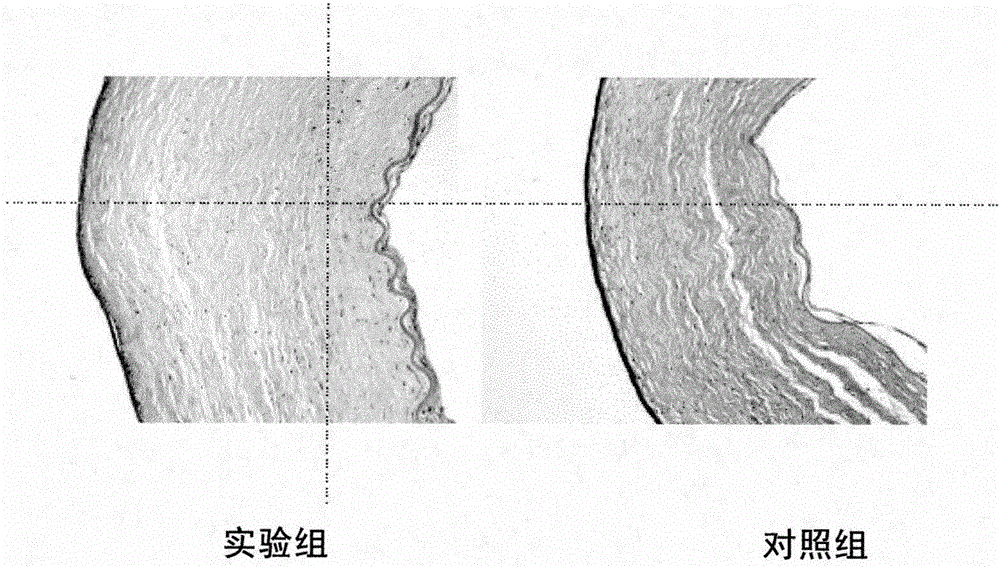
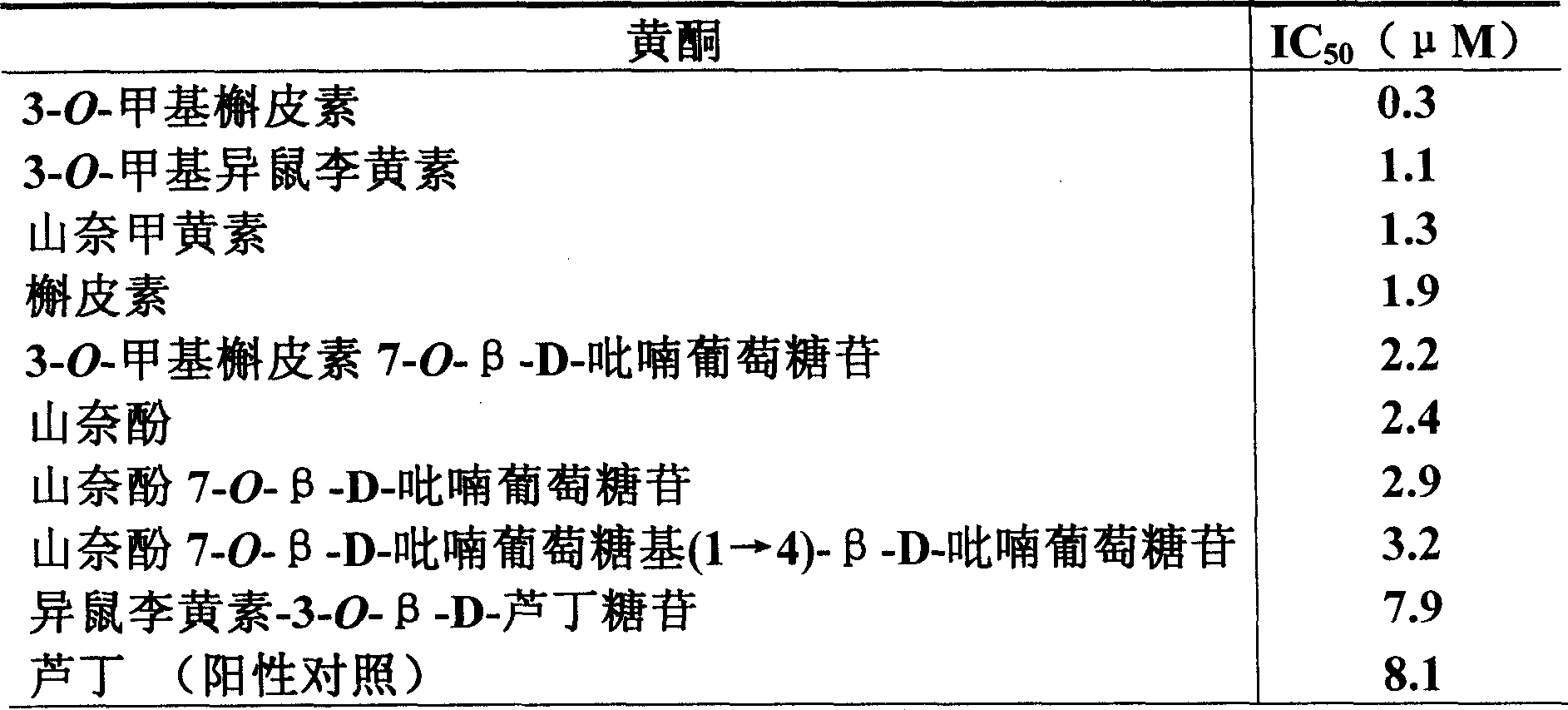
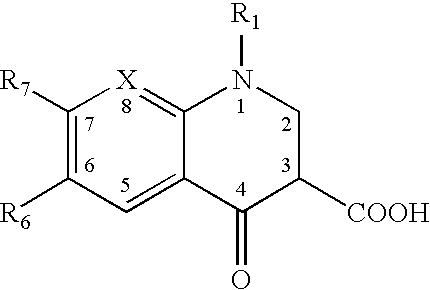
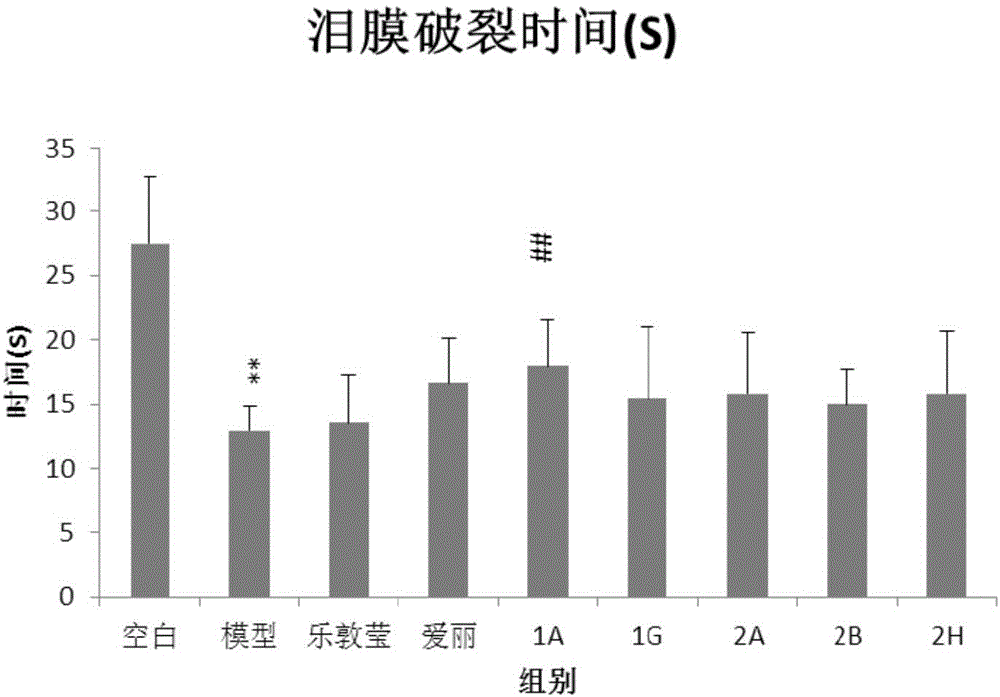
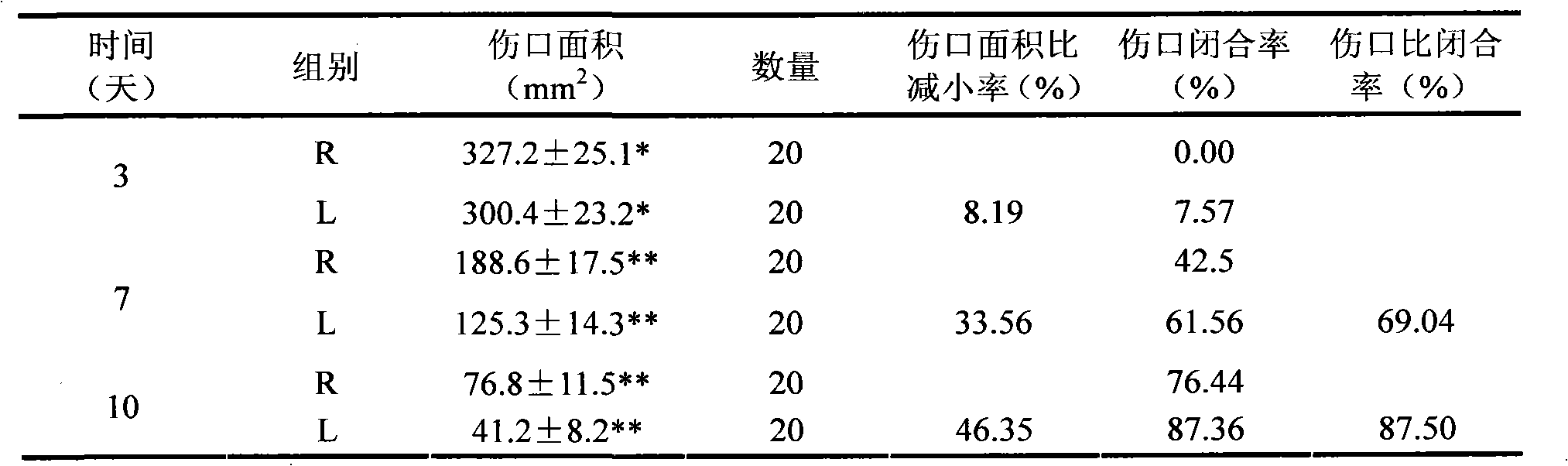
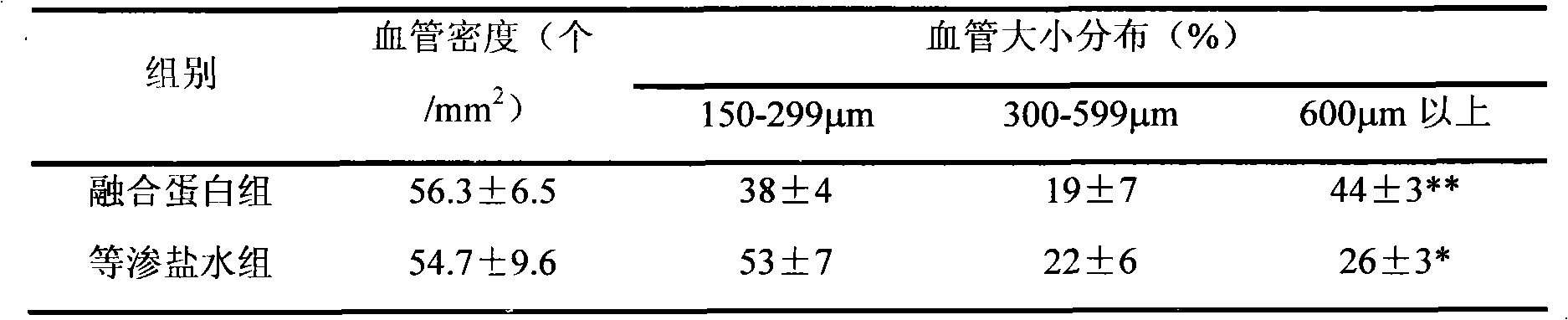
Patents
Literature
86 results about "Corneal Injury" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tissue engineered cornea epithelial transplantation membrane and preparation method and use thereof
InactiveCN101306207AMaintain biological characteristicsElasticEye implantsTransplanted corneaOphthalmology
The invention discloses a tissue engineering cornea epithelial transplantation membrane, a preparing method and an application thereof. The invention aims to provide a tissue engineering cornea epithelial transplantation membrane, the tissue engineering cornea epithelial transplantation membrane comprises a porcine cornea epithelial cell and nonantigenic tissue engineering cornea bracket material, wherein, the porcine cornea epithelial cell and the nonantigenic tissue engineering cornea bracket material are combined into a whole by adopting a tissue engineering method. And meanwhile, The invention further provides a method for preparing the tissue engineering cornea epithelial transplantation membrane, and a use of the tissue engineering cornea epithelial transplantation membrane during the process of preparing medical treatment material used for remedying blind eye disease caused by the pathological state and the damage of corneas.
Owner:PEKING UNIV THIRD HOSPITAL
Eye care composition as well as preparation method and application thereof
ActiveCN111991415APromote recoveryAvoid complicationsAntibacterial agentsSenses disorderStaphyloccocus aureusPseudomonas
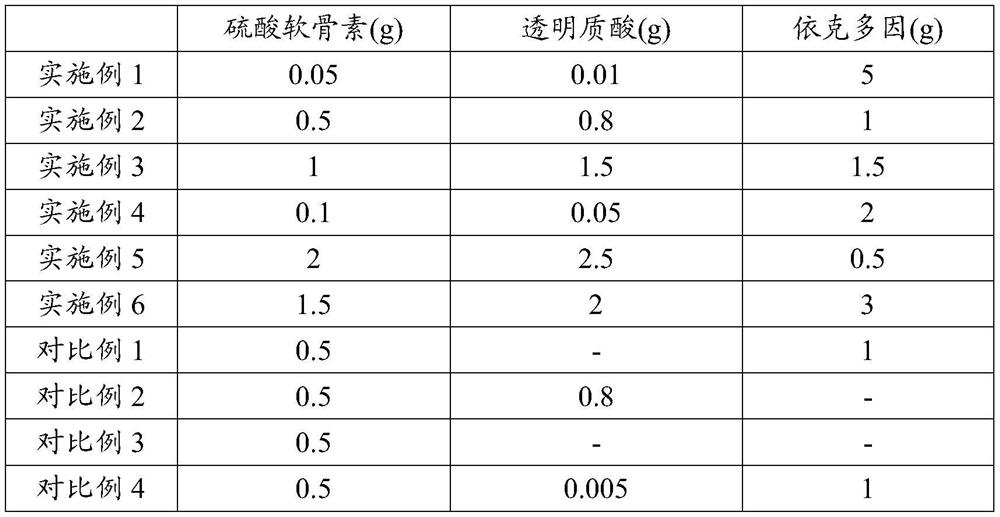
The invention provides an eye care composition. The eye care composition is prepared from chondroitin sulfate, hyaluronic acid and ectoin or an ophthalmologically acceptable ectoin derivative. In theeye care composition, the chondroitin sulfate accounts for 0.05 to 2 percent by mass preferably 0.1%-1%; the mass percentage of the hyaluronic acid is 0.01%-2.5%, preferably 0.05%-1.5%; and the masspercentage of the ectoine or the ophthalmologically acceptable ectoine derivative is 0.5%-5%, preferably 0.5%-2%. Compared with the prior art, according to the eye care composition disclosed by the invention, hyaluronic acid, ectoin and chondroitin sulfate are combined according to a certain weight ratio, so that the repair of corneal injury can be accelerated; the anti-fatigue effect is remarkable, the recovery time of iris congestion and swelling is short, and the growth of escherichia coli, staphylococcus aureus, pseudomonas aeruginosa and candida albicans is well inhibited. More importantly, the irritation of chondroitin sulfate can be obviously relieved.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Bioactive material promoted stem cell secreted exosome in treatment of corneal injuries
ActiveCN108743620AImprove retentionGood treatment effectSenses disorderPharmaceutical delivery mechanismApoptosisBiocompatibility Testing
The invention relates to biomaterial hydrogel eye drops containing stem cell secreted exosome, good in cellular biocompatibility and biological activity. Active hydrogel can serve as the eye drops, and the exosome is slowly released into damaged tissue; the hydrogel can also enhance retention and local concentration of the exosome at injured sites to promote repair of eye tissue injuries, promotecorneal cells to survive and proliferate, reduce apoptosis, promote functional recovery of the injured sites, inhibit pathological angiogenesis and inhibit eye inflammation; meanwhile, after the active hydrogel is combined with the bioactive material, residence time of the exosome can be prolonged, drug treating times can be reduced, and accurate control of drug dosage is facilitated.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
Human keratinized cell growth factor-1 analogue preparation method and application thereof
ActiveCN101220092AHigh expressionHigh activitySenses disorderPeptide/protein ingredientsCorneal woundFibrosis
The invention provides a human keratinocyte growth factor -1 structure analogues KGF-1delta 23KGF(40S), an N end of an amino acid sequence of which lacks 23 amino acids, while the 40-bit cysteine point of which is mutated into a nonpolar amino acid. The invention also relates to a production method of the structure analogues, which carries out the fusion expression with a small ubiquitin related modifier gene mature peptide, while a fusion protein and the ubiquitin related modifier gene protease 1 co-express in the prokaryotes. In the process of fermentation expression, the ubiquitin related modifier gene protease 1 can hydrolyze the fusion protein to produce a soluble KGF-1delta 23KGF (40S). The human keratinocyte growth factor structure analogues can facilitate the proliferation of the keratinocyte cells, the growth of the hair follicle cells and inhibit the growth of the fibroblast cells, and has the functions of anti-scar, anti-fibrosis, epidermis healing facilitation and corneal wound reparation, etc.
Owner:吉林农大生物反应器工程有限公司
Stem cell induced culture solution used for eyes and preparation method and application thereof
ActiveCN105838672APromotes regenerative repairInhibit deteriorationSenses disorderSkeletal/connective tissue cellsVascular endotheliumCulture fluid
The invention belongs to the field of biotechnology and provides a stem cell induced culture solution used for eyes .The stem cell induced culture solution comprises a vascular endothelial cell growth factor (VEGF), a basic fibroblast growth factor (bFGF) and an epidermal growth factor (EGF); the content of the vascular endothelial cell growth factor (VEGF) is 0.5-2.5 ng / ml, the content of the basic fibroblast growth factor (bFGF) is 0.25-0.4 ng / ml, and the content of the epidermal growth factor (EGF) is 0.5-2.0 ng / ml .The stem cell (ASCs) in vitro conditioned culture medium from human eyepit fat is adopted, and a significant reinforcement function is achieved for regeneration and repair of corneal injury.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
New pharmaceutical of cactus total flavone and its preparation method
A medical application of general cactus flavone in preparing the natural medicine or health-care food acting to suppress aldose reductase for preventing and treating the complication of diabetes suchas the healing defect of corneal injury, cataract, neuropathy, retinopathy and renal disfunction. The said general cactus flavone is prepared by the solvent extraction method or resin adsorption method and conventional drying method.
Owner:SHENYANG PHARMA UNIVERSITY
Compositions including antibiotics and methods for using same
InactiveUS20080070908A1Easy to produceEasy to useAntibacterial agentsBiocideQuinoloneCorneal Infection
Compositions including a quinolone component, such as ofloxacin, having fungistatic activity in the compositions, present in an amount effective as an antibiotic when the composition is placed in a mammalian eye, a NSAID component present in an amount to reduce inflammation or pain when the composition is placed in the eye, and a carrier component in an amount effective to act as a carrier for the quinolone component and NSAID component are provided. Methods of using the present compositions, for example, to resolve microbial infections and / or to reduce inflammation and / or pain in a mammalian eye are included within the scope of the present invention. Methods for treating corneal injuries are also included. In addition, methods for treating ocular infections, for example, corneal infections, are included.
Owner:ALLERGAN INC
Application of herba dendrobii extractives for preparing medicine capable of treating xerophthalmia, and medicine
InactiveCN106581446AGood restorativeImprove repair effectSenses disorderInorganic non-active ingredientsXerophthalmiaMedicine use
The invention belongs to the field of traditional Chinese medicines, and particularly relates to application of herba dendrobii extractives for preparing a medicine capable of treating xerophthalmia. The invention also relates to a medicine used for treating the xerophthalmia. The herba dendrobii extractives can be applied in the medicine capable of treating the xerophthalmia. The medicine used for treating the xerophthalmia takes the herba dendrobii extractives as effective ingredients and comprises pharmaceutically acceptable carriers or auxiliary materials. The medicine can be prepared into any pharmaceutical dosage forms including injection preparation, oral preparation, mist spray preparation, ointment preparation, guttae ophthalmicae, patch and the like according to a common method for medicine preparation. According to the herba dendrobii extractives provided by the invention, the breakup time of a tear film is prolonged, and corneal injury is repaired so as to achieve a purpose on treating and preventing the xerophthalmia.
Owner:CHINA TRADITIONAL CHINESE MEDICINE
Recombinant protein PACAP38-NtA, and coding gene and application thereof
ActiveCN103145851AImprove repair effectNeurotrophicSenses disorderBacteriaEscherichia coliPurification methods
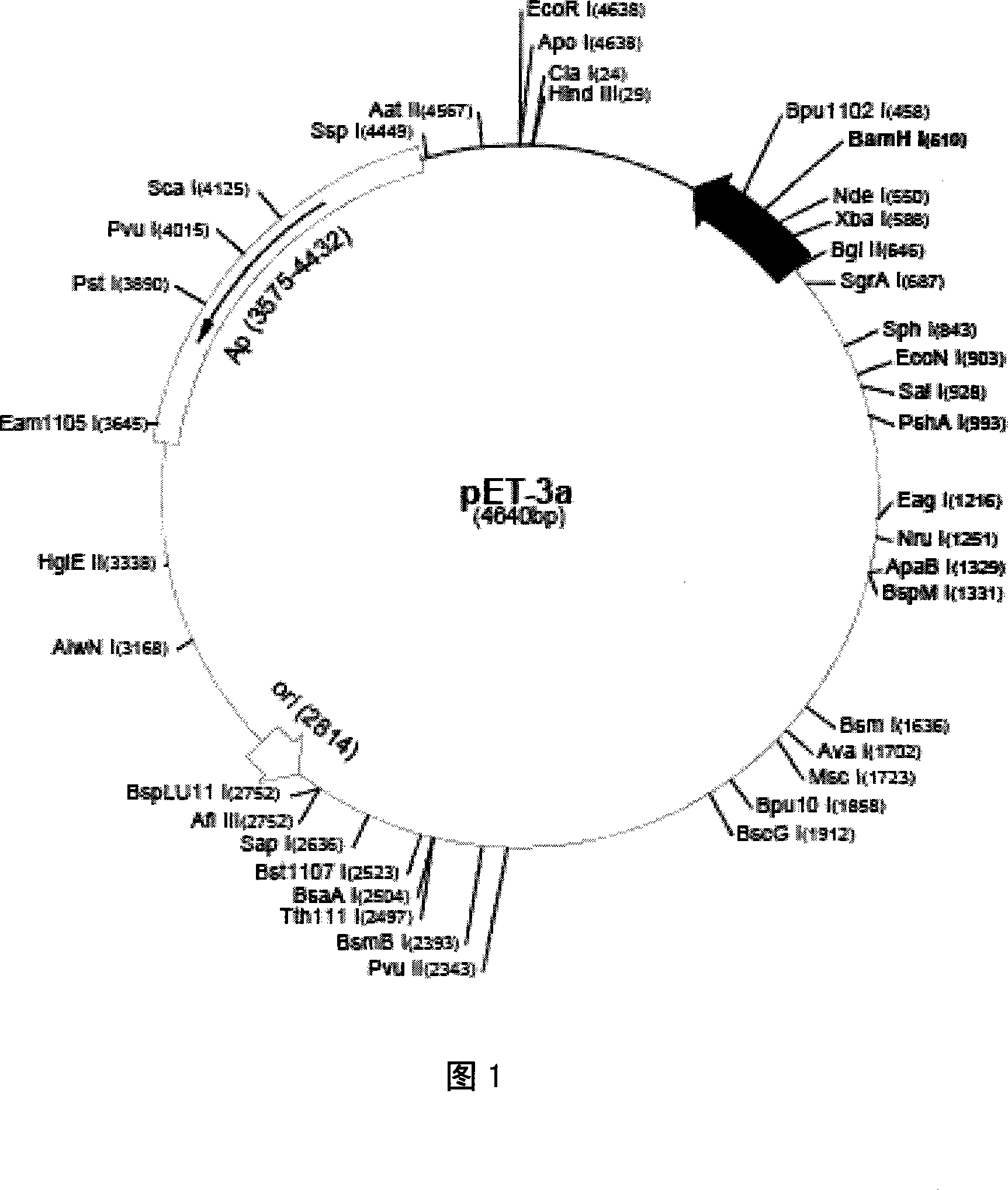
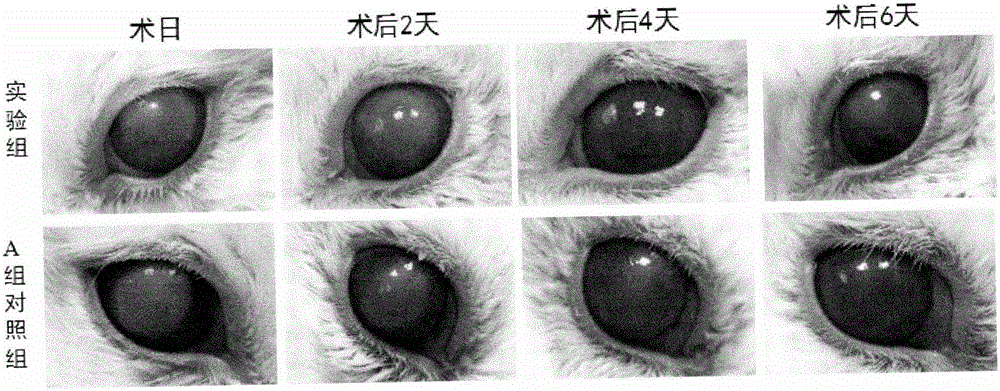
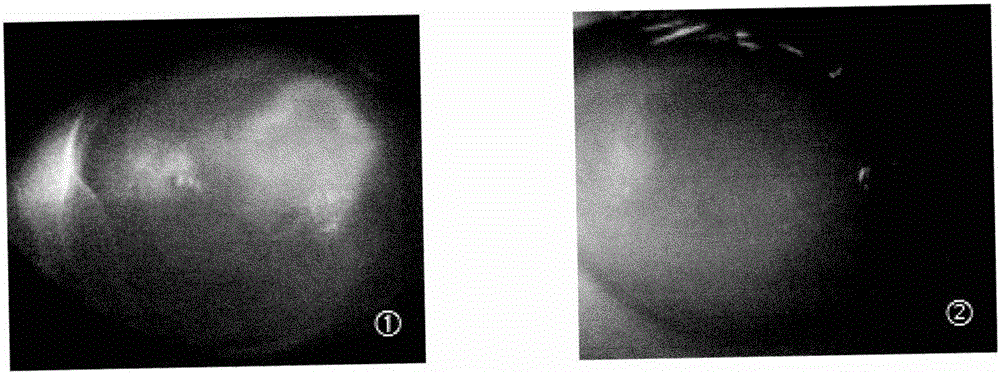
The invention discloses a recombinant protein PACAP38-NtA, and a coding gene and application thereof. The amino acid sequence of the recombinant protein PACAP38-NtA is disclosed as SEQ ID NO.1. The nucleotide sequence for coding the recombinant protein PACAP38-NtA is disclosed as SEQ ID NO.2. The nucleotide sequence disclosed as SEQ ID NO.2 is cloned into a prokaryotic expression vector pET-3c, and transfected into Escherichia coli BL21(DE3) to obtain the expression recombinant protein PACAP38-NtA strain. According to the invention, a minitype bioreactor is utilized to ferment the strain, and a two-step purification method comprising common ion-exchange chromatography and nickel-column affinity chromatography, which is simple to operate, is utilized to obtain the high-purity target protein. The obtained recombinant PACAP38-NtA fusion protein can be specifically combined with laminin, can effectively promote proliferation and differentiation of neuron-like cells PC12, and can enhance the restorability of the injured part when acting on the mouse corneal scratched part; and the recombinant PACAP38-NtA fusion protein can be can be used for preparing medicines for restoring corneal injuries.
Owner:广州恒宁生物科技有限公司
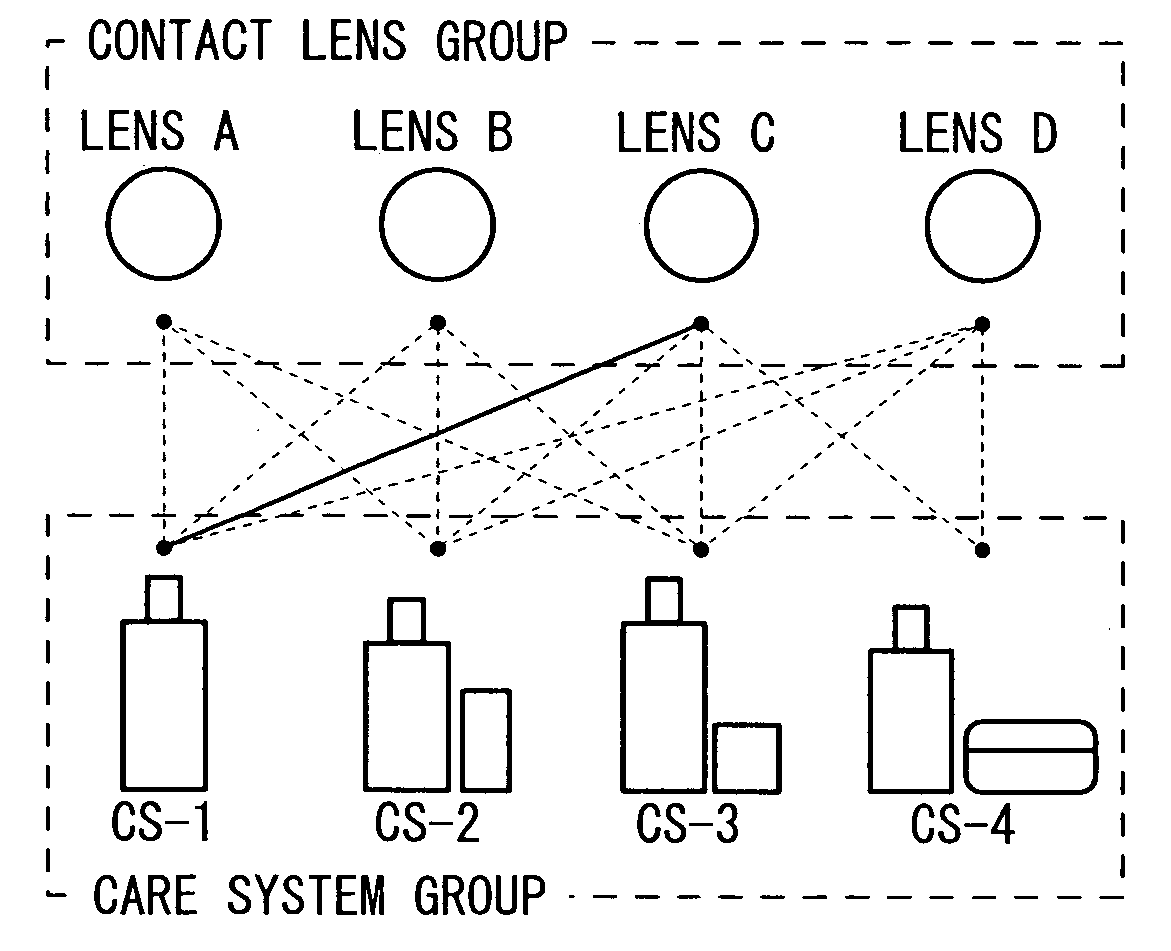
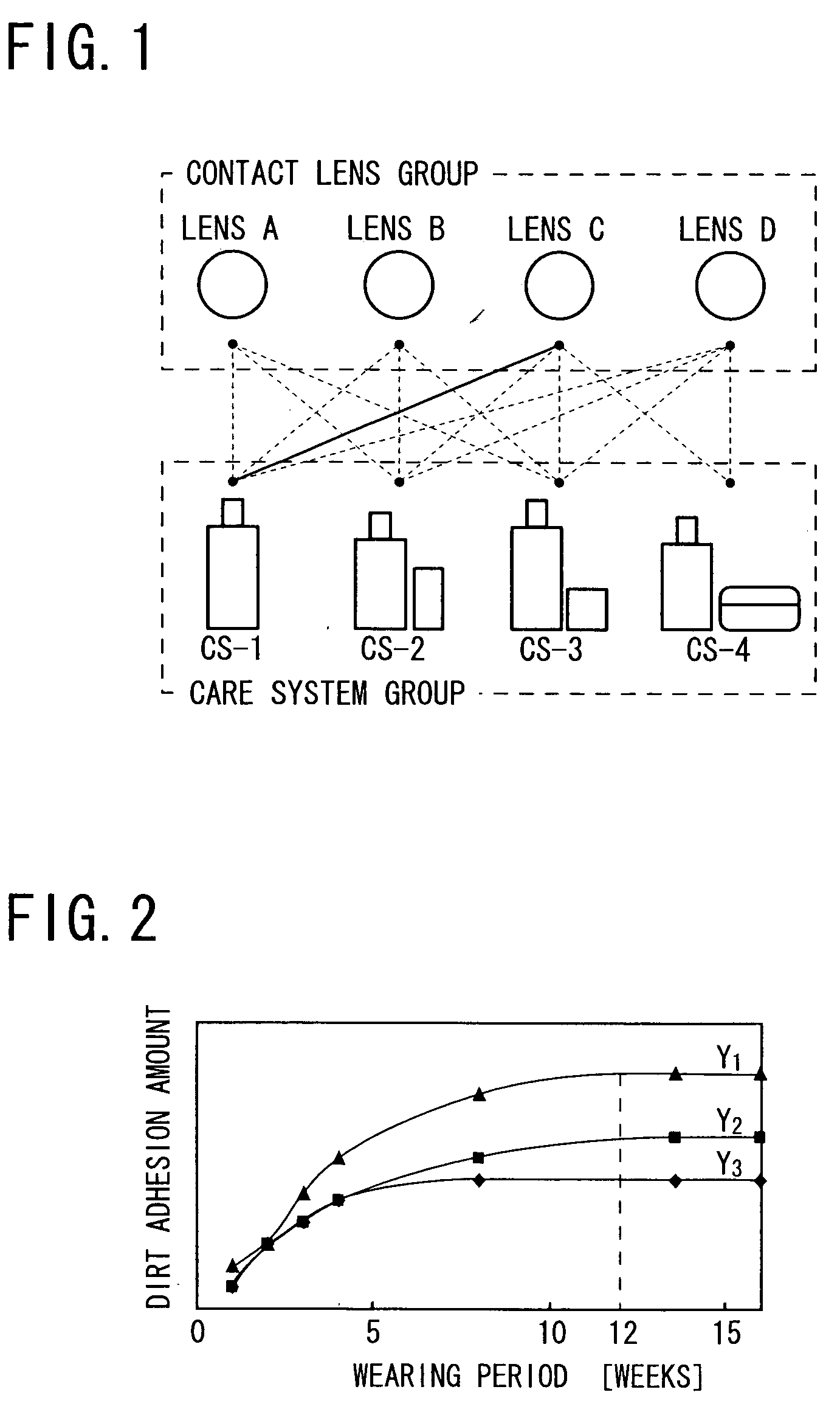
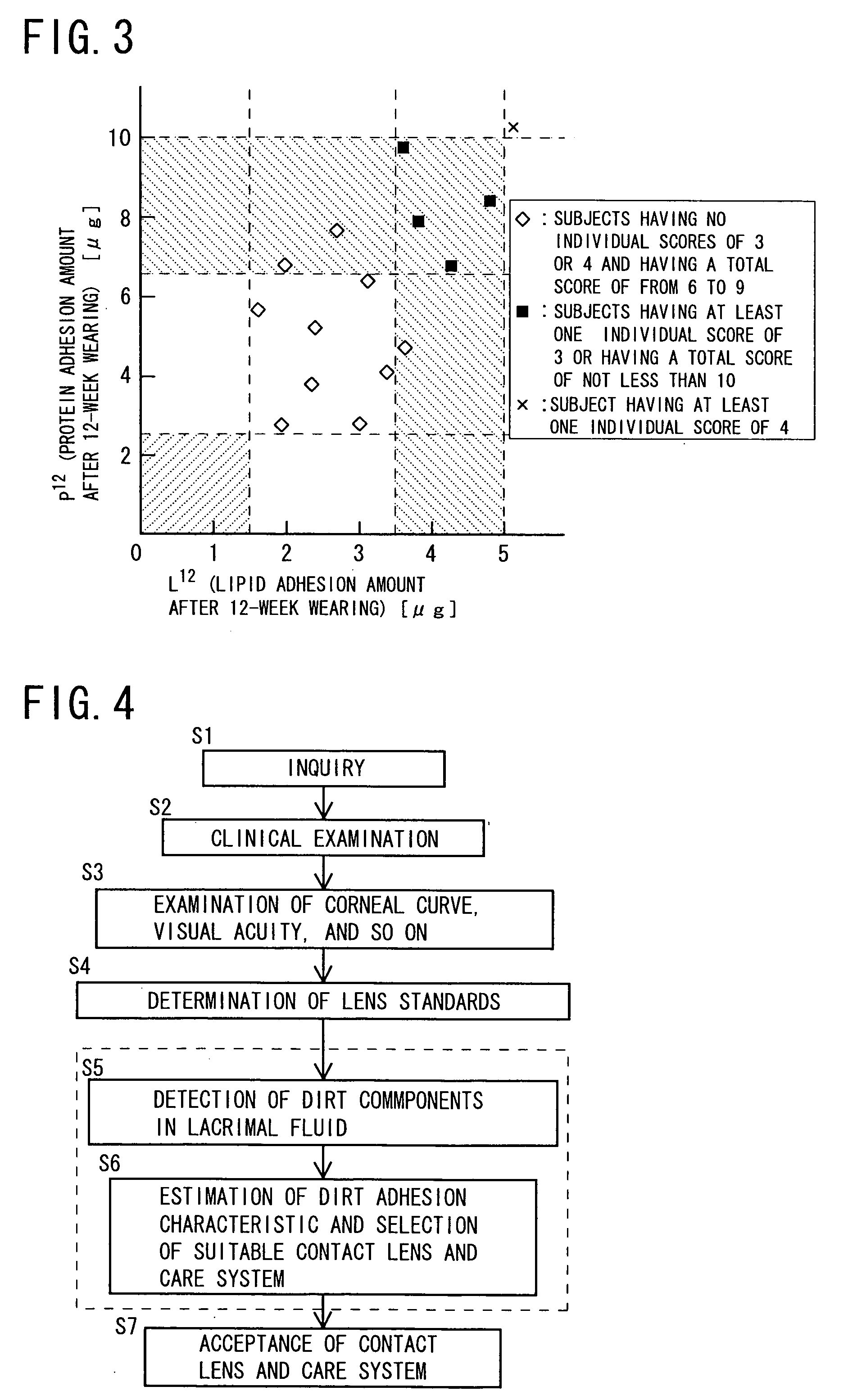
Method of selecting contact lens and/or care system therefor
InactiveUS20060003906A1Shortening in lens lifeLow oxygen permeabilitySpectales/gogglesComponent separationMedicineLipid content
It is intended to provide a method of selecting a contact lens suitable for a wearer and / or a care system therefor, by which the occurrence of problems (for example, worsening in the feel during wearing, lowering in the oxygen permeability of the lens, shortening in the lens life, lowering in the visual acuity, corneal injury, etc.) can be minimized to thereby ensure a high safety for the eye, namely, a method which comprises collecting the lacrimal fluid of a wearer, detecting the protein content and / or the lipid content in the lacrimal fluid thus obtained (Step S5), estimating the dirt adhesion characteristics of the wearer to a contact lens based on the protein content and / or lipid content thus detected, and then selecting a contact lens suitable for the wearer and / or a care system therefor from among a plurality of contact lenses and a plurality of care systems (Step S6).
Owner:MENICON CO LTD
Method for manufacturing and storing corneal injury scar-free repairing device
ActiveCN103989553ABreathe freelyPromote growthMedical applicatorsEye treatmentConjunctivaTissue repair
The invention relates to a method for manufacturing and storing a corneal injury scar-free repairing device. Specially-prepared amnion elements are settled or bonded in or on the surfaces of various biological or synthetic attaching devices such as the invisible contact lens through the novel carrier controlled release technology and the biological tissue engineering technology so as to form the corneal or conjunctiva injury scar-free repairing device. At the early stage of the corneal or conjunctiva injury, the affected corneal tissue or the affected conjunctiva tissue is covered with the amnion elements of the corneal or conjunctiva injury scar-free repairing device, the amnion elements, such as the necessary cell growth factors, the tissue repair factors and protease inhibitors, of the corneal or conjunctiva injury scar-free repairing device are diffused to the affected corneal tissue or the affected conjunctiva tissue due to the special superfine structure of an amnion basilar membrane and the stem cell characteristic of amniotic epithelial cells, and therefore growth of epithelial cells of the corneal or the conjunctiva is promoted, the affected corneal tissue or the affected conjunctiva tissue is stimulated, regulated and controlled to heal orderly, and less scars are formed on the affected corneal tissue or the affected conjunctiva tissue.
Owner:广州睿辰生物科技有限公司
Methods of treating conditions associated with corneal injury
InactiveUS20020128191A1Prevent and reduce adverse effectReduce congestionOrganic active ingredientsSenses disorderCorneal InfectionCorneal Injury
The present invention provides methods of treating a subject suffering from adverse effects, complications or conditions, associated with or resulting from a corneal injury including, corneal infection or ulceration, by topical administration of suitable ophthalmic preparations of bactericidal / permeability-increasing (BPI) protein products.
Owner:XOMA CORP
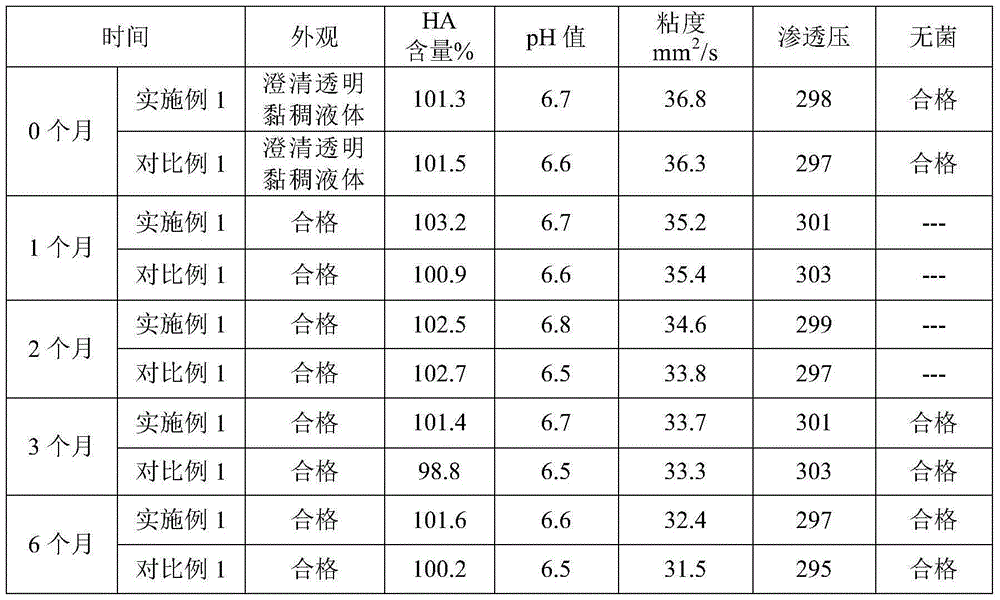
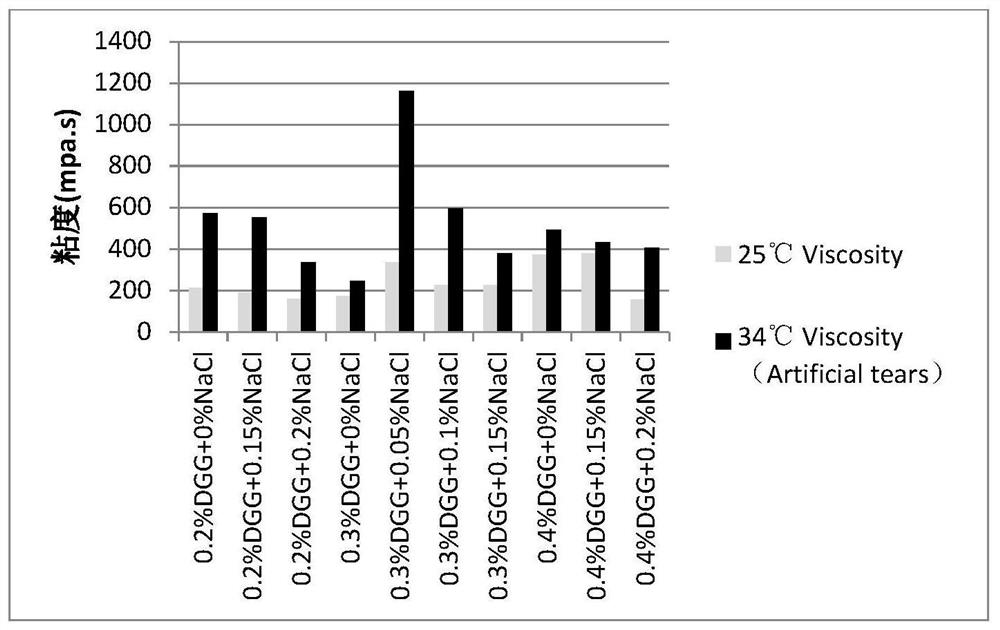
High-fidelity chitosan sodium hyaluronate eye drops and preparation method thereof
ActiveCN105232454AWith anti-corrosion functionPromote wound healingOrganic active ingredientsSenses disorderCorneal woundXerophthalmus
The invention belongs to the technical field of medicines, and in particular relates to high-fidelity chitosan sodium hyaluronate eye drops and a preparation method thereof. Specifically, the high-fidelity chitosan sodium hyaluronate eye drops are prepared from the following components in percentage by weight: 0.03-0.1 wt% of chitosan, 0.1-0.3 wt% of sodium hyaluronate, 0.5wt% of an isoosmotic adjusting agent, 0.5-1.0 wt% of auxiliary material, 0.01-0.03 wt% of borneol and a pH value regulator, and the balance of water for injection. The addition quantity of the pH value regulator enables the pH value of the composition to be 6.0-9.0. The high-fidelity chitosan sodium hyaluronate eye drops intrinsically have the corrosion inhibition function, and the existing experimental data prove that the high-fidelity chitosan sodium hyaluronate eye drops have the functions of effectively preventing and treating the xerophthalmus and promoting the corneal wound healing.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
Sodium hyaluronate eye drops containing 0.01% of atropine and preparation method of sodium hyaluronate eye drops
PendingCN111803441AAvoid side effectsAvoid potential dangerSenses disorderInorganic non-active ingredientsAllergic reactionSodium hyaluronate
The invention discloses sodium hyaluronate eye drops containing 0.01% of atropine. The eye drops comprise sodium hyaluronate, atropine, sodium hydroxide, hydrogen chloride, sodium chloride and water for injection. The mass concentration of sodium hyaluronate is 0.1-10 g / L, the mass concentration of atropine is 0.1 g / L, sodium hydroxide and hydrogen chloride are pH regulators, and sodium chloride is an osmotic pressure regulator. The eye drops contain no traditional Chinese medicinal components, bacteriostatic agent, preservative, cosolvent, thicken agent, complexing agent or the like, the components are simple and are normal substances of human bodies except for atropine, so that corneal damage caused by damage of the microenvironment on the surfaces of eyeballs is avoid, harm to the humanbodies and growth and development due to long-term excessive use of components such as traditional Chinese medicine is also avoided, and the incidence of allergic reaction is effectively reduced. Inaddition, the eye drops have pH and osmotic pressure adjusted, use comfort is improved, small-dose independent packages are also produced based on aseptic filling and a hot pressing sterilization process, and biological safety of the product is ensured.
Owner:SECOND AFFILIATED HOSPITAL OF COLLEGE OF MEDICINEOF XIAN JIAOTONG UNIV
Preparation method of high-strength collagen membrane having curvature
ActiveCN107213510AAvoid bendingAvoid foldingTissue regenerationProsthesisHigh intensityUltimate tensile strength
The invention relates to a collagen membrane, and discloses a preparation method of a high-strength collagen membrane having curvature. The method comprises the following steps: S1, preparing a collagen solution into collagen gel; S2, placing the collagen gel prepared in the step S1 into an annular fixing ring; S3, placing the annular fixing ring and the collagen gel in the annular fixing ring on a lower mold, and compressing the collagen gel with an upper mold, wherein the lower mold comprises a concave part with a curvature, the upper mold comprises a projecting part matched with the concave part with the curvature, and compression of the collagen gel is realized through cooperation of the projecting part and the concave part; S4, after finishing compression, taking the lower mold out, and drying the collagen membrane attached to the upper mold by blowing to obtain the high-strength collagen membrane having curvature. Through a method for compressing the collagen gel, moisture is drained rapidly, preparation time is saved, the prepared collagen membrane having curvature has high strength can be matched with a curved cornea well, the efficiency of corneal injury repairing is increased, and the refractive of the cornea is not influenced.
Owner:JINAN UNIVERSITY
Corneal injury scar-free repairing device and application thereof
InactiveCN103989554ABreathe freelyGood conditionMedical applicatorsEye treatmentConjunctivaTissue repair
The invention relates to a corneal injury scar-free repairing device and application thereof. Specially-prepared amnion elements are settled or bonded in or on the surfaces of various biological or synthetic attaching devices such as the invisible contact lens through the novel carrier controlled release technology and the biological tissue engineering technology so as to form the corneal or conjunctiva injury scar-free repairing device. At the early stage of the corneal or conjunctiva injury, the affected corneal tissue or the affected conjunctiva tissue is covered with the amnion elements of the corneal or conjunctiva injury scar-free repairing device, the amnion elements, such as the necessary cell growth factors, the tissue repair factors and protease inhibitors, of the corneal or conjunctiva injury scar-free repairing device are diffused to the affected corneal tissue or the affected conjunctiva tissue due to the special superfine structure of an amnion basilar membrane and the stem cell characteristic of amniotic epithelial cells, and therefore growth of epithelial cells of the corneal or the conjunctiva is promoted, the affected corneal tissue or the affected conjunctiva tissue is stimulated, regulated and controlled to heal orderly, and less scars are formed on the affected corneal tissue or the affected conjunctiva tissue.
Owner:周辉
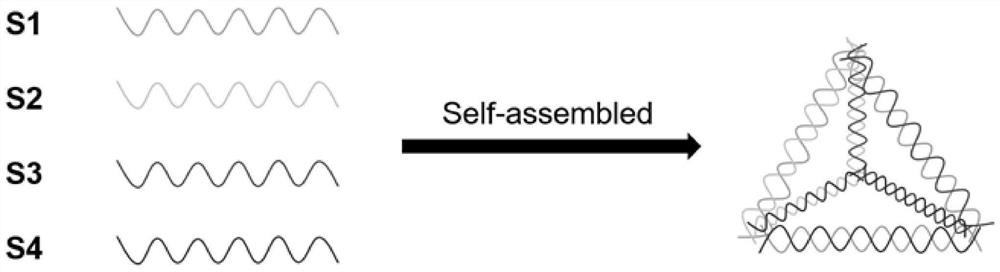
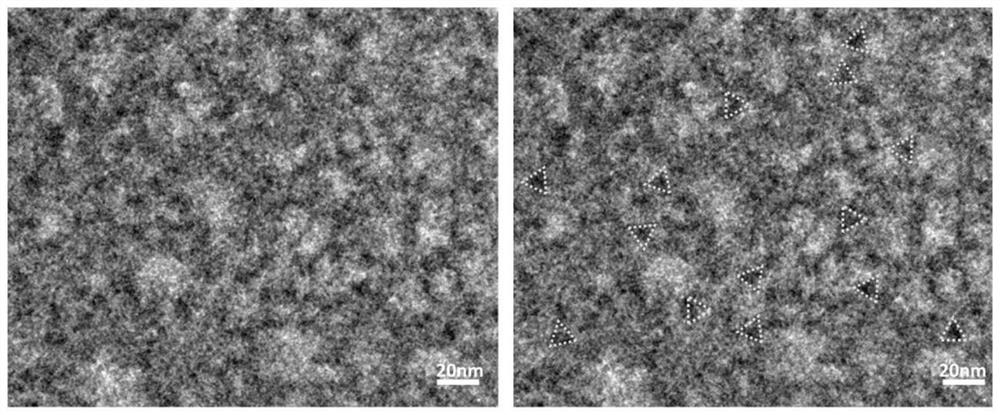
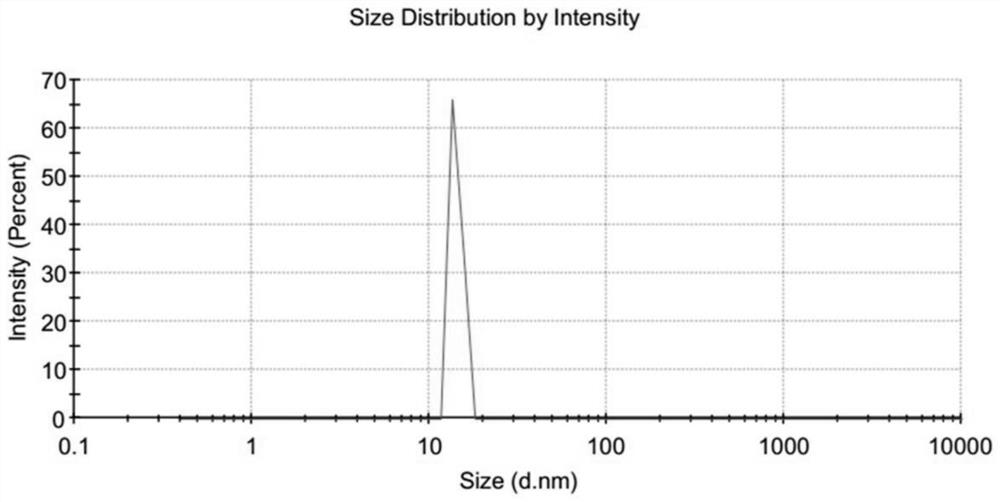
Application of dna tetrahedron in preparation of medicine for treating corneal injury
ActiveCN109646450BPromote proliferationPromote migrationOrganic active ingredientsSenses disorderSide effectOphthalmology
Owner:CHENGDU GENREZE GENE TECH CO LTD
Water-based in-situ gel ophthalmic preparation for treating xerophthalmia
ActiveCN112190542AImprove complianceReduce adverse reactionsOrganic active ingredientsSenses disorderAntiinflammatory drugTear secretion
The present invention discloses a water-based in-situ gel ophthalmic preparation. Thewater-based in-situ gel ophthalmic preparation comprises the following components: non-steroidal anti-inflammatorydrugs, polysaccharide polymers with in-situ gel characteristics, artificial tears and water, in-situ gel containing the non-steroidal anti-inflammatory drugs and the artificial tears is formed under physiological conditions of eyes, and instantaneous viscosity is increased when the drug solution is dripped into the eyes. The water-based in-situ gel ophthalmic preparation can be used for treating xerophthalmia caused by corneal injury, can supplement lacrimal secretion insufficiency during treatments, increases retention and bioavailability of the drug in the eyes, improves compliance of patients, and reduces stimulation of the drug to eyes of the patients and use frequency of the drug.
Owner:IVEW THERAPEUTICS (ZHUHAI) CO LTD
Novel application of sulfated bletilla striata polysaccharide and preparation for treating ocular surface damage
InactiveCN105147722AGood effectRepair damageOrganic active ingredientsSenses disorderSulfationBletilla striata
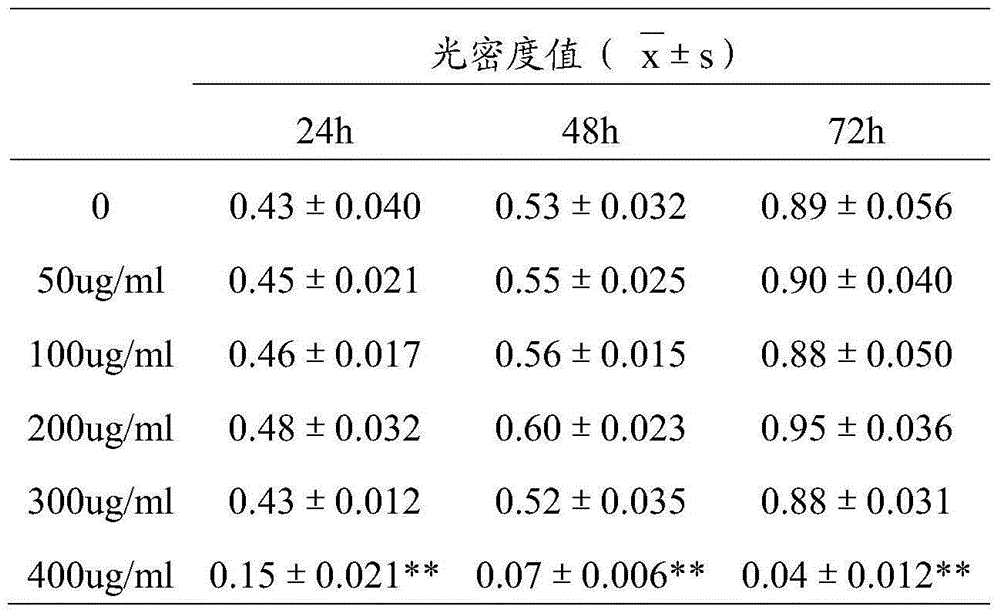
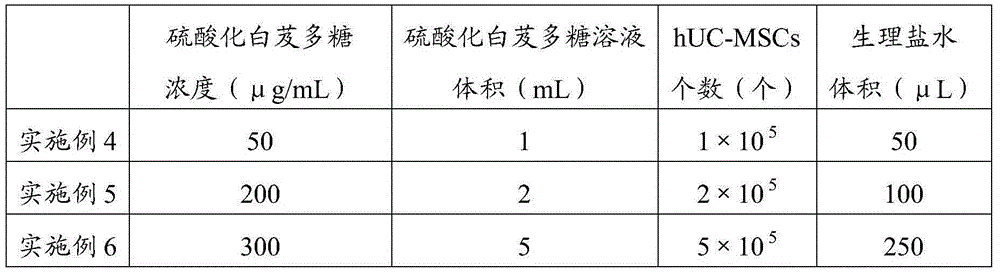
The invention relates to the field of medicines, particularly relates to a novel application of sulfated bletilla striata polysaccharide and a preparation for treating ocular surface damage, and provides an application of sulfated bletilla striata polysaccharide in preparation of a preparation for treating ocular surface damage, a preparation containing sulfated bletilla striata polysaccharide and umbilical cord mesenchymal stem cells and an application of the preparation in treatment of ocular surface damage. Tests prove that the preparation compounded by the umbilical cord mesenchymal stem cells and the sulfated bletilla striata polysaccharide can be used for remarkably promoting reproduction of corneal epithelial cells, and has an effect remarkably superior to independent application of the sulfated bletilla striata polysaccharide or umbilical cord mesenchymal stem cells. Cell migration tests indicate that the preparation and bletilla striata polysaccharide can be used for remarkably promoting migration of the corneal epithelial cells, but the effect for using the preparation is remarkably superior to independent application of the sulfated bletilla striata polysaccharide or umbilical cord mesenchymal stem cells. Therefore, the preparation has an effect of repairing corneal injury.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Ophthalmic preparation containing oxygen micro-capsules and preparation method
ActiveCN102973594ASolve the problem of hypoxiaPrevention and treatment of lesionsSenses disorderInorganic active ingredientsGel preparationActive agent
The invention relates to the field of an ophthalmic preparation containing oxygen micro-capsules. The ophthalmic preparation containing the oxygen micro-capsules is mainly used for preventing and treating various eye diseases caused by oxygen deficiency and is particularly suitable for corneal injury. The ophthalmic preparation containing the oxygen micro-capsules is characterized in that the preparation is a flowing semisolid gel preparation and comprises the following components of: oxygen accounting for 5-70% of the total volume of the preparation and having the concentration of 5-100%, a surface active agent accounting for 0.1-50% of the total weight of the preparation, an isoosmotic adjusting agent accounting for 0.1-2.5% of the total weight of the preparation, a thickening agent accounting for 0.1-40% of the total weight of the preparation, a certain amount of pH adjusting agent capable of adjusting the pH value of the preparation to be 6.8-8.6, and the balance of water. When corneas are injured and are in contact with little oxygen due to wearing of contact lens, the corneas are dropsical and turbid, and the ophthalmic preparation containing the oxygen micro-capsules aims at solving the problem of oxygen deficiency of the corneas by supplying oxygen for eyes through oxygen micro-capsules.
Owner:李洪江
Corneal contact lens cleaning machine
PendingCN111618020AClean upReduce wearDrying gas arrangementsFlexible article cleaningCorneal InfectionSurgery
The invention provides a corneal contact lens cleaning machine. The corneal contact lens cleaning machine aims to solve the problems that people need to pay attention to daily cleaning, sterilization,storage and other care work of corneal contact lenses, otherwise, corneal infection, keratitis and corneal injury are caused very easily; the lenses are difficult and complicated to clean; accordingto a traditional cleaning mode, the corneal contact lenses are rubbed with fingers, many people use improper cleaning methods, the lenses are not stressed evenly, nails are too long, cross contamination of the lenses is easily caused by bacteria left on the fingers and in the nails, the lenses are always scratched and damaged and severely abraded, and accordingly the lenses are not easy to clean or optical characteristics of the lenses are changed. Compared with the prior art, the corneal contact lens cleaning machine has the beneficial effects that the traditional manual cleaning mode of thecorneal contact lenses is changed, a cleaning brush is adopted for automatic cleaning and disinfection, the lenses are automatically sprayed and blown dry, finger contact is avoided in the whole process, abrasion of the lenses is reduced, and the lenses are cleaned better.
Owner:韩佳志
Adipose-derived stem cell preparation eye drops for treating corneal injury and preparation method thereof
InactiveCN107320492AImprove repair effectGood effectSenses disorderPharmaceutical delivery mechanismSerum free mediaTissue repair
The invention discloses adipose-derived stem cell preparation eye drops for treating corneal injury and a preparation method thereof and solves the problem that in the prior art, the tissue repair condition is less than satisfactory, cicatrization is obvious and visual function recovery is not ideal. The adipose-derived stem cell preparation eye drops comprise a cell culture liquid which is a concentrated liquid prepared by the following steps: culturing adipose-derived stem cells through a mesenchymal stem cell serum-free medium; when the cell density in the mesenchymal stem cell serum-free medium reaches 90% or above, performing separation to obtain a culture supernatant fluid; and finally concentrating the culture supernatant fluid to obtain the concentrated liquid. The adipose-derived stem cell preparation eye drops disclosed by the invention have the advantages of remarkably improving the cornea repair effect and the like.
Owner:成都远山博桥生物科技有限公司
Fusogenic peptide containing thrombin fragment
The invention relates to a novel thrombin fragment fusogenic peptide S-TP508 which is obtained by adding a section of stable peptide on the N end of TP508 (the section of stable peptide mainly comprises glutamine and arginine). The fusogenic peptide S-TP508 added with the stable peptide has prolonged half-life period compared with the TP508, overcomes the defect of easiness of enzymolysis of the TP508 and enhances the stability. The invention also relates to a medical composite containing the S-TP508 and application thereof to preparing drugs for treating skin tissue injury and corneal injury.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Application of notoginsenoside extract in preparation of medicinal preparation for eyes
PendingCN110075148AHigh activityPromotes damage repairSenses disorderNervous disorderHyphemaDiabetes retinopathy
The invention discloses application of a notoginsenoside extract in preparation of drugs for treating xerophthalmia, ocular injuries, ocular vascular disease or ocular neurogenic disease; the xerophthalmia, ocular injuries, ocular vascular disease or ocular neurogenic disease include but not limited to xerophthalmia, retina retrogressive disease, retinal vein obstruction, retinal periphlebitis, hyphema, vitreous hemorrhage, corneal injury, retinal contusion, glaucomatous optic atrophy, drusen, age-related macular degeneration and diabetic retinopathy. A mode of eye local drug delivery adoptedby the invention has the advantages of being low in dose, high in safety, less in adverse reaction, and good in patient compliance; the safety risk brought by injection delivery is avoided; furthermore, after changing the drug delivery route, the metabolic pathways, action mechanisms and other aspects thereof are different from the injection, and better clinical application prospect is realized.
Owner:CHINA PHARM UNIV
Application of umbilical cord mesenchymal stem cells to preparation of stem cell preparation for treating corneal injury
InactiveCN106265744APromote healingReduce turbiditySenses disorderPharmaceutical delivery mechanismMesenchymal stem cellSurgery
The invention relates to the technical field of stem cells, in particular to application of umbilical cord mesenchymal stem cells to preparation of a stem cell preparation for treating corneal injury. Studies show that the umbilical cord mesenchymal stem cells can promote the corneal epithelium healing; the cornea turbid degree is low; the generation of cornea blood vessels is reduced; the cornea epithelial tissue can be more effectively repaired; the umbilical cord mesenchymal stem cells can be used for treating cornea alkali burn. The stem cells in the preparation are from the umbilical cord; the collection is convenient and is noninvasive operation; the obtained mesenchymal stem cells are infantile than other mesenchymal stem cells; the multiplication and differentiative potential is great; the umbilical cord resource is rich, and the large-scale production can be realized; the ethic limitation is avoided; the immunogenicity is low; the wide application prospects are realized in the field of corneal injury treatment.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Actin binding peptide and purpose thereof
ActiveCN102924573APromote wound healingGood restorativeSenses disorderPeptide/protein ingredientsCorneal woundSide effect
The invention relates to the field of biological medicine, particularly discloses actin binding peptide and the purpose of the actin binding peptide. The amino acid sequence of the actin binding peptide is LKKTETQ, the synthesis difficulty and the cost are lowered, the actin binding peptide is suitable for batch production in a large scale and has a brilliant preparing function on various corneal injuries, side effects do not appear in the dosing process, and corneal wound healing and transparency recovery are benefited. Compared with a curing method through thymosin beta4, the advantages are more obvious, the side effects are small, and the actin binding peptide can be used for corneal repairing treatment.
Owner:ZHAOKE GUANGZHOU OPTHALMIC DRUG
Serum-free cultured corneal limbus stromal stem cells and method for in-vitro inducing balling and induced differentiation
ActiveCN110564688AGood repeatabilityEasy to purifyDrug screeningCulture processCorneal neoplasmBiology
The invention discloses serum-free cultured corneal limbus stromal stem cells and a method for inducing differentiation and stem cell balling of the corneal limbus stromal stem cells. The invention further provides a culture medium combination for inducing corneal limbus stromal stem cells to be pelletized or differentiated into corneal limbus stromal cells in vitro. The serum-free culture mediumcombination used in the invention can provide sufficient nutrition and a good environment required by cell growth and proliferation, can stably carry out in-vitro amplification culture on corneal limbus stromal stem cells, and can ensure that the dryness and specificity of the corneal limbus stromal stem cells are still maintained after amplification culture. Meanwhile, a system for inducing and differentiating into corneal limbus stromal cells is successfully constructed, can be used for experimental research of the corneal limbus stromal stem cells, cell treatment of corneal lesions and transplantation of corneal injuries, and the serum-free cultured corneal limbus stromal stem cells has wide scientific benefits and social and economic benefits.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
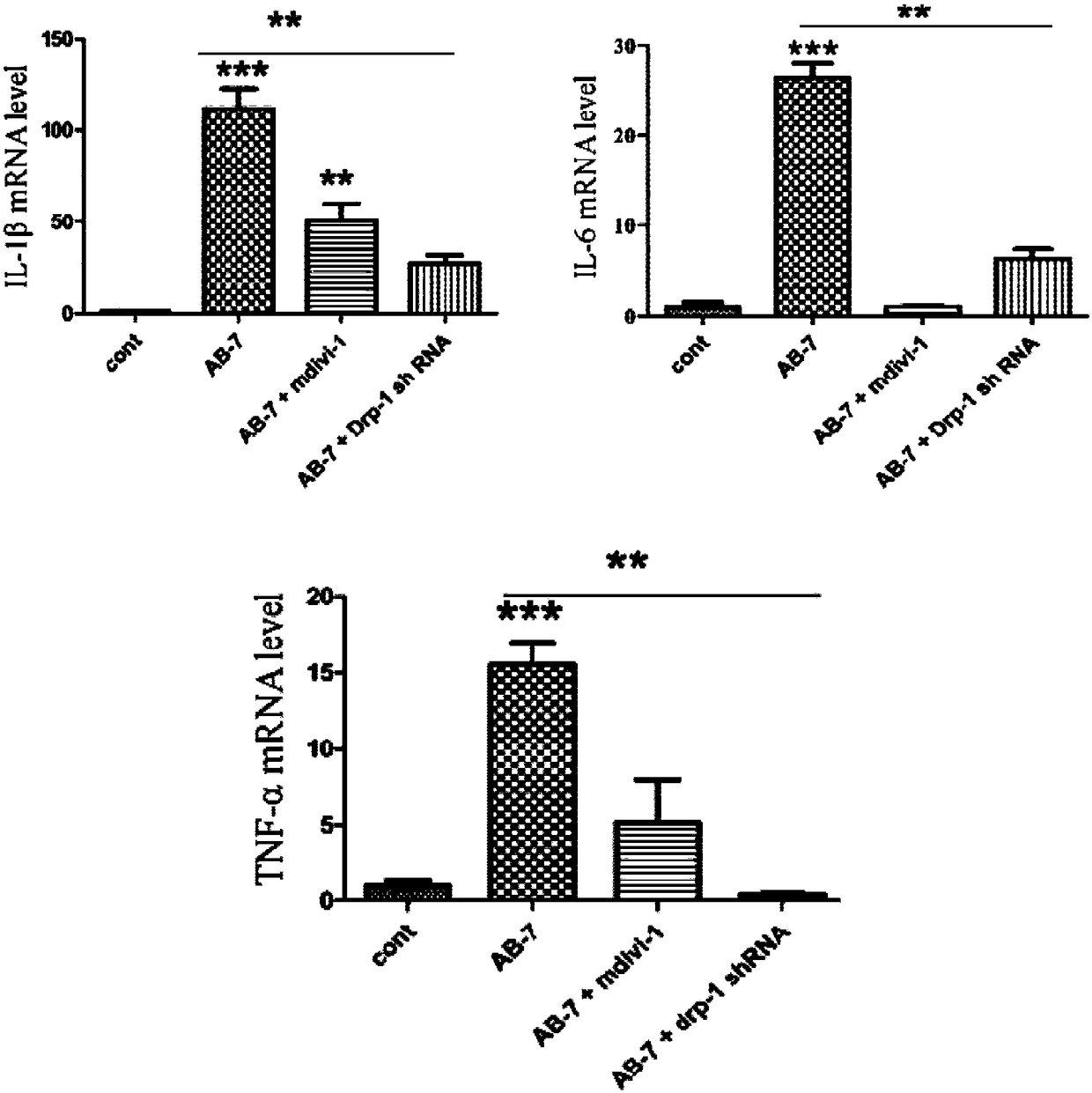
Application of Mdivi-1 in preparation of corneal alkali burn therapeutic drugs
ActiveCN108498514AReduce ROS generationReduce inflammationOrganic active ingredientsSenses disorderInflammatory factorsImpaired visual acuity
The invention relates to application of Mdivi-1 in preparation of corneal alkali burn therapeutic drugs. The animal experiment proves the effectiveness of the Mdivi-1 in treating the corneal alkali burn. The material inhibits chondriokinesis through inhibiting the DRP1 and Dnm1, ROS production, inflammatory factor IL-1 beta and IL-6 and TNF-alpha expression levels are reduced, corneal neovascularization is reduced, and accordingly, the corneal injury and the visual impairment caused by the corneal alkali burn are improved.
Owner:NANCHANG UNIV
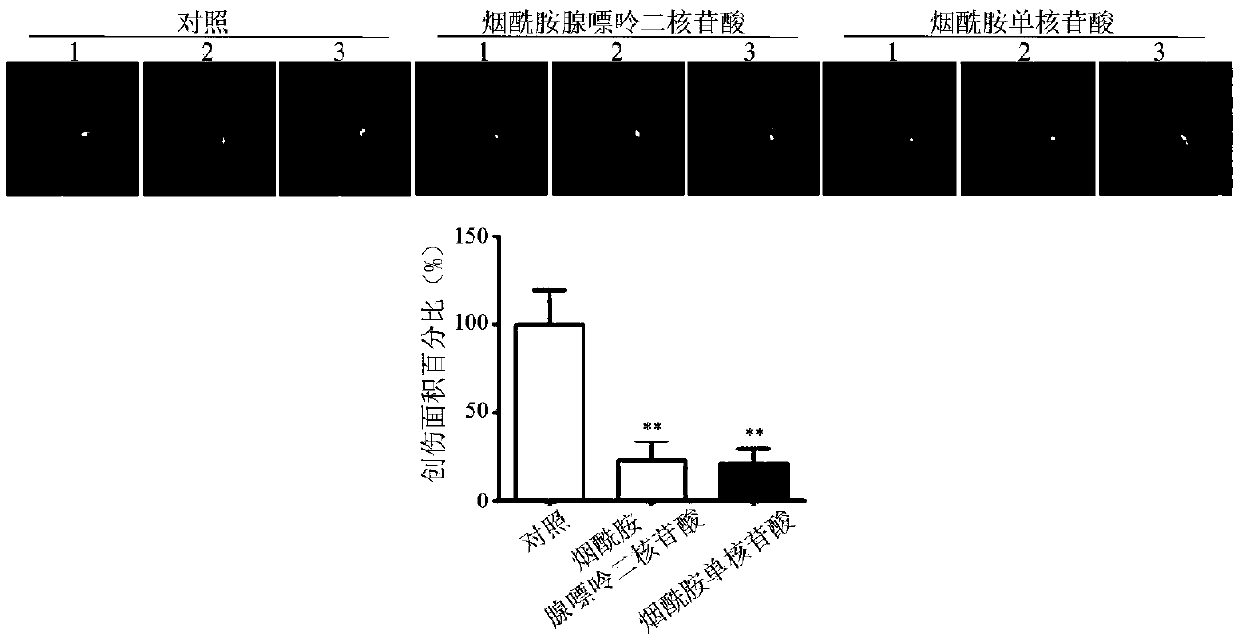
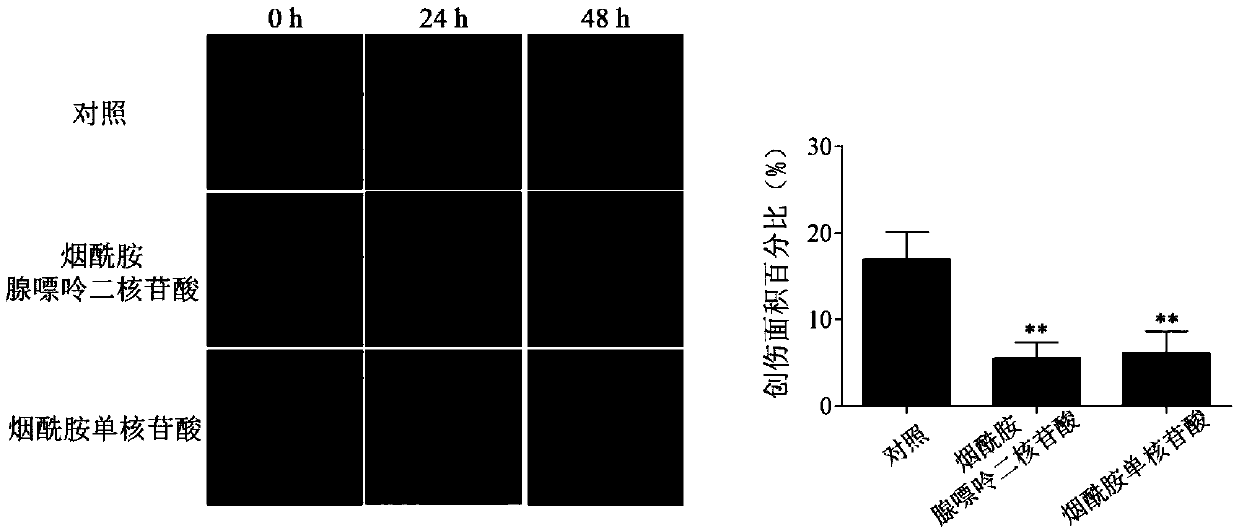
Application of nicotinamide adenine dinucleotide or precursor substance thereof in preparation of medicament for treating corneal epithelial defect
ActiveCN109662973APromote repairEffective treatmentOrganic active ingredientsSenses disorderCorneal epithelial defectPurine
The invention provides an application of nicotinamide adenine dinucleotide or a precursor substance thereof in preparation of a medicament for treating corneal epithelial defect, and relates to the technical field of ophthalmic medicaments. Research shows that the expression of the nicotinamide phosphotransferase in epithelial cells after the corneal injury is abnormal, so that the level of the nicotinamide purine dinucleotide in the corneal epithelial cells is reduced, and the nicotinamide phosphodinucleotide can promote the repair of the corneal epithelial cells, therefore, applying the nicotinamide adenine dinucleotide or the precursor substance thereof to local area of an eye can effectively accelerate the repair of corneal epithelial cells and effectively treat corneal epithelial defects. According to the invention, the nicotinamide adenine dinucleotide or the precursor substance thereof is applied to the preparation of the medicament for treating corneal epithelial defect, and the medicament has wide development and application prospects.
Owner:SHANDONG EYE INST
Pharmaceutical composition of cortex fraxini extract and borneol as well as preparation method and application of pharmaceutical composition
ActiveCN114366769AIncrease contentEnhanced inhibitory effectSenses disorderHydroxy compound active ingredientsMedicinal herbsDisease
The invention provides a pharmaceutical composition of a cortex fraxini extract and borneol as well as a preparation method and application of the pharmaceutical composition. The pharmaceutical composition comprises 3.6-116 parts by weight of the cortex fraxini extract and 1 part by weight of borneol. The cortex fraxini extract mainly takes cortex fraxini total coumarin as an active ingredient, and the cortex fraxini total coumarin comprises aesculin, aesculetin, fraxin and fraxetin; the content of cortex fraxini total coumarin in the cortex fraxini extract is 75-85 wt% or above, the sum of the contents of aesculin, aesculetin, fraxin and fraxetin in the cortex fraxini extract is 55-65 wt% or above, and the content ratio of aesculin to aesculetin to fraxetin is (30.0-40.0): (2.0-4.0): (20.0-30.0): 1. The cortex fraxini extract is prepared by taking traditional Chinese medicine cortex fraxini decoction pieces or a cortex fraxini medicinal material as a raw material and adopting a combined process of alcohol extraction, macroporous resin column purification and the like. The composition disclosed by the invention can be used for remarkably inhibiting the formation of newborn lymphatic vessels in corneas and has a relatively good treatment effect on diseases such as corneal injury.
Owner:SHANGHAI UNIV OF T C M
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com