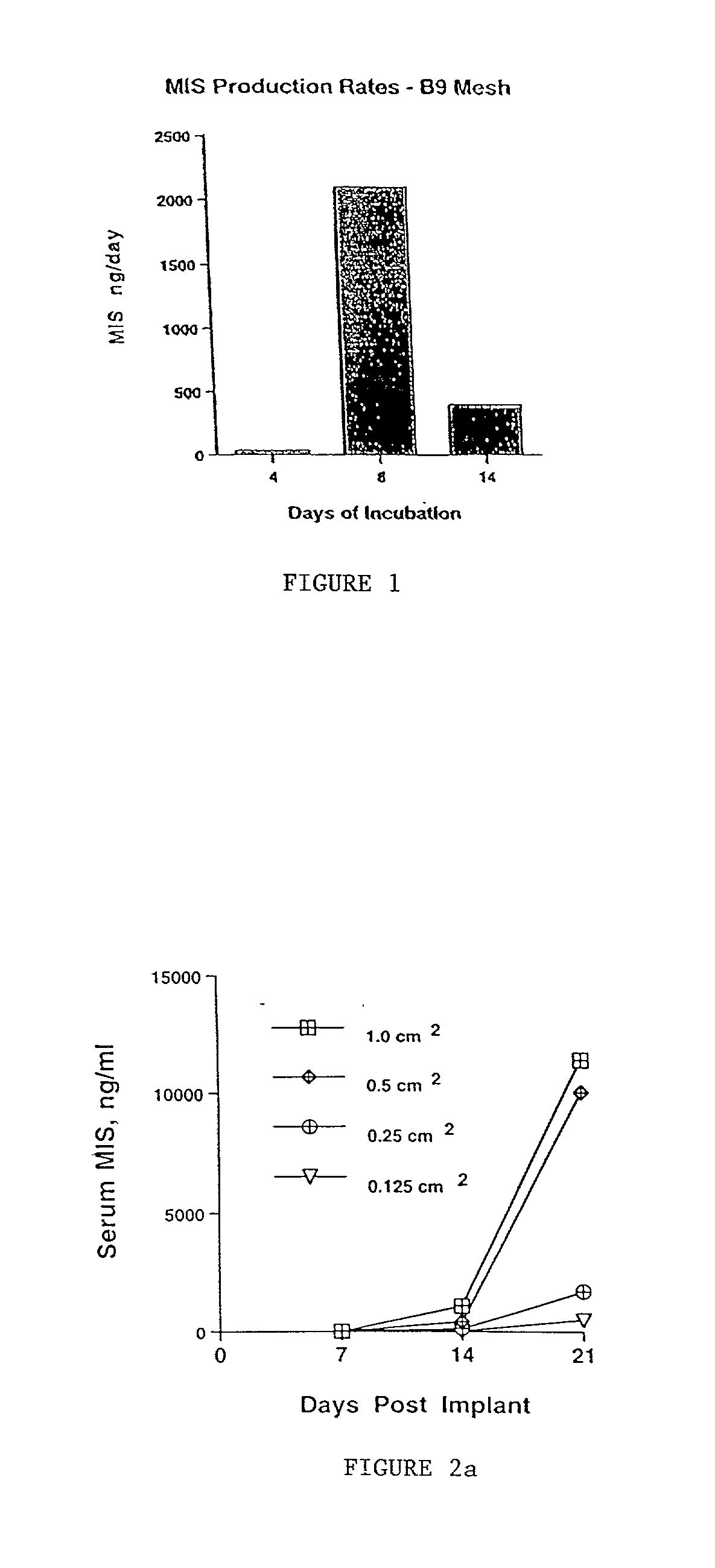
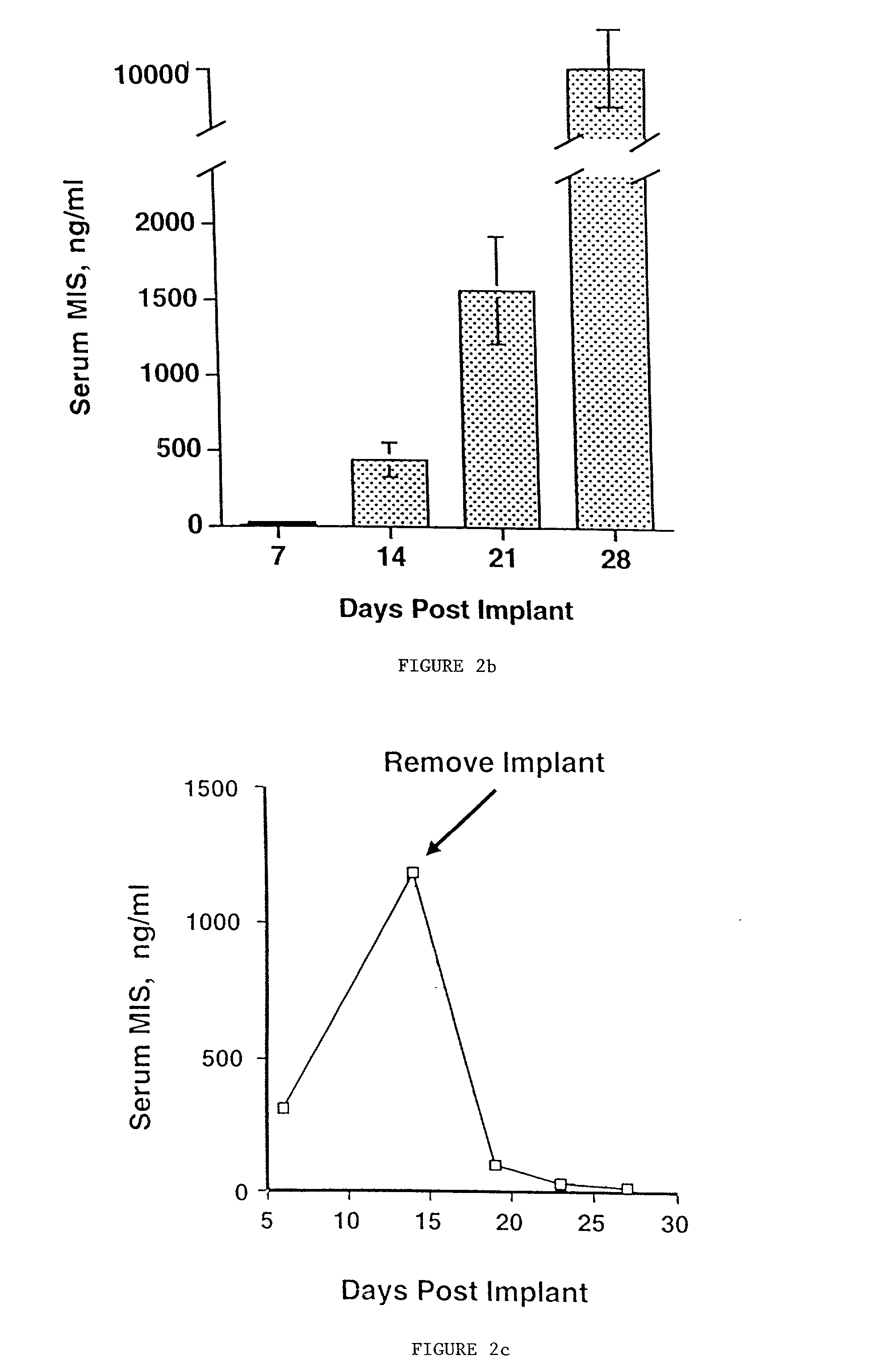
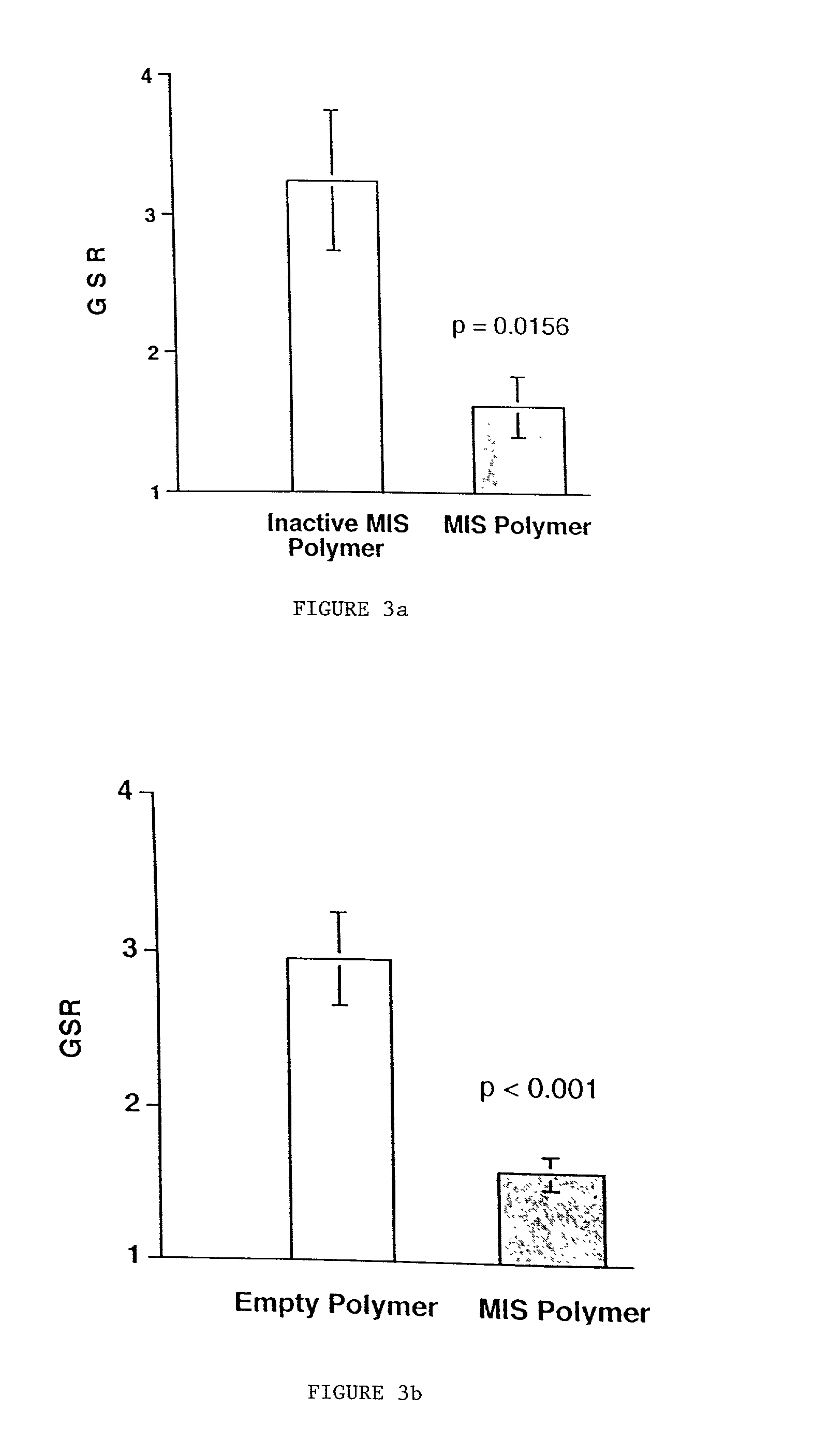
Delivery of therapeutic biologicals from implantable tissue matrices
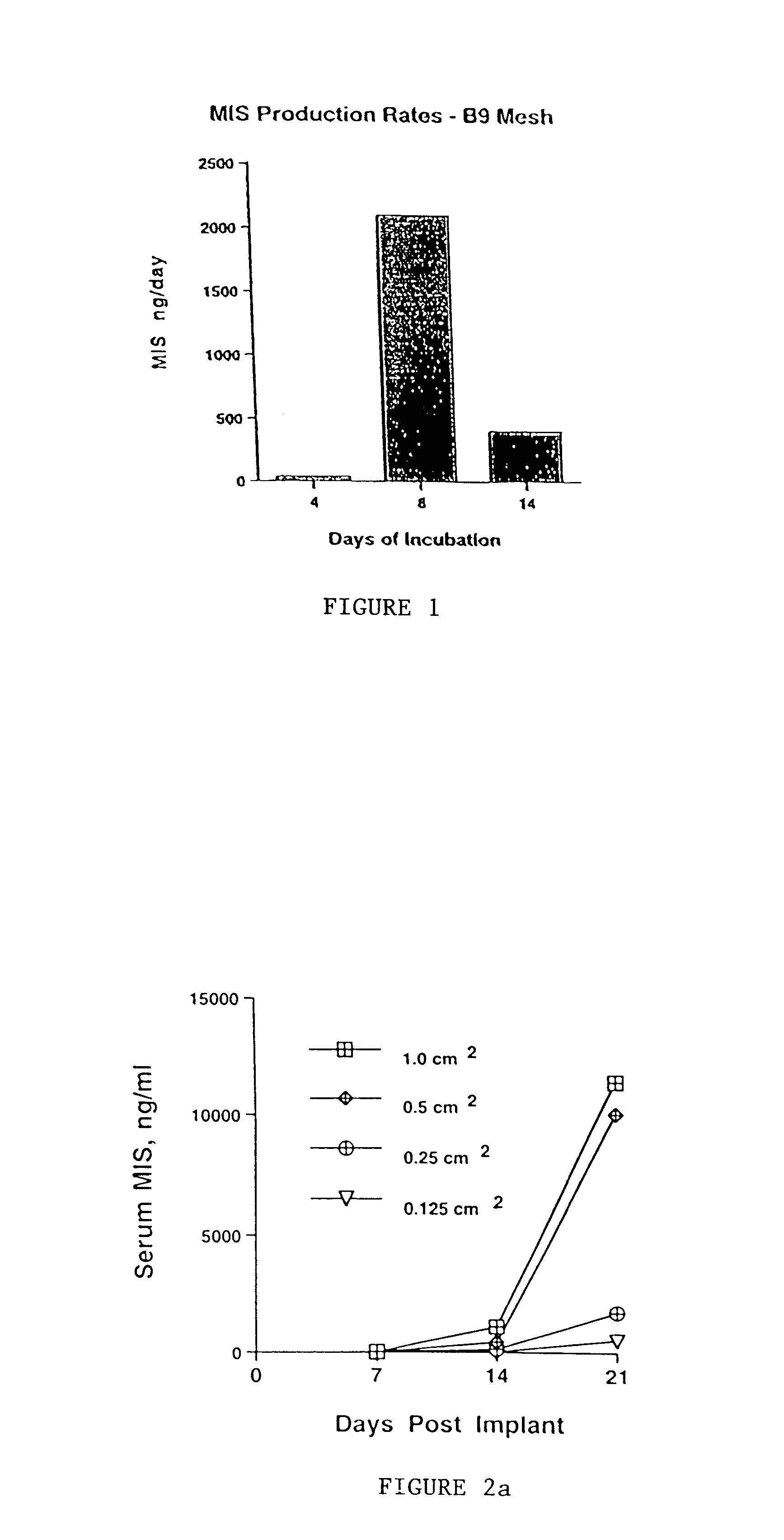
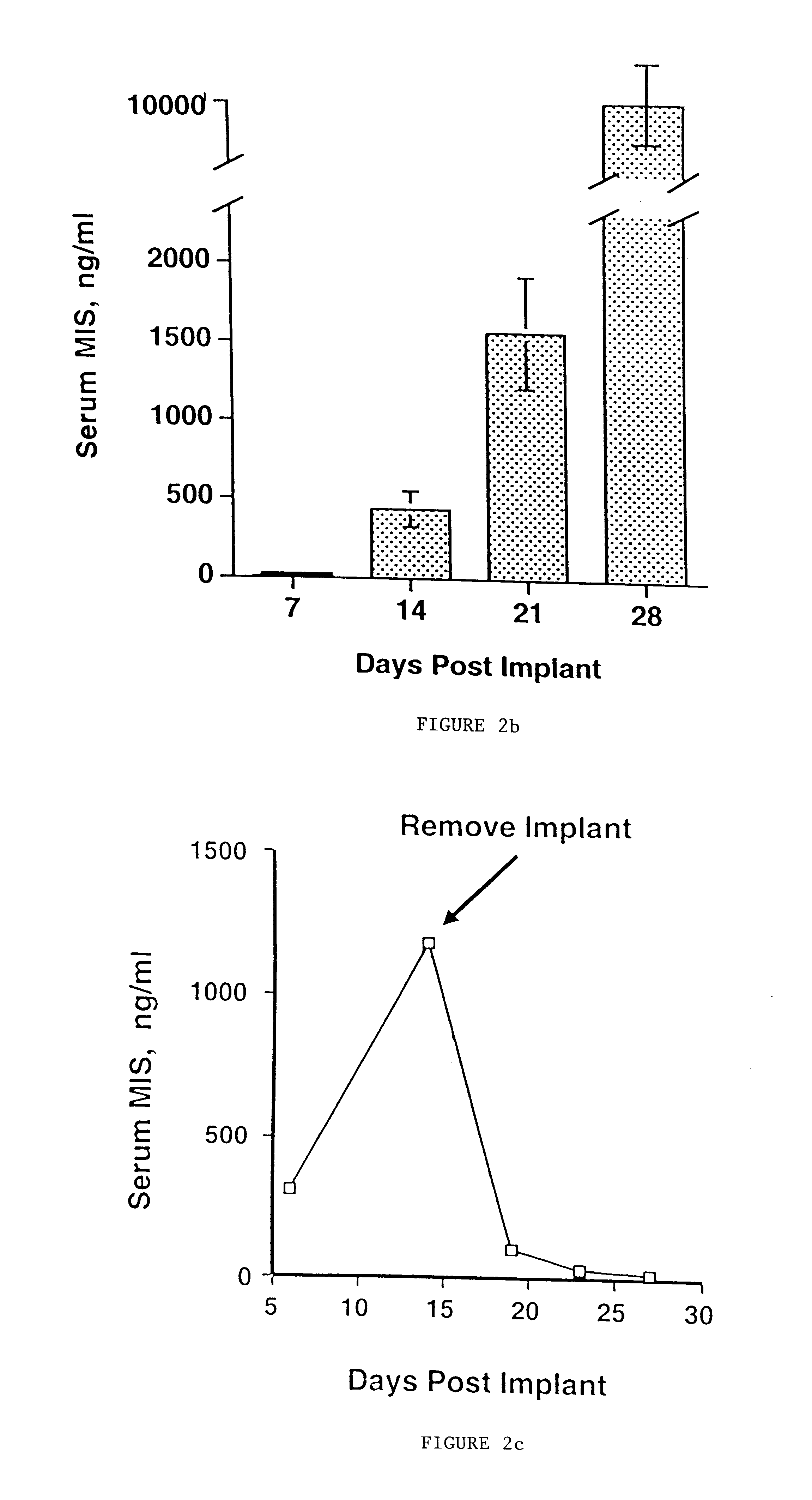
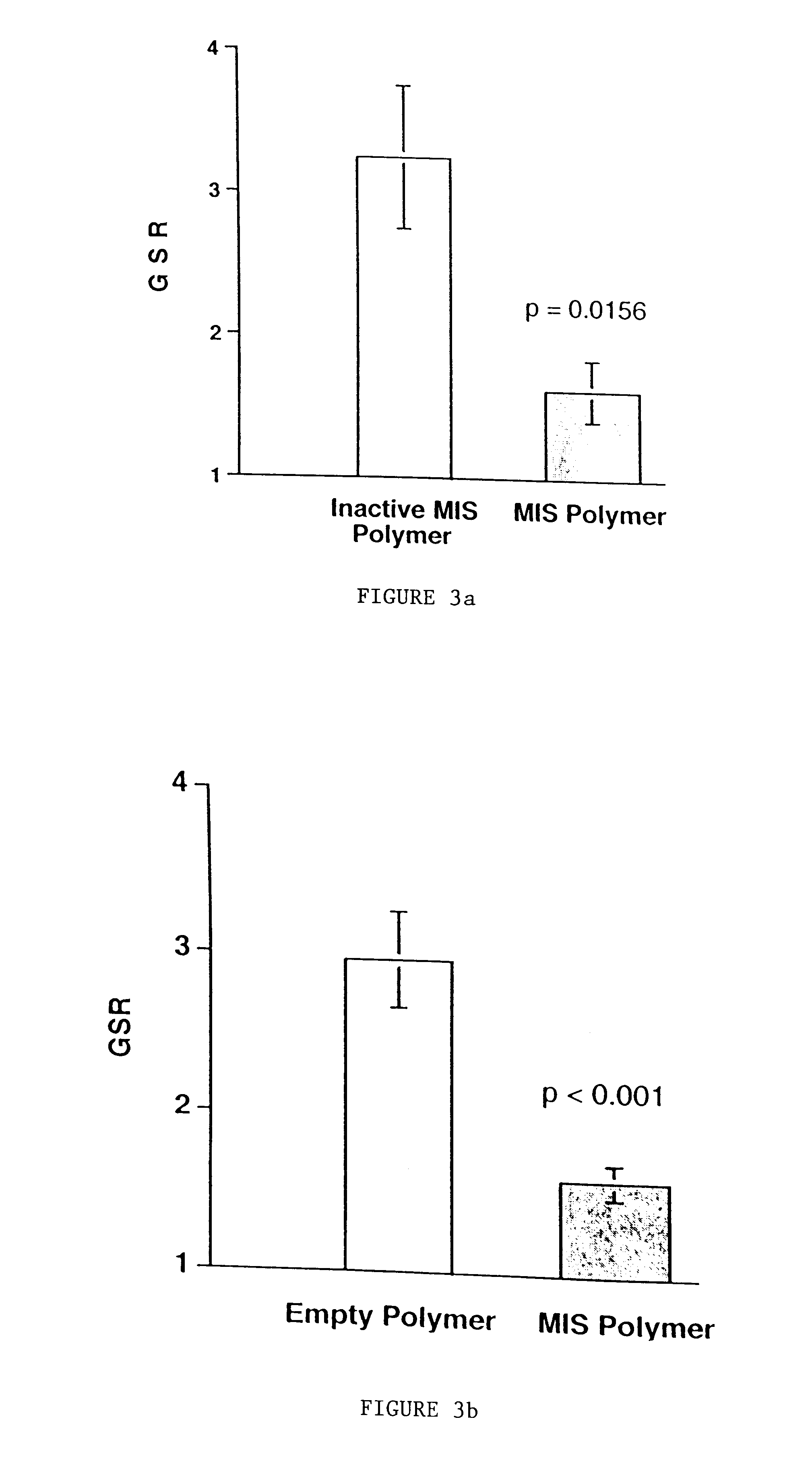
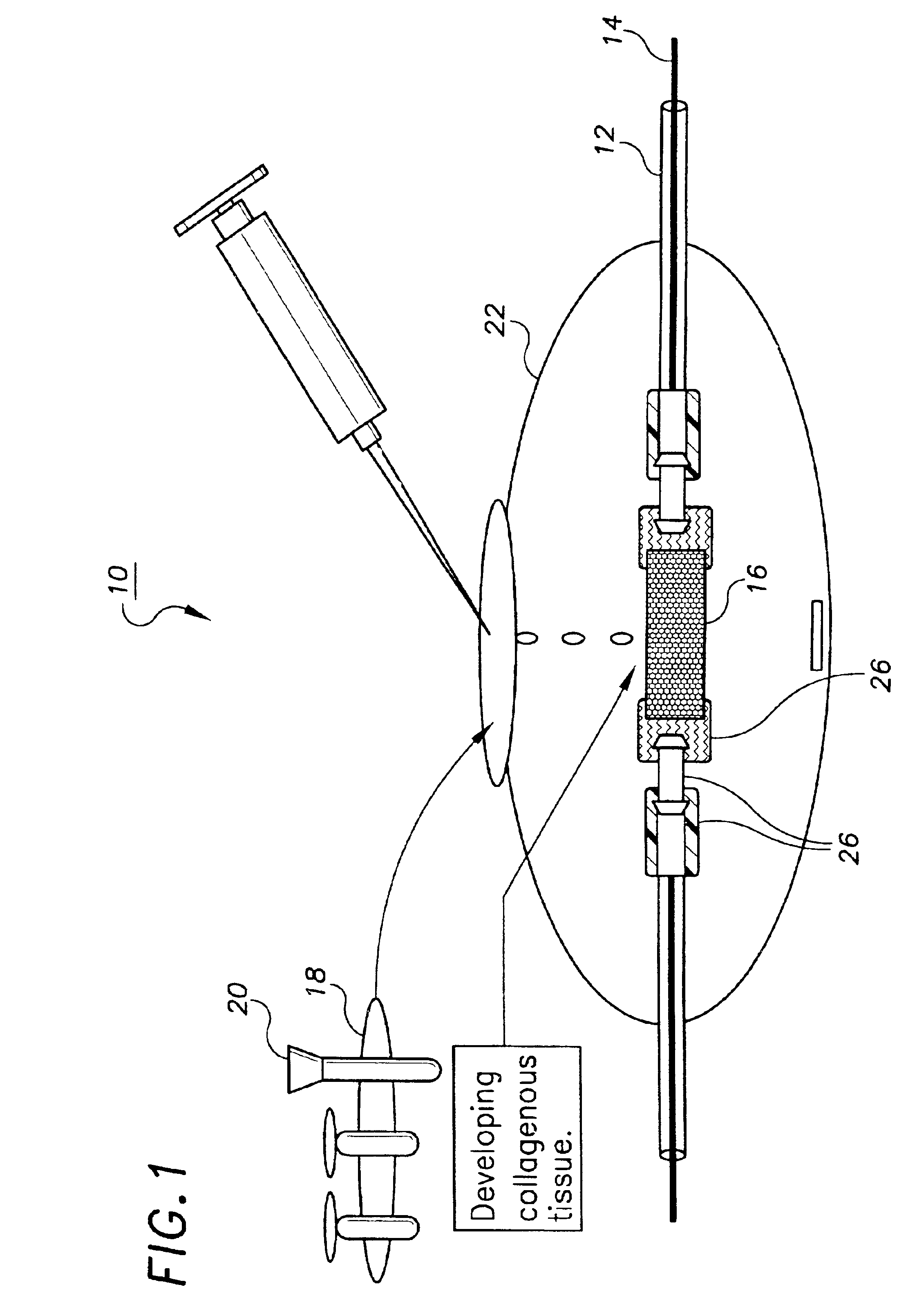
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Decellularized tissue engineered constructs and tissues
InactiveUS6962814B2Low immunogenicityReduction in immuneBiocidePeptide/protein ingredientsGrowth phaseCell-Extracellular Matrix
New methods for producing tissue engineered constructs and engineered native tissues are disclosed. The methods include producing a tissue engineered construct by growing cells in vitro on a substrate and then decellularizing the construct to produce a decellularized construct consisting largely of extracellular matrix components. The construct can be used immediately or stored until needed. The decellularized construct can be used for further tissue engineering, which may include seeding the construct with cells obtained from the intended recipient of the construct. During any of the growth phases required for production of the construct, the developing construct may be subjected to various tissue engineering steps such as application of mechanical stimuli including pulsatile forces. The methods also include producing an engineered native tissue by harvesting tissue from an animal or human, performing one or more tissue engineering steps on the tissue, and subjecting the tissue to decellularization. The decellularized, engineered native tissue may then be subjected to further tissue engineering steps.
Owner:DUKE UNIV
Breast augmentation system
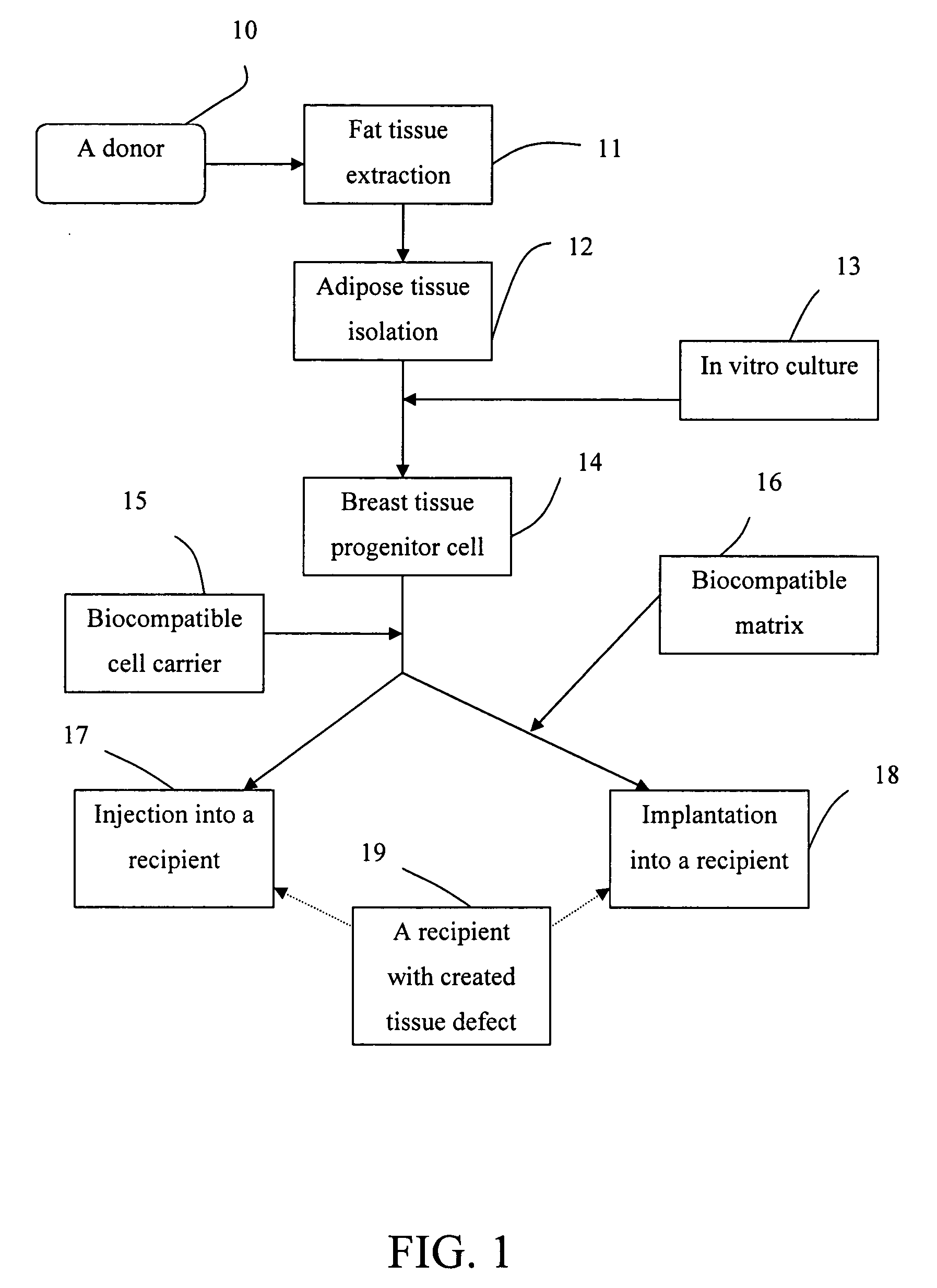
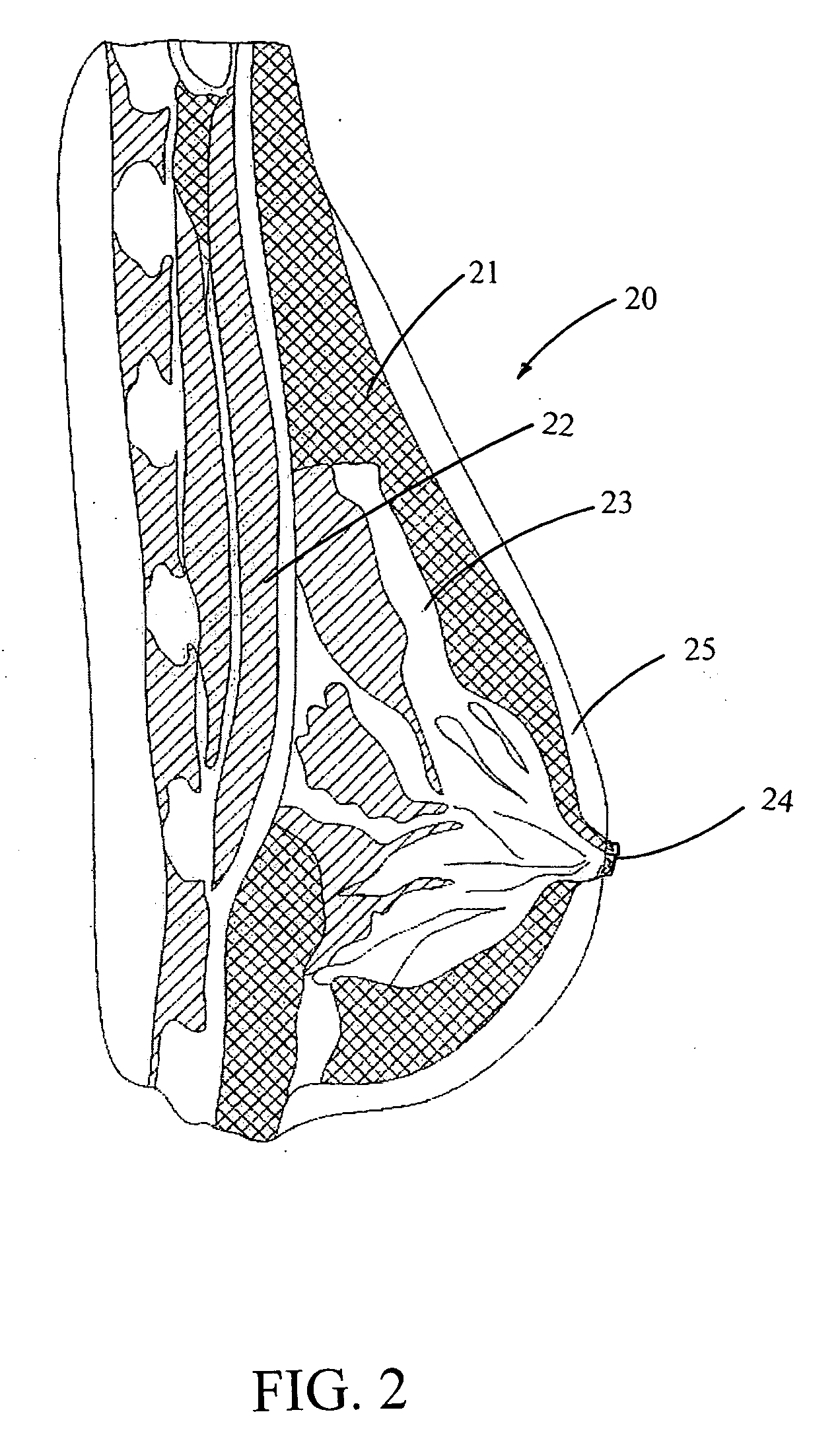
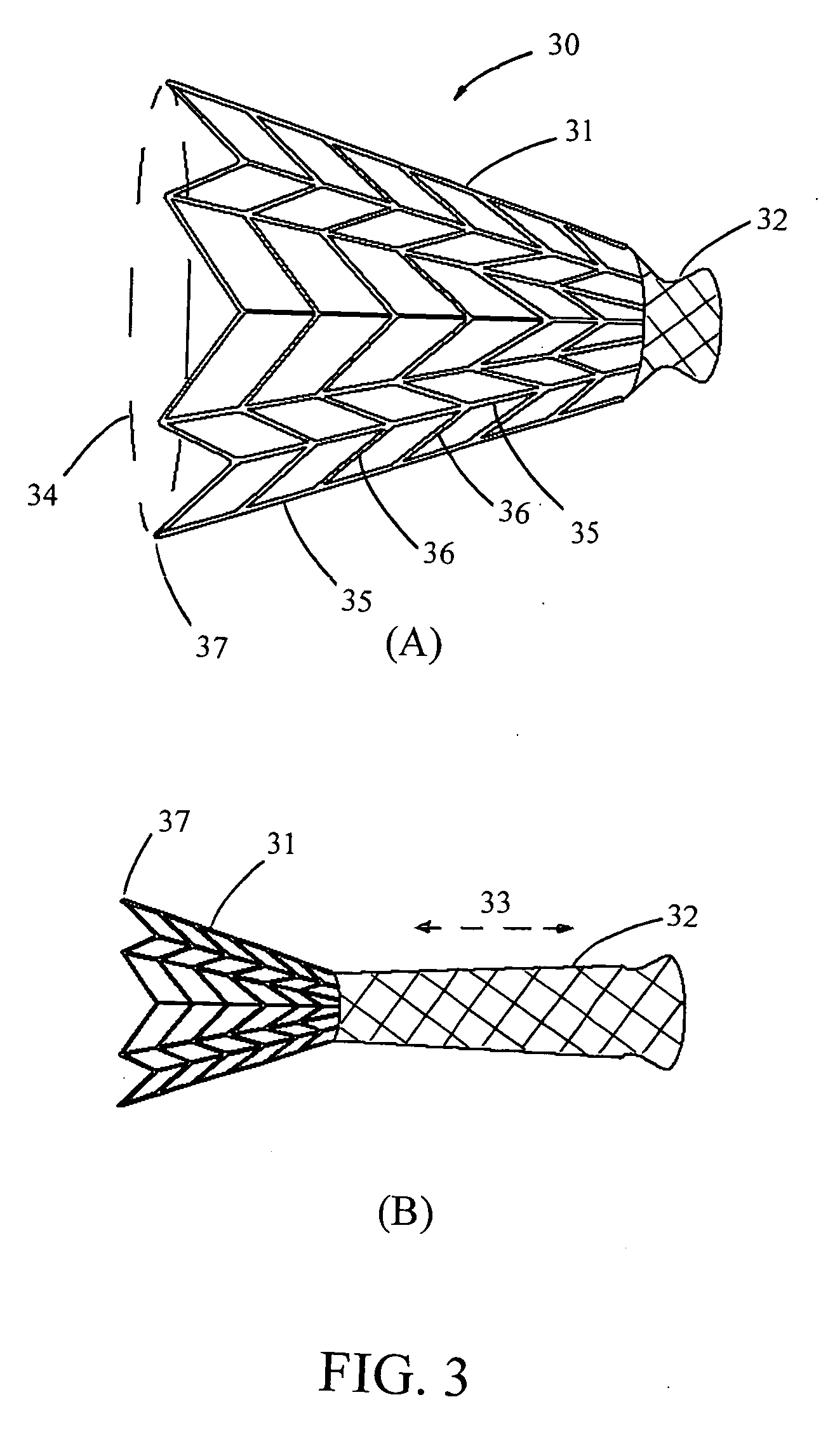
A stem-cell-seeded porous scaffold implant and delivery systems for treating or augmenting a breast tissue defect in a patient.
Owner:JUNOMEDICA
Scaffold for tissue engineering cartilage having outer surface layers of copolymer and ceramic material
InactiveUS6692761B2High mechanical strengthEfficient transportBiocideJoint implantsPolyesterPolytetramethylene terephthalate
A biodegradable, biocompatible porous matrix as a scaffold for tissue engineering cartilage is formed of a copolymer of a polyalkylene glycol and an aromatic polyester such as a polyethylene glycol / polybutylene terephtalate copolymer. A ceramic coating such as a calcium phosphate coating may be provided on the scaffold by soaking the scaffold in a solution containing calcium and phosphate ions. A composite scaffold which is preferably a two-layer system may be formed having an outer surface of a layer of the porous matrix formed of the copolymer, and an outer surface of a layer of a ceramic material. The composite scaffold may be prepared by casting the copolymer on top of the ceramic material in a mould. Cells are preferably seeded on the scaffold prior to implanting, and the scaffold may contain bioactive agents that are released on degradation of the scaffold in vivo.
Owner:OCTOPLUS SCI
Apparatus and Methods for Cell Isolation
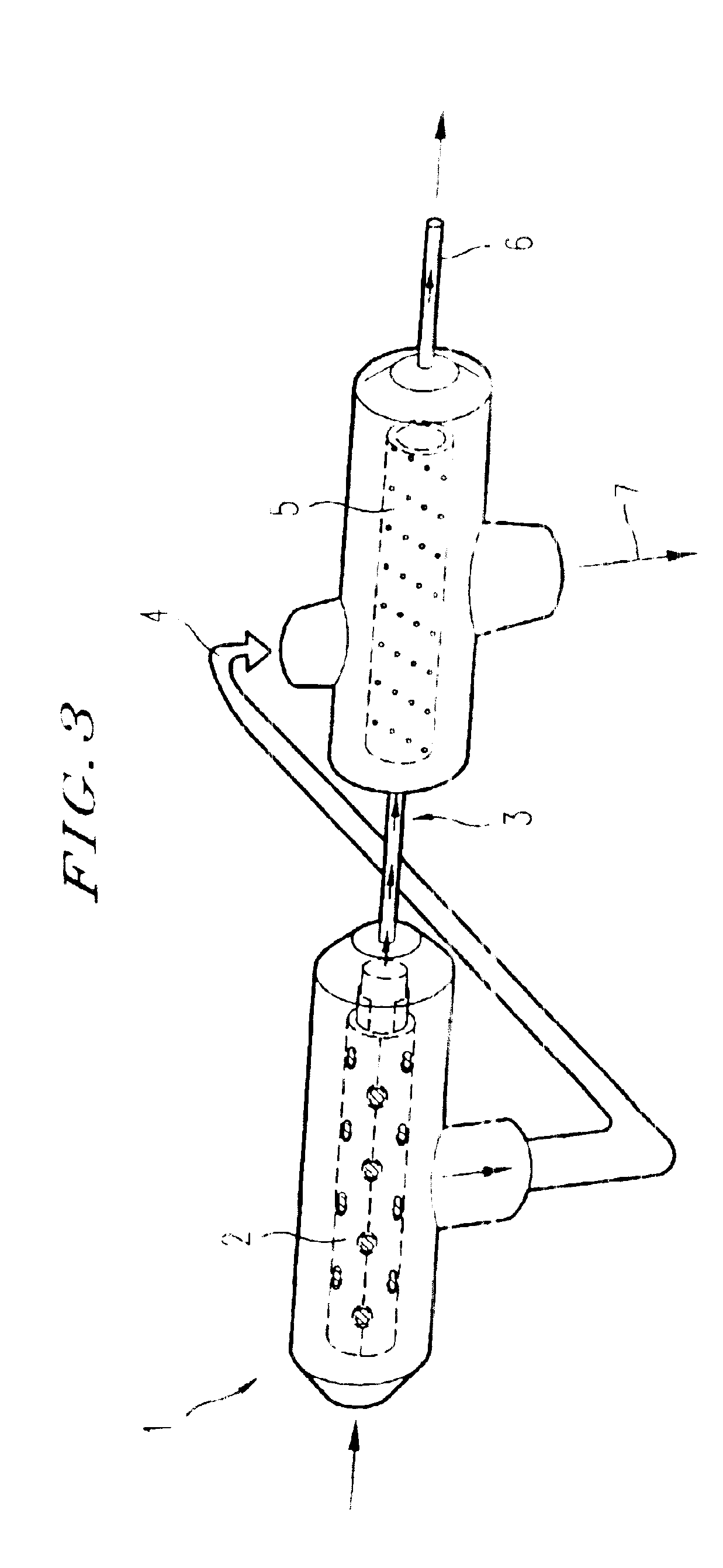
ActiveUS20100285588A1Reduce cloggingMaximize surface areaBioreactor/fermenter combinationsBiological substance pretreatmentsMixed cellMixed Cellular Population
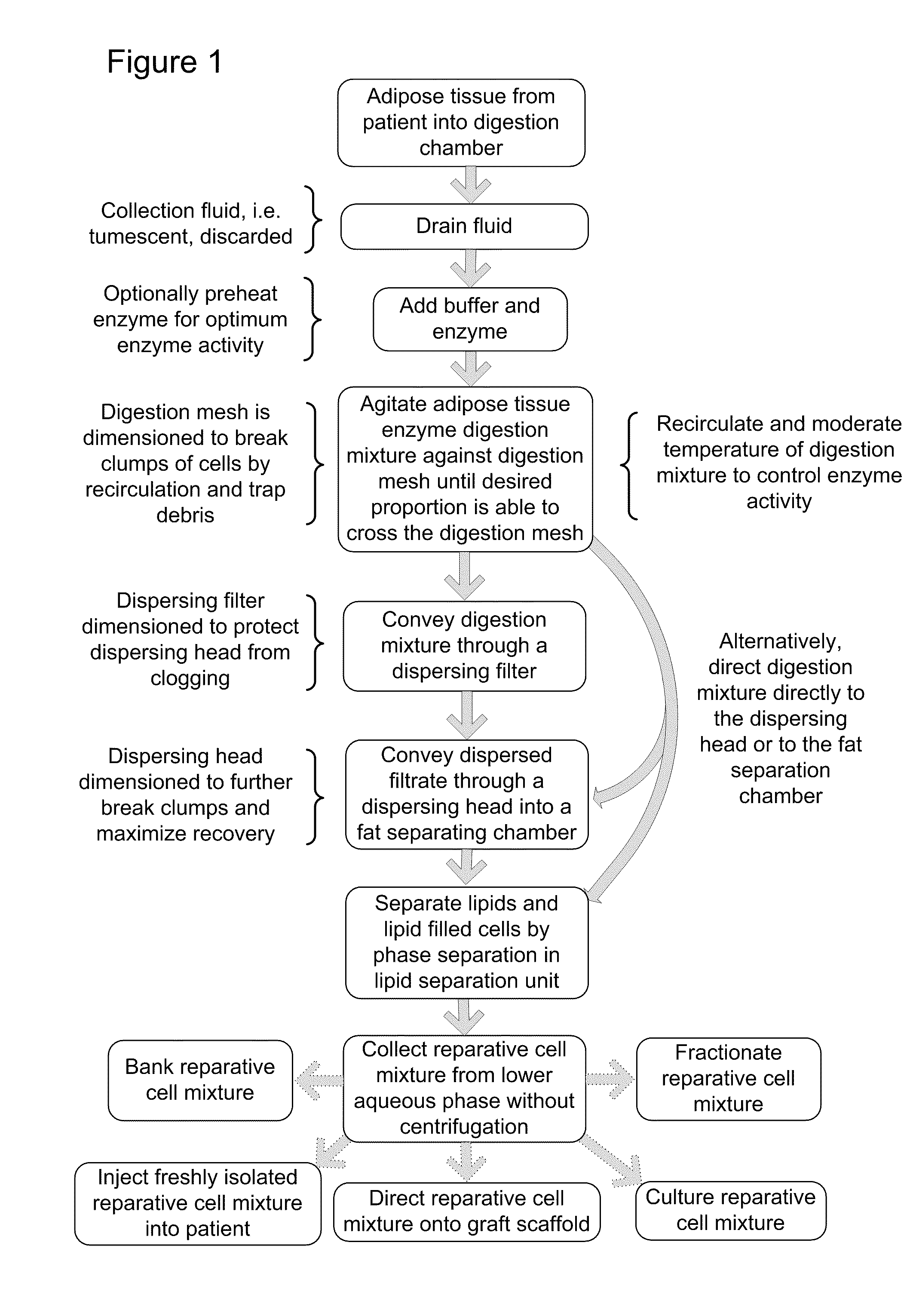
A unitary apparatus for isolating cells from adipose tissue including a lipid separation processor with a dispersing head equipped with a plurality of ports and a digestion chamber for dissociation of the constituent cells disposed in adipose tissue. The lipid separating apparatus is useful for the separation of lipids and adipocytes from a mixed cell population. A cell seeding chamber may be attached to the cell isolation apparatus. The components of the apparatus may be packaged in modular kit form.
Owner:INGENERON
Cryopreservation of cells using cross-linked bioactive hydrogel matrix particles
InactiveUS20090130756A1Simplifies and improves methodRaise transfer toPowder deliveryDead animal preservationParticulatesCross-link
The present invention is directed to methods of cryopreserving cells and cryopreserved cells prepared according to the methods. In specific embodiments, the method comprises combining cells with a cross-linked hydrogel matrix in particulate form, the matrix comprising a polyglycan cross-linked to a polypeptide and subjecting the combination to cryopreservation conditions. In further embodiments, the invention provides cell-seeded compositions comprising cells and a cross-linked bioactive hydrogel matrix in particulate form, the matrix comprising a polyglycan cross-linked to a polypeptide, wherein the composition has been subjected to cryopreservation conditions. The cryopreserved cells can be thawed and used in methods of treatment without the need for intervening steps to make the cells viable for in vivo use.
Owner:PIONEER SURGICAL TECH INC
Isolation and expansion of animal cells in cell cultures
Described are methods for isolating / purifying and expanding animal stem cells and stem-cell-like cells. Isolation methods include conditions comprising preferentially digesting non-stem cells and non-stem-cell-like cells in a population and preferentially adhering stem cells and stem-cell-like cells in a population. Expansion methods include culturing such cells under conditions comprising modulation of TGF-β signaling, inhibition of cell signaling mediated by p38 MAP kinase using small molecular weight inhibitors, expansion of the cells on human amniotic epithelial cells as feeder layers, control of cell seeding density, control of levels of Ca2+ in the culture media, rapid adhesion on a substrate or by a combination of such conditions. More particularly, what is disclosed relates to methods and systems for expanding animal cells in ex vivo cell cultures, while preventing cellular differentiation, and selectively enriching stem cells. The embodiments also disclose a culture system for ex vivo expansion of limbal epithelial cells or mesenchymal cells, as well as surgical grafts made there from.
Owner:TISSUETECH INC
Scaffolds for tissue engineering and regenerative medicine
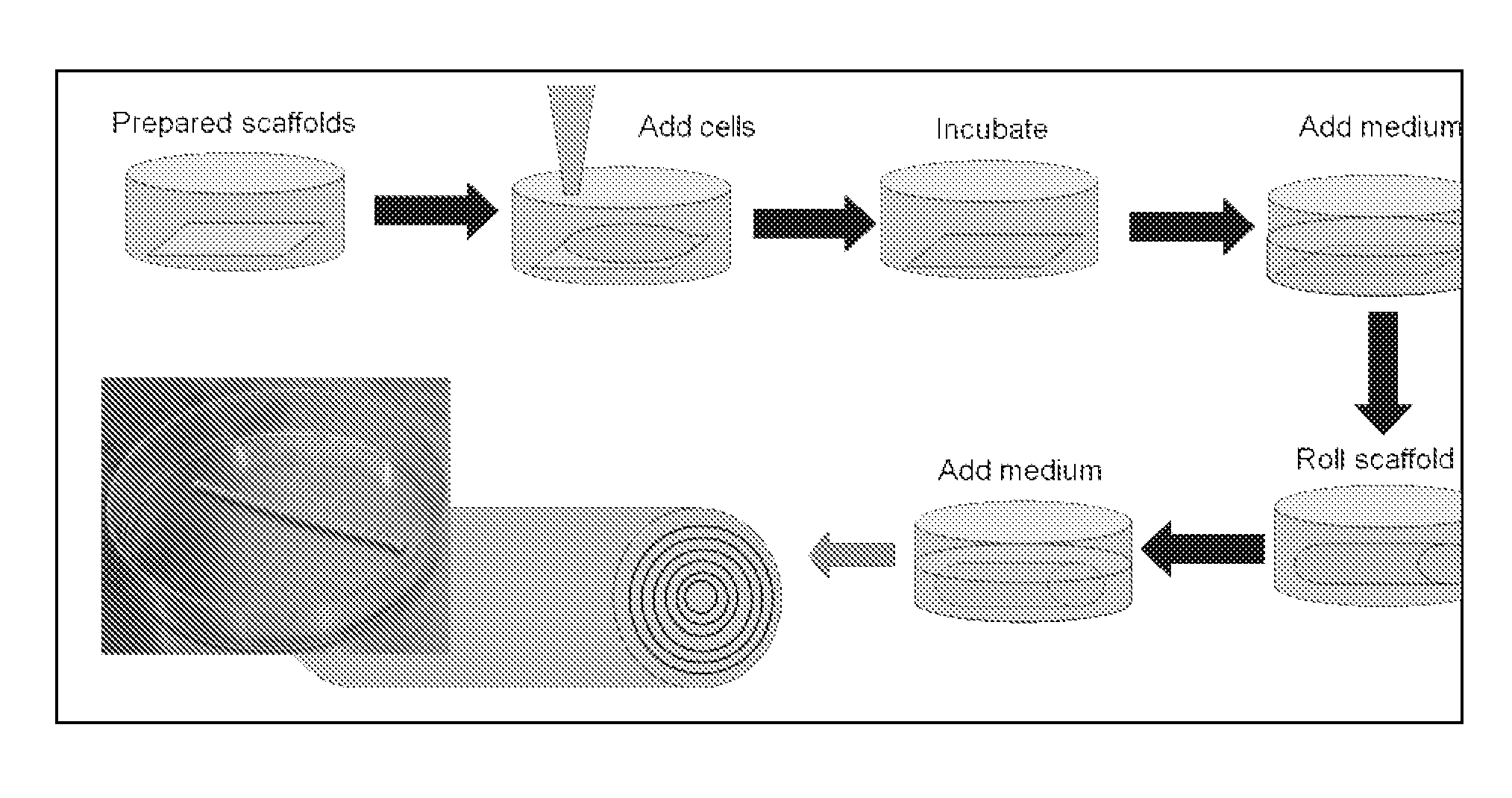
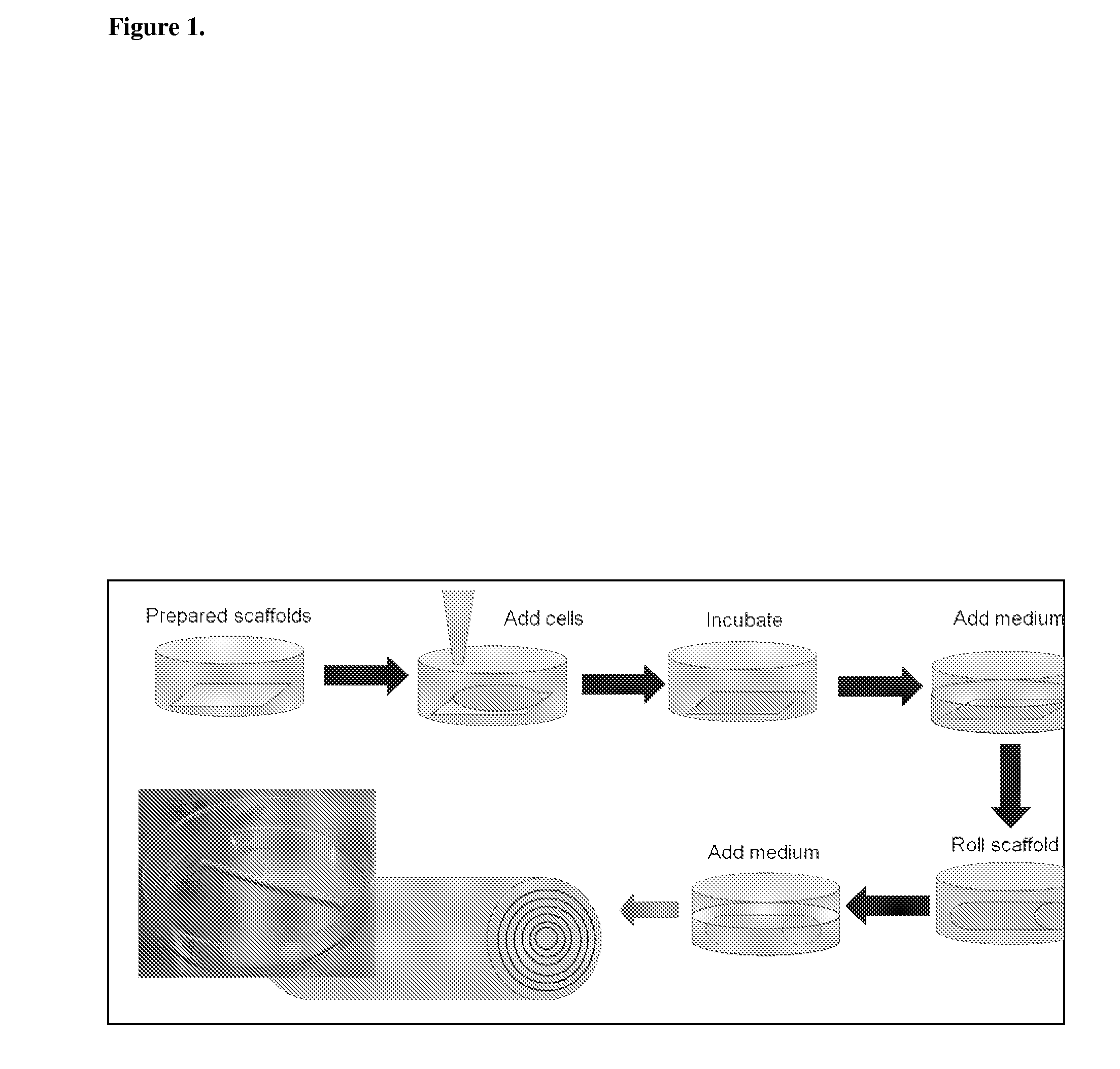
The methods and compositions described herein relate to novel 3-dimensional porous scaffolds useful for tissue re-generation, enhancement, or tissue repair. Electrospinning or other methods are used to create mats comprised of fibers that can be seeded with cells and subsequently rolled into a desired shape / form to replace a desired tissue.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Bioartificial filtration device for filtering blood to mimic kidney function
InactiveUS6942879B2Long life-timeImmobilised enzymesBioreactor/fermenter combinationsBioartificial liver deviceUltrafiltration
A novel cell seeded hollow fiber bioreactor is described as a potential bioartificial kidney. Endothelial cells along with pericyte, vascular smooth muscle, and / or mesangial cells or any mesenchymally derived support cells are seeded along a hollow fiber in a perfused bioreactor to reproduce the ultrafiltration function and transport function of the kidney. Maintenance of tissue specific function and ultrastructure suggest that this bioreactor provides an economical device for treating renal failure.
Owner:RGT UNIV OF MICHIGAN
Biomatrix Composition and Methods of Biomatrix Seeding
ActiveUS20100124563A1Good treatment effectAccelerated remodelingBiocideBioreactor/fermenter combinationsLipid formationPoint of care
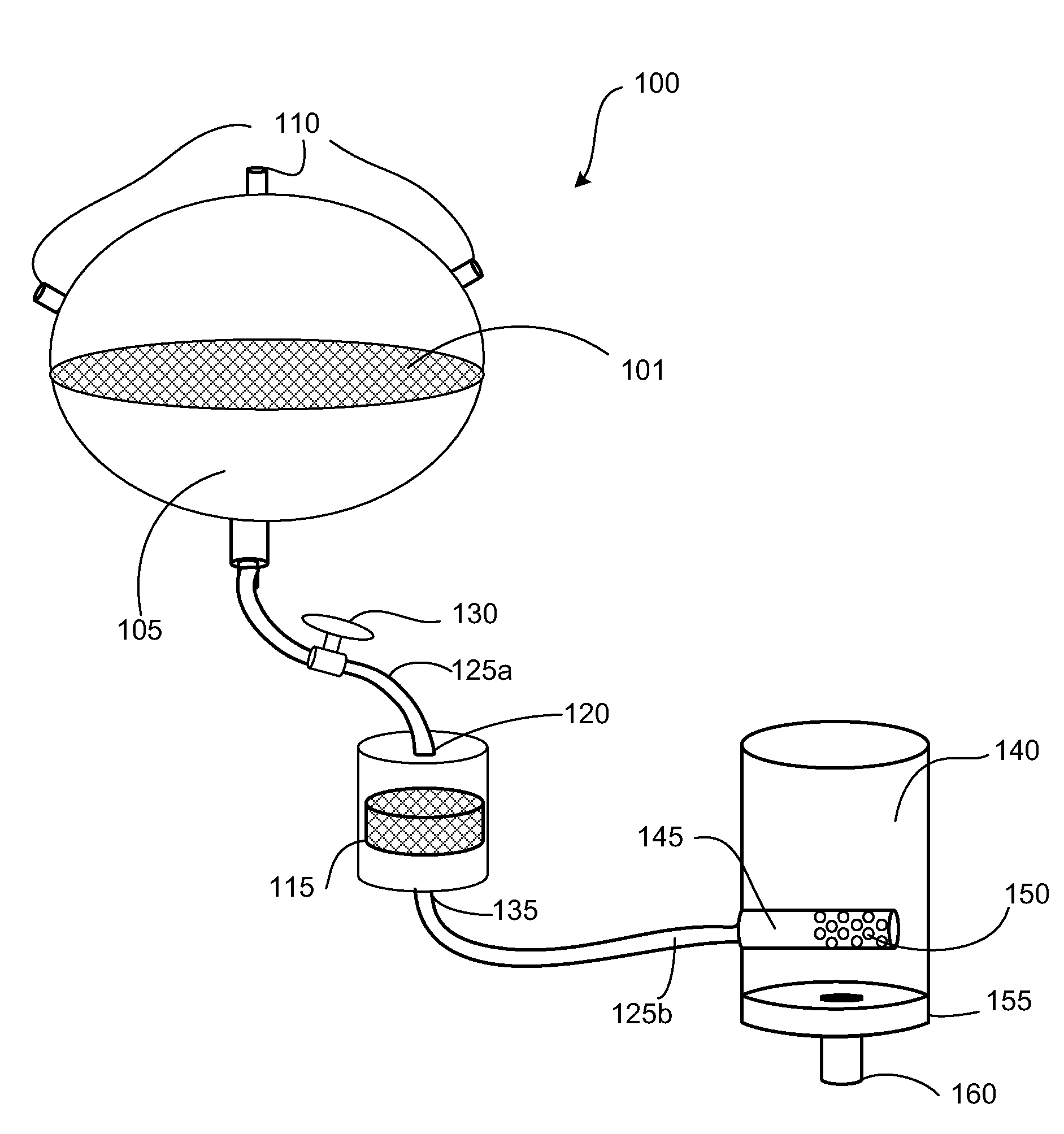
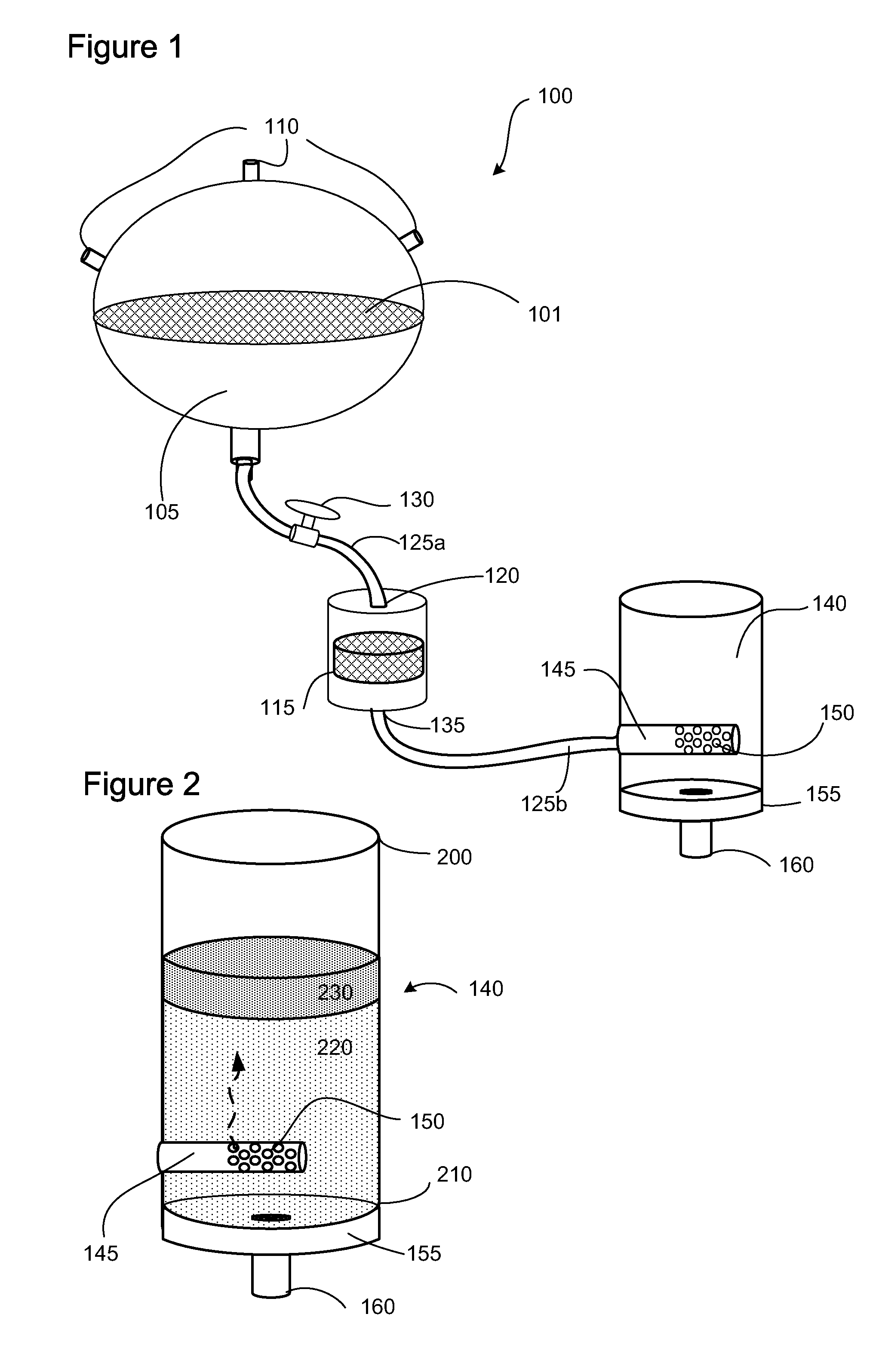
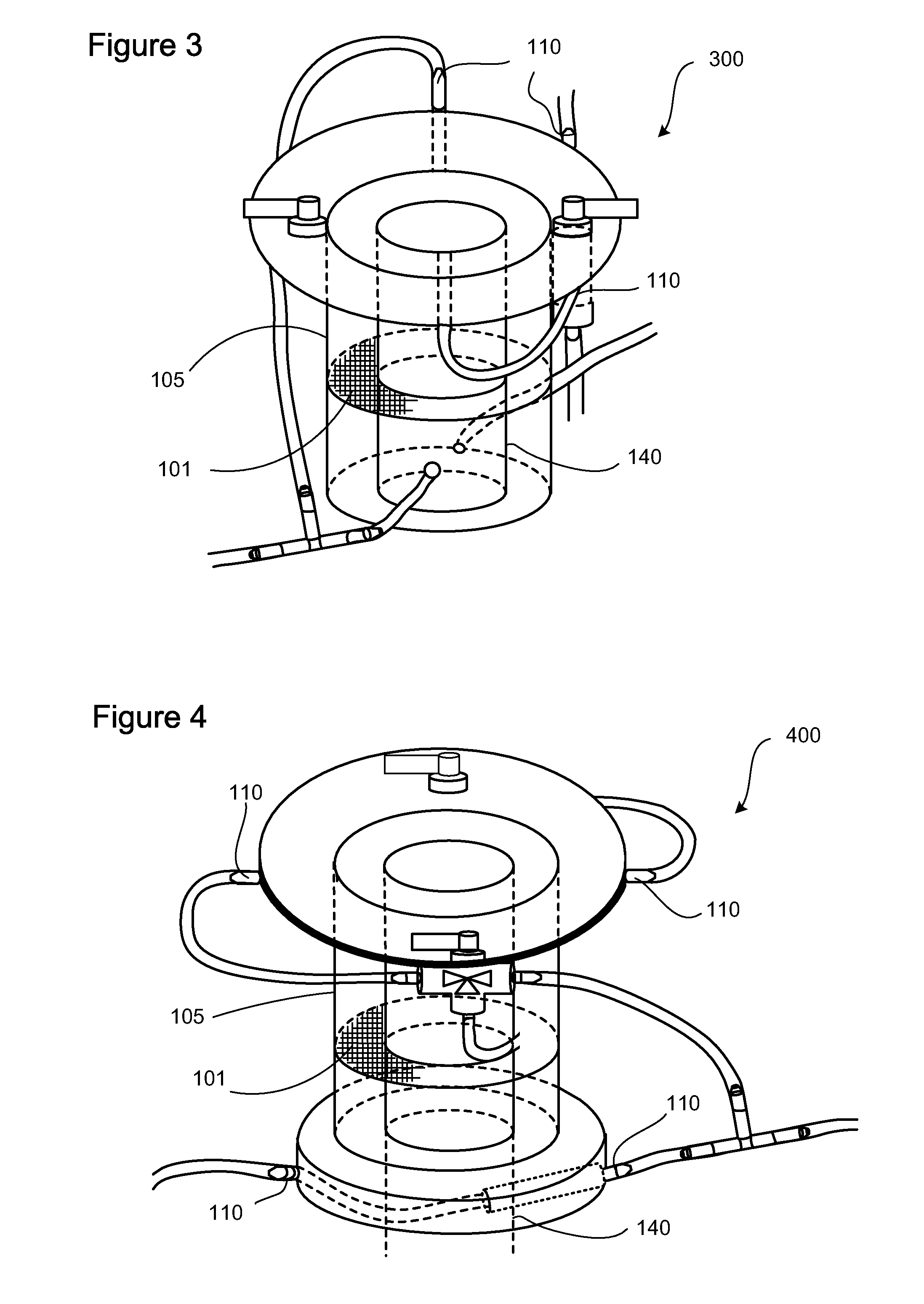
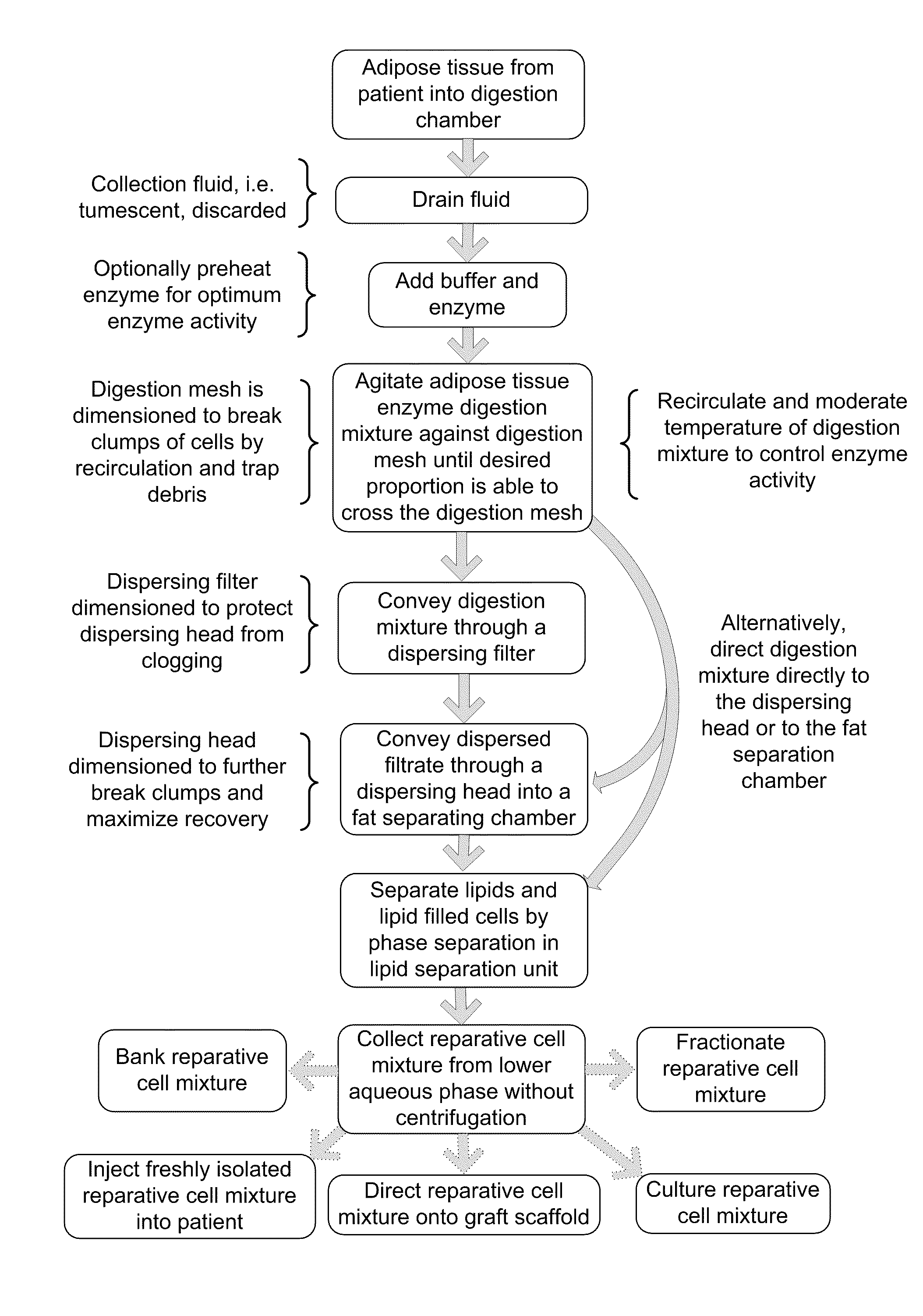
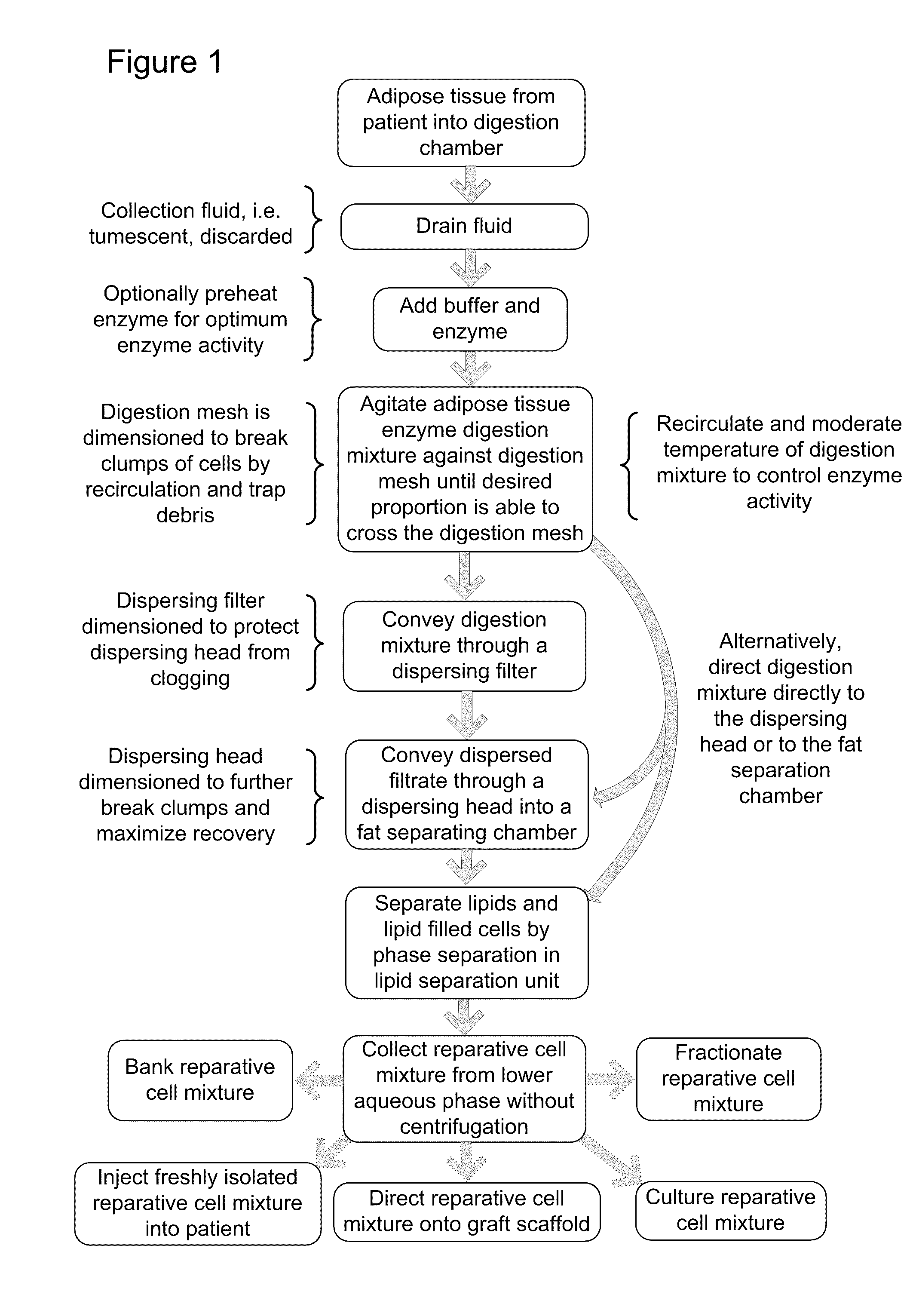
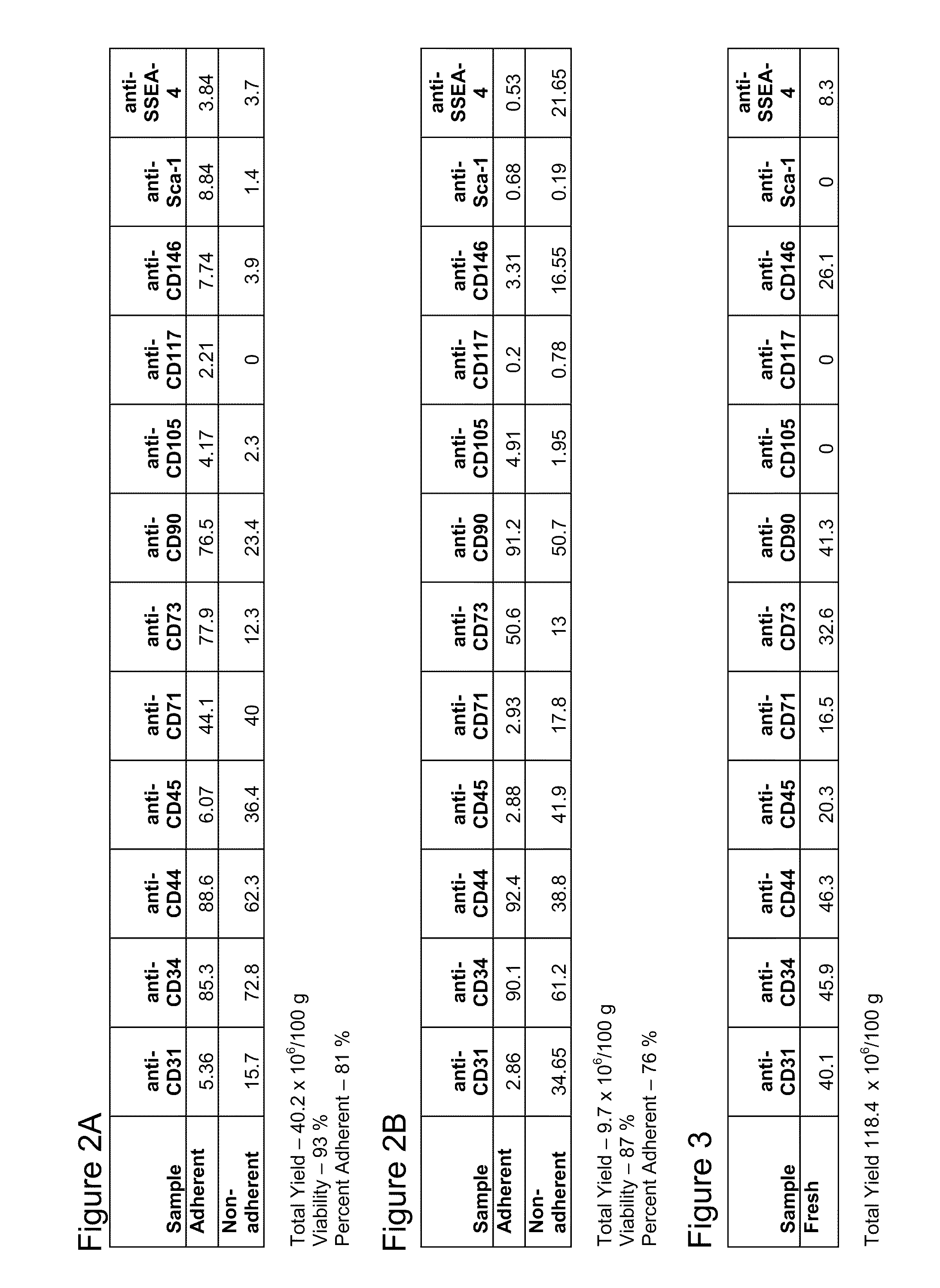
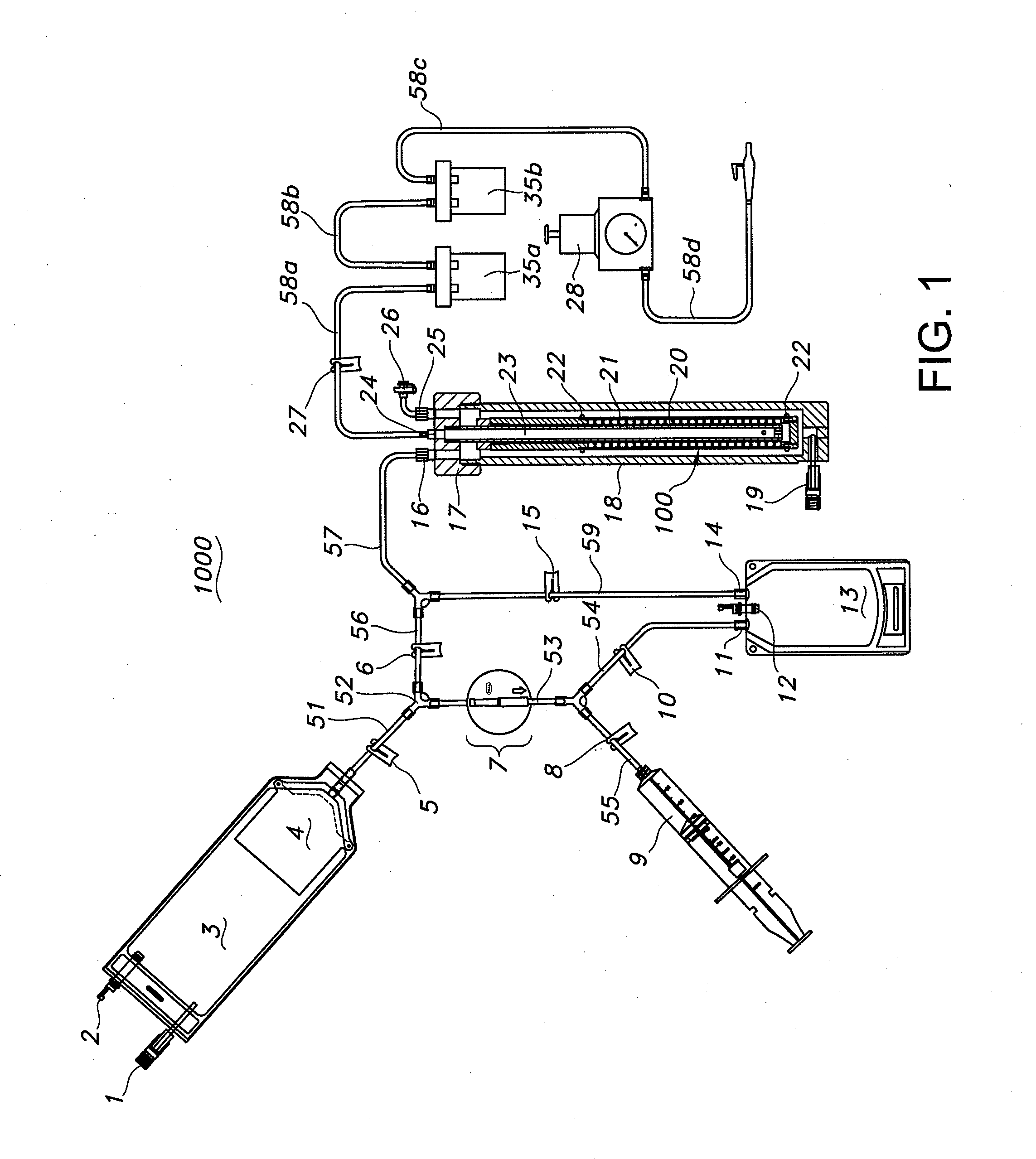
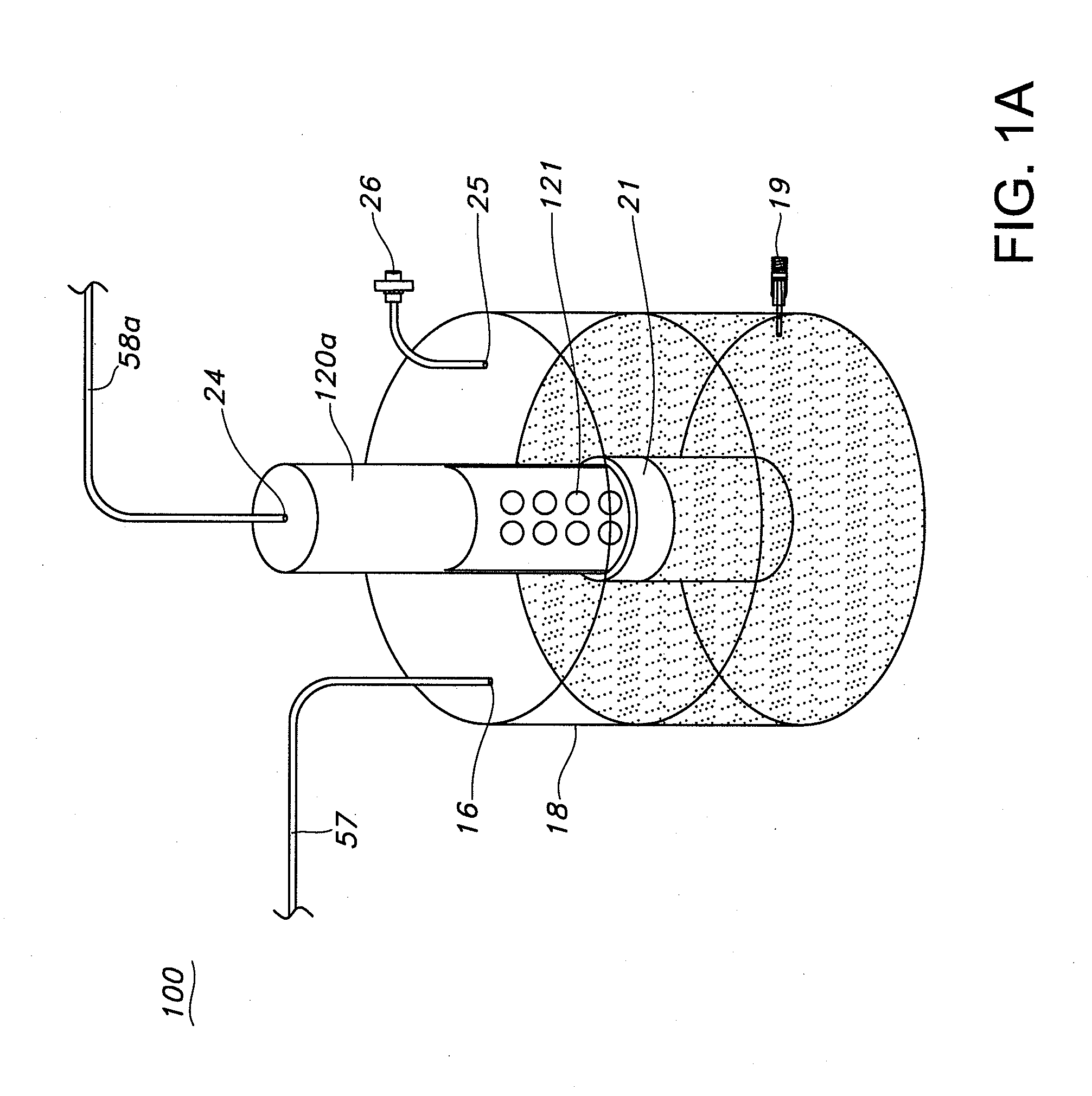
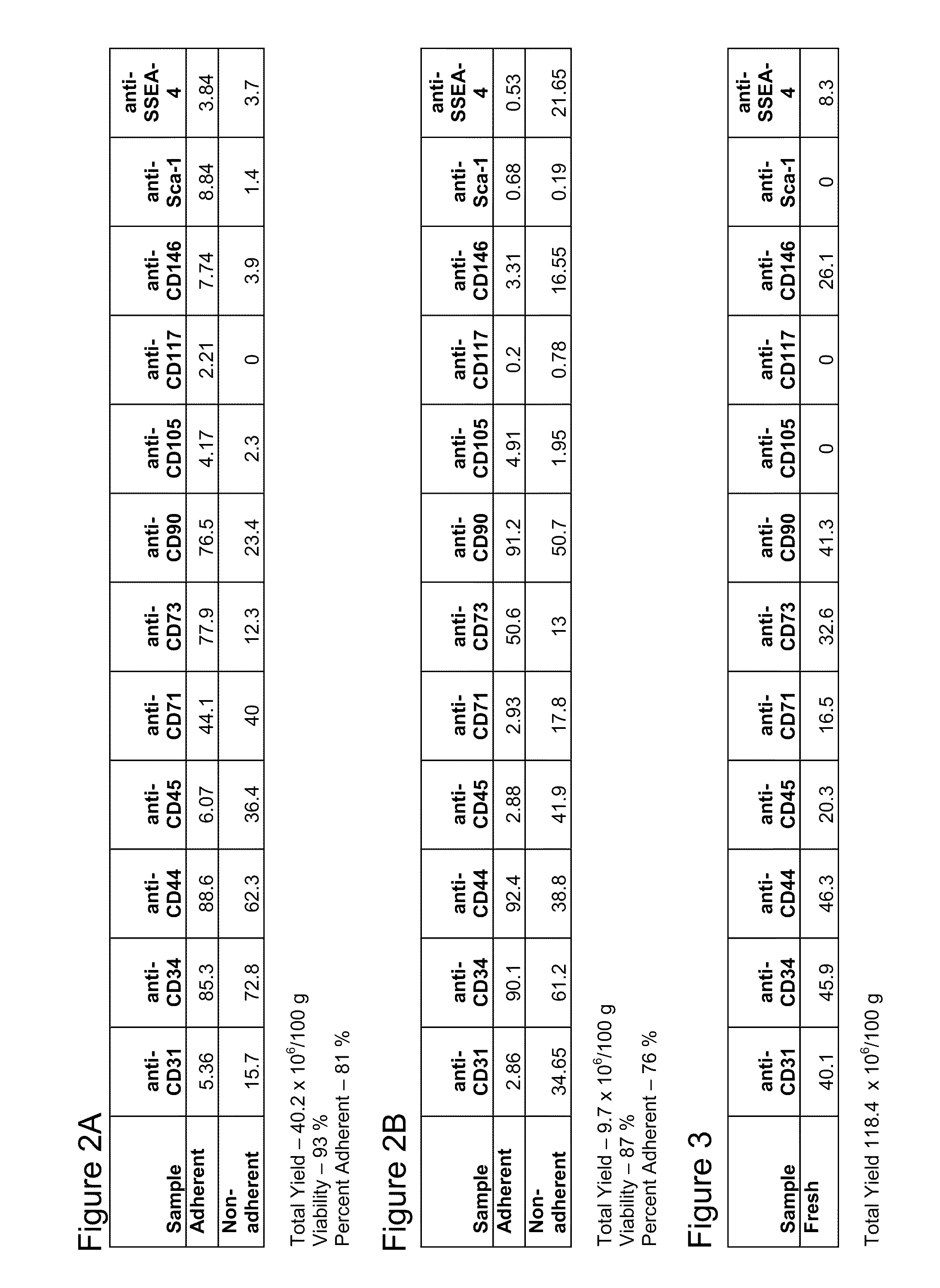
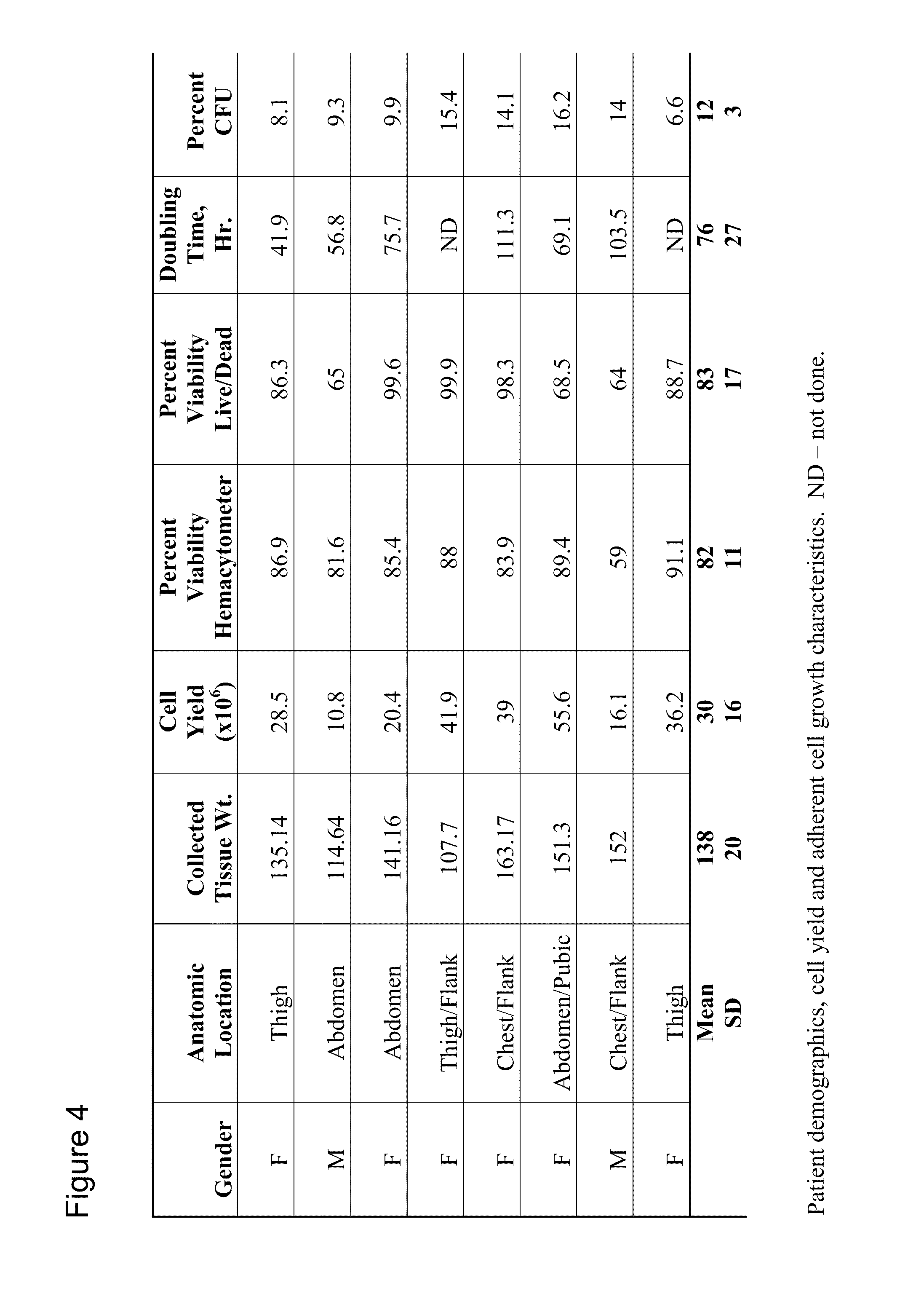
Apparatus and methods are described for generating autologous tissue grafts, the apparatus including a point of care SVF isolation unit that includes a tissue digestion chamber in fluid communication with a lipid separating chamber, whereby SVF cells are isolated without centrifugation; and a cell seeding chamber in fluid communication with the SVF isolation unit, said cell seeding chamber adapted to support a cell scaffold. Methods and materials for cell seeding, including through the provision of micro rough scaffold surfaces, are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Production of tissue engineered heart valves
ActiveUS20060253192A1Avoid it happening againMore of their natural flexibilityHeart valvesNanoinformaticsProgenitorSmooth muscle
The invention is directed to methods for preparing artificial heart valves by preconditioning a matrix seeded with endothelial cells and smooth muscle cells differentiated from isolated progenitor cells. These cell seeded matrices are exposed to fluid conditions that mimic blood flow through the heart to produce tissue engineered heart valves that are analogous to native heart valves.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Tissue engineered biomimetic hair follicle graft
InactiveUS20050214344A1High degreeReduces and inhibits and cure lossBiocideAnimal repellantsBioabsorbable scaffoldAllogeneic cell
The present invention relates to an improved scaffold which is constructed to mimic the architecture of the native hair follicle. The present invention also relates to the use of specific compositions and methods of manufacture to produce scaffolds that combine biocompatibility with the desired rates of bioabsorption. In another embodiment, the present invention relates to a process for manufacturing a biomimetic hair follicle graft and a method for seeding the graft with cells and implanting the graft into the skin where the growth of a new hair shaft is desired. A further embodiment of the present invention relates to a method for hair multiplication in which cells are multiplied in culture and aliquoted into a multitude of bioabsorbable scaffolds in combination with cultured keratinocytes or other allogenic cells.
Owner:ADERANS RES INST
Three dimensional vaginal tissue model containing immune cells
InactiveUS6943021B2Improve survivabilityInduced proliferationBiocideEpidermal cells/skin cellsSerum free mediaAir liquid interface
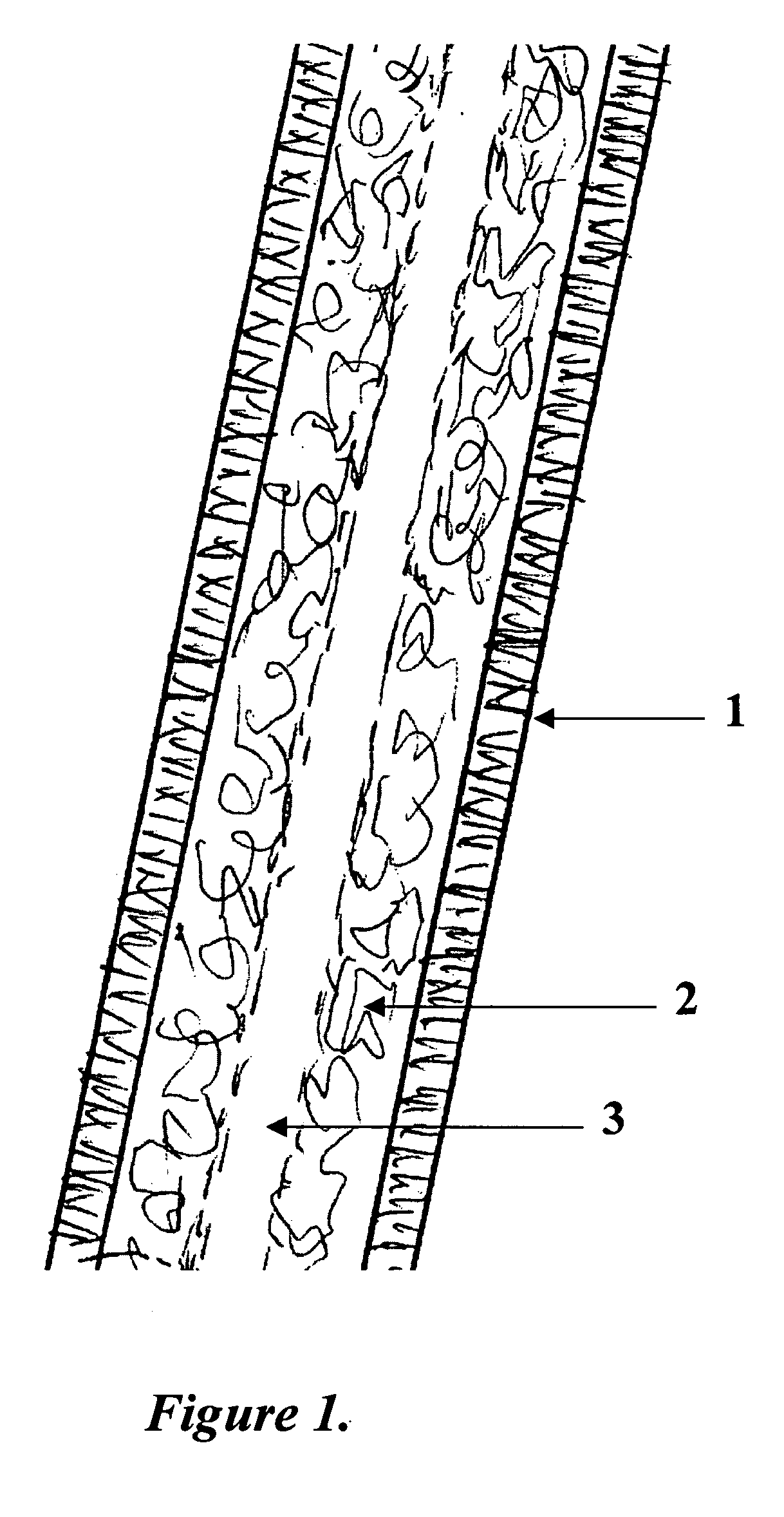
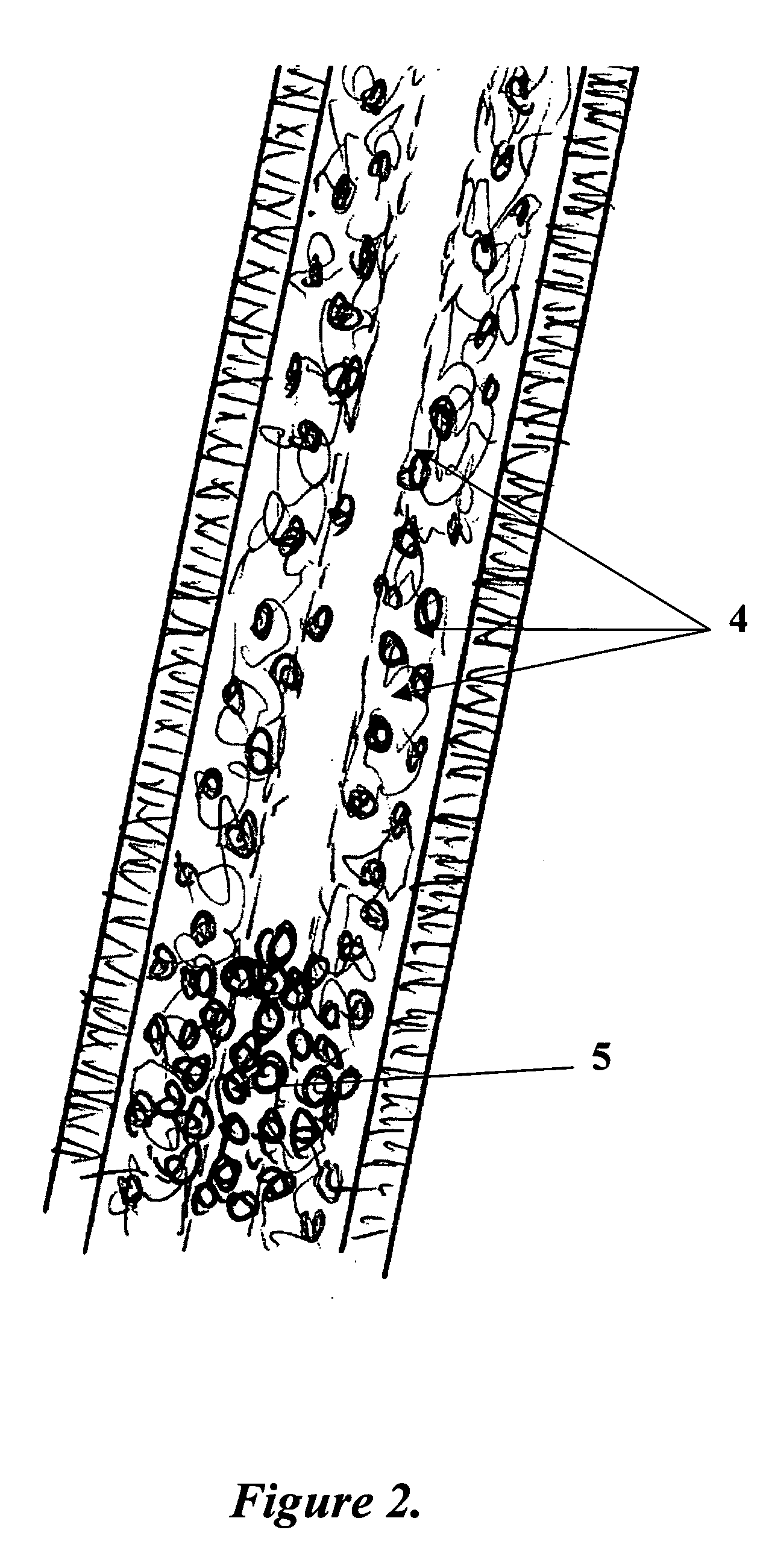
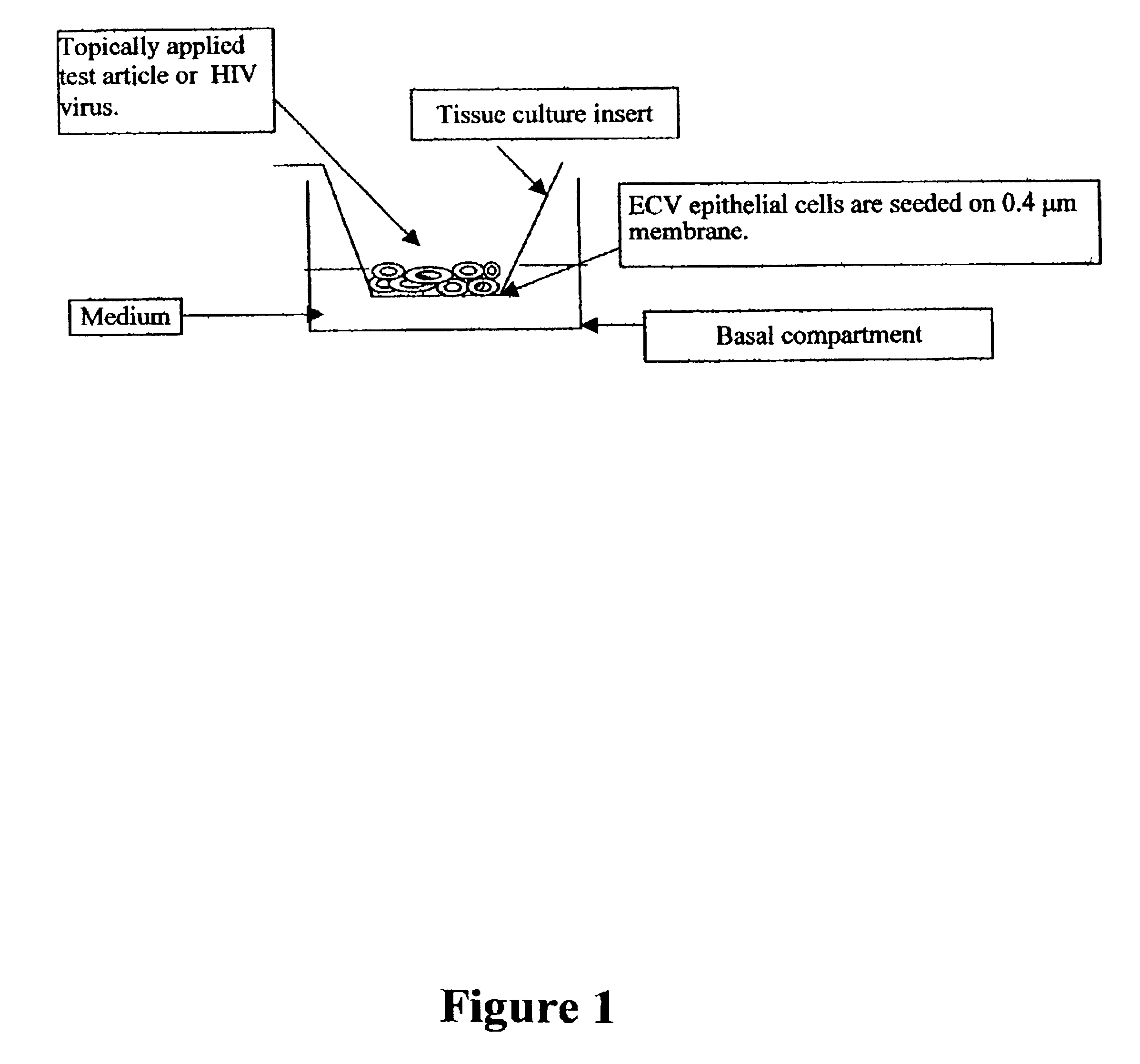
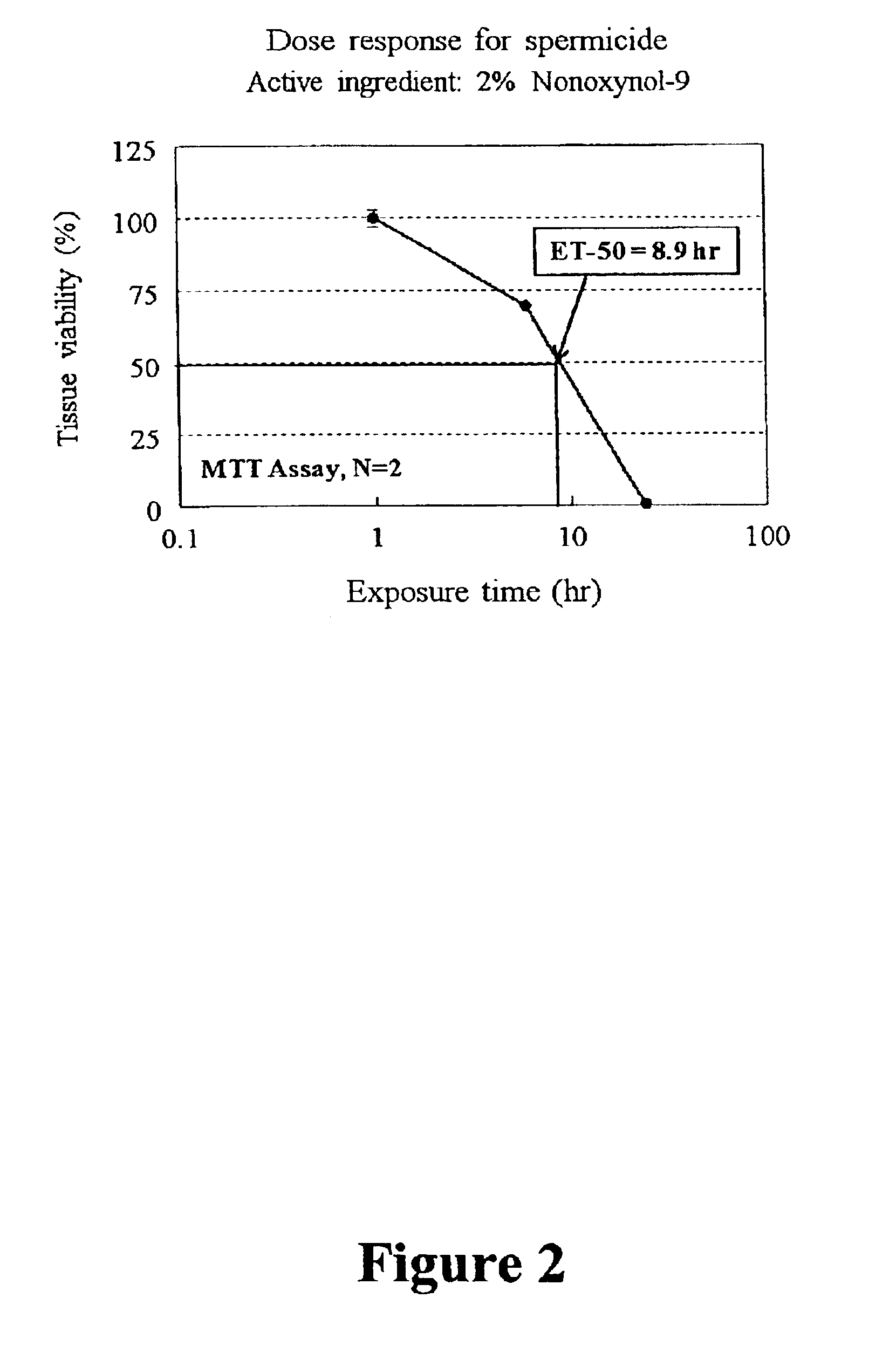
Disclosed is a cervico-vaginal tissue equivalent comprised of vaginal epithelial cells and immune cells, cultured at the air-liquid interface. The tissue equivalent is capable of being infected with a sexually transmitted pathogen such as a virus (e.g., HIV), a bacteria, a helminthic parasite, or a fungus. The tissue equivalent is also capable of undergoing an allergic-type reaction or an irritant-type reaction. The tissue equivalent is characterized as having nucleated basal layer cells and nucleated suprabasal layer cells, and further as having cell layers external to the suprabasal layer progressively increasing in glycogen content and progressively decreasing in nuclei content. Immune cells of the tissue equivalent are primarily located in the basal and suprabasal layers. Also disclosed are methods for producing the tissue equivalent. The methods involve providing vaginal epithelial cells and immune cells, seeding the cells onto a porous support, and co culturing the seeded cells at the air-liquid interface under conditions appropriate for differentiation. One such method disclosed is for generation of the tissue equivalent in serum free medium. Specific cells from which the tissue equivalent is generated, and also specific preferred components of the medium in which the tissue equivalent is generated are provided. Also disclosed is a cervico-vaginal tissue equivalent produced by the methods disclosed herein.
Owner:MATTEK CORP
Delivery of therapeutic biologicals from implantable tissue matrices
InactiveUS20020031500A1Many of effectMany of inconveniencePowder deliveryBiocideProgenitorActive agent
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
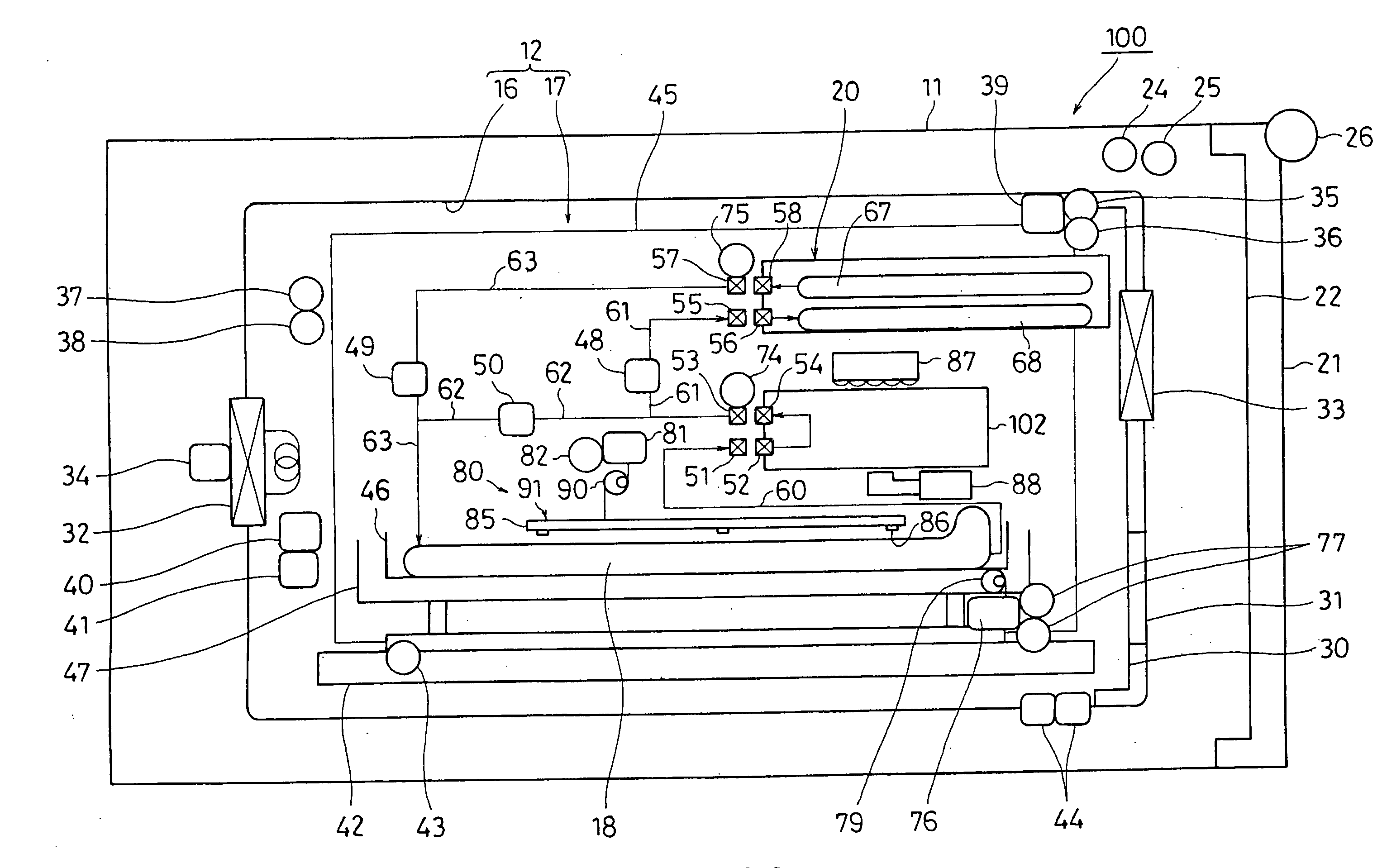
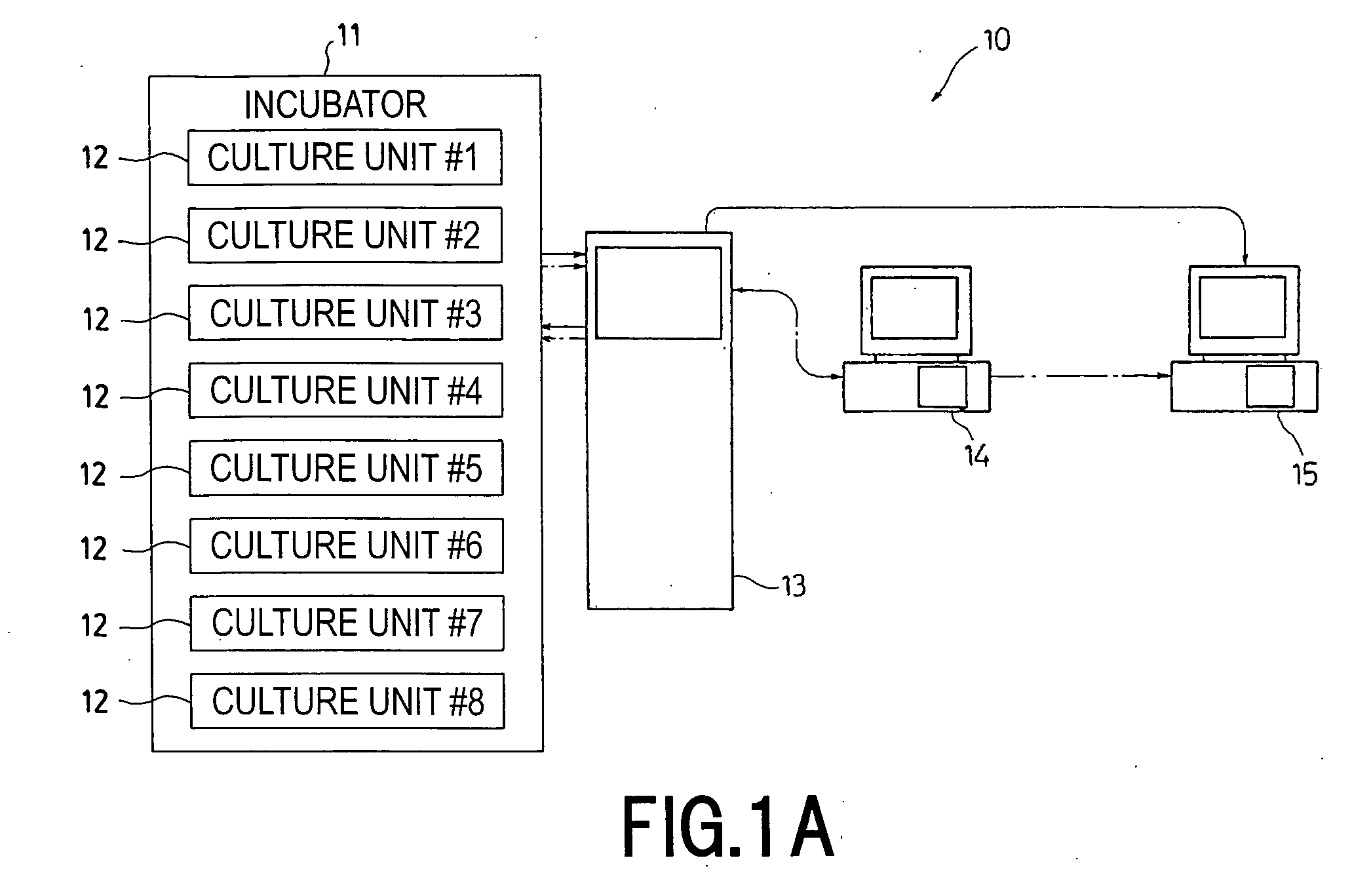
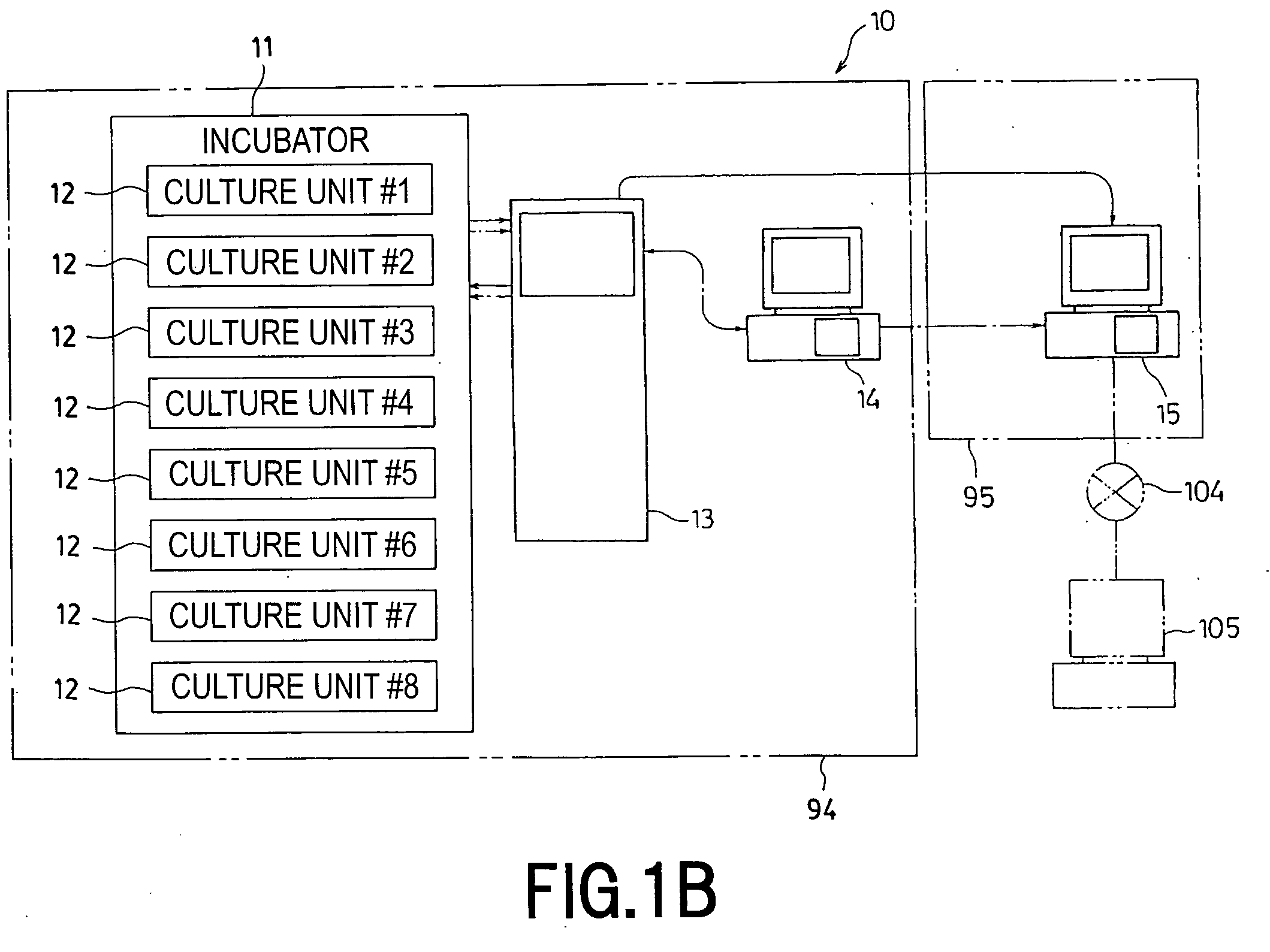
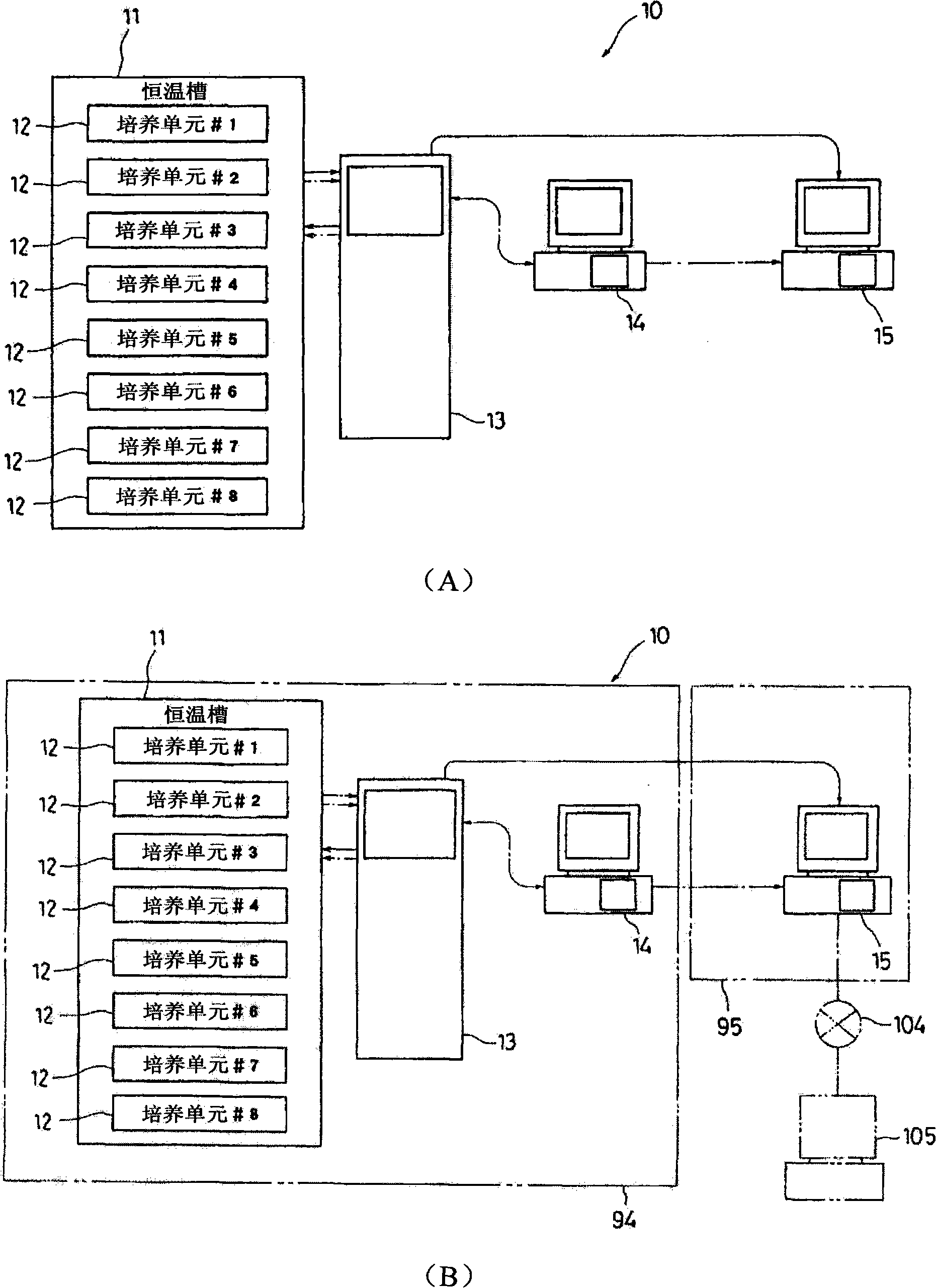
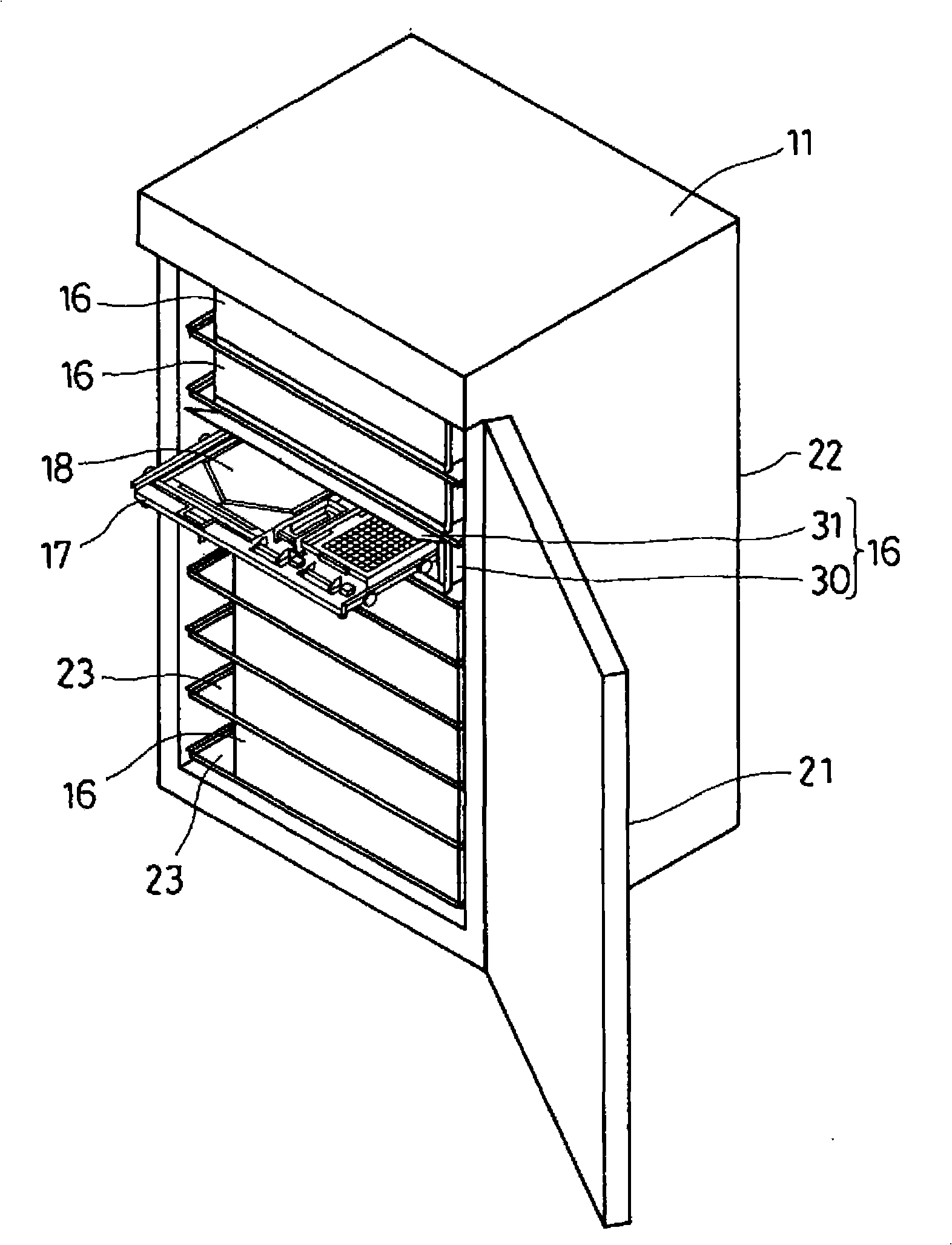
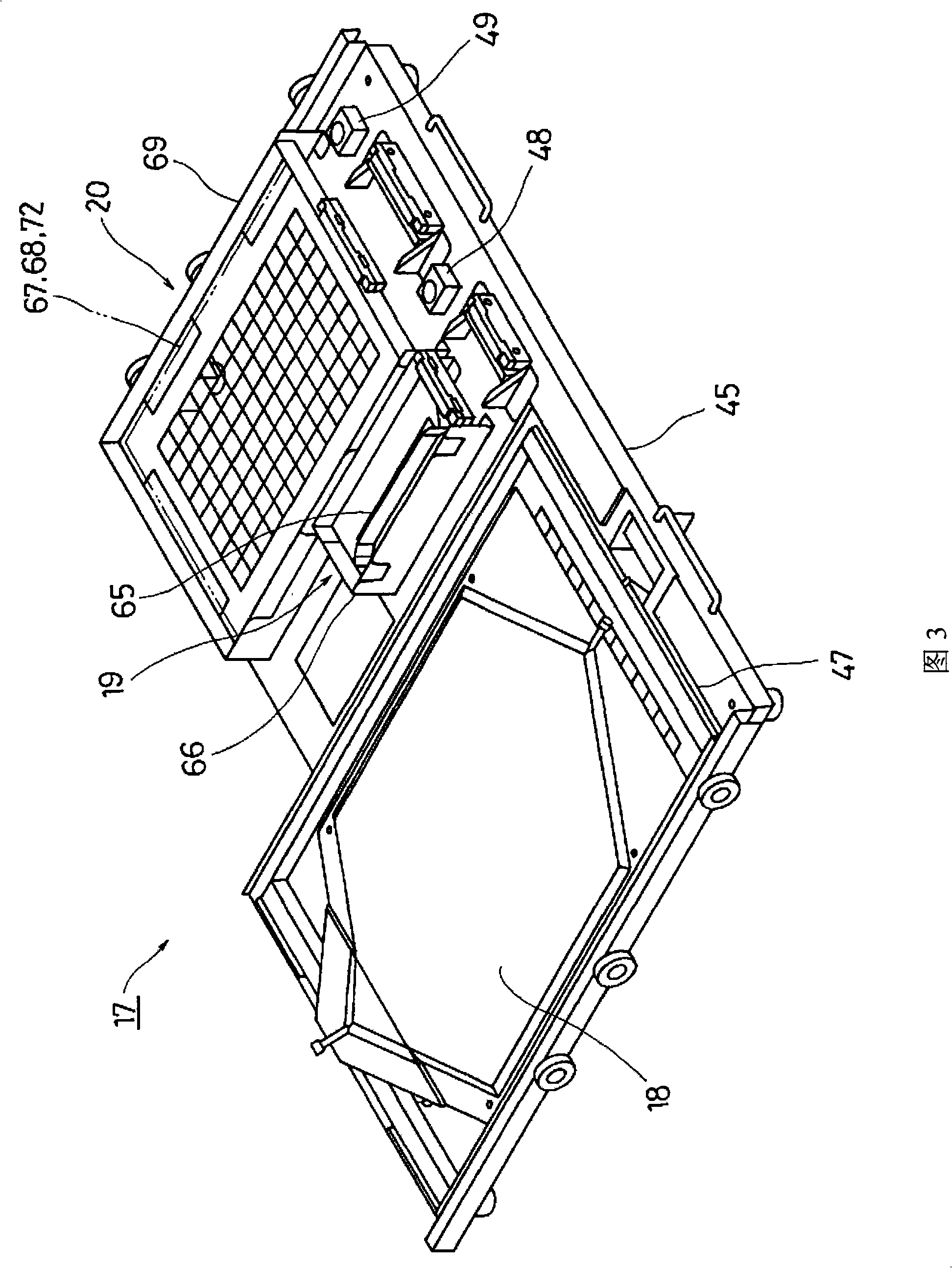
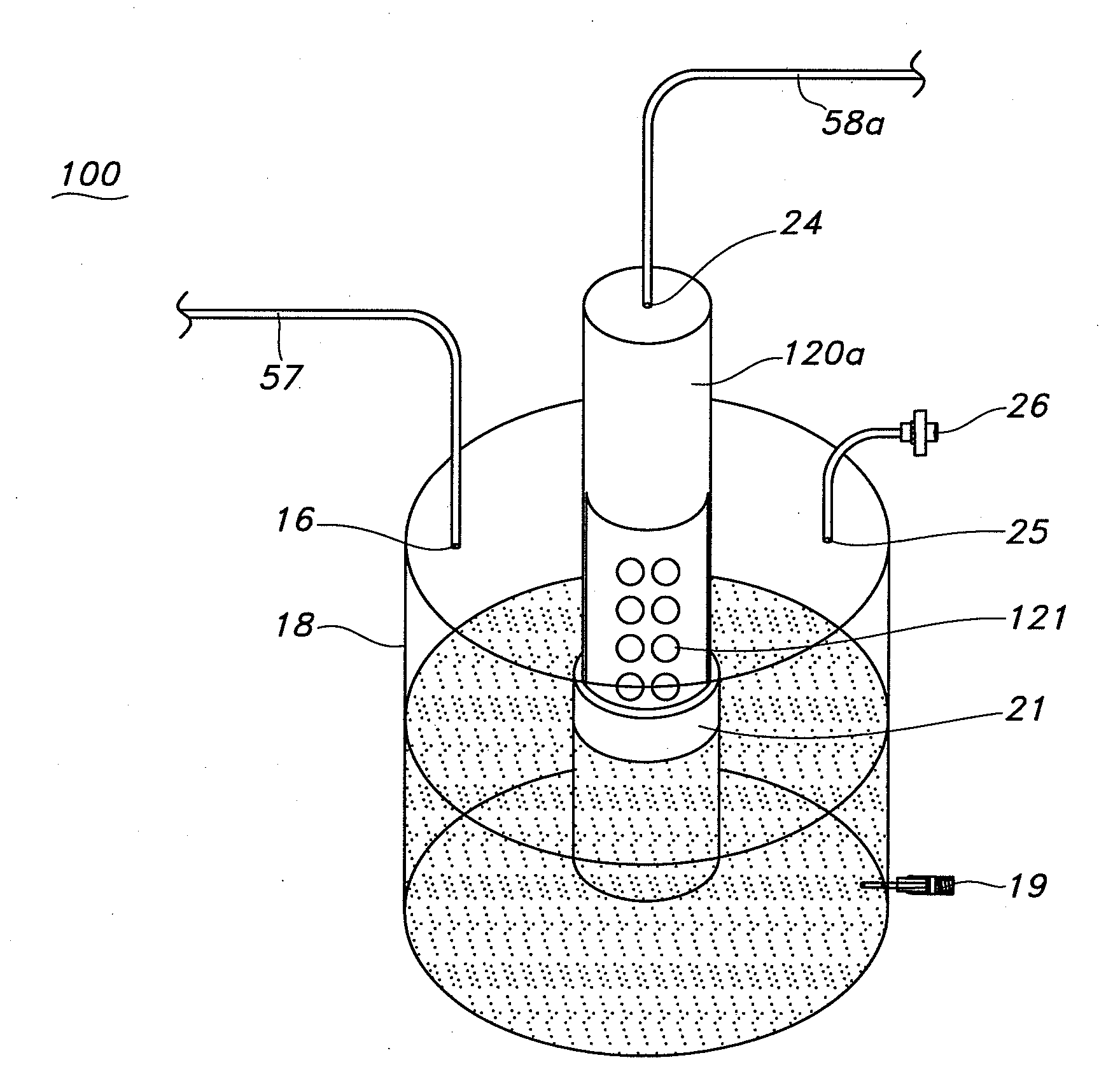
Cell Culture Apparatus, Cell Culture Method, Cell Culture Program and Cell Culture System
InactiveUS20090042293A1Increase cell densityReduce the number of timesBioreactor/fermenter combinationsBiological substance pretreatmentsImaging processingCulture vessel
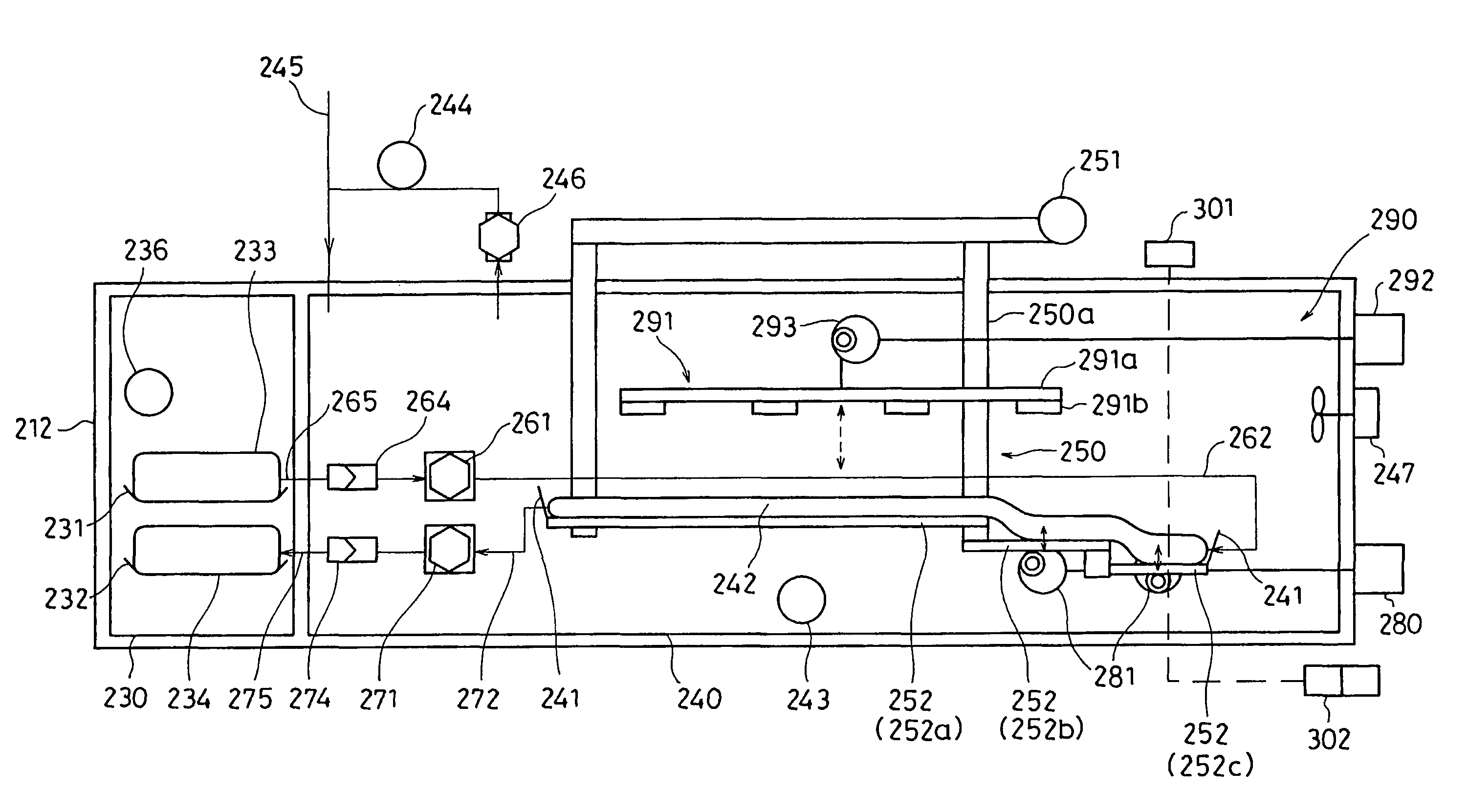
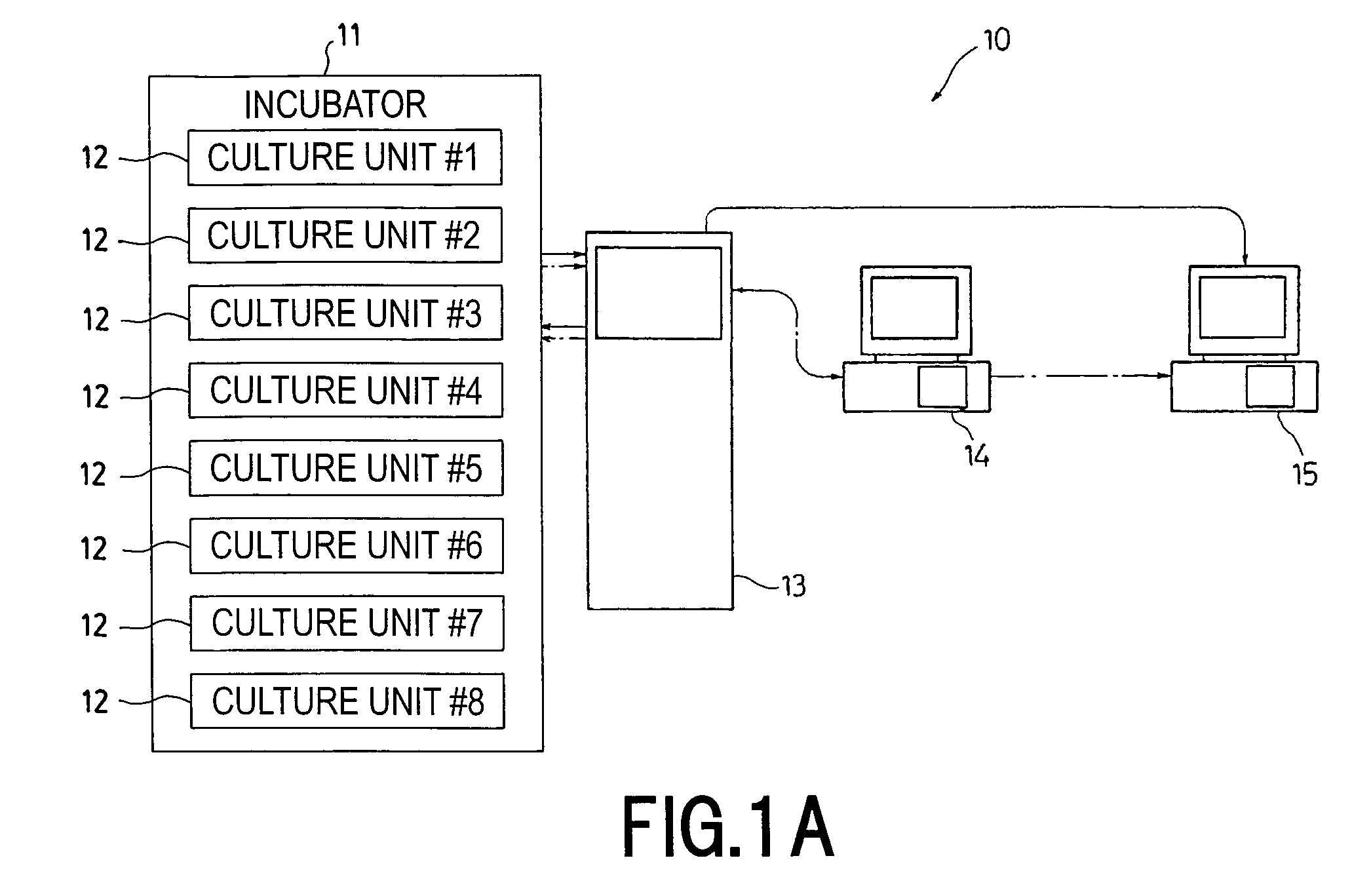
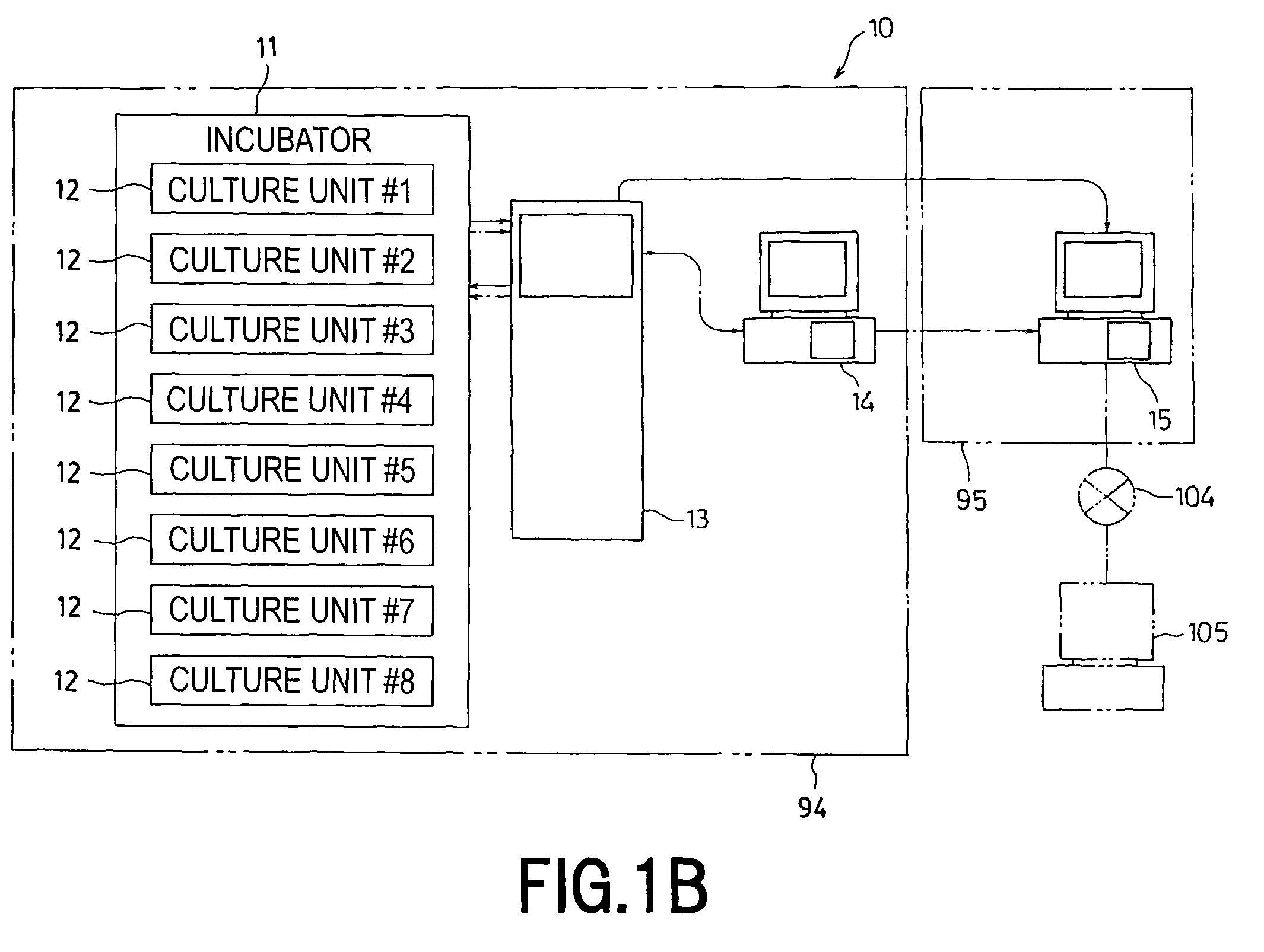
The invention intends to provide a cell culture apparatus which is able to realize an adequate culture according to the culture state of cells while alleviating the labor of an operator. The cell culture apparatus includes a culture bag for causing the cells to proliferate, a cell inoculation cassette (or culture bag as an antibody stimulating and proliferation culture vessel) for stimulating the cells by an inducer for the proliferation, a culture medium cassette for storing a culture medium supplied to the culture bag and the cell inoculation cassette, a CCD camera 88 for acquiring images of the cells in the cell inoculation cassette, and an image processing computer and an operation control computer for determining the culture state (proliferation capability and proliferation ability of the cells) of the cells from the images of the cells acquired by the CCD camera, and causing a culture operation to be carried out on the basis of the determination.
Owner:MEDINET CO LTD
Two-layer cell culture system organ chip and preparation method thereof
InactiveCN103981096AEasy to observeReduce dosageBioreactor/fermenter combinationsBiological substance pretreatmentsHuman bodyMicro structure
The invention relates to a two-layer cell culture system organ chip and a preparation method thereof. The organ chip comprises a two-layer cell culture system, wherein each layer comprises a culture solution micro-fluid channel, a medicament micro-fluid channel, cell culture chambers and a medicament testing tank; a micro structure and micro-fluid channels are designed on the organ chip, two cells are respectively fixed on each specific cell culture chamber, and intercellular signal transmission and interaction between the cells are performed by virtue of the micro-fluid channels. The organ chip realizes the parallel implantation and co-culture of two or more cells, is simple in operation, reduces the dose of practical samples, simplifies the cell implantation process, has the characteristics of portability, economic performance, high efficiency and accuracy, and can be used for independently performing cell seeding and culture and detection of medicament toxicity or pharmacological activity. The two-layer cell culture system organ chip is a novel organ chip with miniaturization, automation and visualization, prepared by simulating the structures and functions of a human organ, and can be used for providing the effective theoretical basis for tissue and regeneration engineering, organ transplantation and medicament evaluation.
Owner:SOUTHEAST UNIV
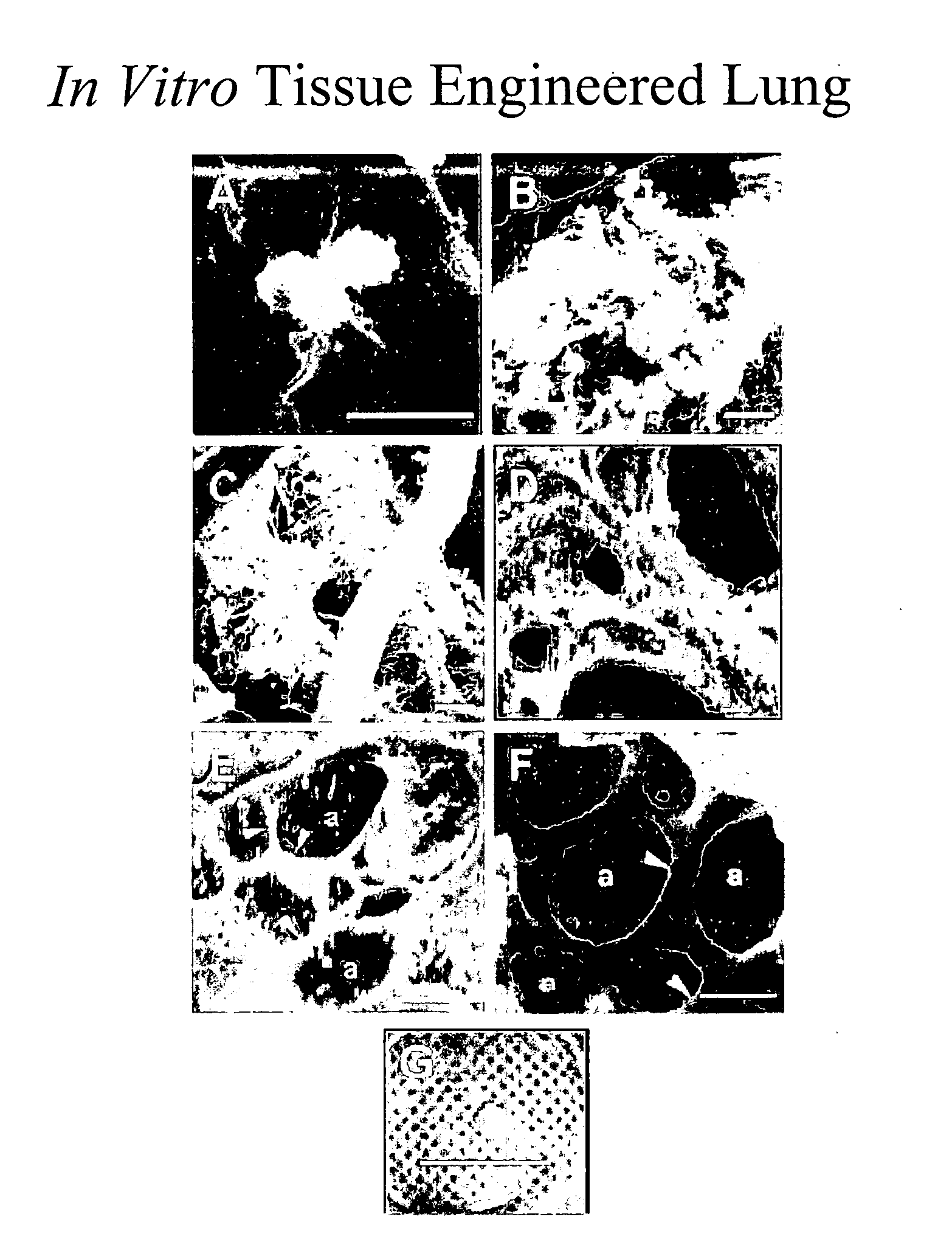
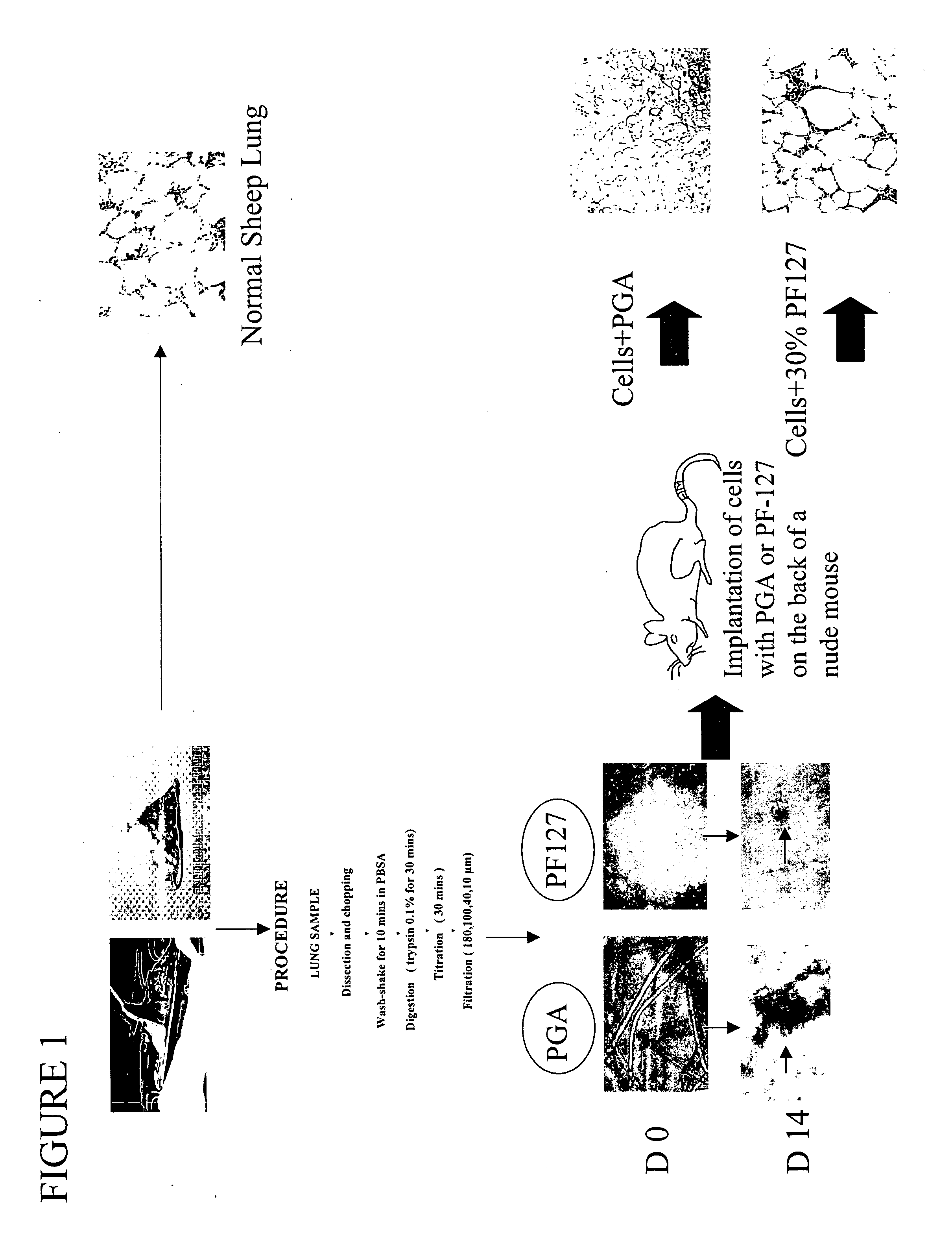
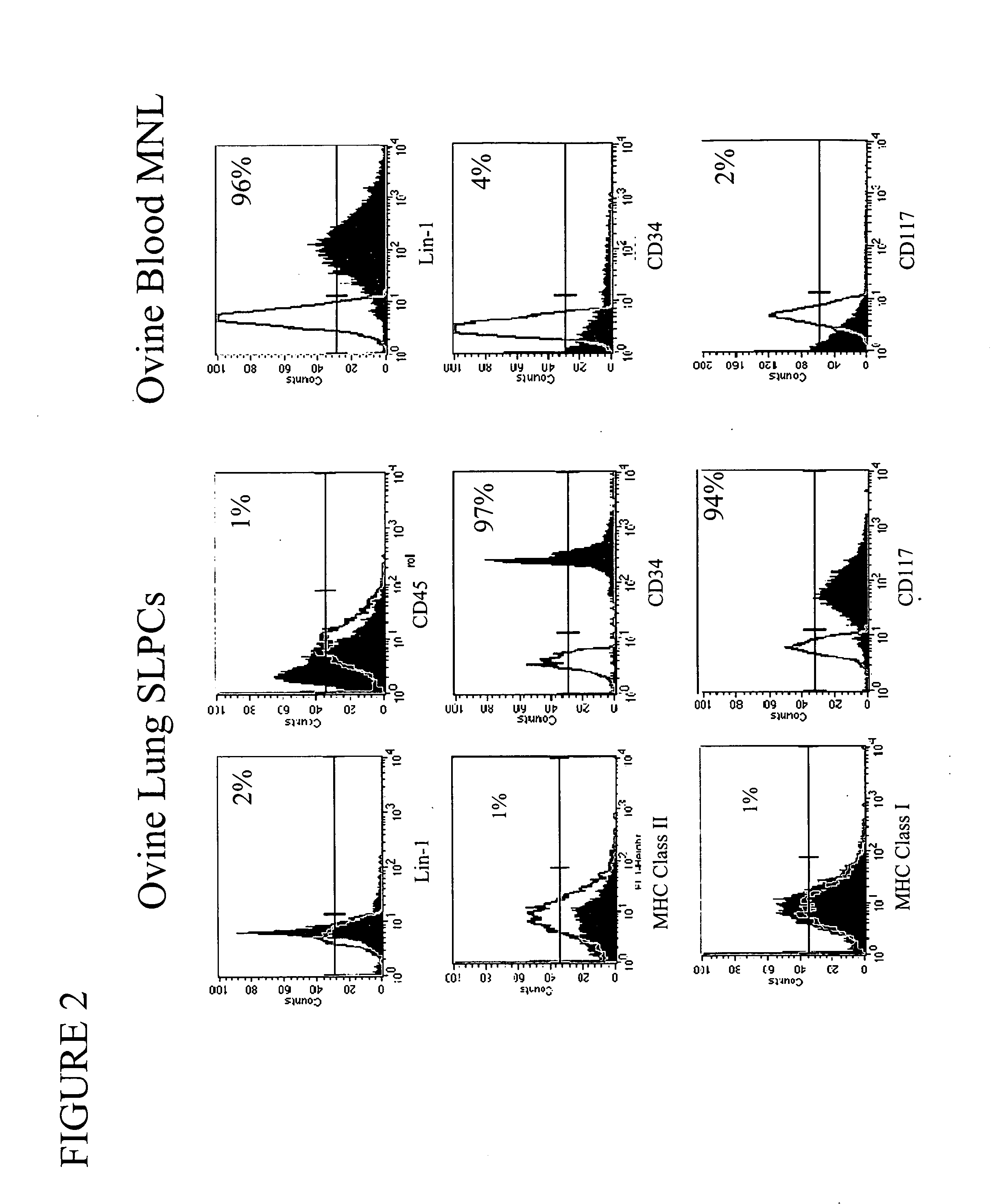
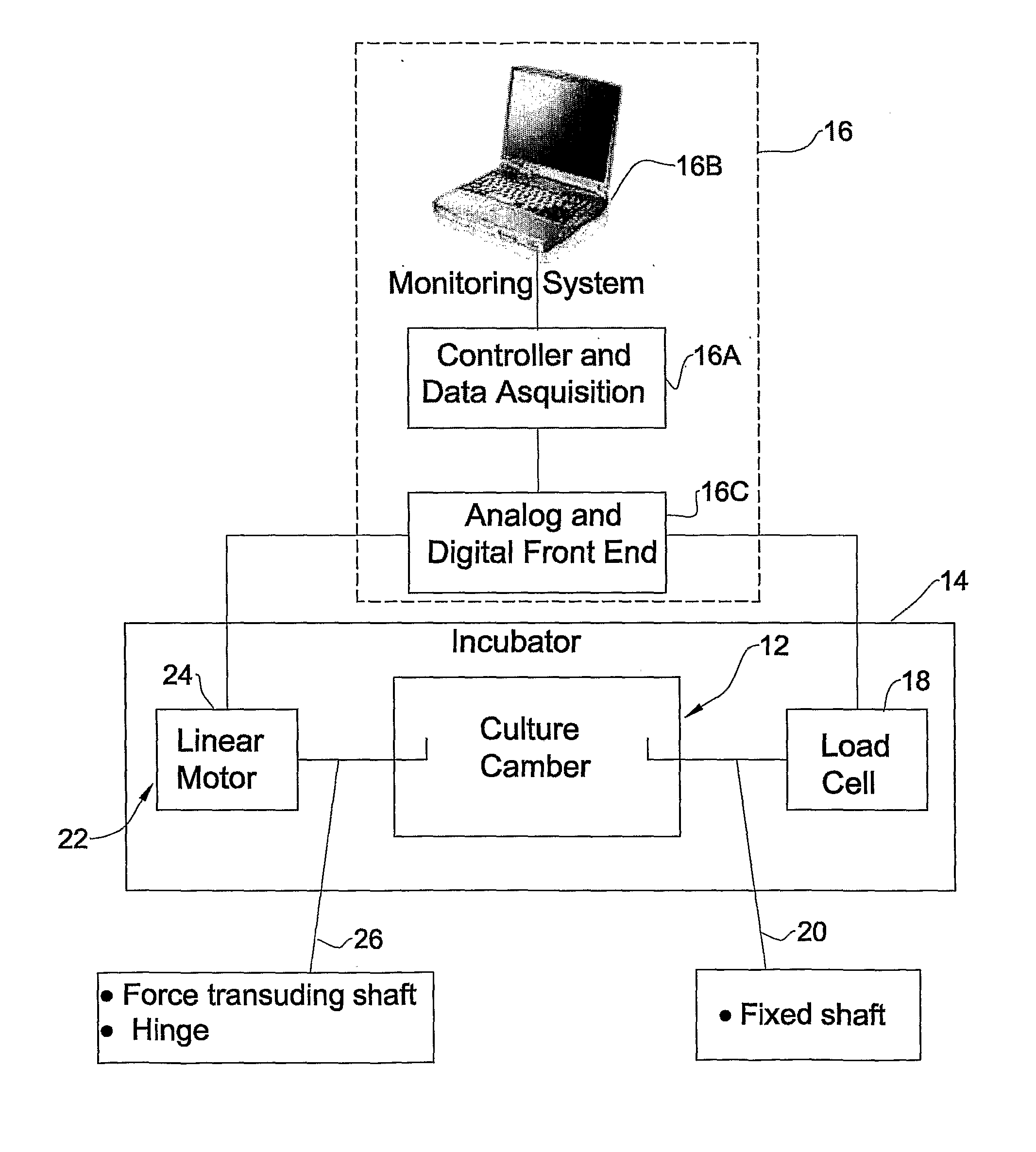
Engineered lung tissue, hydrogel/somatic lung progenitor cell constructs to support tissue growth, and method for making and using same
Somatic lung progenitor cell / polymer constructs are disclosed along with methods for isolating somatic lung progenitor cells from adult mammals, seeding the cells onto or into polymeric scaffolds and allowing the cells to differentiate and proliferate into functional lung tissue / polymer implants. A method for treating lung disease, disorders or injuries is also disclosed.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compartmental extract compositions for tissue engineering
The present invention concerns biologically-active cell-free scaffolds composed of extracts of cellular and / or extracellular compartments for use in tissue regeneration. The present invention also contemplates the novel concept of redesigning the biological scaffold by seeding cells thereon followed by cell elimination. Cells are seeded on the scaffold for a period of time during which a dynamic interaction occurs between the scaffold and the seeded cells, resulting in reshaping of the scaffold architecture and integration of newly synthesized matrix elements. Redesigning may improve the physical and biological characteristics of the scaffold, and also improve the matching of the scaffold to treat a specific target tissue or a specific patient, by seeding tissue-specific cells or by seeding cells which are autologous to a patient, respectively.
Owner:NAYACURE THERAPEUTICS
Methods and compositions of bioartificial kidney suitable for use in vivo or ex vivo
A novel cell seeded hollow fiber bioreactor is described as a potential bioartificial kidney. Endothelial cells along with pericyte, vascular smooth muscle, and / or mesangial cells or any mesenchymally derived support cells are seeded along a hollow fiber in a perfused bioreactor to reproduce the ultrafiltration function and transport function of the kidney. Maintenance of tissue specific function and ultrastructure suggest that this bioreactor provides an economical device for treating renal failure.
Owner:RGT UNIV OF MICHIGAN
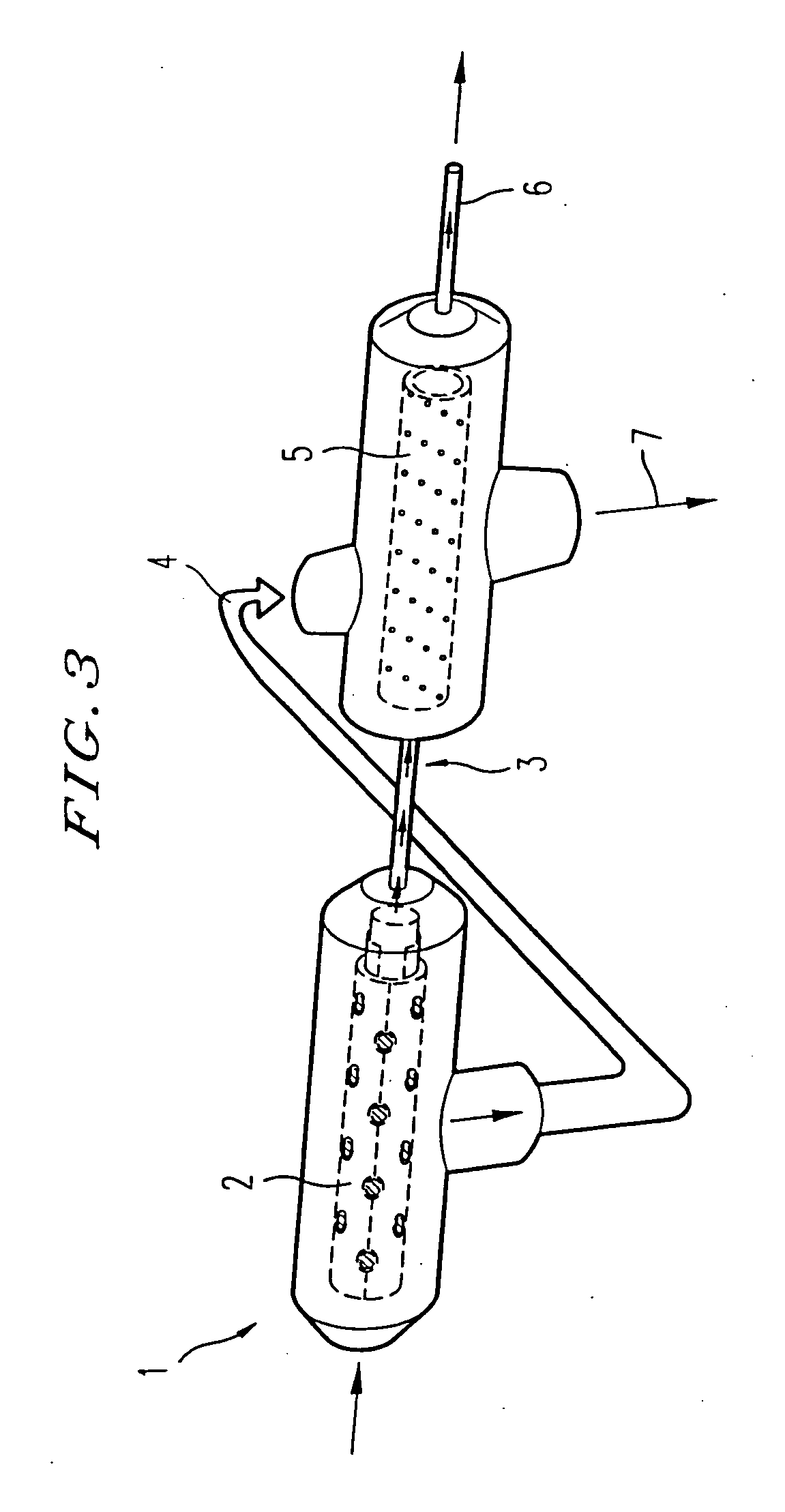
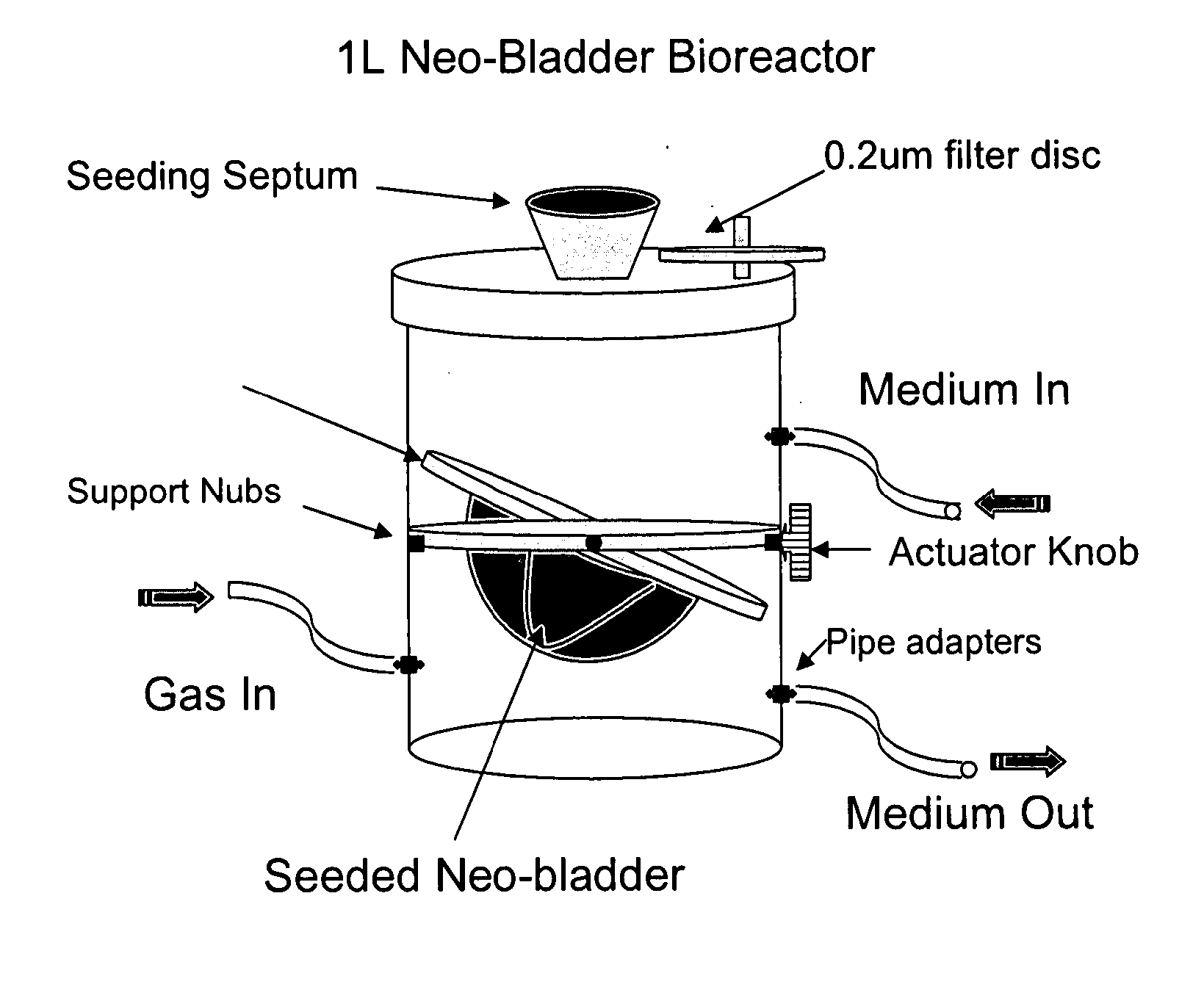
Bioreactor for organ reconstruction and augmentation
InactiveUS20070275363A1Inhibit cell growthBioreactor/fermenter combinationsBiological substance pretreatmentsSufficient timeUrothelial Cell
Bioreactors are used in neo-organ production to allow for an appropriate environment for the maintenance of healthy culturing conditions from pre-wetting to shipment of the neo-organ. The closed system “all-in-one bioreactor” is designed to allow for minimal exposure of the scaffold to the open air in order to maintain sterility. The design allows for the same container to be utilized for sterilization, pre-wetting, cell seeding, medium exchange, and shipment. The “all-in-one” bioreactor also remains completely closed after the urothelial cell seeding step to the implantation at the clinical site. This allows for sufficient time for release testing to occur so the neo-organ can be implanted into the patient.
Owner:TENGION
Dual-chamber perfusion bioreactor for orthopedic tissue interfaces and methods of use
InactiveUS20110229970A1Modulate communicationModulate interactionBioreactor/fermenter combinationsBiological substance pretreatmentsPerfusion bioreactorCultured cell
The subject invention concerns a perfusion bioreactor device and methods of using the same. A bioreactor device of the invention can be used to grow cells and tissue in a controlled in vitro environment. A perfusion bioreactor device of the invention can have multiple perfusion chambers that can be controlled individually. Transverse or parallel flow of a fluid can be provided to each chamber. Cells can be seeded on a hydrogel and / or 3D scaffold to provide a 3D environment in the bioreactor device where the cells can adhere, proliferate, migrate, secrete growth and / or differentiation factors, and / or undergo differentiation, etc. The subject invention also concerns hydrogels and 3D scaffolds that can be used to grow and / or differentiate cells thereon.
Owner:FLORIDA STATE UNIV RES FOUND INC
Engineered osteochondral construct for treatment of articular cartilage defects
ActiveUS20070009610A1Recovery functionRelief the painBiocideMammal material medical ingredientsBone structureFibroblast
A method of growing chondrocytes on an allograft cancellous bone structure which had been previously treated by demineralization to form a cartilage repair implant comprises isolating allograft chondrocytes from articular cartilage of a donor other than the patient on which the implant is to be used, cultivating the isolated chondrocytes in a medium to obtain a dedifferentiated fibroblast-like phenotype, placing an at least partially demineralized allograft cancellous bone structure carrier having at least one surface prepared for cell seeding in a culture vessel; adding the cultivated dedifferentiated chondrocytes to a demineralized surface of the cancellous bone structure carrier, and incubating the cancerous bone structure carrier for a period of time of at least 40 days to obtain an implant with a cartilage layer which ranges from about 2 mm to 5 mm n thickness.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Cell culture apparatus, cell culture method, cell culture program and cell culture system
InactiveUS8383395B2Avoid volatilityCross-contamination of the cells is avoidedBioreactor/fermenter combinationsBiological substance pretreatmentsImaging processingCulture vessel
The invention intends to provide a cell culture apparatus which is able to realize an adequate culture according to the culture state of cells while alleviating the labor of an operator. The cell culture apparatus includes a culture bag for causing the cells to proliferate, a cell inoculation cassette (or culture bag as an antibody stimulating and proliferation culture vessel) for stimulating the cells by an inducer for the proliferation, a culture medium cassette for storing a culture medium supplied to the culture bag and the cell inoculation cassette, a CCD camera 88 for acquiring images of the cells in the cell inoculation cassette, and an image processing computer and an operation control computer for determining the culture state (proliferation capability and proliferation ability of the cells) of the cells from the images of the cells acquired by the CCD camera, and causing a culture operation to be carried out on the basis of the determination.
Owner:MEDINET CO LTD
Cell culture apparatus, cell culture method, cell culture program and cell culture system
InactiveCN101300340AReduce laborProper trainingTissue cultureTissue/virus culture apparatusImaging processing3D cell culture
The invention intends to provide a cell culture apparatus which is able to realize an adequate culture according to the culture state of cells while alleviating the labor of an operator. The apparatus includes: a culture bag (18) for causing the cells to proliferate; a cell inoculation cassette (19) (or culture bag (242) as an antibody stimulating and proliferation culture vessel) for stimulating the cells by an inducer for the proliferation; a culture medium cassette (20) for storing a culture medium supplied to the culture bag (18) and the cell inoculation cassette (19); a CCD camera (88) for acquiring images of the cells in the cell inoculation cassette; and an image processing computer and an operation control computer for determining the culture state (proliferation capability and proliferation ability of the cells) of the cells from the images of the cells acquired by the CCD camera, and causing a culture operation to be carried out on the basis of the determination.
Owner:迈世耐特股份公司
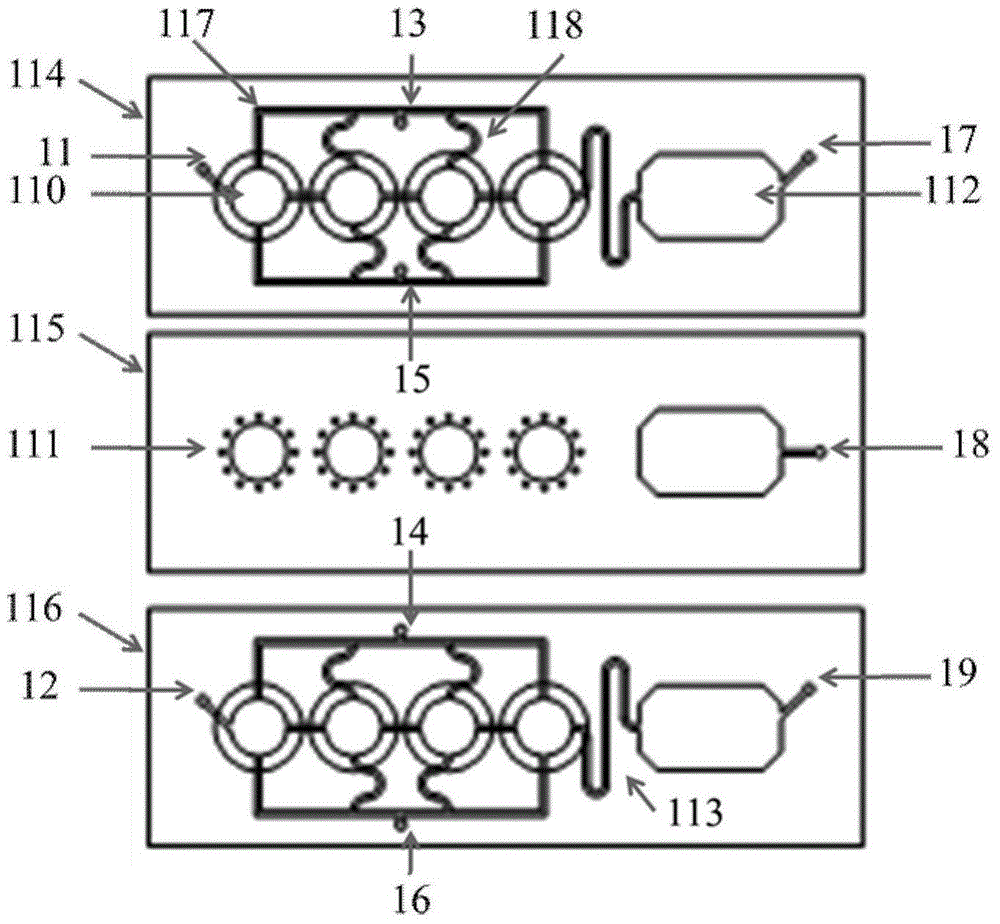
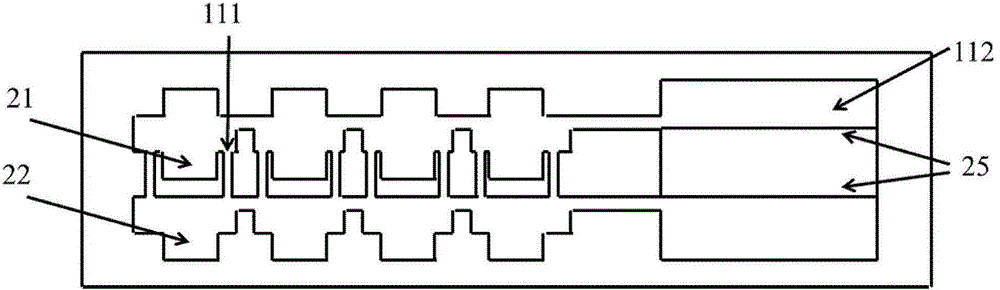
System For Seeding Cells Onto Three Dimensional Scaffolds
ActiveUS20110281358A1Bioreactor/fermenter combinationsBiological substance pretreatmentsTissue GraftThree dimensional scaffolds
Systems are provided for convenient and sterile isolation, collection, and seeding of cells onto a scaffold or tissue graft. The systems may be closed. Methods for use of the disclosed systems for isolation, collection and seeding of cells and generation of tissue engineered vascular grafts are also provided. The systems may be supplied in kits for efficient and expeditious use.
Owner:YALE UNIV +1
Osteoinduction of cortical bone allografts by coating with biopolymers seeded with recipient periosteal bone cells
InactiveUS6899107B2Improve clinical outcomesLower immune responseBone implantDiagnosticsBiopolymerBone Cortex
A method by which immune responses to cortical bone grafts and other substrates (e.g., cement, IPN, etc.) can be minimized and at the same time graft osteoinductive potential can be improved, and improved graft substrate materials are disclosed. The method of the invention provides new types of bone grafts that incorporate into host bone more thoroughly and more rapidly, eliminating long-term complications, such as fracture, non-union, infection, and rejection. In the method of the invention, bone grafts or other substrates are modified to have an osteoinductive surface modification that the recipient's body will accept as its own tissue type and therefore will not reject or otherwise cause to fail. The osteoinductive surface modification comprises a biopolymer matrix coating that is seeded with periosteal cells that have been previously harvested either from the graft recipient or from an allogenic or xenogenic donor source.
Owner:DEPUY MITEK INC
Method for preparing human umbilical cord mesenchymal stem cells
The invention discloses a method for preparing human umbilical mesenchymal stem cells, which comprises the following steps: (1) removing residual blood from the human umbilical cord, shearing the umbilical cord and digesting the umbilical cord by collagenase; (2) adding pancreatin into the umbilical cord to digest the umbilical cord; (3) filtering the digestive solution by using a screen, and removing tissues which are not digested; (4) diluting the filtered digestive solution by using phosphate buffer solution; (5) centrifugating the solution to obtain cells; and (6) inoculating the cells to a culture medium to culture so as to obtain large amount of human umbilical cord mesenchymal stem cells. The obtained cells can meet the requirements of clinical and experimental study, and lay a foundation for the future application.
Owner:TIANJIN AMCELLGENE ENG
Biomatrix composition and methods of biomatrix seeding
ActiveUS8865199B2Good treatment effectAccelerated remodelingBioreactor/fermenter combinationsBiocideLipid formationAutologous tissue
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com