Preparation of honokiol ophthalmic medicine and application of honokiol ophthalmic medicine in treatment of fungal keratitis
A technology of fungal keratitis and honokiol, which is applied in the field of drug preparation, can solve the problems of aggravated corneal tissue damage, low bioavailability, and delayed healing, so as to alleviate keratitis reactions, and the preparation method is easy to control and reduce The effect of intraocular pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
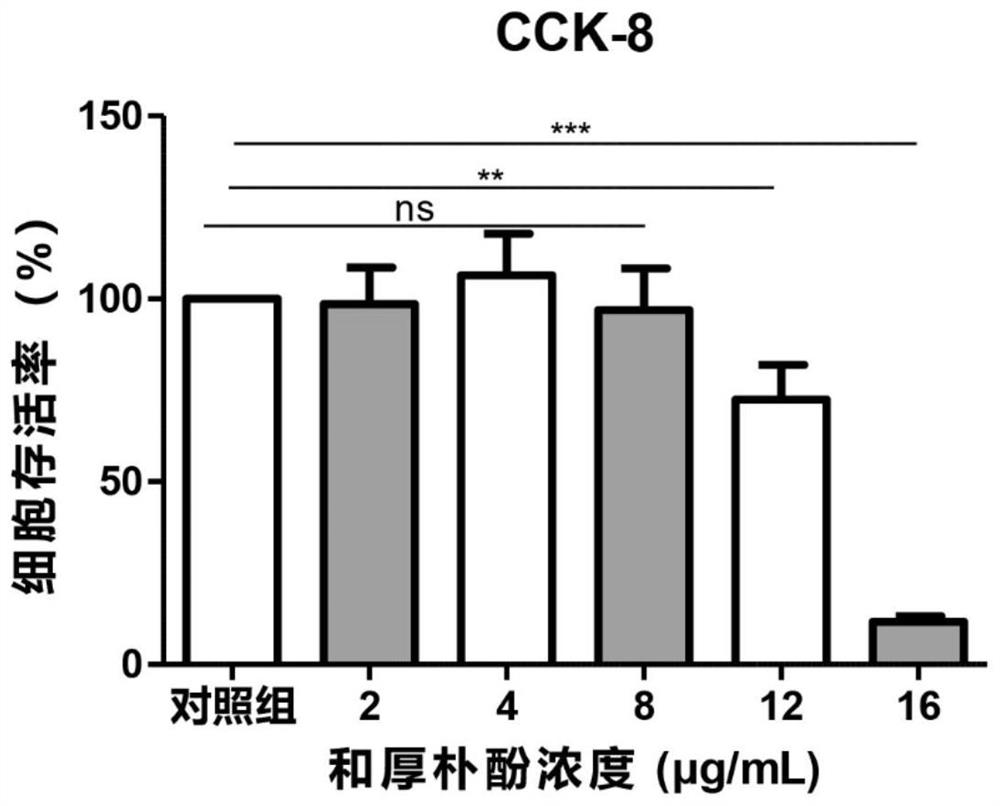
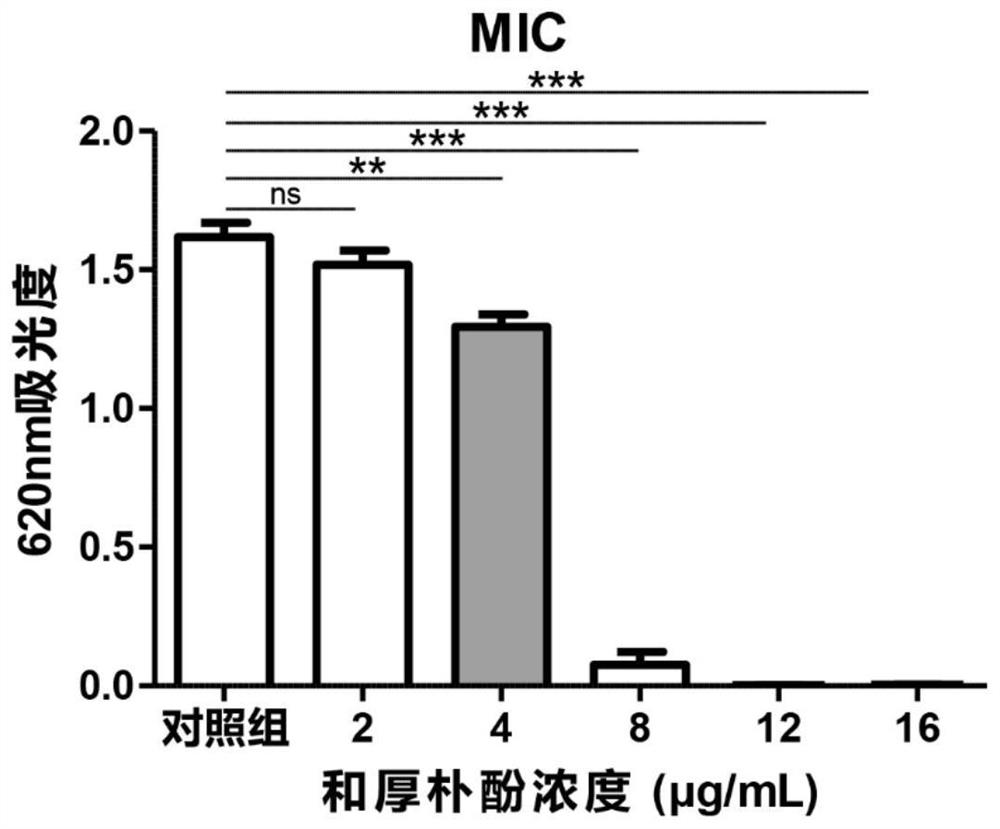
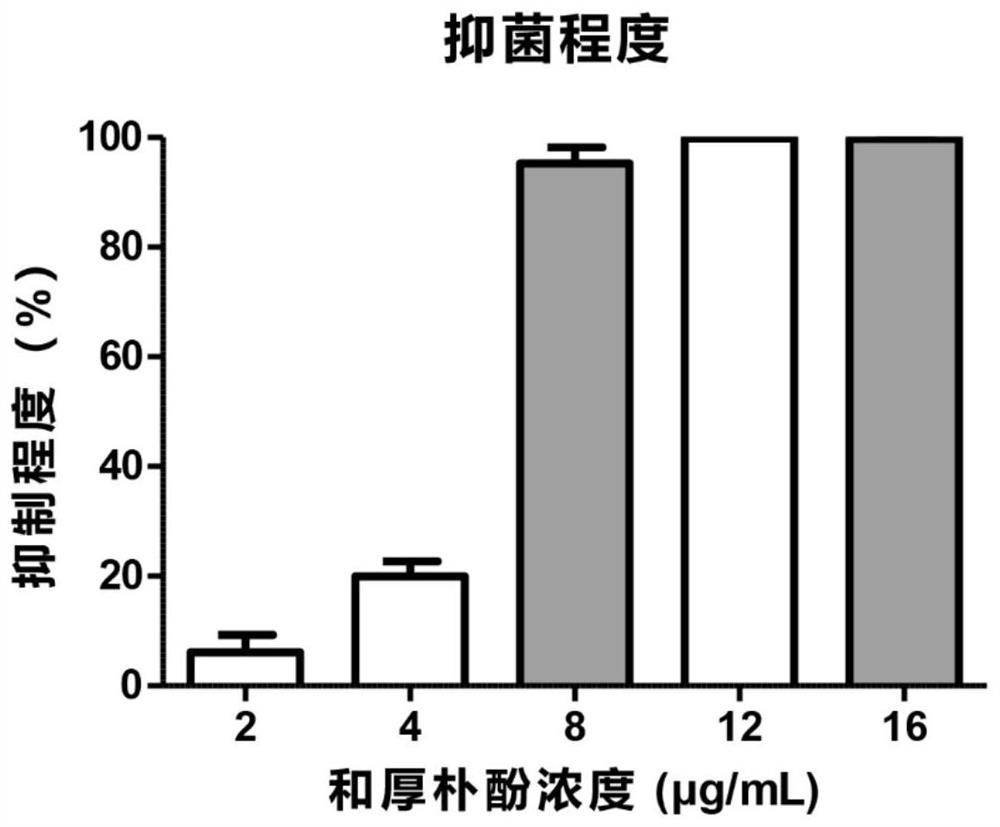
[0036] The embodiment of the present invention provides Honokiol eye drops, specifically: potassium L-aspartate 130 μg / mL, vitamin B6 of 6.5 μg / mL, naphazoline hydrochloride 0.39 μg / mL, methyl sulfate Neostigmine 0.65μg / mL, L-menthol 0.16μg / mL, and honokiol 8μg / mL, the base component is a sterile injection containing 5-10% hydroxyethyl cellulose, eye drops Instill directly into the lower eyelid, 3-6 times a day, and the dosage for each administration should be greater than or equal to the volume of the conjunctival sac. The volume of the human conjunctival sac is usually 20 μL. When the dosage is greater than 20 μL, excess eye drops will overflow. A lot of waste will be caused. The Honokiol ophthalmic medicine of the present invention uses Honokiol as an active ingredient, as a fungal keratitis treatment drug, can effectively kill and kill the pathogenic fungi of fungal keratitis, and at the same time pass the naphthyl hydrochloride hydrochloride. Zolin is a vasoconstrictor to...
Embodiment 2
[0038] The difference between this embodiment and Example 1 is that a kind of Honokiol eye ointment includes Honokiol and excipient, more specifically:
[0039] S1: Put five parts of excipients into a tank water bath and heat to 30-50°C, until the excipients are completely melted, and keep the melting temperature;
[0040] S2: Slowly drop a portion of 40 mg / L Honokiol chloroform solution into the melted excipient, and continue to stir until the dropping is completed, until the solution is completely mixed with the excipient;
[0041] S3: After the mixing is completed, add the mixed medicine into a vacuum chamber with a stirring device, and at the same time as vacuuming, carry out continuous stirring to completely evaporate the chloroform.
[0042] The excipient is 99 parts of the matrix after heating and mixing 60-70% yellow petrolatum and 30-40% lanolin, adding 1 part of antioxidant vitamin E under the condition of 5-70 ℃, fully stirring and mixing to form, about approx. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com