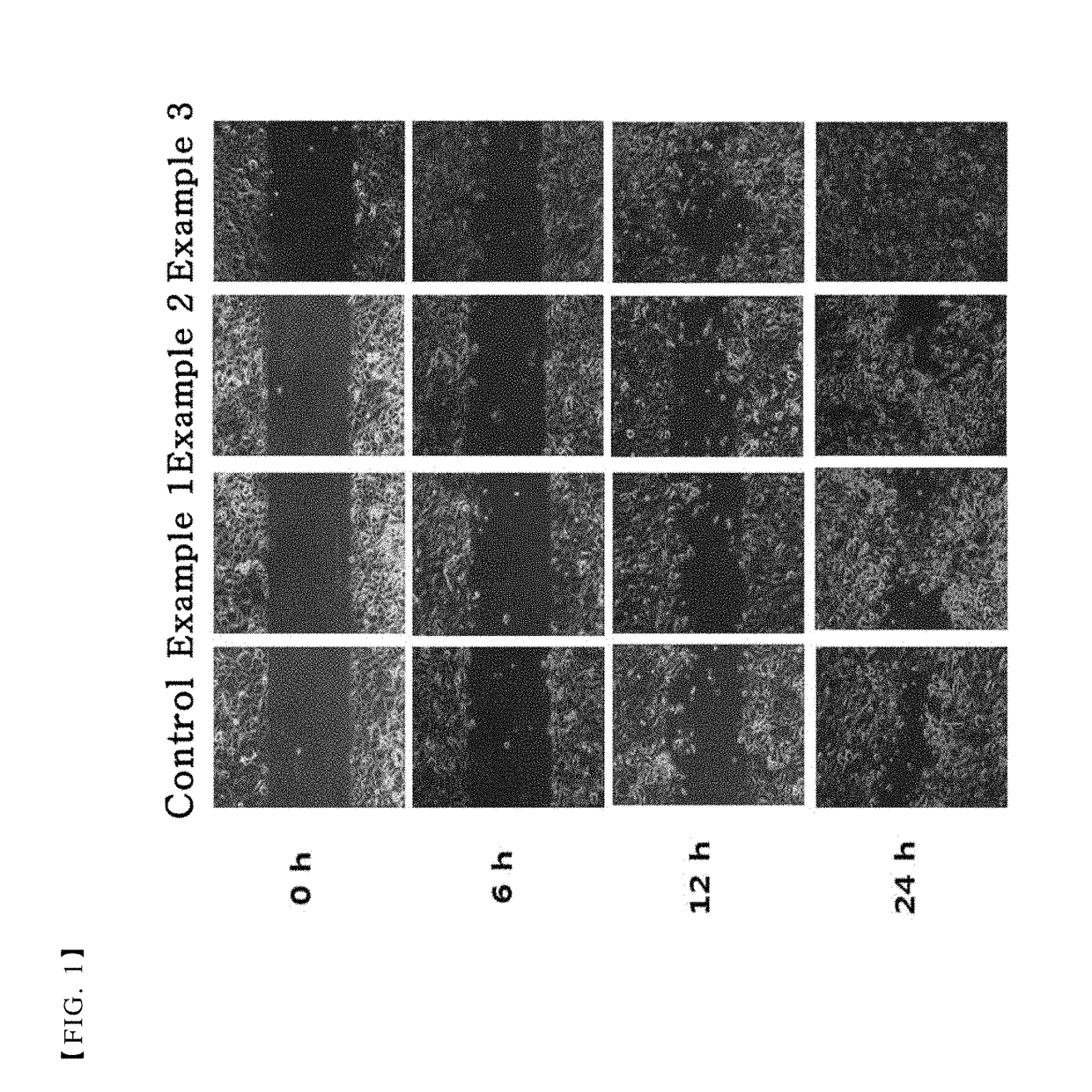
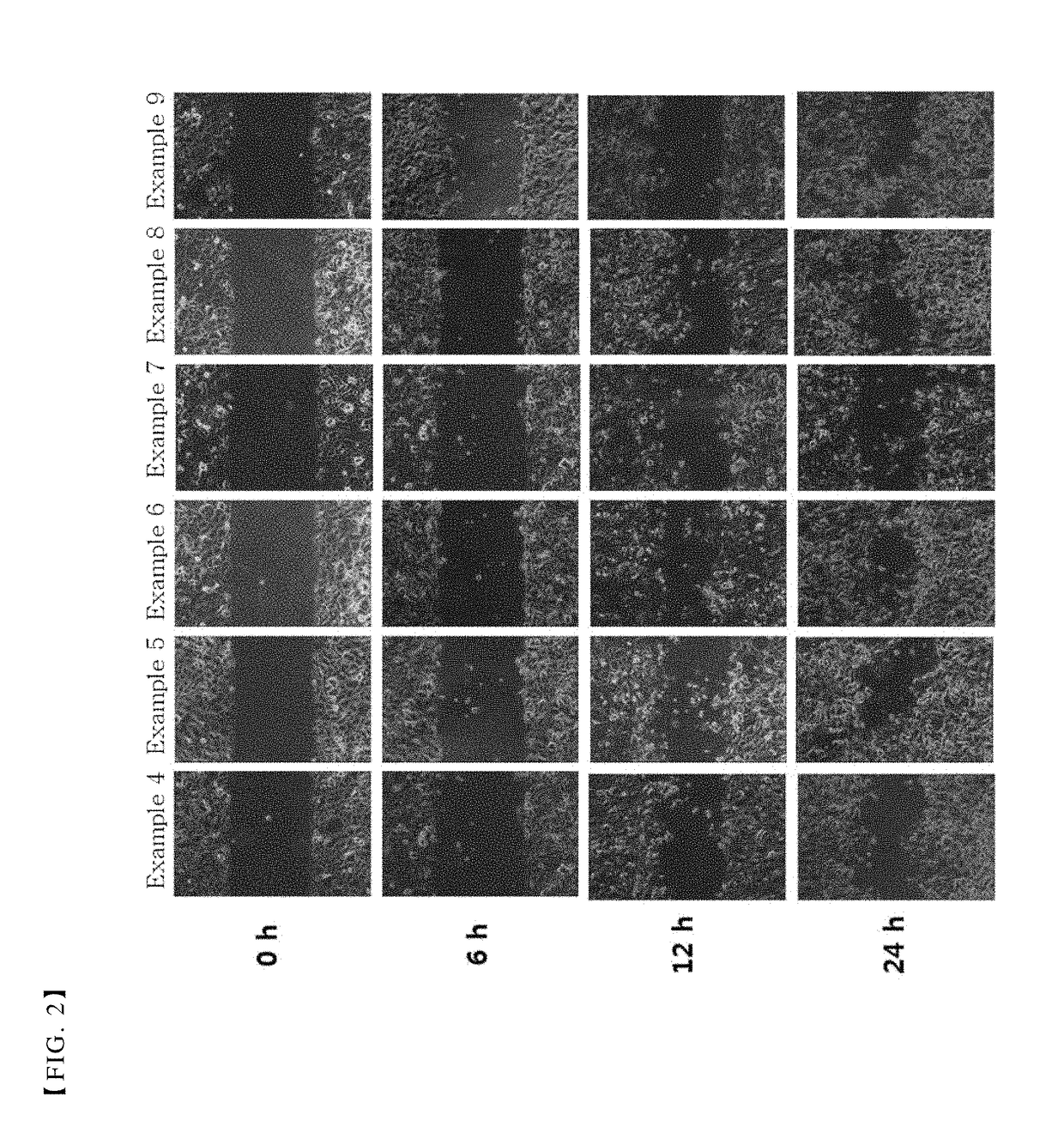
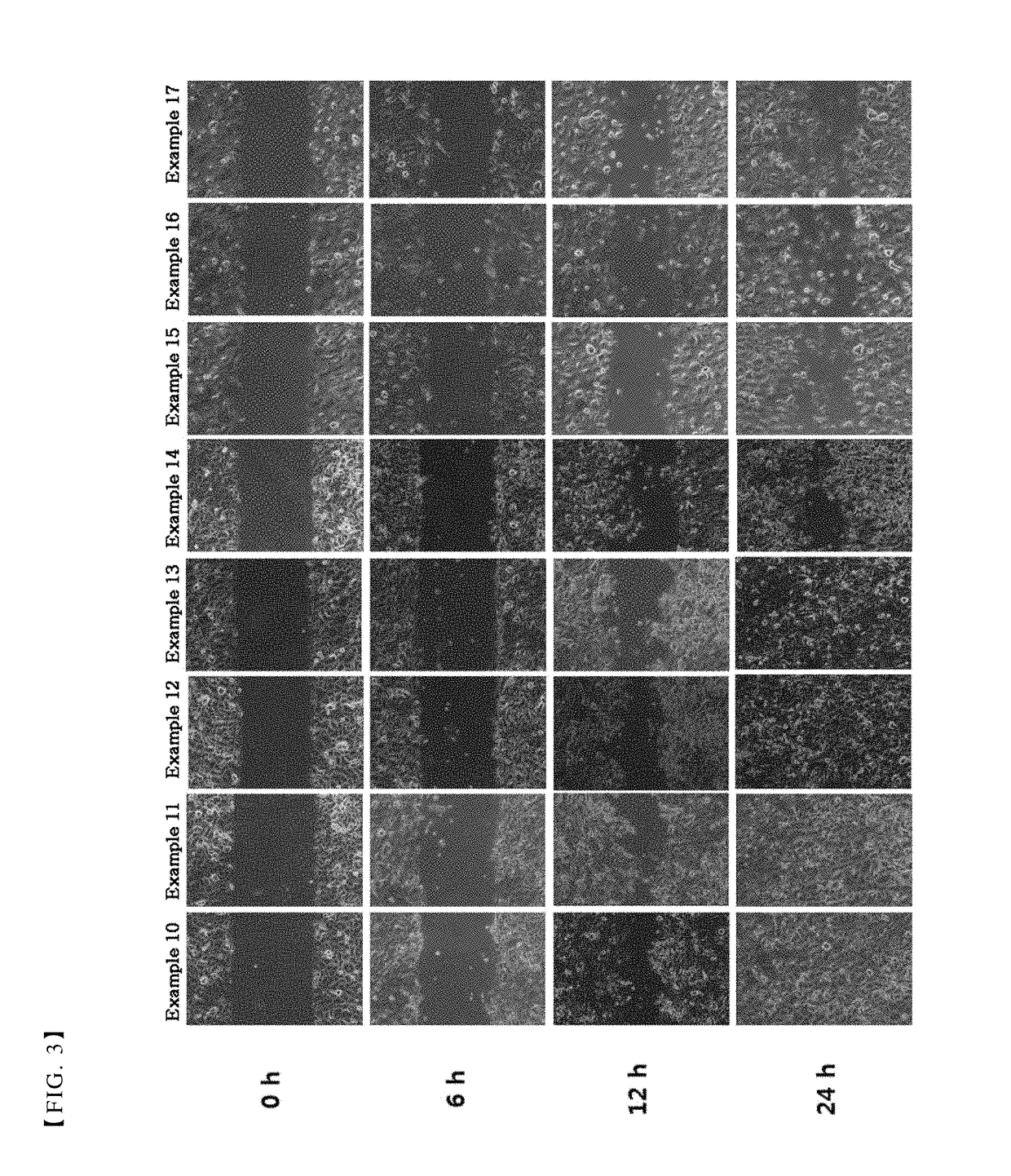
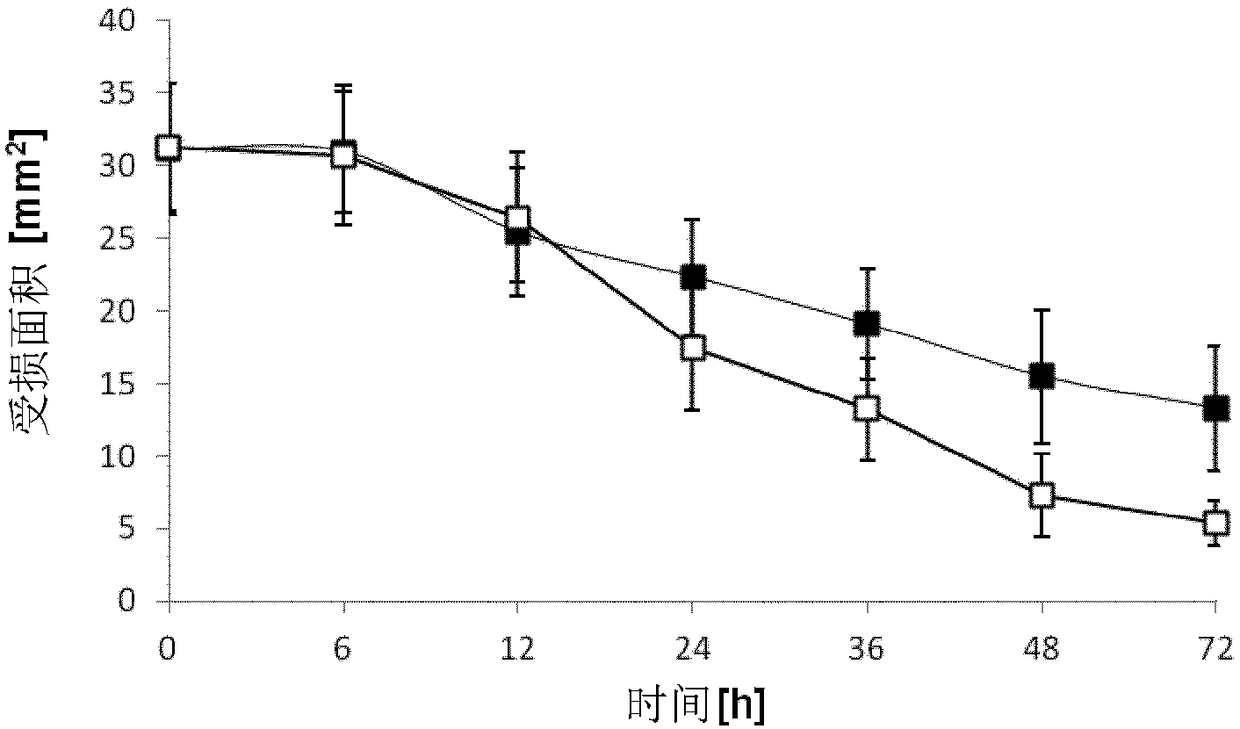
Patents
Literature
33 results about "Corneal wound" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Histatin for Corneal Wound Healing and Ocular Surface Disease
InactiveUS20130310326A1Promote wound healingPromote healingSenses disorderPeptide/protein ingredientsCorneal woundOcular surface disease
Histatins may be used for corneal wound healing and as a treatment for ocular surface disease in humans and other animals. For example, histatins could be included in eye drops, eye gels, ointment, glue, or embedded in (polymer) contact lenses.
Owner:VISUS THERAPEUTICS INC
Histatin for Corneal Wound Healing and Ocular Surface Disease
InactiveUS20130310327A1Promote healingPromote wound healingSenses disorderPeptide/protein ingredientsCorneal woundOcular surface disease
Histatins may be used for corneal wound healing and as a treatment for ocular surface disease in humans and other animals. For example, histatins could be included in eye drops, eye gels, ointment, glue, or embedded in (polymer) contact lenses.
Owner:VISUS THERAPEUTICS INC
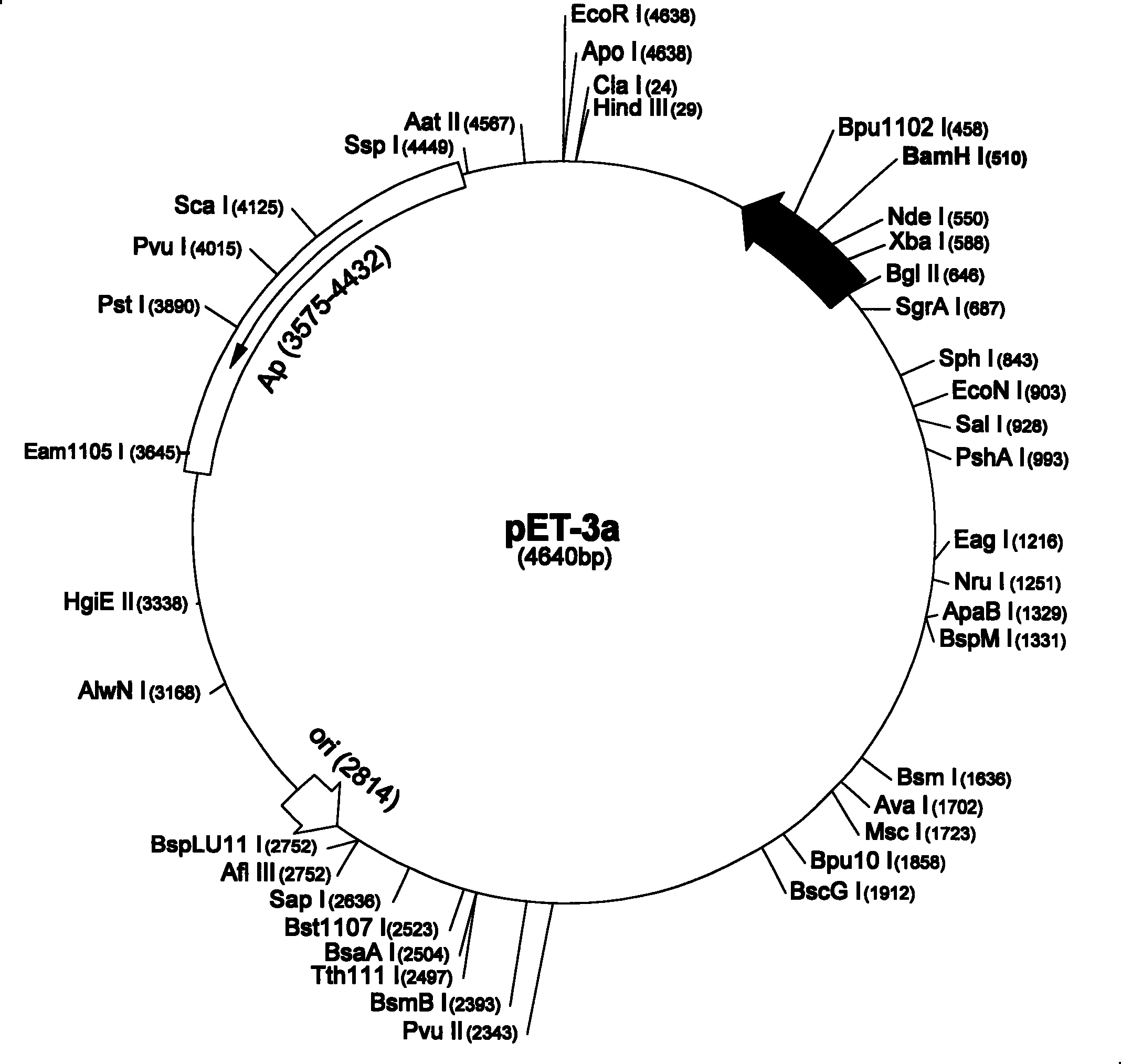
Human keratinized cell growth factor-1 analogue preparation method and application thereof
ActiveCN101220092AHigh expressionHigh activitySenses disorderPeptide/protein ingredientsCorneal woundFibrosis
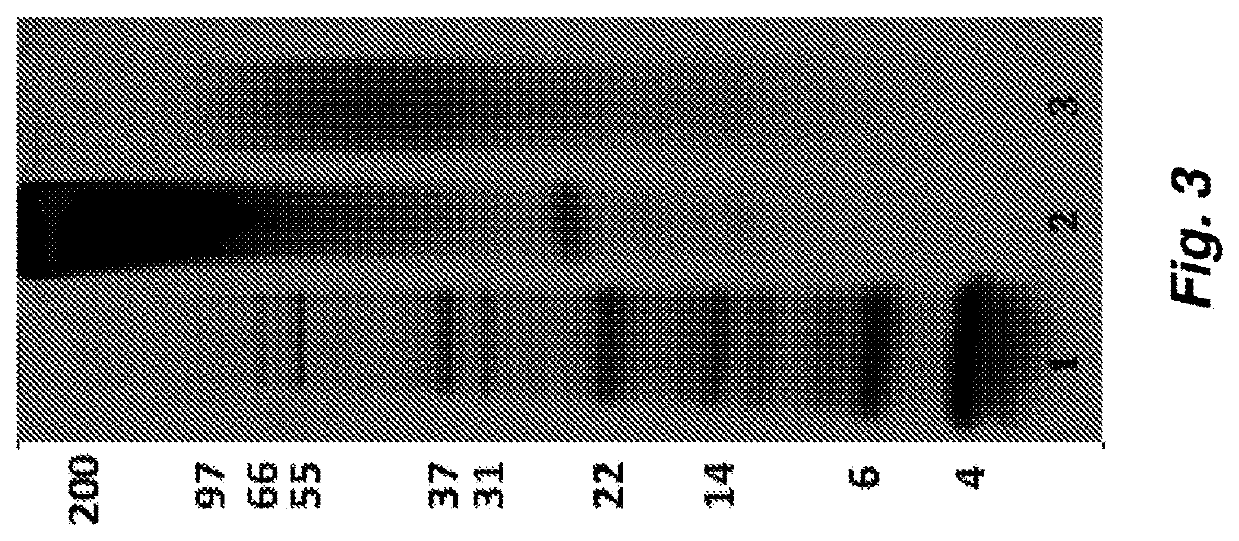
The invention provides a human keratinocyte growth factor -1 structure analogues KGF-1delta 23KGF(40S), an N end of an amino acid sequence of which lacks 23 amino acids, while the 40-bit cysteine point of which is mutated into a nonpolar amino acid. The invention also relates to a production method of the structure analogues, which carries out the fusion expression with a small ubiquitin related modifier gene mature peptide, while a fusion protein and the ubiquitin related modifier gene protease 1 co-express in the prokaryotes. In the process of fermentation expression, the ubiquitin related modifier gene protease 1 can hydrolyze the fusion protein to produce a soluble KGF-1delta 23KGF (40S). The human keratinocyte growth factor structure analogues can facilitate the proliferation of the keratinocyte cells, the growth of the hair follicle cells and inhibit the growth of the fibroblast cells, and has the functions of anti-scar, anti-fibrosis, epidermis healing facilitation and corneal wound reparation, etc.
Owner:吉林农大生物反应器工程有限公司
Histatin for Corneal Wound Healing and Ocular Surface Disease
ActiveUS20160279194A1Promote wound healingPromote healingSenses disorderPeptide/protein ingredientsCorneal woundInjury mouth
Histatins may be used for corneal wound healing and as a treatment for ocular surface disease in humans and other animals. For example, histatins could be included in eye drops, eye gels, ointment, glue, or embedded in (polymer) contact lenses.
Owner:VISUS THERAPEUTICS INC
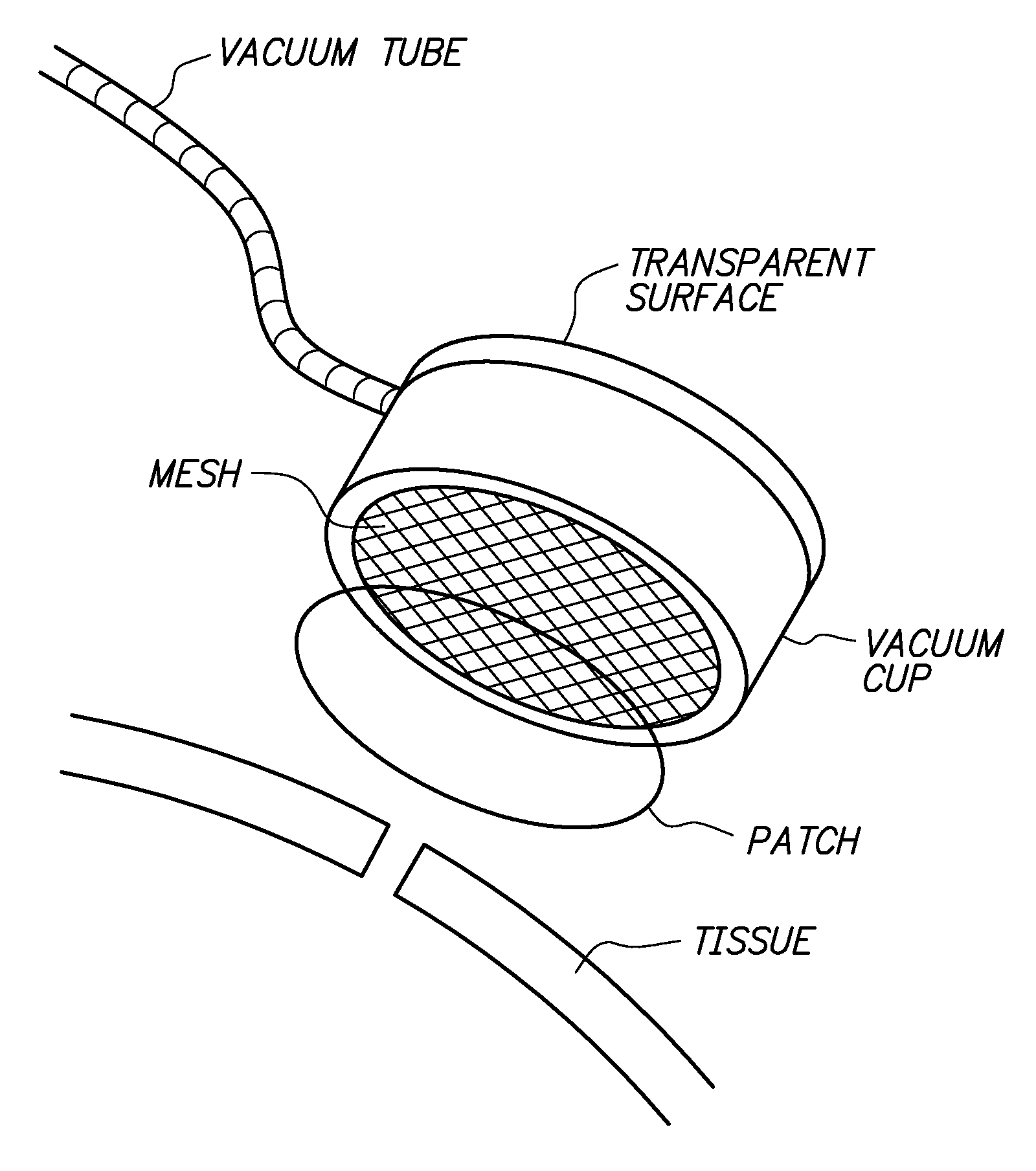
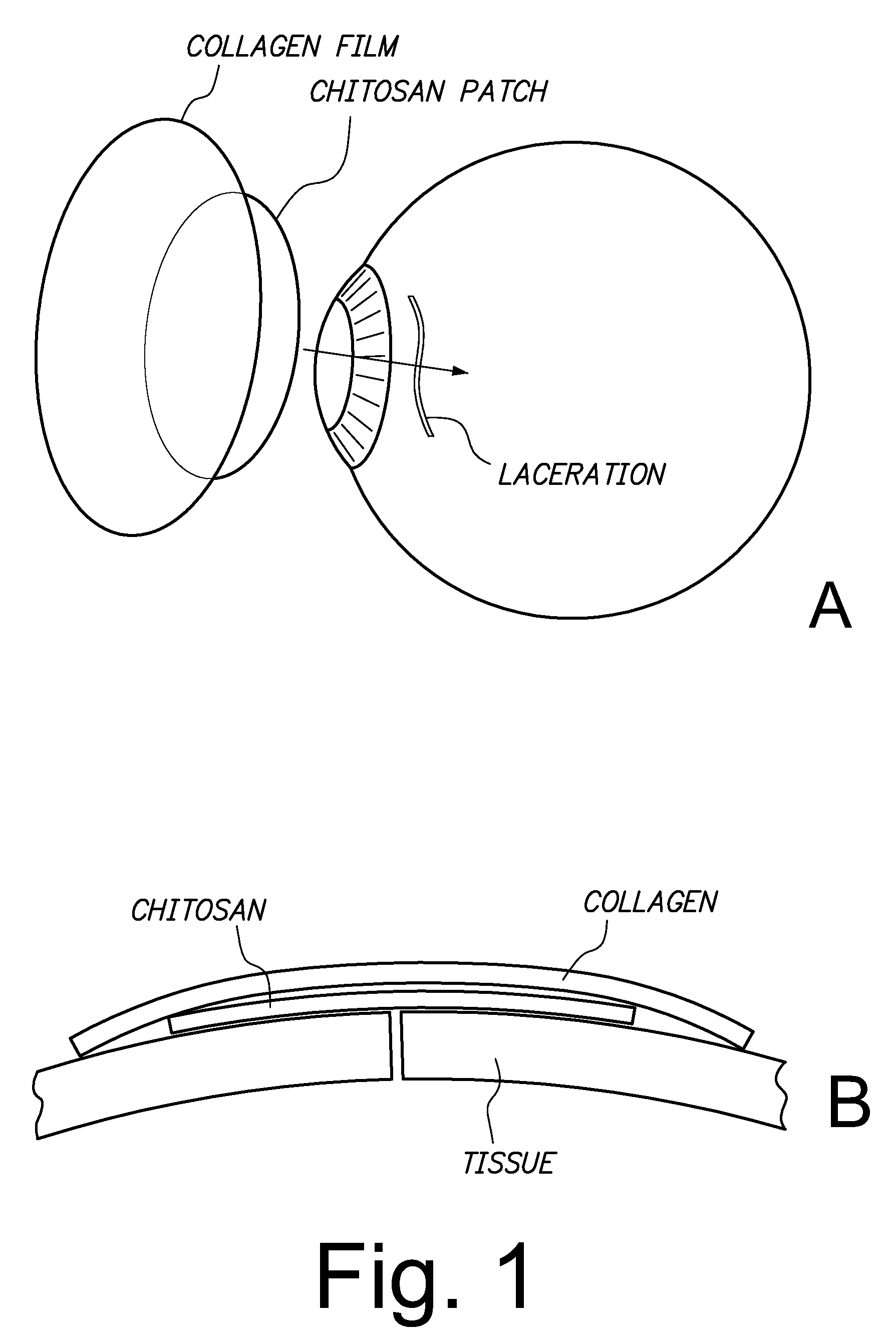
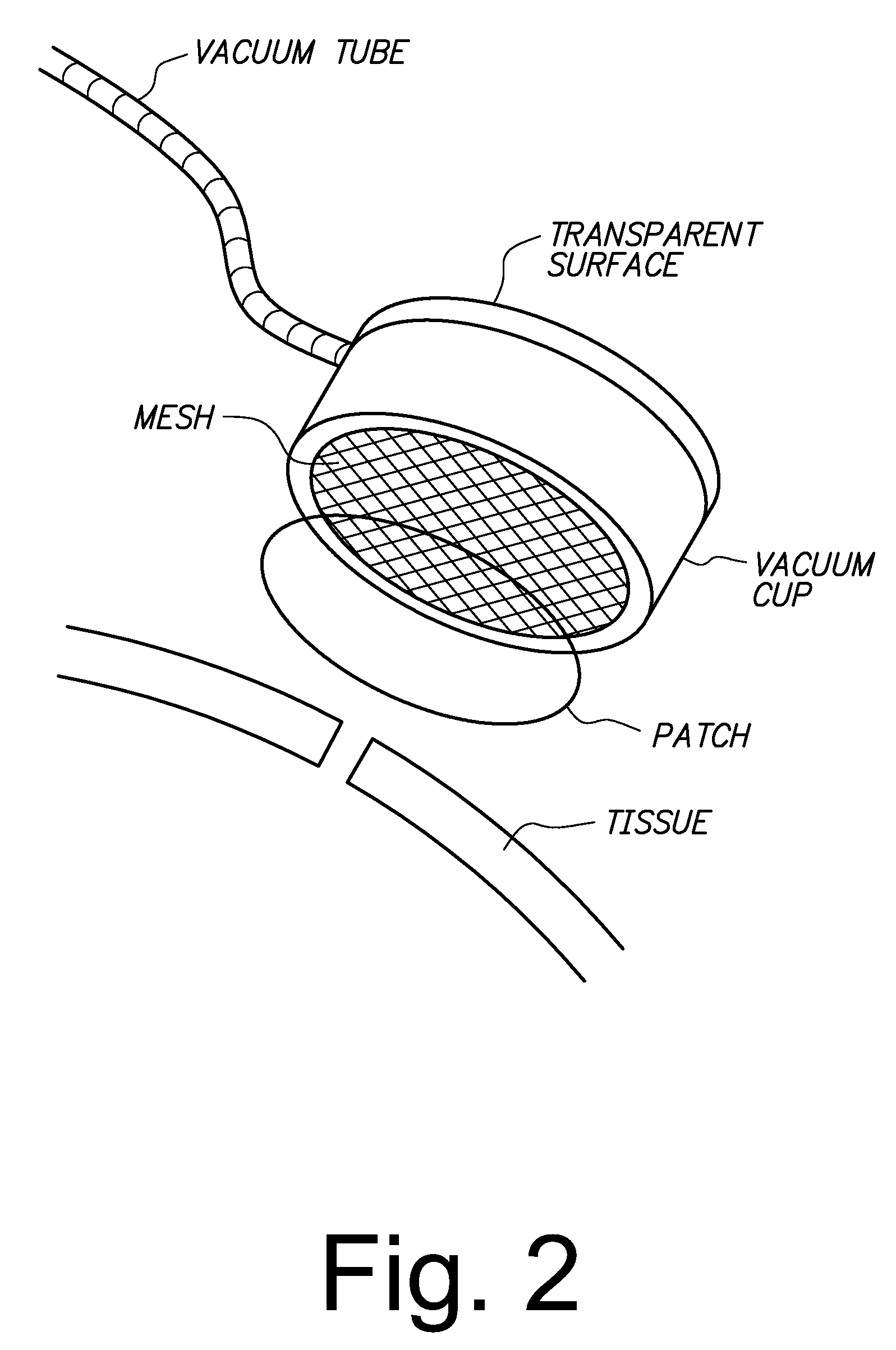
Sutureless methods for laceration closure
Tissue lacerations are closed using a vacuum cup applied to the tissue surface having a tissue-abutting, optically transparent mesh surface that under vacuum conforms with the tissue surface, apposing edges of the wound, and is optionally loaded with a bandage comprising a chitosan film and a collagen backing. An eye tissue surface wound is closed without sutures by closing the wound with a bioadhesive, biocompatible sclera or cornea wound patch comprising a chitosan film and a collagen backing, wherein the backing is bonded to the film without adhesive and protects the film against dissociation when the patch is exposed to a physiological fluid, and the film adheres to the sclera sufficient to retain apposed edges of the wound.
Owner:SRI INTERNATIONAL
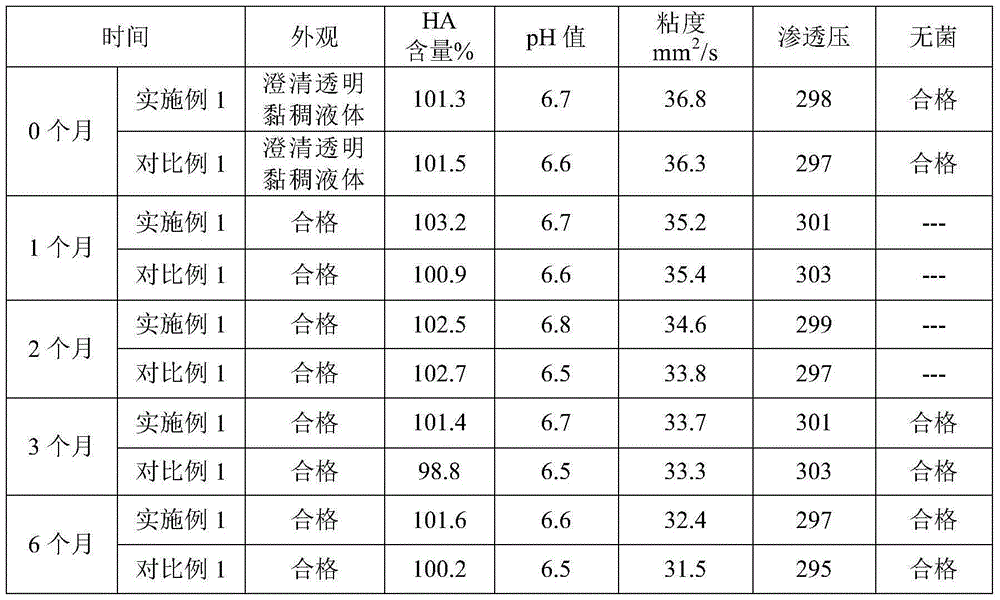
Sodium hyaluronate eyedrop containing deproteinized calf blood extractive and preparation method thereof
InactiveCN102579492AImprove damage repair rateIncrease intakeOrganic active ingredientsSenses disorderCorneal woundXerophthalmia
The invention discloses a sodium hyaluronate eyedrop containing deproteinized calf blood extractive. The components comprise deproteinized calf blood extractive, sodium hyaluronate, pH buffering agent, metal chelating agent, osmotic pressure regulation agent and injection water. The preparation method has the following steps: weighing the sodium hyaluronate, adding the injection water to resolve, and obtaining sodium hyaluronate solution after overnight swelling; fetching the deproteinized calf blood extractive, the pH buffering agent, the metal chelating agent and the osmotic pressure regulation agent, adding the injection water to resolve, mixing with the sodium hyaluronate solution after resolving, and agitating uniformly after supplementing the injection water to full dose; filtering and sterilizing after adjusting the pH of the solution, embedding to single dose packaging containers after being qualified by testing, and sealing and obtaining the product. The eyedrop disclosed by the invention is used for curing corneal wounds and xerophthalmia, can promote the corneal cells to incept and utilize the energy, and improves the recovery rate of the corneal wounds. The preparation method of the eyedrop avoids the stimulus to the surface cells of eyes, and prevents the contamination caused by microorganisms in tears and in air during use and storage.
Owner:ZHAOKE PHARMA GUANGZHOU
High-fidelity chitosan sodium hyaluronate eye drops and preparation method thereof
ActiveCN105232454AWith anti-corrosion functionPromote wound healingOrganic active ingredientsSenses disorderCorneal woundXerophthalmus
The invention belongs to the technical field of medicines, and in particular relates to high-fidelity chitosan sodium hyaluronate eye drops and a preparation method thereof. Specifically, the high-fidelity chitosan sodium hyaluronate eye drops are prepared from the following components in percentage by weight: 0.03-0.1 wt% of chitosan, 0.1-0.3 wt% of sodium hyaluronate, 0.5wt% of an isoosmotic adjusting agent, 0.5-1.0 wt% of auxiliary material, 0.01-0.03 wt% of borneol and a pH value regulator, and the balance of water for injection. The addition quantity of the pH value regulator enables the pH value of the composition to be 6.0-9.0. The high-fidelity chitosan sodium hyaluronate eye drops intrinsically have the corrosion inhibition function, and the existing experimental data prove that the high-fidelity chitosan sodium hyaluronate eye drops have the functions of effectively preventing and treating the xerophthalmus and promoting the corneal wound healing.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
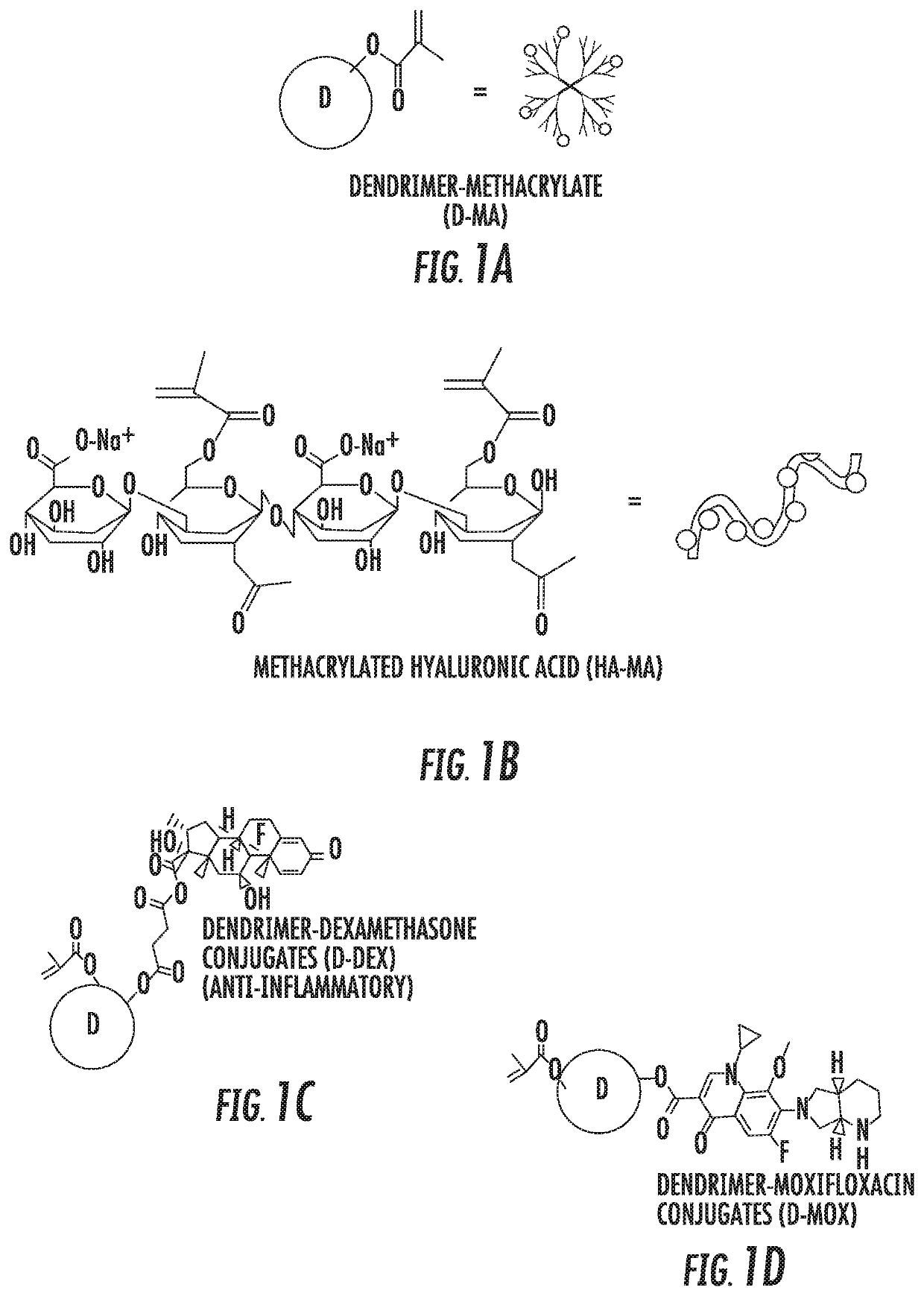
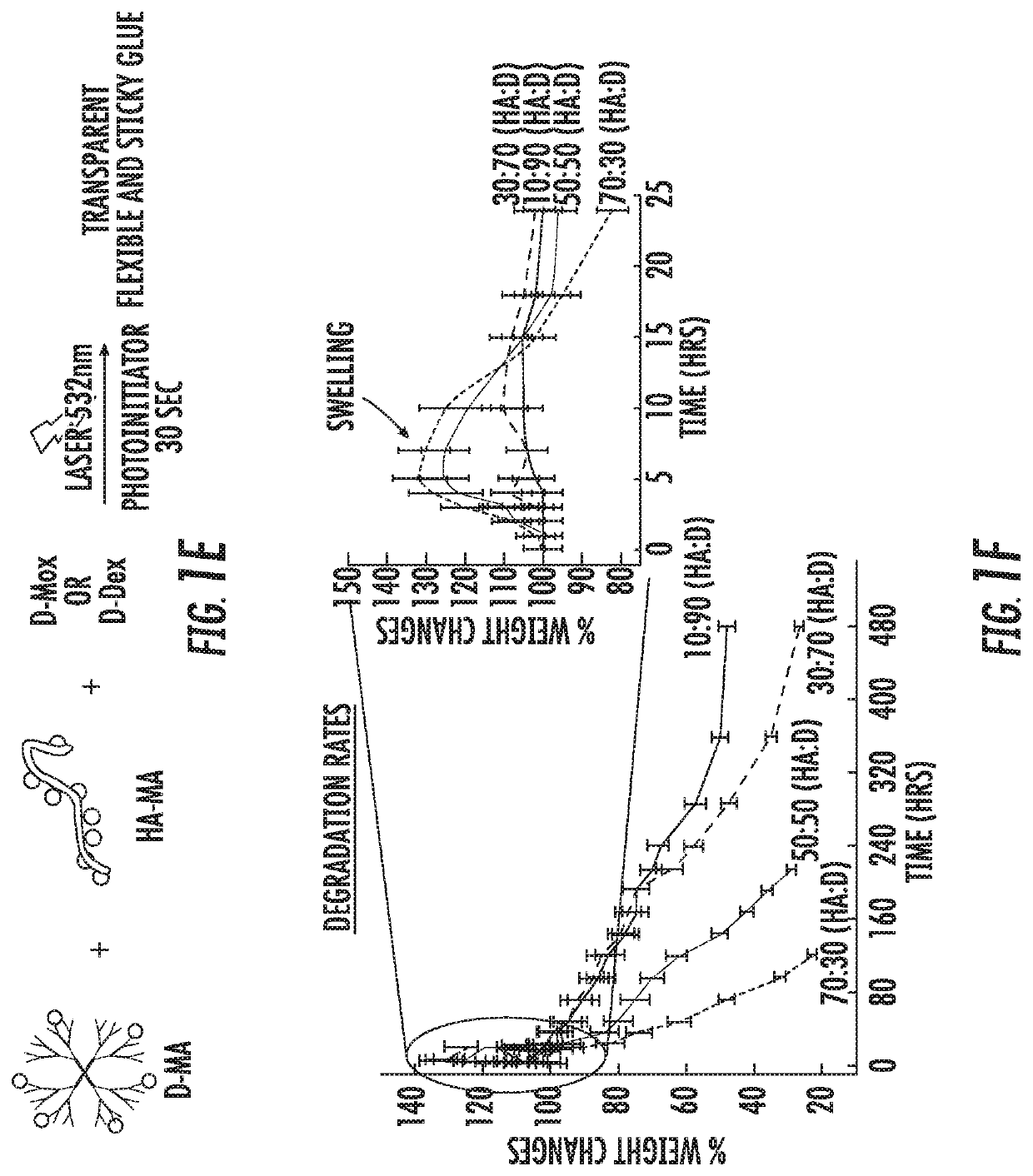
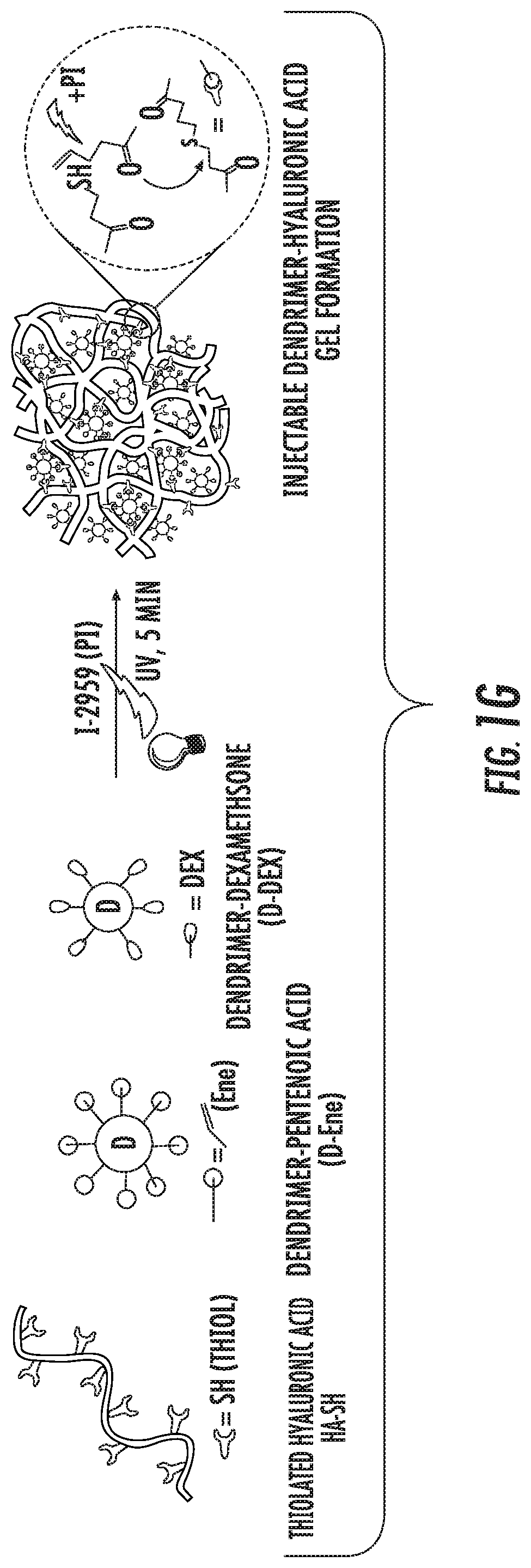
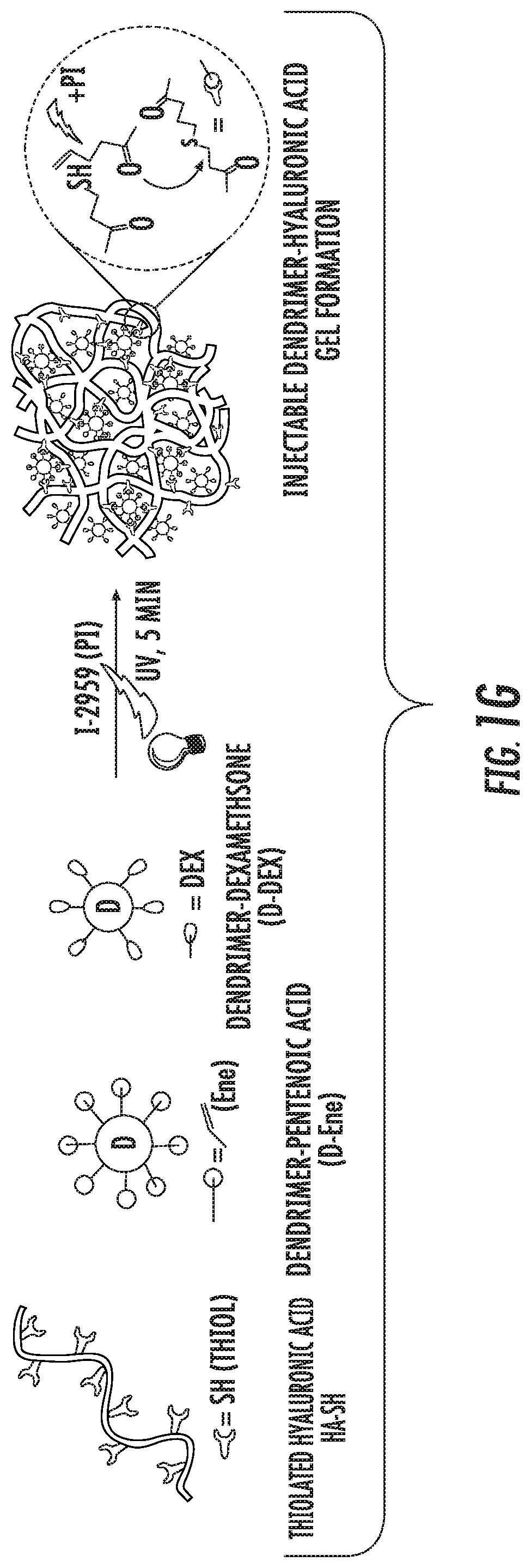
Dendrimer-bioadhesive polymer hydrogel nanoglue and use thereof
PendingUS20200171200A1Desirable mechanical propertyAvoid infectionMaterial nanotechnologyOrganic active ingredientsCorneal woundPharmaceutical drug
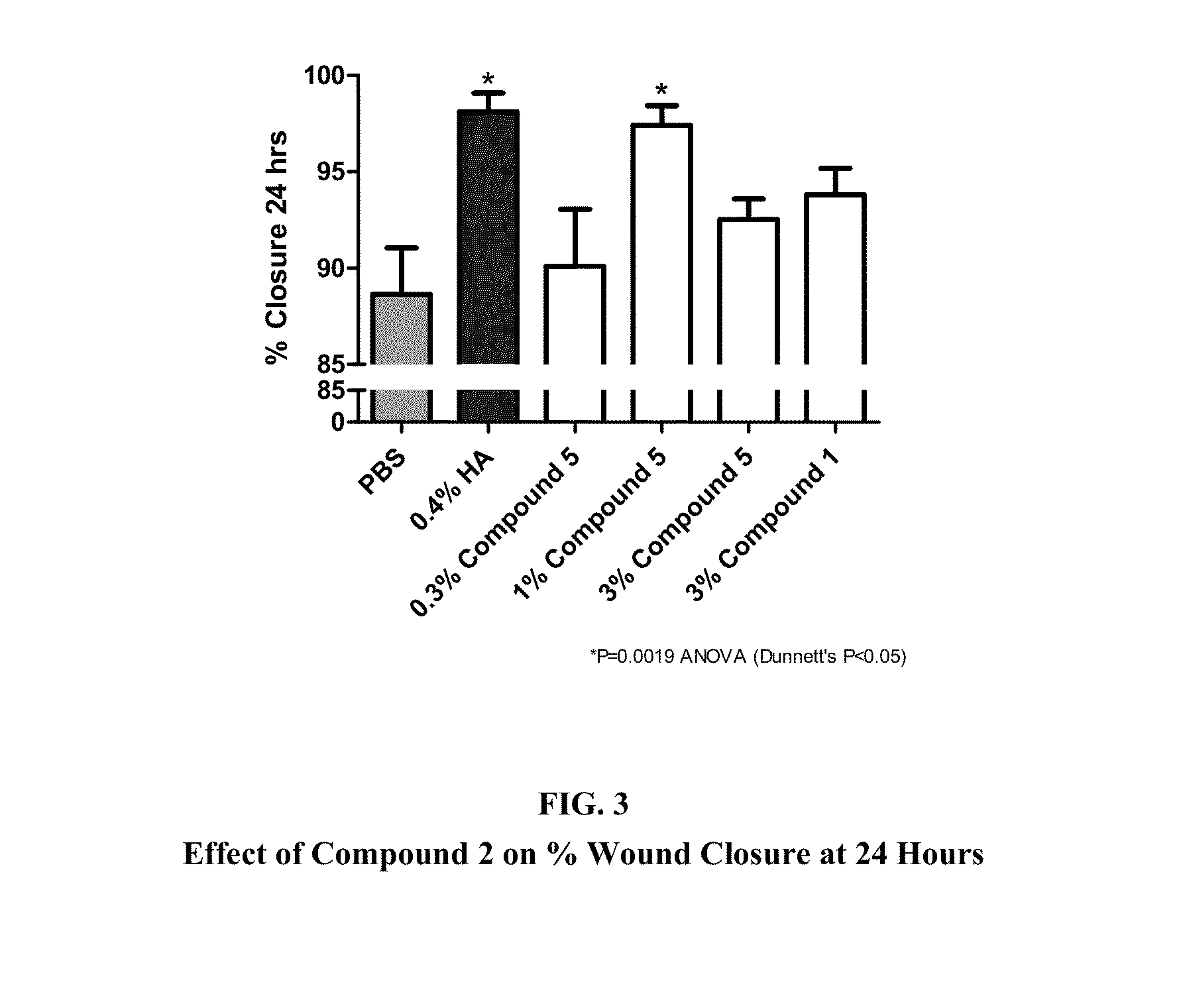
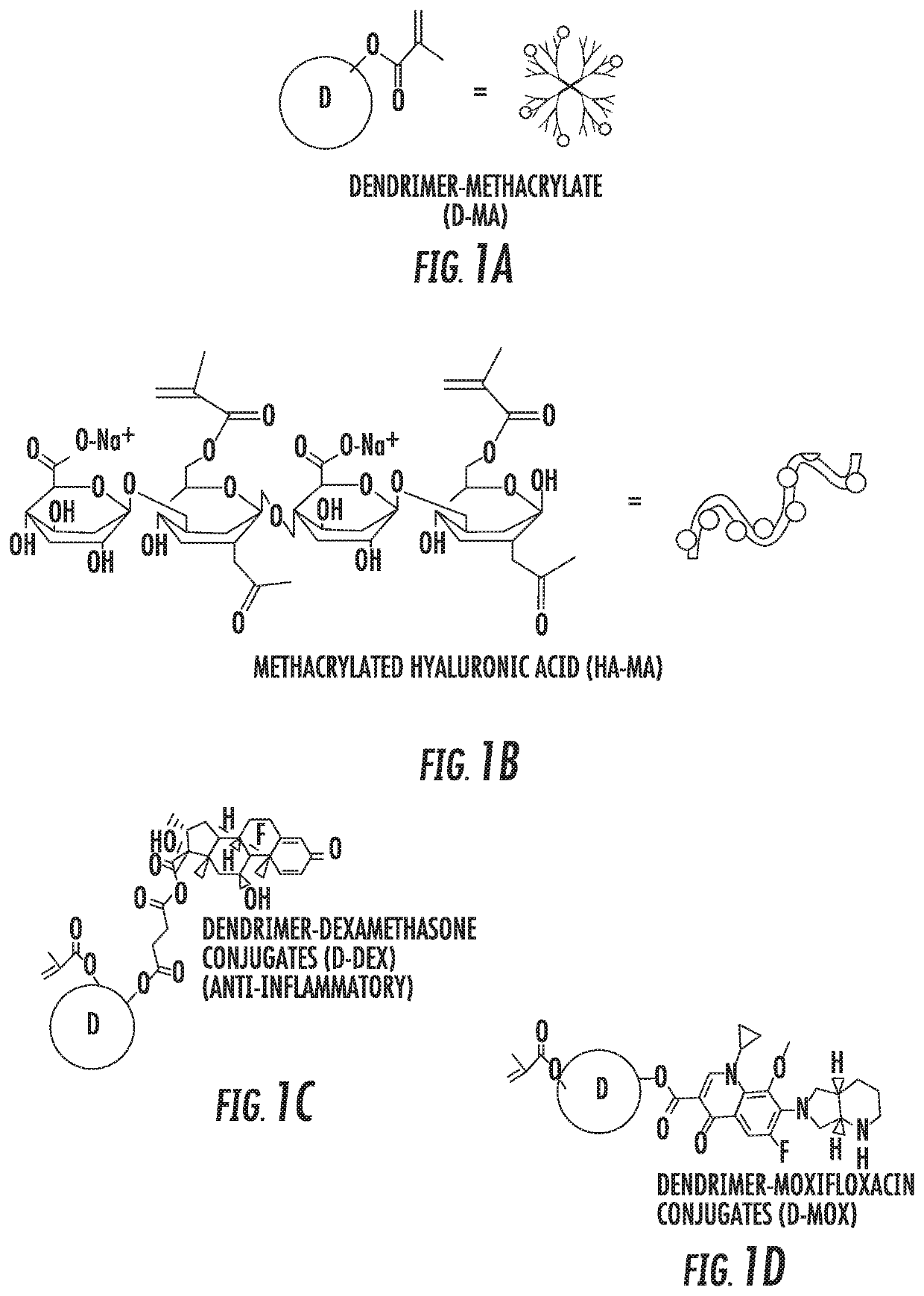
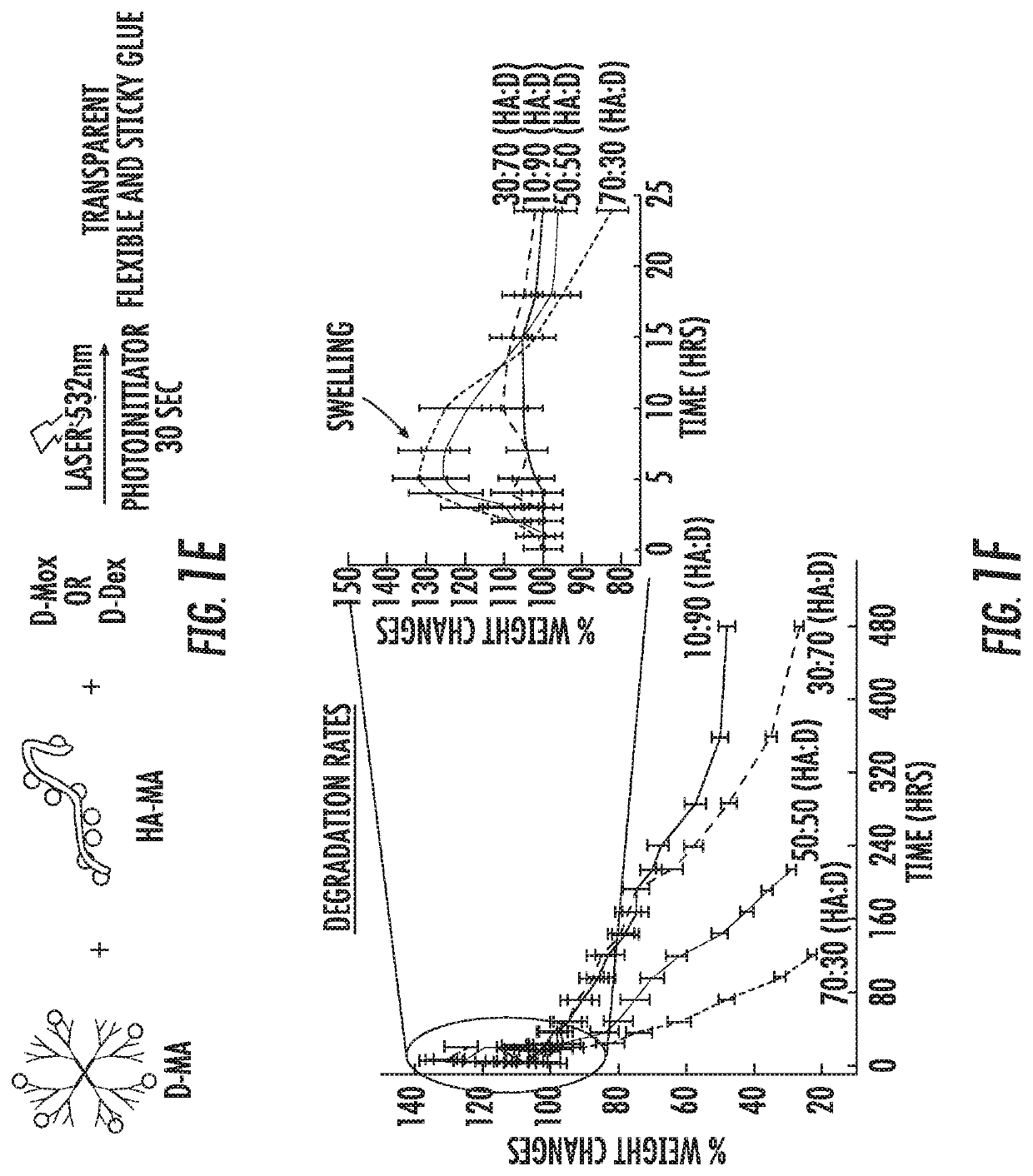
A nanoglue is formed with one or more bioadhesive polymers, one or more dendrimers, and optionally one or more therapeutic, prophylactic, or diagnostic agents. The bioadhesive polymers and dendrimers are modified with functional groups to permit crosslinking upon one or more stimuli, e.g., ultraviolet irradiation, and form hydrogel in situ at tissue sites. In the repair of corneal wounds, the nanoglue leads to improved rate of healing with less scarring and less inflammation, compared to non-treated cornea or ones treated with sutures. Therapeutic agents can be covalently conjugated to the precursor components and be delivered to specific eye compartments, providing a more efficacious treatment formulation of ocular disorders than delivering drugs in their free forms. Methods of making and using the hydrogel and hydrogel precursor compositions are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
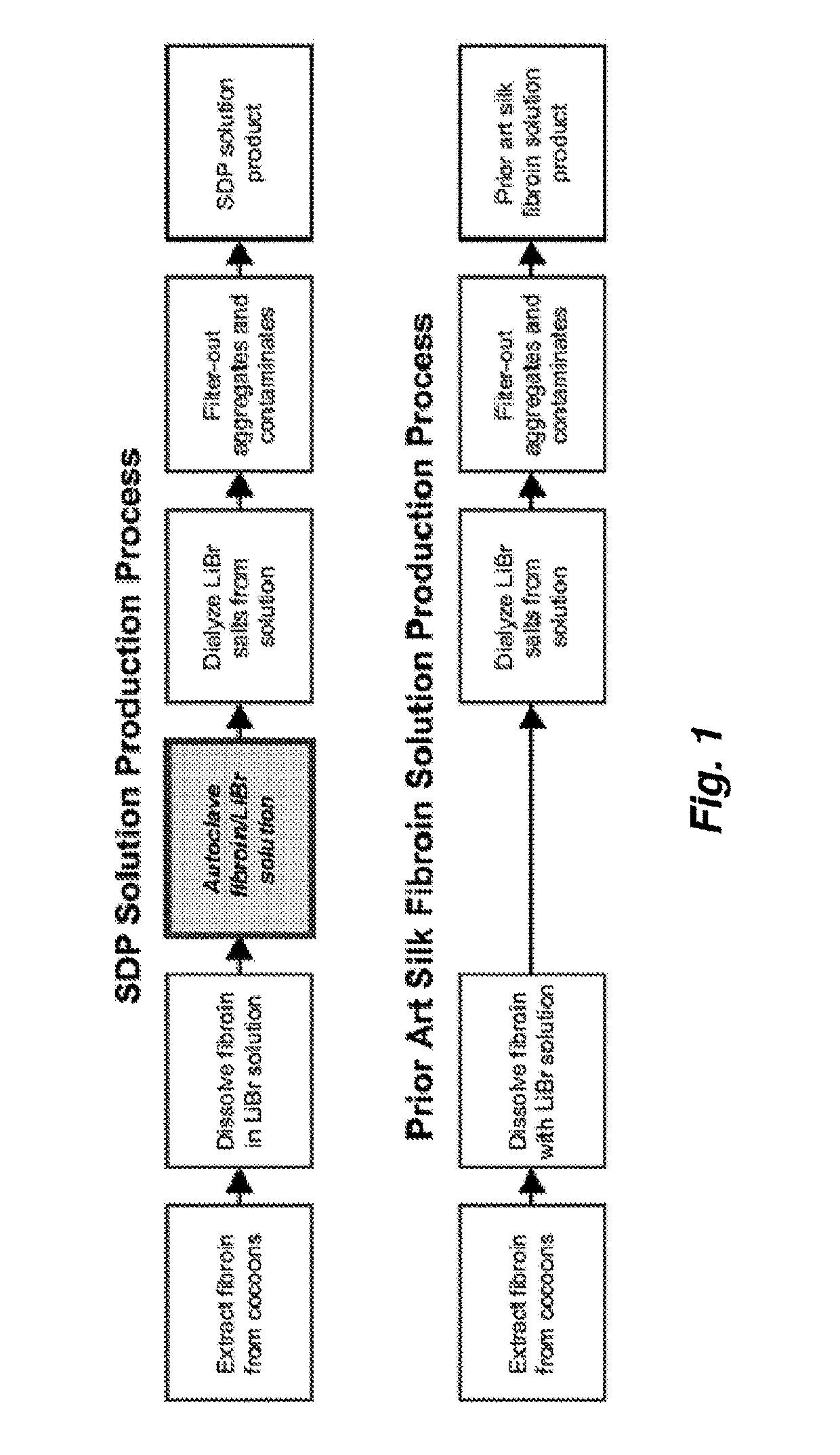
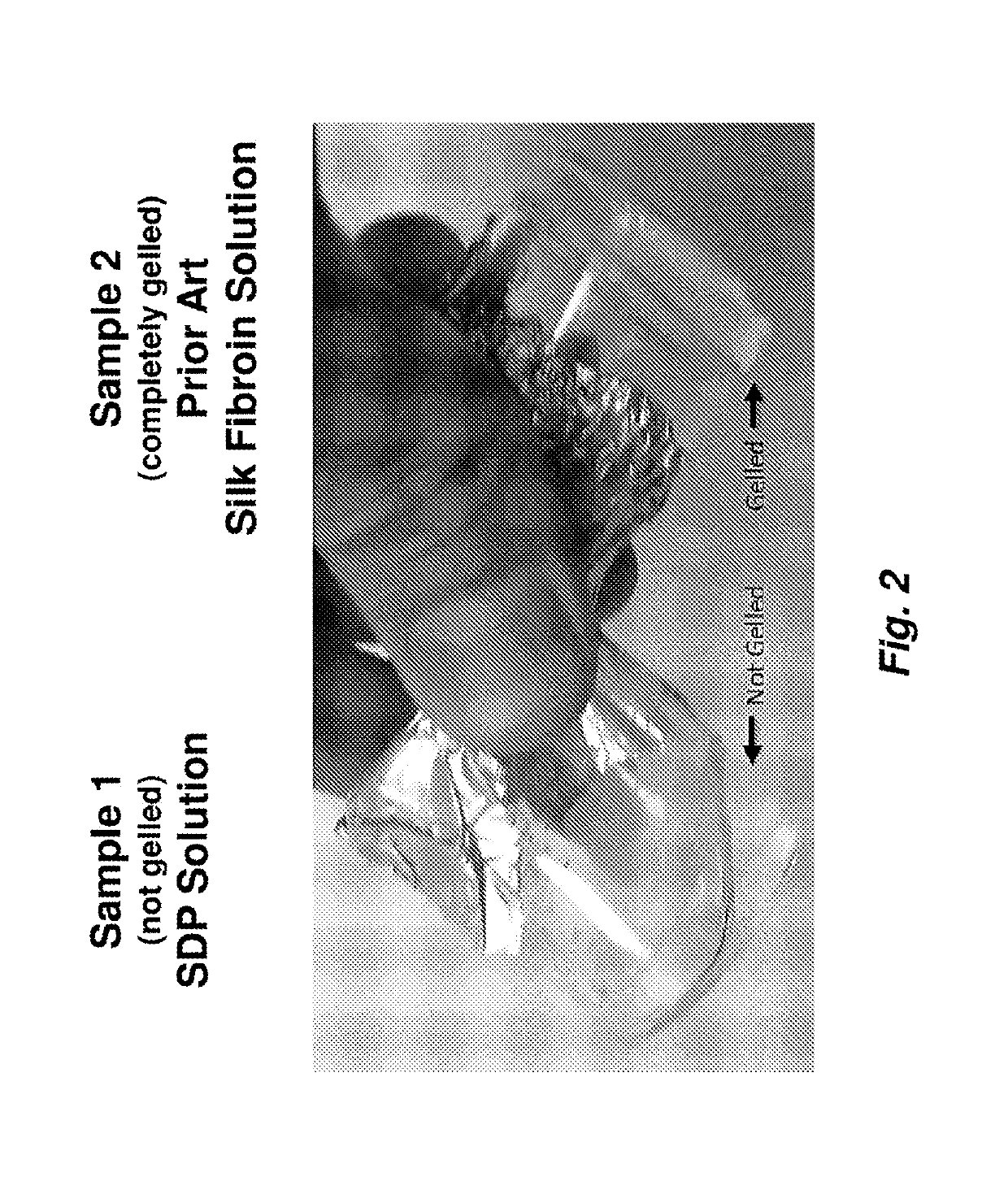
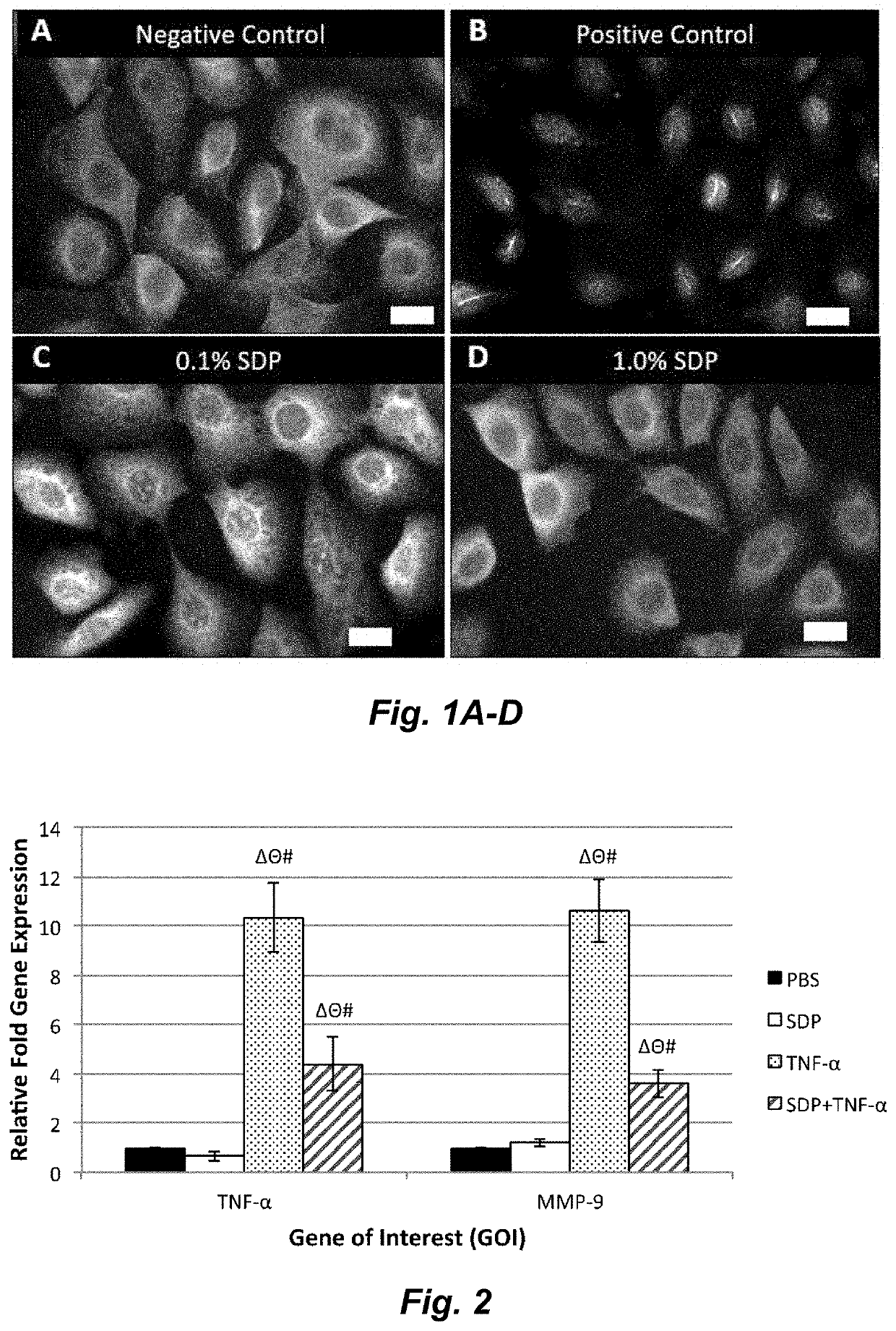
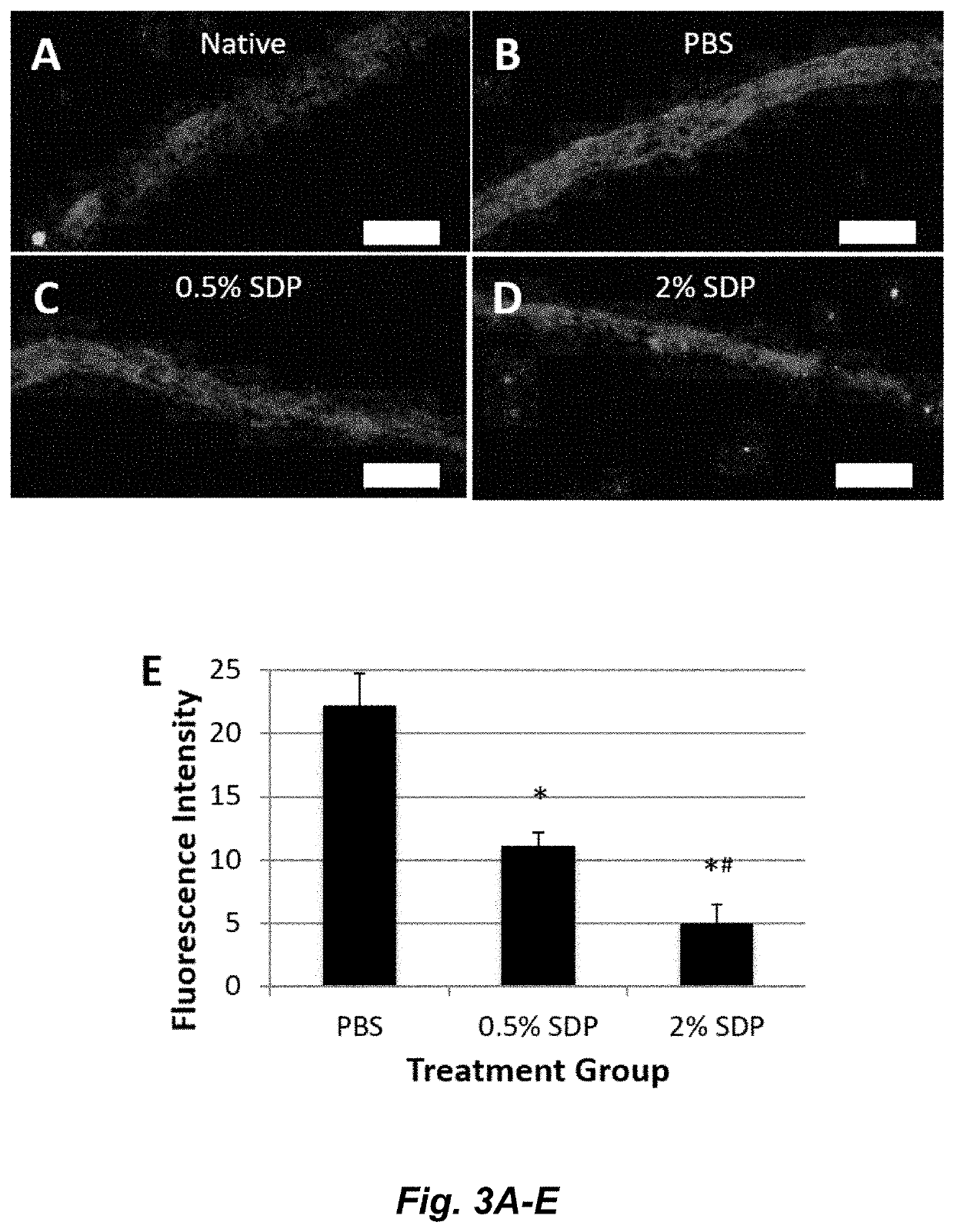
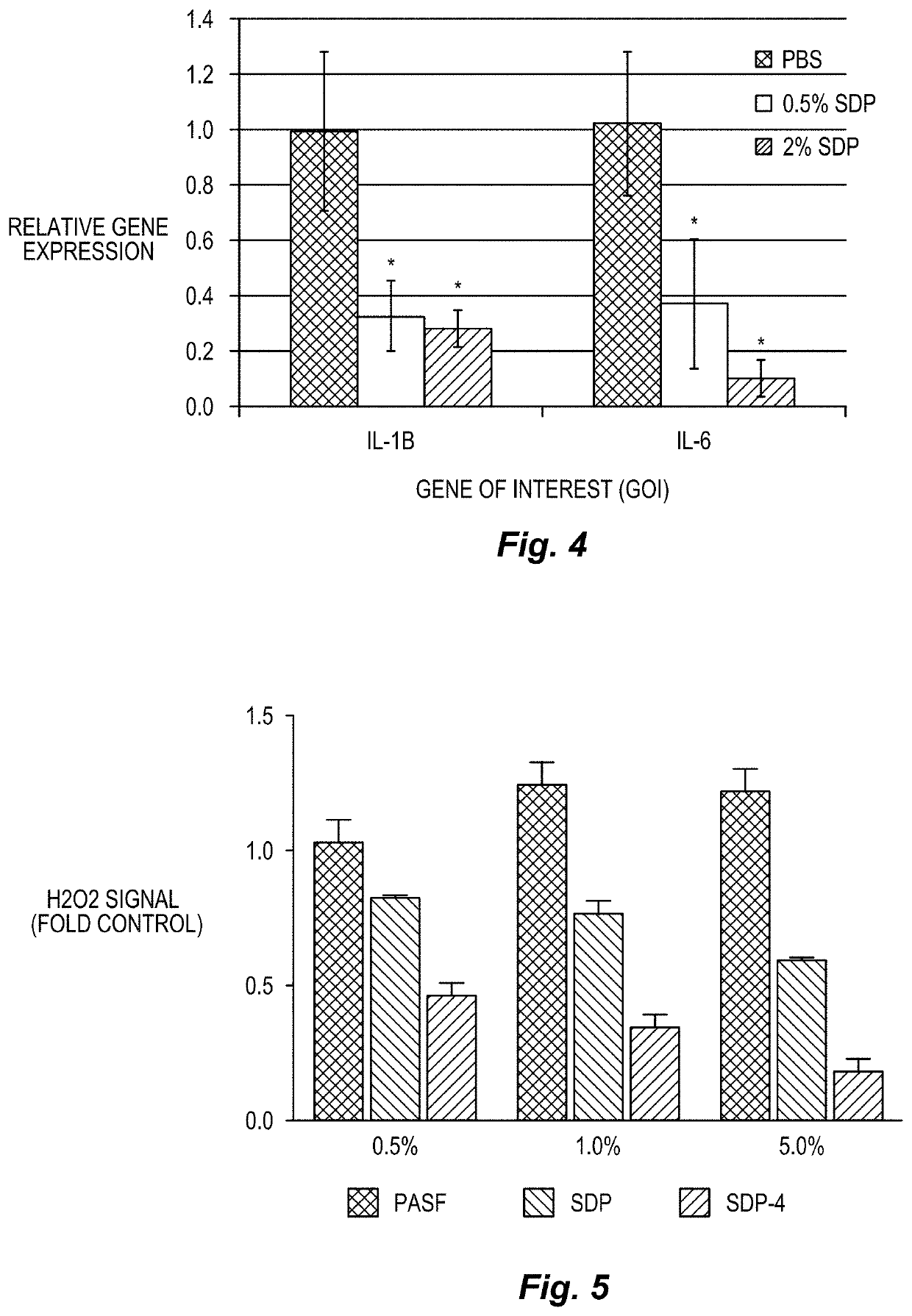
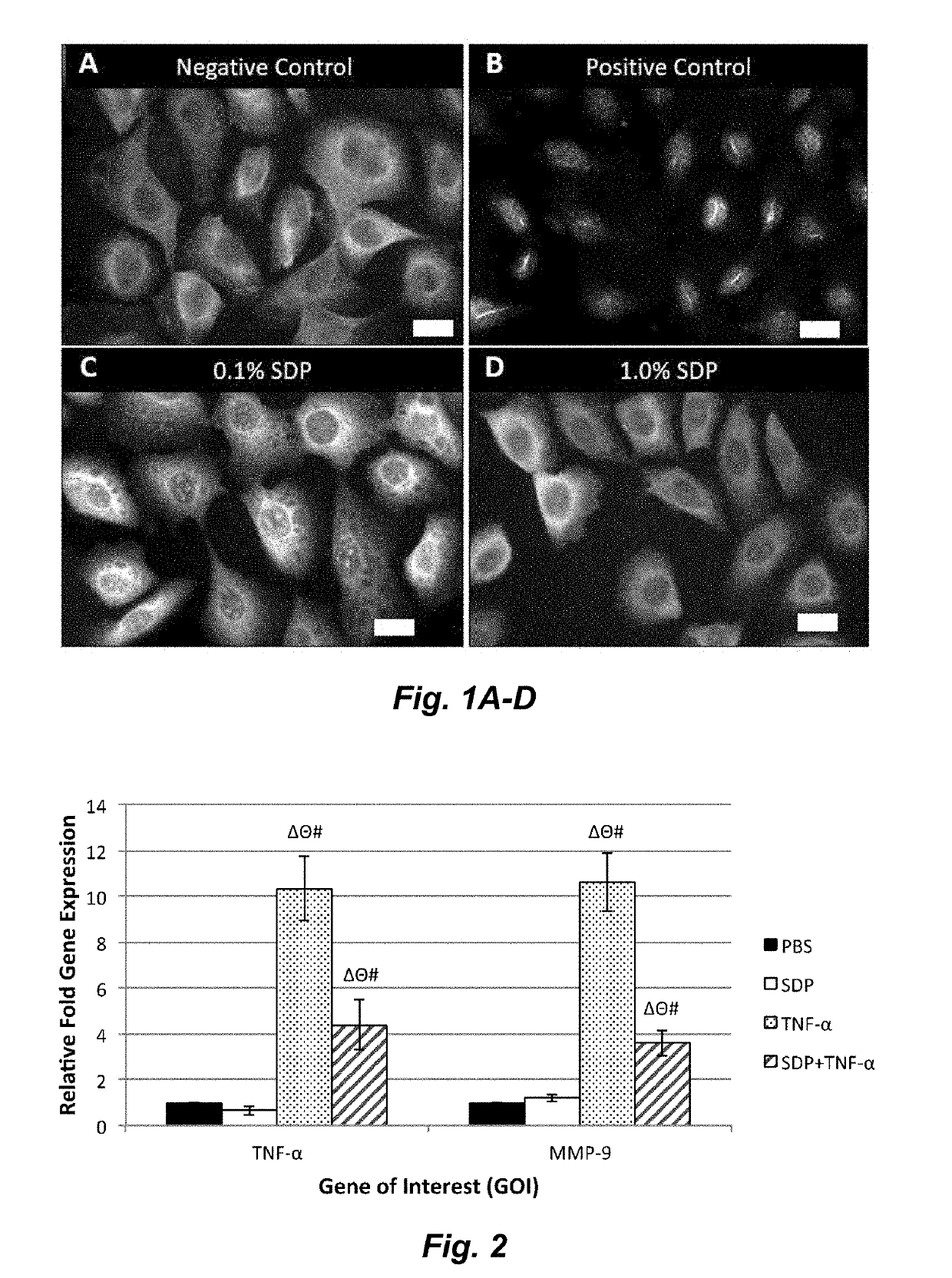
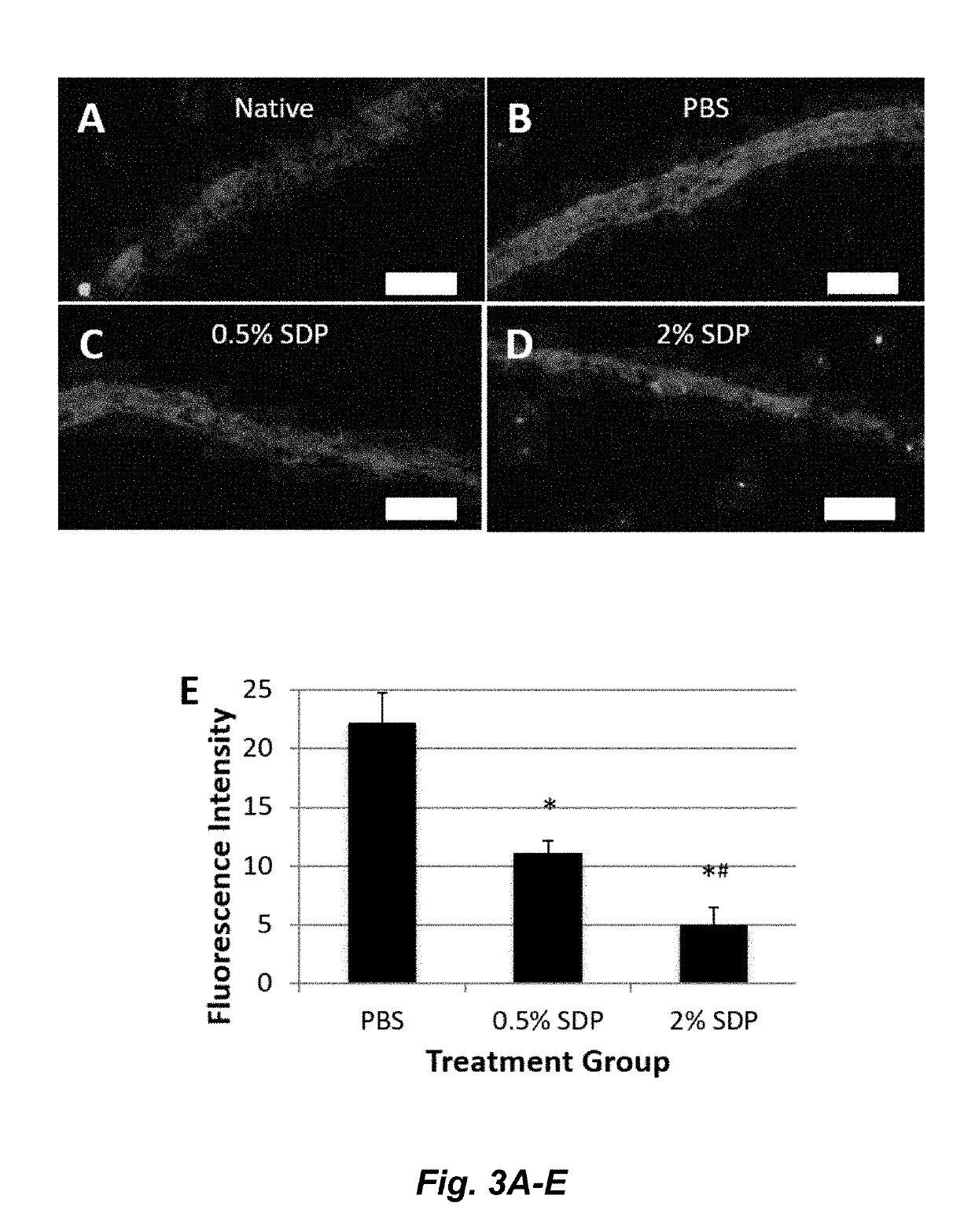
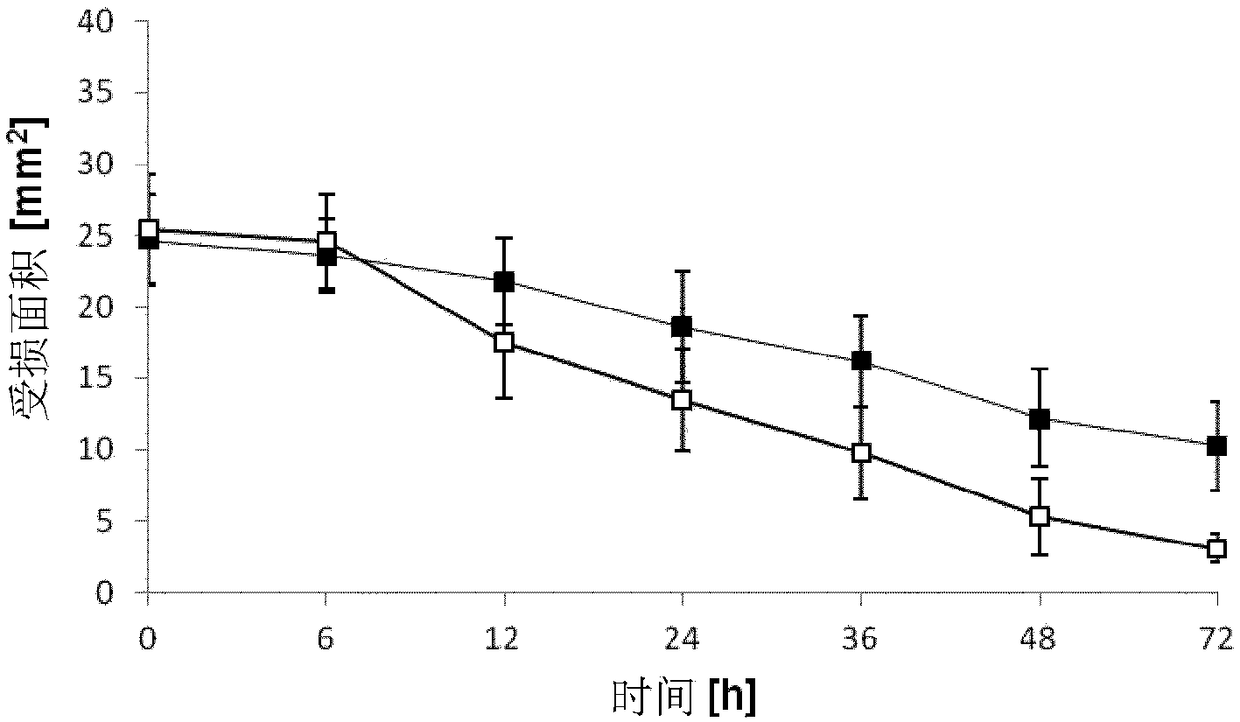
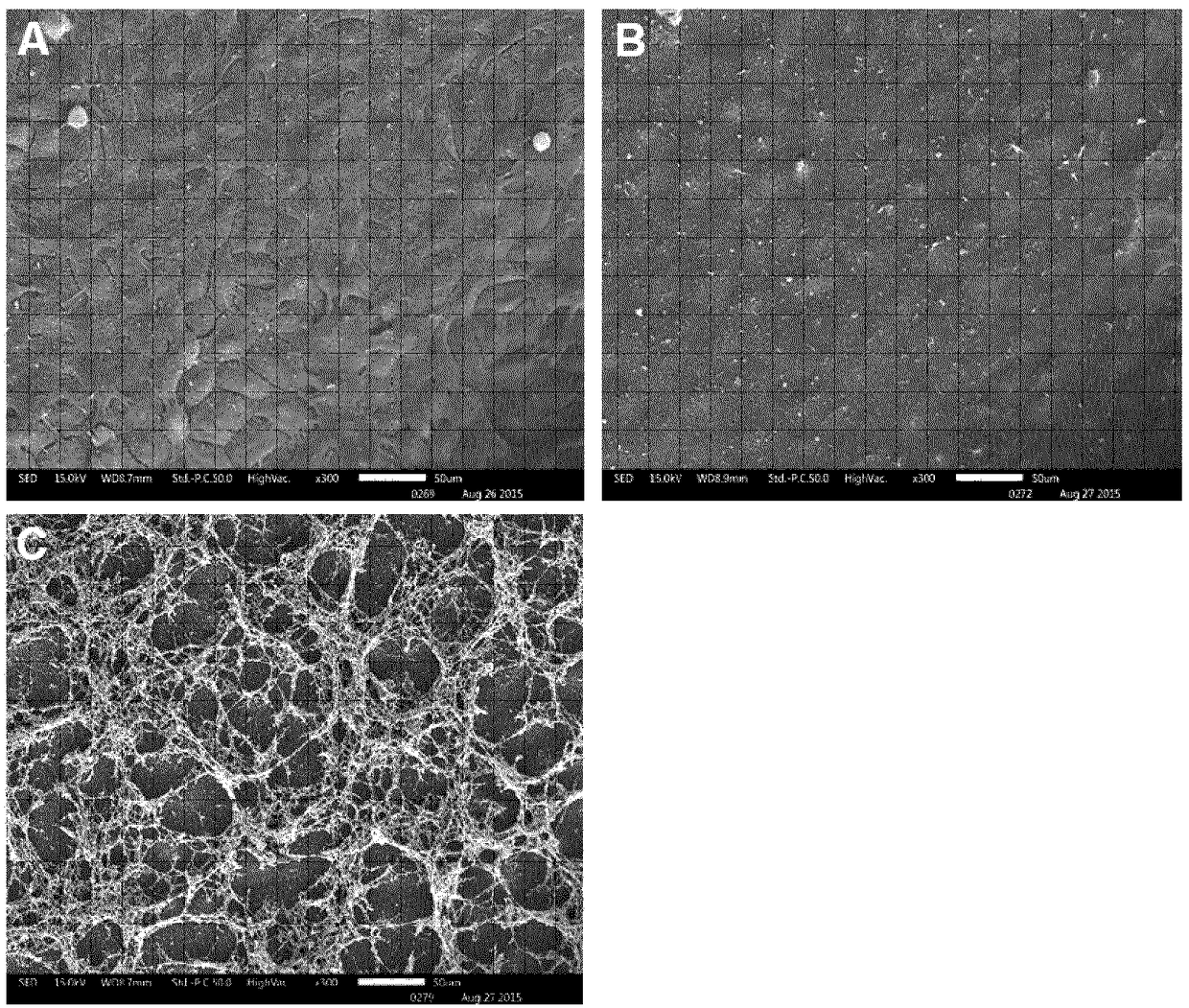
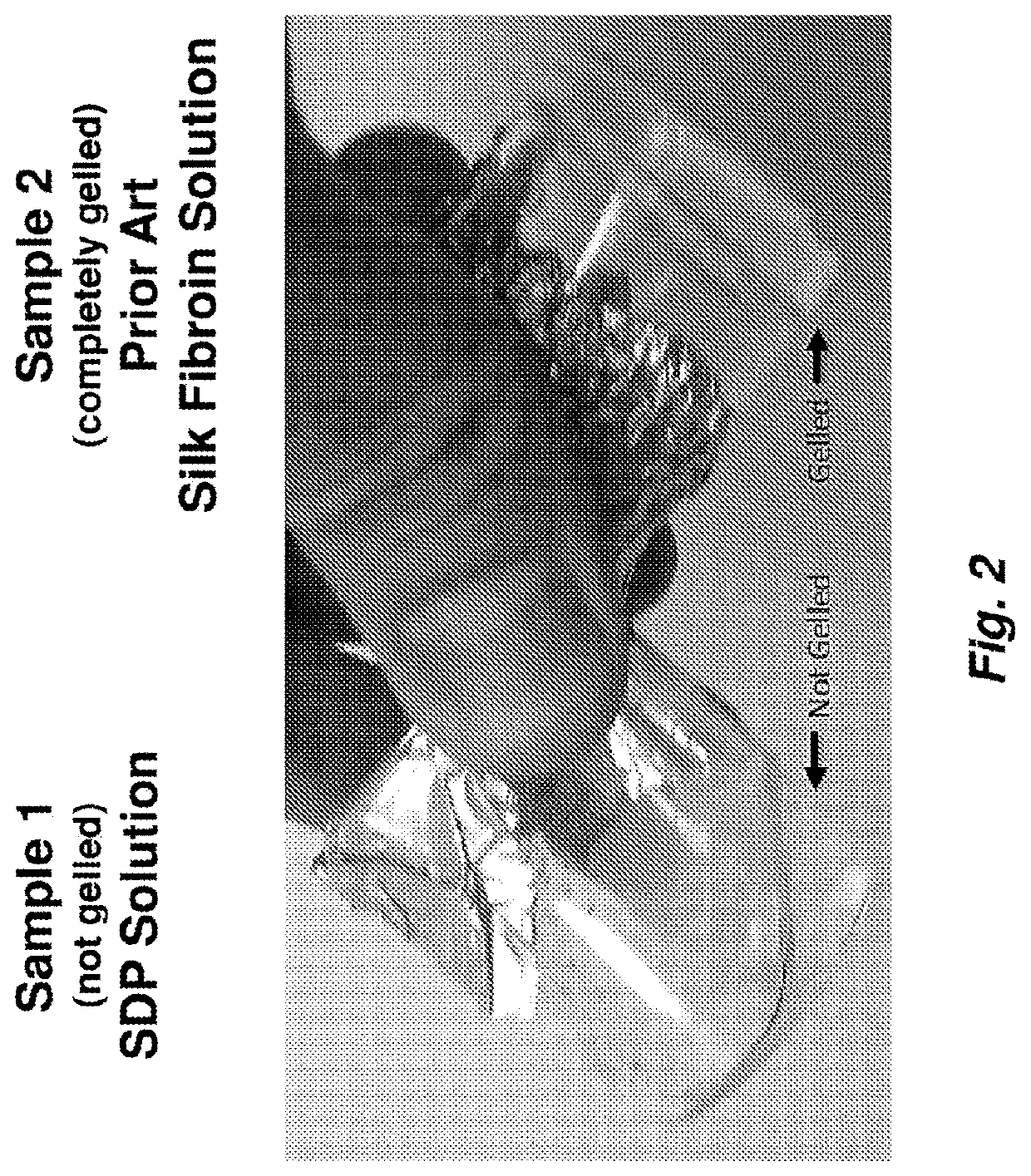
A method to enhance wound healing using silk-derived protein
ActiveUS20190117834A1Promote wound healingImprove stabilitySenses disorderPeptide/protein ingredientsCorneal woundSurgical incision
Described herein are methods of enhancing wound healing using silk-derived proteins (SDP), including low molecular weight SDP fragments. Also described are compositions for the treatment of wounds, including corneal wounds, skin wounds, surgical incisions, burns, and skin ulcers, comprising SDP fragments, including low molecular weight SDP fragments.
Owner:SILK TECH +1
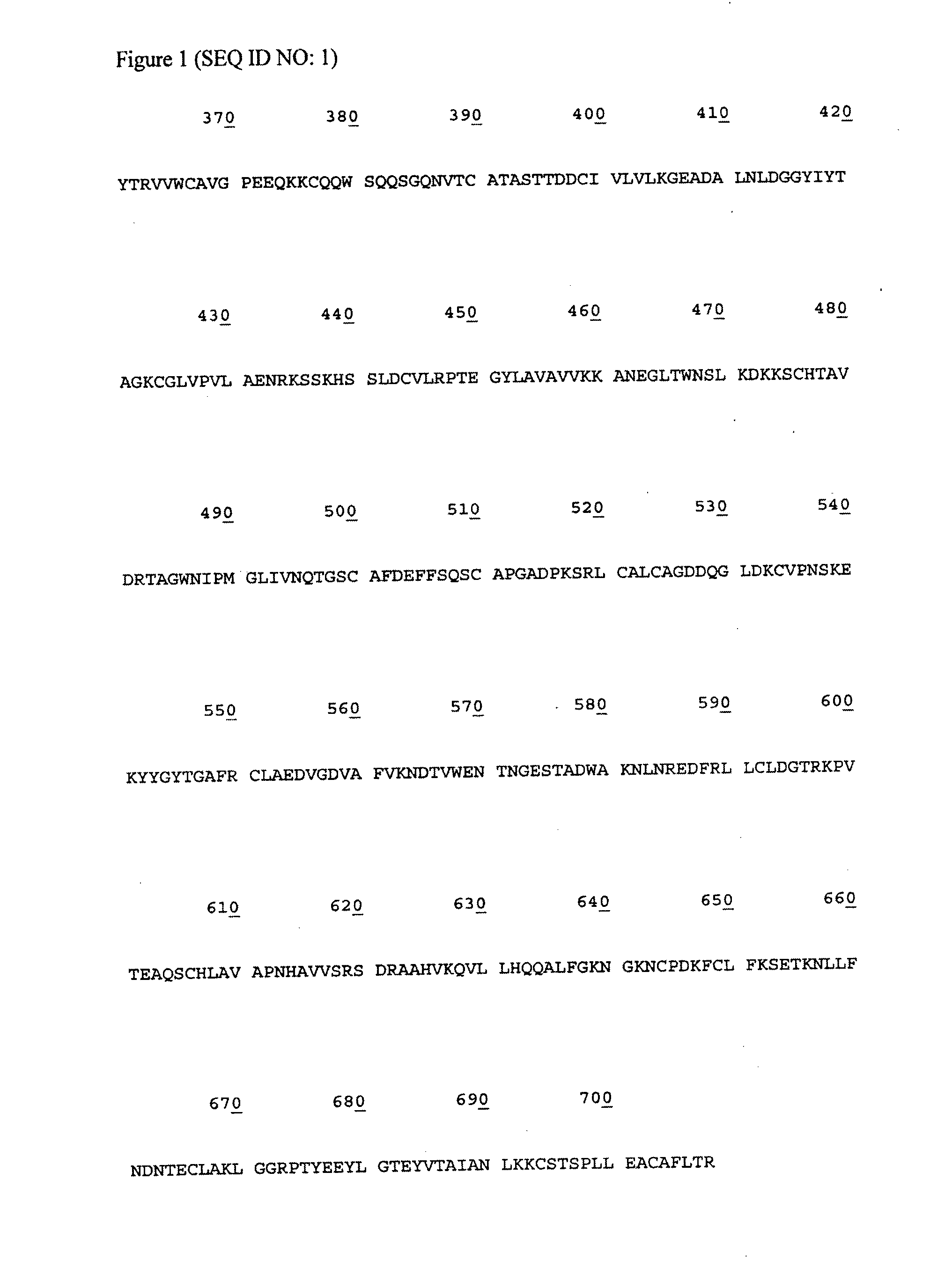
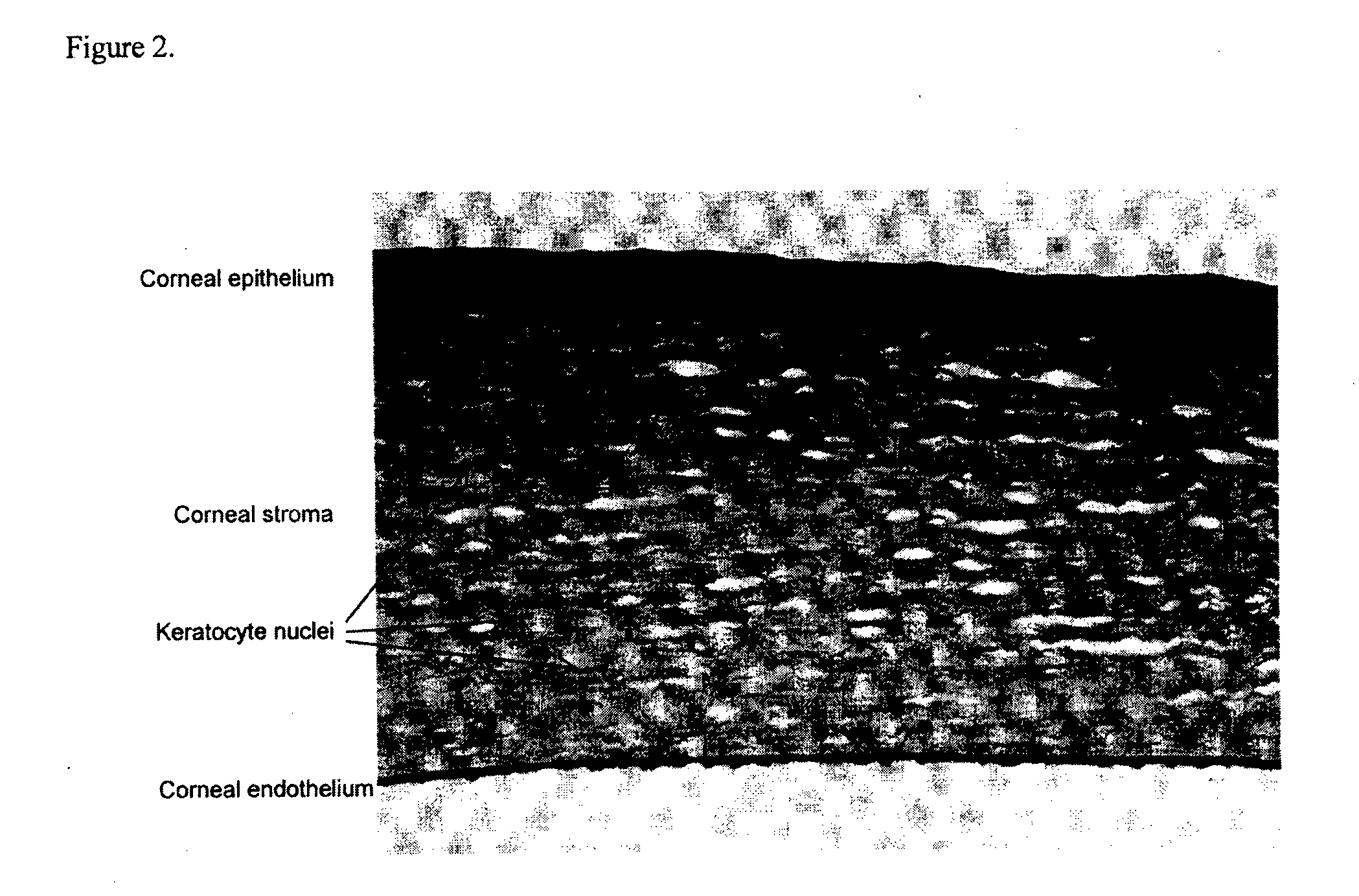
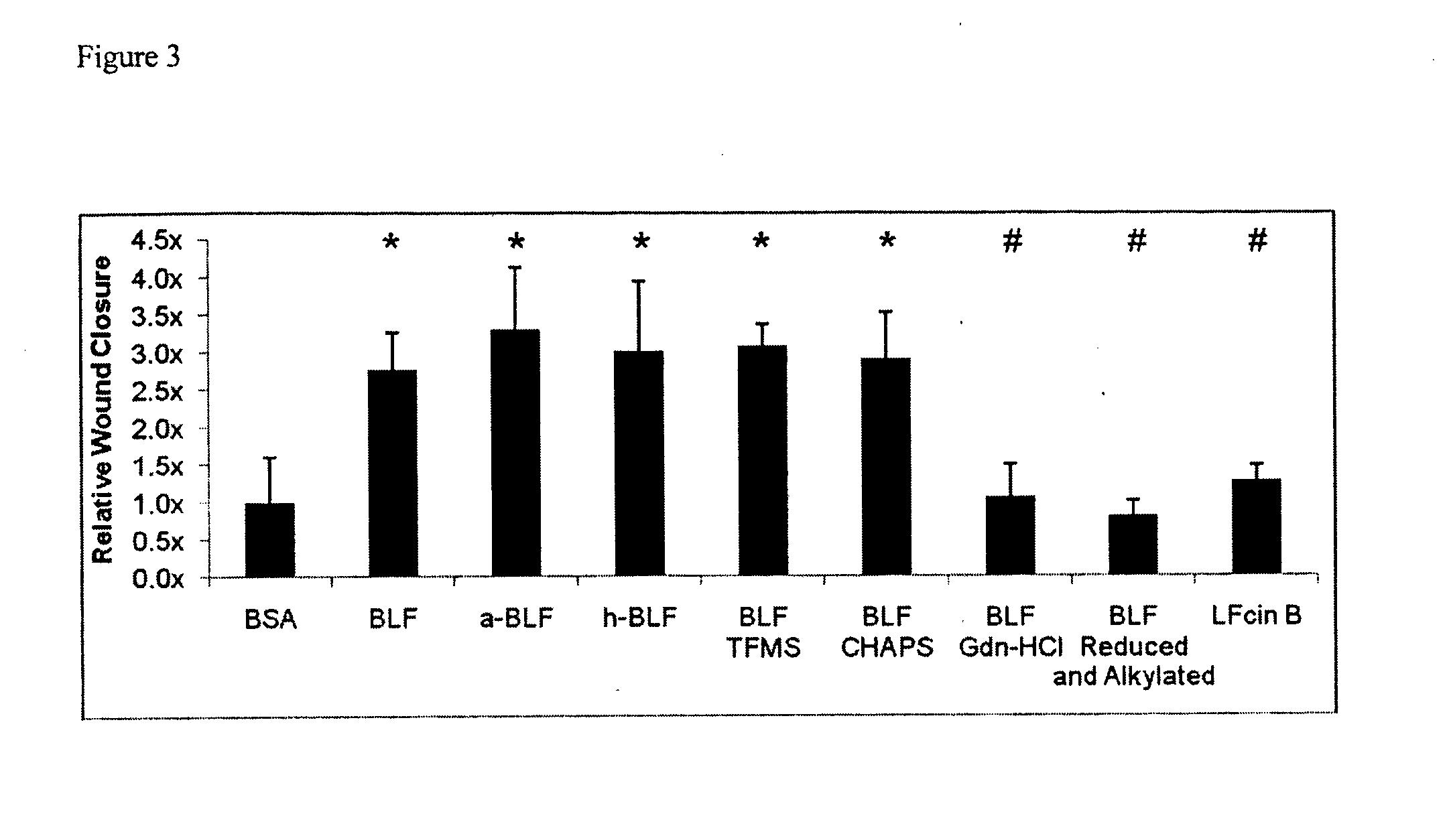
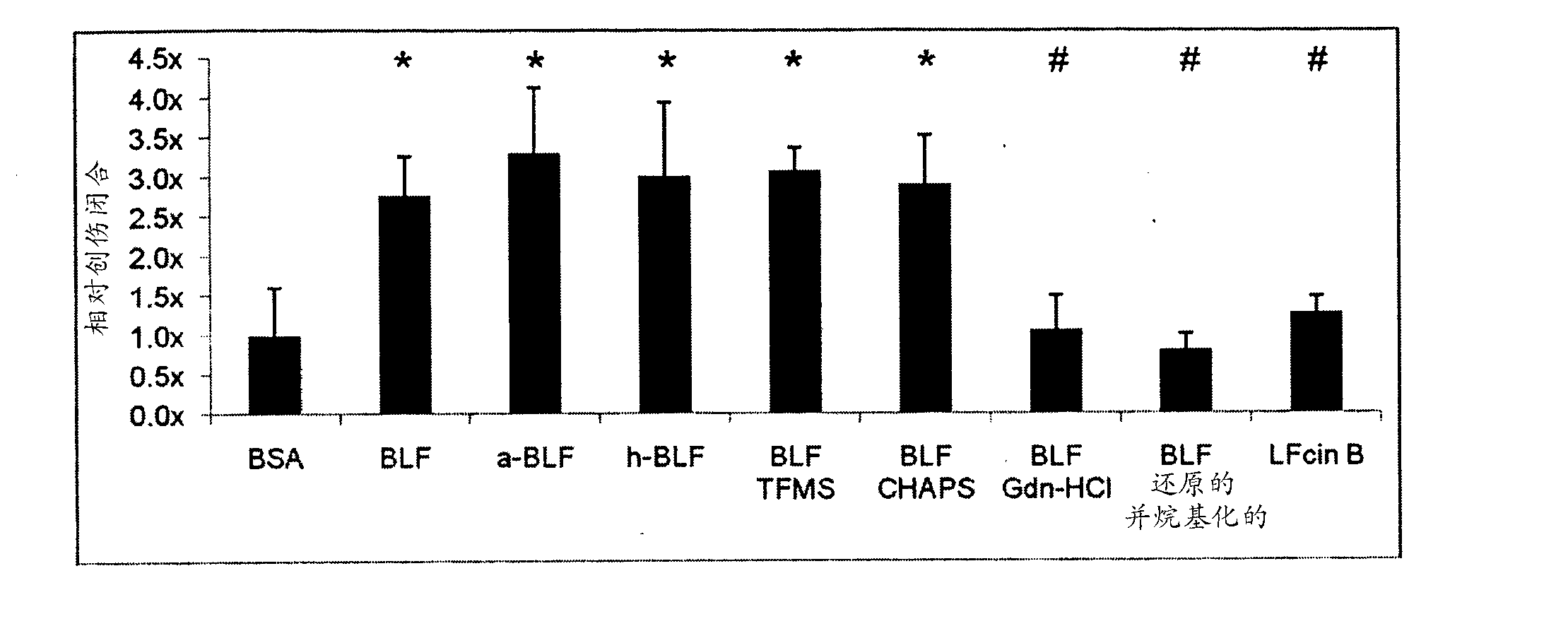
Lactoferrin seqences, compositions and methods for corneal wound treatment
The present invention relates to pharmaceutical compositions containing lactoferrin, or fragments of it, and their use in the treatment of wounds, particularly corneal wounds. The present invention also provides a pharmaceutical composition comprising an effective amount of a polypeptide or peptidomimetic consisting essentially of the C-lobe of lactoferrin, or functionally active fragments or variants thereof.
Owner:BRIEN HOLDEN VISION INST (AU)
Corneal suture nail based on shape memory alloy
InactiveCN102133142BGood biocompatibilitySuture equipmentsEye surgeryCorneal woundMartensite transformation
Owner:BEIHANG UNIV
Actin binding peptide and purpose thereof
ActiveCN102924573APromote wound healingGood restorativeSenses disorderPeptide/protein ingredientsCorneal woundSide effect
The invention relates to the field of biological medicine, particularly discloses actin binding peptide and the purpose of the actin binding peptide. The amino acid sequence of the actin binding peptide is LKKTETQ, the synthesis difficulty and the cost are lowered, the actin binding peptide is suitable for batch production in a large scale and has a brilliant preparing function on various corneal injuries, side effects do not appear in the dosing process, and corneal wound healing and transparency recovery are benefited. Compared with a curing method through thymosin beta4, the advantages are more obvious, the side effects are small, and the actin binding peptide can be used for corneal repairing treatment.
Owner:ZHAOKE GUANGZHOU OPTHALMIC DRUG
High-performance renewable corneal material, reparation method and application thereof
ActiveCN112295018AHigh transparencyImprove mechanical propertiesTissue regenerationProsthesisCorneal woundBiocompatibility
The invention belongs to the field of medical devices, and relates to a high-performance renewable corneal material, a preparation method and an application thereof. The preparation method of the corneal material comprises the following steps: harvesting fresh lamellar corneas from animal or human eyeballs under aseptic conditions, and sequentially performing preliminary mild decellularization, elution and removal of decellularization agent, supplementation of glycosaminoglycan for crosslinking, subsequent repeated thorough decellularization, secondary elution and removal of the decellularization agent, preservation with preserving liquid and sterilization. According to the invention, the high-performance renewable corneal material prepared by a repeated decellularization technology and akey technology of synergistic modification with efficient healing-promoting materials has high transparency, good mechanical property, good degradation resistance and biocompatibility and can promotehealing of corneal wounds. The high-performance renewable corneal material is a major breakthrough in corneal material preparation technology. Meanwhile, the process of the present invention is simple, flexible, good in product reproducibility and easy to realize industrial popularization. The renewable corneal material of the present invention can be widely used for treating blinding eye diseasescaused by corneal lesions or corneal injuries.
Owner:济南金泉生物科技有限公司
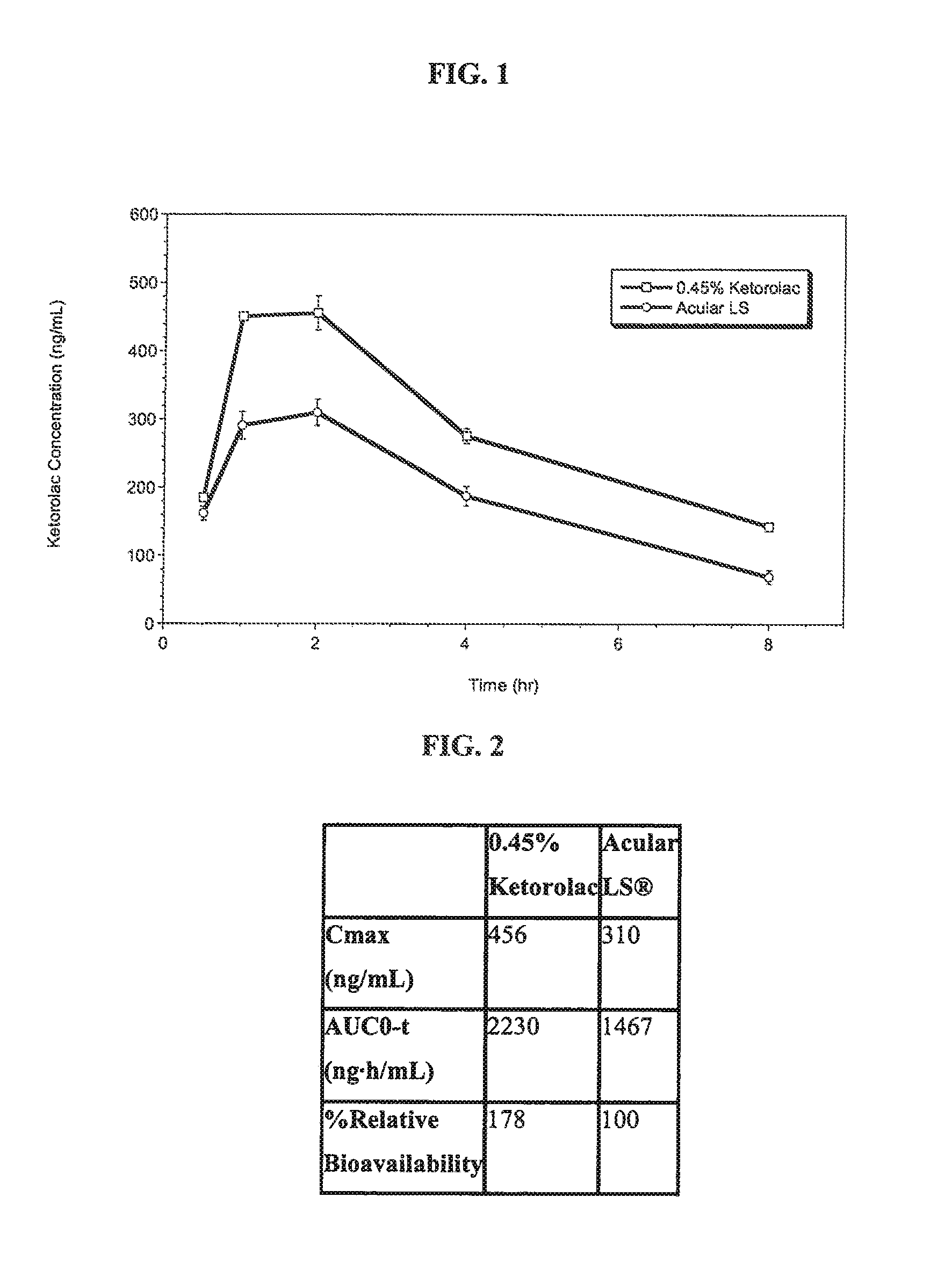
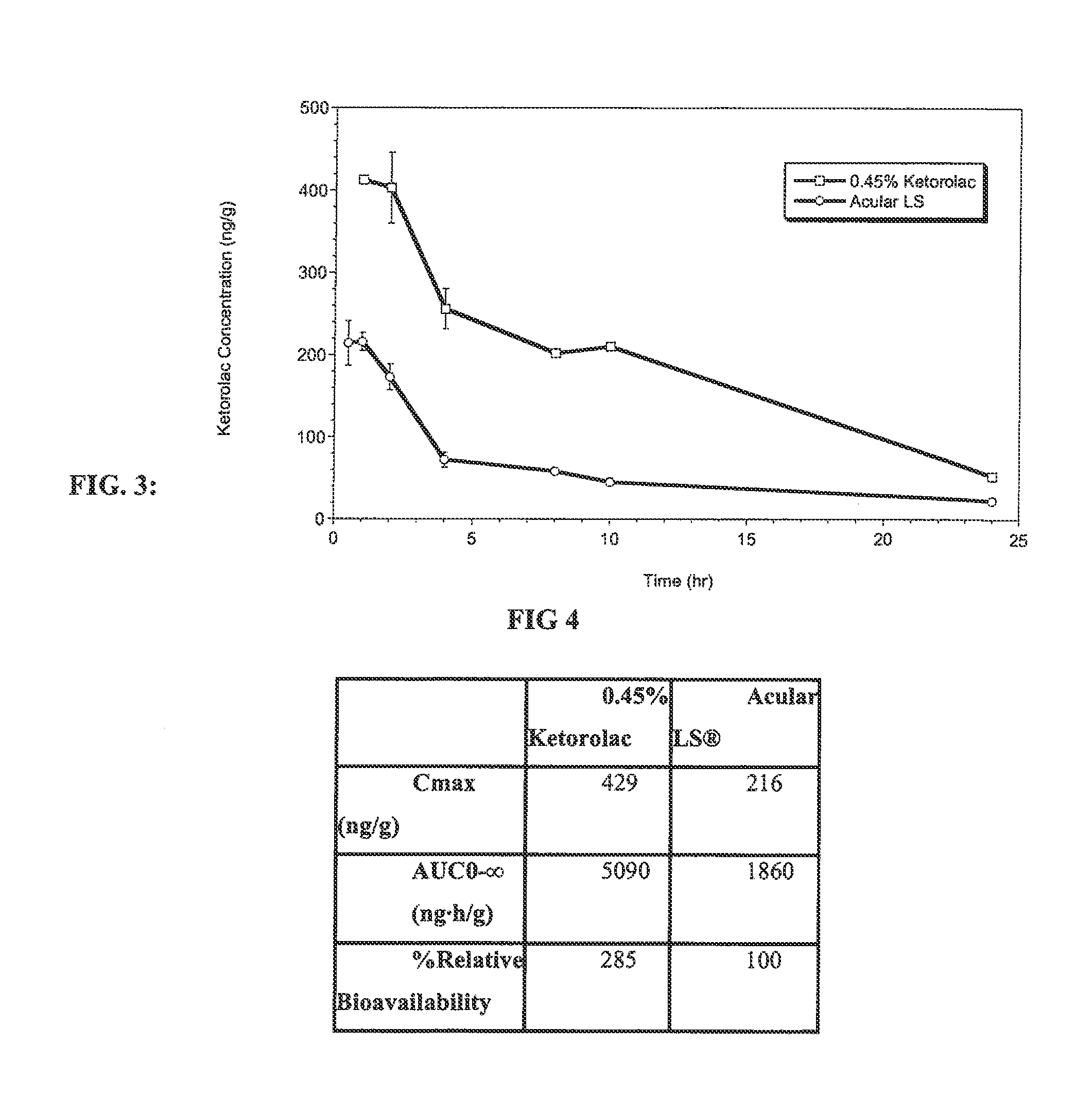
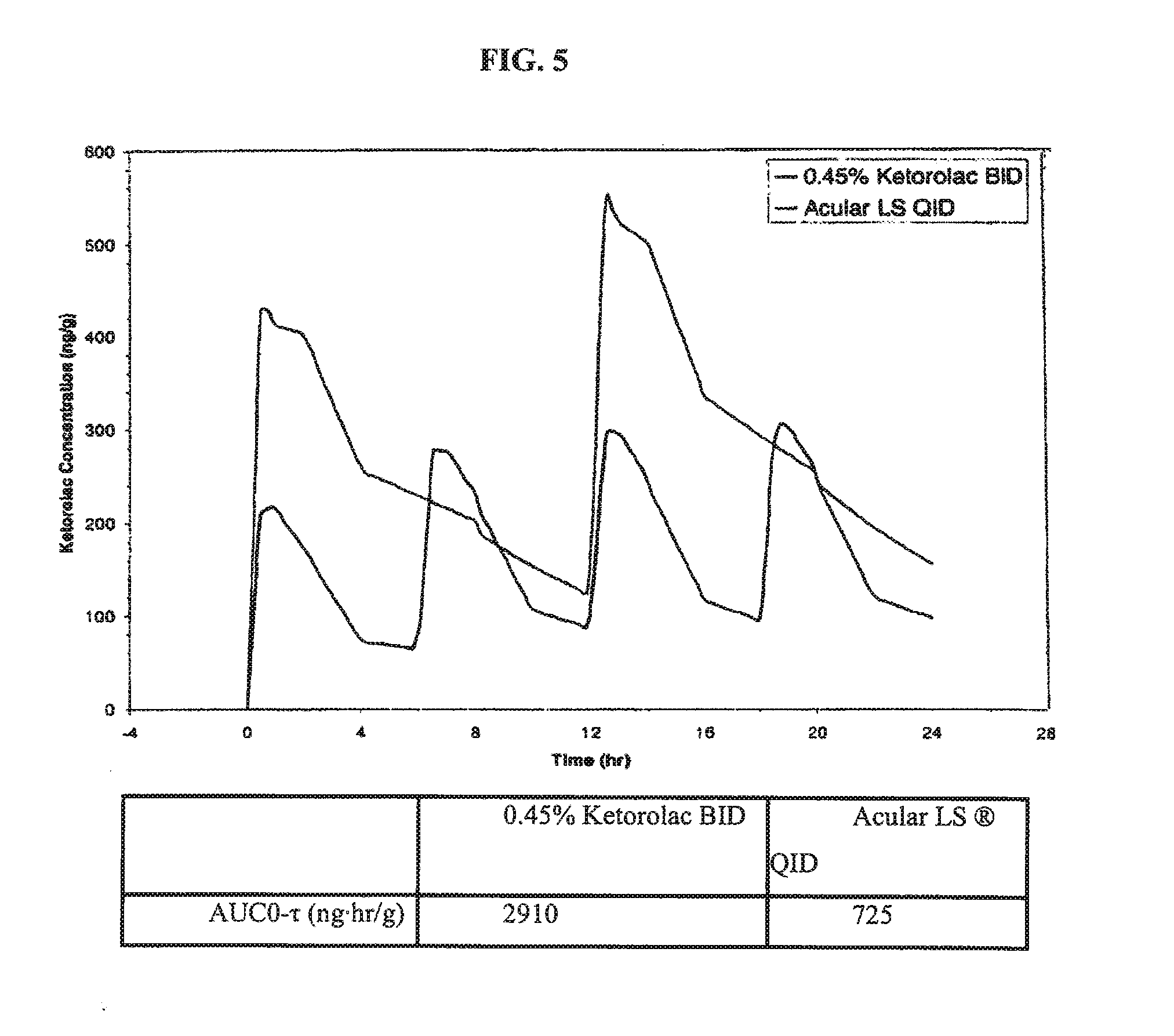
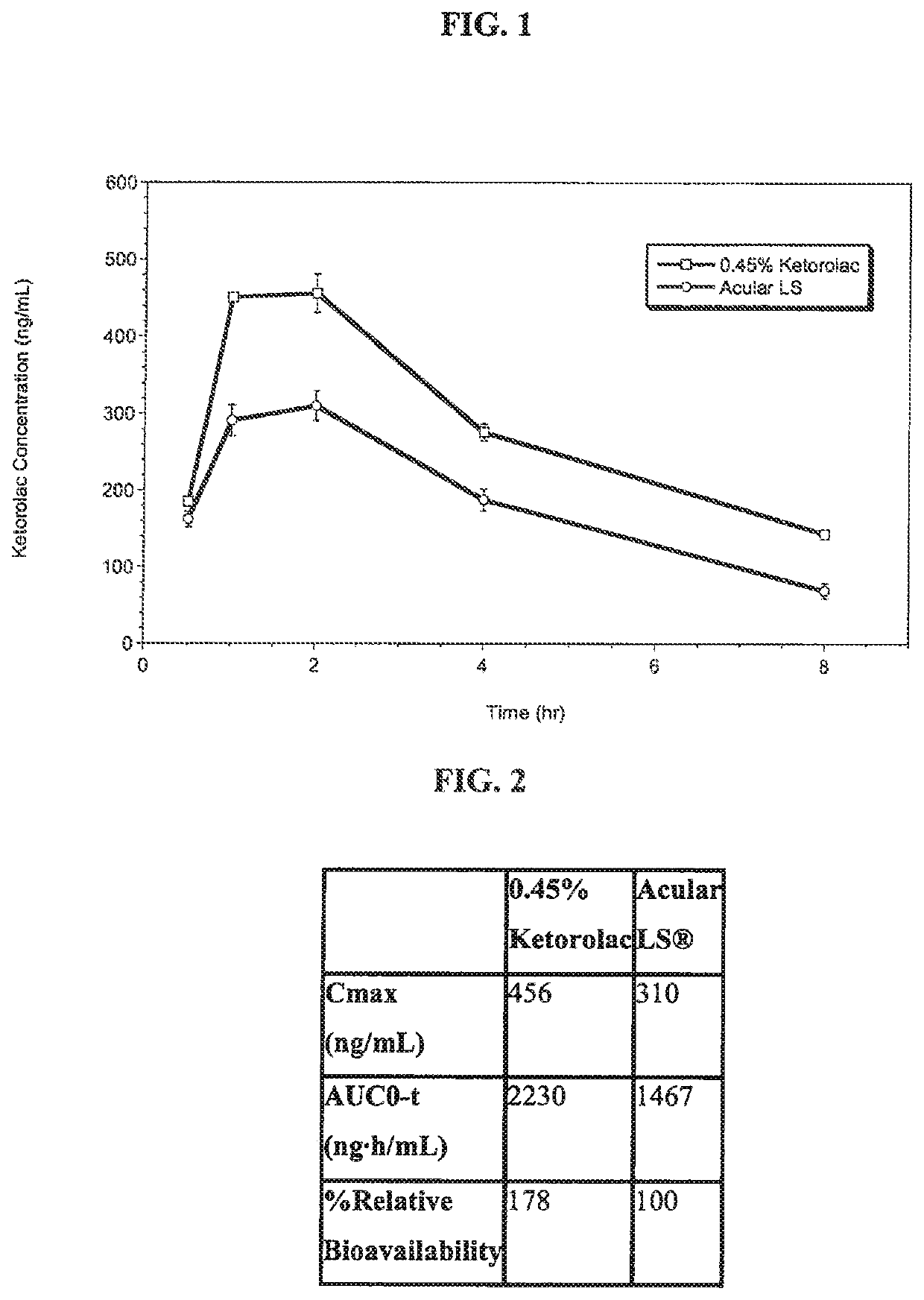
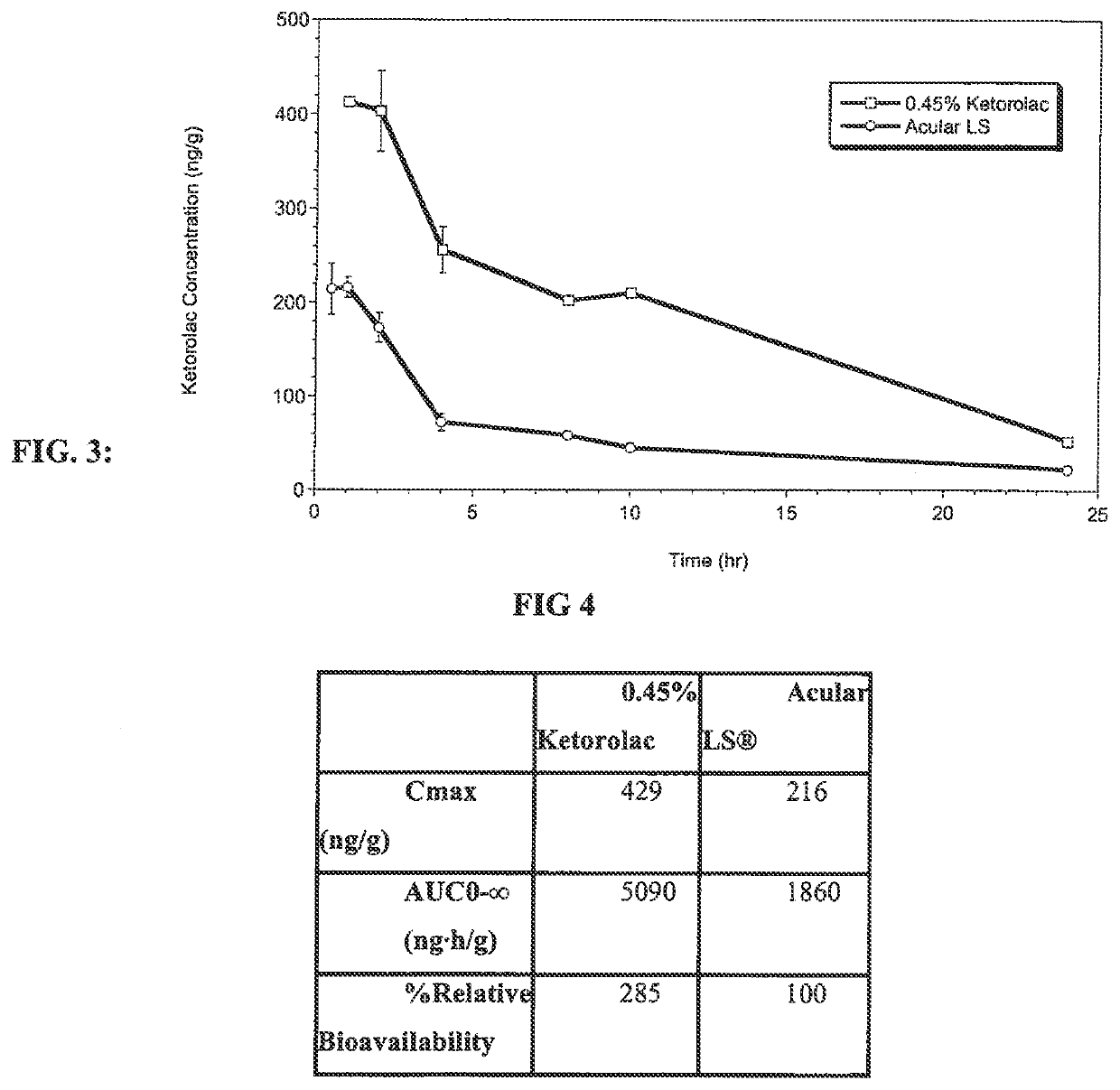
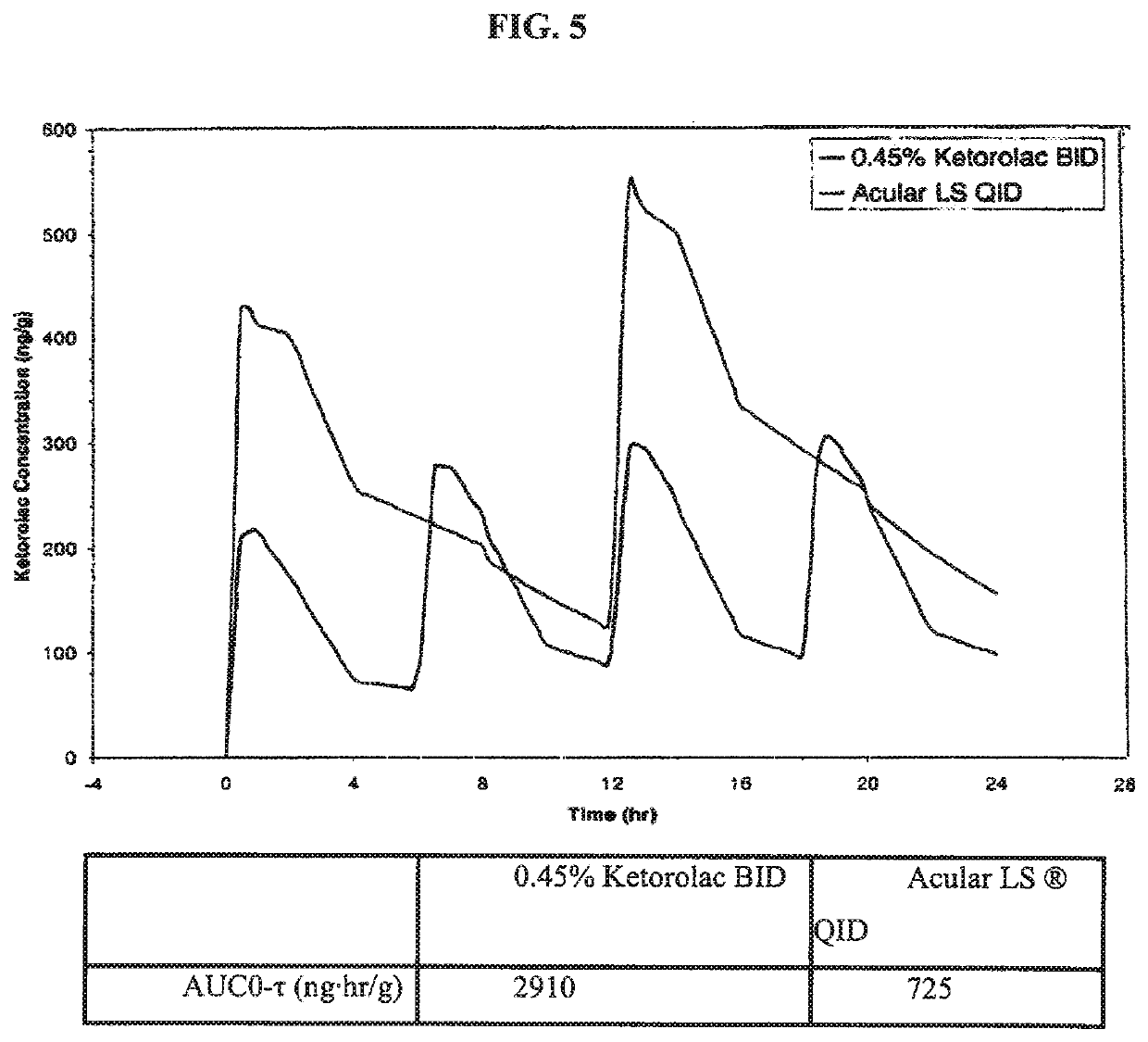
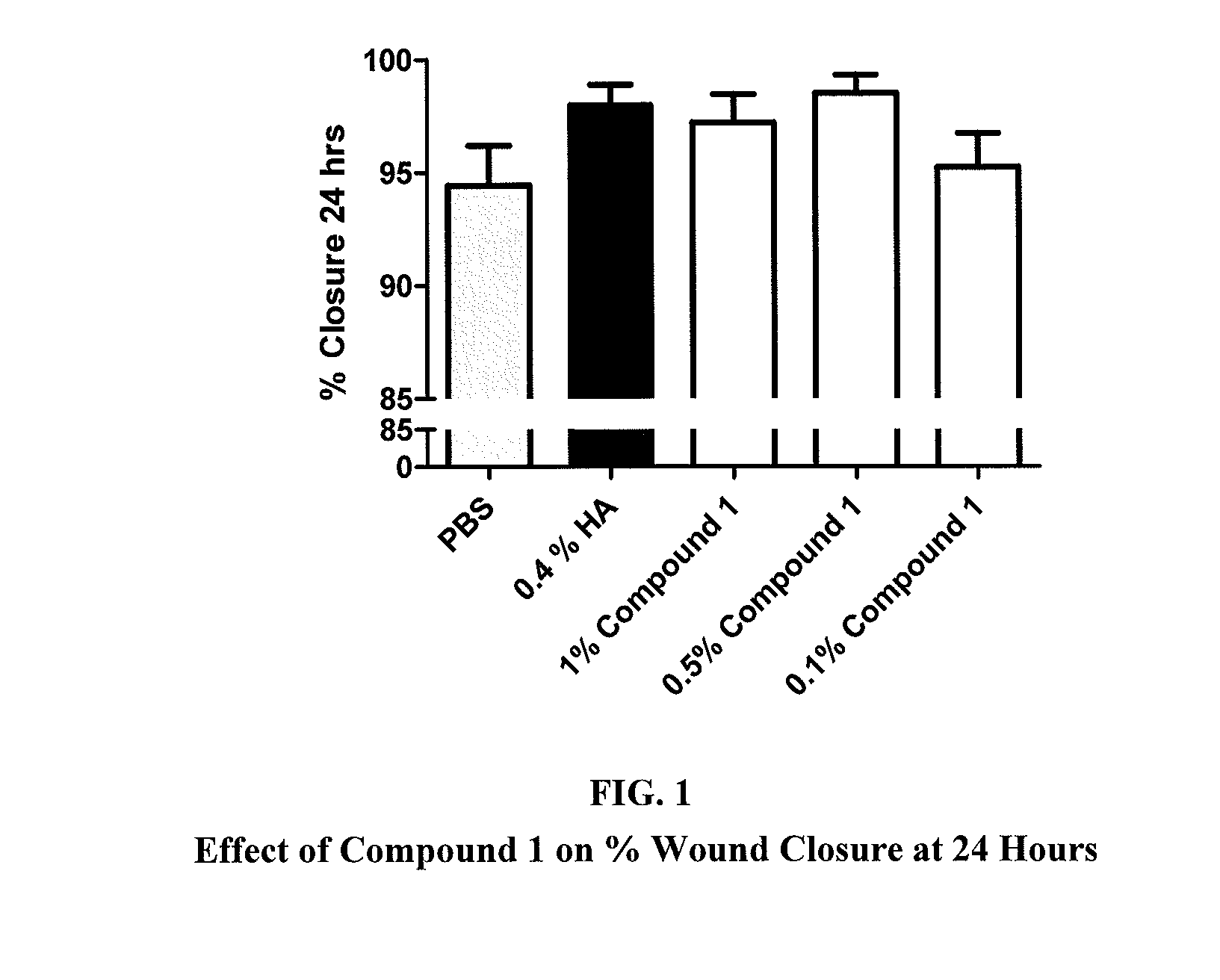
Ketorolac compositions for corneal wound healing
InactiveUS20130245088A1Promote absorptionReduce generationBiocideSenses disorderCarboxymethyl celluloseCorneal wound
The present invention is directed to an aqueous ophthalmic solution comprising an effective amount of ketorolac which comprises carboxymethyl cellulose which promotes epithelial wound healing in a patients cornea.
Owner:ALLERGAN INC
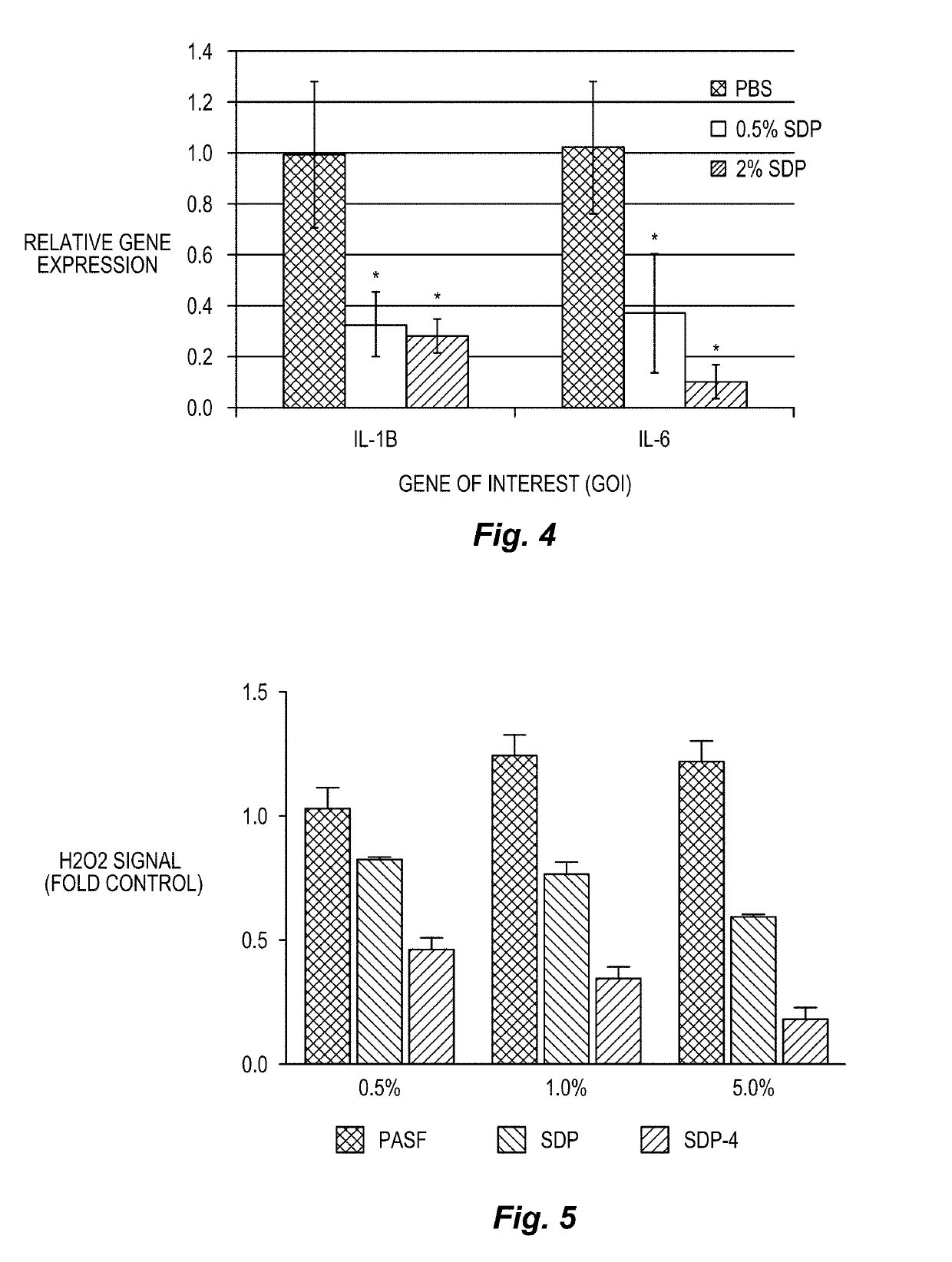
Silk-derived protein for treating inflammation
ActiveUS11242367B2Support corneal epithelial cell attachment and proliferationReduced beta-sheet activitySenses disorderHydrolysed protein ingredientsCorneal woundDermatology
Described herein are methods for reducing inflammation by administration of an effective amount of silk-derived proteins (SDP) or a fraction thereof to a subject having an inflammatory condition. The methods include the treatment of inflammatory conditions and wounds, including corneal wounds, comprising the topical administration of an effective amount of SDP material as described herein.
Owner:SILK TECH
Silk-derived protein for treating inflammation
ActiveUS20190169243A1Support corneal epithelial cell attachment and proliferationReduced beta-sheet activitySenses disorderEye implantsCorneal woundDermatology
Described herein are methods for reducing inflammation by administration of an effective amount of silk-derived proteins (SDP) or a fraction thereof to a subject having an inflammatory condition. The methods include the treatment of inflammatory conditions and wounds, including corneal wounds, comprising the topical administration of an effective amount of SDP material as described herein.
Owner:SILK TECH
Lactoferrin sequences, compositions and methods of corneal wound treatment
The present invention relates to pharmaceutical compositions containing lactoferrin, or fragments of it, and their use in the treatment of wounds, particularly corneal wounds. The present invention also provides a pharmaceutical composition comprising an effective amount of a polypeptide or peptidomimetic consisting essentially of the C-lobe of lactoferrin, or functionally active fragments or variants thereof.
Owner:BRIEN HOLDEN VISION INST (AU)
Method of Accelerating Corneal Wound Healing
The topical ophthalmic use of 5,6,7-trihydroxyheptanoic acid and analogs for the acceleration of corneal wound healing in humans, is disclosed.
Owner:ALCON RES LTD
Actin binding peptide and purpose thereof
ActiveCN102924573BPromote wound healingGood restorativeSenses disorderPeptide/protein ingredientsCorneal woundSide effect
The invention relates to the field of biological medicine, particularly discloses actin binding peptide and the purpose of the actin binding peptide. The amino acid sequence of the actin binding peptide is LKKTETQ, the synthesis difficulty and the cost are lowered, the actin binding peptide is suitable for batch production in a large scale and has a brilliant preparing function on various corneal injuries, side effects do not appear in the dosing process, and corneal wound healing and transparency recovery are benefited. Compared with a curing method through thymosin beta4, the advantages are more obvious, the side effects are small, and the actin binding peptide can be used for corneal repairing treatment.
Owner:ZHAOKE GUANGZHOU OPTHALMIC DRUG
Dendrimer-bioadhesive polymer hydrogel nanoglue and use thereof
PendingUS20210353823A9Desirable mechanical propertyPrevent infection and scar tissue formationMaterial nanotechnologyOrganic active ingredientsCorneal woundPharmaceutical drug
A nanoglue is formed with one or more bioadhesive polymers, one or more dendrimers, and optionally one or more therapeutic, prophylactic, or diagnostic agents. The bioadhesive polymers and dendrimers are modified with functional groups to permit crosslinking upon one or more stimuli, e.g., ultraviolet irradiation, and form hydrogel in situ at tissue sites. In the repair of corneal wounds, the nanoglue leads to improved rate of healing with less scarring and less inflammation, compared to non-treated cornea or ones treated with sutures. Therapeutic agents can be covalently conjugated to the precursor components and be delivered to specific eye compartments, providing a more efficacious treatment formulation of ocular disorders than delivering drugs in their free forms. Methods of making and using the hydrogel and hydrogel precursor compositions are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Ketorolac compositions for corneal wound healing
PendingUS20210315805A1Promote absorptionReduce generationOrganic active ingredientsSenses disorderCelluloseCorneal wound
The present invention is directed to an aqueous ophthalmic solution comprising an effective amount of ketorolac which comprises carboxymethyl cellulose which promotes epithelial wound healing in a patients cornea.
Owner:ALLERGAN INC
Method for preparing tissue engineering cornea
ActiveCN101757690BRetain structureRestore transparencyEye implantsNervous system cellsCorneal woundImmunogenicity
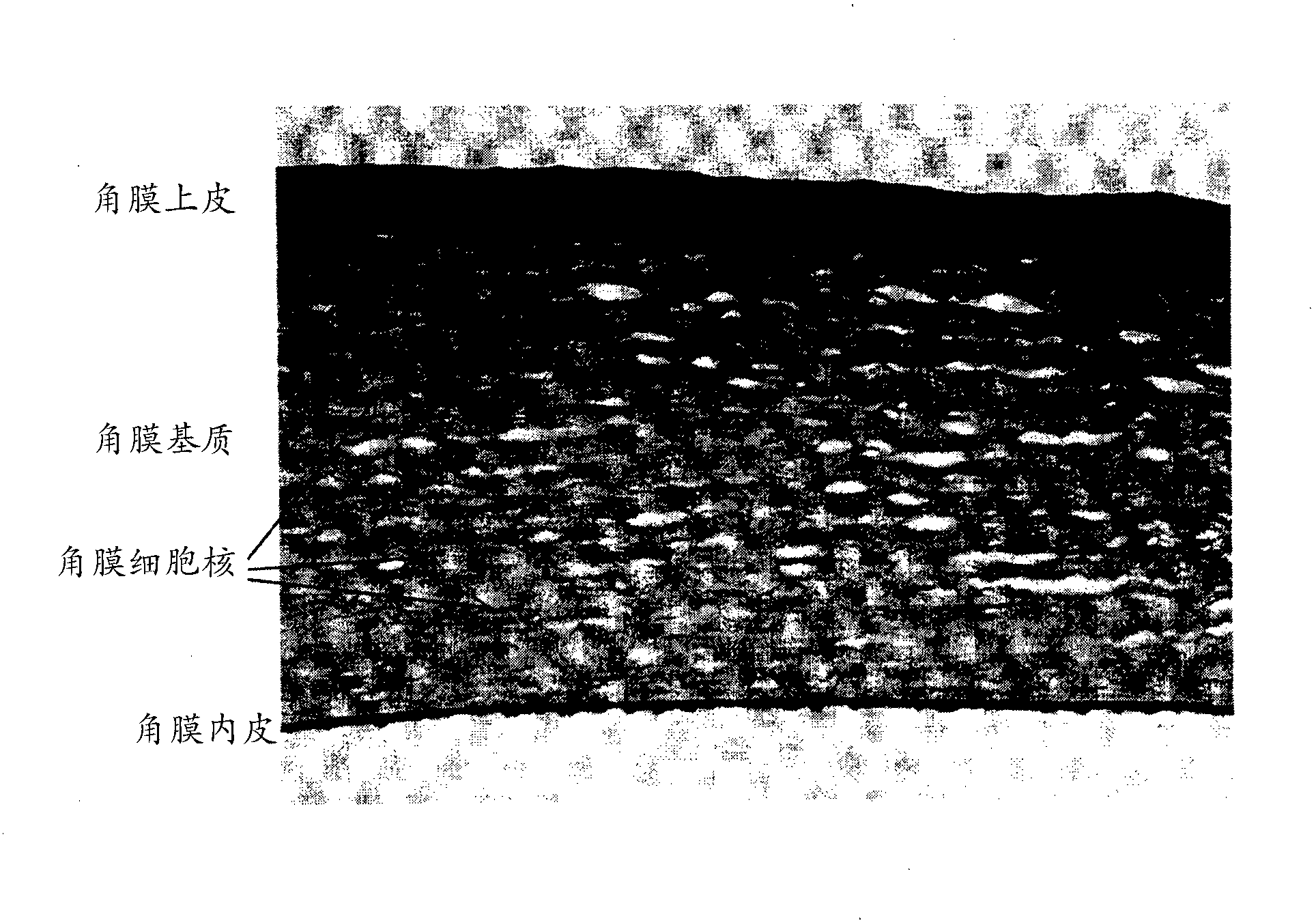
The present invention discloses a producing method for tissue engineering cornea. The method involves using amniotic epithelial cells and amniotic stromal cells as seed cells, planting at two sides of decellularized natural corneal stroma after inducement and differentiation in vitro, forming tissue engineering cornea through organ culture in vitro. The scaffold of present invention not only resolves the problem of strong immunological rejection of heterogenetic corneal stroma, but also sustains the structure (which can restore transparency of cornea) and major components (including growth factors which could promote corneal cell growth, proliferation and differentiation) of natural cornea. Compared with the prior art, present invention has advantages of low cost, easy operation, wide range of sources and easy preservation. The said tissue engineering cornea containing live cells has certain elasticity and tenacity, and its shape, size and thickness can be changed easily. The tissue engineering cornea has very low immunogenicity. It could avoid complications caused by non-corneal material, be reconstructed by recipient cells after being transplanted in body, and be incorporated into organism rapidly to become transparent. It could be used to repair corneal wounds caused by all kinds of reasons.
Owner:SHAANXI RUISHENG BIOTECH
Pharmaceutical composition for treating or preventing corneal wound comprising thymosin β4 and citric acid
ActiveUS9867868B2Promote proliferation and migrationInhibits PMN infiltrationHormone peptidesSenses disorderCorneal woundOrganic acid
The present invention relates to a composition for the treatment or prevention of a corneal injury, which comprises thymosin β4, and citric acid or its salt as active ingredients. The composition may further comprise at least one organic acid selected from acetic acid, ascorbic acid or salts thereof. The composition can maintain or increase the activity of thymosin β4 conventionally used to effectively treat wounds on the cornea, and thus, is useful as an ophthalmic formulation for treating the corneal injury.
Owner:HLB THERAPEUTICS CO LTD
Therapeutic use of a sterile aqueous ophthalmic solution
The present invention relates to a sterile aqueous ophthalmic solution comprising N-(N-acetylcysteinyl-)chitosan or a pharmaceutically acceptable salt thereof in a carrier solution, wherein the N-(N-acetylcysteinyl-)chitosan has a content of free thiol groups in an amount of from 80 [mu]mol / g polymer to 280 [mu]mol / g polymer, for use in the treatment of corneal wounds.
Owner:CROMA PHARMA GMBH
Method to enhance wound healing using silk-derived protein
ActiveUS10953132B2Promote wound healingImprove stabilitySenses disorderPeptide/protein ingredientsCorneal woundSurgical incision
Described herein are methods of enhancing wound healing using silk-derived proteins (SDP), including low molecular weight SDP fragments. Also described are compositions for the treatment of wounds, including corneal wounds, skin wounds, surgical incisions, burns, and skin ulcers, comprising SDP fragments, including low molecular weight SDP fragments.
Owner:SILK TECH +1
Traditional Chinese medicine for improving eyesight and removing nebula and preparation method thereof
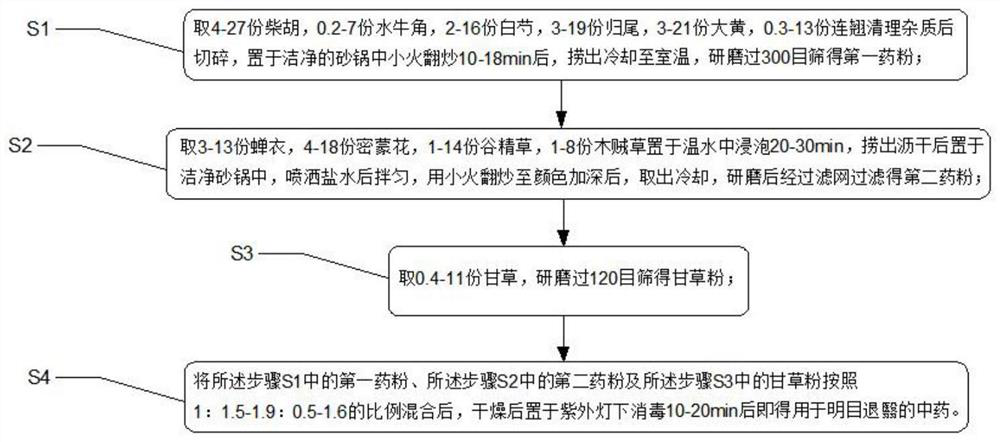
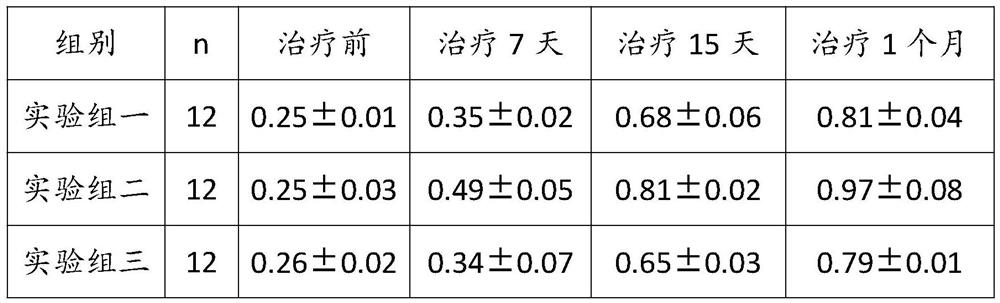
PendingCN114259529AAntipyreticPromote secretionPowder deliverySenses disorderCorneal woundMedicinal herbs
Compared with the prior art, the traditional Chinese medicine is prepared from the following components in parts by weight: 1 to 47 parts of radix bupleuri, 0.1 to 12 parts of cornu bubali, 2 to 38 parts of radix paeoniae alba, 1 to 33 parts of radix angelicae lateralis preparata, 3 to 34 parts of radix et rhizoma rhei, 0.2 to 17 parts of fructus forsythiae, 0.2 to 19 parts of liquorice root, 1 to 16 parts of periostracum cicada, 1 to 23 parts of flos buddlejae, 1 to 27 parts of flos eriocauli and 1 to 15 parts of herba equiseti hiemalis. The traditional Chinese medicine for improving eyesight and removing nebula has the effects of improving eyesight, removing nebula, diminishing inflammation and relieving pain through combination of the medicines, can promote epithelization of corneal wounds, inhibit surface growth of fibrous vascular tissues, block corneal nebula, separate the corneal nebula from the cornea and recover the transparency of the cornea, adopts natural traditional Chinese medicinal materials, and is low in toxic and side effects and good in safety.
Owner:湖南省开元博物馆 +1
Method of accelerating corneal wound healing
The topical ophthalmic use of 5,6,7-trihydroxyheptanoic acid and analogs for the acceleration of corneal wound healing in humans, is disclosed.
Owner:ALCON RES LTD
Pharmaceutical composition for treating or preventing corneal wound comprising thymosin beta 4 and citric acid
ActiveUS20170049857A1Easy to migrateInhibits PMN infiltrationSenses disorderPeptide/protein ingredientsCorneal woundBULK ACTIVE INGREDIENT
The present invention relates to a composition for the treatment or prevention of a corneal injury, which comprises thymosin β4, and citric acid or its salt as active ingredients. The composition may further comprise at least one organic acid selected from acetic acid, ascorbic acid or salts thereof. The composition can maintain or increase the activity of thymosin β4 conventionally used to effectively treat wounds on the cornea, and thus, is useful as an ophthalmic formulation for treating the corneal injury.
Owner:HLB THERAPEUTICS CO LTD
Therapeutic and cosmetic uses and applications of calreticulin
The invention relates to therapeutic and cosmetic uses of calreticulin including reducing or eliminating wrinkles and / or fine lines, tissue repair and reconstruction, repairing damaged bone and / or cartilage, stimulating regeneration of an epidermal appendage, enhancing phagocytosis of bacteria by phagocytes within a wound, treating a wound in a patient suffering from delayed wound healing, treating a corneal wound, and treating or preventing a surgical adhesion.
Owner:NEW YORK UNIV
Human keratinized cell growth factor-1 analogue preparation method and application thereof
ActiveCN101220092BHigh expressionHigh activitySenses disorderPeptide/protein ingredientsCorneal woundFibrosis
Owner:吉林农大生物反应器工程有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com