Medical material for treating fungal keratitis and preparation method thereof
A technology for fungal keratitis and enzyme-producing lysobacteria, which is applied in the field of medical materials, can solve the problems of general patient compliance and satisfaction, frequent eye drops, and low bioavailability, achieving good application prospects and Scientific value, simple equipment, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The present embodiment provides a kind of medical material for the treatment of fungal keratitis, comprising the following steps:
[0044] S1: Preparation of Xanthomonas fermentation broth
[0045](1) Xanthomonas was inoculated in LB solid medium for activation, cultured at 28°C for 24-48 hours, and continuously transferred to obtain strains with good activity. In this step, Xanthomonas include Lysobacter zymogenes and Stenotrophomonas maltophilia, wherein Lysobacter zymogenes: strain number is ATCC29487 or Lysobacter zymogenes subspecies BNCC223409, BNCC203967, BNCC341391, LeYC36; Stenotrophomonas: the strain number is ATCC13637, ATCC17806, ATCC17808 or ATCC13270.
[0046] Preparation of LB medium: 10 g of peptone, 5 g of yeast extract, and 10 g of NaCl were successively dissolved in 1 L of distilled water, and the pH was 7.0-7.5. Raising the concentration of sodium chloride to 2% in the medium can increase the expression of dihydromaltophilin.
[0047] (2) Wash the...
Embodiment 2
[0064] By characterizing the physical and chemical properties of the medical composite material in Example 1, the results are as follows:
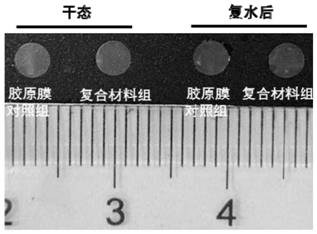
[0065] Depend on Figure 4 It can be seen that through the mechanical characterization of the biomechanical performance testing machine, the composite material has good operability and high tensile strength. The method for testing the tensile strength is: in a dry state, the sample is cut into a rectangular shape with a mold, and the width of the test part is 5mm. After the sample is soaked in deionized water for 30 minutes, it is measured by a Model 5567 biomechanical performance testing machine. The tensile strength of the sample, the tensile rate is 10mm / min. Each sample was tested 5 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com