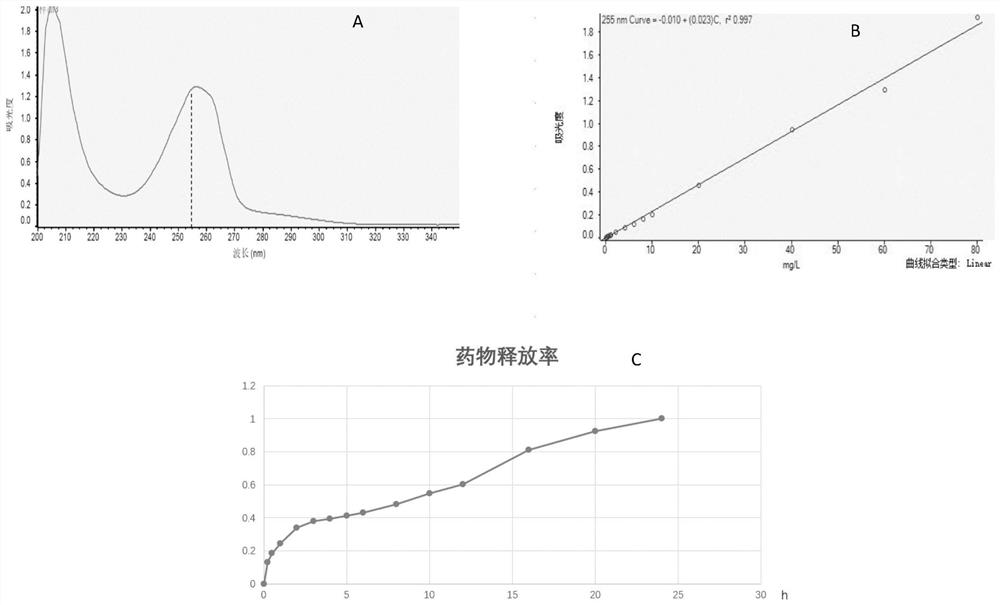
Patents
Literature
50 results about "Fungal keratitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A fungal keratitis is an 'inflammation of the eye's cornea' (called keratitis) that results from infection by a fungal organism. Keratomycosis is the Greek terminology equivalent of fungal keratitis - it is the fungal infection of the cornea, the anterior part of the eye which covers the pupil. Those experiencing these symptoms are typically advised to immediately visit the appropriate eyecare professional.
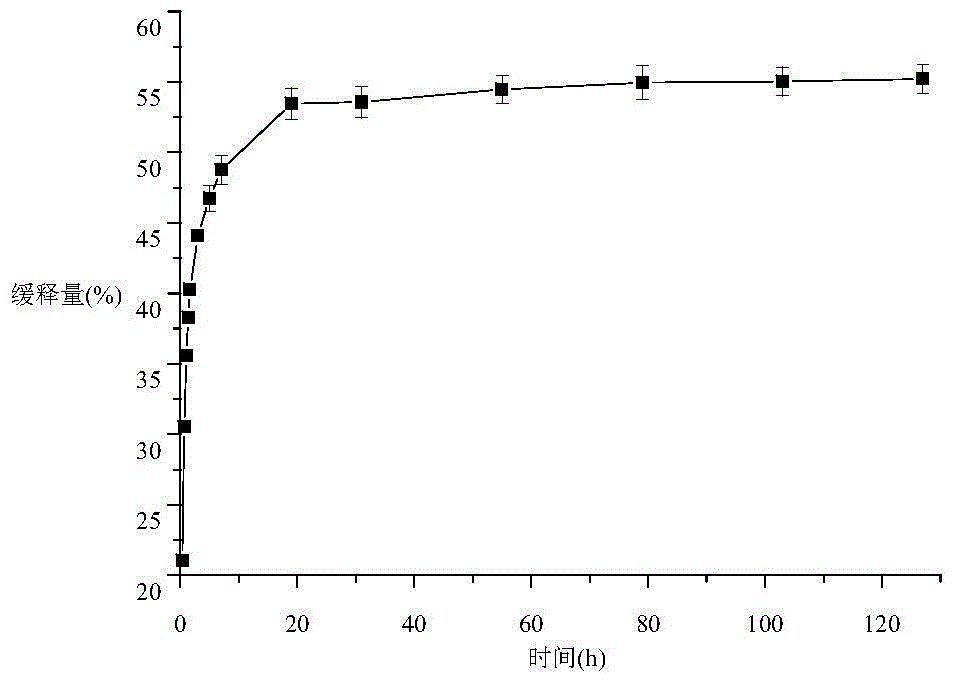
Long-acting slow release preparation for treating keratomycosis as well as preparation method and application thereof
ActiveCN104940936AHigh drug loadingFormulation stabilityOrganic active ingredientsSenses disorderAntifungalSide effect
The invention relates to a preparation for treating treating keratomycosis, and particularly relates to a long-acting slow release preparation for treating keratomycosis as well as a preparation method and application thereof. The substrate of the long-acting slow release preparation is formed through electrostatic bonding of a graphene material and a drug-carried chitosan material, drugs are carried on the sheet structure of the graphene material, the structure is stable, and the loading rate is high. The preparation provided by the invention has excellent bacteriostatic activity on fungus and bacterium, and is excellent in cytocompatibility, toughness, and tensile strength. The preparation can be simply and conveniently applied onto cornea, and has no toxic effects on normal tissue while achieving excellent bacteriostatic activity. The long-acting slow release preparation is taken as a dosage form in ophthalmology, can be adhered onto a cornea of a patient for long-acting slow release, so as to achieve effective drug concentration, and the preparation has the advantages that the preparation is simple and convenient, the drug-carried material self is excellent in bacteriostatic activity and mechanical property, stimulation and toxic or side effects on orbital tissue can be avoided, and the use is convenient.
Owner:SOUTH CHINA AGRI UNIV
Treatment for microbe-induced inflammatory responses in the eye
InactiveUS20130274216A1Reduce inflammationMinimizing suppressionBiocideSenses disorderMicroorganismOphthalmology
The present invention relates to ophthalmic compositions comprising inhibitors of spleen tyrosine kinase (syk). The compositions are particularly well suited for the treatment of ophthalmic infection such as fungal keratitis. The compositions optionally comprise an antiinfective compound such as an antibacterial or antifungal compound. The present invention also relates to methods for treating fungal keratitis using compositions comprising syk inhibitors.
Owner:NOVARTIS AG
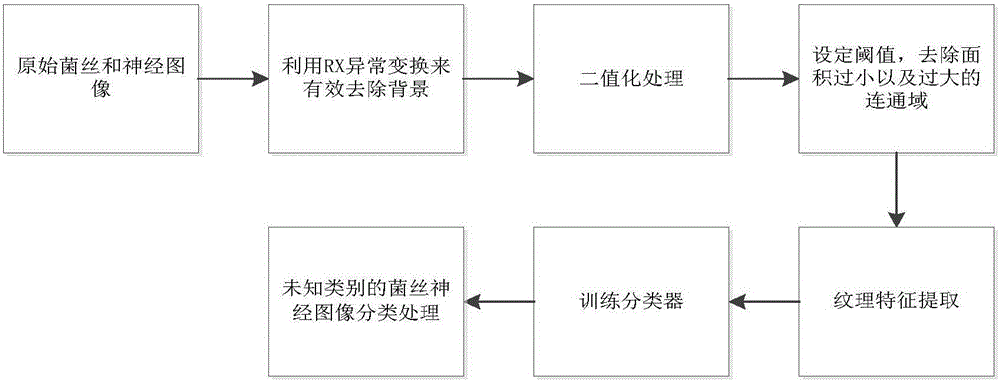
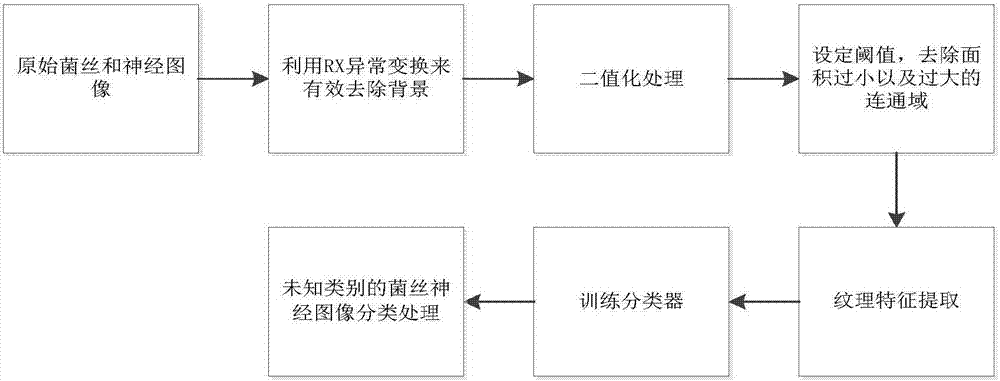
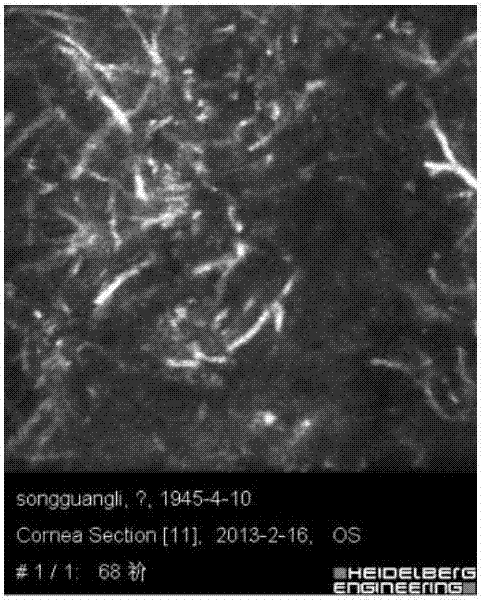
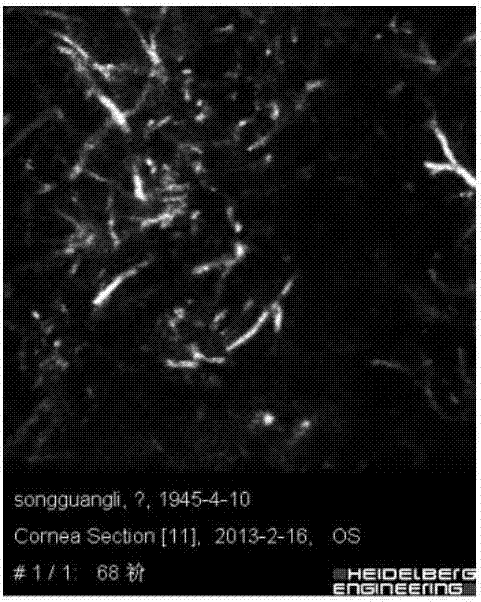
Fungal keratitis image identification method based on AMBP improved algorithm
ActiveCN105809188AEliminate distractionsFacilitating the feature extraction stepRecognition of medical/anatomical patternsPattern recognitionMycelium
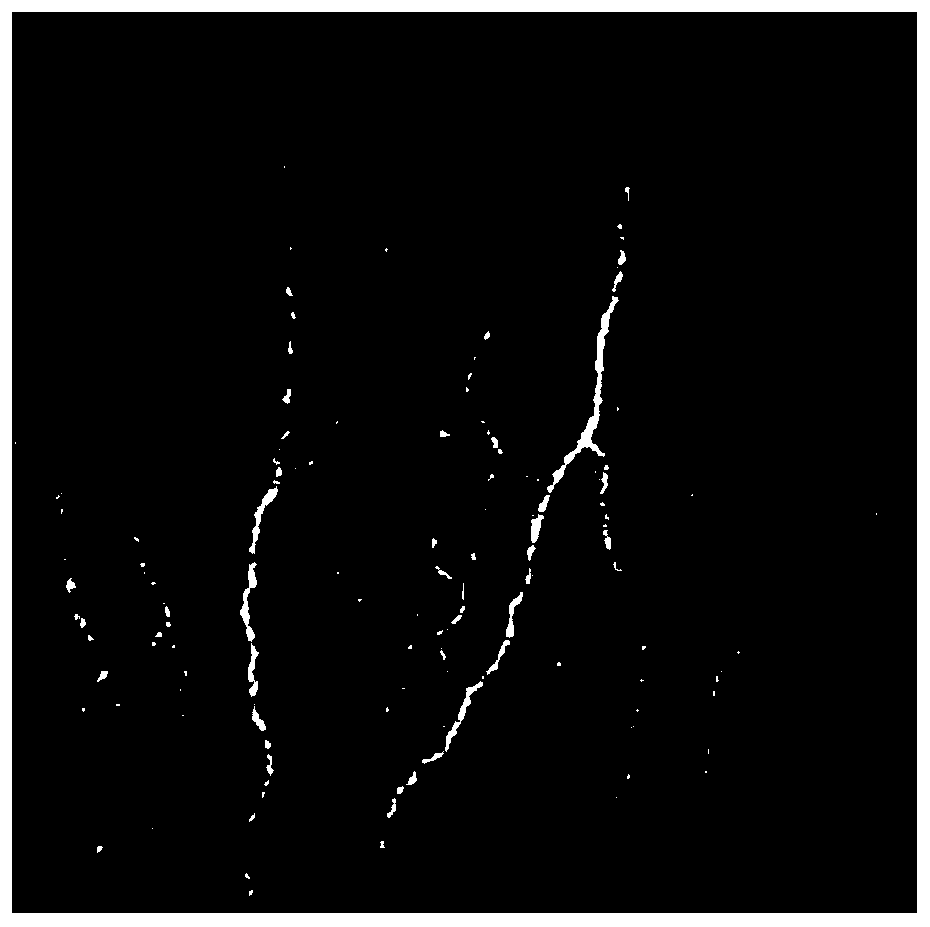
The invention discloses a fungal keratitis image identification method based on an AMBP improved algorithm. The method comprises the following steps: performing preprocessing and binaryzation on a mycelial image and a normal cornea nerve image by use of an RX abnormity detection algorithm, and accordingly, carrying out expansion corrosion processing to reinforce mycelium and nerve characteristic information in the images; improving an AMBP algorithm, calculating a mean value of other pixels apart from a center pixel in an analysis window, and taking the mean value as a new parameter; respectively solving an average value of the pixels in the analysis window and a median pixel, calculating variances between the two and other pixels in the analysis window, and by taking the sizes of differences as newly added discrimination conditions, extracting image texture characteristics; and training a classifier by use of the extracted characteristics, identifying images obtained by a confocal microscope by use of the classifier, and identifying nerves and mycelia in the images.
Owner:SHANDONG UNIV
Eye drop of povidone iodine and preparation process thereof
InactiveCN1965857ASignificant effectNo side effectsAntibacterial agentsSenses disorderSide effectCurative effect
The invention relates to a polyketone iodine eyedrops, and relative production, wherein it adds polyketone iodine into osmotic pressure adjuster, stabilizer, and pH adjuster to be dissolved, and using pH adjuster to realize constant volume; pH adjuster at 100 volume units contain 0.1-0.5 units of polyketone iodine, 0.35-0.65 osmotic pressure adjuster mass units, stabilizer at 0.05 mass units; the pH of osmotic pressure adjuster is 6.0-6.8. The invention can treat kinds of ceratitis, with high safety but no side effect.
Owner:WUHAN YIFAN BIO PHARMA TECH
Convolutional neural network-based fungal keratitis detection method and system
InactiveCN109829901ARealize automatic matching identificationImprove recognition rateImage analysisMedical automated diagnosisBack propagation algorithmData entry
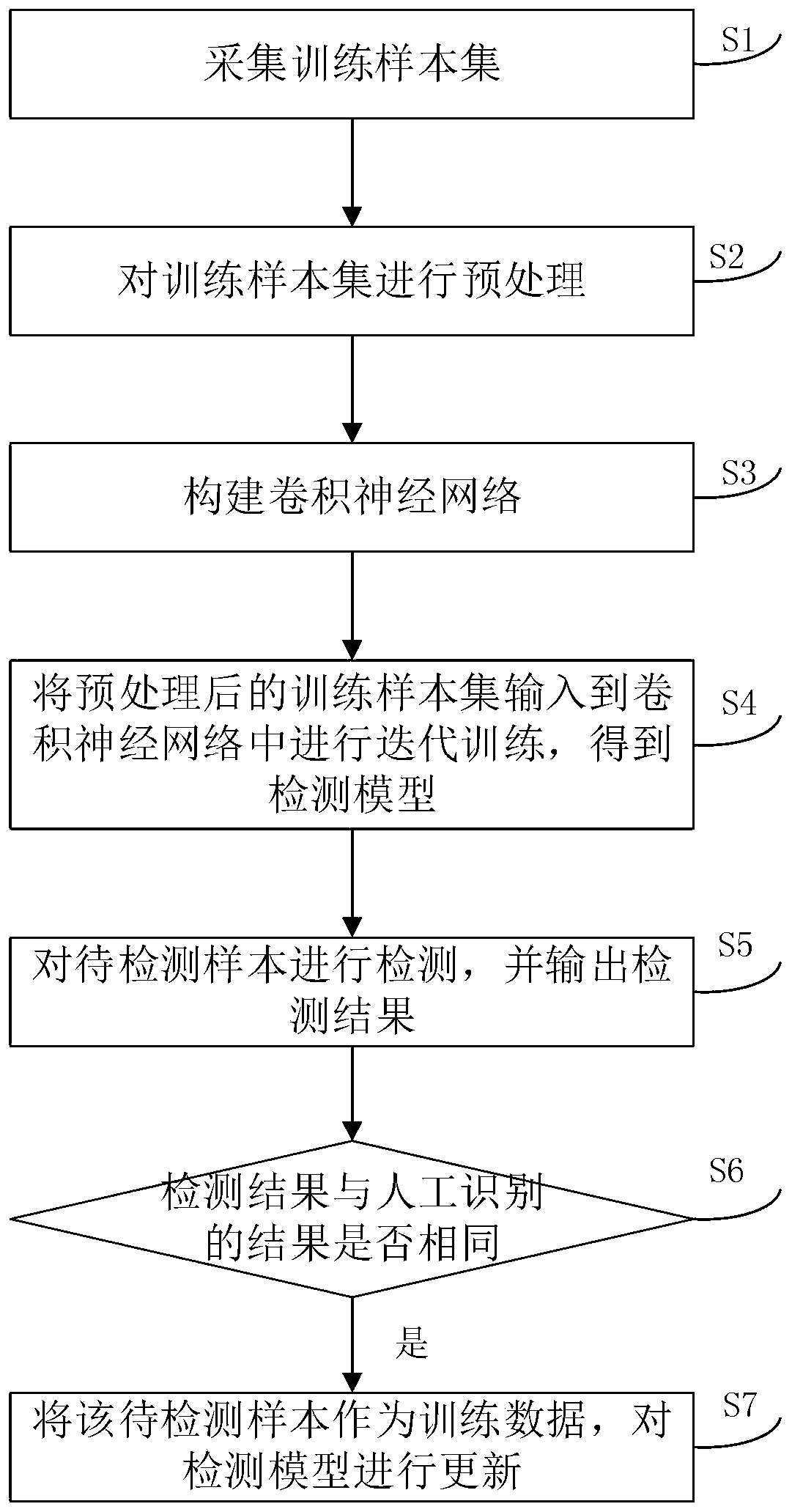
The invention discloses a convolutional neural network-based fungal keratitis detection method and system, and the method comprises the following steps: collecting a training sample set, and dividingthe training sample set into a fungal mycelium picture set and a fungal mycelium-free picture set; preprocessing the training sample set; constructing a convolutional neural network; Inputting the preprocessed training sample set as training data into the convolutional neural network, and performing iterative training through a gradient descent method and a back propagation algorithm to obtain a detection model with a fungal mycelium recognition function; and detecting a to-be-detected sample by using the detection model, and outputting a detection result. The detection method provided by theinvention has the advantages of high recognition speed and high recognition rate.
Owner:武汉爱尔眼科汉口医院有限公司
Composition for treating eye's fungal and bacterial infection
InactiveCN1887352AGood treatment effectAntifungal highAntibacterial agentsHeavy metal active ingredientsBacteroidesAntioxidant
The present invention discloses one kind of composition for treating eye's fungal and bacterrial infection. The composition has at least one of mercury preservatives, including merthiolate, merphenyl nitrate, merphenyl nitrate alkali, phenylmercuric acetate, phenylmercuric borate, etc as well as their salts and derivatives as the main active component; supplementary material including antioxidant, pH regulator and osmotic pressure regulator; and water as the solvent and carrier. The composition may be prepared into different preparation forms for treating eye's fungal and bacterrial infection.
Owner:徐艳
Common ocular surface disease diagnosis system based on intelligent terminal
PendingCN111700582ARapid assessmentConvenient remote self-diagnosisEye diagnosticsDiseaseOcular surface disease
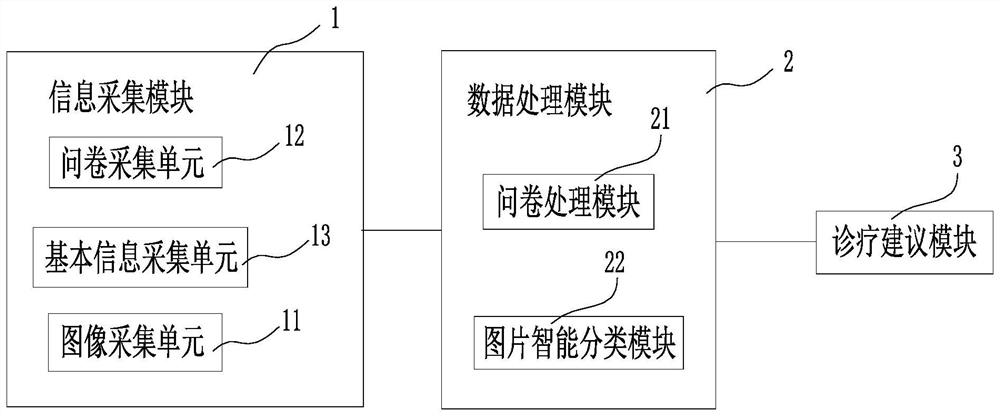
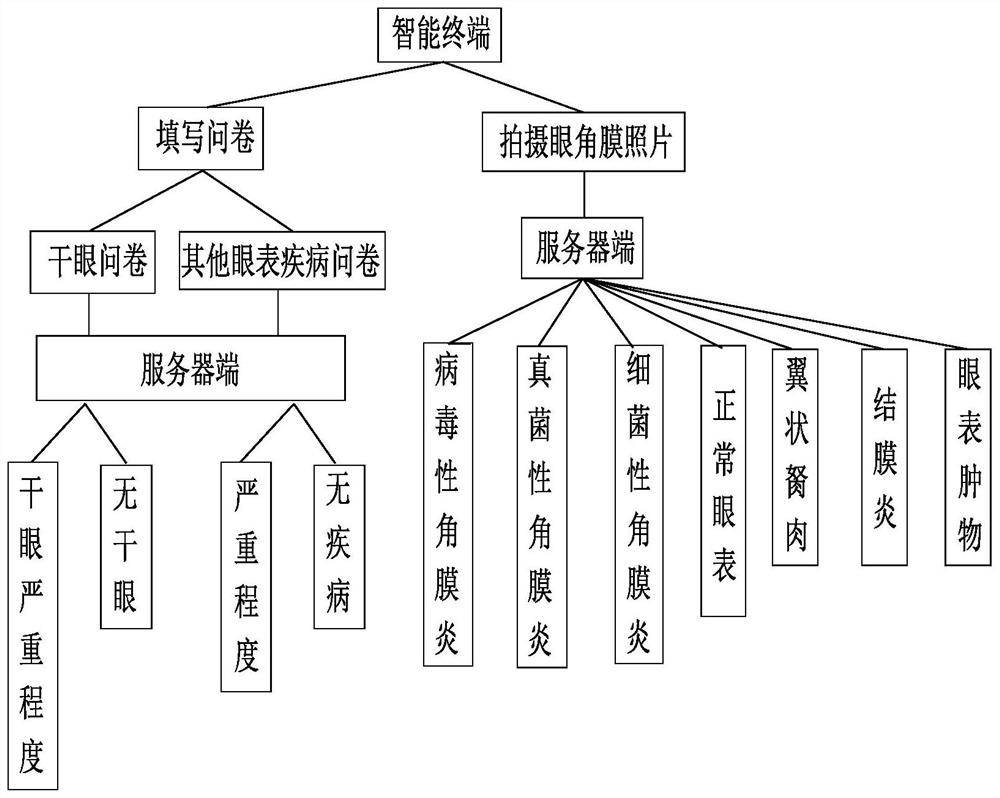
Disclosed is a common ocular surface disease diagnosis system based on an intelligent terminal. The common ocular surface disease diagnosis system comprises an information acquisition module, a data processing module and a diagnosis and treatment suggestion module, wherein the information acquisition module is installed on the intelligent terminal, and comprises an image acquisition unit, a questionnaire acquisition unit and a basic information acquisition unit; the data processing module is connected with the information acquisition module, and comprises a questionnaire processing module andan intelligent picture classification module, wherein the questionnaire processing module is used for receiving the questionnaire information input by the questionnaire acquisition unit and giving a corresponding ocular surface disease evaluation score; the intelligent picture classification module is used for classifying the input ocular surface pictures by using an ocular surface model stored inthe intelligent picture classification module or in a cloud end, and obtaining classification results of normal ocular surface, viral keratitis, bacterial keratitis, fungal keratitis, pterygium, conjunctivitis and ocular surface tumors; and the diagnosis and treatment suggestion module is connected with the data processing module, receives the evaluation score of the questionnaire processing module and / or the classification result of the intelligent picture classification module, and outputs a corresponding diagnosis and treatment suggestion according to the evaluation score and / or the classification result.
Owner:眼小医(温州)生物科技有限公司
Fungal keratitis image recognition method based on RX anomaly detection and texture analysis
ActiveCN104850861AEliminate distractionsFacilitating the feature extraction stepCharacter and pattern recognitionCorneal nerveAnomaly detection
The present invention discloses a fungal keratitis image recognition method based on RX anomaly detection and texture analysis. The method comprises a step of obtaining a normal corneal nerve image and a mycelium image which comprises mycelium only as a training sample, a step of obtaining the fundus image of a fungal keratitis patient as a test sample, a step of carrying out preprocessing, feature extraction and feature integration on the normal corneal nerve image in the training sample to obtain a nerve feature after training sample integration, a step of carrying out preprocessing, feature extraction and feature integration on the mycelium image which comprises the mycelium only in the training sample to obtain a mycelium feature after the training sample integration, a step of carrying out preprocessing, feature extraction and feature integration on the image in the test sample to obtain the nerve feature after test sample integration and the mycelium feature after the test sample integration, and a step of recognizing the nerve and mycelium in the test sample.
Owner:SHANDONG UNIV
Voriconazole-coated carrageenan corneal contact lens and preparation method thereof
PendingCN112999354AGood tissue compatibilityNon-immunogenicOrganic active ingredientsSenses disorderCarrageenanOphthalmic Dosage Form
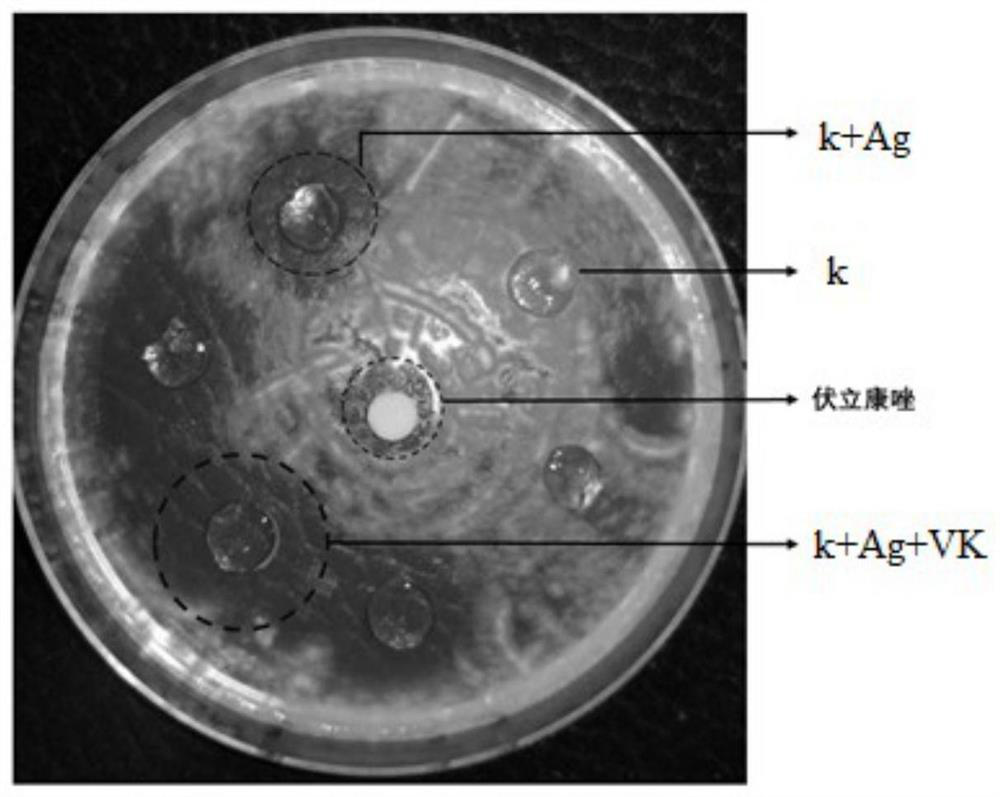
The invention relates to the technical field of ophthalmic dosage forms and medical materials, and relates to a voriconazole-coated carrageenan corneal contact lens which is prepared by the following components according to the content: sequentially adding a silver salt aqueous solution and a voriconazole solution into a carrageenan aqueous solution, wherein the mass volume ratio of the carrageenan aqueous solution to the silver salt aqueous solution to the voriconazole solution is 10 g: (5-15) microliter: (7-13) microliter, the mass percent concentration of the carrageenan aqueous solution is 0.99-10%, the voriconazole solution is a voriconazole DMSO solution with the mass concentration of 0.1-5 mg / microliter, and the silver salt aqueous solution is a silver nitrate aqueous solution with the molar concentration of 0.1 mol / liter; the added silver ions can promote gelation of carrageenan, and the silver elementary substance can kill fungi, so that the dosage of voriconazole is reduced, the drug release time can be prolonged to 24 hours, the frequency of eye administration is reduced, the local eye stimulation symptom is relieved and the treatment effect on fungal keratitis is improved.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Medicinal composition with neticonazole hydrochloride
ActiveCN108186639AReduced responseReduces the appearance of hyperpigmentationOrganic active ingredientsSenses disorderChemical compositionActive component
The invention discloses a medicinal composition with neticonazole hydrochloride. The medicinal composition uses the neticonazole hydrochloride and cetylpyridinium chloride as medicinal active components, wherein the weight ratio of the cetylpyridinium chloride to the neticonazole hydrochloride in the medicinal composition is 1 to (0.1 to 1,000), is preferably 1 to (10 to 100), and is further preferably 1 to (20 to 70). The medicinal composition can be prepared in a suitable medicinal dosage form for administration, which is preferably an eye drop, a cream and a tablet. The medicinal composition is particularly effective for the treatment of fungal keratitis, and not only are the administration dosages of two medicines decreased and is the obvious synergistic effect reflected, but also themedicinal composition has a certain treatment advantage compared with other similar medicines on the market.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD +1
Eye ointment for treating fungal keratitis
InactiveCN102526051AExtended retention timeAntifungal effect is obviousSenses disorderAntimycoticsTreatment effectSide effect
The invention discloses eye ointment for treating fungal keratitis. The eye ointment is a mixture containing terbinafine hydrochloride and atropine. The eye ointment solves the problems that the anti-fungal eye medicine lacks and the conventional anti-fungal eye drop is expensive and short in persistence time in eyes, has a poor anti-fungal curative effect and cannot relieve secondary iridocyclitis of the fungal keratitis and the like. The invention has the advantages that: the eye ointment has long persistence time in the eyes and an obvious anti-fungal curative effect, the effective rate reaches 98 percent, and the eye ointment has a treatment effect on the secondary iridocyclitis, obviously relieves the eye pain symptom of a patient and does not have obvious toxic or side effect; the preparation method is simple, and has the feasibility of industrialized production; and the eye ointment is expected to play an important role in treating the clinical fungal keratitis.
Owner:HARBIN MEDICAL UNIVERSITY
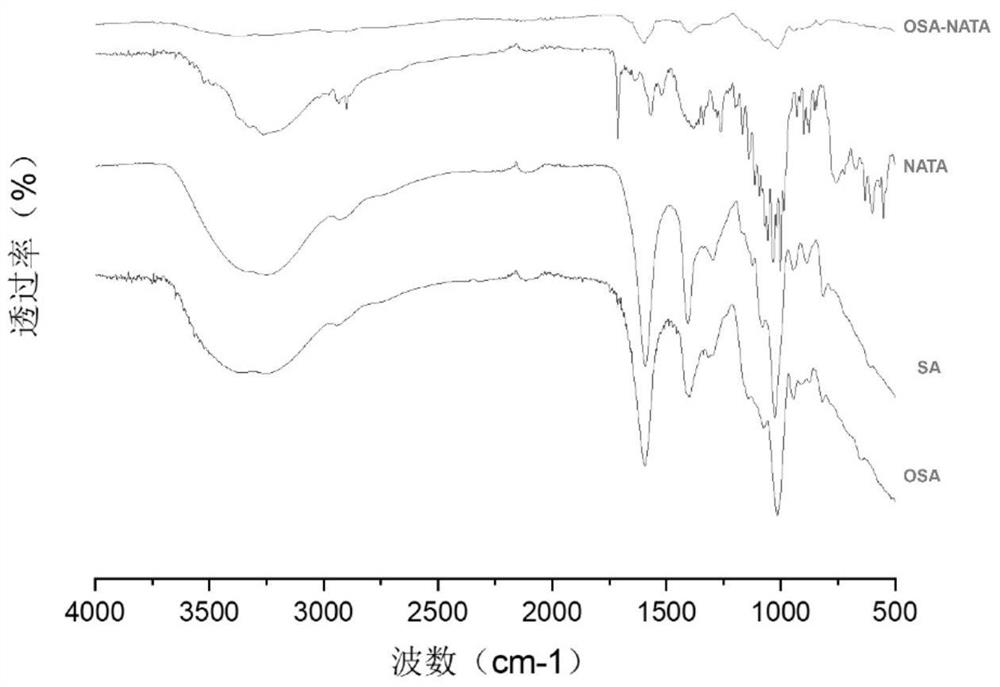
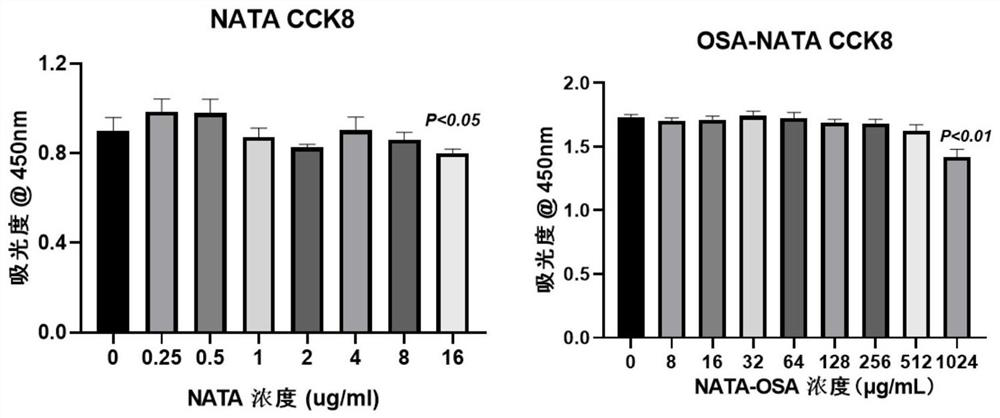
Oxidized sodium alginate modified natamycin eye drops and preparation method thereof
PendingCN113081956AGood tissue compatibilitySimple preparation processOrganic active ingredientsSenses disorderEyes irritationEye drop
The invention relates to the technical field of medical materials, in particular to oxidized sodium alginate modified natamycin eye drops and a preparation method thereof. The oxidized sodium alginate modified natamycin eye drops has the component and content that 1 mL of sterilized deionized water contains 0.5-1.5 mg of oxidized sodium alginate-natamycin freeze-dried powder; the oxidized sodium alginate-natamycin is a product obtained by carrying out a Schiff base reaction on oxidized sodium alginate and natamycin; the preparation method comprises the following specific steps: preparing oxidized sodium alginate solid, preparing oxidized sodium alginate-natamycin medicine powder, and preparing the eye drops. The property of the oxidized sodium alginate is similar to that of a human extracellular matrix, so that the oxidized sodium alginate is good in histocompatibility and can be degraded into non-toxic polysaccharide which does not participate in metabolism; the water solubility and permeability of the natamycin are improved, the eye irritation and discomfort of the natamycin are reduced, and the treatment effect of the fungal keratitis is improved; the preparation process is relatively simple, low in cost and easy to store.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Fungal keratitis prophylactic or therapeutic agent
ActiveUS20180271841A1Organic active ingredientsSenses disorderBULK ACTIVE INGREDIENTPharmaceutical Substances
For preventing or treating keratomycosis, a medicament containing rapamycin or a salt thereof as an active ingredient is used.
Owner:SANTEN PHARMA CO LTD
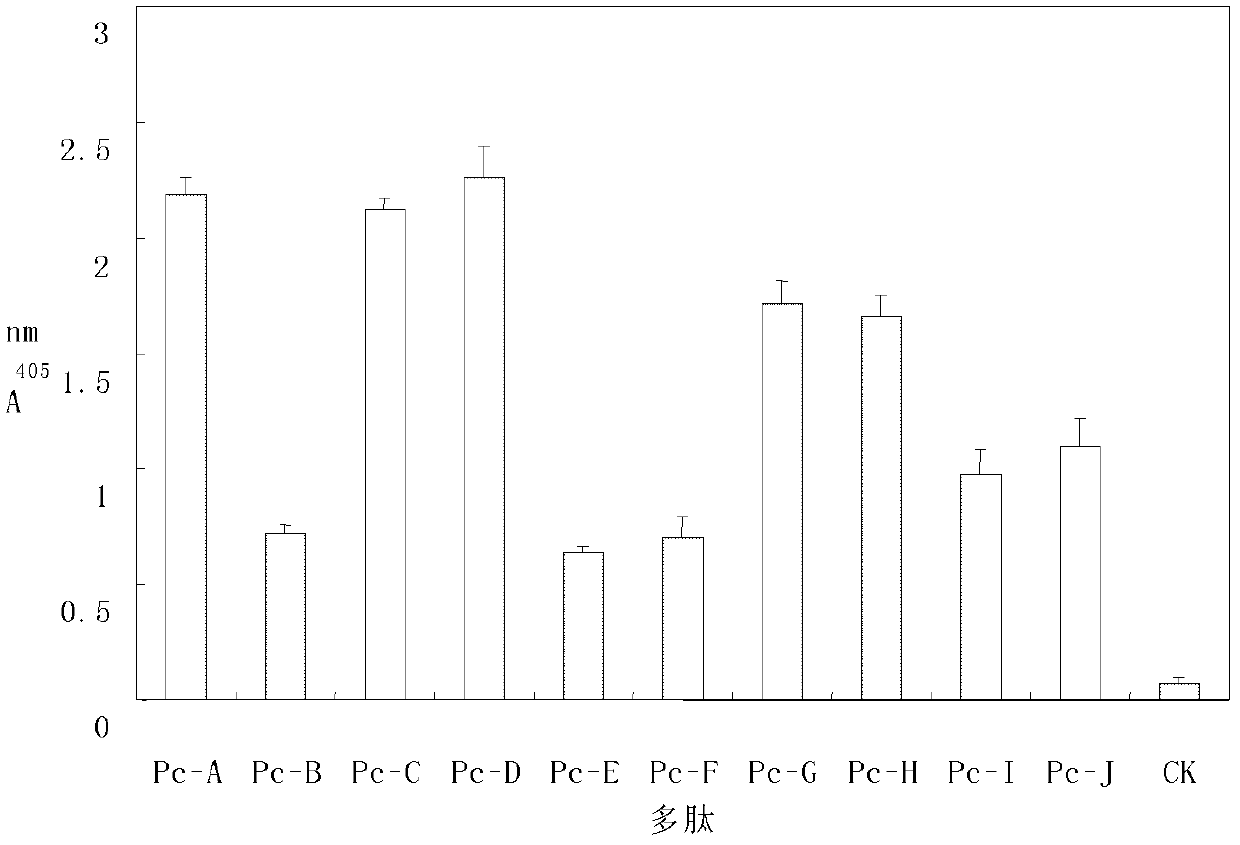
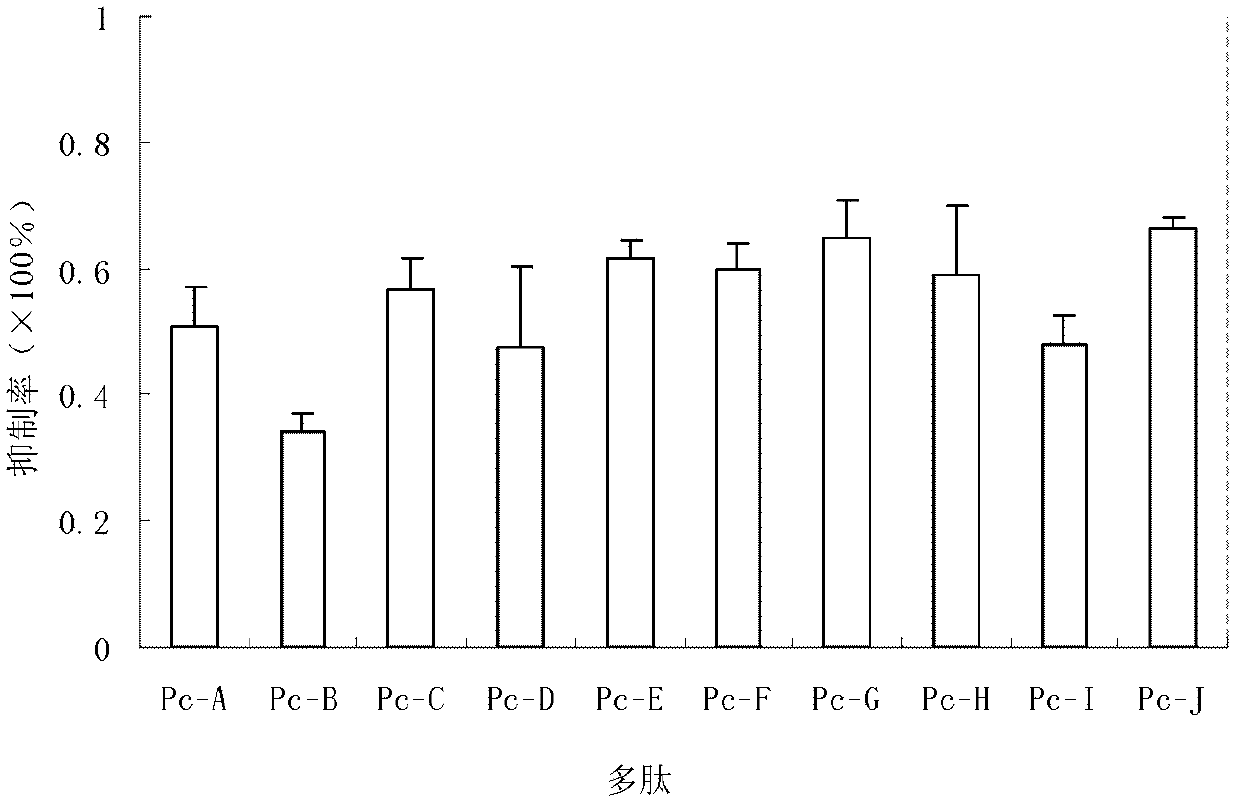
Peptides inhibiting adhesion of aspergillus fumigatus to cornea
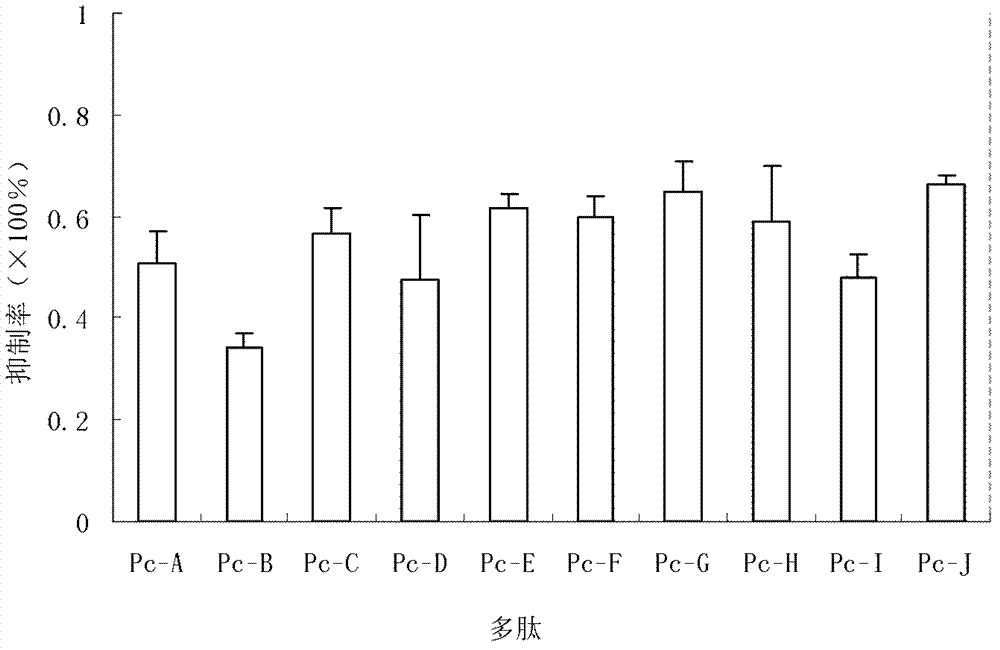
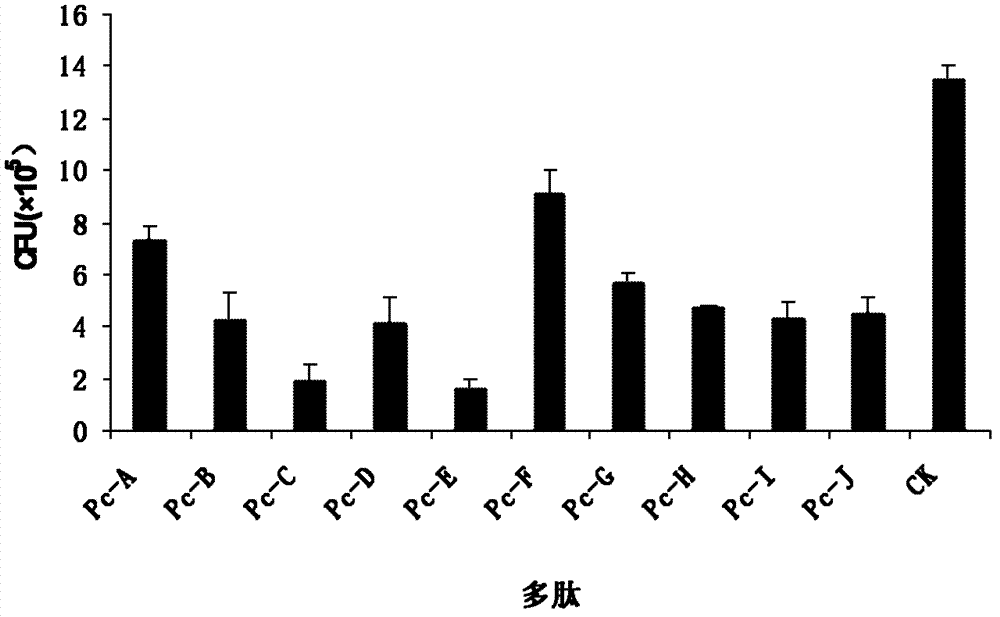
Provided are peptides inhibiting the adhesion of Aspergillus fumigatus to cornea, comprising: (1) peptide with the sequence of SEQ ID NOs: 1-10 respectively; (2) peptide having 70% or above homology to the peptide of (1) respectively, whose function is the same or similar with that of the peptide of (1). Also provided are polynucleotides. The polynucleotides are 1) a nucleotide of any peptide with the sequence of SEQ ID NOs: 1-10; 2) nucleotides of the peptides having 70 % or more homology with the peptide described in (1), and the encoded peptides have the same or the similar functions with the peptide described in (1). 10 peptides selected by the invention have competitive inhibitory effect on the interaction of the Aspergillus fumigatus and epithelial cells of the cornea in different degrees, can specifically block the adherence between ligands on the surface of the Aspergillus fumigatus and the epithelial cells of the cornea, and become new target spots for designing treatment drugs of fungal keretitis, thereby providing new ideas for clinical treatment thereof.
Owner:SHANDONG EYE INST
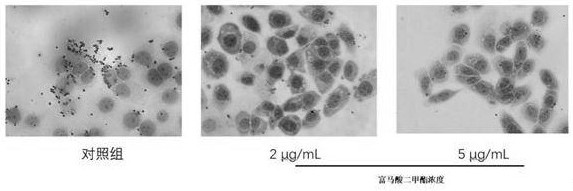
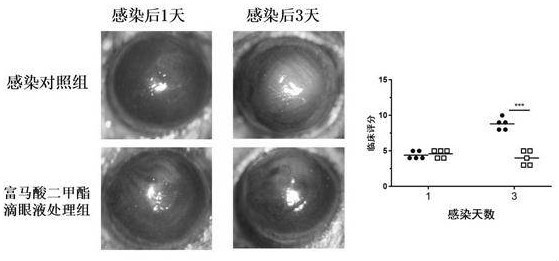
Dimethyl fumarate eye drops, preparation method thereof and application of dimethyl fumarate eye drops as medicine for treating fungal keratitis
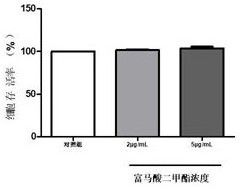
InactiveCN113143862AHigh cure rateNo adverse gastrointestinal reactionsOrganic active ingredientsSenses disorderAnti fungalEye drop
The invention discloses dimethyl fumarate eye drops, a preparation method thereof and application of the dimethyl fumarate eye drops as a medicine for treating fungal keratitis, and belongs to the technical field of pharmacy. According to the dimethyl fumarate eye drops disclosed by the invention, dimethyl fumarate is used as an effective component; the concentration of dimethyl fumarate is less than or equal to 5ug / mL; and the dimethyl fumarate eye drops can effectively kill pathogenic bacteria of fungal keratitis, does not irritate eyes, and does not generate adverse effects. The dimethyl fumarate eye drops disclosed by the invention can be applied as the medicine for treating fungal keratitis; the antifungal function is achieved by inhibiting the growth of fungi and weakening the adhesion ability of fungi to cells; the keratitis reaction is relieved by reducing the inflammatory reaction factor interleukin-1beta and the tumor necrosis factor-alpha, so that the dimethyl fumarate eye drops is greatly beneficial to fungal keratitis patients; and the cure rate on fungal keratitis reaches up to 95.7%.
Owner:顾凌雯 +1
Ocular surface in-situ drug and preparation method thereof
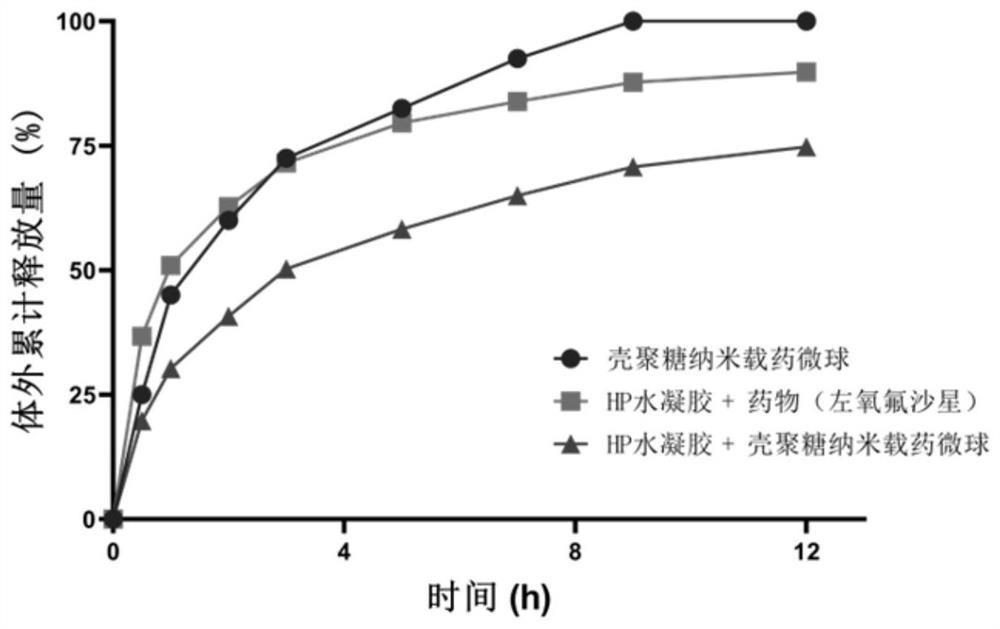
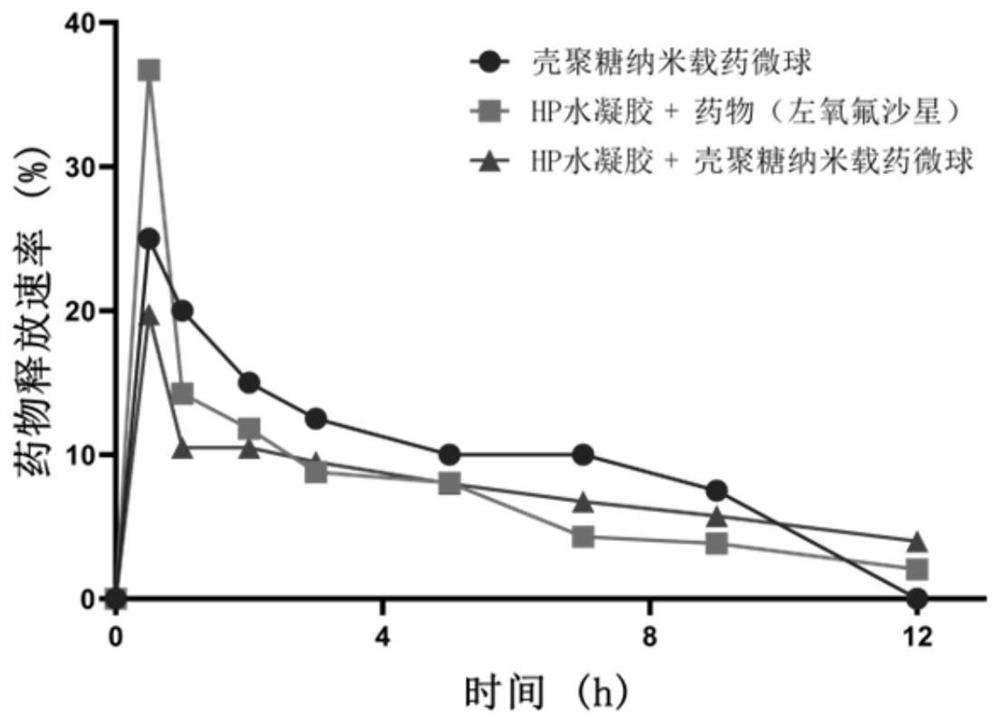
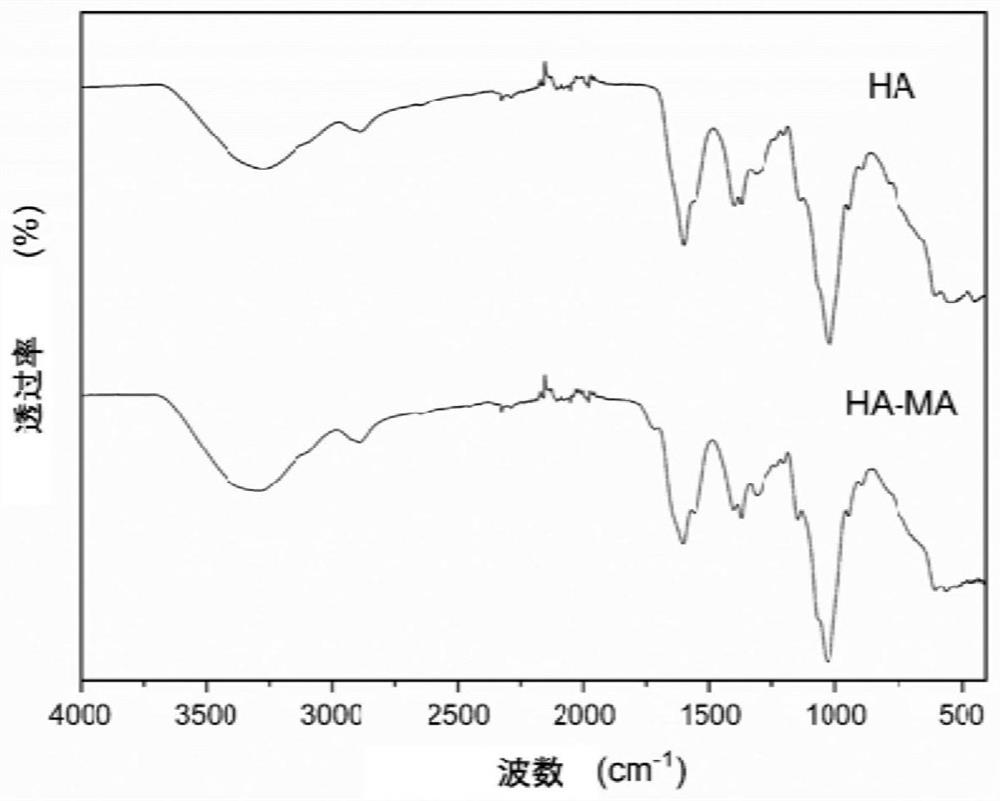
PendingCN113041215AGood biocompatibilityImprove the defect of poor biocompatibilityAntibacterial agentsOrganic active ingredientsMicrosphereCytotoxicity
The invention relates to an ocular surface in-situ drug and a preparation method thereof. The ocular surface in-situ drug comprises a hydrogel system which takes PluronicF127, PluronicF68 and methacrylic acid-hyaluronic acid (HA-MA) as main components and has a temperature response characteristic, and a nano drug-loaded microsphere which contains an active drug or wraps the active drug, in-situ gel is formed at the ocular surface temperature, and the drug release time is prolonged. By combining the hyaluronic acid derivative HA-MA with high biocompatibility and low cytotoxicity, a brand-new temperature-triggered hydrogel sustained-release system formula for eyes is provided, so that drug sustained release on the ocular surface is realized, and the treatment on bacterial / fungal keratitis and conjunctivitis is achieved; and meanwhile, the toxicity of the material is reduced, and the repair of corneal cells can be promoted during treatment.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
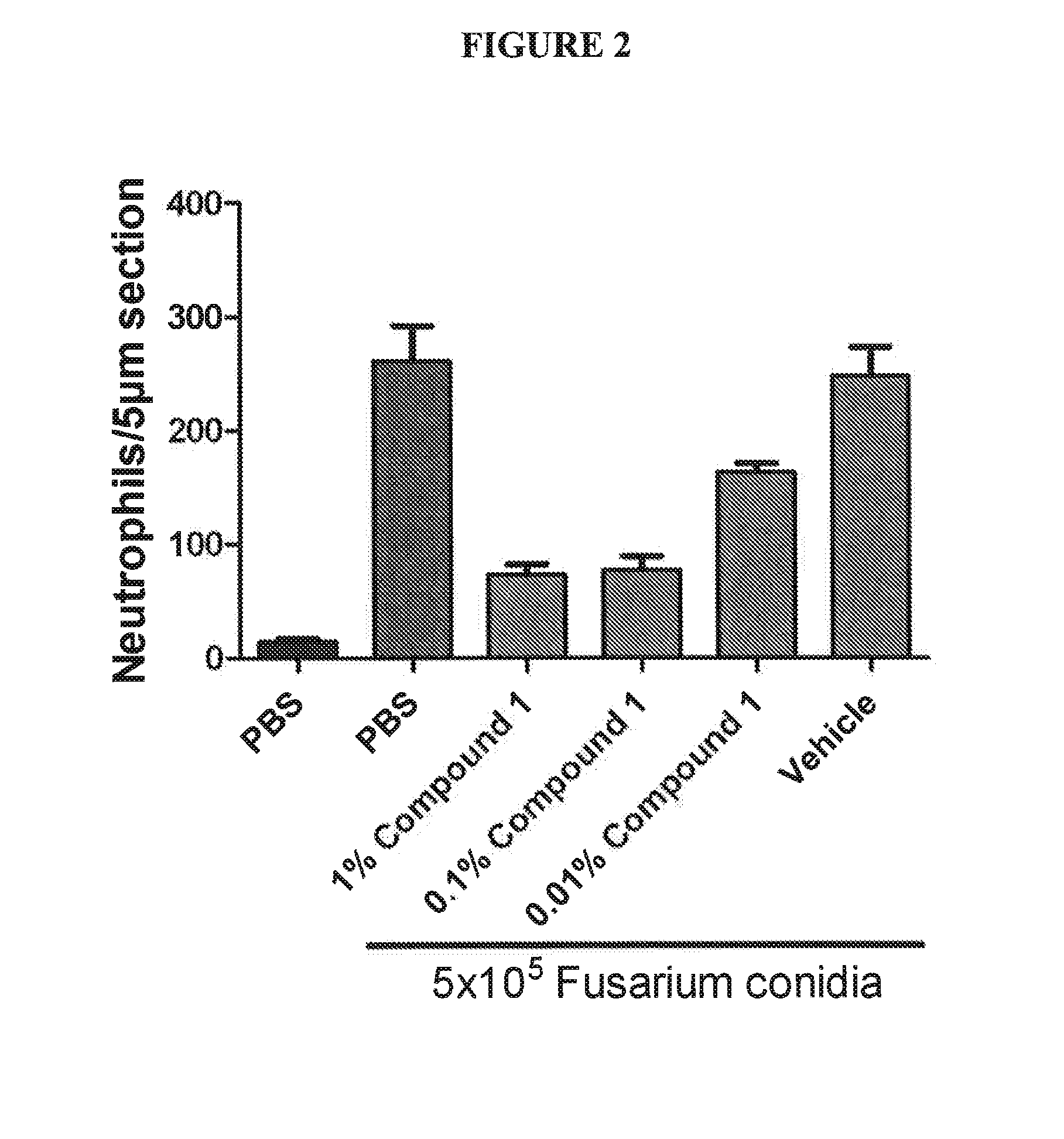
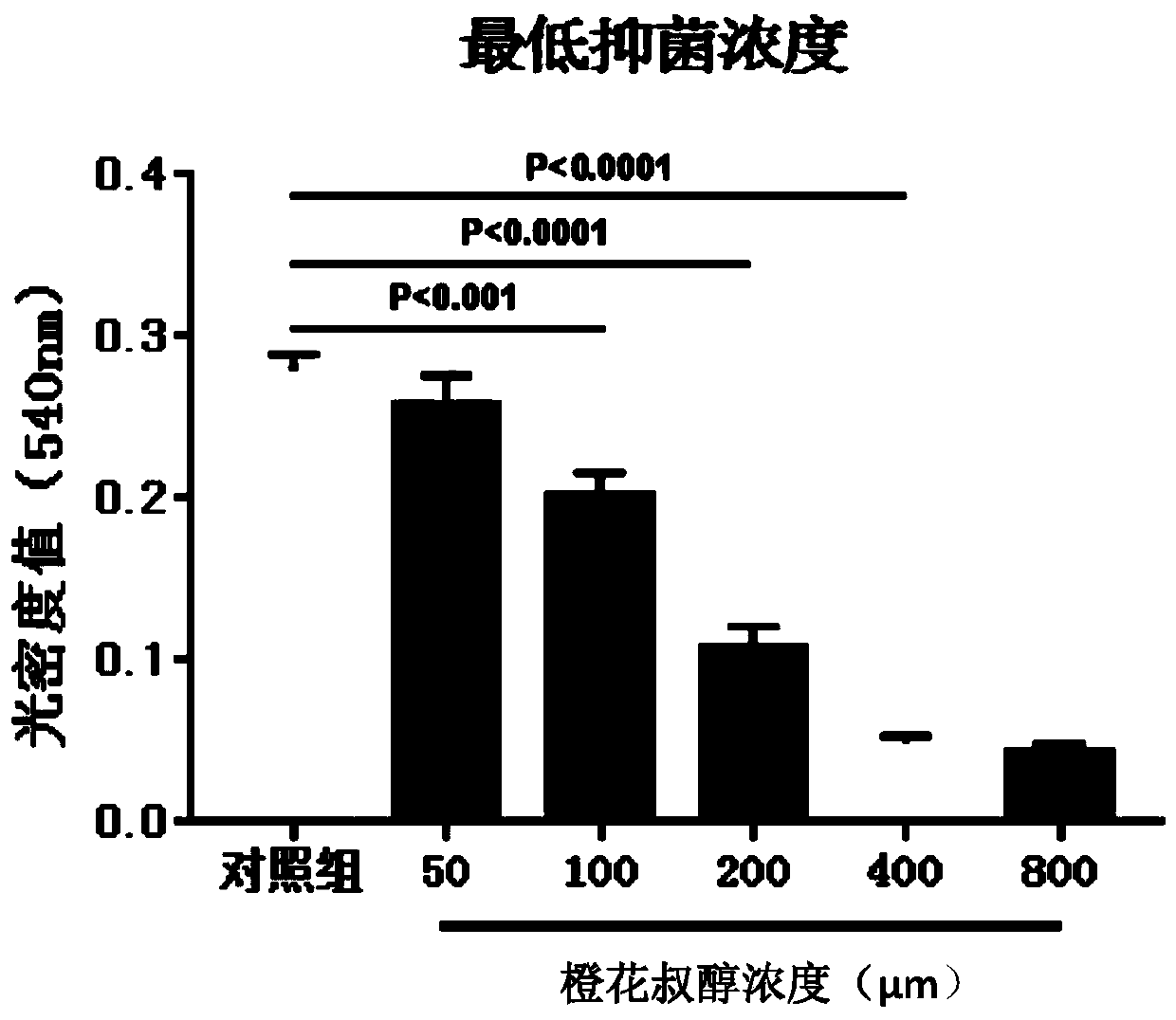
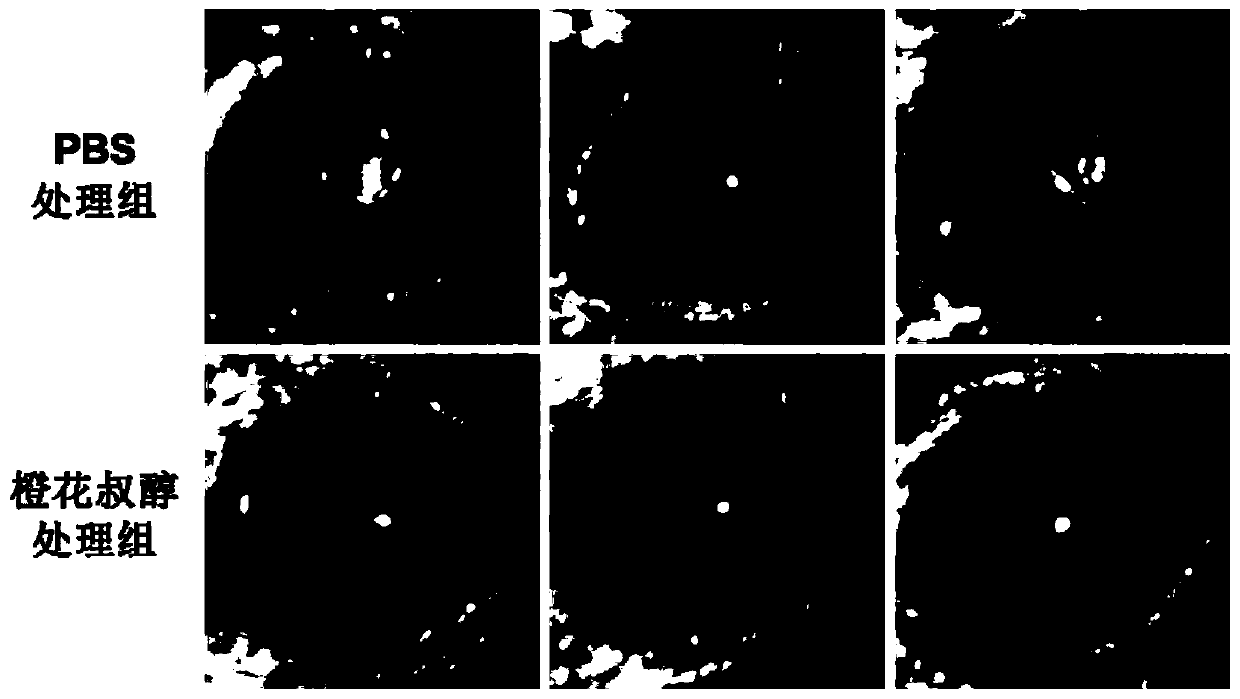
Application of nerolidol in preparing drug for treating fungal keratitis
The invention belongs to the technical field of pharmacy and discloses application of nerolidol in preparing drug for treating fungal keratitis. It is put forward for the first time that nerolidol caninhibit fungus growth and has treatment effect on fungal keratitis in vivo and in vitro. In in-vivo experiments, C57BL / 6 mice are adopted to build a fungal keratitis model to study impact of nerolidol on clinical scoring of model mouse cornea, neutrophil collection and human oxidized low-density lipoprotein receptor 1 and interleukin 1beta expression. In in-vitro experiments, inhibiting effect ofnerolidol at different concentrations on growth of aspergillus fumigatus is studied, and a human corneal epithelial cell model stimulated by aspergillus fumigatus is adopted to verify that nerolidolhas impact on LOX-1 / IL-1beta signal pathway.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
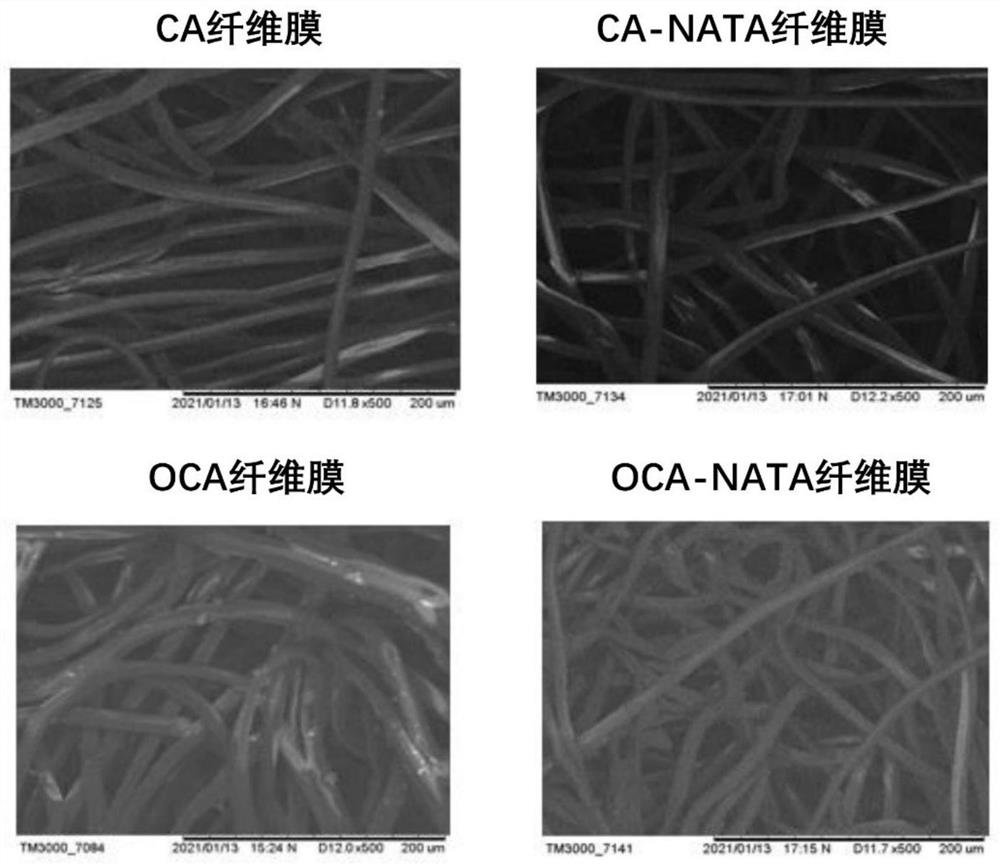
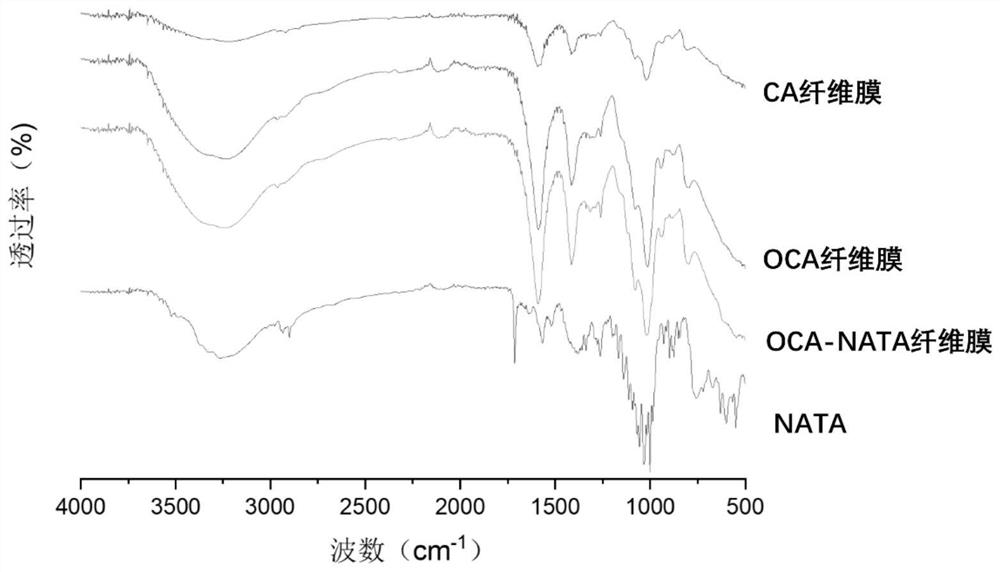
Natamycin-grafted oxidized alginic acid fiber membrane and preparation method thereof
ActiveCN113069556AReduce eye irritationLess discomfortOrganic active ingredientsSenses disorderFiberEye irritation
The invention relates to the technical field of medical materials, and relates to a natamycin-grafted oxidized alginic acid fiber membrane and a preparation method thereof. The preparation method comprises specific preparation process as follows: preparing oxidized alginic acid fiber: reacting sodium periodate with alginic acid fiber to obtain the oxidized alginic acid fiber; putting the oxidized alginic acid fiber into a glycerol aqueous solution to be soaked, conducting rinsing and drying, and putting the oxidized alginic acid fiber into a dry and sterile centrifugal tube to be stored; and soaking the oxidized alginic acid fiber with a natamycin aqueous solution, oscillating and incubating the oxidized alginic acid fiber at 50 DEG C in a dark place for 36 hours, then washing the oxidized alginic acid fiber twice with an ethanol aqueous solution and deionized water, and drying to obtain the oxidized alginic acid fiber membrane. The surface of the oxidized alginic acid fiber membrane is grafted with natamycin, so that the eye irritation and discomfort caused by the natamycin can be reduced, the half-life period of the medicine is prolonged, the utilization rate of the medicine is increased, and the treatment effect of the fungal keratitis is improved; and the preparation process is simple, the cost is low, and the product is easy to preserve and has wide market prospects.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Eye medicine for treating fungal keratitis
InactiveCN1843342AIncreased sensitivityPracticalOrganic active ingredientsSenses disorderCurative effectTerbinafine Hydrochloride
The invention discloses an eye medicament for treating fungal keratitis, which is the aqueous solution containing 0.1-0.5 wt% of terbinafine hydrochloride and 2.0-4.0 wt% of cyclodextrin.
Owner:王香兰
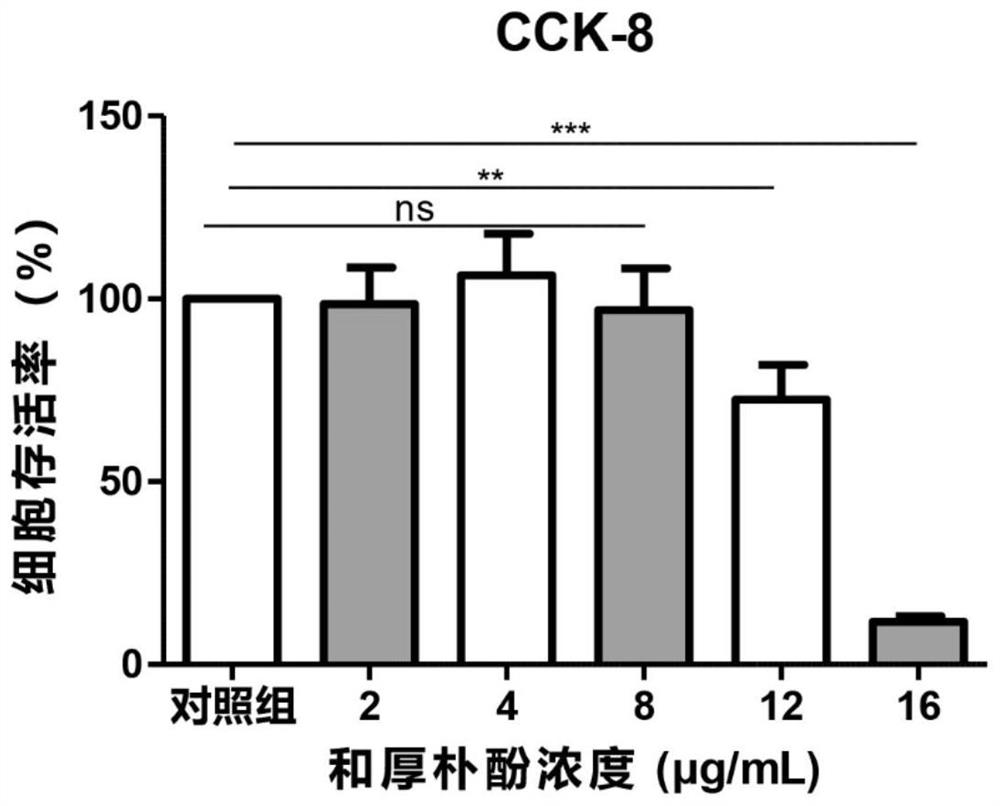
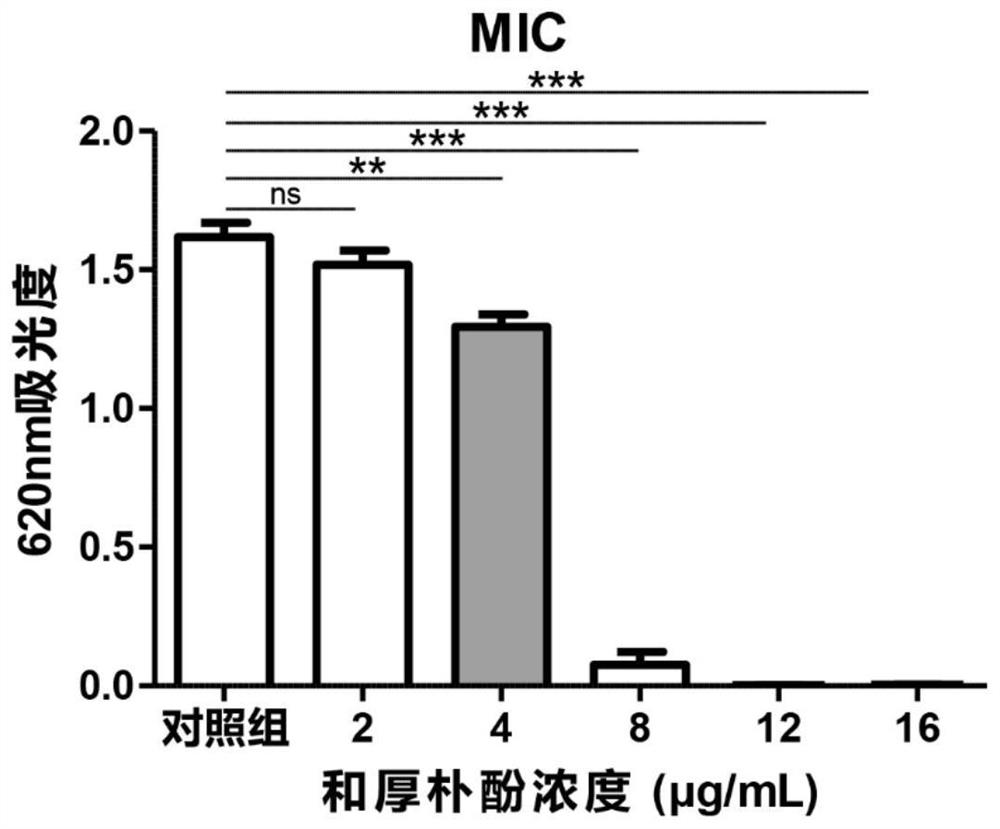
Preparation of honokiol ophthalmic medicine and application of honokiol ophthalmic medicine in treatment of fungal keratitis
PendingCN114869885AEfficient killingReduce inflammationSenses disorderAntimycoticsVitamin b6Tissue repair
The invention provides preparation of honokiol ophthalmic medicine and application of honokiol ophthalmic medicine in treatment of fungal keratitis, and relates to the technical field of medicine preparation. According to the preparation method of the ophthalmic medicine based on honokiol, honokiol eye drops are prepared, specifically, the honokiol eye drops comprise 130 [mu] g / mL of L-potassium aspartate, 6.5 [mu] g / mL of vitamin B6, 0.39 [mu] g / mL of naphazolin hydrochloride, 0.65 [mu] g / mL of neostigmine methyl sulfate, 0.16 [mu] g / mL of L-menthol and 8 [mu] g / mL of honokiol, and a substrate component is a sterile injection containing 5-10% of hydroxyethyl cellulose. The honokiol ophthalmic medicine disclosed by the invention takes honokiol as an effective component, can effectively kill pathogenic fungi of fungal keratitis as a medicine for treating fungal keratitis, does not damage corneal epithelium to generate a bad effect, does not cause toxic damage to ocular surface and promote tissue repair when being reasonably used, and has the advantages of simple preparation process, low cost and the like. The prognosis of a fungal keratitis patient is greatly facilitated.
Owner:战璐
Medical material for treating fungal keratitis and preparation method thereof
PendingCN112773778AHigh expressionEasy to handleOrganic active ingredientsSenses disorderBiotechnologyFreeze-drying
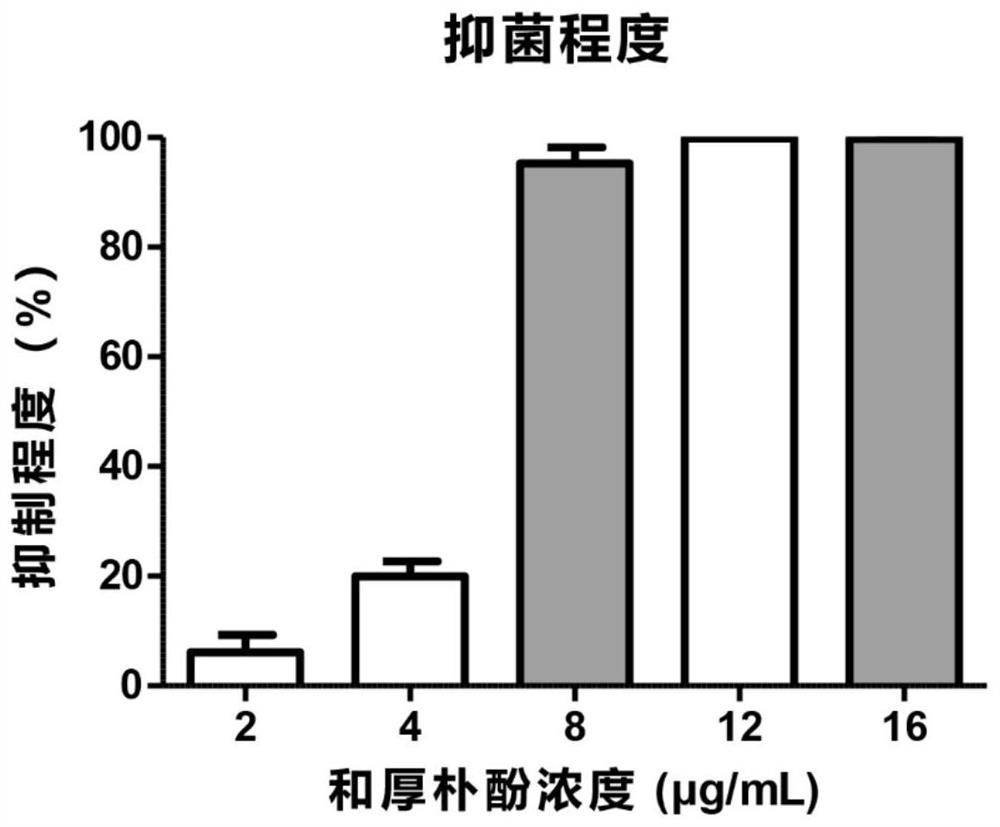
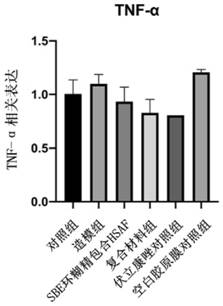
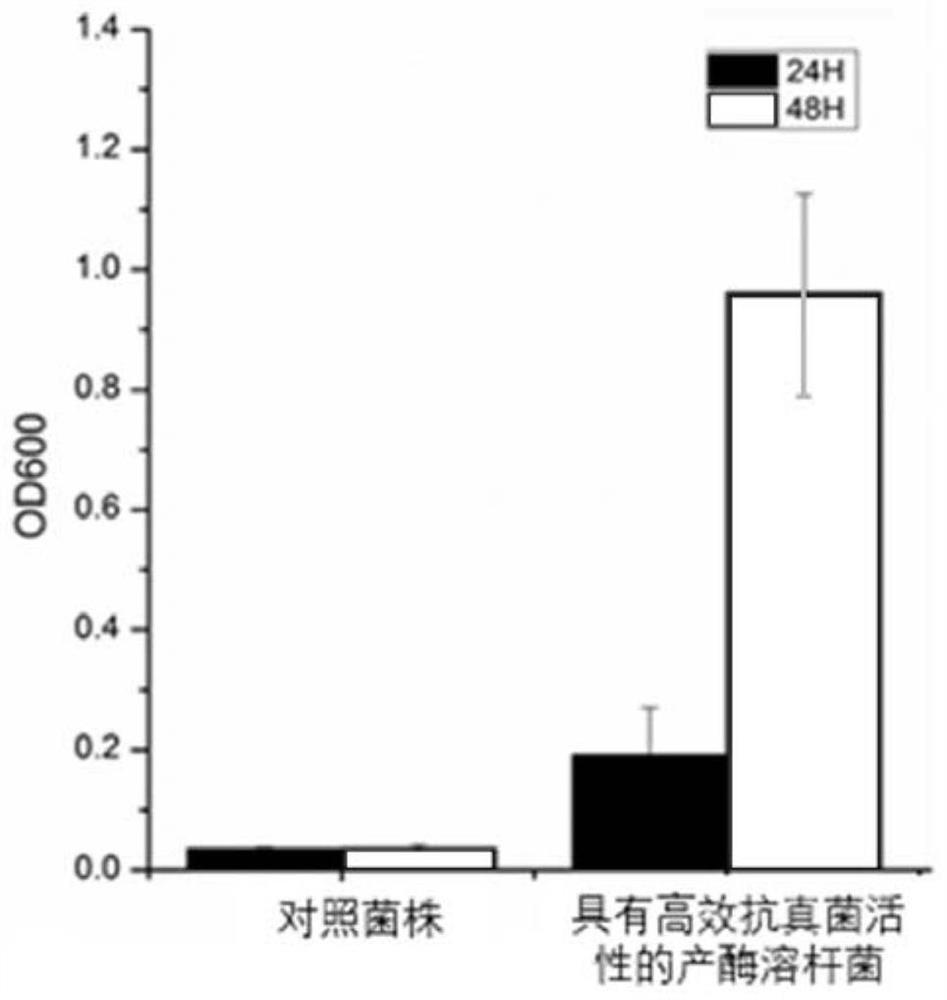
The invention provides a medical material for treating fungal keratitis and a preparation method thereof. The preparation method comprises the following steps: S1, preparing xanthomonas fermentation liquor; S2, separating and purifying the fermentation liquor in the step S1, and extracting dihydromaltophilia; S3, respectively preparing a dihydromaltophilia solution A, a sulfobutyl ether beta cyclodextrin solution B and a collagen solution C; S4, dropwise adding the solution A into the solution B to obtain a mixed solution I, and freeze-drying the mixed solution I to obtain composite mixed powder; S5, dissolving the compound powder obtained in the step S4 into water to obtain a solution D, and then dropwise adding the collagen solution C to prepare a mixed solution II; and S6, pouring the mixed solution II in the step S5 into a mould, and drying in a constant-temperature and constant-humidity environment to obtain the medical material disclosed by the invention. The composite material provided by the invention can realize loading of thermostable antifungal factors, can be released on ocular surfaces in a use process, and can be used for treatment of fungal keratitis in the medical field.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Method for making animal model simulated human cornea fungal infection
InactiveCN101168066AHigh success rate of preparationIn line with the clinical natural infection processOrganic active ingredientsAntimycoticsFungal hyphaeBiology
The invention discloses a making method of an animal model for imitating fungal infection of porcine cornea, composed of steps of preparing fungi strains, preparing inoculum, preparing animals, inoculating animals and processing after the inoculating. The invention has the advantages that model-making success rate is high, generally the success rate of outbred animals reaches over 85% and the success rate of inbred animals reaches over 90%, immunosuppressant is not necessary, fungal hyphaes are observed in pathological section of cornea among the second day and thirteenth day after inoculating, operational method is economical, simple and easy to apply, materials are easy to get, inoculating method is easy to master, researchers need no special technology, the invention is suitable for the process of clinical natural infection of fungal keratitis, and is adaptable for the research of the generation, development and the mechanism of infection.
Owner:王丽娅
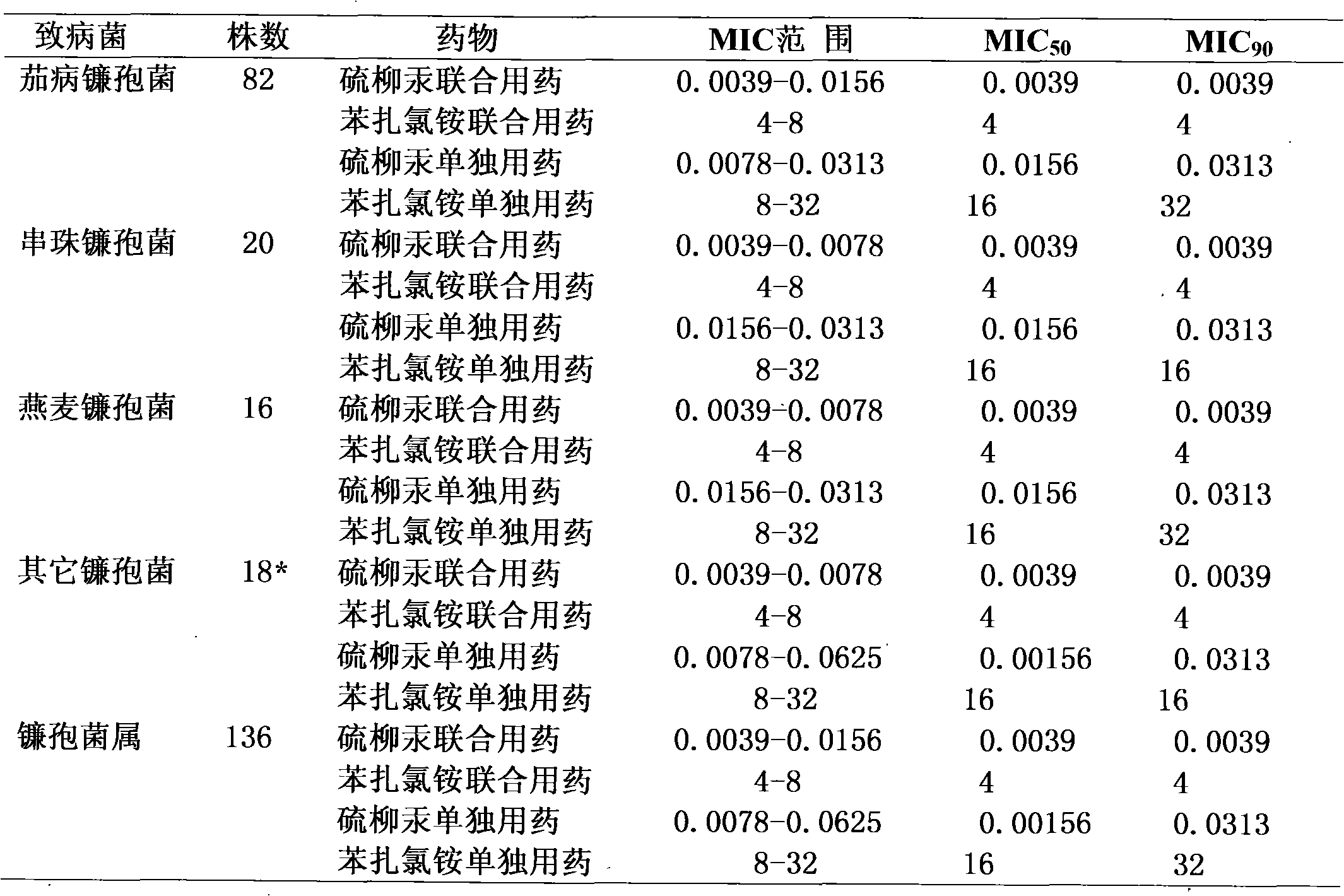
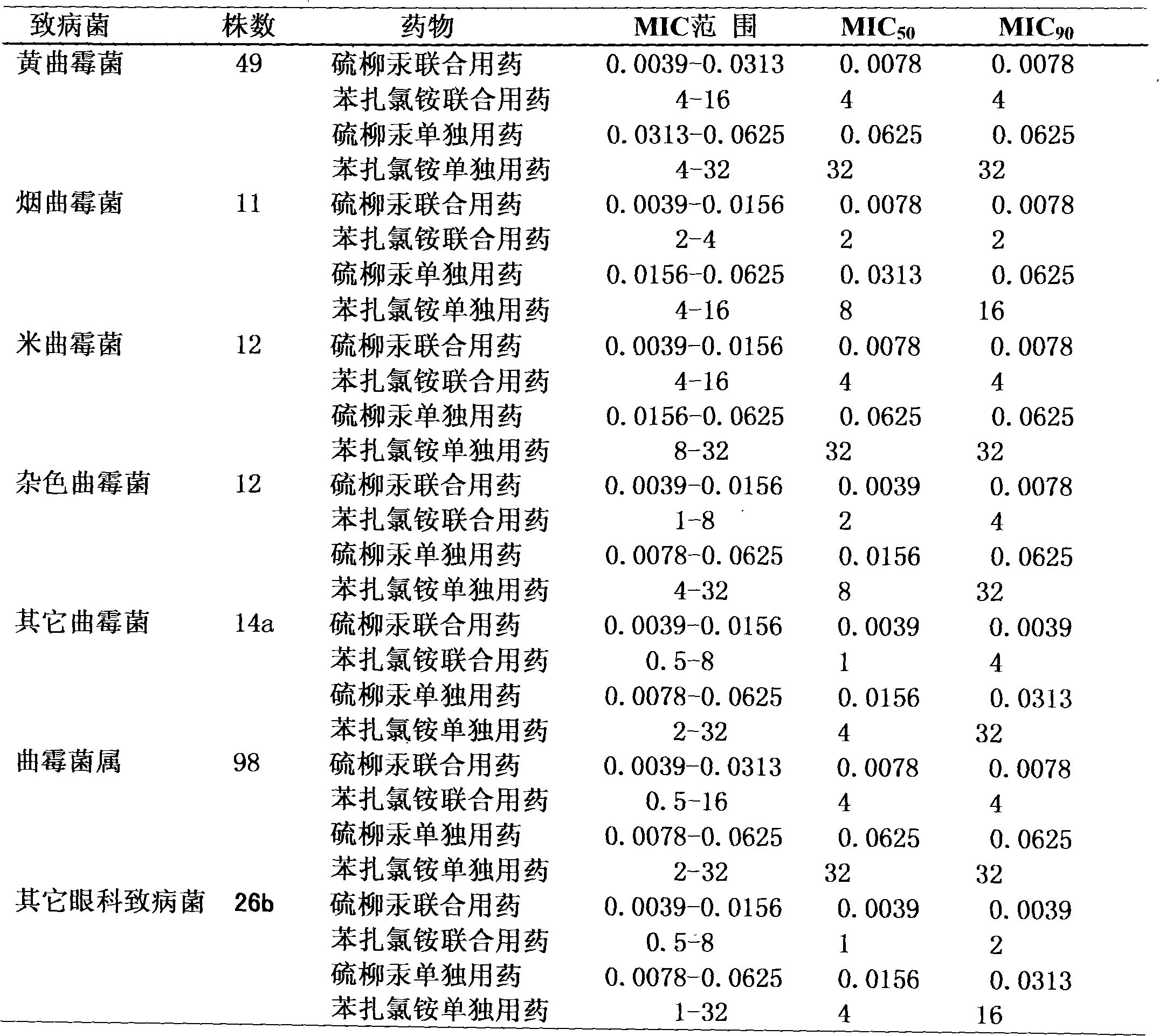
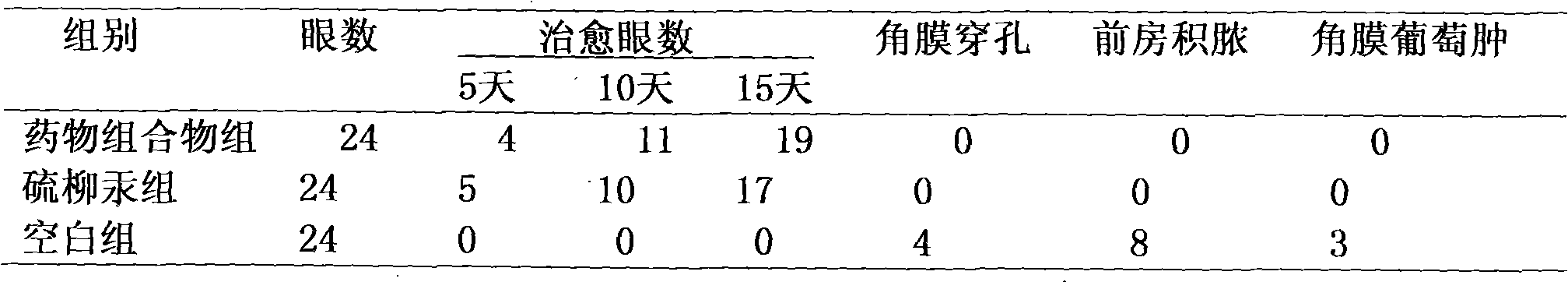
Medicine composition for treating fungal keratitis
InactiveCN102302481AGood treatment effectNo obvious side effectsSenses disorderAntimycoticsAntifungalOphthalmology department
The invention discloses a medicine composition for treating fungal keratitis, which comprises thiomersalate and benzalkonium chloride or benzalkonium bromide. The weight ratio of the thiomersalate to the benzalkonium chloride or the benzalkonium bromide is 1: (1-50). Action mechanisms of the thiomersalate and the benzalkonium chloride or the benzalkonium bromide for inhibiting fungal growth are different, so that during combine medication, different links of fungal growth are inhibited, and thus a synergy effect is achieved; compared with a single medicine, the medicine composition disclosed by the invention has remarkably-enhanced antibacterial effect, remarkably-reduced dosage, better effect on treating the fungal keratitis and no obvious side effect, thereby providing a novel efficient, broad-spectrum and harmfulless antifungal eye medicine for clinically treating the fungal keratitis in the ophthalmology department. Compared with a single medicine, the medicine composition disclosed by the invention has the advantages of enhancing the antibacterial effect in vitro by eight times, remarkably decreasing the dosage in vivo, remarkably reducing the toxic and side effects and preventing the occurrence of drug resistance.
Owner:河南省眼科研究所
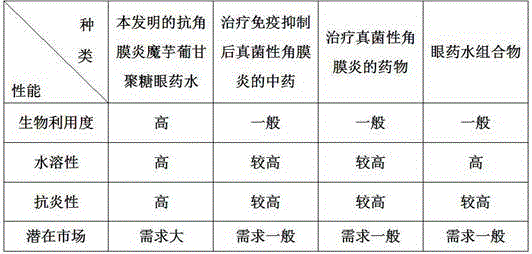
Anti-keratitis konjac glucomannan eye drops and preparation method thereof
InactiveCN106822154AStable performance of drug-loaded microspheresImprove bioavailabilitySenses disorderAntimycoticsParaffin waxSolubility
The invention relates to anti-keratitis konjac glucomannan eye drops and a preparation method thereof. The preparation method of the anti-keratitis konjac glucomannan eye drops comprises the following steps: preparing paraffin wax emulsion, preparing sol, preparing sol emulsion, preparing konjac glucomannan drug-carrying microspheres and preparing the anti-keratitis konjac glucomannan eye drops. According to the anti-keratitis konjac glucomannan eye drops, konjac glucomannan is used as a main raw material and a carrier; the gelling property, water-retaining property, water solubility and anti-keratitis property of the konjac glucomannan are utilized, and voriconazole, ofloxacin and doxycycline are added, so that the anti-keratitis effect is comprehensively expressed; and the anti-keratitis konjac glucomannan eye drops are used for treating fungal keratitis. The konjac glucomannan drug-carrying microspheres have stable performances, and have high bioavailability, good anti-keratitis performance, good water solubility and good anti-inflammatory performance. According to the anti-keratitis konjac glucomannan eye drops, the used materials are simple; and the anti-keratitis konjac glucomannan eye drops have the advantages of convenience for taking the materials, toxin and no harms, no secondary pollution and the like.
Owner:FUJIAN AGRI & FORESTRY UNIV
Eye medicine for treating fungal keratitis
InactiveCN100438864CIncreased sensitivityPracticalOrganic active ingredientsSenses disorderCurative effectTerbinafine Hydrochloride
The invention discloses an eye medicament for treating fungal keratitis, which is the aqueous solution containing 0.1-0.5 wt% of terbinafine hydrochloride and 2.0-4.0 wt% of cyclodextrin.
Owner:王香兰
An Image Recognition Method of Fungal Keratitis Based on Ambp Improved Algorithm
ActiveCN105809188BEliminate distractionsFacilitating the feature extraction stepRecognition of medical/anatomical patternsCorneal nerveMycelium
The invention discloses an image recognition method for fungal keratitis based on the AMBP improved algorithm, which comprises the following steps: using the RX abnormal detection algorithm to preprocess and binarize the mycelium image and the normal corneal nerve image, and then perform expansion and corrosion processing , strengthen the hyphae and neural characteristic information in the image; improve the AMBP algorithm, calculate the mean value of the pixels in the analysis window except the center pixel, and use it as a new parameter; calculate the average value and median value of the pixels in the analysis window respectively pixels, calculate the variance between the two and the rest of the pixels in the analysis window, and use the difference as a new discriminant condition to extract image texture features; use the extracted features to train a classifier, and use this classifier to identify images acquired by confocal microscopes. Identify nerves and hyphae in images.
Owner:SHANDONG UNIV
A kind of chondroitin sulfate modified natamycin eye drops and preparation method thereof
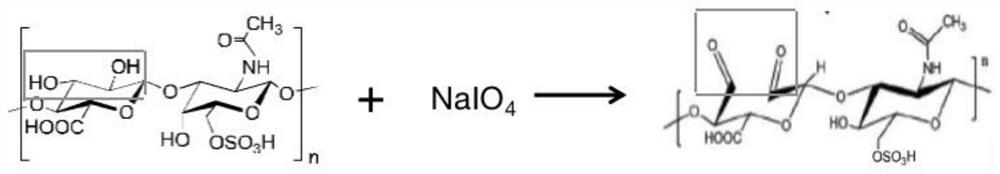
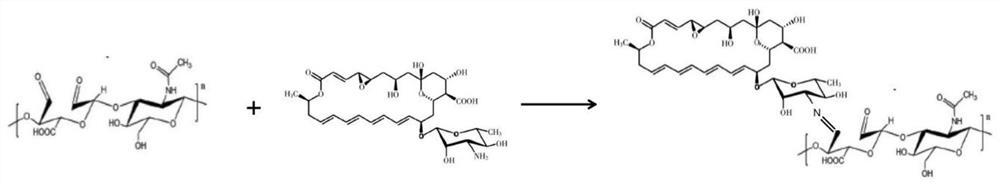
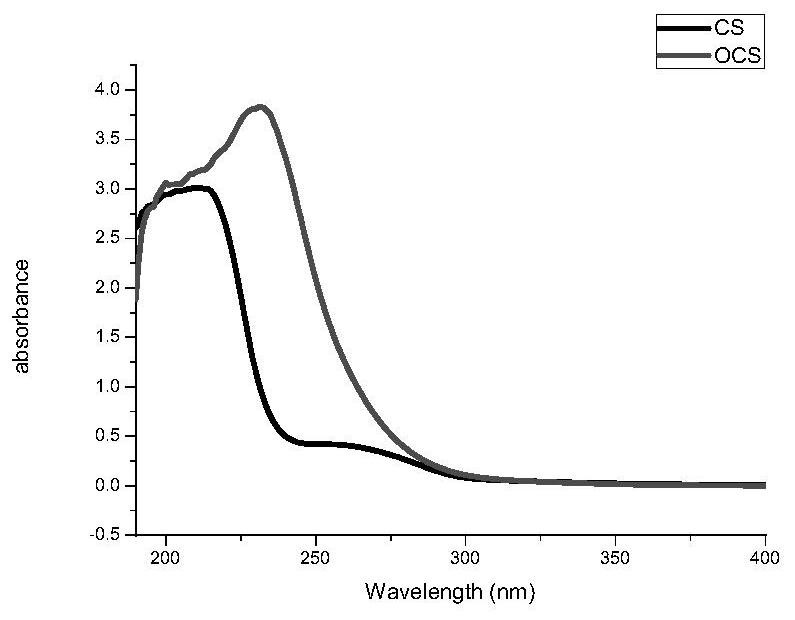
ActiveCN112957321BLow toxicityPromote growthOrganic active ingredientsSenses disorderAntifungalOcular surface
The invention belongs to the technical field of medicine and material chemistry, and relates to a chondroitin sulfate-modified natamycin eye drop and a preparation method thereof. The components of the eye drop and its content are: , the content of the added chondroitin oxysulfate-natamycin freeze-dried powder is 10-80 mg, and the preparation method steps include: preparing chondroitin oxysulfate-natamycin granules, preparing chondroitin oxysulfate-natamycin drug granules, preparing eye drops Three process steps, the prepared chondroitin sulfate-natamycin is applied in ophthalmology to prepare eye drops for the treatment of fungal keratitis, the eye drops have strong water solubility, strong corneal penetration, and toxicity Small, anti-fungal, and anti-inflammatory, it directly acts on the ocular surface without releasing and degrading, and has quick effects, promotes the proliferation and migration of corneal epithelial cells, and promotes corneal epithelial repair. The preparation process is simple, easy to operate, and widely used.
Owner:SHENYANG XINGQI PHARM CO LTD
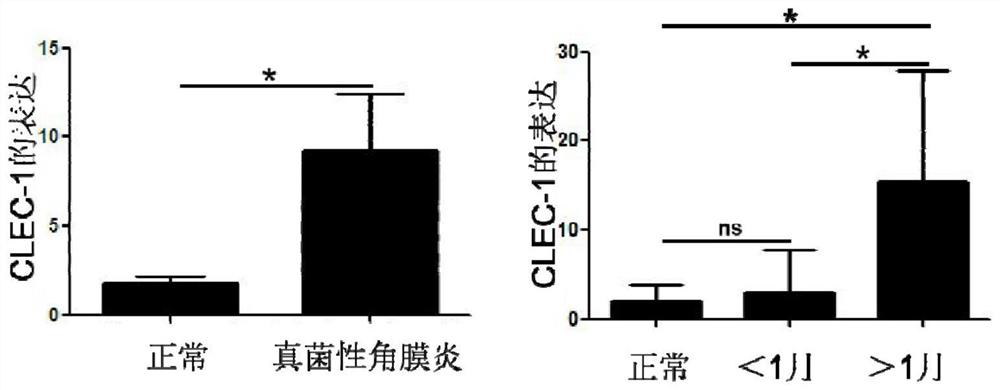
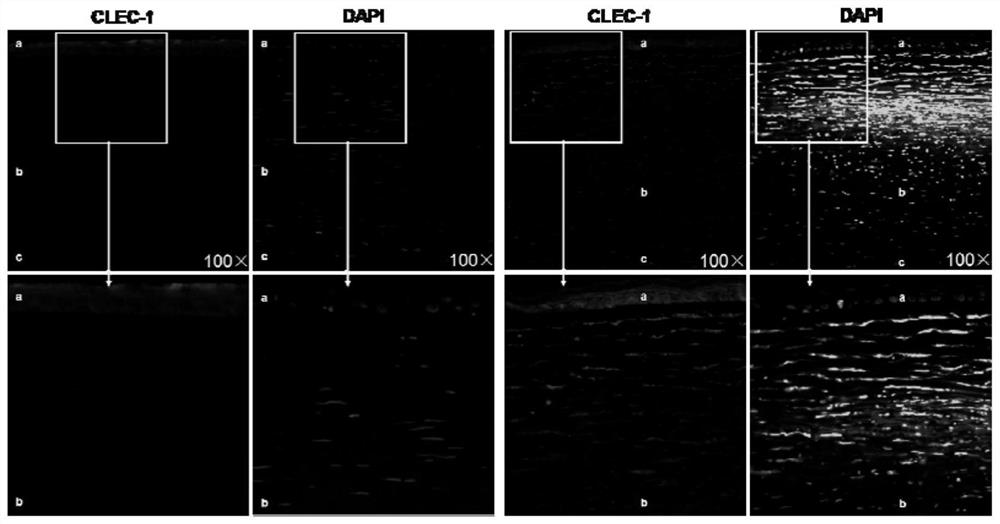
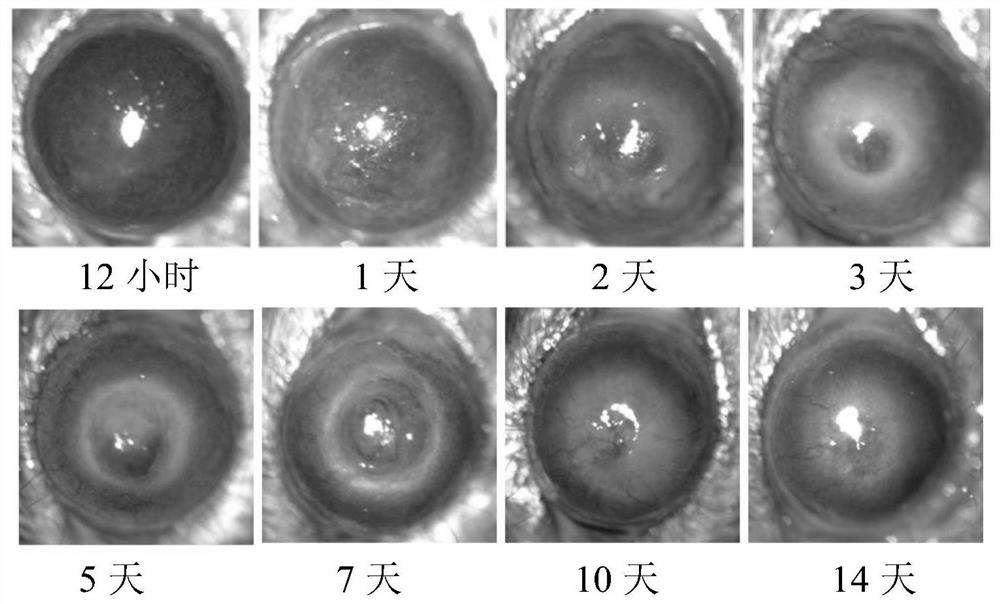
Application of C-type lectin-like receptor-1 as therapeutic marker of fungal keratitis
The invention discloses an application of a C-type lectin-like receptor-1 as a therapeutic marker in fungal keratitis, and provides a kit for evaluating the medication opportunity of glucocorticoid drugs in the process of treating fungal keratitis. The C-type lectin-like receptor-1 can be used as a biomarker for simply, conveniently and safely judging the use opportunity of glucocorticoid drugs during fungal keratitis, and has important significance in the aspect of guiding clinical decision.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Peptides inhibiting adhesion of aspergillus fumigatus to cornea
Owner:SHANDONG EYE INST
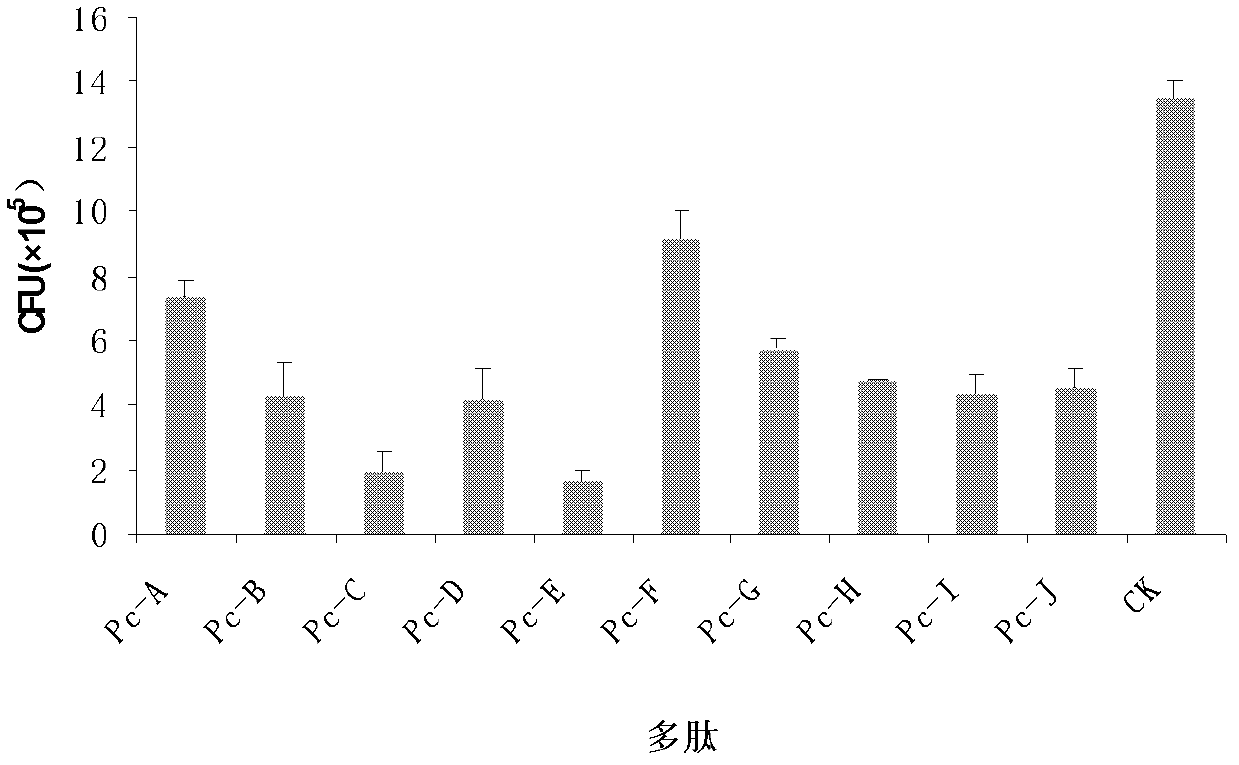
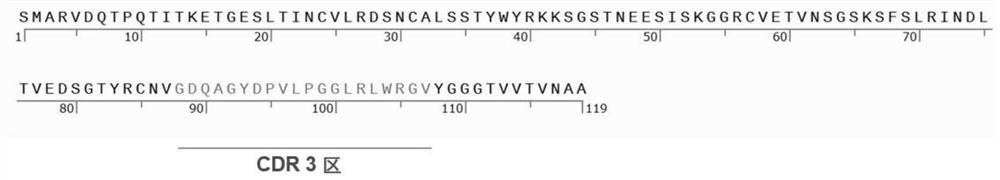
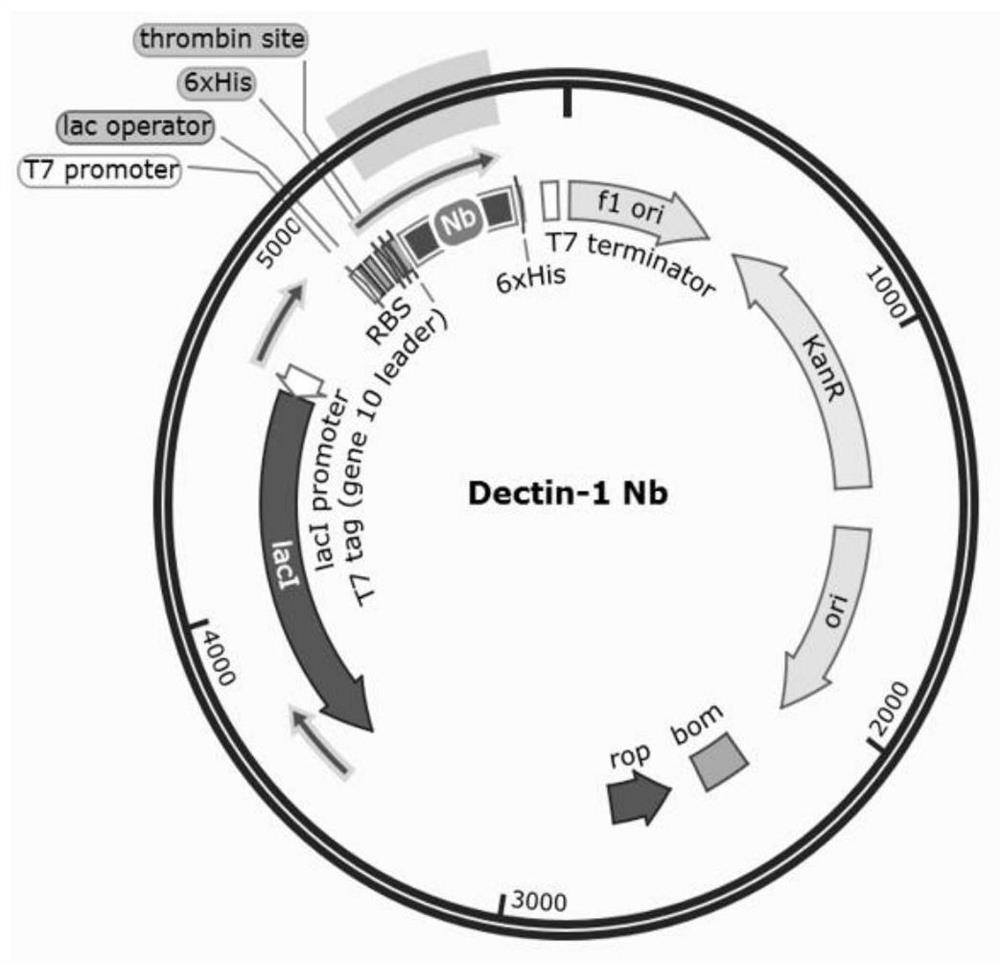
A kind of specific dectin-1 nanobody and its preparation method and application
ActiveCN113354735BImprove bioavailabilityLow toxicitySenses disorderAntimycoticsCorneal toxicityMicrobiology
The invention belongs to the technical field of biology and immunology, and relates to a specific Dectin-1 nanobody and its preparation method and application. The preparation method of the specific Dectin-1 nanobody comprises three process steps: the preparation of the nanobody phage text library Recovery, panning and identification of specific nanobody phage, construction of recombinant pET‑28a plasmid expression vector, induction of expression and purification of specific Dectin‑1 nanobody; prepared specific Dectin‑1 nanobody against Dectin‑1 recombination Protein, HCECs and RAW264.7 cells are specific; Dectin‑1 nanobody has simple production process, low cost, high yield, easy storage, and simple structure; Dectin‑1 nanobody is applied to the treatment of fungal keratitis and is bioavailable High penetration, good specificity, low corneal toxicity, good anti-inflammatory effect.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com