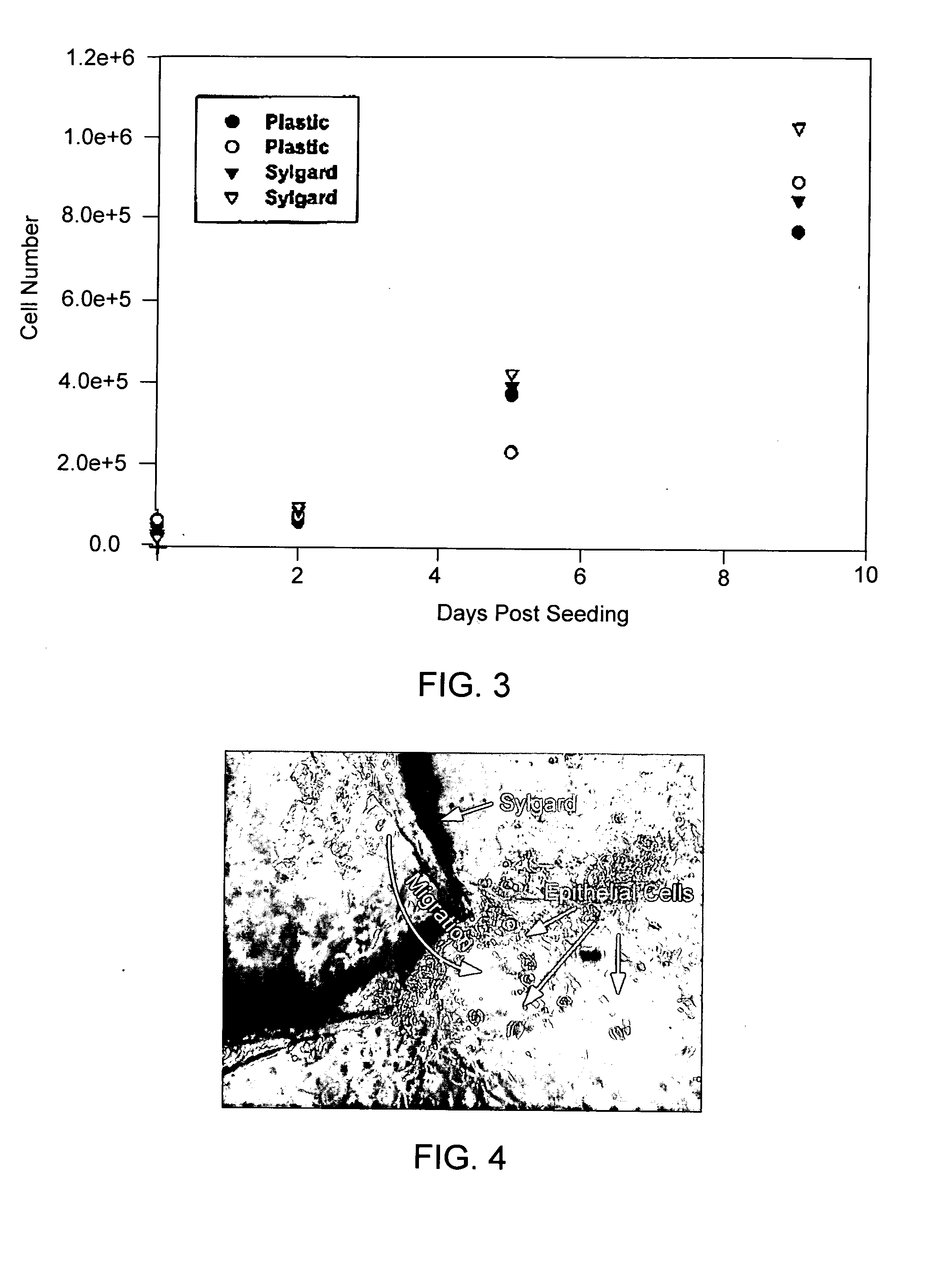
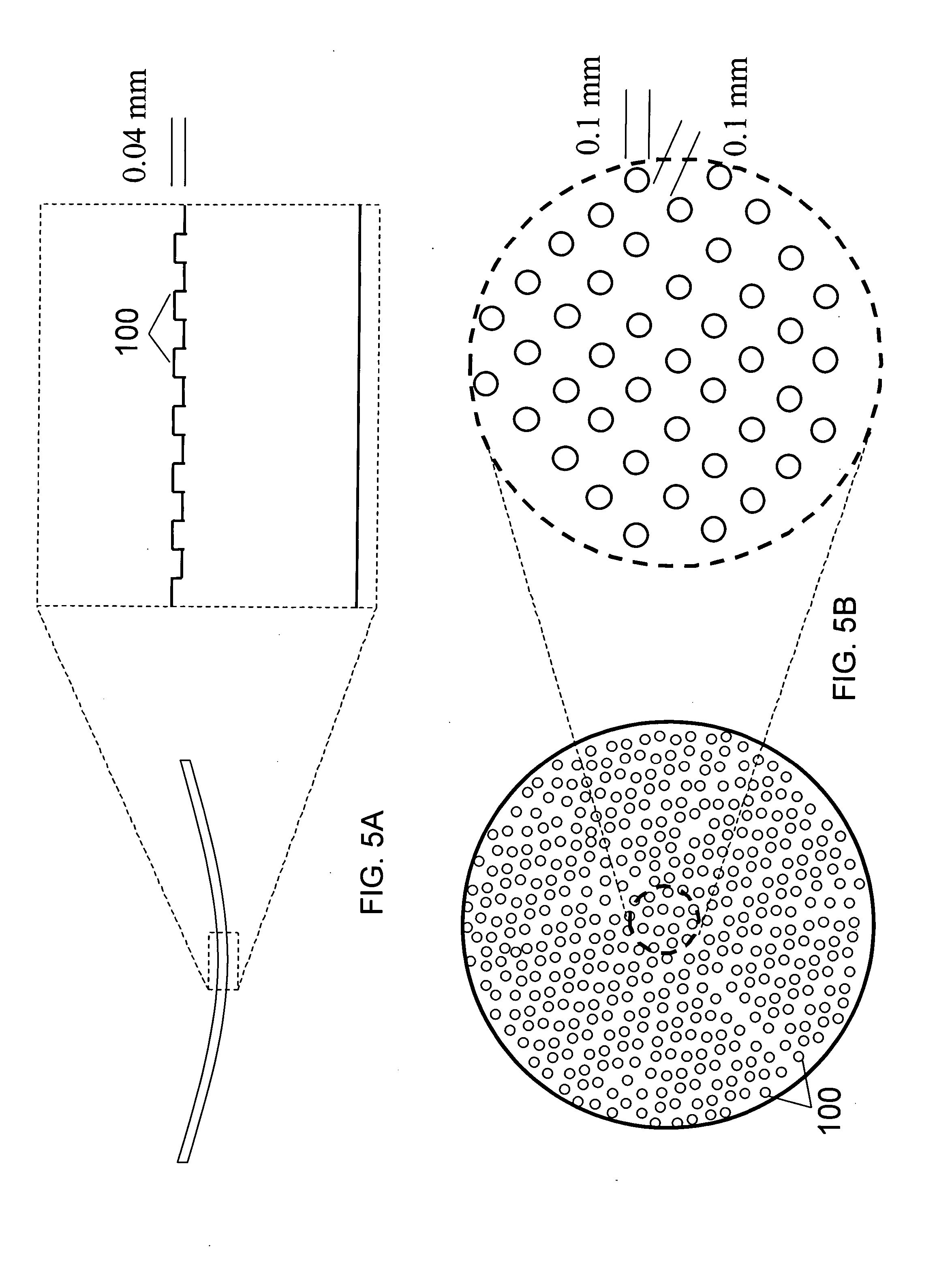
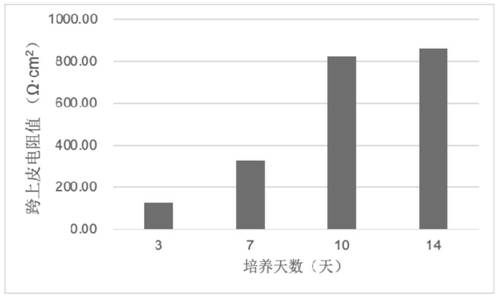
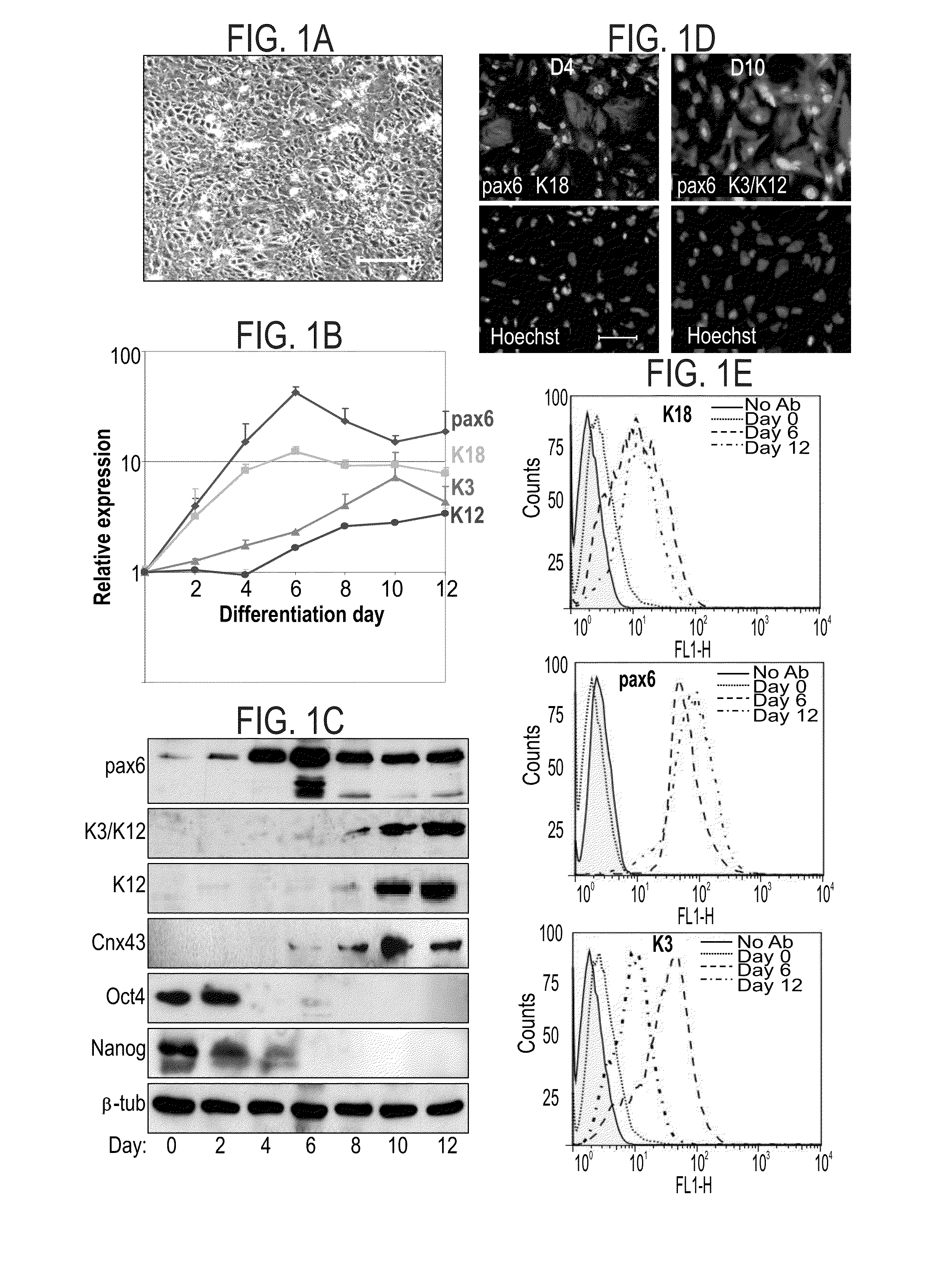
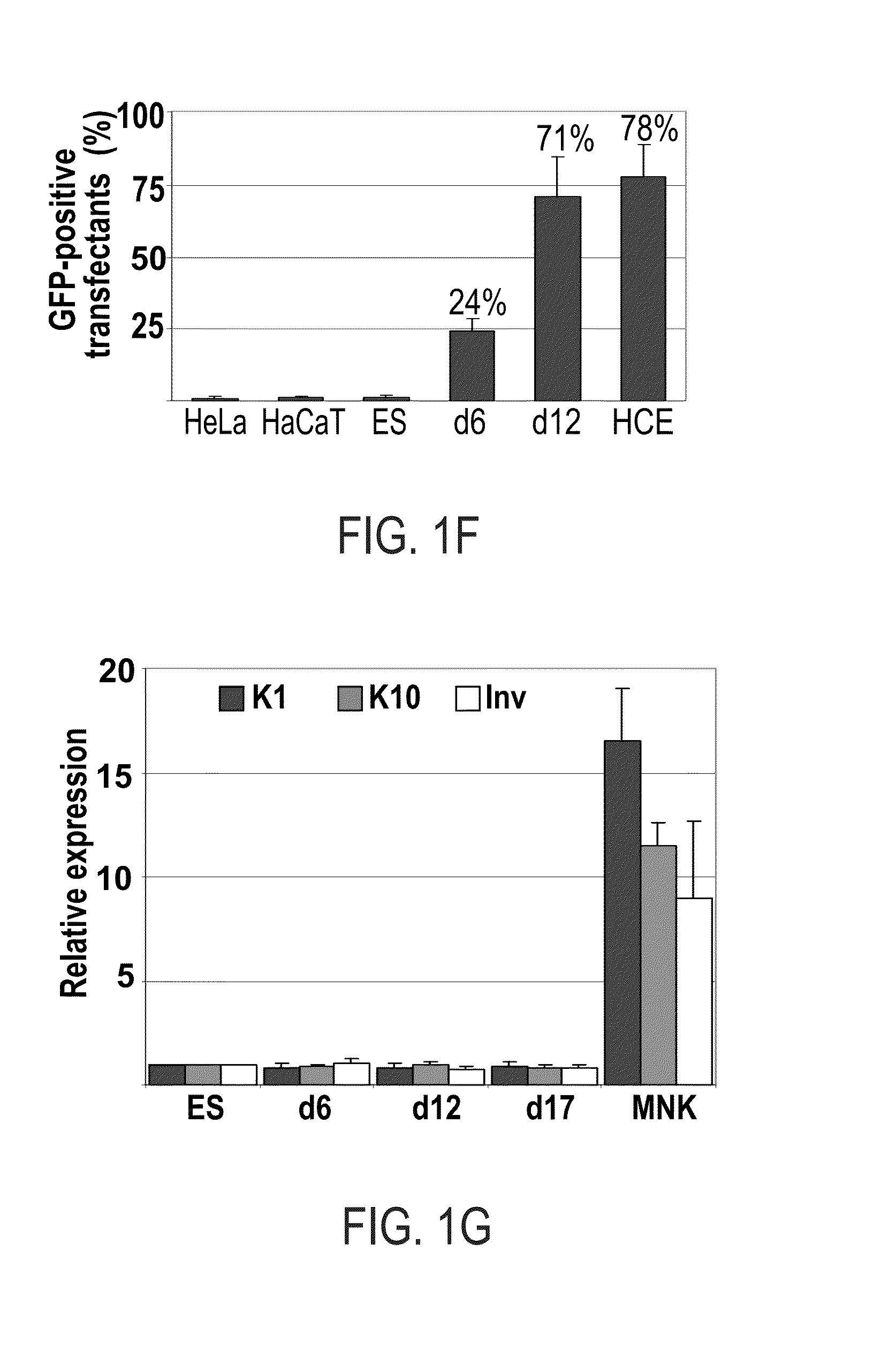
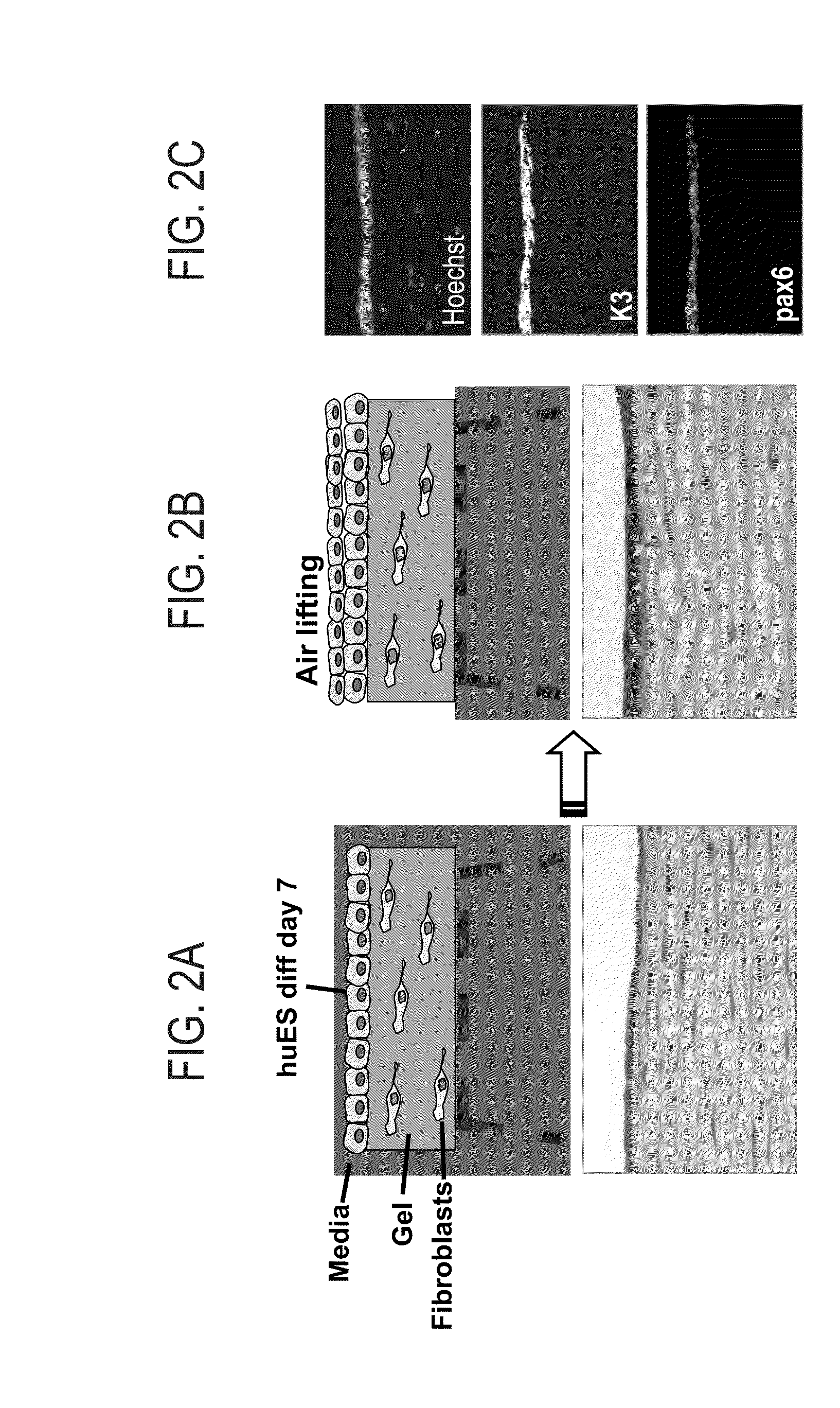
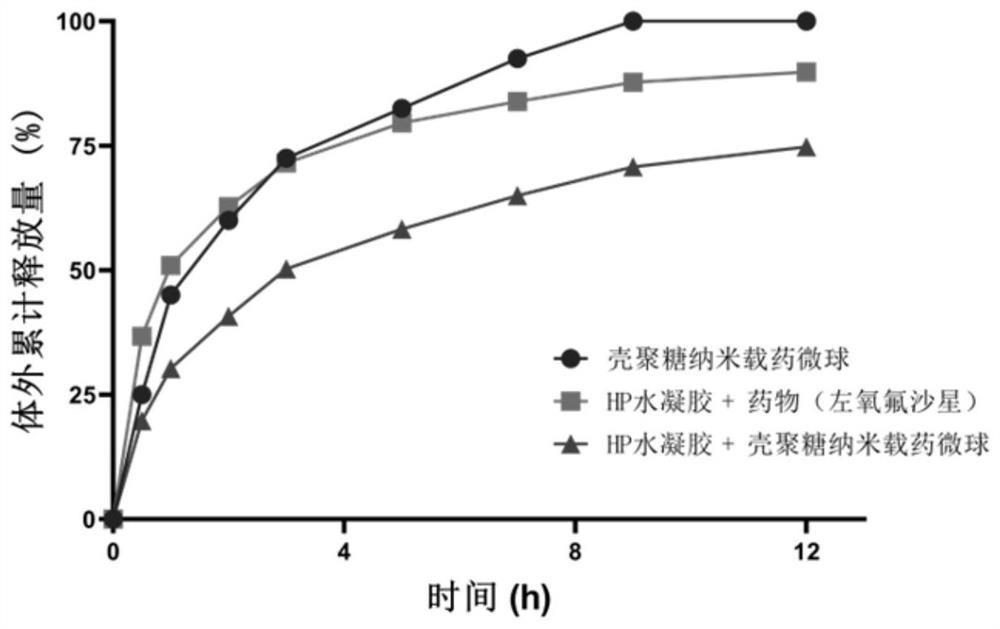
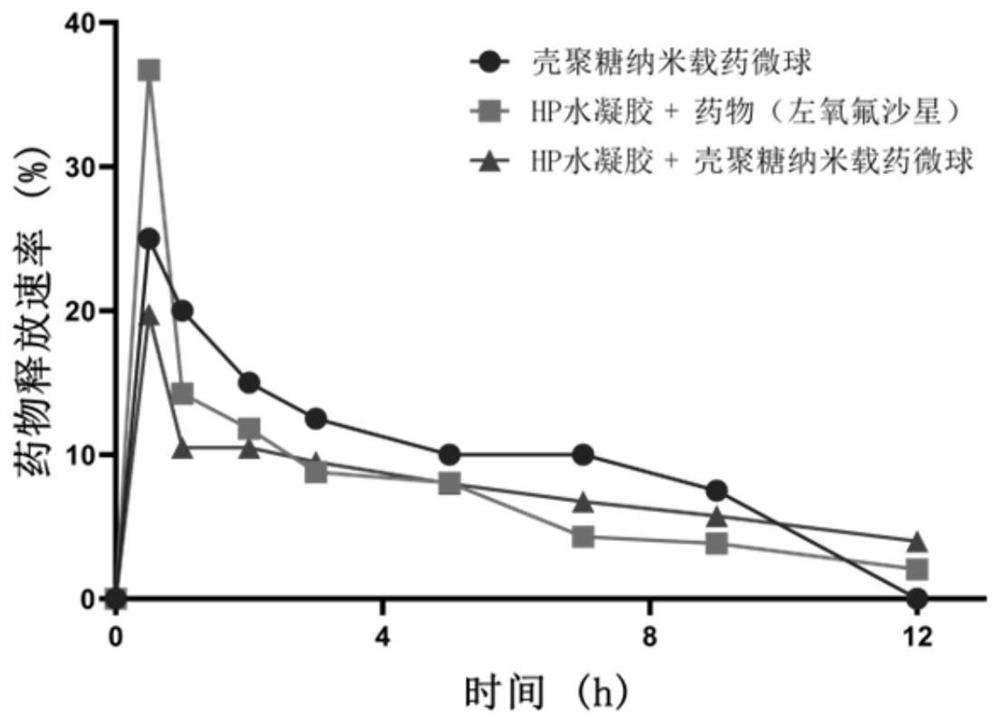
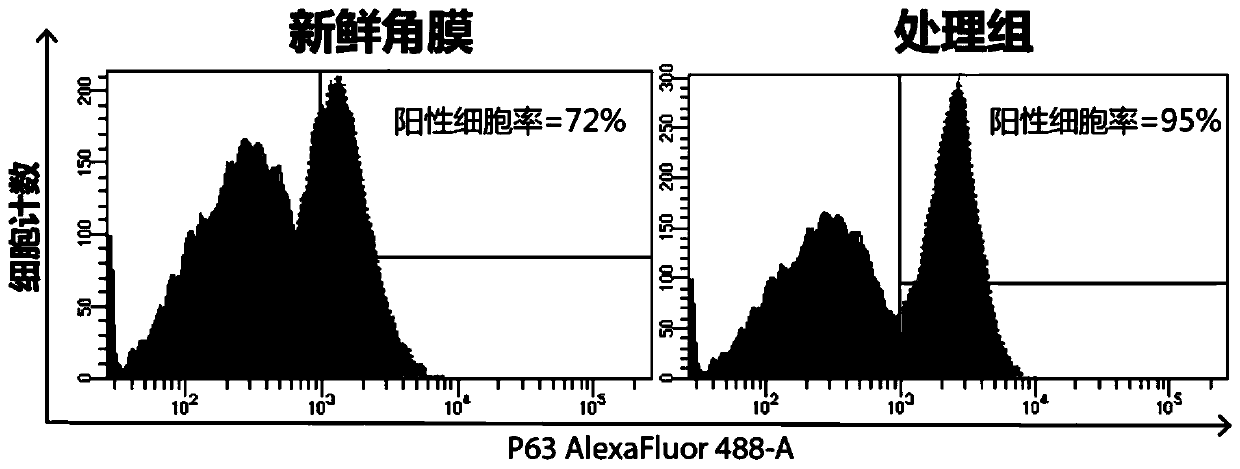
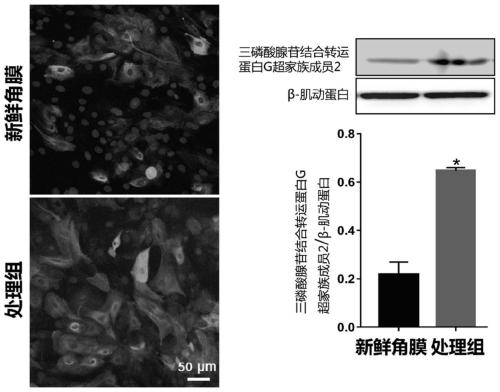
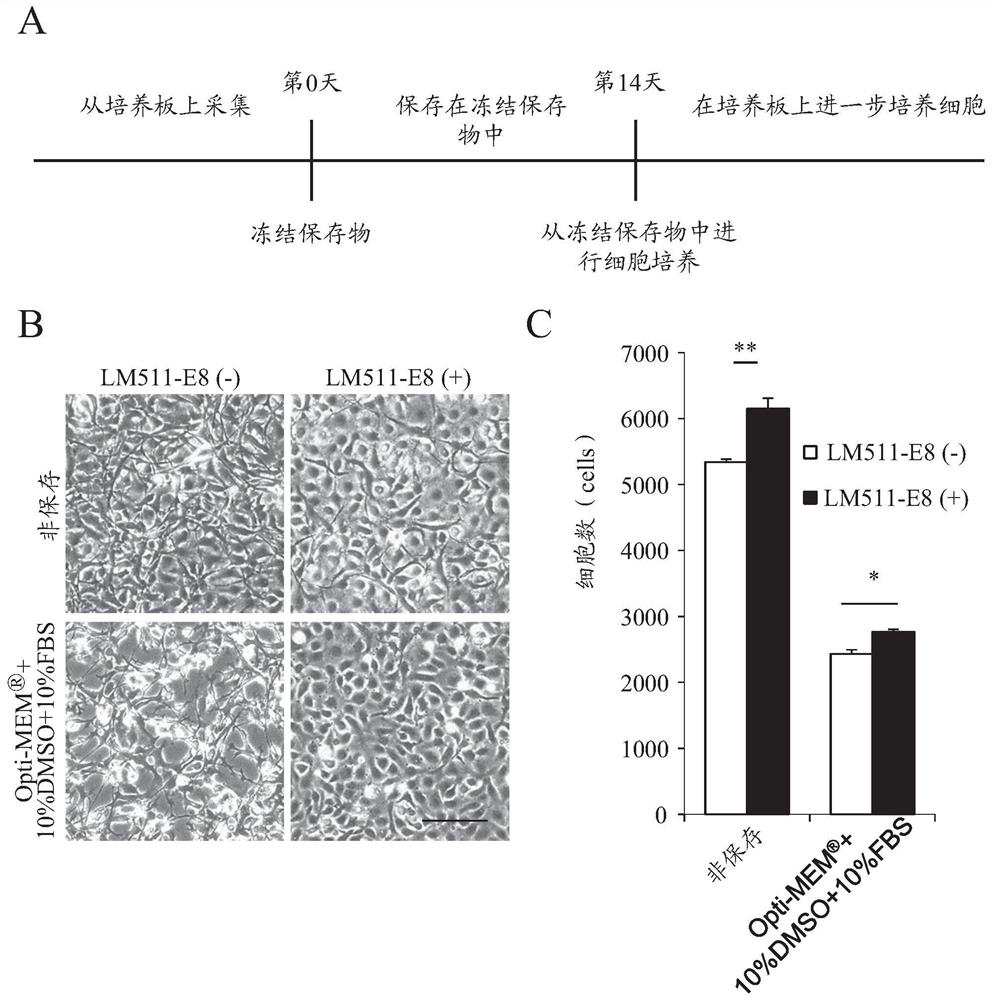
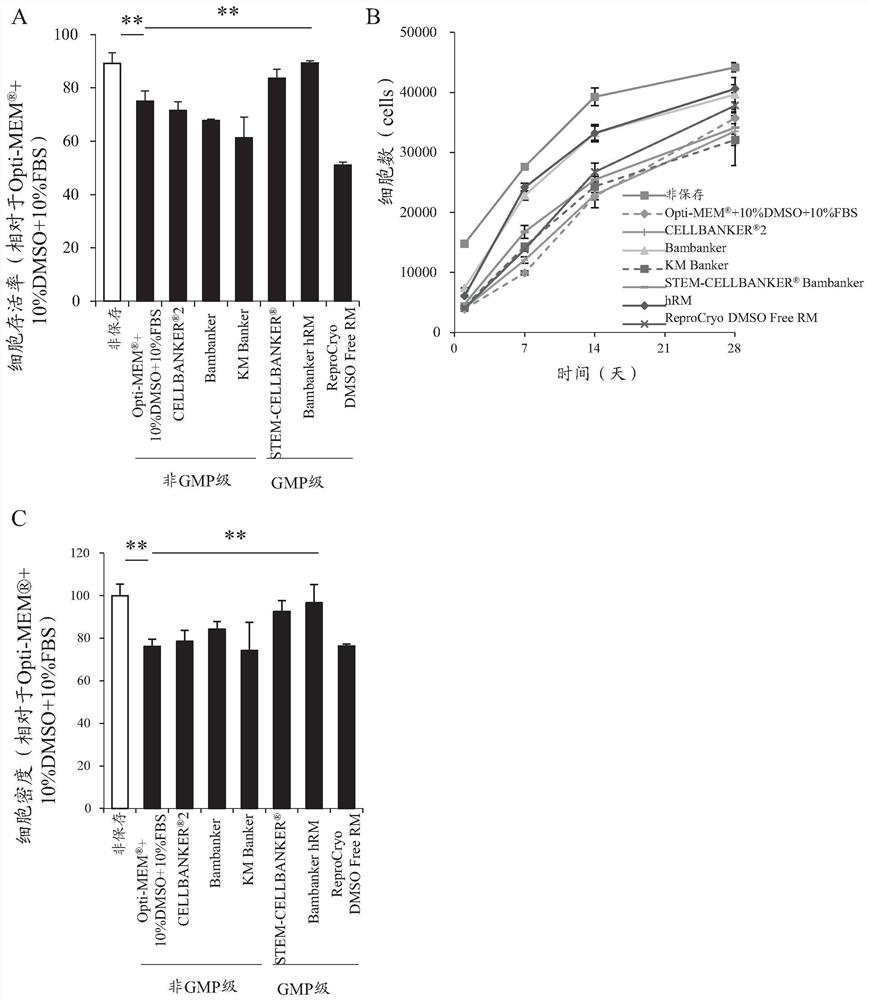
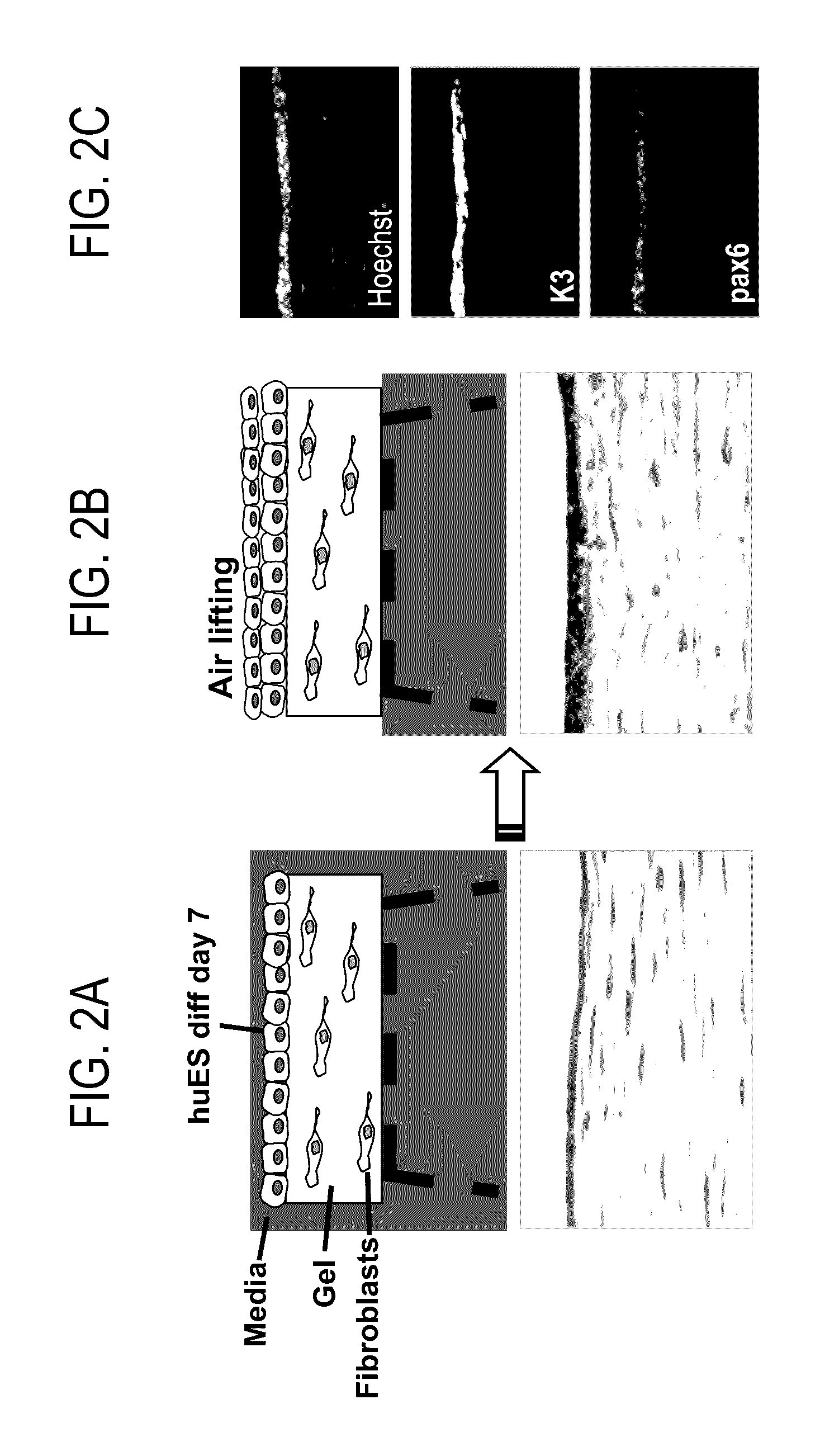
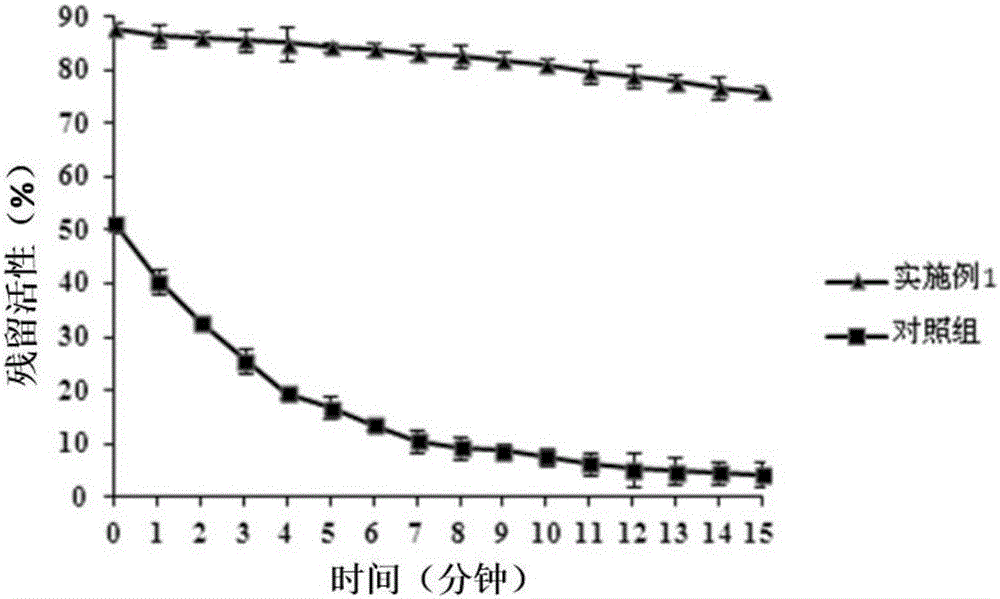
Patents
Literature
54 results about "Corneal cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Human Corneal Epithelial Cells are cryopreserved as secondary cells. The cells have been isolated from human corneal tissue and expanded twice in culture vessels before being harvested for cryopreservation. This product is for Research Use Only.
Ophthalmic composition
InactiveUS20130053374A1Good water solubilitySafely employedBiocideSenses disorderCarteololBeta blocker
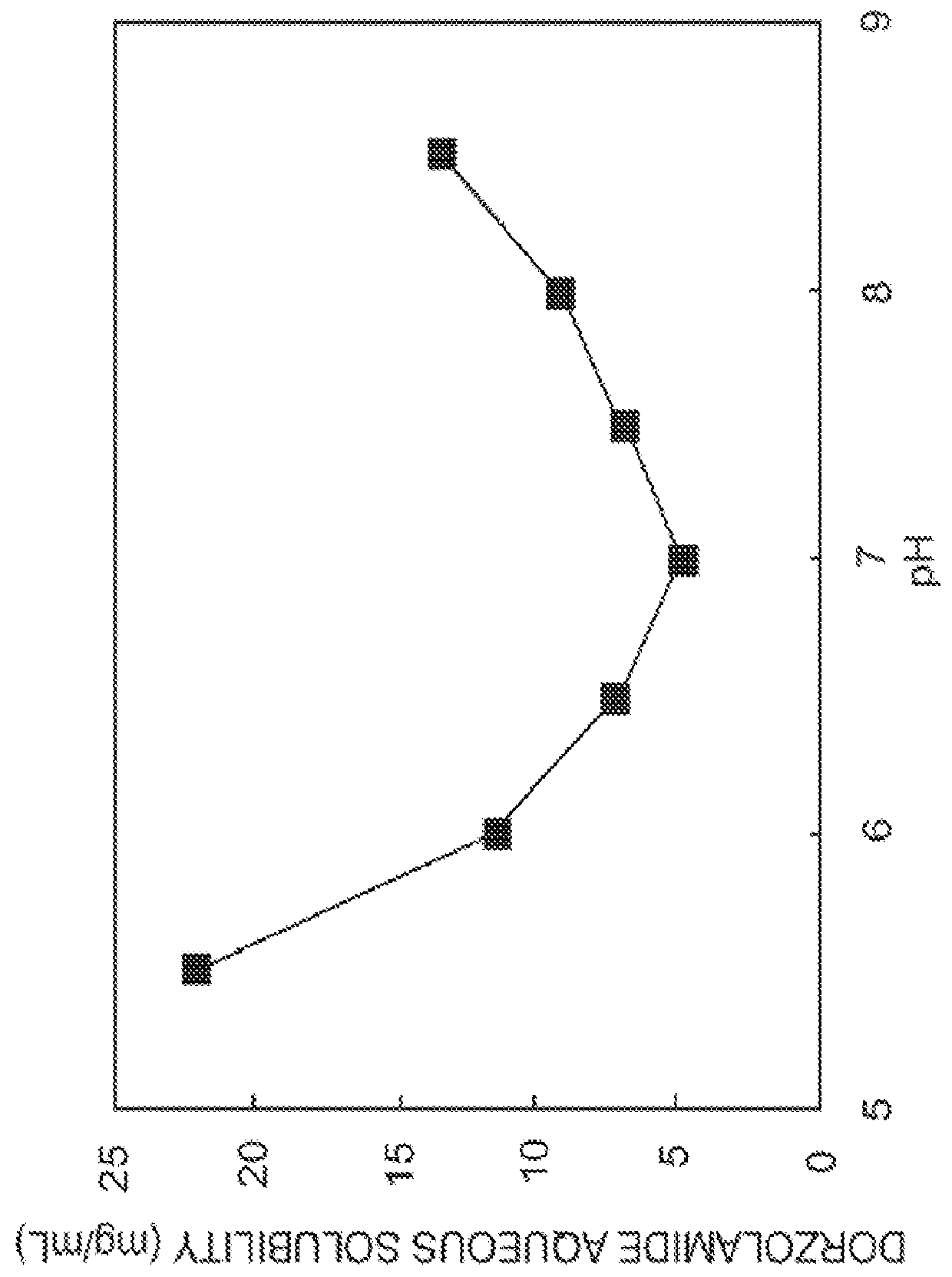
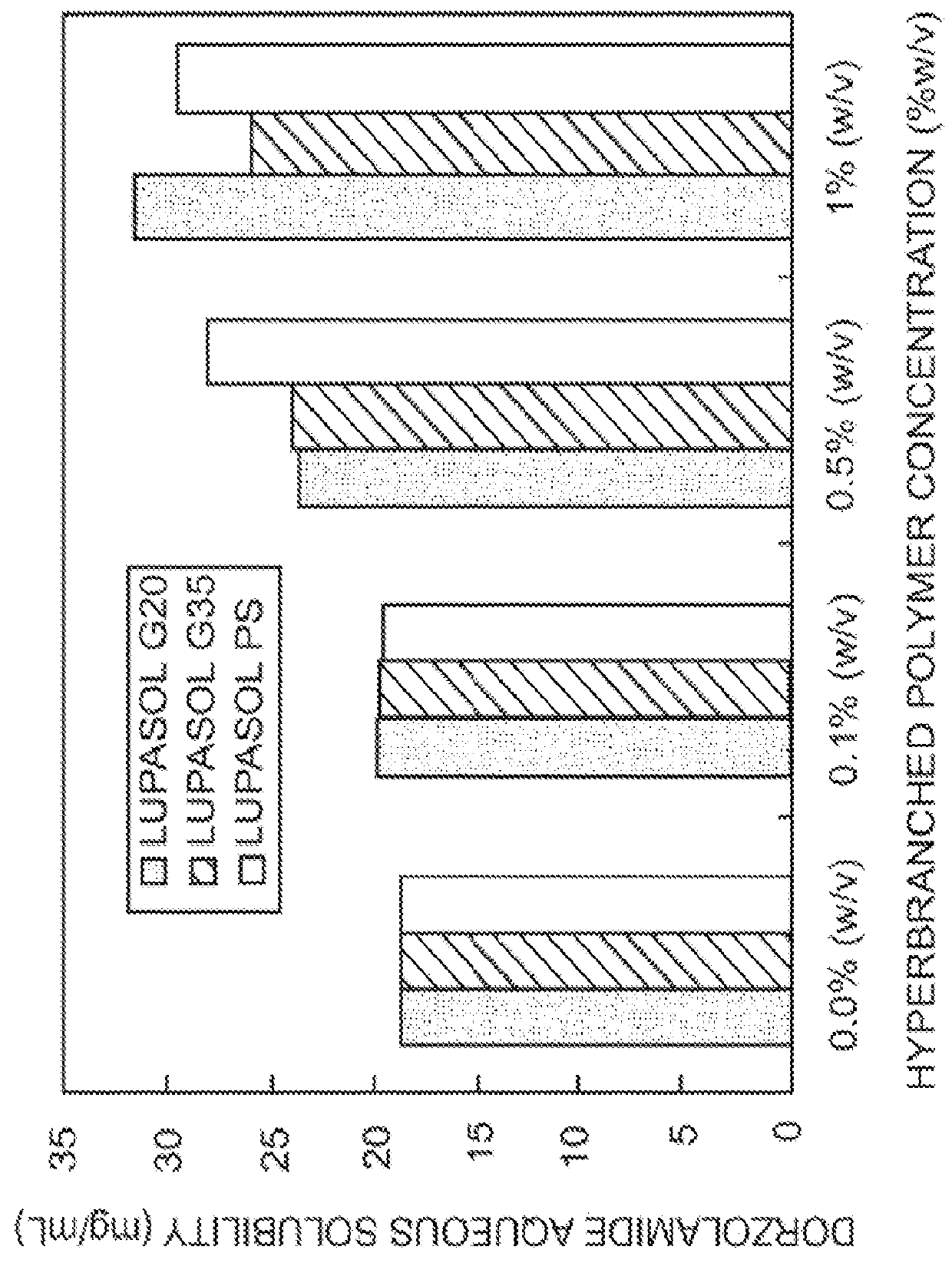
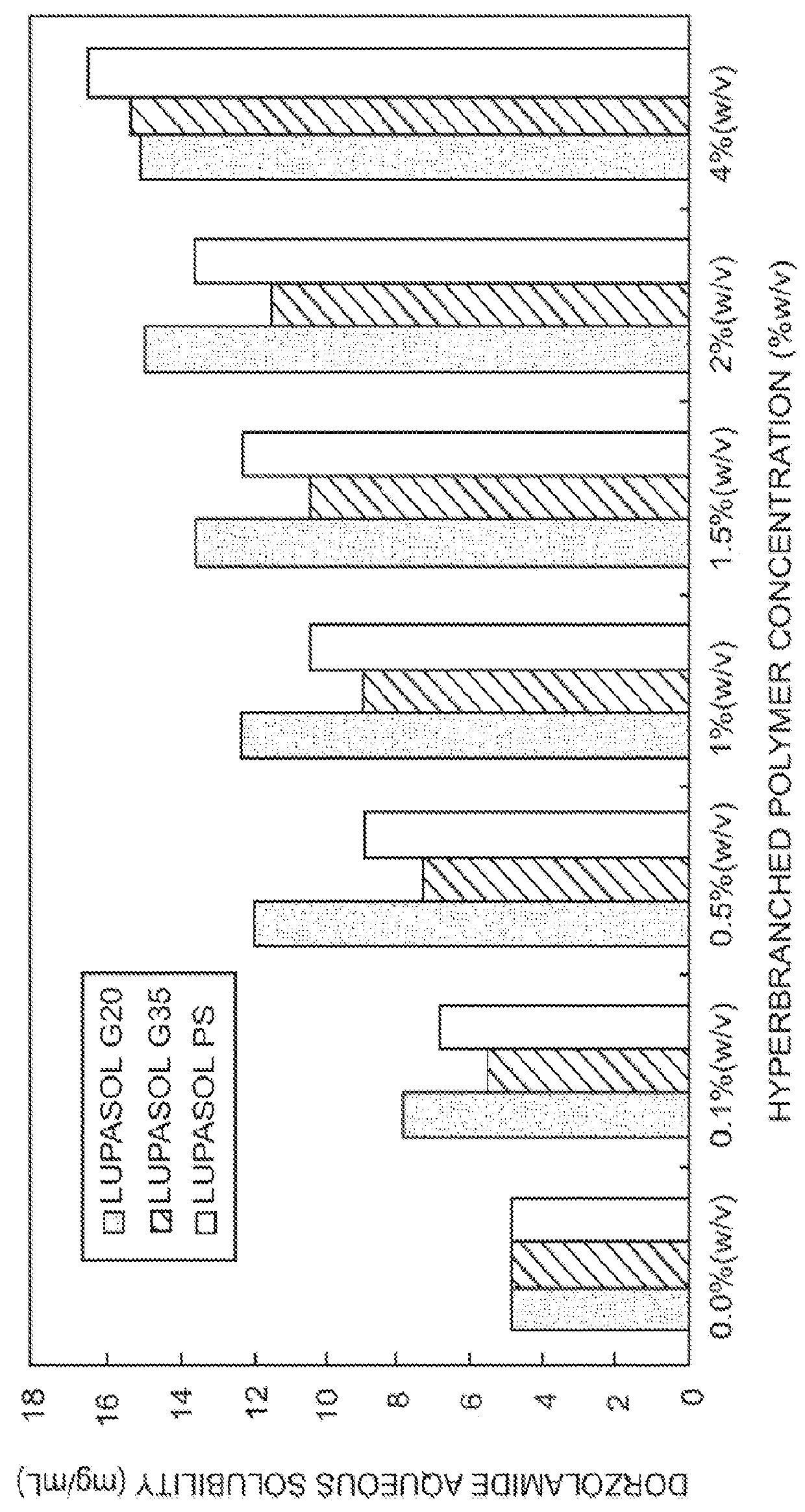
The present invention provides an ophthalmic composition comprising a hyperbranched polyester. The ophthalmic compositions may also comprise carbonic anhydrase inhibitors, wherein the hyperbranched polyester increases the aqueous solubility of the carbonic anhydrase inhibitor, and increases corneal permeation of the active agent. The ophthalmic compositions may also comprise non-ionic surfactants, such as PEG, Polysorbate, HPMC or HEC, and beta-blockers, such as Carteolol, Levobunolol, Betaxolol, Metipranolol, Timolol or Propranolol. The concentration of the hyperbranched polyester in the ophthalmic formulation should be less than or equal to 4% (w / v) in order to avoid any cytotoxic effects on human corneal cells and thus the eye irritation.
Owner:SENJU USA
Use of contact lens for corneal cell transplant
The present invention addresses the need for improved methods and apparatuses of transplanting cells to injured or diseased cornea. The methods disclosed provide for culture of cells on the concave surface of a contact lens, followed by application of the contact lens to the eye, thereby permitting repopulation of the corneal region by the cells. A particular use for the present invention involves the transfer of genetically altered cells.
Owner:VANDERBILT UNIV
Preparation method for artificial corneas
The invention discloses a preparation method for artificial corneas. The preparation method comprises the following steps of (1) collecting age-appropriate pig eyeballs; (2) scraping corneal epithelial cells and corneal endothelial cells of the pig eyeballs, reserving bowman layers, preparing lamellar corneas by using a lamellar blade, and drilling size-specific corneal slices; (3) putting the corneal slices obtained in step (2) in liquid nitrogen for 8-35 minutes, slowly rewarming the corneal slices to room temperature under normal temperature and normal pressure after the corneal slices are taken out, repeating the operation for 4-12 times; (4) putting the corneal slices which are subjected to freezing-thawing treatment in step (3) in normal saline for carrying out ultrasonic cleaning, wherein the ultrasonic working frequency is 20-250 kHz, and the ultrasonic time is 15-65 minutes; (5) putting the corneal slices which are subjected to ultrasonic treatment in a buffer system, carrying out enzymolysis on DNA (Deoxyribose Nucleic Acid) of corneal stromal cells by using endonuclease, and obtaining the artificial corneas. According to the artificial corneas prepared by the preparation method disclosed by the invention, gaps between natural collagen fibers of the artificial corneas are beneficial for inducing the growth of the corneal cells and the growth of nerve fibers of an acceptor and promoting the repairing and the reconstructing of corneas of a donor.
Owner:XIAMEN DAKAI BIOTECH CO LTD
RNAi Methods and Compositions for Stimulating Proliferation of Cells with Adherent Functions
ActiveUS20100003299A1Promotes HCEC adhesionReduce thicknessBiocideOrganic active ingredientsSurgical GraftIn vivo
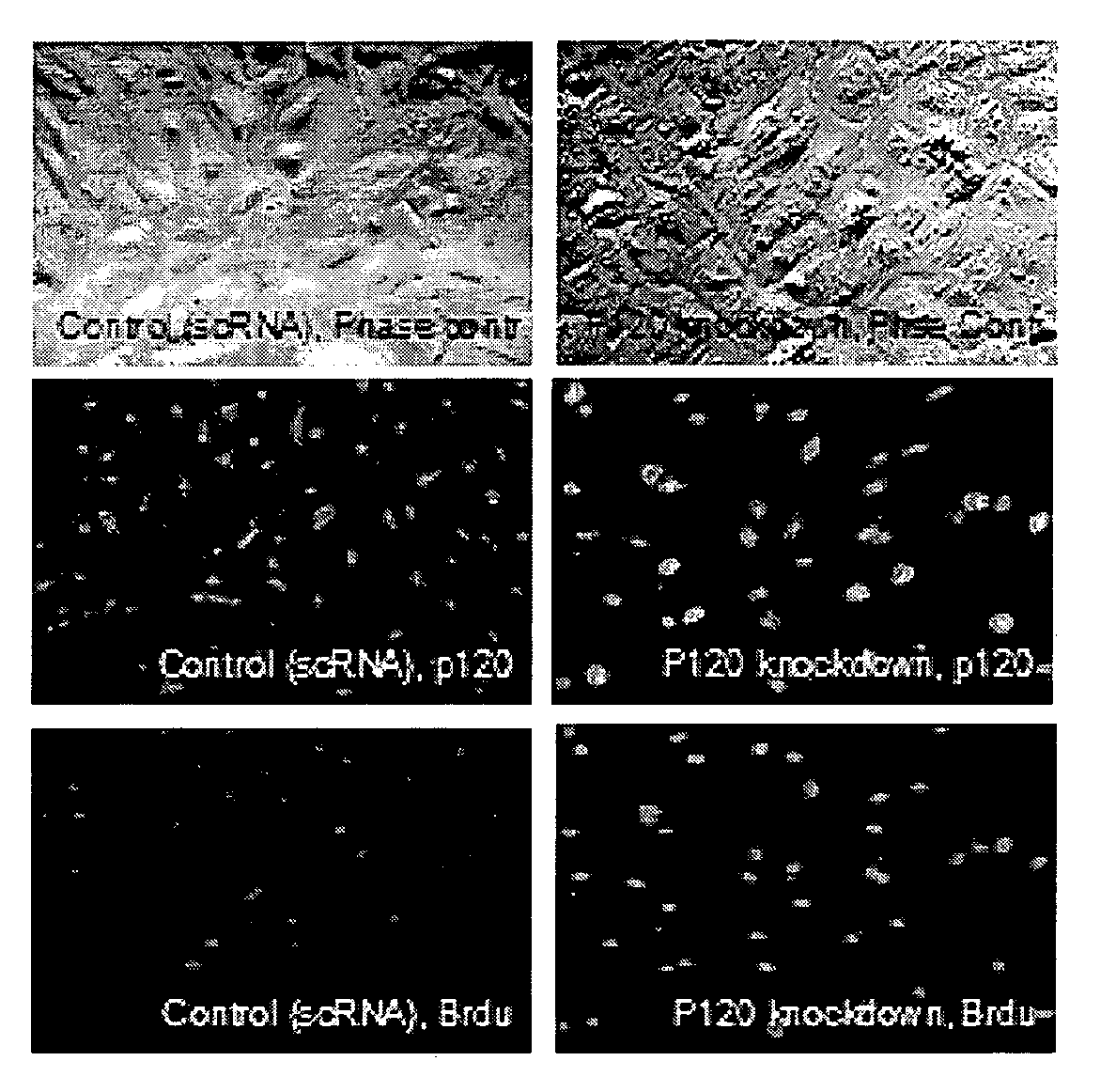
Described herein are methods and compositions for stimulating proliferation of cells that express adherent junctions and cease proliferation, for example, human corneal endothelial cells, by downregulation of certain cell-cell junctions. In one embodiment, downregulation is achieved using RNA interference, and contacting the cells with mitogenic growth factors and an agent that elevates intracytoplasmic cAMP. Furthermore, described herein are methods of isolating human corneal endothelial cells from keratocytes, and methods of preserving and maintaining viability of human corneal endothelial cell aggregates. Also described are surgical grafts comprising human corneal endothelial cells that have been isolated, optionally stored, and transiently contacted with an agent that downregulates expression of p 120, and a biocompatible support. The methods and compositions described herein can be used in novel therapies to help expand human corneal endothelial cells during in vitro tissue engineering and for in vivo treatment of corneal endothelial dysfunction.
Owner:TISSUETECH INC
Degradation controlled tissue engineering comea fibrous scaffold and preparation method thereof
The invention relates to a degradation controlled tissue engineering comea fibrous scaffold and a preparation method thereof. The basic compositions of the scaffold are gelatin and aliphatic polyesters. The preparation method comprises the following steps: dissolving the gelatin in a mixed solvent to prepare gelatin solution, dissolving the aliphatic polyesters in the gelatin solution, and evenlystirring to prepare mixed polymer spinning solution; and preparing the orderly and disorderly arranged fibrous scaffold through electrostatic spinning, and performing vacuum drying to prepare the degradation controlled tissue engineering comea fibrous scaffold. The gelatin and aliphatic polyesters compounded electrostatic spinning is adopted, crosslinking stabilization is not needed, and the degradation speed of the scaffold and growth speed of corneal cells can be controlled to be synchronous. The product has transmittance of 97 percent, wet tensile strength of 4.91MPa, elongation at ruptureof 956.2 percent, water content of 154.1 percent, and performance parameters similar to human comea, and has good bioactivity and biocompatibility, has controlled degradation and no toxicity, is favorable for adhesion and growth of the cells, and has certain strength and toughness, simple preparation process, low cost and easy wide application.
Owner:JILIN UNIV
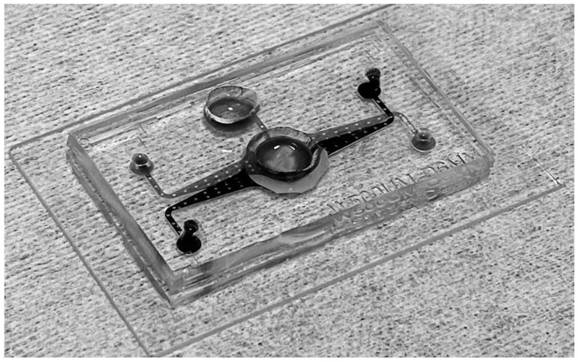
Multifunctional organ chip based on microfluidic technology, preparation method and application thereof
InactiveCN112574884ASolve the problem that it is difficult to correctly reflect the human corneaMeet needsBioreactor/fermenter combinationsBiological substance pretreatmentsMedicineMicrofluidics
The invention belongs to the field of microfluidic technology and biotechnology, and relates to a multifunctional organ chip based on a microfluidic technology, and a preparation method and an application thereof. The multifunctional organ chip based on the microfluidic technology comprises a substrate, the substrate at least comprises two structural layers, each structural layer is provided witha micro-channel and a cell culture area communicated with the micro-channel, the cell culture areas of every two adjacent structural layers are communicated, and a porous membrane is arranged at the communication position. The invention provides the multifunctional organ chip based on the microfluidic technology, which not only can stably culture human corneal cells in a culture area to form three-dimensional corneal tissues, but also can detect the integrity of an established corneal barrier, and solves the problem that in-vitro two-dimensional culture and in-vivo animal experiments are difficult to correctly reflect human corneas, and the requirements of scientific research and clinical application on an in-vitro bionic research model are met.
Owner:SHENZHEN INST OF ADVANCED TECH
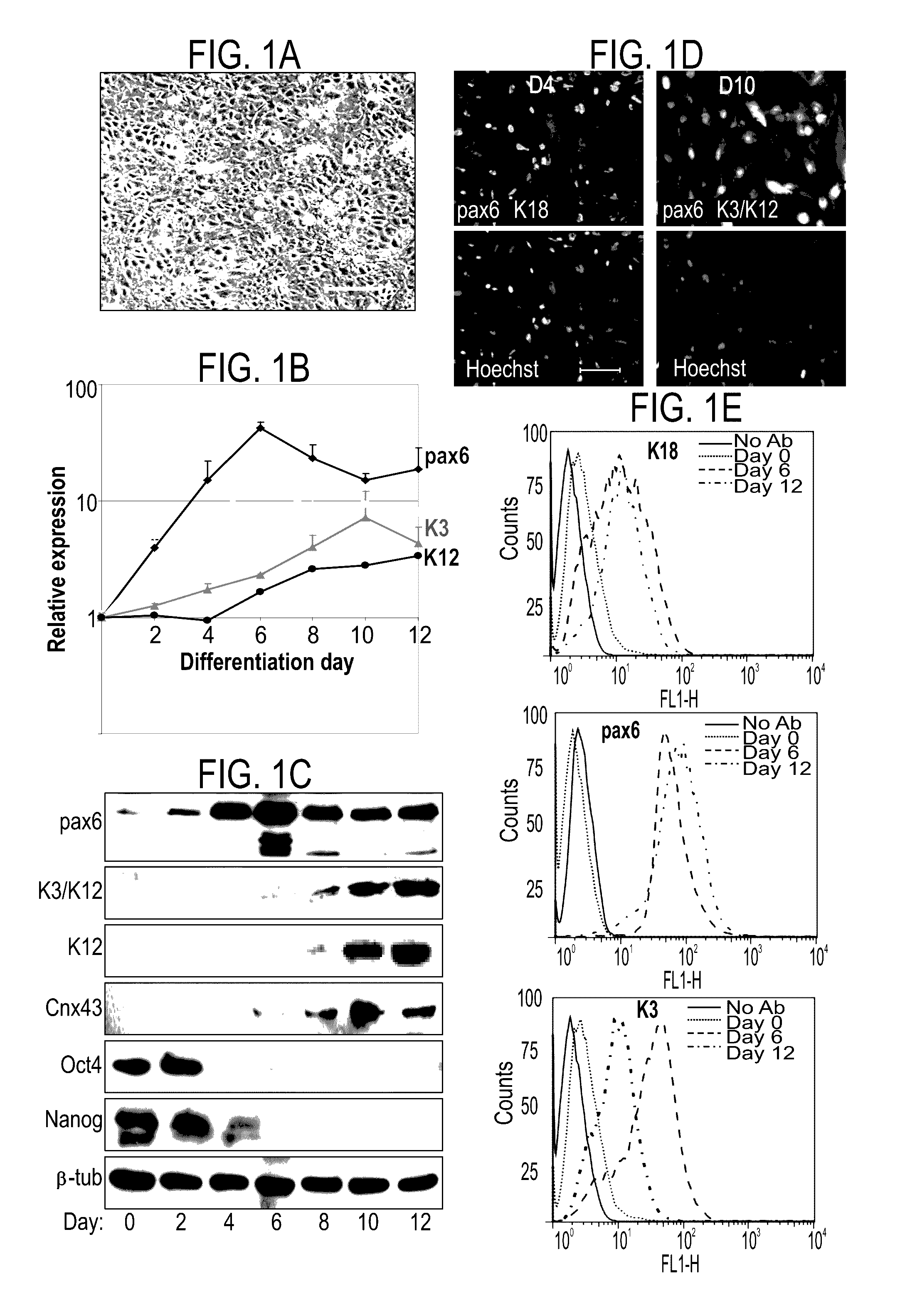
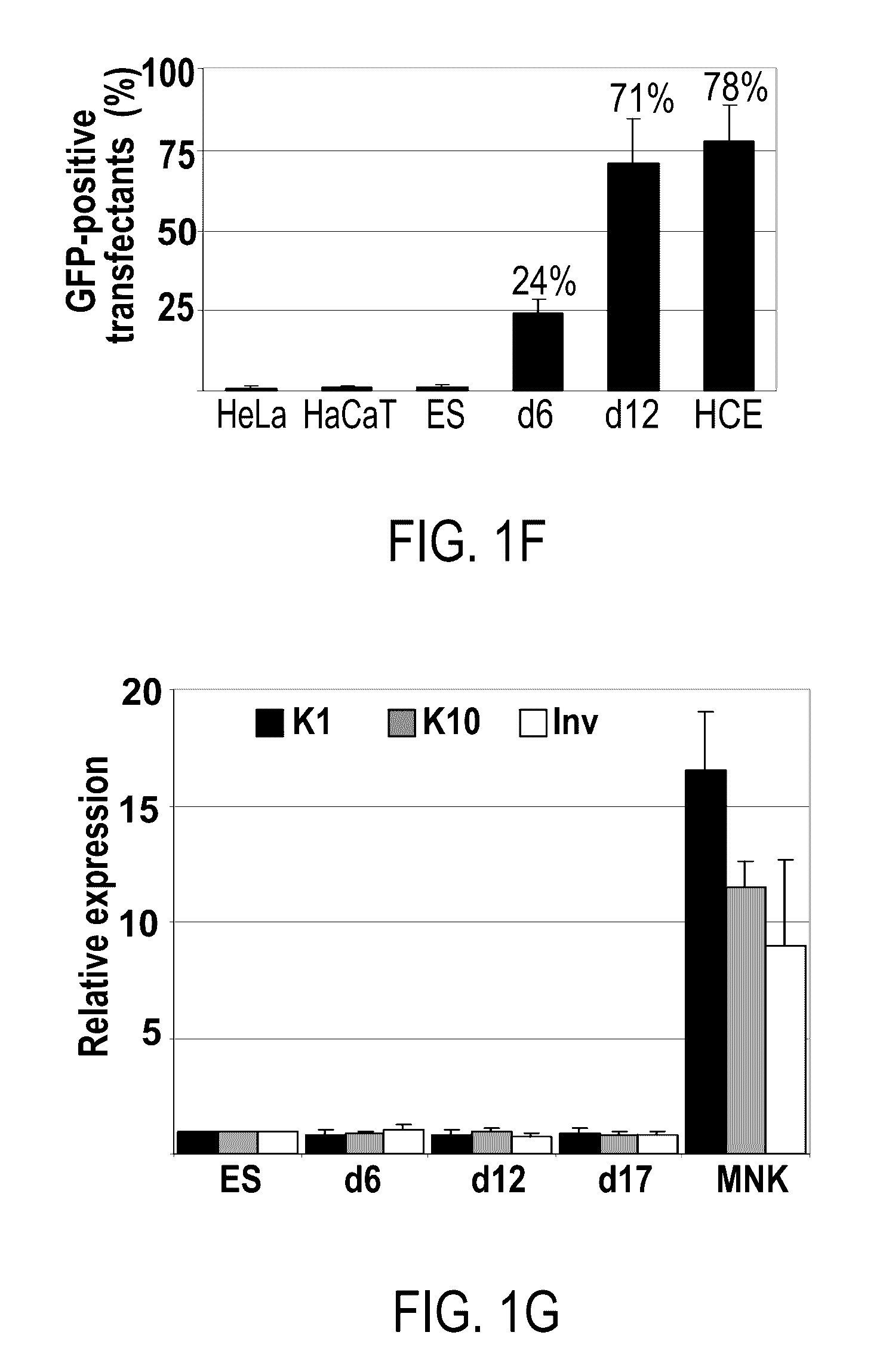
Methods of generating corneal cells and cell populations comprising same
A method of generating a population of corneal epithelial cells is disclosed. The method comprises culturing human pluripotent stem cells in corneal fibroblast-conditioned medium on a solid surface comprising an extracellular matrix component thereby generating the population of corneal epithelial cells. Isolated cell populations and corneal tissues are also disclosed, as well as uses thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Synthetic graft
The present invention relates to the use of a plastically-compacted collagen gel as a substrate for the growth of corneal cells, particularly limbal corneal epithelial stem cells. Cells grown on such a substrate can be cultured to produce artificial ocular epithelia which can be used in ocular toxicity testing or for transplantation.
Owner:THE UNIV OF READING
Corneal cells expressing active agents and methods of use thereof
InactiveUS20060257382A1Improving corneal graft healingImprove graft survivalBiocideVirusesActive agentNucleotide
The invention relates to methods of modifying cells of corneal tissue to express an active agent, to modified corneal tissue, to vectors utilised in such methods and to methods of xeno- and allo-transplantation utilising the modified corneal tissue. The method of modifying cells of corneal tissue to express an active agent involves exposing harvested corneal tissue to an effective concentration for transfection of an expression vector which comprises a nucleotide sequence encoding for the active agent for a period sufficient to allow infection, such that cells of said the corneal tissue will express the active agent.
Owner:THE FLINDERS UNIV OF SOUTH AUSTRALIA
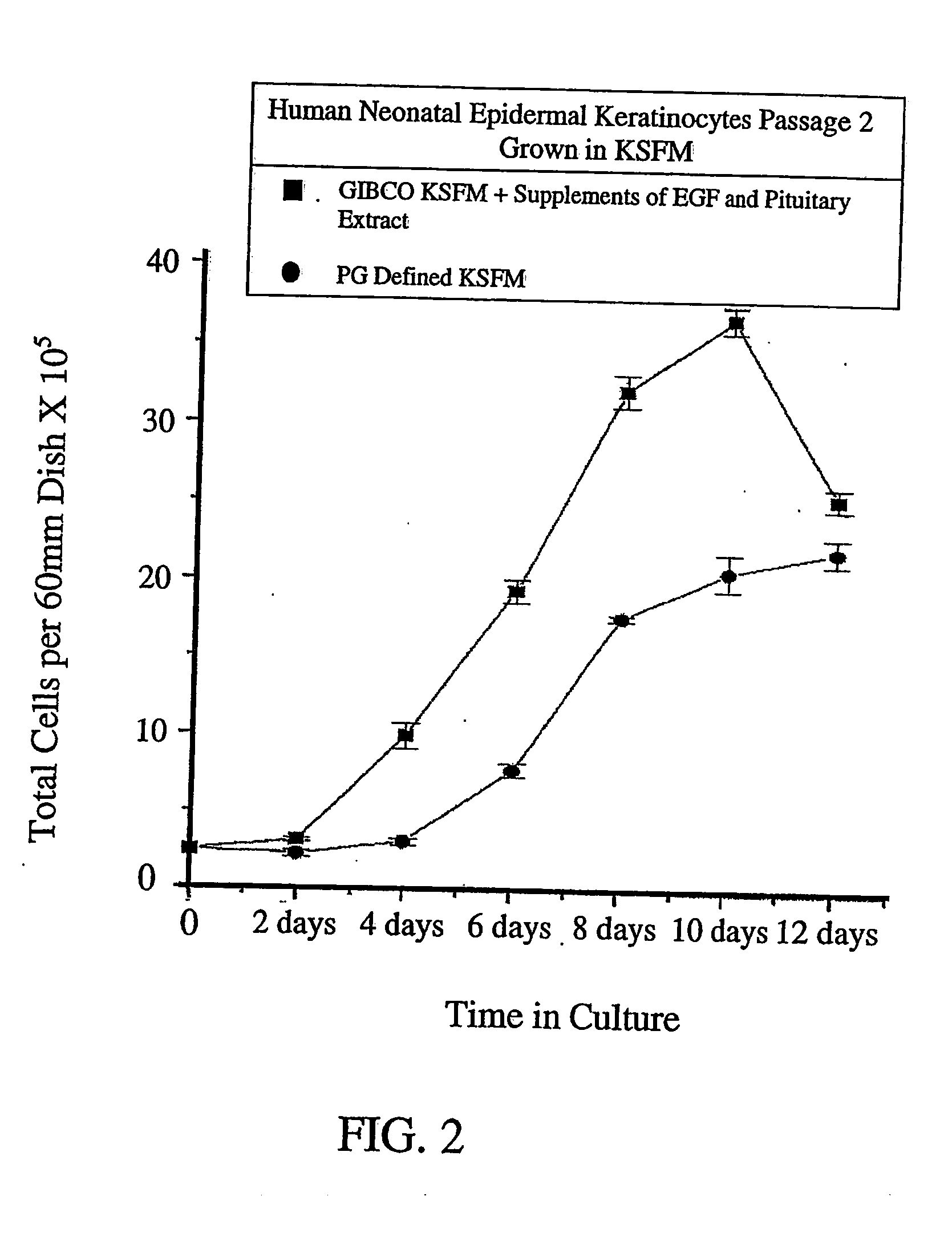
Method for culturing and expansion of mammalian undifferentiated epidermal kerainocytes exhibiting stem cell characteristics
InactiveUS20050019310A1Increase the number ofPromote optimal healingBiocidePeptide/protein ingredientsMammalCulture mediums
The invention disclosed relates to the culturing of mammalian e.g. human cells, and in particular to the culturing and expansion of substantially undifferentiated human epidermal keratinocytes exhibiting stem cell-like characteristics, and in co-culture with human dermal fibroblasts utilizing a low calcium serum free and animal by-product free medium derived from a commercially available medium. The medium consists of a commercially available basal medium, and a single fibroblast growth factor (FGF) or a mimic thereof e.g. FGF7 / KGF. A method is also disclosed for treating mammalian e.g. human skin wound injuries, by applying to the wound an effective amount of substantially pure mammalian e.g. human epidermal stem cell-like keratinocytes in a substantially undifferentiated state, and optionally additionally, dermal fibroblasts e.g. human dermal fibroblasts. Both cell types can be grown separately or in a co-culture for application purposes.
Owner:PHARMAGAP
Collagen-based composite cornea substitute with bioactivity and preparation method thereof
InactiveCN101543643BHigh biosecurityPromote growth and reproductionEye implantsFreeze-dryingCross linker
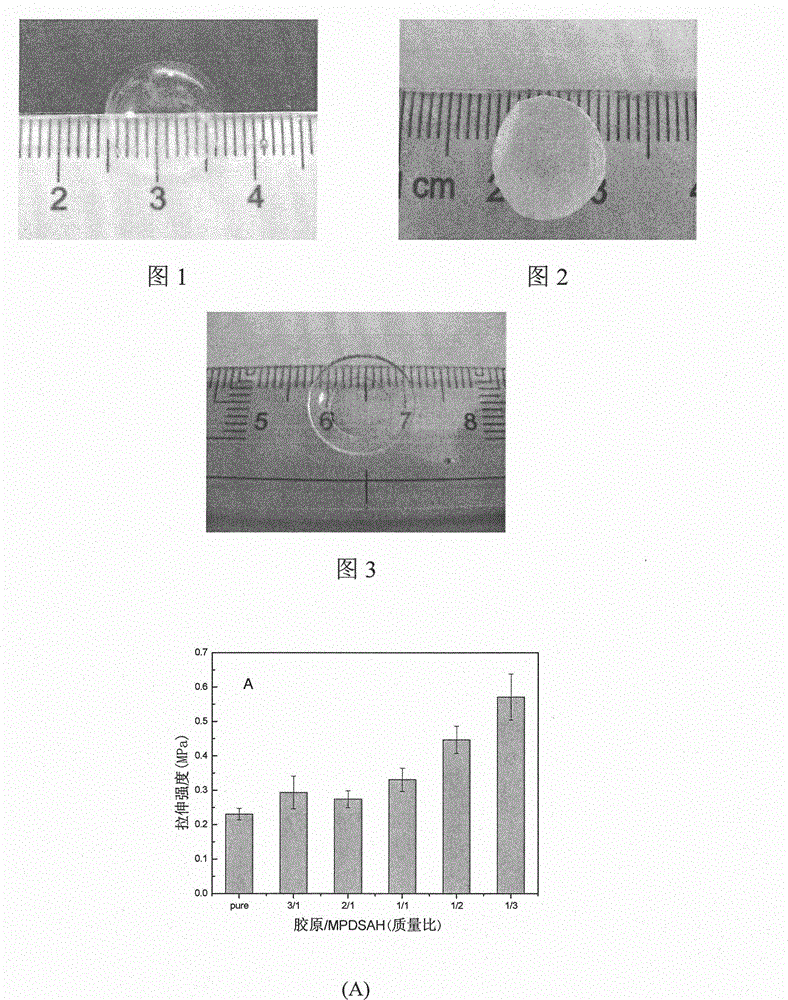
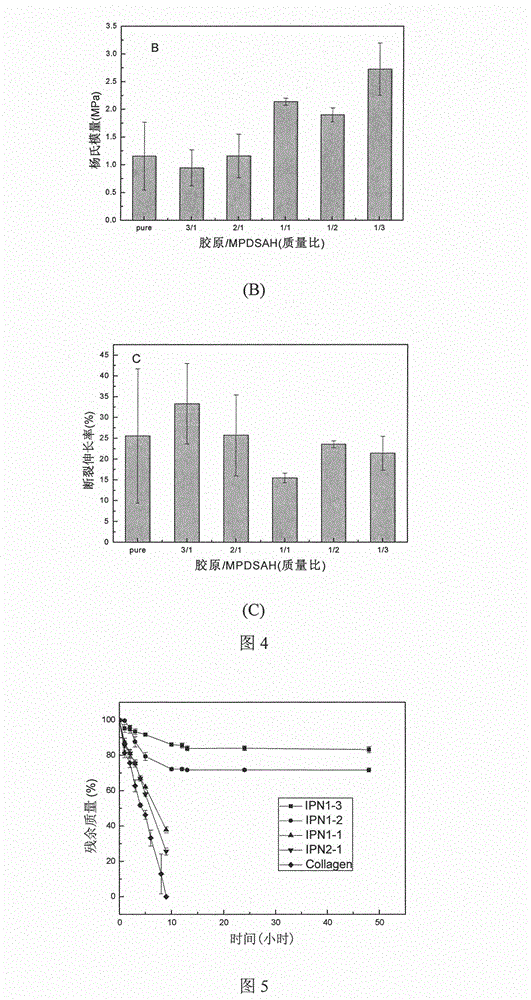
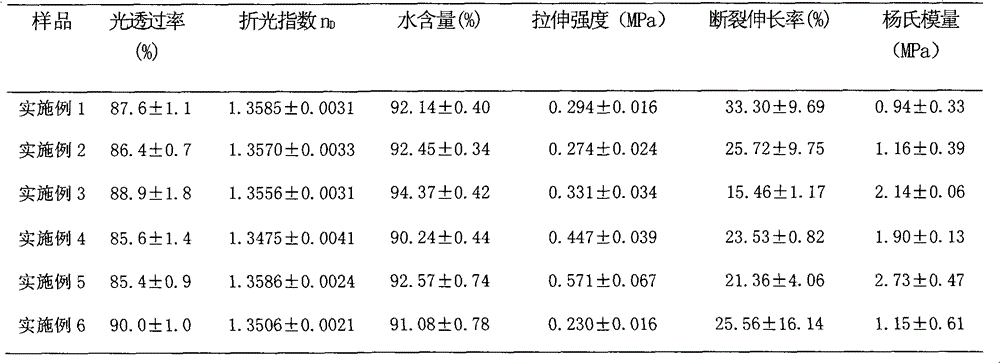
The invention provides a collagen-based composite cornea substitute with bioactivity and a preparation method thereof. The composite cornea substitute is prepared by the following steps: taking collagen and 3-(methacrylamide)propyl-dimethyl(3-sulfopropyl)amide (MPDSAH) as raw materials and water as a solvent, crosslinking the collagen with zero-length cross linker carbodiimide (EDC); taking Irgacure 2959 as a photo initiator and polyethyleneglycol diacrylate (PEGDA) as a crosslinking agent to perform initiated polymerization and crosslinking for the MPDSAH; and after even mixing, injecting a die with the mixture for cure forming. After freeze drying, the composite cornea constitute has a three-dimensional porous structure, can adsorb a large amount of cornea cell growth factors, can be stored for a long time, and can recover the primary shape and performance like before freeze drying after water absorption and expansion. The prepared composite cornea substitute has good histocompatibility, can induce and promote the cornea regeneration, and can be biodegraded along with the cornea regeneration, and the introduction of the growth factors can promote the enrichment of self growth factors and reduce the occurrence of postoperative complications.
Owner:TIANJIN UNIV
Sodium hyaluronate eyedrop containing deproteinized calf blood extractive and preparation method thereof
InactiveCN102579492AImprove damage repair rateIncrease intakeOrganic active ingredientsSenses disorderCorneal woundXerophthalmia
The invention discloses a sodium hyaluronate eyedrop containing deproteinized calf blood extractive. The components comprise deproteinized calf blood extractive, sodium hyaluronate, pH buffering agent, metal chelating agent, osmotic pressure regulation agent and injection water. The preparation method has the following steps: weighing the sodium hyaluronate, adding the injection water to resolve, and obtaining sodium hyaluronate solution after overnight swelling; fetching the deproteinized calf blood extractive, the pH buffering agent, the metal chelating agent and the osmotic pressure regulation agent, adding the injection water to resolve, mixing with the sodium hyaluronate solution after resolving, and agitating uniformly after supplementing the injection water to full dose; filtering and sterilizing after adjusting the pH of the solution, embedding to single dose packaging containers after being qualified by testing, and sealing and obtaining the product. The eyedrop disclosed by the invention is used for curing corneal wounds and xerophthalmia, can promote the corneal cells to incept and utilize the energy, and improves the recovery rate of the corneal wounds. The preparation method of the eyedrop avoids the stimulus to the surface cells of eyes, and prevents the contamination caused by microorganisms in tears and in air during use and storage.
Owner:ZHAOKE PHARMA GUANGZHOU
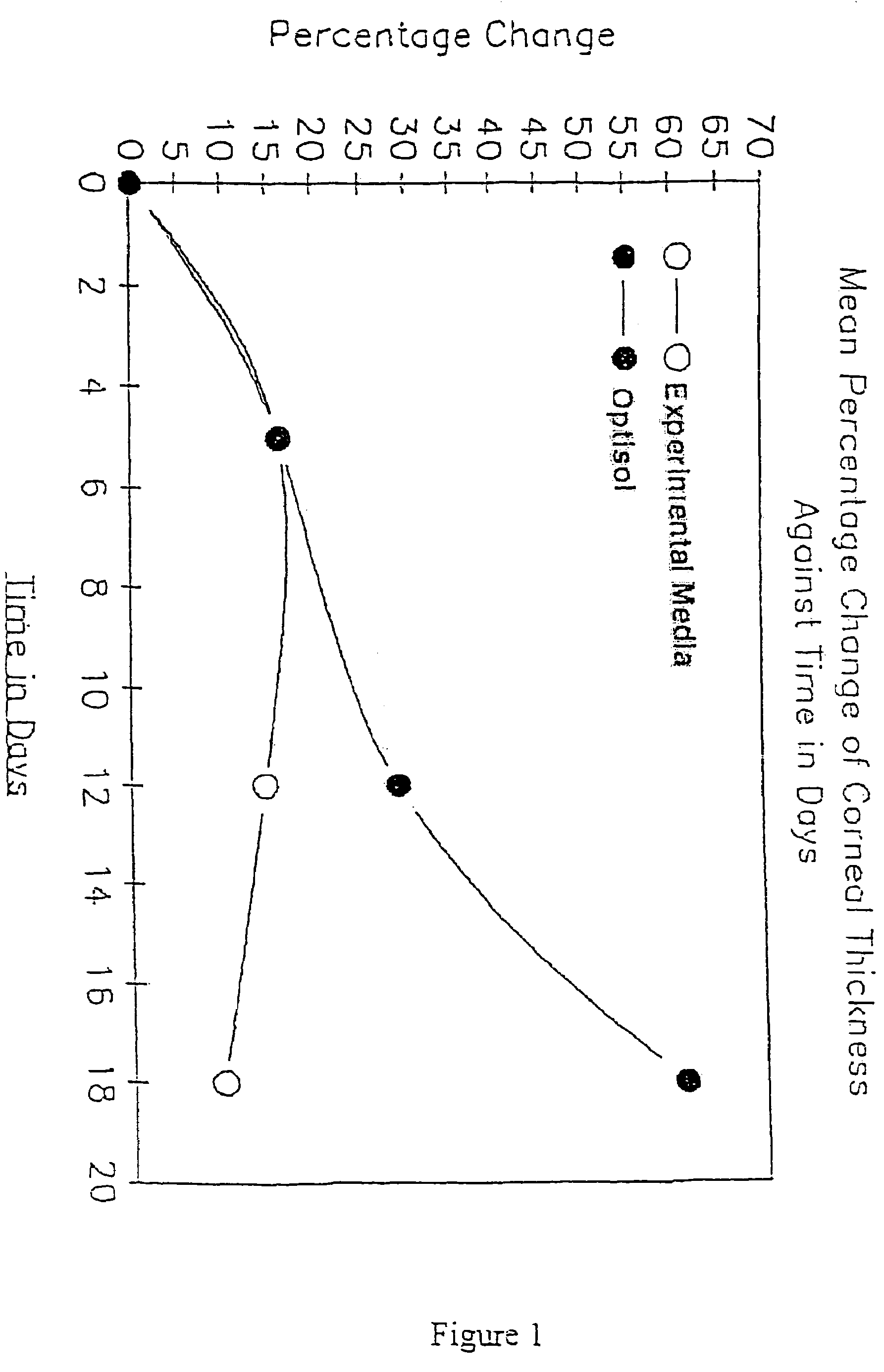
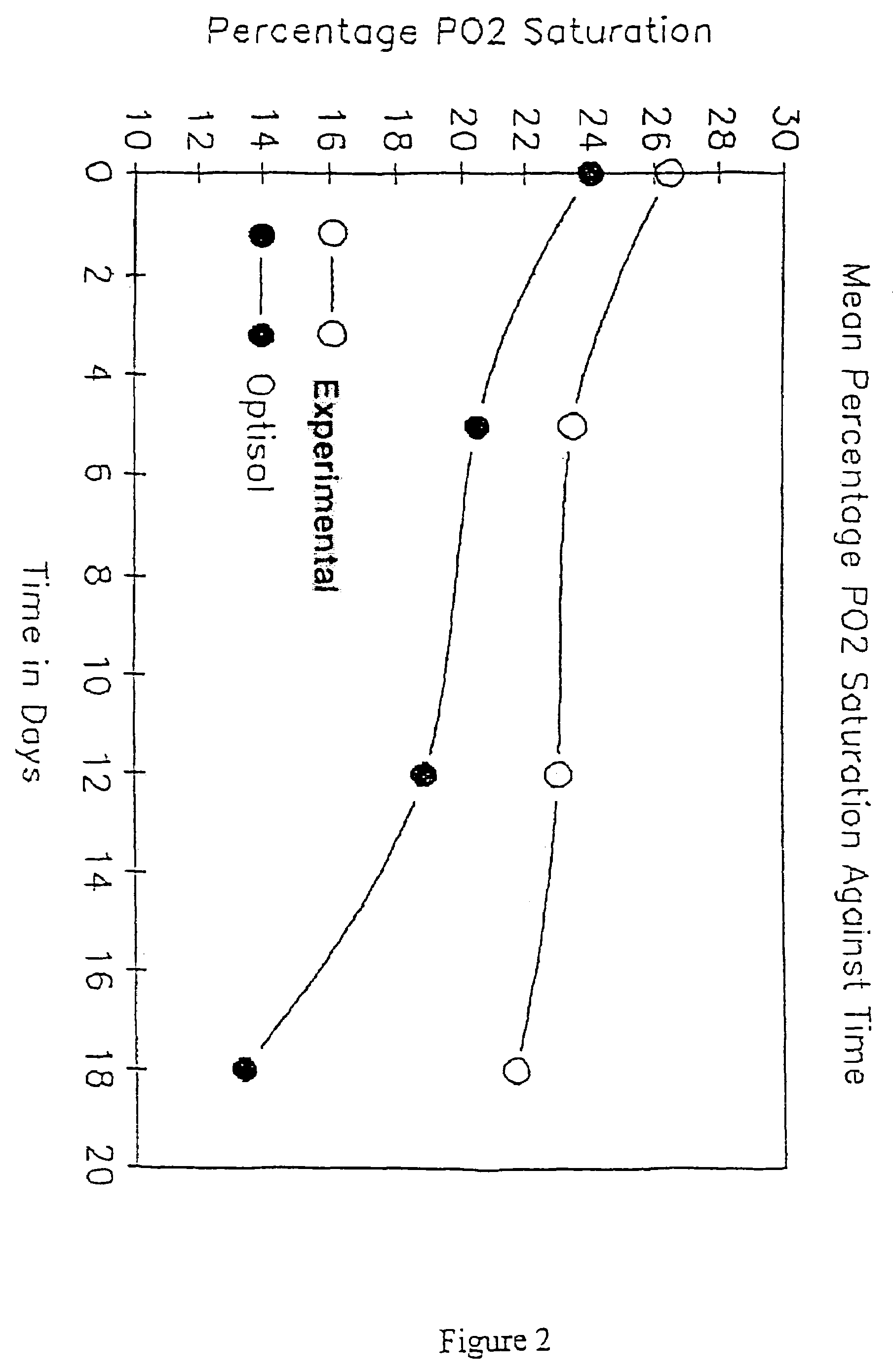
Extending tissue preservation
InactiveUS20040142466A1Keep clearAnti-freeze proteins can be further enhancedDead animal preservationArtificial cell constructsMicrobiologyCorneal cell
Owner:UNIV OF SOUTH FLORIDA
Collagen film with 3D micro-patterns, and preparation method and application thereof
The invention belongs to the field of biomedical materials, and discloses a collagen film with 3D micro-patterns as well as a preparation method and application thereof. The collagen film with the 3Dmicro-patterns is prepared by the following steps: forming 3D micro-patterns on a silicon wafer by adopting a photolithography technology by using a mask with 3D micro-patterns; pouring PDMS prepolymers onto the silicon wafer so as to be subjected to cross-linking and curing, thereby obtaining a PDMS base with the 3D micro-patterns; dissolving collagen in an acidic solution, adding water, and carrying out uniform stirring so as to obtain a collagen solution; adding a cross-linking agent into the collagen solution so as to be subjected to cross-linking, thereby obtaining cross-linking reactionproducts; and then, removing bubbles from the cross-linking reaction products, pouring the bubble-removed cross-linking reaction products onto the PDMS base, carrying out air-drying so as to have a film formed, and carrying out stripping so as to obtain the collagen film with the 3D micro-patterns. The preparation technology of the collagen film with the 3D micro-patterns is simple in processes and relatively low in cost; and the prepared film combines advantages of collagen and patterned surface, thereby having good physicochemical properties and biocompatibility. The collagen film with the 3D micro-patterns can be used in corneal tissue engineering so as to induce corneal tissue regeneration; moreover, the collagen film with the 3D micro-patterns is capable of performing regulation on corneal cell behavior, thereby forming a structure similar to native tissues.
Owner:SOUTH CHINA UNIV OF TECH
Acellular corneal stroma and preparing method for acellular corneal stroma
The invention discloses an acellular corneal stroma and a preparing method for the acellular corneal stroma. The preparing method for the acellular corneal stroma includes the following steps: (1) placing a picked animal cornea into water, and carrying out vibration processing to enable the epithelial layer to fall off; (2) replacing the water with acellular reagents, and carrying out vibration processing, wherein during the period, the acellular reagents and the water are alternately used to enable stroma cells and endothelial cells to fall off; (3) adding dehydrating agents, and enabling the mixture to stand or carrying out vibration processing to obtain the acellular cornea; (4) cutting the acellular cornea to obtain the acellular corneal stroma, wherein the acellular corneal stroma only comprises a front elastic layer and a stroma layer. The acellular reagents contain NaCl and EDTA, and the dehydrating agents contain glycerin. By means of the preparing method for the acellular corneal stroma, corneal cell components and soluble protein which are prone to causing the immune response can be effectively removed, and the complete acellular corneal stroma only containing the front elastic layer and the stroma layer can be obtained.
Owner:SHENZHEN AINEAR CORNEA ENG
Pharmaceutical composition for corneal tissue repair of insulin nano system and application of pharmaceutical composition
PendingCN114099645AReduce dosing frequencyImprove complianceSenses disorderPeptide/protein ingredientsTissue repairOphthalmology
The invention discloses a pharmaceutical composition for corneal tissue repair by an insulin nano system and application of the pharmaceutical composition, and belongs to the technical field of pharmaceutical compositions, and the pharmaceutical composition is characterized by comprising nano materials such as lipidosome and insulin wrapped in the nano materials such as lipidosome; the pharmaceutical composition for corneal tissue repair by the insulin nano system is applied to corneal repair. The invention is mainly used for providing a novel preparation ophthalmic pharmaceutical composition which has the advantages of safety, effectiveness, side effect and low administration frequency, and can effectively improve the compliance of patients; meanwhile, the ophthalmic pharmaceutical composition has important biological effects of resisting inflammation, resisting apoptosis and promoting corneal cell and nerve repair, and has more advantages in the treatment effects of resisting inflammation, resisting infection, promoting corneal repair and strengthening amniotic transplantation compared with a traditional treatment method.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
Ocular surface in-situ drug and preparation method thereof
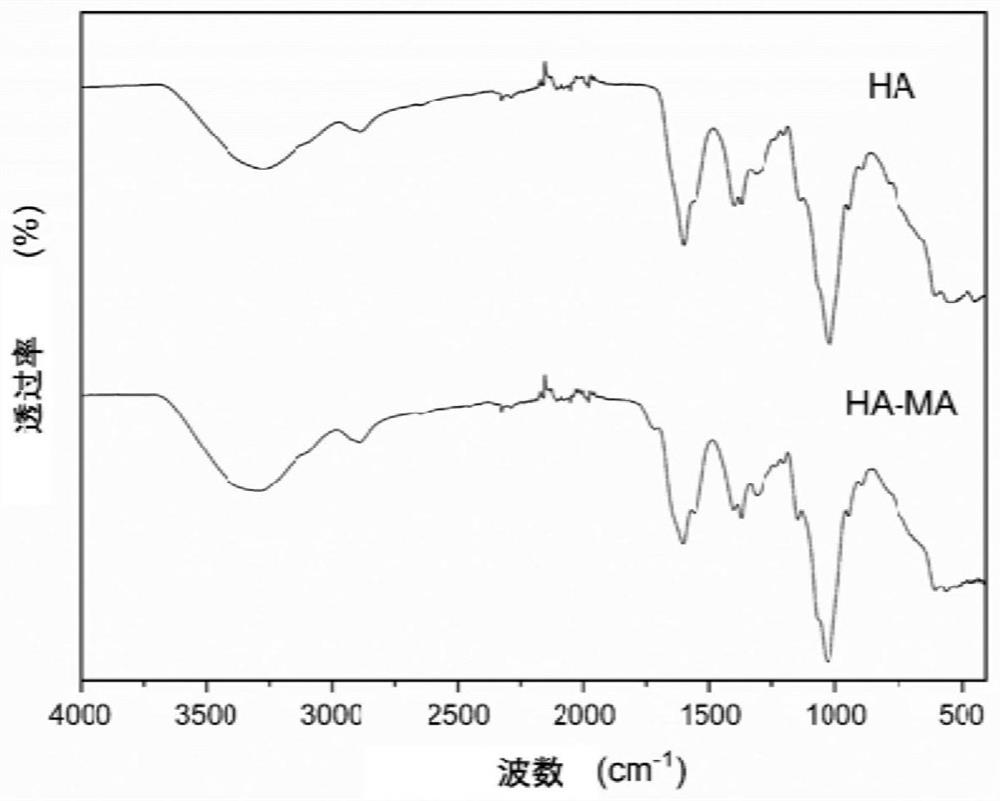
PendingCN113041215AGood biocompatibilityImprove the defect of poor biocompatibilityAntibacterial agentsOrganic active ingredientsMicrosphereCytotoxicity
The invention relates to an ocular surface in-situ drug and a preparation method thereof. The ocular surface in-situ drug comprises a hydrogel system which takes PluronicF127, PluronicF68 and methacrylic acid-hyaluronic acid (HA-MA) as main components and has a temperature response characteristic, and a nano drug-loaded microsphere which contains an active drug or wraps the active drug, in-situ gel is formed at the ocular surface temperature, and the drug release time is prolonged. By combining the hyaluronic acid derivative HA-MA with high biocompatibility and low cytotoxicity, a brand-new temperature-triggered hydrogel sustained-release system formula for eyes is provided, so that drug sustained release on the ocular surface is realized, and the treatment on bacterial / fungal keratitis and conjunctivitis is achieved; and meanwhile, the toxicity of the material is reduced, and the repair of corneal cells can be promoted during treatment.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Differentiation of pluripotent stem cells into corneal cells
The present description relates to differentiation of stem cells into eye precursor cells and further into differentiated corneal cells, such as corneal epithelial cells. Differentiated corneal cells may contribute to treatment and research of corneal conditions, diseases, and pathologies, as well as to toxicological studies and drug development.
Owner:STEMSIGHT OY
Cell culture substrate and application and use method thereof
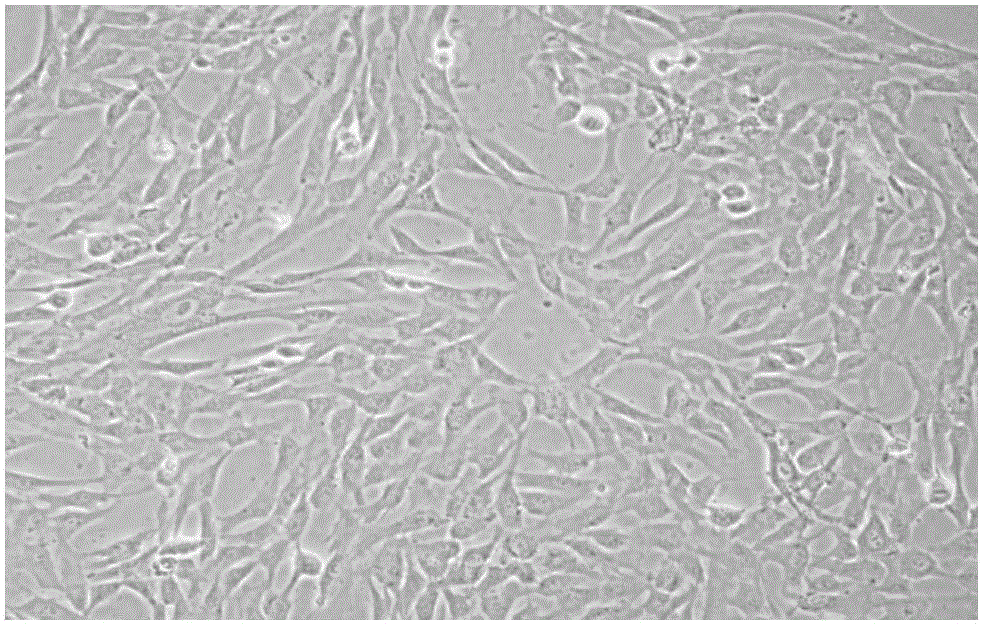
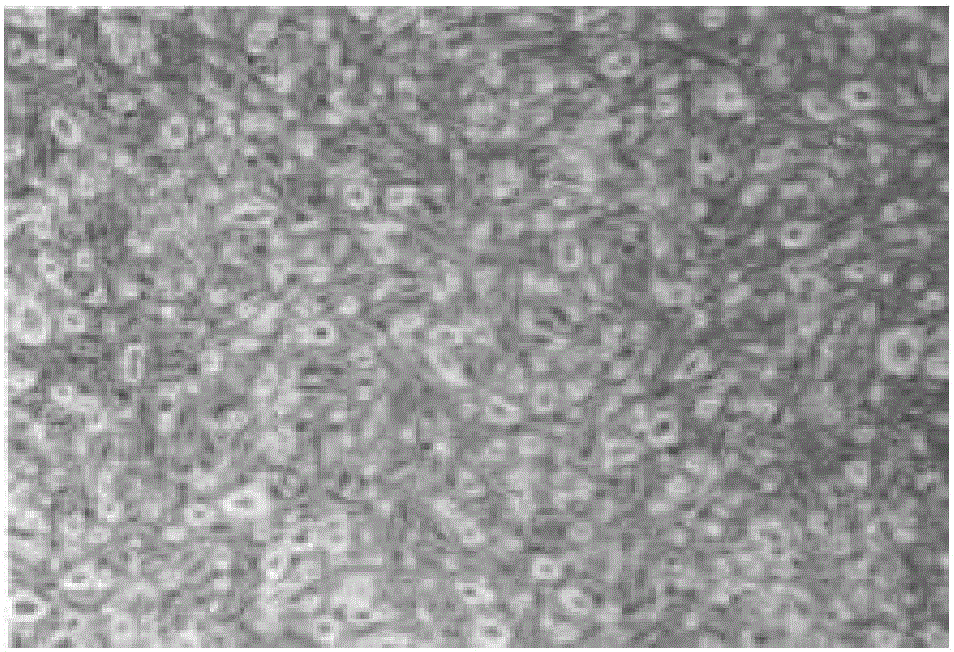
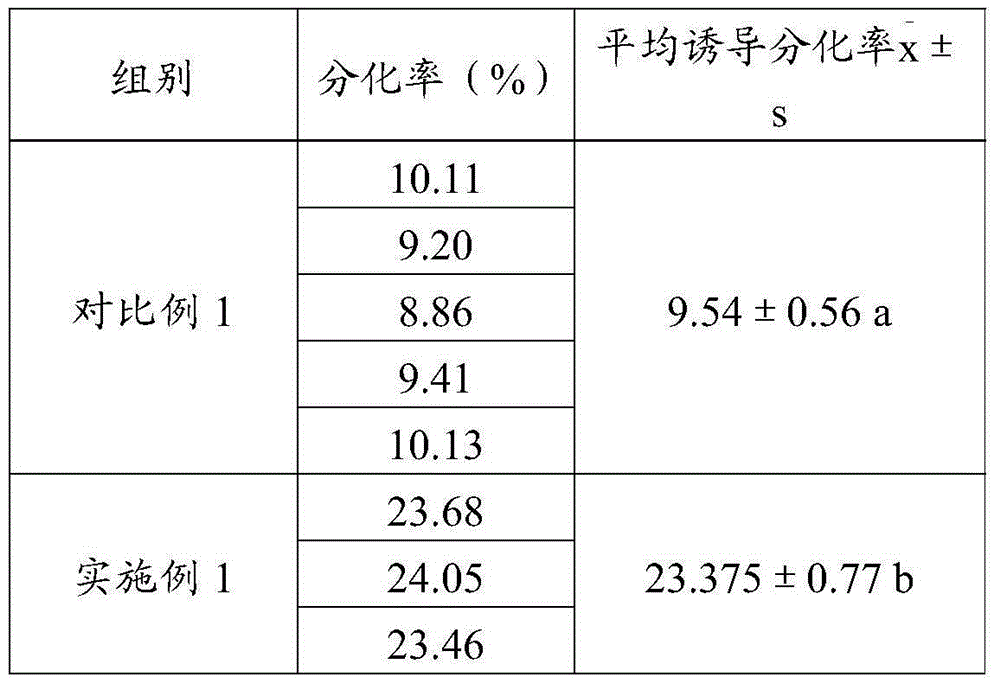
ActiveCN105087482AHigh differentiation efficiencyPromote differentiationArtificial cell constructsSkeletal/connective tissue cellsHuc mscsCell culture media
The invention relates to the field of stem cell culture, in particular to a cell culture substrate and an application and a use method thereof. The cell culture substrate comprises a collagen membrane and a culture liquid. The cell culture substrate has the advantages that the collagen membrane is used as a carrier and is matched with the effective culture liquid, so the differentiation of hUC-MSCs (human umbilical cord mesenchymal stem cells) to cornea epithelial cells is promoted; after being proofed by experiments, when the hUC-MSCs are inoculated to the collagen membrane and are induced for seven days by the culture liquid, the differentiation efficiency of hUC-MSCs can reach 32%, but the differentiation efficiency is only 9.54% when the collagen membrane is not used as the carrier for inducing, so the collagen membrane can obviously improve the differentiation efficiency of the hUC-MSCs to the cornea cell.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
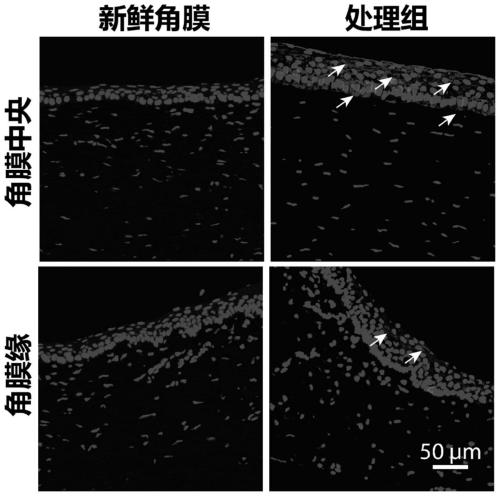
Limbal stem cells and preparation method thereof
ActiveCN110079500AHigh activityRaise the ratioNervous system cellsCell culture active agentsCryopreservationInducer
The invention relates to limbal stem cells and a preparation method thereof. The preparation method comprises the following steps: obtaining corneal tissues, and pre-treating the to-be-used corneal tissues; adding a cell cryopreservation solution containing a cell inducer into the pre-treated corneal tissues; freezing the corneal tissues with the cell cryopreservation solution, wherein the cell inducer is a reagent which can induce inflammation or stress response of part of cells in the corneal tissues, but does not cause cell death directly. The method can achieve screening of the limbal stemcells and various kinds of maturely differentiated corneal cells in the corneal tissues through simple low-temperature refrigeration operation before cell culture, can achieve long-term cryopreservation of the high-activity limbal stem cells at the same time, does not need specific instruments or equipment, is simple to operate, high in screening efficiency and high in purity of screened cells, and can improve the ratio and activity of the limbal stem cells.
Owner:广州市亮目生物科技有限公司
Composition and method for preserving or culturing ocular cells
PendingCN113056556AIncrease the number of cellsImprove work efficiencyPowder deliverySenses disorderCorneal endothelial cellOphthalmology
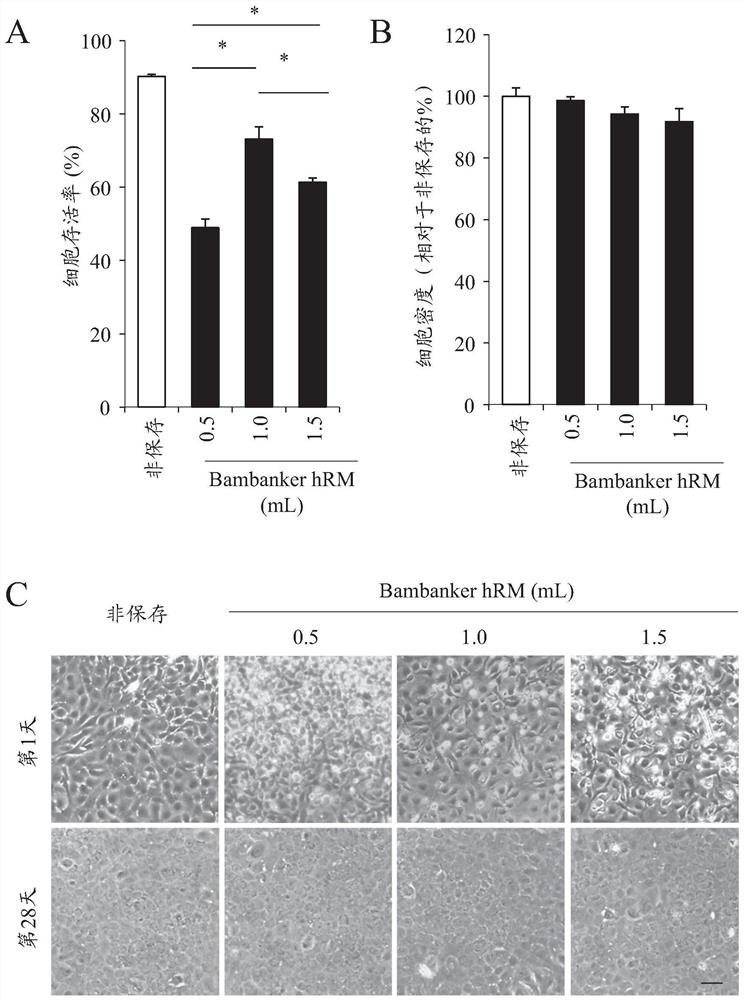
The present disclosure provides a composition and method for preserving ocular cells. More specifically, the present disclosure provides: a composition for preserving ocular cells, or culturing the same after preservation, the composition containing albumin and dimethyl sulfoxide; a cell preparation containing said ocular cells, albumin, and dimethyl sulfoxide; and a therapeutic / preventive method using the same. The present disclosure also provides a method for preserving corneal endothelial cells, the method including a step of suspending ocular cells in said composition to provide a suspension, and a step of freezing the suspension. In some embodiments, the ocular cells are corneal cells (for example, corneal endothelial cells).
Owner:DOSHISHA CO LTD
Extending tissue preservation
InactiveUS7498169B2Improve survivabilityLess increase in cell thicknessVertebrate cellsDead animal preservationMicrobiologyCorneal cell
A method of sustaining cells is provided. The method can include providing a non-perfluorocarbon cell storage medium, providing a pre-oxygenated liquid perfluorocarbon in contact with the storage medium, and placing the cells in contact with the storage medium but not in contact with the perfluorocarbon. Additionally, the method can result in increased corneal cell viability compared to corneal cells placed in a non-perfluorocarbon cell storage medium without being in contact with a pre-oxygenated liquid perfluorocarbon.
Owner:UNIV OF SOUTH FLORIDA
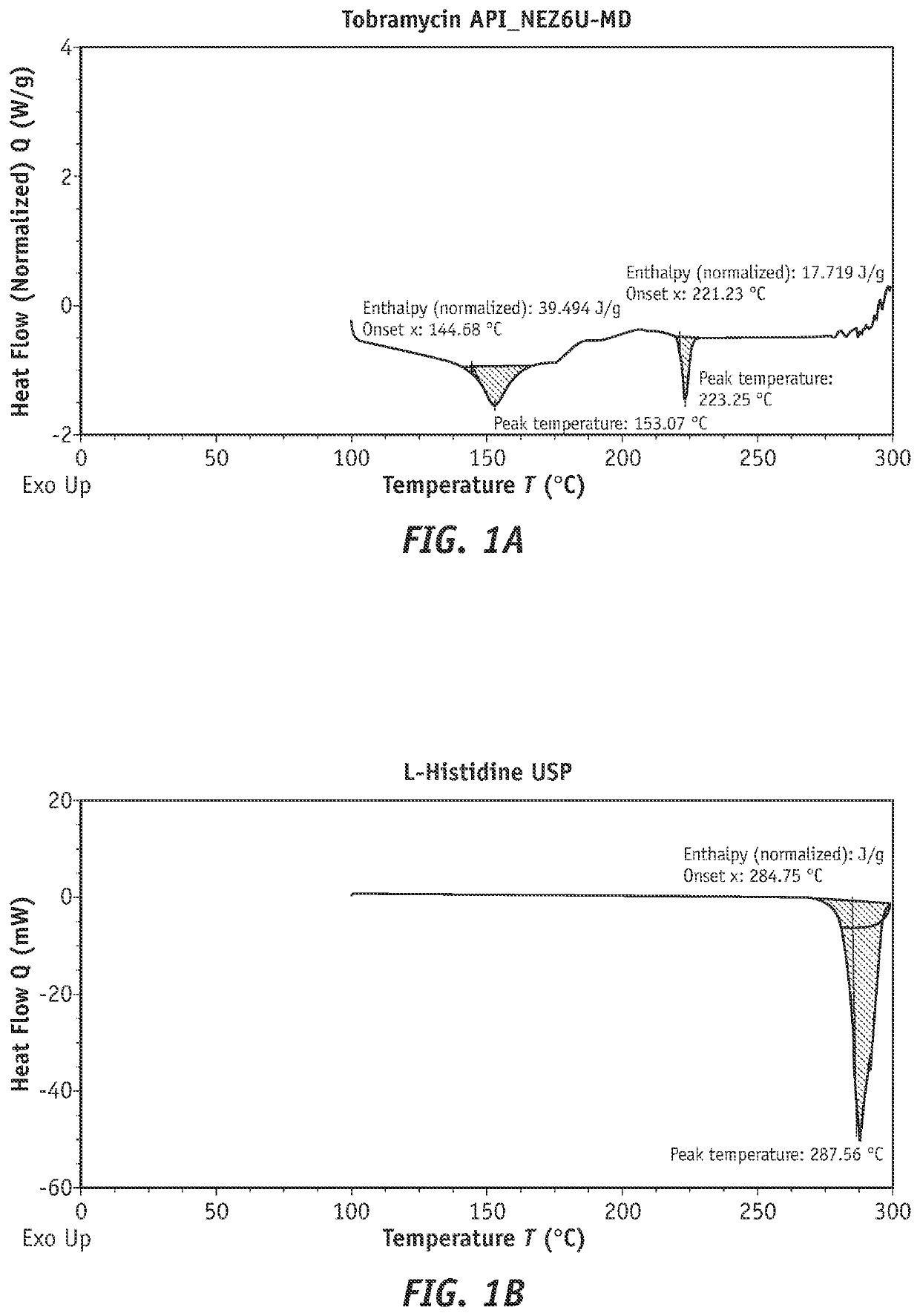
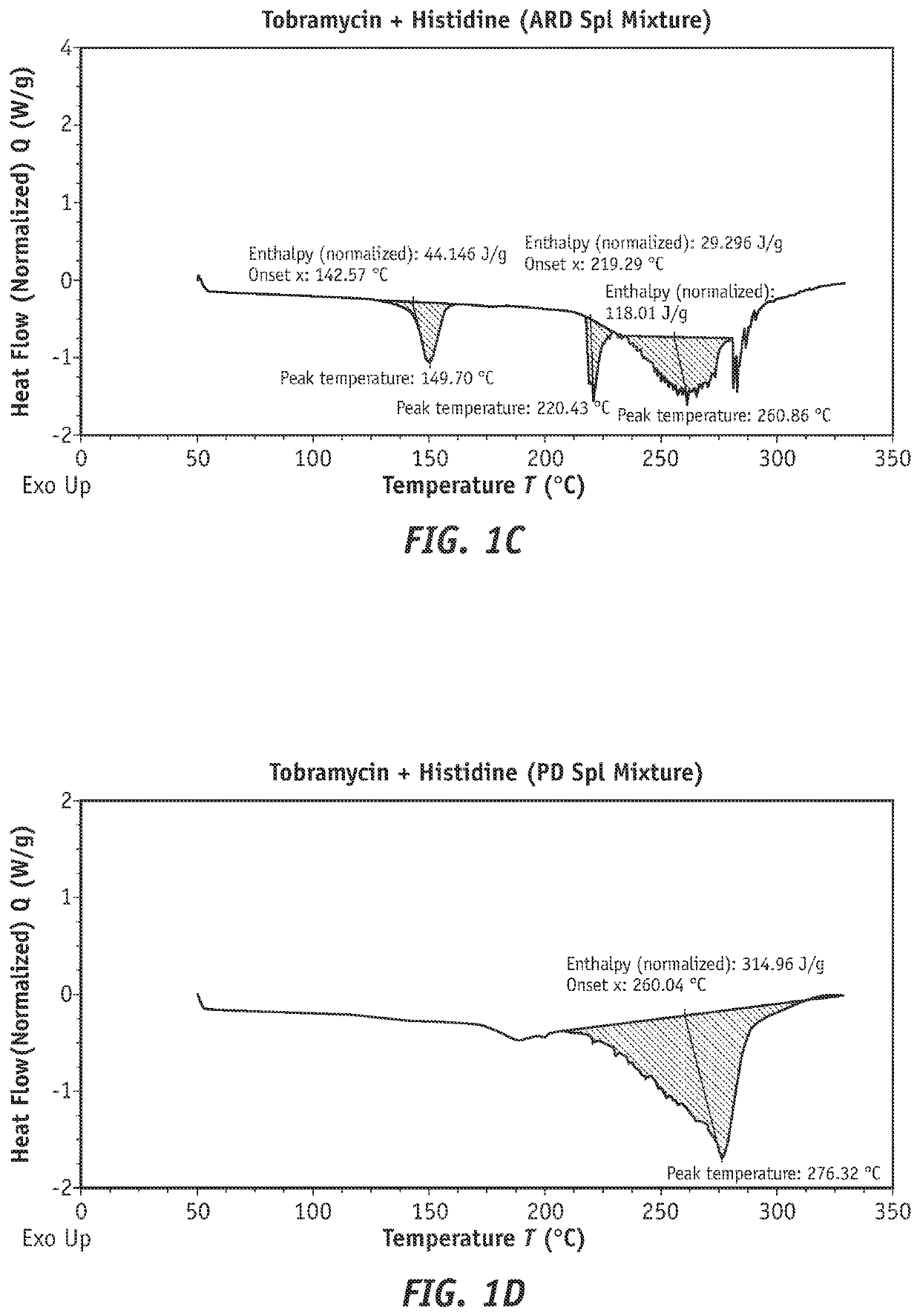
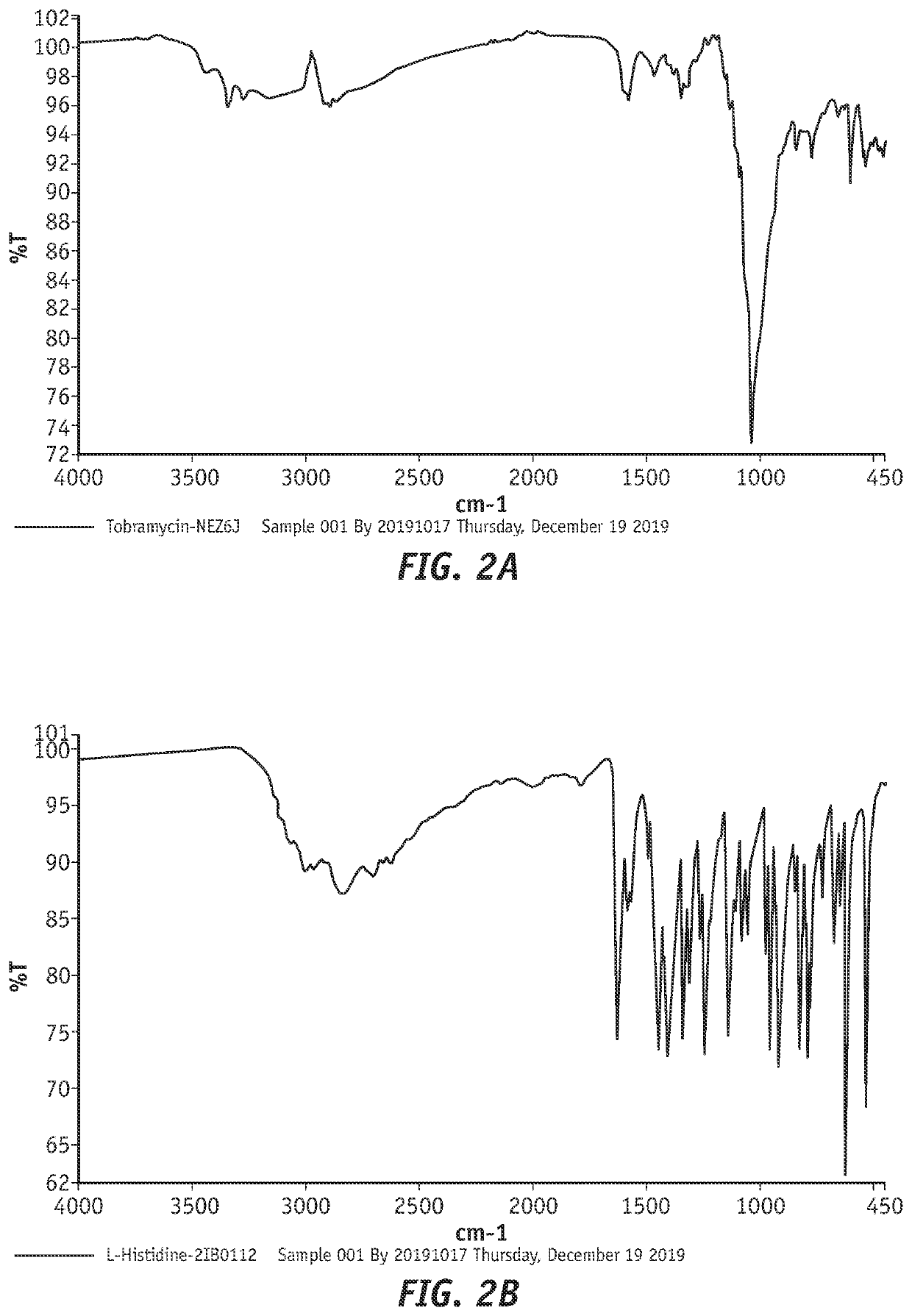
Combination products of tobramycin compounds and dexamethasone compounds
ActiveUS20210361687A1Promote formationReduce dosing frequencyAntibacterial agentsOrganic active ingredientsPharmaceutical drugPerylene derivatives
Disclosed herein are new compositions comprising (1) an antibacterial component comprising (a) a complex comprising (i) an active pharmaceutical ingredient (API) wherein the active pharmaceutical ingredient is tobramycin, a similar compound, or a derivative of either thereof and (ii) a complexing agent, or (b) a derivative of tobramycin or a similar compound comprising one or more conjugates / derivatizing groups, wherein the complexing agent or conjugate(s) cause a detectable increase in API permeation of corneal cells, retention in corneal cells, or both, as compared to the non-complexed or non-derivatized API, and (2) an effective amount of an anti-inflammatory agent, such as dexamethasone or another dexamethasone compound. This disclosure also describes new methods of using such compositions, producing such compositions, and the like.
Owner:SOMERSET THERAPEUTICS LLC
Methods of generating corneal cells and cell populations comprising same
A method of generating a population of corneal epithelial cells is disclosed. The method comprises culturing human pluripotent stem cells in corneal fibroblast-conditioned medium on a solid surface comprising an extracellular matrix component thereby generating the population of corneal epithelial cells. Isolated cell populations and corneal tissues are also disclosed, as well as uses thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Moxifloxacin eye drop having both bionic function and targeting function and preparation method thereof
InactiveCN106236704ABionic with tearsFunctionalAntibacterial agentsOrganic active ingredientsForeign matterIrritation
The invention discloses a moxifloxacin eye drop having both a bionic function and a targeting function and a preparation method of the moxifloxacin eye drop. Specifically, the moxifloxacin eye drop comprises the following components according to mass concentration: 5-6g / L of moxifloxacin hydrochloride, 3-5g / L of xanthan gum, 1-4g / L of sodium hyaluronate, 6-8g / L of an isoosmotic adjusting agent, and the balance of distilled water; the pH value is regulated through a pH regulator to 6.0-8.0. A xanthan gum / sodium hyaluronate gel system is creatively applied compositely to achieve both the bionic function of tears and the targeting function of a cornea; the xanthan gum has the bionic rheological property similar to that of the tear, so that higher viscosity is achieved when the shearing force is low, and the xanthan gum is evenly coated on the surface of an eyeball when the shearing force is high; the sodium hyaluronate acts on a receptor CD44 of a corneal cell, so that the cell intake is increased, and the targeting absorption is achieved. Compared with a common moxifloxacin eye drop, the moxifloxacin eye drop having both the bionic function and the targeting function has the characteristics of compatibility with the tear, no foreign-matter irritation, long residence time, higher bioavailability and the like.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
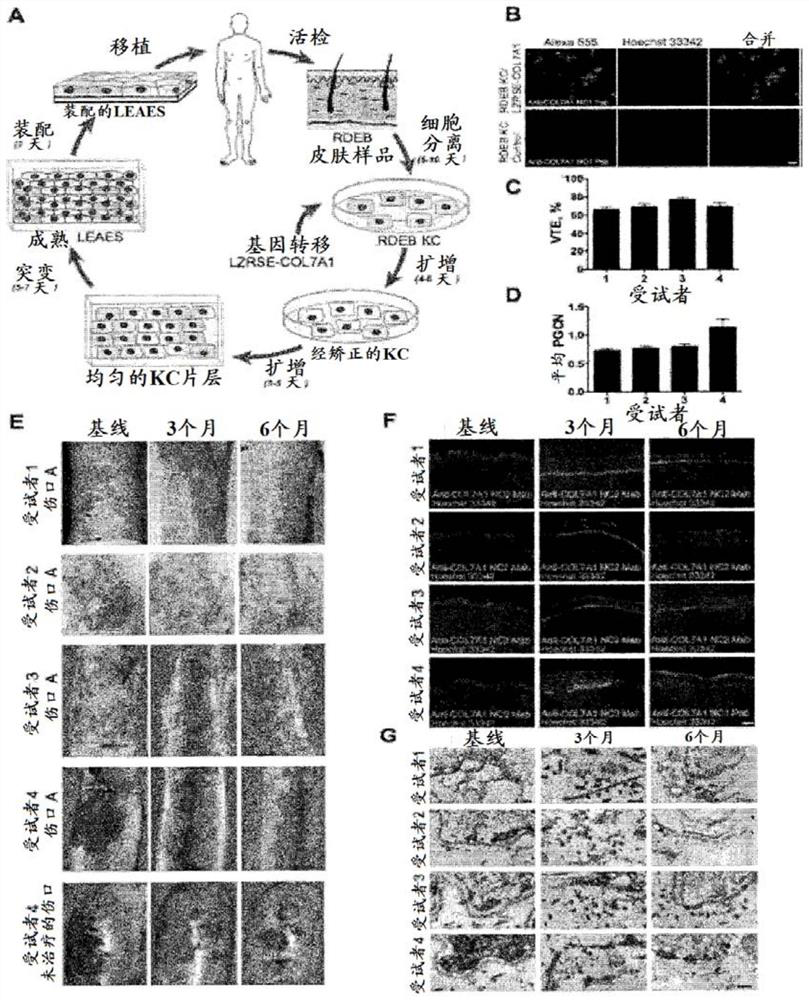
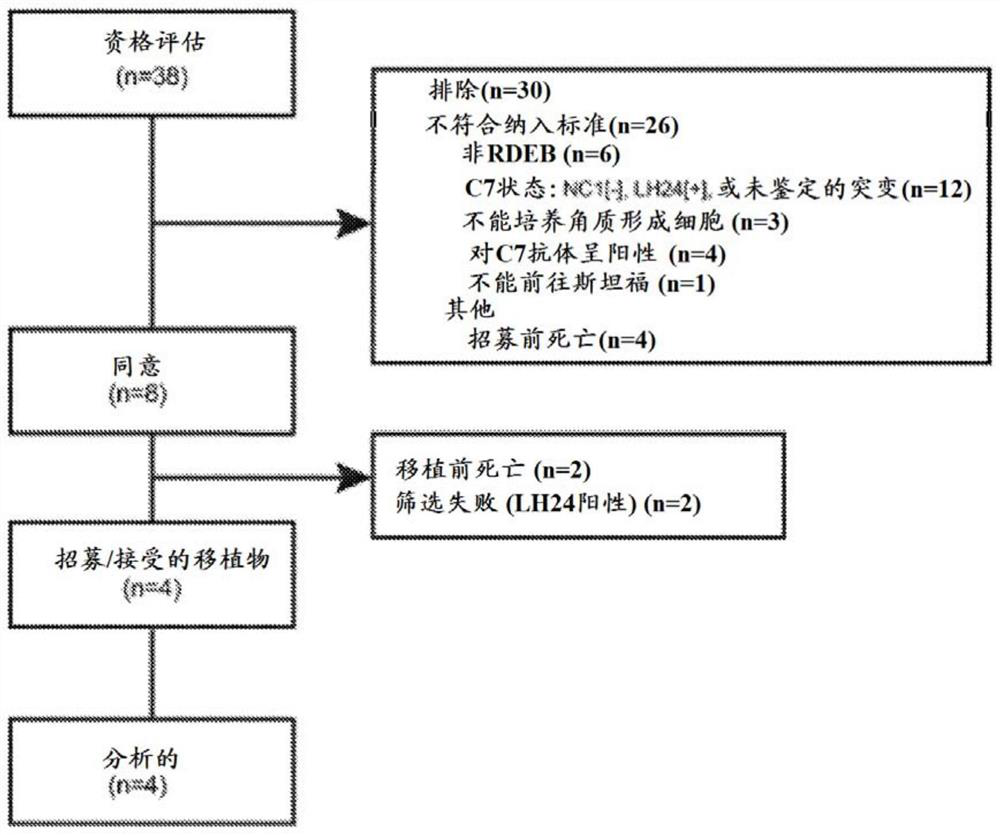
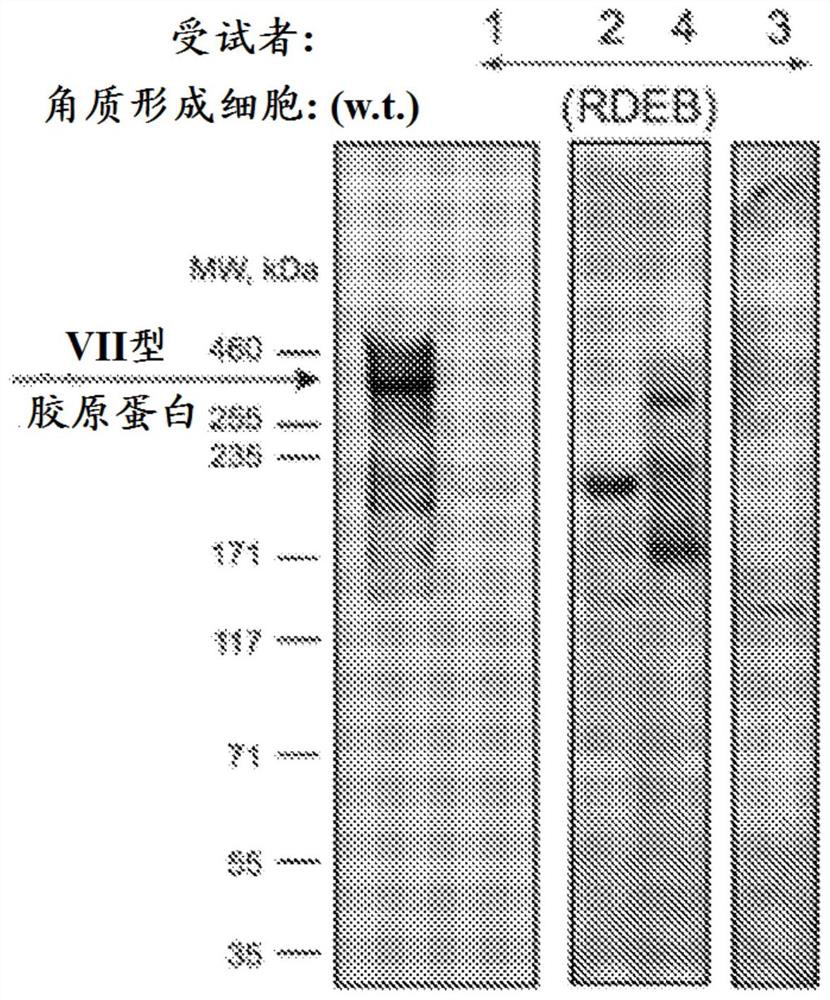
Gene therapy for recessive dystrophy epidermis bullosa using genetically corrected autologous keratinocytes
Methods for cell-based delivery of collagen VII to treat epidermis bullosa and corneal erosion are provided. The present disclosure also provides compositions and pharmaceutical compositions comprising, or consisting essentially of, or further consisting of, a keratinocyte sheet or a corneal cell sheet.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
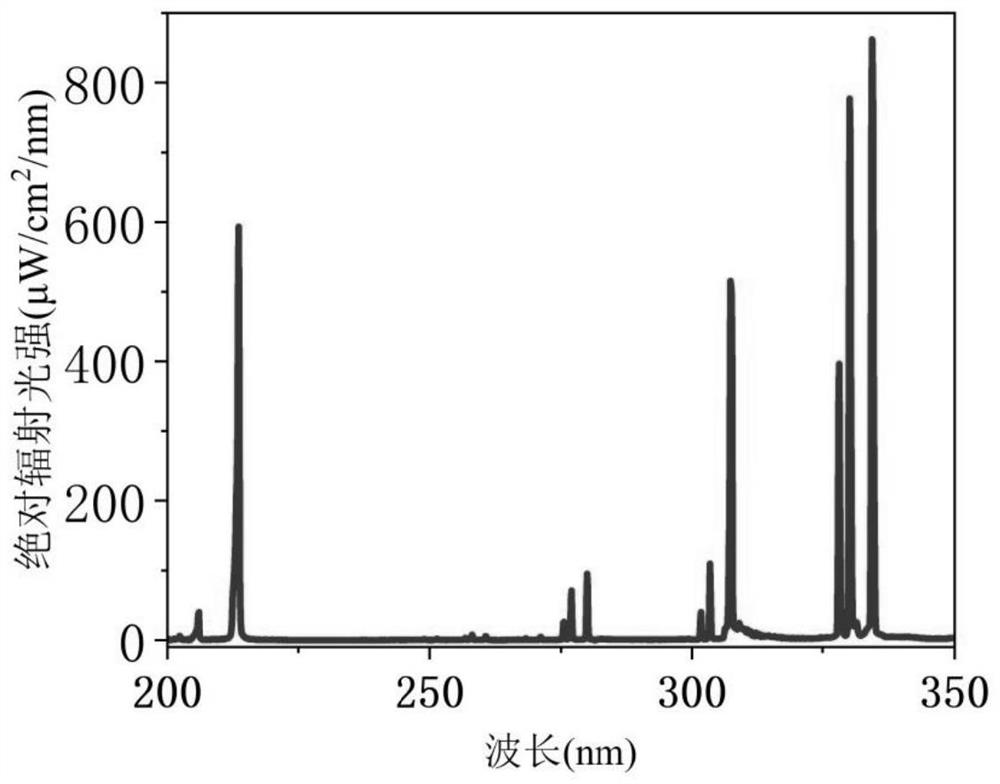
Microwave electrodeless ultraviolet light source, system and application
PendingCN114464522AWill not harmExtend your lifeElectric discharge lampsRadiationMetallic electrodeUltraviolet lights
The invention discloses a microwave electrodeless ultraviolet light source, a system and application, and belongs to the technical field of ultraviolet sterilization. According to the ultraviolet light source, inert gas and Zn and / or a Zn compound are filled into a quartz lamp tube, and the inert gas in the quartz lamp tube is excited by a microwave device to generate plasma. Then, the Zn and / or the Zn compound are heated and evaporated, the Zn atoms collide with the electrons or the inert gas atoms in the excited state, transition from the ground state to the excited state is achieved, and then ultraviolet light with the wavelength of 213 nm is radiated when the Zn atoms return to the ground state. Compared with the prior art, the ultraviolet light source provided by the invention has the advantages that Zn and / or a Zn compound is used for replacing mercury as a luminous substance, the raw material is cleaner, and microwave excitation is used for replacing a metal electrode, so that the problem that the service life of the light source is shortened due to electrode consumption is avoided. In addition, the dominant wavelength of the ultraviolet light source is 213nm, so that the skin and corneal cells of a human body are not damaged while bacteria are effectively killed, and the application range is wider.
Owner:SICHUAN UNIV
Efficient tobramycin compound conjugate compositions
ActiveUS20220054519A1Promote formationReduce dosing frequencyAntibacterial agentsOrganic active ingredientsOphthalmologyEngineering
Disclosed herein are compositions comprising tobramycin or a tobramycin derivative that exhibit improvements in permeation of corneal cells, retention in corneal cells, or both. Such compositions can comprise tobramycin or tobramycin derivative(s) complexed to an agent which facilitates improved permeation of corneal cells, retention in corneal cells, or both. The invention also relates to a process of preparing such compositions.
Owner:SOMERSET THERAPEUTICS LLC
Health-care nebula-eliminating eye drops
InactiveCN113456677ARelieve pressureRelieve fatigueSenses disorderHydroxy compound active ingredientsTobramycinEye drop
The invention discloses a health-care nebula-eliminating eye drops. The health-care nebula-eliminating eye drops are prepared from the following components in parts by weight: 300 to 500 parts of distilled water, 15 to 25 parts of potassium chloride, 1 to 3 parts of zinc sulfate, 2 to 4 parts of menthol, 0.005 to 0.015 part of tobramycin, 0.005 to 0.015 part of propolis and 5 to 10 parts of traditional Chinese medicine extract. Compared with the prior art, the health-care nebula-eliminating eye drops have the advantages that intraocular pressure can be reduced, eye fatigue can be relieved, corneal cell death can be reduced, saponin containing the traditional Chinese medicine extract can melt dead corneal cells, accumulation of dead cells can be reduced, generation of nebula can be promoted to be reduced, and the ealth-care nebula-eliminating eye drops have a very small amount of a mixture of traditional Chinese medicines and antibiotics, the effect can be amplified by more than 100 times, bacterial breeding can be reduced, virus infection can be prevented, and aged corneal cells can be promoted to recover and grow.
Owner:张坤镛
Preparation method of tissue engineering cornea
The invention relates to a preparation method of a tissue engineering cornea, comprising the following steps of: by using epidermal stem cells and pluripotent stromal stem cells as seed cells, planting on the two surfaces of an acellular natural cornea stroma after in-vitro amplification culture; and then forming the tissue engineering cornea through in-vitro induction culture. A scaffold used inthe preparation method not only overcomes the problem of serious immunological rejection of an xenogeneic cornea stroma, but also retains the structure (capable of recovering the transparency of the cornea) and main components (comprising a growth factor capable of promoting the growth, the proliferation and the differentiation of cornea cells) of a natural cornea. Compared with a product of the prior art, the invention has low cost, simple and convenient operation, extensive sources and easy storage; and in addition, the tissue engineering cornea containing living cells has certain elasticity and toughness, easy changes on shape, size and thickness and extremely low immunogenicity, can prevent complications caused by non-corneal materials and be rebuilt by recipient cells when implanted in vivo and rapidly integrated with an organism to realize transparency and is suitable for repairing corneal injury caused by various reasons.
Owner:SHAANXI RUISHENG BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com