Synthetic graft
A composition and compound technology, applied in the direction of eye implants, microorganisms, animal cells, etc., can solve the problems of uncharacterized, amnion lack of polymer structure scalability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Isolation of Keratocytes Using the Initial Spheroid Formation Assay
[0108] Normal bovine eyes were obtained from a local abattoir (Chity Wholesale Abattoir, Guildford, UK) within 2 hours of death, transported to the laboratory at 4°C and used immediately. The corneoscleral button was incised using standard eye bank techniques. Briefly, corneal-scleral tissue was rinsed three times with Dulbecco's minimal essential medium (DMEM, GIBCO). After careful removal of the central cornea, excess sclera, iris, corneal endothelium, conjunctiva, and eye capsule, the remaining rim (limbal rim) is cut to approximately 25mm 2 small pieces. Limbal stromal keratocytes and epithelial cells were subsequently isolated from these sheets. For limbal stromal keratocyte isolation, limbal pieces were incubated overnight at 37°C with 0.02% collagenase (GIBCO). The remaining limbal stroma sheets were then collected and treated with 0.2% EDTA (Sigma, UK) for 5 minutes at 37°C, fol...
Embodiment 2
[0109] Example 2: Differentiation of sphere colonies
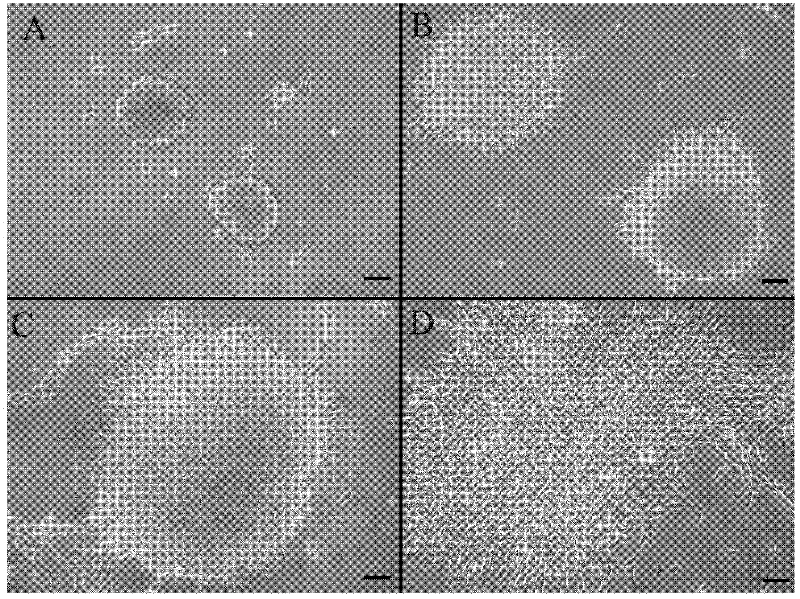
[0110] Initial spheres were formed from suspended limbal keratocytes and were transferred to glass coverslips coated with 50 μg / ml poly-L-lysine (Sigma, UK) and 10 μg / ml fibronectin (Sigma, UK) after 7 days in culture slices for microscopy studies. In order to promote the differentiation of limbal keratocytes, 1% fetal bovine serum was added to the basal medium, and the culture was continued for 7 days. The resulting differentiated keratocytes were digested in 0.25% trypsin and 0.02% EDTA (Sigma, UK) and 2.0 × 10 5 The density of cells is resuspended in basal medium.
[0111] When the limbic stroma was disaggregated into individual cells and cultured for 9 days, spheres of viable cells grew during this time. Photographs of representative spheres cultured at 5, 7, and 9 days, respectively, are in figure 1 Shown in A, 1B and 1C. Differentiated progeny from each initial sphere displayed typical fibroblast-like morphology...
Embodiment 3
[0112] Example 3: Formation of acellular collagen gels
[0113] Eagle's minimum essential Cell-free collagen gels were prepared with 4 mL of sterile rat tail type I collagen (First Link Ltd. West Midlands, UK) in medium (Gibco, Paisley, UK). The gel was cast into a rectangular model (33mm×13mm×4mm) and heated at 37°C in 0.5% CO 2 Freeze / stabilize for 30 minutes in the incubator. After solidification and incubation, the gel was compressed by a combination of compression and absorption using layers of nylon mesh and paper sheets (no additional wire mesh as used by Brown et al.). To achieve the compression of the gel, a layer of nylon mesh (50 μm mesh size) was placed on a double layer of absorbent paper, the collagen gel was placed on the nylon mesh and covered with a second nylon mesh, and a load of 50 g weight was added at room temperature Up to 5 minutes resulted in the formation of a flat collagen sheet (20-40 μm thick) protected between a double nylon mesh.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com